User login
Melasma is a complex, long-lasting, acquired dermatologic pigmentation disorder resulting in grey-brown patches that last for more than 3 months. Sun-exposed areas including the nose, cheeks, forehead, and forearms are most likely to be affected.1 In Southeast Asia, 0.25% to 4% of the population affected by melasma is aged 30 to 40 years.2 In particular, melasma is a concern among pregnant women due to increased levels of melanocyte-stimulating hormones (MSHs) and is impacted by genetics, hormonal influence, and exposure to UV light.3,4 In Pakistan, approximately 46% of women are affected by melasma during pregnancy.2,5 Although few studies have focused on the clinical approaches to melasma in darker skin types, it continues to disproportionately affect the skin of color population.4
The areas of hyperpigmentation seen in melasma exhibit increased deposition of melanin in the epidermis and dermis, but melanocytes are not elevated. However, in areas of hyperpigmentation, the melanocytes are larger and more dendritic and demonstrate an increased level of melanogenesis.6 During pregnancy, especially in the third trimester, elevated levels of estrogen, progesterone, and MSH often are found in association with melasma.7 Tyrosinase (TYR) activity increases and cellular proliferation is reduced after treatment of melanocytes in culture with β-estradiol.8 Sex steroids increase transcription of genes encoding melanogenic enzymes in normal human melanocytes, especially TYR.9 These results are consistent with the notable increases in melanin synthesis and TYR activity reported for normal human melanocytes under similar conditions in culture.10 Because melanocytes contain both cytosolic and nuclear estrogen receptors, melanocytes in patients with melasma may be inherently more sensitive to the stimulatory effects of estrogens and possibly other steroid hormones.11
The current treatment options for melasma have varying levels of success and include topical depigmenting agents such as hydroquinone, tretinoin, azelaic acid, kojic acid, and corticosteroids; dermabrasion; and chemical peels.12-14 Chemical peels with glycolic acid, salicylic acid, lactic acid, trichloroacetic acid, and phenol, as well as laser therapy, are reliable management options.13,14 Traditionally, melasma has been treated with a combination of modalities along with photoprotection and trigger avoidance.12
The efficacy and safety of the available therapies for melasma are still controversial and require further exploration. In recent years, off-label tranexamic acid (TA) has emerged as a potential therapy for melasma. Although the mechanism of action remains unclear, TA may inhibit melanin synthesis by blocking the interaction between melanocytes and keratinocytes.15 Tranexamic acid also may reverse the abnormal dermal changes associated with melasma by inhibiting melanogenesis and angiogenesis.16
Although various therapeutic options exist for melasma, the search for a reliable option in patients with darker skin types continues.13 We sought to evaluate the efficacy of TA solution 5% in reducing the severity of melasma in South Asian patients, thereby improving patient outcomes and maximizing patient satisfaction. Topical TA is inexpensive and readily accessible and does not cause systemic side effects. These qualities make it a promising treatment compared to traditional therapies.
Methods
We conducted a randomized controlled trial at Rawalpindi Medical Institute (Punjab, Pakistan). The researchers obtained informed consent for all enrolled patients. Cases were sampled from the original patient population seen at the office using nonprobability consecutive sampling. The sample size was calculated with a 95% CI, margin of error of 9%, and expected percentage of efficacy of 86.1% by using TA solution 5%. South Asian male and female patients aged 20 to 45 years with melasma were included in the analysis. Patients were excluded if they were already taking TA, oral contraceptive pills, or photosensitizing drugs (eg, nonsteroidal anti-inflammatory drugs, tetracyclines, phenytoin, carbamazepine); were pregnant; had chronic kidney disease (creatinine >2.0 mg/dL); had cardiac abnormalities (abnormal electrocardiogram); had hematologic disorders (international normalized ratio >2); or had received another melasma treatment within the last 3 to 6 months.
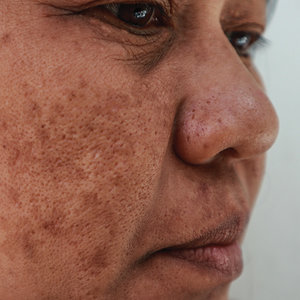
All enrolled patients underwent a detailed history and physical examination. Patient demographics were subsequently noted, including age, sex, history of diabetes mellitus or hypertension, and duration of melasma. The melasma area and severity index (MASI) score of each patient was calculated at baseline, and a corresponding photograph was taken.
The topical solution was prepared with 5 g of TA dissolved in 10 cc of ethanol at 96 °F, 10 cc of 1,3-butanediol, and distilled water up to 100 cc. The TA solution was applied to the affected areas once daily by the patient for 12 weeks. Each application covered the affected areas completely. Patients were instructed to apply sunscreen with sun protection factor 60 to those same areas for UV protection after 15 minutes of TA application. Biweekly follow-ups were scheduled during the trial, and the MASI score was recorded at these visits. If the mean MASI score was reduced by half after 12 weeks of treatment, then the treatment was considered efficacious with a 95% CI.
The percentage reduction from baseline was calculated as follows: percentage reduction=(baseline score– follow-up score)/baseline score×100.
Statistical Analysis—Data were analyzed in SPSS Statistics 25 (IBM). The quantitative variables of age, duration of melasma, and body mass index were presented as mean (SD). Qualitative variables such as sex, history of diabetes mellitus or hypertension, site of melasma, and efficacy were presented as frequencies and percentages. Mean MASI scores at baseline and 12 weeks posttreatment were compared using a paired t test (P≤.05). Data were stratified for age, sex, history of diabetes mellitus or hypertension, site of melasma, and duration of melasma, and a χ2 test was applied to compare efficacy in stratified groups (P≤.05).
Results
Sixty patients were enrolled in the study. Of them, 17 (28.33%) were male, and 43 (71.67%) were female (2:5 ratio). They ranged in age from 20 to 45 years (mean [SD], 31.93 [6.26] years). Thirty-seven patients (61.67%) were aged 31 to 45 years of age (Table 1). The mean (SD) duration of disease was 10.18 (2.10) months. The response to TA was recorded based on patient distribution according to the site of melasma as well as history of diabetes mellitus and hypertension.

