User login
The benefits of integrating hysteroscopy into office practice are compelling. Most importantly, patients appreciate the comfort and convenience of having hysteroscopic procedures done in a familiar setting. Patients can generally be in and out of the office in less than 30 minutes for a diagnostic procedure, and in less than 1-2 hours for an operative procedure.
Not only is an in-office approach patient centered and clinically valuable, but it is more efficient and economically favorable for the gynecologic surgeon. Physicians earn higher reimbursement for diagnostic hysteroscopies, as well as many therapeutic and operative hysteroscopies, when these procedures are done in the office rather than when they’re performed in a hospital or an outpatient center.
Transitioning to in-office hysteroscopy need not be daunting: The setup is relatively simple and does not require an operating suite, just a dedicated exam room. And the need for premedication and local anesthesia can be low, particularly when a vaginoscopic approach to hysteroscopy is employed. For most gynecologic surgeons, the necessary skills and comfort levels fall into place after only a few vaginoscopic procedures.
A vaginoscopic approach avoids the use of a vaginal speculum or cervical tenaculum, significantly decreasing discomfort or pain. Not using these instruments is the only difference between this and traditional hysteroscopy. It is a less invasive approach that is much more tolerable for patients. And for the surgeon, it can be easier and quicker and provides equally good visualization without any impairment in cervical passage.
Described in the literature as far back as the 1950s, vaginoscopy has its roots in the pediatric/adolescent population, where it was used for the removal of foreign bodies and evaluation of the vagina and external cervical os.
More recently, Stefano Bettocchi, MD, and Luigi Selvaggi, MD, in Italy were the first to describe a vaginoscopic approach to hysteroscopy for evaluating the endocervical canal and uterine cavity.
In a series of papers from 1997 to 2004, Dr. Bettocchi and Dr. Selvaggi documented their efforts to improve patient tolerance during diagnostic hysteroscopies. When they used both the speculum and tenaculum in 163 patients, with local anesthesia, 8% reported severe pain, 11% reported moderate pain, and 69% reported mild pain. Only 12% reported no discomfort. With speculum use only, and no anesthesia, in 308 patients, none reported severe pain, 2% reported moderate pain, 32% reported mild pain, and 66% reported no discomfort. When neither instrument was used (again, no anesthesia), patient discomfort was nearly eliminated: In 680 procedures, patients had a 96% no-discomfort rate (J Am Assoc Gynecol Laparosc. 1997 Feb;4[2]:255-8; Curr Opin Obstet Gynecol. 2003 Aug;15[4]:303-8; Obstet Gynecol Clin North Am. 2004 Sep;31[3]:641-54, xi).
Since then, research has affirmed the differences in patient tolerance and has shown that there is no significant difference between traditional and vaginoscopic hysteroscopy in the rate of procedure failure (0%-10%).
In my practice, in addition to vaginal or cervical examination and evaluation of the uterine cavity, I utilize a vaginoscopic approach to perform minor therapeutic and operative procedures such as biopsies, polypectomies, tubal occlusion using the Essure system, and removal of lost intrauterine devices. I can assess infertility, trauma, abnormal uterine bleeding, and mesh erosion, and provide pre- and postsurgical evaluations. In all of these cases, I use minimal premedication and only rarely need any local anesthetic and/or sedation.
Instrumentation and technique
There are a variety of hysteroscopes available on the market, from single-channel flexible diagnostic hysteroscopes that are 3 mm to 4 mm in diameter, to “see-and-treat” operative hysteroscopes that are rigid and have various diameters and camera lens angles.
A hysteroscope with a 5.5-mm outer diameter works well for a vaginoscopic approach that avoids cervical dilation. Accessory instrumentation includes semirigid 5 Fr 35-cm–long biopsy forceps, scissors, and alligator forceps.
In timing the procedure, our main goal is a thin uterine lining. This can be achieved by scheduling the procedure during the early proliferative phase of the menstrual cycle or by using a gonadotropin-releasing hormone agonist or a transdermal or transvaginal contraceptive medication.
By far the most important element of pain control and analgesia is the time spent with each patient to thoroughly discuss the experience of hysteroscopy and to set expectations about what she will hear, see, and feel. An unexpected experience can worsen anxiety, which in turn can worsen pain. If everything is as familiar and relaxed as possible, there will be little need for analgesia.
