User login
The number of patients who undergo total knee arthroplasty (TKA) and total hip arthroplasty (THA) procedures has significantly increased over the past 2 to 3 decades. As life expectancy in the U.S. increases and medical advances allow patients with preexisting conditions to successfully undergo joint replacements, the demand for these procedures is expected to grow.1 In 2010, the CDC estimated that 719,000 patients underwent TKA procedures and 332,000 patients underwent THA procedures in the U.S.2 Kurtz and colleagues have projected that by 2030 the annual number of TKA procedures will increase to 3.5 million and the number of THA procedures will increase to 572,000.1
Although becoming more prevalent, these procedures are still associated with considerable intra- and postoperative blood loss that may lead to complications and blood transfusions.3 Previous studies have shown that perioperative anemia and red blood cell transfusions are associated with negative outcomes, including increased health care resource utilization; length of hospitalization; pulmonary, septic, wound, or thromboembolic complications; and mortality.4,5
In order to prevent excessive blood loss during TKA and THA procedures, antifibrinolytic agents, such as tranexamic acid, have been used. Tranexamic acid is a synthetic form of lysine that binds to the lysine-binding sites on plasminogen and slows the conversion of plasminogen to plasmin. This interaction inhibits fibrinolysis and theoretically decreases bleeding.6 Because tranexamic acid is an antifibrinolytic, its mechanism of action has raised concerns that it could increase the risk of clotting complications, such as venous thromboembolism and myocardial infarction.7
Several published meta-analyses and systematic reviews have shown that tranexamic acid reduces blood loss in patients undergoing orthopedic surgery. Despite positive results, many study authors have acknowledged limitations in their analyses, such as heterogeneity of study results, small trial size, and varied dosing strategies.3,7-12 There is no FDA-approved tranexamic acid dosing strategy for orthopedic procedures; therefore, its use in TKA and THA procedures is off label. This lack of guidance results in the medication being used at varied doses, timing of doses, and routes of administration with no clear dosing strategy showing the best outcomes.
Tranexamic acid was first used in TKA and THA surgical procedures at the Sioux Falls VA Health Care System (SFVAHCS) in South Dakota in October 2012. The dose used during these procedures was 1 g IV at first surgical incision and 1 g IV at incision closure. The objective of this study was to determine whether this tranexamic acid dosing strategy utilized at SFVAHCS safely improved outcomes related to blood loss.
Methods
A single-center retrospective chart review was performed on all patients who underwent TKA and THA procedures by 4 orthopedic surgeons between January 2010 and August 2015 at SFVAHCS. This study received approval by the local institutional review board and research and development committee in September 2015.
Patients were included in the study if they were aged ≥ 18 years and underwent primary unilateral TKA or THA procedures during the study time frame. Patients were excluded if they underwent bilateral or revision TKA or THA procedures, did not have recorded blood loss measurements during and/or after the procedure, or did not receive tranexamic acid between October 2012 and August 2015 at the standard dosing strategy utilized at SFVAHCS.
Patients who underwent surgery between January 2010 and October 2012 and did not receive tranexamic acid were included in the control groups. The treatment groups contained patients who underwent surgery between October 2012 and August 2015 and received tranexamic acid at the standard SFVAHCS dosing strategy. Patients in the control and treatment groups were divided and compared with patients who underwent the same type of surgery.
The primary endpoint of this study was total blood loss, which included intraoperative and postoperative blood loss. Intraoperative blood loss was measured by a suctioning device that the surgical physician’s assistant used to keep the surgical site clear of bodily fluids. The suctioning device collected blood as well as irrigation fluids used throughout the surgical procedure. The volume of irrigation fluids used during the procedure was subtracted from the total volume collected by the suctioning device to estimate the total blood volume lost during surgery. Sponges and other surgical materials that may have collected blood were not included in the intraoperative blood loss calculation. Postoperative blood loss was collected by a drain that was placed in the surgical site prior to incision closure. The drain collected postoperative blood loss until it was removed 1 day after surgery.
The secondary endpoints for the study were changes in hemoglobin (Hgb) and hematocrit (Hct) from before surgery to after surgery. These changes were calculated by subtracting the lowest measured postoperative Hgb or Hct level within 21 days postsurgery from the closest measured Hgb or Hct level obtained presurgery.
