User login
Once it’s determined that a growth requires a biopsy, there is often uncertainty about which type of biopsy to perform. Insufficient knowledge of, and/or experience with, the various biopsy modalities may deter FPs from performing skin biopsies when they are indicated. To help fill the knowledge gaps and better position FPs to tackle skin cancer in its earliest stages, this article identifies and dispels 5 of the most common myths surrounding skin biopsies for the detection of basal and squamous cell carcinoma and melanoma.
MYTH #1
A punch biopsy is always preferred for suspected melanoma because it gets full depth.
A deep shave biopsy (saucerization)—not a punch biopsy—is usually the procedure of choice when biopsying a lesion suspected to be melanoma.2 The National Comprehensive Cancer Network (NCCN) "Melanoma Clinical Practice Guidelines in Oncology" state that an excisional biopsy (elliptical, punch, or saucerization) with a 1- to 3-mm margin is the preferred method of biopsy for suspected melanoma.3 However, a punch biopsy should be performed only if a 1- to 3-mm margin all around a suspected melanoma can be obtained. Otherwise, a saucerization or elliptical excision is preferred.3
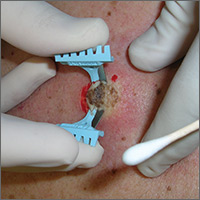
The saucerization technique generally permits optimal sampling in terms of both the breadth and depth of the growth, providing the pathologist with sufficient tissue from both the epidermis and dermis (FIGURE 1).
Why are breadth/depth important? Breadth is important because showing the pathologist the epidermis (especially the edge) of a suspected melanocytic tumor allows for detection of pagetoid spread (upward movement through the epidermis) of melanocytes and of single melanocytes at the edge of a tumor. Single melanocytes at the edge of a tumor and pagetoid spread are histologic features of melanoma that help to distinguish these lesions from nevi, which tend to have nested melanocytes.2
Depth is important because it predicts prognosis and impacts management. For tumors 0.8 mm to 1 mm deep, a sentinel lymph node biopsy (SLNB) should be considered.3,4 Although the tumor depth threshold for a SLNB is still debated, most skin cancer experts in the United States agree that a melanoma thicker than 1 mm qualifies for this procedure. Some melanomas with high-risk features (such as ulceration) qualify for an SNLB even if they are <1 mm in depth.5 An SLNB provides prognostic information, and a positive SLNB directly affects staging.
[polldaddy:9990508]
Avoid partial biopsies. For tumors that have been partially biopsied with a punch or shallow shave biopsy, evaluation of the remaining neoplasm after subsequent excision leads to tumor upstaging in 21% of patients, with 10% qualifying for an SLNB.6 Thus, the goal should always be to obtain the entire depth of the tumor with the initial biopsy.
In addition, surgical margins are determined by primary tumor depth. To ensure a depth greater than 1 mm, aim to obtain a tissue specimen that is at least as thick as a dime (1.3 mm).
Because the goal is to avoid partial sampling, a challenge exists when the suspicious growth is large. Many melanomas are broader than a centimeter. And while punch biopsies ensure a depth of 1 mm or more, they risk missing the thickest portion of the tumor.7
Partial sampling of large melanocytic tumors with punch biopsies can lead to sampling error.8 Ng et al9 found there was a significant increase in histopathologic misdiagnosis with a punch biopsy of part of a melanoma (odds ratio [OR]=16.6; 95% confidence interval [CI], 10-27; P<.001) and with shallow shave biopsy (OR=2.6; 95% CI, 1.2-5.7; P=.02) compared with excisional biopsy (including saucerization).9 Punch biopsy of part of a melanoma was also associated with increased odds of misdiagnosis with an adverse outcome (OR=20; 95% CI, 10-41; P<.001).
Punch biopsies do, however, offer a reasonable alternative when the melanoma is too broad for a complete saucerization. In these cases, consider multiple 4- to 6-mm punch biopsies to reduce the risk of sampling error.
Avoid performing punch biopsies <4 mm, as the breadth of tissue is inadequate. For example, even with dermoscopy, facial lentigo maligna melanoma is often difficult to differentiate from pigmented actinic keratosis and solar lentigines. (See JFP’s Watch and Learn Video on dermoscopy.) A broad shave biopsy is the preferred method of biopsy for lentigo maligna melanoma in situ according to the NCCN.3 And there have been several reports showing that the results of shave biopsies of melanocytic lesions are cosmetically acceptable to patients.10,11
If the biopsy confirms malignancy, a larger surgery with suturing will be needed. The most important issue to keep in mind is that if partial sampling leads to a benign diagnosis of a suspicious lesion, then the remainder of the lesion must be excised and sent for pathology.
Saucerization is also the preferred biopsy type for basal cell and squamous cell carcinomas (SCCs). Studies have shown tumor depth is the most important factor in predicting metastasis of SCC, as well as tumor relapse rate, making accurate identification of the depth of the tumor important for both management and prognosis.12,13 Determining the thickness of the SCC is important for guiding management. SCC in situ is more amenable than invasive SCC to topical therapy or electrodesiccation and curettage.
What you’ll need. FPs can perform saucerization quickly and easily in the office during a standard 15-minute visit. Of course, it is essential to have all the necessary materials available. The key materials needed are lidocaine and epinephrine, a sharp razor blade such as a DermaBlade, and something for hemostasis (aluminum chloride and/or an electrosurgical instrument). Cotton-tipped applicators to apply the aluminum chloride and needles and syringes to administer the local anesthetic are also needed. (See JFP’s Watch and Learn Video on shave biopsy.) A quick saucerization eliminates the need for the patient to return for an elliptical excision and prevents a delayed diagnosis that can occur as a result of a long wait to see a dermatologist.
As a final note, the pathology order form should be completed with information on biopsy type, clinical presentation, differential diagnosis, and whether or not the full lesion was excised.
MYTH #2
A wide excisional biopsy is required for a suspected melanoma.
While complete excision of the entire tumor does allow the pathologist to evaluate the entire growth, wide (>3 mm) margins on the initial biopsy are not necessary. In fact, there are potential disadvantages to full excisional biopsy.
For example, seborrheic keratoses and other benign growths can mimic melanoma. Neither the physician nor the patient wants to learn that a large elliptical wound was created for a growth that turned out to be a benign seborrheic keratosis. Saucerization provides the pathologist with the entire lesion, and the resulting shallow wound heals as a round scar that is most often acceptable to patients.10,11,14 In addition, excisional biopsies carry a higher risk of infection than does saucerization.