I tell patients in preprocedure counseling that the distention of the uterine walls usually causes some cramping, and that NSAIDs can minimize this cramping. In rare cases, when a patient is very worried about her pain tolerance, I will prescribe diazepam. However, many of my patients opt to do nothing other than take ibuprofen. On a case-by-case basis, you can determine with your patient what type and level of analgesia and preprocedure medication will be best.
Paracervical blocks are an option for some surgical patients, but I advise my patients to move forward without the block and assure them that it can be administered later if needed. Thus far, I’ve never proceeded with a paracervical block. There are other methods and sites for introducing local anesthesia, including intracervical, by injection or topical, or topical intracavitary techniques. Nevertheless, it is unclear from randomized controlled trials whether local anesthesia is effective. Trials of paracervical blocks similarly have had inconsistent outcomes.
I do commonly premedicate patients – mainly nulliparous patients and postmenopausal patients – with misoprostol, which softens the cervix and facilitates an easier entry of the hysteroscope into the cervix.
Published studies on misoprostol administration before hysteroscopy have had mixed results. A Cochrane review from 2015 concluded there is moderate-quality evidence in support of preoperative ripening with the agent, while another meta-analysis also published in 2015 concluded that data are poor and do not support its use. Recently, however, there appear to be more supportive studies demonstrating or suggesting that misoprostol is effective in reducing discomfort.
Patient discomfort is also minimized when there is little manipulation of the hysteroscope. Scopes that are angled (12, 25, or 30 degrees) allow optimal visualization with minimal movement; the scope can be brought to the midline of the uterine cavity and the light cord rotated to the 3:00 and 9:00 o’clock positions to enable visualization of the cornu. A 0-degree scope, on the other hand, must be manipulated quite a bit for the same degree of visualization, potentially increasing patient discomfort.
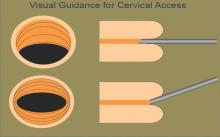
Prior to hysteroscopy, the cervix and vagina are cleaned with a small-diameter swab dipped in povidone-iodine or chlorhexidine gluconate in the case of allergies. One or two 1,000-cc bags of saline inserted into pressure bags are attached to Y-type tubing. (A diagnostic procedure rarely will require two bags.) I spread the labia initially while guiding the scope into the posterior fornix of the vagina. If the leakage of fluid causes inadequate distension of the vaginal walls, I will gently pinch the labia together with gauze.
I then gently pull back the scope and manipulate it posteriorly to visualize the external cervical os anteriorly. The hysteroscope may then be introduced through the cervical os, endocervical canal, and uterine cavity, with care taken so that the instrument does not rub against the cervix or the uterine tissue and cause trauma, pain, and bleeding. The uterus will progressively align with the cervix and vagina, thereby eliminating the need for a tenaculum to straighten the uterine axis.
Fluid monitoring is important, especially during operative hysteroscopy. In my practice, a nurse watches inflow and outflow amounts while I explain what I am doing and visualizing. Some patients like to be able to view the surgery, so I am always ready to tilt the screen accordingly.
The economics
How do you know if office hysteroscopy is right for you? Your own surgical skill and the skills of your staff, who must be trained to handle and sterilize equipment and to consistently assist you, are major factors, as is ensurance of a return on your investment.
One manufacturer contacted for this Master Class lists the price of a complete office tower (light source, camera, and monitor) at approximately $9,700 and the price of a rigid hysteroscope, sheath, and hand instruments at about $6,300. A complete setup for office hysteroscopy, including a standard operative (rigid) hysteroscope, should therefore cost between $15,000 and $17,000. Companies also offer leasing options for about $300-400/month.
Flexible hysteroscopes cost about $6,000 more, which prompts many gynecologic surgeons to focus their investment on a rigid scope that can be used for both diagnostic and therapeutic procedures. Disposables cost $10 or less, and $40-50 or less, for each diagnostic and operative hysteroscopy, respectively.