Follow-up appointments were routinely conducted 2 weeks after surgery, so the 21-day time frame would include any laboratory results drawn at these appointments. Other secondary endpoints included the number of patients receiving at least 1 blood transfusion during hospitalization and the number of patients experiencing clotting complications within 30 days of surgery. Postoperative and progress notes were reviewed by a single study investigator in order to record blood transfusions and clotting complications.
All patients who underwent TKA or THA procedures were instructed to stop taking antiplatelet agents 7 days prior to surgery and warfarin 5 days prior to surgery. If patients were determined to be at high risk for thromboembolic complications following warfarin discontinuation, therapeutic doses of low-molecular weight heparin or unfractionated heparin were used as a bridging therapy pre- and postsurgery. Enoxaparin 30 mg twice daily was started the day after surgery in all patients not previously on warfarin therapy prior to surgery to prevent clotting complications. If a patient was on warfarin therapy prior to the procedure but not considered to be at high risk of thromboembolic complications by the surgeon, warfarin was restarted after surgery, and enoxaparin 30 mg twice daily was used until therapeutic international normalized ratio values were obtained. If the patient had ongoing bleeding after the procedure or was determined to be at high risk for bleeding complications, the provider may have delayed anticoagulant use.
Some patients who underwent TKA or THA procedures during the study time frame received periarticular pain injections during surgery. These pain injections included a combination of ropivacaine 200 mg, ketorolac 30 mg, epinephrine 0.5 mg, and clonidine 0.08 mg and were compounded in a sterile mixture with normal saline. Several injections of this mixture were administered into the surgical site to reduce postoperative pain. These periarticular pain injections were first implemented into TKA and THA procedures in August 2012 and were used in patients at the surgeon’s discretion.
Baseline characteristics were analyzed using a chi-square test for categoric variables and an unpaired t test for continuous variables to determine whether any differences were present. Total blood loss, change in Hgb, and change in Hct were analyzed using an unpaired t test. Patients receiving at least 1 blood transfusion during hospitalization and patients experiencing a clotting complication were analyzed using a chi-square test. P values < .05 were considered to indicate statistical significance. Descriptive statistics were calculated using Microsoft Excel (Redmond, WA), and GraphPad Prism (La Jolla, CA) was used for all statistical analyses.
Results
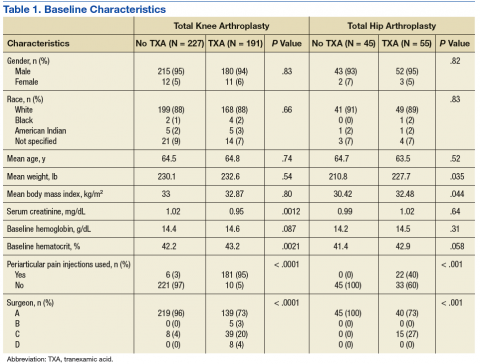
Initially, a total of 443 TKA patients and 111 THA patients were reviewed. Of these patients, 418 TKA patients and 100 THA patients met the inclusion criteria. Due to the retrospective design of this study, not all of the baseline characteristics were equal between groups (Table 1). Most notably, the number of patients who received the periarticular pain injection and the distribution of surgeons performing the procedures were different between groups in both the TKA and THA procedures.
Baseline Hgb levels were not found to be different between groups in either type of procedure; however, the baseline Hct levels of patients undergoing TKA who received tranexamic acid were found to be statistically higher when compared with those who did not receive tranexamic acid. Other baseline characteristics with statistically higher values included average weight and BMI in patients who received tranexamic acid and underwent THA and serum creatinine in patients who did not receive tranexamic acid and underwent TKA.
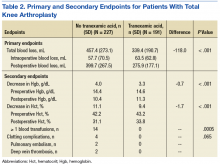
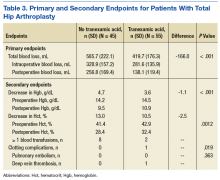
In the primary analysis (Tables 2 and 3), the mean estimated total blood loss in TKA patients was lower in patients who received tranexamic acid than it was in the control group (339.4 mL vs 457.4 mL, P < .001). Patients who underwent THA receiving tranexamic acid similarly had significantly less total blood loss than that of the control group (419.7 mL vs 585.7 mL, P < .001). Consistent with previous studies, patients undergoing TKA procedures in the treatment group when compared to the control group, respectively, were likely to have more blood loss postoperatively (275.9 mL vs 399.7 mL) than intraoperatively (63.5 mL vs 57.7 mL) regardless of tranexamic acid administration.6 On the other hand, patients who had undergone THA were more likely to experience more intraoperative blood loss (281.6 mL in treatment group vs 328.9 mL in control group) than postoperative blood loss (138.1 mL in treatment group vs 256.8 mL in control group) regardless of tranexamic acid administration.