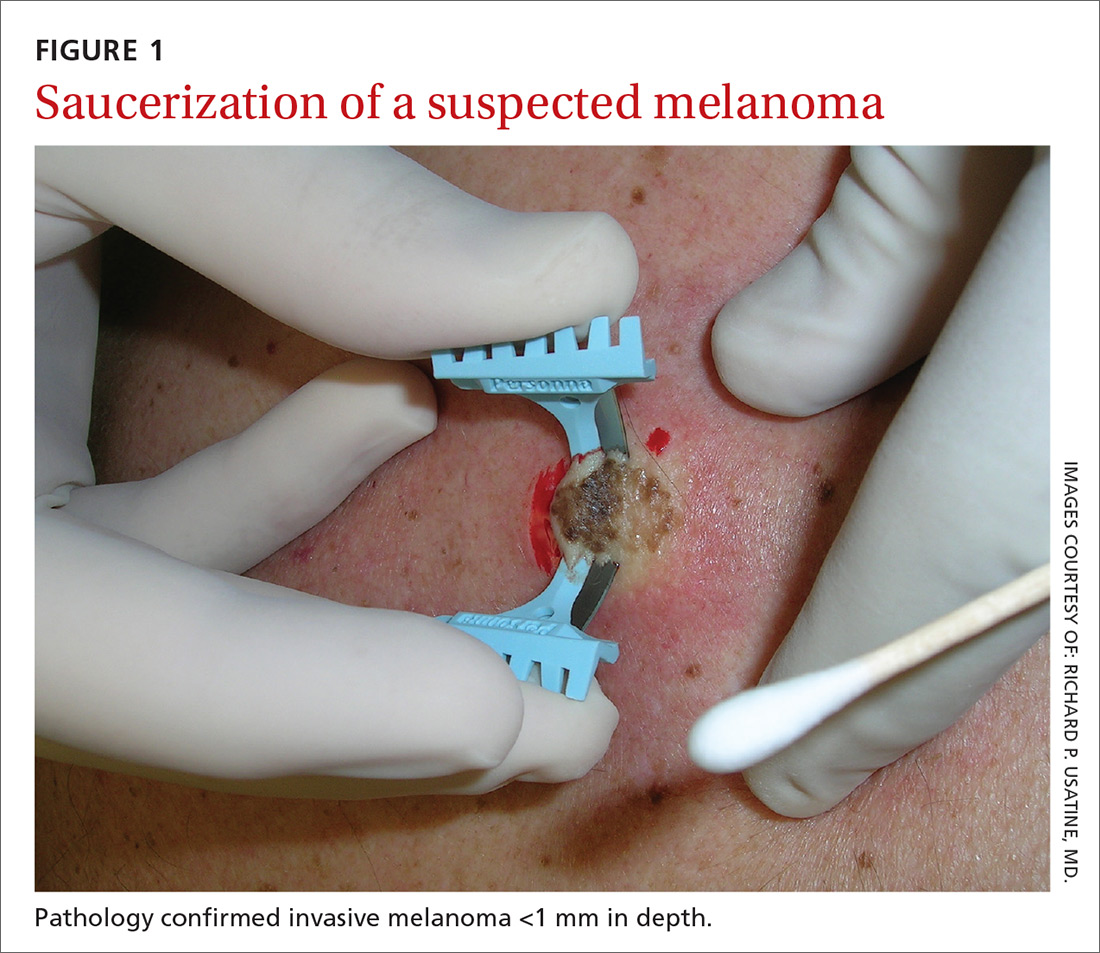
Even when the index of suspicion is high for melanoma, a wide margin is not indicated. NCCN guidelines suggest that the margins around a suspected melanoma on initial biopsy not exceed 3 mm to avoid disrupting the accuracy of an SLNB (FIGURE 2).3
In addition, time constraints (elliptical excisional biopsies can take up to one hour, especially when a layered closure is performed) and a lack of surgical training may prohibit FPs from performing excisions.
One study found that while dermatologists prefer shave biopsies (80.5%), surgeons prefer excisional biopsies (46.3%) and primary care physicians prefer punch biopsies (44%) for biopsy of a growth suspicious for melanoma.7 In fact, of the biopsies FPs perform, only 29% are of the shave variety.7
However, deep shave biopsies can be performed quickly, with the whole process taking less than 5 minutes. We advocate performing them at the time of presentation, as the evidence shows that deep shave biopsies of suspected melanoma are reliable and accurate in 97% of cases.15
MYTH #3
A partial biopsy can make the cancer spread.
There is no evidence to support that a partial biopsy has any effect on the local recurrence or metastatic potential of malignant melanoma.16 In fact, a biopsy elicits an inflammatory response that activates the patient’s immune system and often causes tumor lysis. Some tumors may even resolve after biopsy. In our clinical practice, we have had several cases of basal cell carcinoma resolve after a biopsy without additional treatment.
MYTH #4
If after performing a deep shave biopsy, tumor or pigment remains, you must leave it because a second biopsy specimen can’t be added to the first.