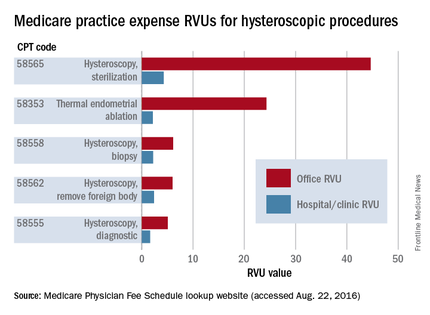
A look at the Medicare Relative Value Units (RVUs) – a key component of the Medicare reimbursement system and a standard for many payers in determining compensation – shows higher reimbursement for quite a few hysteroscopic codes when these procedures are performed in the office.
Total RVUs have three components:
1. Physician work, including time and the technical skill and intensity of effort it takes to perform a service or procedure.
2. Practice expenses, such as rent, equipment and supplies, and nonphysician salaries.
3. Malpractice insurance premiums.
Each component is multiplied by a factor that accounts for geographic cost variations, and each total RVU is multiplied by a dollar amount known as the conversion factor.
Practice expense (PE) RVUs for services provided in a “facility” (e.g., hospital or outpatient clinic) are often lower than office-based PE RVUs for the same services. Hysteroscopy is no exception. The PE RVU value for diagnostic hysteroscopy performed in the office, for instance, is approximately 5 units, compared with 1.64 units for diagnostic hysteroscopy performed in a facility.
Information on hysteroscopic procedures, and their associated RVUs, on geographic practice cost indices and on pricing, can be accessed using Medicare’s Physician Fee Schedule lookup tool (www.cms.gov/apps/physician-fee-schedule/overview.aspx).
This tool is useful for calculating returns on investment. According to national payment amounts listed in August, a diagnostic hysteroscopy performed in the office will earn an average of $315.08 vs. $192.27 for each case performed in the hospital. If you perform 12 such procedures a year, that’s about $3,781 in the office, compared with $2,307 in the hospital.
This difference alone might not be worth an investment of $15,000 or more, but if you anticipate performing additional procedures with higher margins and higher reimbursement, such as 12 thermal endometrial ablations a year in combination with diagnostic hysteroscopy (which, according to the Medicare national fee schedule averages would earn $15,962 in the office vs. $4,971 in the hospital), or 12 Essure tubal occlusions ($22,595 vs. $5,263), the investment will look more favorable.
And if your patients are largely privately insured, your return on investment will occur much more quickly. In metropolitan Chicago, Blue Cross Blue Shield is reimbursing in-office diagnostic hysteroscopy at approximately $568, hysteroscopic ablations at $3,844, and Essure tubal occlusions at $3,885.
In addition to reimbursement levels, it’s important to consider the efficiencies of in-office hysteroscopy. You can perform an annual exam while the assistant sets up the room and greets each patient, for instance, or see another established patient while the assistant discharges your patient and turns the room over. Our patients, in turn, benefit from increased accessibility, with less time spent away from work or family, as well as more familiarity and comfort and reduced out-of-pocket expenses.
Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois in Chicago and is director of gynecologic surgical education and associate director of minimally invasive gynecology at Advocate Lutheran General Hospital. She is in private practice with Dr. Charles Miller and Dr. Kristen Sasaki at the Advanced Gynecologic Surgical Institute in Chicago. She is a consultant for DySIS Medical, Hologic, and Bayer HealthCare.
The benefits of integrating hysteroscopy into office practice are compelling. Most importantly, patients appreciate the comfort and convenience of having hysteroscopic procedures done in a familiar setting. Patients can generally be in and out of the office in less than 30 minutes for a diagnostic procedure, and in less than 1-2 hours for an operative procedure.
Not only is an in-office approach patient centered and clinically valuable, but it is more efficient and economically favorable for the gynecologic surgeon. Physicians earn higher reimbursement for diagnostic hysteroscopies, as well as many therapeutic and operative hysteroscopies, when these procedures are done in the office rather than when they’re performed in a hospital or an outpatient center.
Transitioning to in-office hysteroscopy need not be daunting: The setup is relatively simple and does not require an operating suite, just a dedicated exam room. And the need for premedication and local anesthesia can be low, particularly when a vaginoscopic approach to hysteroscopy is employed. For most gynecologic surgeons, the necessary skills and comfort levels fall into place after only a few vaginoscopic procedures.