In the secondary analysis, the change between preoperative and postoperative Hgb and Hct had results consistent with the total blood loss results. Patients receiving tranexamic acid in TKA procedures had a lower decrease in Hgb compared with the control group (3.3 mg/dL vs 4.0 mg/dL, P < .001). Similarly, patients undergoing TKA who received tranexamic acid had a smaller decrease in Hct than that of the control group (9.4% vs 11.1%, P < .001). Consistent with the TKA procedure results, patients undergoing THA who received tranexamic acid had a smaller decrease in Hgb (3.6 mg/dL vs 4.7 mg/dL, P < .001) and Hct (10.5% vs 13.0%, P = .0012) than that of the control group.
Patients who did not receive tranexamic acid were more likely to require at least 1 blood transfusion than were patients who received tranexamic acid in TKA (14 patients vs 0 patients, P = .0005) and THA procedures (8 patients vs 2 patients, P = .019). Despite the theoretically increased likelihood of clotting complications with tranexamic acid, no significant differences were observed between the treatment and control groups in either TKA (0 vs 4, P = .065) or THA (1 vs 0, P = .363) procedures.
Discussion
Patients undergoing total joint arthroplasty are at risk for significant blood loss with a potential need for postoperative blood transfusions.6,13 The use of tranexamic acid during these procedures offers a possible solution to decrease blood loss and minimize blood transfusions. This study retrospectively evaluated whether tranexamic acid safely decreased blood loss and the need for blood transfusions at SFVAHCS with the dosing strategy of 1 g IV at first incision and 1 g IV at incision closure.
Patients who received tranexamic acid in TKA and THA procedures had significantly less blood loss than did patients who did not receive tranexamic acid. Patients receiving tranexamic acid also had a significantly lower change from preoperative to postoperative Hgb and Hct levels than did patients who did not receive tranexamic acid in TKA and THA procedures. In addition to decreasing blood loss, the tranexamic acid groups had significantly fewer patients who required blood transfusions than that of the control groups.
This reduction in blood transfusions should be considered clinically significant.
Even though baseline Hct levels were found to be significantly different between the tranexamic acid group and the control group for patients who underwent TKA, medical record documentation indicated that the determination whether postoperative blood transfusions were needed was based primarily on Hgb levels. Baseline Hgb levels were found not to be significantly different between the tranexamic acid and control groups for either TKA or THA procedures. This suggests that at baseline the tranexamic acid and control groups had the same risk of reaching the Hgb threshold where blood transfusions would be required.
There was a significant difference in the proportion of patients receiving the periarticular pain injection of ropivacaine, ketorolac, epinephrine, and clonidine between the groups who received tranexamic acid and those who did not. Originally, this baseline characteristic was theorized to be a major confounder in the primary and secondary analyses because epinephrine is a vasoconstrictor and ketorolac is a reversible antiplatelet agent. However, Vendittoli and colleagues showed that patients receiving these periarticular pain injections during surgery actually had greater total blood loss than did patients who did not receive the injections, although this comparison did not reach statistical significance.14 The Vendittoli and colleagues results suggest that the pain injections did not confound the primary and secondary analyses by aiding in the process of reducing blood loss in these procedures.
Limitations
There are several limitations for extrapolating the results from this study to the general population. Due to the retrospective study design, there was no way to actively control potentially confounding variables during the TKA and THA procedures. Surgeons and surgery teams likely had slightly different techniques and protocols during and after surgery. Several baseline characteristics were not equal between patients who received tranexamic acid and those who did not. Therefore, it is unknown whether these baseline characteristics affected the results of this study. Postoperative anticoagulant use was not recorded and may have differed between study groups, depending on the patient’s risk of thromboembolic complications; however, the drains that collected blood loss were removed prior to the first dose of enoxaparin, which was administered the day after surgery.