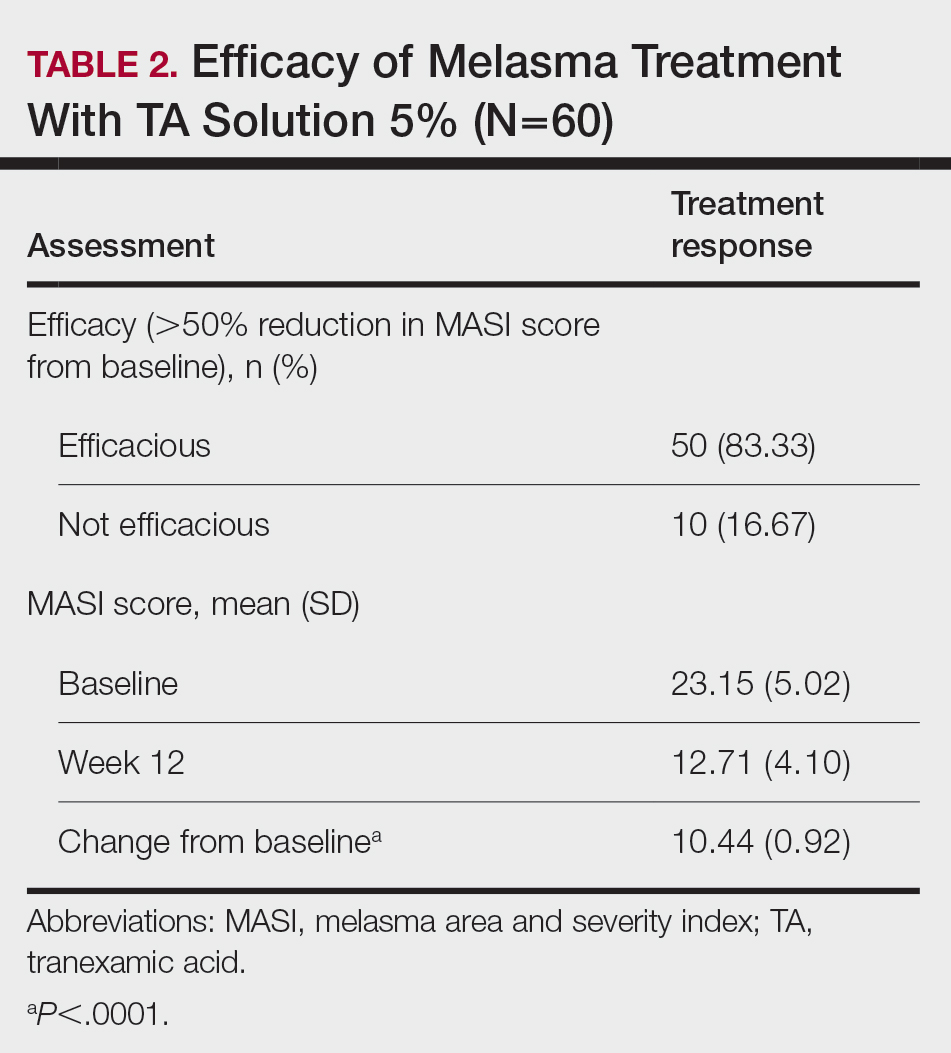
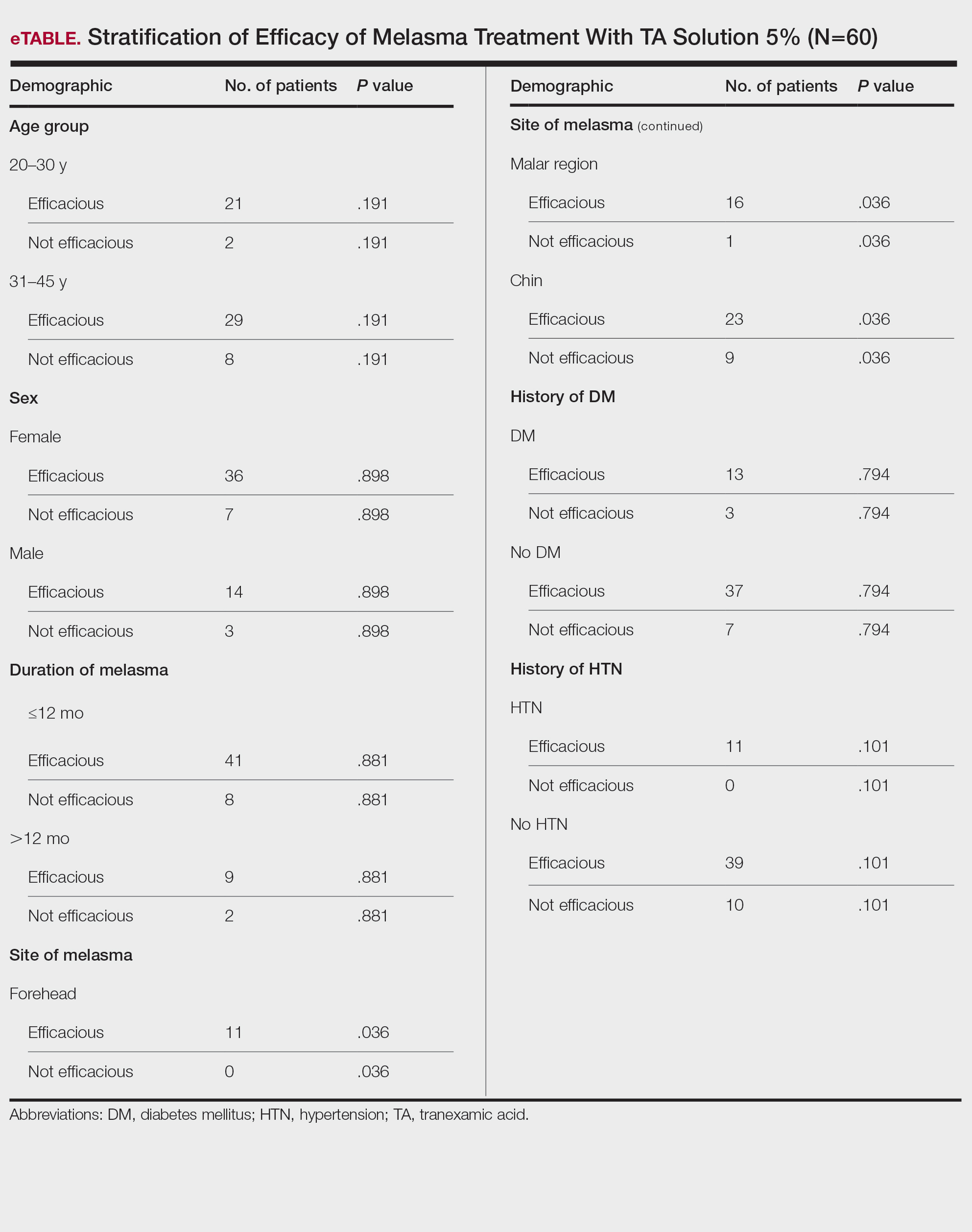
Topical TA was found to be efficacious for melasma in 50 (83.33%) patients. The mean (SD) baseline and week 12 MASI scores were 23.15 (5.02) and 12.71 (4.10)(P<.0001), respectively (Table 2). The stratification of efficacy with respect to age, sex, duration of melasma, site of melasma, and history of diabetes mellitus or hypertension is shown in the eTable. The site of melasma was significant with respect to stratification of efficacy. On the forehead, TA was found to be efficacious in 11 patients and nonefficacious in 0 patients (P=.036). In the malar region, it was efficacious in 16 patients and nonefficacious in 1 patient (P=.036). Finally, on the chin, it was efficacious in 23 patients and nonefficacious in 9 patients (P=.036).