A vaginoscopic approach avoids the use of a vaginal speculum or cervical tenaculum, significantly decreasing discomfort or pain. Not using these instruments is the only difference between this and traditional hysteroscopy. It is a less invasive approach that is much more tolerable for patients. And for the surgeon, it can be easier and quicker and provides equally good visualization without any impairment in cervical passage.
Described in the literature as far back as the 1950s, vaginoscopy has its roots in the pediatric/adolescent population, where it was used for the removal of foreign bodies and evaluation of the vagina and external cervical os.
More recently, Stefano Bettocchi, MD, and Luigi Selvaggi, MD, in Italy were the first to describe a vaginoscopic approach to hysteroscopy for evaluating the endocervical canal and uterine cavity.
In a series of papers from 1997 to 2004, Dr. Bettocchi and Dr. Selvaggi documented their efforts to improve patient tolerance during diagnostic hysteroscopies. When they used both the speculum and tenaculum in 163 patients, with local anesthesia, 8% reported severe pain, 11% reported moderate pain, and 69% reported mild pain. Only 12% reported no discomfort. With speculum use only, and no anesthesia, in 308 patients, none reported severe pain, 2% reported moderate pain, 32% reported mild pain, and 66% reported no discomfort. When neither instrument was used (again, no anesthesia), patient discomfort was nearly eliminated: In 680 procedures, patients had a 96% no-discomfort rate (J Am Assoc Gynecol Laparosc. 1997 Feb;4[2]:255-8; Curr Opin Obstet Gynecol. 2003 Aug;15[4]:303-8; Obstet Gynecol Clin North Am. 2004 Sep;31[3]:641-54, xi).
Since then, research has affirmed the differences in patient tolerance and has shown that there is no significant difference between traditional and vaginoscopic hysteroscopy in the rate of procedure failure (0%-10%).
In my practice, in addition to vaginal or cervical examination and evaluation of the uterine cavity, I utilize a vaginoscopic approach to perform minor therapeutic and operative procedures such as biopsies, polypectomies, tubal occlusion using the Essure system, and removal of lost intrauterine devices. I can assess infertility, trauma, abnormal uterine bleeding, and mesh erosion, and provide pre- and postsurgical evaluations. In all of these cases, I use minimal premedication and only rarely need any local anesthetic and/or sedation.
Instrumentation and technique
There are a variety of hysteroscopes available on the market, from single-channel flexible diagnostic hysteroscopes that are 3 mm to 4 mm in diameter, to “see-and-treat” operative hysteroscopes that are rigid and have various diameters and camera lens angles.
A hysteroscope with a 5.5-mm outer diameter works well for a vaginoscopic approach that avoids cervical dilation. Accessory instrumentation includes semirigid 5 Fr 35-cm–long biopsy forceps, scissors, and alligator forceps.
In timing the procedure, our main goal is a thin uterine lining. This can be achieved by scheduling the procedure during the early proliferative phase of the menstrual cycle or by using a gonadotropin-releasing hormone agonist or a transdermal or transvaginal contraceptive medication.
By far the most important element of pain control and analgesia is the time spent with each patient to thoroughly discuss the experience of hysteroscopy and to set expectations about what she will hear, see, and feel. An unexpected experience can worsen anxiety, which in turn can worsen pain. If everything is as familiar and relaxed as possible, there will be little need for analgesia.
I tell patients in preprocedure counseling that the distention of the uterine walls usually causes some cramping, and that NSAIDs can minimize this cramping. In rare cases, when a patient is very worried about her pain tolerance, I will prescribe diazepam. However, many of my patients opt to do nothing other than take ibuprofen. On a case-by-case basis, you can determine with your patient what type and level of analgesia and preprocedure medication will be best.
Paracervical blocks are an option for some surgical patients, but I advise my patients to move forward without the block and assure them that it can be administered later if needed. Thus far, I’ve never proceeded with a paracervical block. There are other methods and sites for introducing local anesthesia, including intracervical, by injection or topical, or topical intracavitary techniques. Nevertheless, it is unclear from randomized controlled trials whether local anesthesia is effective. Trials of paracervical blocks similarly have had inconsistent outcomes.