Another limitation is that the method of measuring blood loss during and after the procedure was imprecise. Blood not suctioned through the suctioning device during surgery or not collected in the drain after surgery was not measured and may have increased the total blood loss. Hemoglobin and Hct levels also are sensitive to intravascular volume changes. If a patient required more IV fluids during or after a procedure, the fluids may have lowered the Hgb and/or Hct levels by dilution.
Conclusion
This study suggests that using tranexamic acid at a dose of 1 g IV at first incision and 1 g IV at incision closure safely and effectively reduced blood loss and the need for transfusions in patients undergoing TKA and THA procedures at SFVAHCS. Further prospective studies are needed to compare different tranexamic dosing strategies to minimize blood loss during these procedures. ˜
Acknowledgments
This study is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System in South Dakota.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Centers for Disease Control and Prevention. Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. https://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf. Published 2010. Accessed March 15, 2017.
3. Wei Z, Liu M. The effectiveness and safety of tranexamic acid in total hip or knee arthroplasty: a meta-analysis of 2720 cases. Transfus Med. 2015;25(3):151-162.
4. Glance LG, Dick AW, Mukamel DB, et al. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114(2):283-292.
5. Wu WC, Smith TS, Henderson WG, et al. Operative blood loss, blood transfusion, and 30-day mortality in older patients after major noncardiac surgery. Ann Surg. 2010;252(1):283-292.
6. Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86(4):561-565.
7. Gandhi R, Evans HMK, Mahomed SR, Mahomed NN. Tranexamic acid and the reduction of blood loss in total knee and hip arthroplasty: a meta-analysis. BMC Res Notes. 2013;6:184.
8. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
9. Yang ZG, Chen WP, Wu LE. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
10. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
11. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46.
12. Zhou XD, Tao LJ, Li J, Wu LD. Do we really need tranexamic acid in total hip arthroplasty? A meta-analysis of nineteen randomized controlled trials. Arch Orthop Trauma Surg. 2013;133(7):1017-1027.
13. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
14. Vendittoli PA, Makinen P, Drolet P, et al. A multimodal analgesia protocol for total knee arthroplasty: a randomized, controlled study. J Bone Joint Surg Am. 2006;88(2):282-289.
The number of patients who undergo total knee arthroplasty (TKA) and total hip arthroplasty (THA) procedures has significantly increased over the past 2 to 3 decades. As life expectancy in the U.S. increases and medical advances allow patients with preexisting conditions to successfully undergo joint replacements, the demand for these procedures is expected to grow.1 In 2010, the CDC estimated that 719,000 patients underwent TKA procedures and 332,000 patients underwent THA procedures in the U.S.2 Kurtz and colleagues have projected that by 2030 the annual number of TKA procedures will increase to 3.5 million and the number of THA procedures will increase to 572,000.1
Although becoming more prevalent, these procedures are still associated with considerable intra- and postoperative blood loss that may lead to complications and blood transfusions.3 Previous studies have shown that perioperative anemia and red blood cell transfusions are associated with negative outcomes, including increased health care resource utilization; length of hospitalization; pulmonary, septic, wound, or thromboembolic complications; and mortality.4,5
In order to prevent excessive blood loss during TKA and THA procedures, antifibrinolytic agents, such as tranexamic acid, have been used. Tranexamic acid is a synthetic form of lysine that binds to the lysine-binding sites on plasminogen and slows the conversion of plasminogen to plasmin. This interaction inhibits fibrinolysis and theoretically decreases bleeding.6 Because tranexamic acid is an antifibrinolytic, its mechanism of action has raised concerns that it could increase the risk of clotting complications, such as venous thromboembolism and myocardial infarction.7
Several published meta-analyses and systematic reviews have shown that tranexamic acid reduces blood loss in patients undergoing orthopedic surgery. Despite positive results, many study authors have acknowledged limitations in their analyses, such as heterogeneity of study results, small trial size, and varied dosing strategies.3,7-12 There is no FDA-approved tranexamic acid dosing strategy for orthopedic procedures; therefore, its use in TKA and THA procedures is off label. This lack of guidance results in the medication being used at varied doses, timing of doses, and routes of administration with no clear dosing strategy showing the best outcomes.