Comment
Melasma Presentation and Development—Melasma is a chronic skin condition that more often affects patients with darker skin types. This condition is characterized by hyperpigmentation of skin that is directly exposed to the sun, such as the cheek, nose, forehead, and above the upper lip.17 Although the mechanism behind how melasma develops is unknown, one theory suggests that UV light can lead to increased plasmin in keratinocytes.18 This increased plasmin will thereby increase the arachidonic acid and α-MSH, leading to the observed uneven hyperpigmentation that is notable in melasma. Melasma is common in patients using oral contraceptives or expired cosmetic drugs; in those who are pregnant; and in those with liver dysfunction.18 Melasma has a negative impact on patients’ quality of life because of substantial psychological and social distress. Thus, finding an accessible treatment is imperative.19
Melasma Management—The most common treatments for melasma have been topical bleaching agents and photoprotection. Combination therapy options include chemical peels, dermabrasion, and laser treatments, though they present with limited efficacy.17,20 Because melasma focuses on pigmentation correction, topical treatments work to disturb melanocyte pigment production at the enzymatic level.21 Tyrosinase is rate limiting in melanin production, as it converts L-tyrosinase to L-3,4-dihydroxyphenylalanine, using copper to interact with L-3,4-dihydroxyphenylalanine as a cofactor in the active site.22 Therefore, tyrosine is a major target for many drugs that have been developed for melasma to decrease melaninization.21
Recently, research has focused on the effects of topical, intradermal, and oral TA for melasma.17 Tranexamic acid most commonly has been used in medicine as a fibrinolytic agent because of its antiplasmin properties. It has been hypothesized that TA can inhibit the release of paracrine melanogenic factors that normally act to stimulate melanocytes.17 Although studies have supported the safety and efficacy of TA, there remains a lack of clinical studies that are sufficiently powered. No definitive consensus on the use of TA for melasma currently exists, which indicates the need for large-scale, randomized, controlled trials.23
One trial (N=25) found that TA solution 5% achieved efficacy (>50% reduction in MASI score from baseline) in 86.1% of patients with melasma.24 In another study (N=18), topical TA 5% achieved efficacy (>50% reduction in MASI score) in 86% of patients with melasma.25
Melasma Comorbidities—To determine if certain comorbidities, such as diabetes mellitus or hypertension, influenced the progression of melasma, we stratified the efficacy results for patients with these 2 comorbidities, which showed no significant difference (P=.794 and P=.101, respectively). Thus, the relatively higher prevalence of diabetes mellitus (16 patients) and hypertension (11 patients) did not contribute to the efficacy of TA in lowering MASI scores over the 12-week period, which supports the findings of Doolan and Gupta,26 who investigated the endocrinologic conditions associated with melasma and found no such association with diabetes mellitus or hypertension.
TA Formulations for Melasma—The efficacy of topical TA has been explored in several studies. Six studies with sample sizes of 13 to 50 patients each showed statistically significant differences in MASI scores between baseline and following TA treatment (P<.001).27-32 Several formulations and regimens were utilized, including TA cream 3% for 12 weeks, TA gel 5% for 12 weeks, TA solution 3% for 12 weeks, TA liposome 5% for 12 weeks, and TA solution 2% for 12 weeks.18 Additionally, these studies found TA to be effective in limiting dyschromia and decreasing MASI scores. There were no statistically significant differences between formulations and method of application. Topical TA has been found to be just as effective as other treatments for melasma, including intradermal TA injections, topical hydroquinone, and a combination of topical hydroquinone and dexamethasone.18
Further study of the efficacy of intradermal TA is necessary because many human trials have lacked statistical significance or a control group. Lee et al32 conducted a trial of 100 female patients who received weekly intradermal TA microinjections for 12 weeks. After 8 and 12 weeks, MASI scores decreased significantly (P<.01).32 Similarly, Badran et al33 observed 60 female patients in 3 trial groups: group A received TA (4 mg/mL) intradermal injections every 2 weeks, group B received TA (10 mg/mL) intradermal injections every 2 weeks, and group C received TA cream 10% twice daily. Although all groups showed improvement in MASI, group B, which had the highest intradermal TA concentration, exhibited the most improvement. Thus, it was determined that intradermal application led to better results, but the cream was still effective.33
Saki et al34 conducted a randomized, split-face trial of 37 patients comparing the efficacy of intradermal TA and topical hydroquinone. Each group was treated with either monthly intradermal TA injections or nightly hydroquinone for 3 months. After 4 weeks of treatment, TA initially had a greater improvement. However, after 20 weeks, the overall changes were not significant between the 2 groups.34 Pazyar et al35 conducted a randomized, split-face trial of 49 patients comparing the efficacy of intradermal TA and hydroquinone cream. After 24 weeks of biweekly TA injections or twice-daily hydroquinone, there were no statistically significant differences in the decreased MASI scores between treatments.35 Additional large, double-blind, controlled trials are needed to thoroughly assess the role of intradermal TA in comparison to its treatment counterpart of hydroquinone.
Ebrahimi and Naeini29 conducted a 12-week, double-blind, split-phase trial of 50 Iranian melasma patients, which showed that 27.3% of patients rated the improvement in melasma as excellent, 42.4% as good, and 30.3% as fair after using TA solution 3%. Wu et al36 also showed a total melasma improvement rate of 80.9% in 256 patients with long-term oral use of TA. In a study by Kim et al31 (N=245), the mean MASI score considerably decreased after topical TA use, with a total response rate of 95.6%. In another study, Atefi et al37 presented significantly increased levels of satisfaction in patients treated with topical TA 5% vs hydroquinone (P=.015).
Melasma in Patients With Darker Skin Types—Special attention must be given to choosing the appropriate medication in melasma patients with darker skin types, as there is an increased risk for postinflammatory hyperpigmentation. Currently, few randomized controlled trials exist that fulfill the criteria of evaluating pharmacologic options for patients with melasma, and even fewer studies solely focus on patients with darker skin types.38 In addition to treatment advances, patients must be educated on the need to avoid sun exposure when possible or to use photoprotection, especially in the South Asian region, where these practices rarely are taught. Our study provided a unique analysis regarding the efficacy of TA solution 5% for the treatment of melasma in patients of South Asian descent. Clinicians can use these findings as a foundation for treating all patients with melasma but particularly those with darker skin types.
Study Limitations—Our study consisted of 60 patients; although our study had more patients than similar trials, larger studies are needed. Additionally, other variables were excluded from our analysis, such as comorbidities beyond diabetes mellitus and hypertension.
Conclusion
This study contributes to the growing field of melasma therapeutics by evaluating the efficacy of using TA solution 5% for the treatment of melasma in South Asian patients with darker skin types. Clinicians may use our study to broaden their treatment options for a common condition while also addressing the lack of clinical options for patients with darker skin types. Further studies investigating the effectiveness of TA in large clinical trials in humans are warranted to understand the efficacy and the risk for any complications.
- Espósito ACC, Brianezi G, De Souza NP, et al. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32:101-108.
- Janney MS, Subramaniyan R, Dabas R, et al. A randomized controlled study comparing the efficacy of topical 5% tranexamic acid solution versus 3% hydroquinone cream in melasma. J Cutan Aesthet Surg. 2019;12:63-67.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasmaJ Cosmet Laser Ther. 2018;20:134-139.
- Grimes PE, Ijaz S, Nashawati R, et al. New oral and topical approaches for the treatment of melasma. Int J Womens Dermatol. 2019;5:30-36.
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Barankin B, Silver SG, Carruthers A. The skin in pregnancy. J Cutan Med Surg. 2002;6:236-240.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Smith AG, Shuster S, Thody AJ, et al. Chloasma, oral contraceptives, and plasma immunoreactive beta-melanocyte-stimulating hormone. J Invest Dermatol. 1977;68:169-170.
- Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593-598.
- Kippenberger S, Loitsch S, Solano F, et al. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR—regulation by steroid hormones. J Invest Dermatol. 1998;110:364-367.
- McLeod SD, Ranson M, Mason RS. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49:9-14.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasma. J Cosmet Laser Ther. 2018;20:134-139.
- Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis. 2018;101:E7-E8.
- Tian B. The Asian problem of frequent laser toning for melasma. J Clin Aesthet Dermatol. 2017;10:40-42.
- Zhang L, Tan WQ, Fang QQ, et al. Tranexamic acid for adults with melasma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1683414.
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911.
- Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma. J Cosmet Dermatol. 2019;18:1495-1501.
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30:19-26.
- Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Kim YJ, Kim MJ, Kweon DK, et al. Quantification of hypopigmentation activity in vitro. J Vis Exp. 2019;145:20-25.
- Cardoso R, Valente R, Souza da Costa CH, et al. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: a molecular modeling approach. Molecules. 2021;26:2875.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Khuraiya S, Kachhawa D, Chouhan B, et al. A comparative study of topical 5% tranexamic acid and triple combination therapy for the treatment of melasma in Indian population. Pigment International. 2019;6:18-23.
- Steiner D, Feola C, Bialeski N, et al. Study evaluating the efficacy of topical and injected tranexamic acid in treatment of melasma. Surg Cosmet Dermatol. 2009;1:174-177.
- Doolan B, Gupta M. Melasma. Aust J Gen Pract. 2021;50:880-885.
- Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol. 2015;14:174-177.
- Chung JY, Lee JH, Lee JH. Topical tranexamic acid as an adjuvant treatment in melasma: side-by-side comparison clinical study. J Dermatolog Treat. 2016;27:373-377.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, et al. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150-154.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32:626-631.
- Badran AY, Ali AU, Gomaa AS. Efficacy of topical versus intradermal injection of tranexamic acid in Egyptian melasma patients: a randomised clinical trial. Australas J Dermatol. 2021;62:E373-E379.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clin Cosmet Investig Dermatol. 2019;12:115-122.
- Wu S, Shi H, Wu H, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964-970.
- Atefi N, Dalvand B, Ghassemi M, et al. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of women with melasma. Dermatol Ther (Heidelb). 2017;7:417-424.
- Cestari T, Arellano I, Hexsel D, et al. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:760-772.
Melasma is a complex, long-lasting, acquired dermatologic pigmentation disorder resulting in grey-brown patches that last for more than 3 months. Sun-exposed areas including the nose, cheeks, forehead, and forearms are most likely to be affected.1 In Southeast Asia, 0.25% to 4% of the population affected by melasma is aged 30 to 40 years.2 In particular, melasma is a concern among pregnant women due to increased levels of melanocyte-stimulating hormones (MSHs) and is impacted by genetics, hormonal influence, and exposure to UV light.3,4 In Pakistan, approximately 46% of women are affected by melasma during pregnancy.2,5 Although few studies have focused on the clinical approaches to melasma in darker skin types, it continues to disproportionately affect the skin of color population.4
The areas of hyperpigmentation seen in melasma exhibit increased deposition of melanin in the epidermis and dermis, but melanocytes are not elevated. However, in areas of hyperpigmentation, the melanocytes are larger and more dendritic and demonstrate an increased level of melanogenesis.6 During pregnancy, especially in the third trimester, elevated levels of estrogen, progesterone, and MSH often are found in association with melasma.7 Tyrosinase (TYR) activity increases and cellular proliferation is reduced after treatment of melanocytes in culture with β-estradiol.8 Sex steroids increase transcription of genes encoding melanogenic enzymes in normal human melanocytes, especially TYR.9 These results are consistent with the notable increases in melanin synthesis and TYR activity reported for normal human melanocytes under similar conditions in culture.10 Because melanocytes contain both cytosolic and nuclear estrogen receptors, melanocytes in patients with melasma may be inherently more sensitive to the stimulatory effects of estrogens and possibly other steroid hormones.11
The current treatment options for melasma have varying levels of success and include topical depigmenting agents such as hydroquinone, tretinoin, azelaic acid, kojic acid, and corticosteroids; dermabrasion; and chemical peels.12-14 Chemical peels with glycolic acid, salicylic acid, lactic acid, trichloroacetic acid, and phenol, as well as laser therapy, are reliable management options.13,14 Traditionally, melasma has been treated with a combination of modalities along with photoprotection and trigger avoidance.12
The efficacy and safety of the available therapies for melasma are still controversial and require further exploration. In recent years, off-label tranexamic acid (TA) has emerged as a potential therapy for melasma. Although the mechanism of action remains unclear, TA may inhibit melanin synthesis by blocking the interaction between melanocytes and keratinocytes.15 Tranexamic acid also may reverse the abnormal dermal changes associated with melasma by inhibiting melanogenesis and angiogenesis.16
Although various therapeutic options exist for melasma, the search for a reliable option in patients with darker skin types continues.13 We sought to evaluate the efficacy of TA solution 5% in reducing the severity of melasma in South Asian patients, thereby improving patient outcomes and maximizing patient satisfaction. Topical TA is inexpensive and readily accessible and does not cause systemic side effects. These qualities make it a promising treatment compared to traditional therapies.
Methods
We conducted a randomized controlled trial at Rawalpindi Medical Institute (Punjab, Pakistan). The researchers obtained informed consent for all enrolled patients. Cases were sampled from the original patient population seen at the office using nonprobability consecutive sampling. The sample size was calculated with a 95% CI, margin of error of 9%, and expected percentage of efficacy of 86.1% by using TA solution 5%. South Asian male and female patients aged 20 to 45 years with melasma were included in the analysis. Patients were excluded if they were already taking TA, oral contraceptive pills, or photosensitizing drugs (eg, nonsteroidal anti-inflammatory drugs, tetracyclines, phenytoin, carbamazepine); were pregnant; had chronic kidney disease (creatinine >2.0 mg/dL); had cardiac abnormalities (abnormal electrocardiogram); had hematologic disorders (international normalized ratio >2); or had received another melasma treatment within the last 3 to 6 months.
All enrolled patients underwent a detailed history and physical examination. Patient demographics were subsequently noted, including age, sex, history of diabetes mellitus or hypertension, and duration of melasma. The melasma area and severity index (MASI) score of each patient was calculated at baseline, and a corresponding photograph was taken.
The topical solution was prepared with 5 g of TA dissolved in 10 cc of ethanol at 96 °F, 10 cc of 1,3-butanediol, and distilled water up to 100 cc. The TA solution was applied to the affected areas once daily by the patient for 12 weeks. Each application covered the affected areas completely. Patients were instructed to apply sunscreen with sun protection factor 60 to those same areas for UV protection after 15 minutes of TA application. Biweekly follow-ups were scheduled during the trial, and the MASI score was recorded at these visits. If the mean MASI score was reduced by half after 12 weeks of treatment, then the treatment was considered efficacious with a 95% CI.
The percentage reduction from baseline was calculated as follows: percentage reduction=(baseline score– follow-up score)/baseline score×100.