I do commonly premedicate patients – mainly nulliparous patients and postmenopausal patients – with misoprostol, which softens the cervix and facilitates an easier entry of the hysteroscope into the cervix.
Published studies on misoprostol administration before hysteroscopy have had mixed results. A Cochrane review from 2015 concluded there is moderate-quality evidence in support of preoperative ripening with the agent, while another meta-analysis also published in 2015 concluded that data are poor and do not support its use. Recently, however, there appear to be more supportive studies demonstrating or suggesting that misoprostol is effective in reducing discomfort.
Patient discomfort is also minimized when there is little manipulation of the hysteroscope. Scopes that are angled (12, 25, or 30 degrees) allow optimal visualization with minimal movement; the scope can be brought to the midline of the uterine cavity and the light cord rotated to the 3:00 and 9:00 o’clock positions to enable visualization of the cornu. A 0-degree scope, on the other hand, must be manipulated quite a bit for the same degree of visualization, potentially increasing patient discomfort.
Prior to hysteroscopy, the cervix and vagina are cleaned with a small-diameter swab dipped in povidone-iodine or chlorhexidine gluconate in the case of allergies. One or two 1,000-cc bags of saline inserted into pressure bags are attached to Y-type tubing. (A diagnostic procedure rarely will require two bags.) I spread the labia initially while guiding the scope into the posterior fornix of the vagina. If the leakage of fluid causes inadequate distension of the vaginal walls, I will gently pinch the labia together with gauze.
I then gently pull back the scope and manipulate it posteriorly to visualize the external cervical os anteriorly. The hysteroscope may then be introduced through the cervical os, endocervical canal, and uterine cavity, with care taken so that the instrument does not rub against the cervix or the uterine tissue and cause trauma, pain, and bleeding. The uterus will progressively align with the cervix and vagina, thereby eliminating the need for a tenaculum to straighten the uterine axis.
Fluid monitoring is important, especially during operative hysteroscopy. In my practice, a nurse watches inflow and outflow amounts while I explain what I am doing and visualizing. Some patients like to be able to view the surgery, so I am always ready to tilt the screen accordingly.
The economics
How do you know if office hysteroscopy is right for you? Your own surgical skill and the skills of your staff, who must be trained to handle and sterilize equipment and to consistently assist you, are major factors, as is ensurance of a return on your investment.
One manufacturer contacted for this Master Class lists the price of a complete office tower (light source, camera, and monitor) at approximately $9,700 and the price of a rigid hysteroscope, sheath, and hand instruments at about $6,300. A complete setup for office hysteroscopy, including a standard operative (rigid) hysteroscope, should therefore cost between $15,000 and $17,000. Companies also offer leasing options for about $300-400/month.

Flexible hysteroscopes cost about $6,000 more, which prompts many gynecologic surgeons to focus their investment on a rigid scope that can be used for both diagnostic and therapeutic procedures. Disposables cost $10 or less, and $40-50 or less, for each diagnostic and operative hysteroscopy, respectively.
A look at the Medicare Relative Value Units (RVUs) – a key component of the Medicare reimbursement system and a standard for many payers in determining compensation – shows higher reimbursement for quite a few hysteroscopic codes when these procedures are performed in the office.
Total RVUs have three components:
1. Physician work, including time and the technical skill and intensity of effort it takes to perform a service or procedure.
2. Practice expenses, such as rent, equipment and supplies, and nonphysician salaries.
3. Malpractice insurance premiums.
Each component is multiplied by a factor that accounts for geographic cost variations, and each total RVU is multiplied by a dollar amount known as the conversion factor.
Practice expense (PE) RVUs for services provided in a “facility” (e.g., hospital or outpatient clinic) are often lower than office-based PE RVUs for the same services. Hysteroscopy is no exception. The PE RVU value for diagnostic hysteroscopy performed in the office, for instance, is approximately 5 units, compared with 1.64 units for diagnostic hysteroscopy performed in a facility.
Information on hysteroscopic procedures, and their associated RVUs, on geographic practice cost indices and on pricing, can be accessed using Medicare’s Physician Fee Schedule lookup tool (www.cms.gov/apps/physician-fee-schedule/overview.aspx).