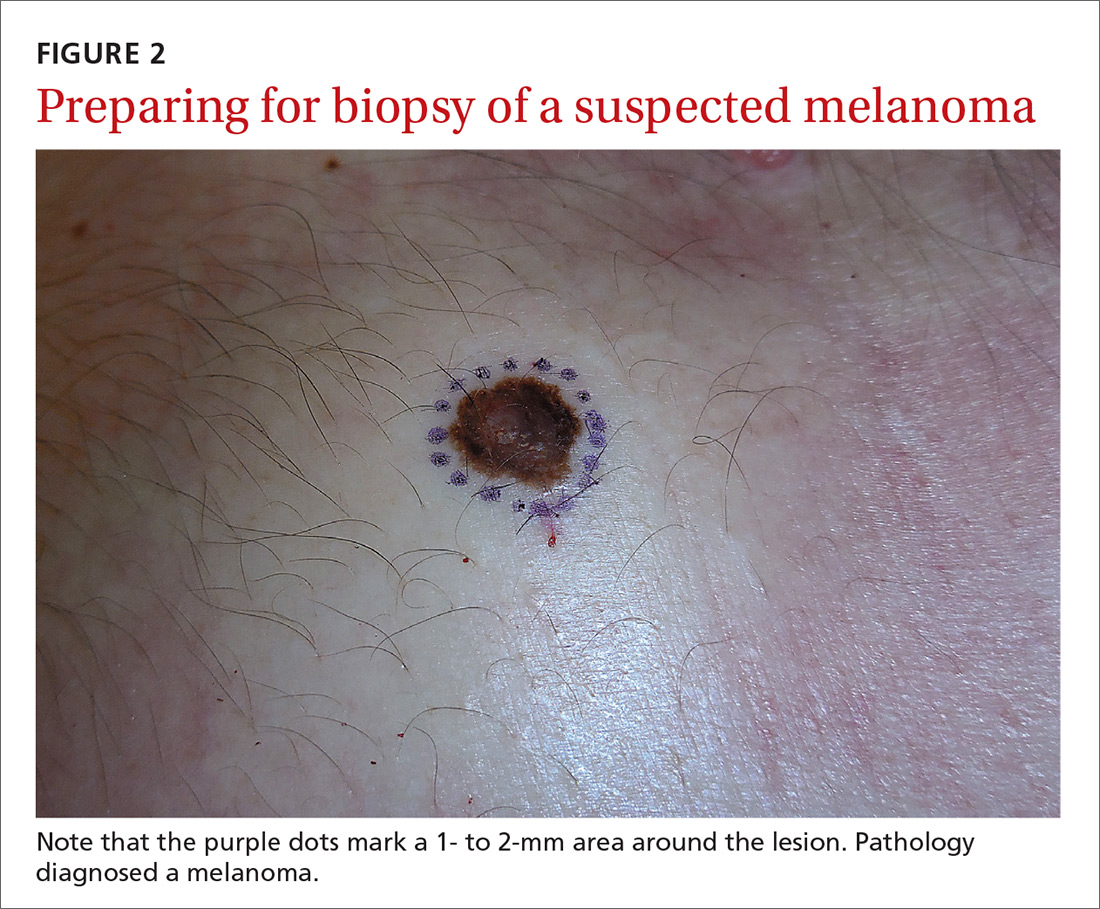
If pigment is visible after an initial shave or punch biopsy, it is reasonable to obtain additional tissue from the base of the biopsy site. While the deeper tissue cannot be added to the initial specimen for the purposes of Breslow’s depth, it is still helpful for the pathologist to have the sample so that he or she can analyze the tumor cells in the dermis. (Melanoma tumor depth is measured as the maximum distance between malignant cells and the top of the granular layer.17) In these situations, be sure to let the pathologist know that there are 2 specimens in the container.
In general, it is valuable to get as much of the tissue as possible at the time of the initial biopsy. One way to avoid leaving tumor at the base of the biopsy is to look at
MYTH #5
Epinephrine cannot be used for biopsies on the fingers, toes, nose, or penis.
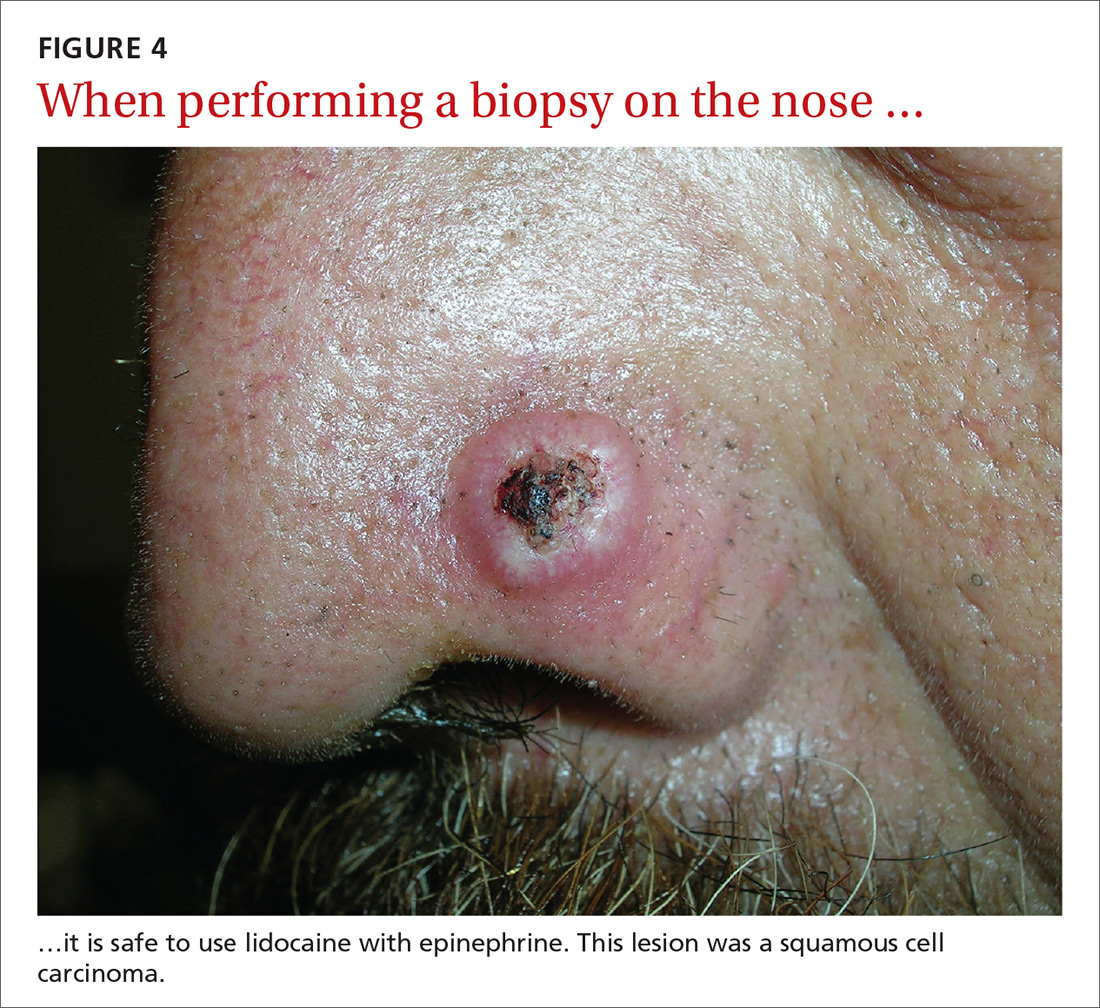
Lidocaine with epinephrine is safe to use in areas with end-arteries, such as the fingers, toes, nose (FIGURE 4), and penis. There is no evidence to support the notion that local anesthesia with vasoconstriction can cause necrosis in these areas, and no case of necrosis has been reported since the introduction of commercial lidocaine with epinephrine in 1948.18
In addition to an absence of complications, epinephrine supplementation results in a relatively bloodless operating field and longer effectiveness of local anesthesia, as a study of more than 10,000 ear and nose surgeries using epinephrine-supplemented local anesthetics showed.19 The relative absence of blood in the operating field significantly reduces the duration of surgery and increases the healing rate because less electrocautery is needed.19
Similarly, the addition of epinephrine in digital blocks minimizes the need for tourniquets and large volumes of anesthetic and provides better and longer pain control during procedures.20 This topic was addressed by Prabhakar et al in a Cochrane Review in 2015.21 While digital surgeries are common, there were only 4 randomized controlled studies addressing the use of epinephrine in digital blocks. In these studies, there were no reports of adverse events, such as ischemia distal to the injection site. Evidence suggests that epinephrine in digital blocks can even be used safely in patients with vascular disease.22
And while the use of epinephrine with lidocaine in sites with end-arteries is beneficial for hemostasis and does not seem to pose a risk of ischemia, it is prudent to use the smallest volume of epinephrine (with lidocaine) needed to achieve anesthesia for the site.