Statistical Analysis—Data were analyzed in SPSS Statistics 25 (IBM). The quantitative variables of age, duration of melasma, and body mass index were presented as mean (SD). Qualitative variables such as sex, history of diabetes mellitus or hypertension, site of melasma, and efficacy were presented as frequencies and percentages. Mean MASI scores at baseline and 12 weeks posttreatment were compared using a paired t test (P≤.05). Data were stratified for age, sex, history of diabetes mellitus or hypertension, site of melasma, and duration of melasma, and a χ2 test was applied to compare efficacy in stratified groups (P≤.05).
Results
Sixty patients were enrolled in the study. Of them, 17 (28.33%) were male, and 43 (71.67%) were female (2:5 ratio). They ranged in age from 20 to 45 years (mean [SD], 31.93 [6.26] years). Thirty-seven patients (61.67%) were aged 31 to 45 years of age (Table 1). The mean (SD) duration of disease was 10.18 (2.10) months. The response to TA was recorded based on patient distribution according to the site of melasma as well as history of diabetes mellitus and hypertension.

Topical TA was found to be efficacious for melasma in 50 (83.33%) patients. The mean (SD) baseline and week 12 MASI scores were 23.15 (5.02) and 12.71 (4.10)(P<.0001), respectively (Table 2). The stratification of efficacy with respect to age, sex, duration of melasma, site of melasma, and history of diabetes mellitus or hypertension is shown in the eTable. The site of melasma was significant with respect to stratification of efficacy. On the forehead, TA was found to be efficacious in 11 patients and nonefficacious in 0 patients (P=.036). In the malar region, it was efficacious in 16 patients and nonefficacious in 1 patient (P=.036). Finally, on the chin, it was efficacious in 23 patients and nonefficacious in 9 patients (P=.036).

Comment
Melasma Presentation and Development—Melasma is a chronic skin condition that more often affects patients with darker skin types. This condition is characterized by hyperpigmentation of skin that is directly exposed to the sun, such as the cheek, nose, forehead, and above the upper lip.17 Although the mechanism behind how melasma develops is unknown, one theory suggests that UV light can lead to increased plasmin in keratinocytes.18 This increased plasmin will thereby increase the arachidonic acid and α-MSH, leading to the observed uneven hyperpigmentation that is notable in melasma. Melasma is common in patients using oral contraceptives or expired cosmetic drugs; in those who are pregnant; and in those with liver dysfunction.18 Melasma has a negative impact on patients’ quality of life because of substantial psychological and social distress. Thus, finding an accessible treatment is imperative.19

Melasma Management—The most common treatments for melasma have been topical bleaching agents and photoprotection. Combination therapy options include chemical peels, dermabrasion, and laser treatments, though they present with limited efficacy.17,20 Because melasma focuses on pigmentation correction, topical treatments work to disturb melanocyte pigment production at the enzymatic level.21 Tyrosinase is rate limiting in melanin production, as it converts L-tyrosinase to L-3,4-dihydroxyphenylalanine, using copper to interact with L-3,4-dihydroxyphenylalanine as a cofactor in the active site.22 Therefore, tyrosine is a major target for many drugs that have been developed for melasma to decrease melaninization.21
Recently, research has focused on the effects of topical, intradermal, and oral TA for melasma.17 Tranexamic acid most commonly has been used in medicine as a fibrinolytic agent because of its antiplasmin properties. It has been hypothesized that TA can inhibit the release of paracrine melanogenic factors that normally act to stimulate melanocytes.17 Although studies have supported the safety and efficacy of TA, there remains a lack of clinical studies that are sufficiently powered. No definitive consensus on the use of TA for melasma currently exists, which indicates the need for large-scale, randomized, controlled trials.23
One trial (N=25) found that TA solution 5% achieved efficacy (>50% reduction in MASI score from baseline) in 86.1% of patients with melasma.24 In another study (N=18), topical TA 5% achieved efficacy (>50% reduction in MASI score) in 86% of patients with melasma.25
Melasma Comorbidities—To determine if certain comorbidities, such as diabetes mellitus or hypertension, influenced the progression of melasma, we stratified the efficacy results for patients with these 2 comorbidities, which showed no significant difference (P=.794 and P=.101, respectively). Thus, the relatively higher prevalence of diabetes mellitus (16 patients) and hypertension (11 patients) did not contribute to the efficacy of TA in lowering MASI scores over the 12-week period, which supports the findings of Doolan and Gupta,26 who investigated the endocrinologic conditions associated with melasma and found no such association with diabetes mellitus or hypertension.
TA Formulations for Melasma—The efficacy of topical TA has been explored in several studies. Six studies with sample sizes of 13 to 50 patients each showed statistically significant differences in MASI scores between baseline and following TA treatment (P<.001).27-32 Several formulations and regimens were utilized, including TA cream 3% for 12 weeks, TA gel 5% for 12 weeks, TA solution 3% for 12 weeks, TA liposome 5% for 12 weeks, and TA solution 2% for 12 weeks.18 Additionally, these studies found TA to be effective in limiting dyschromia and decreasing MASI scores. There were no statistically significant differences between formulations and method of application. Topical TA has been found to be just as effective as other treatments for melasma, including intradermal TA injections, topical hydroquinone, and a combination of topical hydroquinone and dexamethasone.18
Further study of the efficacy of intradermal TA is necessary because many human trials have lacked statistical significance or a control group. Lee et al32 conducted a trial of 100 female patients who received weekly intradermal TA microinjections for 12 weeks. After 8 and 12 weeks, MASI scores decreased significantly (P<.01).32 Similarly, Badran et al33 observed 60 female patients in 3 trial groups: group A received TA (4 mg/mL) intradermal injections every 2 weeks, group B received TA (10 mg/mL) intradermal injections every 2 weeks, and group C received TA cream 10% twice daily. Although all groups showed improvement in MASI, group B, which had the highest intradermal TA concentration, exhibited the most improvement. Thus, it was determined that intradermal application led to better results, but the cream was still effective.33
Saki et al34 conducted a randomized, split-face trial of 37 patients comparing the efficacy of intradermal TA and topical hydroquinone. Each group was treated with either monthly intradermal TA injections or nightly hydroquinone for 3 months. After 4 weeks of treatment, TA initially had a greater improvement. However, after 20 weeks, the overall changes were not significant between the 2 groups.34 Pazyar et al35 conducted a randomized, split-face trial of 49 patients comparing the efficacy of intradermal TA and hydroquinone cream. After 24 weeks of biweekly TA injections or twice-daily hydroquinone, there were no statistically significant differences in the decreased MASI scores between treatments.35 Additional large, double-blind, controlled trials are needed to thoroughly assess the role of intradermal TA in comparison to its treatment counterpart of hydroquinone.
Ebrahimi and Naeini29 conducted a 12-week, double-blind, split-phase trial of 50 Iranian melasma patients, which showed that 27.3% of patients rated the improvement in melasma as excellent, 42.4% as good, and 30.3% as fair after using TA solution 3%. Wu et al36 also showed a total melasma improvement rate of 80.9% in 256 patients with long-term oral use of TA. In a study by Kim et al31 (N=245), the mean MASI score considerably decreased after topical TA use, with a total response rate of 95.6%. In another study, Atefi et al37 presented significantly increased levels of satisfaction in patients treated with topical TA 5% vs hydroquinone (P=.015).