This tool is useful for calculating returns on investment. According to national payment amounts listed in August, a diagnostic hysteroscopy performed in the office will earn an average of $315.08 vs. $192.27 for each case performed in the hospital. If you perform 12 such procedures a year, that’s about $3,781 in the office, compared with $2,307 in the hospital.
This difference alone might not be worth an investment of $15,000 or more, but if you anticipate performing additional procedures with higher margins and higher reimbursement, such as 12 thermal endometrial ablations a year in combination with diagnostic hysteroscopy (which, according to the Medicare national fee schedule averages would earn $15,962 in the office vs. $4,971 in the hospital), or 12 Essure tubal occlusions ($22,595 vs. $5,263), the investment will look more favorable.
And if your patients are largely privately insured, your return on investment will occur much more quickly. In metropolitan Chicago, Blue Cross Blue Shield is reimbursing in-office diagnostic hysteroscopy at approximately $568, hysteroscopic ablations at $3,844, and Essure tubal occlusions at $3,885.
In addition to reimbursement levels, it’s important to consider the efficiencies of in-office hysteroscopy. You can perform an annual exam while the assistant sets up the room and greets each patient, for instance, or see another established patient while the assistant discharges your patient and turns the room over. Our patients, in turn, benefit from increased accessibility, with less time spent away from work or family, as well as more familiarity and comfort and reduced out-of-pocket expenses.
Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois in Chicago and is director of gynecologic surgical education and associate director of minimally invasive gynecology at Advocate Lutheran General Hospital. She is in private practice with Dr. Charles Miller and Dr. Kristen Sasaki at the Advanced Gynecologic Surgical Institute in Chicago. She is a consultant for DySIS Medical, Hologic, and Bayer HealthCare.
The benefits of integrating hysteroscopy into office practice are compelling. Most importantly, patients appreciate the comfort and convenience of having hysteroscopic procedures done in a familiar setting. Patients can generally be in and out of the office in less than 30 minutes for a diagnostic procedure, and in less than 1-2 hours for an operative procedure.
Not only is an in-office approach patient centered and clinically valuable, but it is more efficient and economically favorable for the gynecologic surgeon. Physicians earn higher reimbursement for diagnostic hysteroscopies, as well as many therapeutic and operative hysteroscopies, when these procedures are done in the office rather than when they’re performed in a hospital or an outpatient center.
Transitioning to in-office hysteroscopy need not be daunting: The setup is relatively simple and does not require an operating suite, just a dedicated exam room. And the need for premedication and local anesthesia can be low, particularly when a vaginoscopic approach to hysteroscopy is employed. For most gynecologic surgeons, the necessary skills and comfort levels fall into place after only a few vaginoscopic procedures.
A vaginoscopic approach avoids the use of a vaginal speculum or cervical tenaculum, significantly decreasing discomfort or pain. Not using these instruments is the only difference between this and traditional hysteroscopy. It is a less invasive approach that is much more tolerable for patients. And for the surgeon, it can be easier and quicker and provides equally good visualization without any impairment in cervical passage.
Described in the literature as far back as the 1950s, vaginoscopy has its roots in the pediatric/adolescent population, where it was used for the removal of foreign bodies and evaluation of the vagina and external cervical os.
More recently, Stefano Bettocchi, MD, and Luigi Selvaggi, MD, in Italy were the first to describe a vaginoscopic approach to hysteroscopy for evaluating the endocervical canal and uterine cavity.
In a series of papers from 1997 to 2004, Dr. Bettocchi and Dr. Selvaggi documented their efforts to improve patient tolerance during diagnostic hysteroscopies. When they used both the speculum and tenaculum in 163 patients, with local anesthesia, 8% reported severe pain, 11% reported moderate pain, and 69% reported mild pain. Only 12% reported no discomfort. With speculum use only, and no anesthesia, in 308 patients, none reported severe pain, 2% reported moderate pain, 32% reported mild pain, and 66% reported no discomfort. When neither instrument was used (again, no anesthesia), patient discomfort was nearly eliminated: In 680 procedures, patients had a 96% no-discomfort rate (J Am Assoc Gynecol Laparosc. 1997 Feb;4[2]:255-8; Curr Opin Obstet Gynecol. 2003 Aug;15[4]:303-8; Obstet Gynecol Clin North Am. 2004 Sep;31[3]:641-54, xi).