CORRESPONDENCE
Elizabeth V. Seiverling, MD, 300 Southborough Drive, Suite 201, South Portland, ME 04106; [email protected].
1. Kerr OA, Tidman MJ, Walker JJ, et al. The profile of dermatological problems in primary care. Clin Exp Dermatol. 2010;35:380-383.
2. Hosler GA, Patterson JW. Lentigines, nevi, and melanomas. In: Patterson JW, ed. Weedon’s Skin Pathology. Elsevier; 2015:32,837-901.
3. Coit DG, Andtbacka R, Bichakjian CK, et al. Melanoma. J Natl Compr Canc Netw. 2009;7:250-275.
4. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge S, Greene F, et al, eds. AJCC Cancer Staging Manual. Springer International Publishing; 2017;8:563-585.
5. American Joint Committee on Cancer. Implementation of AJCC 8th Edition Cancer Staging System. Available at: https://cancerstaging.org/About/news/Pages/Implementation-of-AJCC-8th-Edition-Cancer-Staging-System.aspx. Accessed April 2, 2018.
6. Karimipour DJ, Schwartz JL, Wang TS, et al. Microstaging accuracy after subtotal incisional biopsy of cutaneous melanoma. J Am Acad Dermatol. 2005;52:798-802.
7. Kaiser S, Vassell R, Pinckney RG, et al. Clinical impact of biopsy method on the quality of surgical management in melanoma. J Surg Oncol. 2014;109:775-779.
8. Montgomery BD, Sadler GM. Punch biopsy of pigmented lesions is potentially hazardous. Can Fam Physician. 2009;55:24.
9. Ng JC, Swain S, Dowling JP, et al. The impact of partial biopsy on histopathologic diagnosis of cutaneous melanoma: experience of an Australian tertiary referral service. Arch Dermatol. 2010;146:234-239.
10. Gambichler T, Senger E, Rapp S, et al. Deep shave excision of macular melanocytic nevi with the razor blade biopsy technique. Dermatol Surg. 2000;26:662-666.
11. Ferrandiz L, Moreno-Ramirez D, Camacho FM. Shave excision of common acquired melanocytic nevi: cosmetic outcome, recurrences, and complications. Dermatol Surg. 2005;31(9 Pt 1):1112-1115.
12. D'souza G, Carey TE, William WN Jr, et al. Epidemiology of head and neck squamous cell cancer among HIV-infected patients. J Acquir Immune Defic Syndr. 2014;65:603-610.
13. Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Handbook. 7th ed. New York, NY: Springer; 2010.
14. Elston DM, Stratman EJ, Miller SJ, et al. Skin biopsy. J Am Acad Dermatol. 2016;74:1-16.
15. Zager JS, Hochwald SN, Marzban SS, et al. Shave biopsy is a safe and accurate method for the initial evaluation of melanoma. J Am Coll Surg. 2011;212:454-460.
16. Chanda JJ, Callen JP. Adverse effect of melanoma incision. J Am Acad Dermatol. 1985;13:519-522.
17. Noroozi N, Zakerolhosseini A. Computerized measurement of melanocytic tumor depth in skin histopathological images. Micron. 2015;77:44-56.
18. Nielsen LJ, Lumholt P, Hölmich LR. [Local anaesthesia with vasoconstrictor is safe to use in areas with end-arteries in fingers, toes, noses and ears]. Ugeskr Laeger. 2014;176(44).
19. Häfner HM, Röcken M, Breuninger H. Epinephrine-supplemented local anesthetics for ear and nose surgery: clinical use without complications in more than 10,000 surgical procedures. J Dtsch Dermatol Ges. 2005;3:195-199.
20. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
21. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
22. Ilicki J. Safety of epinephrine in digital nerve blocks: a literature review. J Emerg Med. 2015;49:799-809.
Once it’s determined that a growth requires a biopsy, there is often uncertainty about which type of biopsy to perform. Insufficient knowledge of, and/or experience with, the various biopsy modalities may deter FPs from performing skin biopsies when they are indicated. To help fill the knowledge gaps and better position FPs to tackle skin cancer in its earliest stages, this article identifies and dispels 5 of the most common myths surrounding skin biopsies for the detection of basal and squamous cell carcinoma and melanoma.
MYTH #1
A punch biopsy is always preferred for suspected melanoma because it gets full depth.
A deep shave biopsy (saucerization)—not a punch biopsy—is usually the procedure of choice when biopsying a lesion suspected to be melanoma.2 The National Comprehensive Cancer Network (NCCN) "Melanoma Clinical Practice Guidelines in Oncology" state that an excisional biopsy (elliptical, punch, or saucerization) with a 1- to 3-mm margin is the preferred method of biopsy for suspected melanoma.3 However, a punch biopsy should be performed only if a 1- to 3-mm margin all around a suspected melanoma can be obtained. Otherwise, a saucerization or elliptical excision is preferred.3
The saucerization technique generally permits optimal sampling in terms of both the breadth and depth of the growth, providing the pathologist with sufficient tissue from both the epidermis and dermis (FIGURE 1).