Melasma in Patients With Darker Skin Types—Special attention must be given to choosing the appropriate medication in melasma patients with darker skin types, as there is an increased risk for postinflammatory hyperpigmentation. Currently, few randomized controlled trials exist that fulfill the criteria of evaluating pharmacologic options for patients with melasma, and even fewer studies solely focus on patients with darker skin types.38 In addition to treatment advances, patients must be educated on the need to avoid sun exposure when possible or to use photoprotection, especially in the South Asian region, where these practices rarely are taught. Our study provided a unique analysis regarding the efficacy of TA solution 5% for the treatment of melasma in patients of South Asian descent. Clinicians can use these findings as a foundation for treating all patients with melasma but particularly those with darker skin types.
Study Limitations—Our study consisted of 60 patients; although our study had more patients than similar trials, larger studies are needed. Additionally, other variables were excluded from our analysis, such as comorbidities beyond diabetes mellitus and hypertension.
Conclusion
This study contributes to the growing field of melasma therapeutics by evaluating the efficacy of using TA solution 5% for the treatment of melasma in South Asian patients with darker skin types. Clinicians may use our study to broaden their treatment options for a common condition while also addressing the lack of clinical options for patients with darker skin types. Further studies investigating the effectiveness of TA in large clinical trials in humans are warranted to understand the efficacy and the risk for any complications.
Melasma is a complex, long-lasting, acquired dermatologic pigmentation disorder resulting in grey-brown patches that last for more than 3 months. Sun-exposed areas including the nose, cheeks, forehead, and forearms are most likely to be affected.1 In Southeast Asia, 0.25% to 4% of the population affected by melasma is aged 30 to 40 years.2 In particular, melasma is a concern among pregnant women due to increased levels of melanocyte-stimulating hormones (MSHs) and is impacted by genetics, hormonal influence, and exposure to UV light.3,4 In Pakistan, approximately 46% of women are affected by melasma during pregnancy.2,5 Although few studies have focused on the clinical approaches to melasma in darker skin types, it continues to disproportionately affect the skin of color population.4
The areas of hyperpigmentation seen in melasma exhibit increased deposition of melanin in the epidermis and dermis, but melanocytes are not elevated. However, in areas of hyperpigmentation, the melanocytes are larger and more dendritic and demonstrate an increased level of melanogenesis.6 During pregnancy, especially in the third trimester, elevated levels of estrogen, progesterone, and MSH often are found in association with melasma.7 Tyrosinase (TYR) activity increases and cellular proliferation is reduced after treatment of melanocytes in culture with β-estradiol.8 Sex steroids increase transcription of genes encoding melanogenic enzymes in normal human melanocytes, especially TYR.9 These results are consistent with the notable increases in melanin synthesis and TYR activity reported for normal human melanocytes under similar conditions in culture.10 Because melanocytes contain both cytosolic and nuclear estrogen receptors, melanocytes in patients with melasma may be inherently more sensitive to the stimulatory effects of estrogens and possibly other steroid hormones.11
The current treatment options for melasma have varying levels of success and include topical depigmenting agents such as hydroquinone, tretinoin, azelaic acid, kojic acid, and corticosteroids; dermabrasion; and chemical peels.12-14 Chemical peels with glycolic acid, salicylic acid, lactic acid, trichloroacetic acid, and phenol, as well as laser therapy, are reliable management options.13,14 Traditionally, melasma has been treated with a combination of modalities along with photoprotection and trigger avoidance.12
The efficacy and safety of the available therapies for melasma are still controversial and require further exploration. In recent years, off-label tranexamic acid (TA) has emerged as a potential therapy for melasma. Although the mechanism of action remains unclear, TA may inhibit melanin synthesis by blocking the interaction between melanocytes and keratinocytes.15 Tranexamic acid also may reverse the abnormal dermal changes associated with melasma by inhibiting melanogenesis and angiogenesis.16
Although various therapeutic options exist for melasma, the search for a reliable option in patients with darker skin types continues.13 We sought to evaluate the efficacy of TA solution 5% in reducing the severity of melasma in South Asian patients, thereby improving patient outcomes and maximizing patient satisfaction. Topical TA is inexpensive and readily accessible and does not cause systemic side effects. These qualities make it a promising treatment compared to traditional therapies.
Methods
We conducted a randomized controlled trial at Rawalpindi Medical Institute (Punjab, Pakistan). The researchers obtained informed consent for all enrolled patients. Cases were sampled from the original patient population seen at the office using nonprobability consecutive sampling. The sample size was calculated with a 95% CI, margin of error of 9%, and expected percentage of efficacy of 86.1% by using TA solution 5%. South Asian male and female patients aged 20 to 45 years with melasma were included in the analysis. Patients were excluded if they were already taking TA, oral contraceptive pills, or photosensitizing drugs (eg, nonsteroidal anti-inflammatory drugs, tetracyclines, phenytoin, carbamazepine); were pregnant; had chronic kidney disease (creatinine >2.0 mg/dL); had cardiac abnormalities (abnormal electrocardiogram); had hematologic disorders (international normalized ratio >2); or had received another melasma treatment within the last 3 to 6 months.
All enrolled patients underwent a detailed history and physical examination. Patient demographics were subsequently noted, including age, sex, history of diabetes mellitus or hypertension, and duration of melasma. The melasma area and severity index (MASI) score of each patient was calculated at baseline, and a corresponding photograph was taken.
The topical solution was prepared with 5 g of TA dissolved in 10 cc of ethanol at 96 °F, 10 cc of 1,3-butanediol, and distilled water up to 100 cc. The TA solution was applied to the affected areas once daily by the patient for 12 weeks. Each application covered the affected areas completely. Patients were instructed to apply sunscreen with sun protection factor 60 to those same areas for UV protection after 15 minutes of TA application. Biweekly follow-ups were scheduled during the trial, and the MASI score was recorded at these visits. If the mean MASI score was reduced by half after 12 weeks of treatment, then the treatment was considered efficacious with a 95% CI.
The percentage reduction from baseline was calculated as follows: percentage reduction=(baseline score– follow-up score)/baseline score×100.
Statistical Analysis—Data were analyzed in SPSS Statistics 25 (IBM). The quantitative variables of age, duration of melasma, and body mass index were presented as mean (SD). Qualitative variables such as sex, history of diabetes mellitus or hypertension, site of melasma, and efficacy were presented as frequencies and percentages. Mean MASI scores at baseline and 12 weeks posttreatment were compared using a paired t test (P≤.05). Data were stratified for age, sex, history of diabetes mellitus or hypertension, site of melasma, and duration of melasma, and a χ2 test was applied to compare efficacy in stratified groups (P≤.05).
Results
Sixty patients were enrolled in the study. Of them, 17 (28.33%) were male, and 43 (71.67%) were female (2:5 ratio). They ranged in age from 20 to 45 years (mean [SD], 31.93 [6.26] years). Thirty-seven patients (61.67%) were aged 31 to 45 years of age (Table 1). The mean (SD) duration of disease was 10.18 (2.10) months. The response to TA was recorded based on patient distribution according to the site of melasma as well as history of diabetes mellitus and hypertension.