Tranexamic acid was first used in TKA and THA surgical procedures at the Sioux Falls VA Health Care System (SFVAHCS) in South Dakota in October 2012. The dose used during these procedures was 1 g IV at first surgical incision and 1 g IV at incision closure. The objective of this study was to determine whether this tranexamic acid dosing strategy utilized at SFVAHCS safely improved outcomes related to blood loss.
Methods
A single-center retrospective chart review was performed on all patients who underwent TKA and THA procedures by 4 orthopedic surgeons between January 2010 and August 2015 at SFVAHCS. This study received approval by the local institutional review board and research and development committee in September 2015.
Patients were included in the study if they were aged ≥ 18 years and underwent primary unilateral TKA or THA procedures during the study time frame. Patients were excluded if they underwent bilateral or revision TKA or THA procedures, did not have recorded blood loss measurements during and/or after the procedure, or did not receive tranexamic acid between October 2012 and August 2015 at the standard dosing strategy utilized at SFVAHCS.
Patients who underwent surgery between January 2010 and October 2012 and did not receive tranexamic acid were included in the control groups. The treatment groups contained patients who underwent surgery between October 2012 and August 2015 and received tranexamic acid at the standard SFVAHCS dosing strategy. Patients in the control and treatment groups were divided and compared with patients who underwent the same type of surgery.
The primary endpoint of this study was total blood loss, which included intraoperative and postoperative blood loss. Intraoperative blood loss was measured by a suctioning device that the surgical physician’s assistant used to keep the surgical site clear of bodily fluids. The suctioning device collected blood as well as irrigation fluids used throughout the surgical procedure. The volume of irrigation fluids used during the procedure was subtracted from the total volume collected by the suctioning device to estimate the total blood volume lost during surgery. Sponges and other surgical materials that may have collected blood were not included in the intraoperative blood loss calculation. Postoperative blood loss was collected by a drain that was placed in the surgical site prior to incision closure. The drain collected postoperative blood loss until it was removed 1 day after surgery.
The secondary endpoints for the study were changes in hemoglobin (Hgb) and hematocrit (Hct) from before surgery to after surgery. These changes were calculated by subtracting the lowest measured postoperative Hgb or Hct level within 21 days postsurgery from the closest measured Hgb or Hct level obtained presurgery.
Follow-up appointments were routinely conducted 2 weeks after surgery, so the 21-day time frame would include any laboratory results drawn at these appointments. Other secondary endpoints included the number of patients receiving at least 1 blood transfusion during hospitalization and the number of patients experiencing clotting complications within 30 days of surgery. Postoperative and progress notes were reviewed by a single study investigator in order to record blood transfusions and clotting complications.
All patients who underwent TKA or THA procedures were instructed to stop taking antiplatelet agents 7 days prior to surgery and warfarin 5 days prior to surgery. If patients were determined to be at high risk for thromboembolic complications following warfarin discontinuation, therapeutic doses of low-molecular weight heparin or unfractionated heparin were used as a bridging therapy pre- and postsurgery. Enoxaparin 30 mg twice daily was started the day after surgery in all patients not previously on warfarin therapy prior to surgery to prevent clotting complications. If a patient was on warfarin therapy prior to the procedure but not considered to be at high risk of thromboembolic complications by the surgeon, warfarin was restarted after surgery, and enoxaparin 30 mg twice daily was used until therapeutic international normalized ratio values were obtained. If the patient had ongoing bleeding after the procedure or was determined to be at high risk for bleeding complications, the provider may have delayed anticoagulant use.
Some patients who underwent TKA or THA procedures during the study time frame received periarticular pain injections during surgery. These pain injections included a combination of ropivacaine 200 mg, ketorolac 30 mg, epinephrine 0.5 mg, and clonidine 0.08 mg and were compounded in a sterile mixture with normal saline. Several injections of this mixture were administered into the surgical site to reduce postoperative pain. These periarticular pain injections were first implemented into TKA and THA procedures in August 2012 and were used in patients at the surgeon’s discretion.
Baseline characteristics were analyzed using a chi-square test for categoric variables and an unpaired t test for continuous variables to determine whether any differences were present. Total blood loss, change in Hgb, and change in Hct were analyzed using an unpaired t test. Patients receiving at least 1 blood transfusion during hospitalization and patients experiencing a clotting complication were analyzed using a chi-square test. P values < .05 were considered to indicate statistical significance. Descriptive statistics were calculated using Microsoft Excel (Redmond, WA), and GraphPad Prism (La Jolla, CA) was used for all statistical analyses.