Why are breadth/depth important? Breadth is important because showing the pathologist the epidermis (especially the edge) of a suspected melanocytic tumor allows for detection of pagetoid spread (upward movement through the epidermis) of melanocytes and of single melanocytes at the edge of a tumor. Single melanocytes at the edge of a tumor and pagetoid spread are histologic features of melanoma that help to distinguish these lesions from nevi, which tend to have nested melanocytes.2
Depth is important because it predicts prognosis and impacts management. For tumors 0.8 mm to 1 mm deep, a sentinel lymph node biopsy (SLNB) should be considered.3,4 Although the tumor depth threshold for a SLNB is still debated, most skin cancer experts in the United States agree that a melanoma thicker than 1 mm qualifies for this procedure. Some melanomas with high-risk features (such as ulceration) qualify for an SNLB even if they are <1 mm in depth.5 An SLNB provides prognostic information, and a positive SLNB directly affects staging.
[polldaddy:9990508]
Avoid partial biopsies. For tumors that have been partially biopsied with a punch or shallow shave biopsy, evaluation of the remaining neoplasm after subsequent excision leads to tumor upstaging in 21% of patients, with 10% qualifying for an SLNB.6 Thus, the goal should always be to obtain the entire depth of the tumor with the initial biopsy.
In addition, surgical margins are determined by primary tumor depth. To ensure a depth greater than 1 mm, aim to obtain a tissue specimen that is at least as thick as a dime (1.3 mm).
Because the goal is to avoid partial sampling, a challenge exists when the suspicious growth is large. Many melanomas are broader than a centimeter. And while punch biopsies ensure a depth of 1 mm or more, they risk missing the thickest portion of the tumor.7
Partial sampling of large melanocytic tumors with punch biopsies can lead to sampling error.8 Ng et al9 found there was a significant increase in histopathologic misdiagnosis with a punch biopsy of part of a melanoma (odds ratio [OR]=16.6; 95% confidence interval [CI], 10-27; P<.001) and with shallow shave biopsy (OR=2.6; 95% CI, 1.2-5.7; P=.02) compared with excisional biopsy (including saucerization).9 Punch biopsy of part of a melanoma was also associated with increased odds of misdiagnosis with an adverse outcome (OR=20; 95% CI, 10-41; P<.001).
Punch biopsies do, however, offer a reasonable alternative when the melanoma is too broad for a complete saucerization. In these cases, consider multiple 4- to 6-mm punch biopsies to reduce the risk of sampling error.
Avoid performing punch biopsies <4 mm, as the breadth of tissue is inadequate. For example, even with dermoscopy, facial lentigo maligna melanoma is often difficult to differentiate from pigmented actinic keratosis and solar lentigines. (See JFP’s Watch and Learn Video on dermoscopy.) A broad shave biopsy is the preferred method of biopsy for lentigo maligna melanoma in situ according to the NCCN.3 And there have been several reports showing that the results of shave biopsies of melanocytic lesions are cosmetically acceptable to patients.10,11
If the biopsy confirms malignancy, a larger surgery with suturing will be needed. The most important issue to keep in mind is that if partial sampling leads to a benign diagnosis of a suspicious lesion, then the remainder of the lesion must be excised and sent for pathology.
Saucerization is also the preferred biopsy type for basal cell and squamous cell carcinomas (SCCs). Studies have shown tumor depth is the most important factor in predicting metastasis of SCC, as well as tumor relapse rate, making accurate identification of the depth of the tumor important for both management and prognosis.12,13 Determining the thickness of the SCC is important for guiding management. SCC in situ is more amenable than invasive SCC to topical therapy or electrodesiccation and curettage.
What you’ll need. FPs can perform saucerization quickly and easily in the office during a standard 15-minute visit. Of course, it is essential to have all the necessary materials available. The key materials needed are lidocaine and epinephrine, a sharp razor blade such as a DermaBlade, and something for hemostasis (aluminum chloride and/or an electrosurgical instrument). Cotton-tipped applicators to apply the aluminum chloride and needles and syringes to administer the local anesthetic are also needed. (See JFP’s Watch and Learn Video on shave biopsy.) A quick saucerization eliminates the need for the patient to return for an elliptical excision and prevents a delayed diagnosis that can occur as a result of a long wait to see a dermatologist.
As a final note, the pathology order form should be completed with information on biopsy type, clinical presentation, differential diagnosis, and whether or not the full lesion was excised.
MYTH #2
A wide excisional biopsy is required for a suspected melanoma.
While complete excision of the entire tumor does allow the pathologist to evaluate the entire growth, wide (>3 mm) margins on the initial biopsy are not necessary. In fact, there are potential disadvantages to full excisional biopsy.
For example, seborrheic keratoses and other benign growths can mimic melanoma. Neither the physician nor the patient wants to learn that a large elliptical wound was created for a growth that turned out to be a benign seborrheic keratosis. Saucerization provides the pathologist with the entire lesion, and the resulting shallow wound heals as a round scar that is most often acceptable to patients.10,11,14 In addition, excisional biopsies carry a higher risk of infection than does saucerization.
Even when the index of suspicion is high for melanoma, a wide margin is not indicated. NCCN guidelines suggest that the margins around a suspected melanoma on initial biopsy not exceed 3 mm to avoid disrupting the accuracy of an SLNB (FIGURE 2).3

In addition, time constraints (elliptical excisional biopsies can take up to one hour, especially when a layered closure is performed) and a lack of surgical training may prohibit FPs from performing excisions.
One study found that while dermatologists prefer shave biopsies (80.5%), surgeons prefer excisional biopsies (46.3%) and primary care physicians prefer punch biopsies (44%) for biopsy of a growth suspicious for melanoma.7 In fact, of the biopsies FPs perform, only 29% are of the shave variety.7
However, deep shave biopsies can be performed quickly, with the whole process taking less than 5 minutes. We advocate performing them at the time of presentation, as the evidence shows that deep shave biopsies of suspected melanoma are reliable and accurate in 97% of cases.15
MYTH #3
A partial biopsy can make the cancer spread.
There is no evidence to support that a partial biopsy has any effect on the local recurrence or metastatic potential of malignant melanoma.16 In fact, a biopsy elicits an inflammatory response that activates the patient’s immune system and often causes tumor lysis. Some tumors may even resolve after biopsy. In our clinical practice, we have had several cases of basal cell carcinoma resolve after a biopsy without additional treatment.
MYTH #4
If after performing a deep shave biopsy, tumor or pigment remains, you must leave it because a second biopsy specimen can’t be added to the first.
If pigment is visible after an initial shave or punch biopsy, it is reasonable to obtain additional tissue from the base of the biopsy site. While the deeper tissue cannot be added to the initial specimen for the purposes of Breslow’s depth, it is still helpful for the pathologist to have the sample so that he or she can analyze the tumor cells in the dermis. (Melanoma tumor depth is measured as the maximum distance between malignant cells and the top of the granular layer.17) In these situations, be sure to let the pathologist know that there are 2 specimens in the container.