Topical TA was found to be efficacious for melasma in 50 (83.33%) patients. The mean (SD) baseline and week 12 MASI scores were 23.15 (5.02) and 12.71 (4.10)(P<.0001), respectively (Table 2). The stratification of efficacy with respect to age, sex, duration of melasma, site of melasma, and history of diabetes mellitus or hypertension is shown in the eTable. The site of melasma was significant with respect to stratification of efficacy. On the forehead, TA was found to be efficacious in 11 patients and nonefficacious in 0 patients (P=.036). In the malar region, it was efficacious in 16 patients and nonefficacious in 1 patient (P=.036). Finally, on the chin, it was efficacious in 23 patients and nonefficacious in 9 patients (P=.036).

Comment
Melasma Presentation and Development—Melasma is a chronic skin condition that more often affects patients with darker skin types. This condition is characterized by hyperpigmentation of skin that is directly exposed to the sun, such as the cheek, nose, forehead, and above the upper lip.17 Although the mechanism behind how melasma develops is unknown, one theory suggests that UV light can lead to increased plasmin in keratinocytes.18 This increased plasmin will thereby increase the arachidonic acid and α-MSH, leading to the observed uneven hyperpigmentation that is notable in melasma. Melasma is common in patients using oral contraceptives or expired cosmetic drugs; in those who are pregnant; and in those with liver dysfunction.18 Melasma has a negative impact on patients’ quality of life because of substantial psychological and social distress. Thus, finding an accessible treatment is imperative.19

Melasma Management—The most common treatments for melasma have been topical bleaching agents and photoprotection. Combination therapy options include chemical peels, dermabrasion, and laser treatments, though they present with limited efficacy.17,20 Because melasma focuses on pigmentation correction, topical treatments work to disturb melanocyte pigment production at the enzymatic level.21 Tyrosinase is rate limiting in melanin production, as it converts L-tyrosinase to L-3,4-dihydroxyphenylalanine, using copper to interact with L-3,4-dihydroxyphenylalanine as a cofactor in the active site.22 Therefore, tyrosine is a major target for many drugs that have been developed for melasma to decrease melaninization.21
Recently, research has focused on the effects of topical, intradermal, and oral TA for melasma.17 Tranexamic acid most commonly has been used in medicine as a fibrinolytic agent because of its antiplasmin properties. It has been hypothesized that TA can inhibit the release of paracrine melanogenic factors that normally act to stimulate melanocytes.17 Although studies have supported the safety and efficacy of TA, there remains a lack of clinical studies that are sufficiently powered. No definitive consensus on the use of TA for melasma currently exists, which indicates the need for large-scale, randomized, controlled trials.23
One trial (N=25) found that TA solution 5% achieved efficacy (>50% reduction in MASI score from baseline) in 86.1% of patients with melasma.24 In another study (N=18), topical TA 5% achieved efficacy (>50% reduction in MASI score) in 86% of patients with melasma.25
Melasma Comorbidities—To determine if certain comorbidities, such as diabetes mellitus or hypertension, influenced the progression of melasma, we stratified the efficacy results for patients with these 2 comorbidities, which showed no significant difference (P=.794 and P=.101, respectively). Thus, the relatively higher prevalence of diabetes mellitus (16 patients) and hypertension (11 patients) did not contribute to the efficacy of TA in lowering MASI scores over the 12-week period, which supports the findings of Doolan and Gupta,26 who investigated the endocrinologic conditions associated with melasma and found no such association with diabetes mellitus or hypertension.
TA Formulations for Melasma—The efficacy of topical TA has been explored in several studies. Six studies with sample sizes of 13 to 50 patients each showed statistically significant differences in MASI scores between baseline and following TA treatment (P<.001).27-32 Several formulations and regimens were utilized, including TA cream 3% for 12 weeks, TA gel 5% for 12 weeks, TA solution 3% for 12 weeks, TA liposome 5% for 12 weeks, and TA solution 2% for 12 weeks.18 Additionally, these studies found TA to be effective in limiting dyschromia and decreasing MASI scores. There were no statistically significant differences between formulations and method of application. Topical TA has been found to be just as effective as other treatments for melasma, including intradermal TA injections, topical hydroquinone, and a combination of topical hydroquinone and dexamethasone.18
Further study of the efficacy of intradermal TA is necessary because many human trials have lacked statistical significance or a control group. Lee et al32 conducted a trial of 100 female patients who received weekly intradermal TA microinjections for 12 weeks. After 8 and 12 weeks, MASI scores decreased significantly (P<.01).32 Similarly, Badran et al33 observed 60 female patients in 3 trial groups: group A received TA (4 mg/mL) intradermal injections every 2 weeks, group B received TA (10 mg/mL) intradermal injections every 2 weeks, and group C received TA cream 10% twice daily. Although all groups showed improvement in MASI, group B, which had the highest intradermal TA concentration, exhibited the most improvement. Thus, it was determined that intradermal application led to better results, but the cream was still effective.33
Saki et al34 conducted a randomized, split-face trial of 37 patients comparing the efficacy of intradermal TA and topical hydroquinone. Each group was treated with either monthly intradermal TA injections or nightly hydroquinone for 3 months. After 4 weeks of treatment, TA initially had a greater improvement. However, after 20 weeks, the overall changes were not significant between the 2 groups.34 Pazyar et al35 conducted a randomized, split-face trial of 49 patients comparing the efficacy of intradermal TA and hydroquinone cream. After 24 weeks of biweekly TA injections or twice-daily hydroquinone, there were no statistically significant differences in the decreased MASI scores between treatments.35 Additional large, double-blind, controlled trials are needed to thoroughly assess the role of intradermal TA in comparison to its treatment counterpart of hydroquinone.
Ebrahimi and Naeini29 conducted a 12-week, double-blind, split-phase trial of 50 Iranian melasma patients, which showed that 27.3% of patients rated the improvement in melasma as excellent, 42.4% as good, and 30.3% as fair after using TA solution 3%. Wu et al36 also showed a total melasma improvement rate of 80.9% in 256 patients with long-term oral use of TA. In a study by Kim et al31 (N=245), the mean MASI score considerably decreased after topical TA use, with a total response rate of 95.6%. In another study, Atefi et al37 presented significantly increased levels of satisfaction in patients treated with topical TA 5% vs hydroquinone (P=.015).
Melasma in Patients With Darker Skin Types—Special attention must be given to choosing the appropriate medication in melasma patients with darker skin types, as there is an increased risk for postinflammatory hyperpigmentation. Currently, few randomized controlled trials exist that fulfill the criteria of evaluating pharmacologic options for patients with melasma, and even fewer studies solely focus on patients with darker skin types.38 In addition to treatment advances, patients must be educated on the need to avoid sun exposure when possible or to use photoprotection, especially in the South Asian region, where these practices rarely are taught. Our study provided a unique analysis regarding the efficacy of TA solution 5% for the treatment of melasma in patients of South Asian descent. Clinicians can use these findings as a foundation for treating all patients with melasma but particularly those with darker skin types.
Study Limitations—Our study consisted of 60 patients; although our study had more patients than similar trials, larger studies are needed. Additionally, other variables were excluded from our analysis, such as comorbidities beyond diabetes mellitus and hypertension.