Results
Initially, a total of 443 TKA patients and 111 THA patients were reviewed. Of these patients, 418 TKA patients and 100 THA patients met the inclusion criteria. Due to the retrospective design of this study, not all of the baseline characteristics were equal between groups (Table 1). Most notably, the number of patients who received the periarticular pain injection and the distribution of surgeons performing the procedures were different between groups in both the TKA and THA procedures.
Baseline Hgb levels were not found to be different between groups in either type of procedure; however, the baseline Hct levels of patients undergoing TKA who received tranexamic acid were found to be statistically higher when compared with those who did not receive tranexamic acid. Other baseline characteristics with statistically higher values included average weight and BMI in patients who received tranexamic acid and underwent THA and serum creatinine in patients who did not receive tranexamic acid and underwent TKA.
In the primary analysis (Tables 2 and 3), the mean estimated total blood loss in TKA patients was lower in patients who received tranexamic acid than it was in the control group (339.4 mL vs 457.4 mL, P < .001). Patients who underwent THA receiving tranexamic acid similarly had significantly less total blood loss than that of the control group (419.7 mL vs 585.7 mL, P < .001). Consistent with previous studies, patients undergoing TKA procedures in the treatment group when compared to the control group, respectively, were likely to have more blood loss postoperatively (275.9 mL vs 399.7 mL) than intraoperatively (63.5 mL vs 57.7 mL) regardless of tranexamic acid administration.6 On the other hand, patients who had undergone THA were more likely to experience more intraoperative blood loss (281.6 mL in treatment group vs 328.9 mL in control group) than postoperative blood loss (138.1 mL in treatment group vs 256.8 mL in control group) regardless of tranexamic acid administration.
In the secondary analysis, the change between preoperative and postoperative Hgb and Hct had results consistent with the total blood loss results. Patients receiving tranexamic acid in TKA procedures had a lower decrease in Hgb compared with the control group (3.3 mg/dL vs 4.0 mg/dL, P < .001). Similarly, patients undergoing TKA who received tranexamic acid had a smaller decrease in Hct than that of the control group (9.4% vs 11.1%, P < .001). Consistent with the TKA procedure results, patients undergoing THA who received tranexamic acid had a smaller decrease in Hgb (3.6 mg/dL vs 4.7 mg/dL, P < .001) and Hct (10.5% vs 13.0%, P = .0012) than that of the control group.
Patients who did not receive tranexamic acid were more likely to require at least 1 blood transfusion than were patients who received tranexamic acid in TKA (14 patients vs 0 patients, P = .0005) and THA procedures (8 patients vs 2 patients, P = .019). Despite the theoretically increased likelihood of clotting complications with tranexamic acid, no significant differences were observed between the treatment and control groups in either TKA (0 vs 4, P = .065) or THA (1 vs 0, P = .363) procedures.
Discussion
Patients undergoing total joint arthroplasty are at risk for significant blood loss with a potential need for postoperative blood transfusions.6,13 The use of tranexamic acid during these procedures offers a possible solution to decrease blood loss and minimize blood transfusions. This study retrospectively evaluated whether tranexamic acid safely decreased blood loss and the need for blood transfusions at SFVAHCS with the dosing strategy of 1 g IV at first incision and 1 g IV at incision closure.
Patients who received tranexamic acid in TKA and THA procedures had significantly less blood loss than did patients who did not receive tranexamic acid. Patients receiving tranexamic acid also had a significantly lower change from preoperative to postoperative Hgb and Hct levels than did patients who did not receive tranexamic acid in TKA and THA procedures. In addition to decreasing blood loss, the tranexamic acid groups had significantly fewer patients who required blood transfusions than that of the control groups.
This reduction in blood transfusions should be considered clinically significant.
Even though baseline Hct levels were found to be significantly different between the tranexamic acid group and the control group for patients who underwent TKA, medical record documentation indicated that the determination whether postoperative blood transfusions were needed was based primarily on Hgb levels. Baseline Hgb levels were found not to be significantly different between the tranexamic acid and control groups for either TKA or THA procedures. This suggests that at baseline the tranexamic acid and control groups had the same risk of reaching the Hgb threshold where blood transfusions would be required.