In general, it is valuable to get as much of the tissue as possible at the time of the initial biopsy. One way to avoid leaving tumor at the base of the biopsy is to look at

MYTH #5
Epinephrine cannot be used for biopsies on the fingers, toes, nose, or penis.
Lidocaine with epinephrine is safe to use in areas with end-arteries, such as the fingers, toes, nose (FIGURE 4), and penis. There is no evidence to support the notion that local anesthesia with vasoconstriction can cause necrosis in these areas, and no case of necrosis has been reported since the introduction of commercial lidocaine with epinephrine in 1948.18

In addition to an absence of complications, epinephrine supplementation results in a relatively bloodless operating field and longer effectiveness of local anesthesia, as a study of more than 10,000 ear and nose surgeries using epinephrine-supplemented local anesthetics showed.19 The relative absence of blood in the operating field significantly reduces the duration of surgery and increases the healing rate because less electrocautery is needed.19
Similarly, the addition of epinephrine in digital blocks minimizes the need for tourniquets and large volumes of anesthetic and provides better and longer pain control during procedures.20 This topic was addressed by Prabhakar et al in a Cochrane Review in 2015.21 While digital surgeries are common, there were only 4 randomized controlled studies addressing the use of epinephrine in digital blocks. In these studies, there were no reports of adverse events, such as ischemia distal to the injection site. Evidence suggests that epinephrine in digital blocks can even be used safely in patients with vascular disease.22
And while the use of epinephrine with lidocaine in sites with end-arteries is beneficial for hemostasis and does not seem to pose a risk of ischemia, it is prudent to use the smallest volume of epinephrine (with lidocaine) needed to achieve anesthesia for the site.
CORRESPONDENCE
Elizabeth V. Seiverling, MD, 300 Southborough Drive, Suite 201, South Portland, ME 04106; [email protected].
Once it’s determined that a growth requires a biopsy, there is often uncertainty about which type of biopsy to perform. Insufficient knowledge of, and/or experience with, the various biopsy modalities may deter FPs from performing skin biopsies when they are indicated. To help fill the knowledge gaps and better position FPs to tackle skin cancer in its earliest stages, this article identifies and dispels 5 of the most common myths surrounding skin biopsies for the detection of basal and squamous cell carcinoma and melanoma.
MYTH #1
A punch biopsy is always preferred for suspected melanoma because it gets full depth.
A deep shave biopsy (saucerization)—not a punch biopsy—is usually the procedure of choice when biopsying a lesion suspected to be melanoma.2 The National Comprehensive Cancer Network (NCCN) "Melanoma Clinical Practice Guidelines in Oncology" state that an excisional biopsy (elliptical, punch, or saucerization) with a 1- to 3-mm margin is the preferred method of biopsy for suspected melanoma.3 However, a punch biopsy should be performed only if a 1- to 3-mm margin all around a suspected melanoma can be obtained. Otherwise, a saucerization or elliptical excision is preferred.3
The saucerization technique generally permits optimal sampling in terms of both the breadth and depth of the growth, providing the pathologist with sufficient tissue from both the epidermis and dermis (FIGURE 1).

Why are breadth/depth important? Breadth is important because showing the pathologist the epidermis (especially the edge) of a suspected melanocytic tumor allows for detection of pagetoid spread (upward movement through the epidermis) of melanocytes and of single melanocytes at the edge of a tumor. Single melanocytes at the edge of a tumor and pagetoid spread are histologic features of melanoma that help to distinguish these lesions from nevi, which tend to have nested melanocytes.2
Depth is important because it predicts prognosis and impacts management. For tumors 0.8 mm to 1 mm deep, a sentinel lymph node biopsy (SLNB) should be considered.3,4 Although the tumor depth threshold for a SLNB is still debated, most skin cancer experts in the United States agree that a melanoma thicker than 1 mm qualifies for this procedure. Some melanomas with high-risk features (such as ulceration) qualify for an SNLB even if they are <1 mm in depth.5 An SLNB provides prognostic information, and a positive SLNB directly affects staging.
[polldaddy:9990508]
Avoid partial biopsies. For tumors that have been partially biopsied with a punch or shallow shave biopsy, evaluation of the remaining neoplasm after subsequent excision leads to tumor upstaging in 21% of patients, with 10% qualifying for an SLNB.6 Thus, the goal should always be to obtain the entire depth of the tumor with the initial biopsy.
In addition, surgical margins are determined by primary tumor depth. To ensure a depth greater than 1 mm, aim to obtain a tissue specimen that is at least as thick as a dime (1.3 mm).
Because the goal is to avoid partial sampling, a challenge exists when the suspicious growth is large. Many melanomas are broader than a centimeter. And while punch biopsies ensure a depth of 1 mm or more, they risk missing the thickest portion of the tumor.7
Partial sampling of large melanocytic tumors with punch biopsies can lead to sampling error.8 Ng et al9 found there was a significant increase in histopathologic misdiagnosis with a punch biopsy of part of a melanoma (odds ratio [OR]=16.6; 95% confidence interval [CI], 10-27; P<.001) and with shallow shave biopsy (OR=2.6; 95% CI, 1.2-5.7; P=.02) compared with excisional biopsy (including saucerization).9 Punch biopsy of part of a melanoma was also associated with increased odds of misdiagnosis with an adverse outcome (OR=20; 95% CI, 10-41; P<.001).
Punch biopsies do, however, offer a reasonable alternative when the melanoma is too broad for a complete saucerization. In these cases, consider multiple 4- to 6-mm punch biopsies to reduce the risk of sampling error.
Avoid performing punch biopsies <4 mm, as the breadth of tissue is inadequate. For example, even with dermoscopy, facial lentigo maligna melanoma is often difficult to differentiate from pigmented actinic keratosis and solar lentigines. (See JFP’s Watch and Learn Video on dermoscopy.) A broad shave biopsy is the preferred method of biopsy for lentigo maligna melanoma in situ according to the NCCN.3 And there have been several reports showing that the results of shave biopsies of melanocytic lesions are cosmetically acceptable to patients.10,11
If the biopsy confirms malignancy, a larger surgery with suturing will be needed. The most important issue to keep in mind is that if partial sampling leads to a benign diagnosis of a suspicious lesion, then the remainder of the lesion must be excised and sent for pathology.