Conclusion
This study contributes to the growing field of melasma therapeutics by evaluating the efficacy of using TA solution 5% for the treatment of melasma in South Asian patients with darker skin types. Clinicians may use our study to broaden their treatment options for a common condition while also addressing the lack of clinical options for patients with darker skin types. Further studies investigating the effectiveness of TA in large clinical trials in humans are warranted to understand the efficacy and the risk for any complications.
- Espósito ACC, Brianezi G, De Souza NP, et al. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32:101-108.
- Janney MS, Subramaniyan R, Dabas R, et al. A randomized controlled study comparing the efficacy of topical 5% tranexamic acid solution versus 3% hydroquinone cream in melasma. J Cutan Aesthet Surg. 2019;12:63-67.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasmaJ Cosmet Laser Ther. 2018;20:134-139.
- Grimes PE, Ijaz S, Nashawati R, et al. New oral and topical approaches for the treatment of melasma. Int J Womens Dermatol. 2019;5:30-36.
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Barankin B, Silver SG, Carruthers A. The skin in pregnancy. J Cutan Med Surg. 2002;6:236-240.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Smith AG, Shuster S, Thody AJ, et al. Chloasma, oral contraceptives, and plasma immunoreactive beta-melanocyte-stimulating hormone. J Invest Dermatol. 1977;68:169-170.
- Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593-598.
- Kippenberger S, Loitsch S, Solano F, et al. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR—regulation by steroid hormones. J Invest Dermatol. 1998;110:364-367.
- McLeod SD, Ranson M, Mason RS. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49:9-14.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasma. J Cosmet Laser Ther. 2018;20:134-139.
- Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis. 2018;101:E7-E8.
- Tian B. The Asian problem of frequent laser toning for melasma. J Clin Aesthet Dermatol. 2017;10:40-42.
- Zhang L, Tan WQ, Fang QQ, et al. Tranexamic acid for adults with melasma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1683414.
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911.
- Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma. J Cosmet Dermatol. 2019;18:1495-1501.
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30:19-26.
- Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Kim YJ, Kim MJ, Kweon DK, et al. Quantification of hypopigmentation activity in vitro. J Vis Exp. 2019;145:20-25.
- Cardoso R, Valente R, Souza da Costa CH, et al. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: a molecular modeling approach. Molecules. 2021;26:2875.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Khuraiya S, Kachhawa D, Chouhan B, et al. A comparative study of topical 5% tranexamic acid and triple combination therapy for the treatment of melasma in Indian population. Pigment International. 2019;6:18-23.
- Steiner D, Feola C, Bialeski N, et al. Study evaluating the efficacy of topical and injected tranexamic acid in treatment of melasma. Surg Cosmet Dermatol. 2009;1:174-177.
- Doolan B, Gupta M. Melasma. Aust J Gen Pract. 2021;50:880-885.
- Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol. 2015;14:174-177.
- Chung JY, Lee JH, Lee JH. Topical tranexamic acid as an adjuvant treatment in melasma: side-by-side comparison clinical study. J Dermatolog Treat. 2016;27:373-377.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, et al. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150-154.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32:626-631.
- Badran AY, Ali AU, Gomaa AS. Efficacy of topical versus intradermal injection of tranexamic acid in Egyptian melasma patients: a randomised clinical trial. Australas J Dermatol. 2021;62:E373-E379.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clin Cosmet Investig Dermatol. 2019;12:115-122.
- Wu S, Shi H, Wu H, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964-970.
- Atefi N, Dalvand B, Ghassemi M, et al. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of women with melasma. Dermatol Ther (Heidelb). 2017;7:417-424.
- Cestari T, Arellano I, Hexsel D, et al. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:760-772.
- Espósito ACC, Brianezi G, De Souza NP, et al. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32:101-108.
- Janney MS, Subramaniyan R, Dabas R, et al. A randomized controlled study comparing the efficacy of topical 5% tranexamic acid solution versus 3% hydroquinone cream in melasma. J Cutan Aesthet Surg. 2019;12:63-67.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasmaJ Cosmet Laser Ther. 2018;20:134-139.
- Grimes PE, Ijaz S, Nashawati R, et al. New oral and topical approaches for the treatment of melasma. Int J Womens Dermatol. 2019;5:30-36.
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Barankin B, Silver SG, Carruthers A. The skin in pregnancy. J Cutan Med Surg. 2002;6:236-240.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Smith AG, Shuster S, Thody AJ, et al. Chloasma, oral contraceptives, and plasma immunoreactive beta-melanocyte-stimulating hormone. J Invest Dermatol. 1977;68:169-170.
- Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593-598.
- Kippenberger S, Loitsch S, Solano F, et al. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR—regulation by steroid hormones. J Invest Dermatol. 1998;110:364-367.
- McLeod SD, Ranson M, Mason RS. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49:9-14.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasma. J Cosmet Laser Ther. 2018;20:134-139.
- Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis. 2018;101:E7-E8.
- Tian B. The Asian problem of frequent laser toning for melasma. J Clin Aesthet Dermatol. 2017;10:40-42.
- Zhang L, Tan WQ, Fang QQ, et al. Tranexamic acid for adults with melasma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1683414.
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911.
- Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma. J Cosmet Dermatol. 2019;18:1495-1501.
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30:19-26.
- Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Kim YJ, Kim MJ, Kweon DK, et al. Quantification of hypopigmentation activity in vitro. J Vis Exp. 2019;145:20-25.
- Cardoso R, Valente R, Souza da Costa CH, et al. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: a molecular modeling approach. Molecules. 2021;26:2875.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Khuraiya S, Kachhawa D, Chouhan B, et al. A comparative study of topical 5% tranexamic acid and triple combination therapy for the treatment of melasma in Indian population. Pigment International. 2019;6:18-23.
- Steiner D, Feola C, Bialeski N, et al. Study evaluating the efficacy of topical and injected tranexamic acid in treatment of melasma. Surg Cosmet Dermatol. 2009;1:174-177.
- Doolan B, Gupta M. Melasma. Aust J Gen Pract. 2021;50:880-885.
- Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol. 2015;14:174-177.
- Chung JY, Lee JH, Lee JH. Topical tranexamic acid as an adjuvant treatment in melasma: side-by-side comparison clinical study. J Dermatolog Treat. 2016;27:373-377.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, et al. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150-154.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32:626-631.
- Badran AY, Ali AU, Gomaa AS. Efficacy of topical versus intradermal injection of tranexamic acid in Egyptian melasma patients: a randomised clinical trial. Australas J Dermatol. 2021;62:E373-E379.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clin Cosmet Investig Dermatol. 2019;12:115-122.
- Wu S, Shi H, Wu H, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964-970.
- Atefi N, Dalvand B, Ghassemi M, et al. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of women with melasma. Dermatol Ther (Heidelb). 2017;7:417-424.
- Cestari T, Arellano I, Hexsel D, et al. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:760-772.
PRATICE POINTS
- Tranexamic acid (TA) solution 5% is an efficacious treatment for skin of color patients with melasma.
- Topical TA is a treatment alternative for patients who may not be able to tolerate oral TA.
- Our study revealed the greatest efficacy for TA solution 5% was seen on the forehead and malar region, with less efficacy on the chin.