There was a significant difference in the proportion of patients receiving the periarticular pain injection of ropivacaine, ketorolac, epinephrine, and clonidine between the groups who received tranexamic acid and those who did not. Originally, this baseline characteristic was theorized to be a major confounder in the primary and secondary analyses because epinephrine is a vasoconstrictor and ketorolac is a reversible antiplatelet agent. However, Vendittoli and colleagues showed that patients receiving these periarticular pain injections during surgery actually had greater total blood loss than did patients who did not receive the injections, although this comparison did not reach statistical significance.14 The Vendittoli and colleagues results suggest that the pain injections did not confound the primary and secondary analyses by aiding in the process of reducing blood loss in these procedures.
Limitations
There are several limitations for extrapolating the results from this study to the general population. Due to the retrospective study design, there was no way to actively control potentially confounding variables during the TKA and THA procedures. Surgeons and surgery teams likely had slightly different techniques and protocols during and after surgery. Several baseline characteristics were not equal between patients who received tranexamic acid and those who did not. Therefore, it is unknown whether these baseline characteristics affected the results of this study. Postoperative anticoagulant use was not recorded and may have differed between study groups, depending on the patient’s risk of thromboembolic complications; however, the drains that collected blood loss were removed prior to the first dose of enoxaparin, which was administered the day after surgery.
Another limitation is that the method of measuring blood loss during and after the procedure was imprecise. Blood not suctioned through the suctioning device during surgery or not collected in the drain after surgery was not measured and may have increased the total blood loss. Hemoglobin and Hct levels also are sensitive to intravascular volume changes. If a patient required more IV fluids during or after a procedure, the fluids may have lowered the Hgb and/or Hct levels by dilution.
Conclusion
This study suggests that using tranexamic acid at a dose of 1 g IV at first incision and 1 g IV at incision closure safely and effectively reduced blood loss and the need for transfusions in patients undergoing TKA and THA procedures at SFVAHCS. Further prospective studies are needed to compare different tranexamic dosing strategies to minimize blood loss during these procedures. ˜
Acknowledgments
This study is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System in South Dakota.
The number of patients who undergo total knee arthroplasty (TKA) and total hip arthroplasty (THA) procedures has significantly increased over the past 2 to 3 decades. As life expectancy in the U.S. increases and medical advances allow patients with preexisting conditions to successfully undergo joint replacements, the demand for these procedures is expected to grow.1 In 2010, the CDC estimated that 719,000 patients underwent TKA procedures and 332,000 patients underwent THA procedures in the U.S.2 Kurtz and colleagues have projected that by 2030 the annual number of TKA procedures will increase to 3.5 million and the number of THA procedures will increase to 572,000.1
Although becoming more prevalent, these procedures are still associated with considerable intra- and postoperative blood loss that may lead to complications and blood transfusions.3 Previous studies have shown that perioperative anemia and red blood cell transfusions are associated with negative outcomes, including increased health care resource utilization; length of hospitalization; pulmonary, septic, wound, or thromboembolic complications; and mortality.4,5
In order to prevent excessive blood loss during TKA and THA procedures, antifibrinolytic agents, such as tranexamic acid, have been used. Tranexamic acid is a synthetic form of lysine that binds to the lysine-binding sites on plasminogen and slows the conversion of plasminogen to plasmin. This interaction inhibits fibrinolysis and theoretically decreases bleeding.6 Because tranexamic acid is an antifibrinolytic, its mechanism of action has raised concerns that it could increase the risk of clotting complications, such as venous thromboembolism and myocardial infarction.7
Several published meta-analyses and systematic reviews have shown that tranexamic acid reduces blood loss in patients undergoing orthopedic surgery. Despite positive results, many study authors have acknowledged limitations in their analyses, such as heterogeneity of study results, small trial size, and varied dosing strategies.3,7-12 There is no FDA-approved tranexamic acid dosing strategy for orthopedic procedures; therefore, its use in TKA and THA procedures is off label. This lack of guidance results in the medication being used at varied doses, timing of doses, and routes of administration with no clear dosing strategy showing the best outcomes.