Saucerization is also the preferred biopsy type for basal cell and squamous cell carcinomas (SCCs). Studies have shown tumor depth is the most important factor in predicting metastasis of SCC, as well as tumor relapse rate, making accurate identification of the depth of the tumor important for both management and prognosis.12,13 Determining the thickness of the SCC is important for guiding management. SCC in situ is more amenable than invasive SCC to topical therapy or electrodesiccation and curettage.
What you’ll need. FPs can perform saucerization quickly and easily in the office during a standard 15-minute visit. Of course, it is essential to have all the necessary materials available. The key materials needed are lidocaine and epinephrine, a sharp razor blade such as a DermaBlade, and something for hemostasis (aluminum chloride and/or an electrosurgical instrument). Cotton-tipped applicators to apply the aluminum chloride and needles and syringes to administer the local anesthetic are also needed. (See JFP’s Watch and Learn Video on shave biopsy.) A quick saucerization eliminates the need for the patient to return for an elliptical excision and prevents a delayed diagnosis that can occur as a result of a long wait to see a dermatologist.
As a final note, the pathology order form should be completed with information on biopsy type, clinical presentation, differential diagnosis, and whether or not the full lesion was excised.
MYTH #2
A wide excisional biopsy is required for a suspected melanoma.
While complete excision of the entire tumor does allow the pathologist to evaluate the entire growth, wide (>3 mm) margins on the initial biopsy are not necessary. In fact, there are potential disadvantages to full excisional biopsy.
For example, seborrheic keratoses and other benign growths can mimic melanoma. Neither the physician nor the patient wants to learn that a large elliptical wound was created for a growth that turned out to be a benign seborrheic keratosis. Saucerization provides the pathologist with the entire lesion, and the resulting shallow wound heals as a round scar that is most often acceptable to patients.10,11,14 In addition, excisional biopsies carry a higher risk of infection than does saucerization.
Even when the index of suspicion is high for melanoma, a wide margin is not indicated. NCCN guidelines suggest that the margins around a suspected melanoma on initial biopsy not exceed 3 mm to avoid disrupting the accuracy of an SLNB (FIGURE 2).3

In addition, time constraints (elliptical excisional biopsies can take up to one hour, especially when a layered closure is performed) and a lack of surgical training may prohibit FPs from performing excisions.
One study found that while dermatologists prefer shave biopsies (80.5%), surgeons prefer excisional biopsies (46.3%) and primary care physicians prefer punch biopsies (44%) for biopsy of a growth suspicious for melanoma.7 In fact, of the biopsies FPs perform, only 29% are of the shave variety.7
However, deep shave biopsies can be performed quickly, with the whole process taking less than 5 minutes. We advocate performing them at the time of presentation, as the evidence shows that deep shave biopsies of suspected melanoma are reliable and accurate in 97% of cases.15
MYTH #3
A partial biopsy can make the cancer spread.
There is no evidence to support that a partial biopsy has any effect on the local recurrence or metastatic potential of malignant melanoma.16 In fact, a biopsy elicits an inflammatory response that activates the patient’s immune system and often causes tumor lysis. Some tumors may even resolve after biopsy. In our clinical practice, we have had several cases of basal cell carcinoma resolve after a biopsy without additional treatment.
MYTH #4
If after performing a deep shave biopsy, tumor or pigment remains, you must leave it because a second biopsy specimen can’t be added to the first.
If pigment is visible after an initial shave or punch biopsy, it is reasonable to obtain additional tissue from the base of the biopsy site. While the deeper tissue cannot be added to the initial specimen for the purposes of Breslow’s depth, it is still helpful for the pathologist to have the sample so that he or she can analyze the tumor cells in the dermis. (Melanoma tumor depth is measured as the maximum distance between malignant cells and the top of the granular layer.17) In these situations, be sure to let the pathologist know that there are 2 specimens in the container.
In general, it is valuable to get as much of the tissue as possible at the time of the initial biopsy. One way to avoid leaving tumor at the base of the biopsy is to look at

MYTH #5
Epinephrine cannot be used for biopsies on the fingers, toes, nose, or penis.
Lidocaine with epinephrine is safe to use in areas with end-arteries, such as the fingers, toes, nose (FIGURE 4), and penis. There is no evidence to support the notion that local anesthesia with vasoconstriction can cause necrosis in these areas, and no case of necrosis has been reported since the introduction of commercial lidocaine with epinephrine in 1948.18

In addition to an absence of complications, epinephrine supplementation results in a relatively bloodless operating field and longer effectiveness of local anesthesia, as a study of more than 10,000 ear and nose surgeries using epinephrine-supplemented local anesthetics showed.19 The relative absence of blood in the operating field significantly reduces the duration of surgery and increases the healing rate because less electrocautery is needed.19
Similarly, the addition of epinephrine in digital blocks minimizes the need for tourniquets and large volumes of anesthetic and provides better and longer pain control during procedures.20 This topic was addressed by Prabhakar et al in a Cochrane Review in 2015.21 While digital surgeries are common, there were only 4 randomized controlled studies addressing the use of epinephrine in digital blocks. In these studies, there were no reports of adverse events, such as ischemia distal to the injection site. Evidence suggests that epinephrine in digital blocks can even be used safely in patients with vascular disease.22
And while the use of epinephrine with lidocaine in sites with end-arteries is beneficial for hemostasis and does not seem to pose a risk of ischemia, it is prudent to use the smallest volume of epinephrine (with lidocaine) needed to achieve anesthesia for the site.
CORRESPONDENCE
Elizabeth V. Seiverling, MD, 300 Southborough Drive, Suite 201, South Portland, ME 04106; [email protected].
1. Kerr OA, Tidman MJ, Walker JJ, et al. The profile of dermatological problems in primary care. Clin Exp Dermatol. 2010;35:380-383.
2. Hosler GA, Patterson JW. Lentigines, nevi, and melanomas. In: Patterson JW, ed. Weedon’s Skin Pathology. Elsevier; 2015:32,837-901.
3. Coit DG, Andtbacka R, Bichakjian CK, et al. Melanoma. J Natl Compr Canc Netw. 2009;7:250-275.
4. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge S, Greene F, et al, eds. AJCC Cancer Staging Manual. Springer International Publishing; 2017;8:563-585.
5. American Joint Committee on Cancer. Implementation of AJCC 8th Edition Cancer Staging System. Available at: https://cancerstaging.org/About/news/Pages/Implementation-of-AJCC-8th-Edition-Cancer-Staging-System.aspx. Accessed April 2, 2018.