Since then, research has affirmed the differences in patient tolerance and has shown that there is no significant difference between traditional and vaginoscopic hysteroscopy in the rate of procedure failure (0%-10%).
In my practice, in addition to vaginal or cervical examination and evaluation of the uterine cavity, I utilize a vaginoscopic approach to perform minor therapeutic and operative procedures such as biopsies, polypectomies, tubal occlusion using the Essure system, and removal of lost intrauterine devices. I can assess infertility, trauma, abnormal uterine bleeding, and mesh erosion, and provide pre- and postsurgical evaluations. In all of these cases, I use minimal premedication and only rarely need any local anesthetic and/or sedation.
Instrumentation and technique
There are a variety of hysteroscopes available on the market, from single-channel flexible diagnostic hysteroscopes that are 3 mm to 4 mm in diameter, to “see-and-treat” operative hysteroscopes that are rigid and have various diameters and camera lens angles.
A hysteroscope with a 5.5-mm outer diameter works well for a vaginoscopic approach that avoids cervical dilation. Accessory instrumentation includes semirigid 5 Fr 35-cm–long biopsy forceps, scissors, and alligator forceps.
In timing the procedure, our main goal is a thin uterine lining. This can be achieved by scheduling the procedure during the early proliferative phase of the menstrual cycle or by using a gonadotropin-releasing hormone agonist or a transdermal or transvaginal contraceptive medication.
By far the most important element of pain control and analgesia is the time spent with each patient to thoroughly discuss the experience of hysteroscopy and to set expectations about what she will hear, see, and feel. An unexpected experience can worsen anxiety, which in turn can worsen pain. If everything is as familiar and relaxed as possible, there will be little need for analgesia.
I tell patients in preprocedure counseling that the distention of the uterine walls usually causes some cramping, and that NSAIDs can minimize this cramping. In rare cases, when a patient is very worried about her pain tolerance, I will prescribe diazepam. However, many of my patients opt to do nothing other than take ibuprofen. On a case-by-case basis, you can determine with your patient what type and level of analgesia and preprocedure medication will be best.
Paracervical blocks are an option for some surgical patients, but I advise my patients to move forward without the block and assure them that it can be administered later if needed. Thus far, I’ve never proceeded with a paracervical block. There are other methods and sites for introducing local anesthesia, including intracervical, by injection or topical, or topical intracavitary techniques. Nevertheless, it is unclear from randomized controlled trials whether local anesthesia is effective. Trials of paracervical blocks similarly have had inconsistent outcomes.
I do commonly premedicate patients – mainly nulliparous patients and postmenopausal patients – with misoprostol, which softens the cervix and facilitates an easier entry of the hysteroscope into the cervix.
Published studies on misoprostol administration before hysteroscopy have had mixed results. A Cochrane review from 2015 concluded there is moderate-quality evidence in support of preoperative ripening with the agent, while another meta-analysis also published in 2015 concluded that data are poor and do not support its use. Recently, however, there appear to be more supportive studies demonstrating or suggesting that misoprostol is effective in reducing discomfort.
Patient discomfort is also minimized when there is little manipulation of the hysteroscope. Scopes that are angled (12, 25, or 30 degrees) allow optimal visualization with minimal movement; the scope can be brought to the midline of the uterine cavity and the light cord rotated to the 3:00 and 9:00 o’clock positions to enable visualization of the cornu. A 0-degree scope, on the other hand, must be manipulated quite a bit for the same degree of visualization, potentially increasing patient discomfort.
Prior to hysteroscopy, the cervix and vagina are cleaned with a small-diameter swab dipped in povidone-iodine or chlorhexidine gluconate in the case of allergies. One or two 1,000-cc bags of saline inserted into pressure bags are attached to Y-type tubing. (A diagnostic procedure rarely will require two bags.) I spread the labia initially while guiding the scope into the posterior fornix of the vagina. If the leakage of fluid causes inadequate distension of the vaginal walls, I will gently pinch the labia together with gauze.
I then gently pull back the scope and manipulate it posteriorly to visualize the external cervical os anteriorly. The hysteroscope may then be introduced through the cervical os, endocervical canal, and uterine cavity, with care taken so that the instrument does not rub against the cervix or the uterine tissue and cause trauma, pain, and bleeding. The uterus will progressively align with the cervix and vagina, thereby eliminating the need for a tenaculum to straighten the uterine axis.
Fluid monitoring is important, especially during operative hysteroscopy. In my practice, a nurse watches inflow and outflow amounts while I explain what I am doing and visualizing. Some patients like to be able to view the surgery, so I am always ready to tilt the screen accordingly.
The economics
How do you know if office hysteroscopy is right for you? Your own surgical skill and the skills of your staff, who must be trained to handle and sterilize equipment and to consistently assist you, are major factors, as is ensurance of a return on your investment.
One manufacturer contacted for this Master Class lists the price of a complete office tower (light source, camera, and monitor) at approximately $9,700 and the price of a rigid hysteroscope, sheath, and hand instruments at about $6,300. A complete setup for office hysteroscopy, including a standard operative (rigid) hysteroscope, should therefore cost between $15,000 and $17,000. Companies also offer leasing options for about $300-400/month.

Flexible hysteroscopes cost about $6,000 more, which prompts many gynecologic surgeons to focus their investment on a rigid scope that can be used for both diagnostic and therapeutic procedures. Disposables cost $10 or less, and $40-50 or less, for each diagnostic and operative hysteroscopy, respectively.
A look at the Medicare Relative Value Units (RVUs) – a key component of the Medicare reimbursement system and a standard for many payers in determining compensation – shows higher reimbursement for quite a few hysteroscopic codes when these procedures are performed in the office.
Total RVUs have three components:
1. Physician work, including time and the technical skill and intensity of effort it takes to perform a service or procedure.
2. Practice expenses, such as rent, equipment and supplies, and nonphysician salaries.
3. Malpractice insurance premiums.
Each component is multiplied by a factor that accounts for geographic cost variations, and each total RVU is multiplied by a dollar amount known as the conversion factor.
Practice expense (PE) RVUs for services provided in a “facility” (e.g., hospital or outpatient clinic) are often lower than office-based PE RVUs for the same services. Hysteroscopy is no exception. The PE RVU value for diagnostic hysteroscopy performed in the office, for instance, is approximately 5 units, compared with 1.64 units for diagnostic hysteroscopy performed in a facility.
Information on hysteroscopic procedures, and their associated RVUs, on geographic practice cost indices and on pricing, can be accessed using Medicare’s Physician Fee Schedule lookup tool (www.cms.gov/apps/physician-fee-schedule/overview.aspx).
This tool is useful for calculating returns on investment. According to national payment amounts listed in August, a diagnostic hysteroscopy performed in the office will earn an average of $315.08 vs. $192.27 for each case performed in the hospital. If you perform 12 such procedures a year, that’s about $3,781 in the office, compared with $2,307 in the hospital.
This difference alone might not be worth an investment of $15,000 or more, but if you anticipate performing additional procedures with higher margins and higher reimbursement, such as 12 thermal endometrial ablations a year in combination with diagnostic hysteroscopy (which, according to the Medicare national fee schedule averages would earn $15,962 in the office vs. $4,971 in the hospital), or 12 Essure tubal occlusions ($22,595 vs. $5,263), the investment will look more favorable.
And if your patients are largely privately insured, your return on investment will occur much more quickly. In metropolitan Chicago, Blue Cross Blue Shield is reimbursing in-office diagnostic hysteroscopy at approximately $568, hysteroscopic ablations at $3,844, and Essure tubal occlusions at $3,885.
In addition to reimbursement levels, it’s important to consider the efficiencies of in-office hysteroscopy. You can perform an annual exam while the assistant sets up the room and greets each patient, for instance, or see another established patient while the assistant discharges your patient and turns the room over. Our patients, in turn, benefit from increased accessibility, with less time spent away from work or family, as well as more familiarity and comfort and reduced out-of-pocket expenses.
Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois in Chicago and is director of gynecologic surgical education and associate director of minimally invasive gynecology at Advocate Lutheran General Hospital. She is in private practice with Dr. Charles Miller and Dr. Kristen Sasaki at the Advanced Gynecologic Surgical Institute in Chicago. She is a consultant for DySIS Medical, Hologic, and Bayer HealthCare.