6. Karimipour DJ, Schwartz JL, Wang TS, et al. Microstaging accuracy after subtotal incisional biopsy of cutaneous melanoma. J Am Acad Dermatol. 2005;52:798-802.
7. Kaiser S, Vassell R, Pinckney RG, et al. Clinical impact of biopsy method on the quality of surgical management in melanoma. J Surg Oncol. 2014;109:775-779.
8. Montgomery BD, Sadler GM. Punch biopsy of pigmented lesions is potentially hazardous. Can Fam Physician. 2009;55:24.
9. Ng JC, Swain S, Dowling JP, et al. The impact of partial biopsy on histopathologic diagnosis of cutaneous melanoma: experience of an Australian tertiary referral service. Arch Dermatol. 2010;146:234-239.
10. Gambichler T, Senger E, Rapp S, et al. Deep shave excision of macular melanocytic nevi with the razor blade biopsy technique. Dermatol Surg. 2000;26:662-666.
11. Ferrandiz L, Moreno-Ramirez D, Camacho FM. Shave excision of common acquired melanocytic nevi: cosmetic outcome, recurrences, and complications. Dermatol Surg. 2005;31(9 Pt 1):1112-1115.
12. D'souza G, Carey TE, William WN Jr, et al. Epidemiology of head and neck squamous cell cancer among HIV-infected patients. J Acquir Immune Defic Syndr. 2014;65:603-610.
13. Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Handbook. 7th ed. New York, NY: Springer; 2010.
14. Elston DM, Stratman EJ, Miller SJ, et al. Skin biopsy. J Am Acad Dermatol. 2016;74:1-16.
15. Zager JS, Hochwald SN, Marzban SS, et al. Shave biopsy is a safe and accurate method for the initial evaluation of melanoma. J Am Coll Surg. 2011;212:454-460.
16. Chanda JJ, Callen JP. Adverse effect of melanoma incision. J Am Acad Dermatol. 1985;13:519-522.
17. Noroozi N, Zakerolhosseini A. Computerized measurement of melanocytic tumor depth in skin histopathological images. Micron. 2015;77:44-56.
18. Nielsen LJ, Lumholt P, Hölmich LR. [Local anaesthesia with vasoconstrictor is safe to use in areas with end-arteries in fingers, toes, noses and ears]. Ugeskr Laeger. 2014;176(44).
19. Häfner HM, Röcken M, Breuninger H. Epinephrine-supplemented local anesthetics for ear and nose surgery: clinical use without complications in more than 10,000 surgical procedures. J Dtsch Dermatol Ges. 2005;3:195-199.
20. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
21. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
22. Ilicki J. Safety of epinephrine in digital nerve blocks: a literature review. J Emerg Med. 2015;49:799-809.
1. Kerr OA, Tidman MJ, Walker JJ, et al. The profile of dermatological problems in primary care. Clin Exp Dermatol. 2010;35:380-383.
2. Hosler GA, Patterson JW. Lentigines, nevi, and melanomas. In: Patterson JW, ed. Weedon’s Skin Pathology. Elsevier; 2015:32,837-901.
3. Coit DG, Andtbacka R, Bichakjian CK, et al. Melanoma. J Natl Compr Canc Netw. 2009;7:250-275.
4. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge S, Greene F, et al, eds. AJCC Cancer Staging Manual. Springer International Publishing; 2017;8:563-585.
5. American Joint Committee on Cancer. Implementation of AJCC 8th Edition Cancer Staging System. Available at: https://cancerstaging.org/About/news/Pages/Implementation-of-AJCC-8th-Edition-Cancer-Staging-System.aspx. Accessed April 2, 2018.
6. Karimipour DJ, Schwartz JL, Wang TS, et al. Microstaging accuracy after subtotal incisional biopsy of cutaneous melanoma. J Am Acad Dermatol. 2005;52:798-802.
7. Kaiser S, Vassell R, Pinckney RG, et al. Clinical impact of biopsy method on the quality of surgical management in melanoma. J Surg Oncol. 2014;109:775-779.
8. Montgomery BD, Sadler GM. Punch biopsy of pigmented lesions is potentially hazardous. Can Fam Physician. 2009;55:24.
9. Ng JC, Swain S, Dowling JP, et al. The impact of partial biopsy on histopathologic diagnosis of cutaneous melanoma: experience of an Australian tertiary referral service. Arch Dermatol. 2010;146:234-239.
10. Gambichler T, Senger E, Rapp S, et al. Deep shave excision of macular melanocytic nevi with the razor blade biopsy technique. Dermatol Surg. 2000;26:662-666.
11. Ferrandiz L, Moreno-Ramirez D, Camacho FM. Shave excision of common acquired melanocytic nevi: cosmetic outcome, recurrences, and complications. Dermatol Surg. 2005;31(9 Pt 1):1112-1115.
12. D'souza G, Carey TE, William WN Jr, et al. Epidemiology of head and neck squamous cell cancer among HIV-infected patients. J Acquir Immune Defic Syndr. 2014;65:603-610.
13. Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Handbook. 7th ed. New York, NY: Springer; 2010.
14. Elston DM, Stratman EJ, Miller SJ, et al. Skin biopsy. J Am Acad Dermatol. 2016;74:1-16.
15. Zager JS, Hochwald SN, Marzban SS, et al. Shave biopsy is a safe and accurate method for the initial evaluation of melanoma. J Am Coll Surg. 2011;212:454-460.
16. Chanda JJ, Callen JP. Adverse effect of melanoma incision. J Am Acad Dermatol. 1985;13:519-522.
17. Noroozi N, Zakerolhosseini A. Computerized measurement of melanocytic tumor depth in skin histopathological images. Micron. 2015;77:44-56.
18. Nielsen LJ, Lumholt P, Hölmich LR. [Local anaesthesia with vasoconstrictor is safe to use in areas with end-arteries in fingers, toes, noses and ears]. Ugeskr Laeger. 2014;176(44).
19. Häfner HM, Röcken M, Breuninger H. Epinephrine-supplemented local anesthetics for ear and nose surgery: clinical use without complications in more than 10,000 surgical procedures. J Dtsch Dermatol Ges. 2005;3:195-199.
20. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
21. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
22. Ilicki J. Safety of epinephrine in digital nerve blocks: a literature review. J Emerg Med. 2015;49:799-809.
From The Journal of Family Practice | 2018;67(5):270-274.