Tranexamic acid was first used in TKA and THA surgical procedures at the Sioux Falls VA Health Care System (SFVAHCS) in South Dakota in October 2012. The dose used during these procedures was 1 g IV at first surgical incision and 1 g IV at incision closure. The objective of this study was to determine whether this tranexamic acid dosing strategy utilized at SFVAHCS safely improved outcomes related to blood loss.
Methods
A single-center retrospective chart review was performed on all patients who underwent TKA and THA procedures by 4 orthopedic surgeons between January 2010 and August 2015 at SFVAHCS. This study received approval by the local institutional review board and research and development committee in September 2015.
Patients were included in the study if they were aged ≥ 18 years and underwent primary unilateral TKA or THA procedures during the study time frame. Patients were excluded if they underwent bilateral or revision TKA or THA procedures, did not have recorded blood loss measurements during and/or after the procedure, or did not receive tranexamic acid between October 2012 and August 2015 at the standard dosing strategy utilized at SFVAHCS.
Patients who underwent surgery between January 2010 and October 2012 and did not receive tranexamic acid were included in the control groups. The treatment groups contained patients who underwent surgery between October 2012 and August 2015 and received tranexamic acid at the standard SFVAHCS dosing strategy. Patients in the control and treatment groups were divided and compared with patients who underwent the same type of surgery.
The primary endpoint of this study was total blood loss, which included intraoperative and postoperative blood loss. Intraoperative blood loss was measured by a suctioning device that the surgical physician’s assistant used to keep the surgical site clear of bodily fluids. The suctioning device collected blood as well as irrigation fluids used throughout the surgical procedure. The volume of irrigation fluids used during the procedure was subtracted from the total volume collected by the suctioning device to estimate the total blood volume lost during surgery. Sponges and other surgical materials that may have collected blood were not included in the intraoperative blood loss calculation. Postoperative blood loss was collected by a drain that was placed in the surgical site prior to incision closure. The drain collected postoperative blood loss until it was removed 1 day after surgery.
The secondary endpoints for the study were changes in hemoglobin (Hgb) and hematocrit (Hct) from before surgery to after surgery. These changes were calculated by subtracting the lowest measured postoperative Hgb or Hct level within 21 days postsurgery from the closest measured Hgb or Hct level obtained presurgery.
Follow-up appointments were routinely conducted 2 weeks after surgery, so the 21-day time frame would include any laboratory results drawn at these appointments. Other secondary endpoints included the number of patients receiving at least 1 blood transfusion during hospitalization and the number of patients experiencing clotting complications within 30 days of surgery. Postoperative and progress notes were reviewed by a single study investigator in order to record blood transfusions and clotting complications.
All patients who underwent TKA or THA procedures were instructed to stop taking antiplatelet agents 7 days prior to surgery and warfarin 5 days prior to surgery. If patients were determined to be at high risk for thromboembolic complications following warfarin discontinuation, therapeutic doses of low-molecular weight heparin or unfractionated heparin were used as a bridging therapy pre- and postsurgery. Enoxaparin 30 mg twice daily was started the day after surgery in all patients not previously on warfarin therapy prior to surgery to prevent clotting complications. If a patient was on warfarin therapy prior to the procedure but not considered to be at high risk of thromboembolic complications by the surgeon, warfarin was restarted after surgery, and enoxaparin 30 mg twice daily was used until therapeutic international normalized ratio values were obtained. If the patient had ongoing bleeding after the procedure or was determined to be at high risk for bleeding complications, the provider may have delayed anticoagulant use.
Some patients who underwent TKA or THA procedures during the study time frame received periarticular pain injections during surgery. These pain injections included a combination of ropivacaine 200 mg, ketorolac 30 mg, epinephrine 0.5 mg, and clonidine 0.08 mg and were compounded in a sterile mixture with normal saline. Several injections of this mixture were administered into the surgical site to reduce postoperative pain. These periarticular pain injections were first implemented into TKA and THA procedures in August 2012 and were used in patients at the surgeon’s discretion.
Baseline characteristics were analyzed using a chi-square test for categoric variables and an unpaired t test for continuous variables to determine whether any differences were present. Total blood loss, change in Hgb, and change in Hct were analyzed using an unpaired t test. Patients receiving at least 1 blood transfusion during hospitalization and patients experiencing a clotting complication were analyzed using a chi-square test. P values < .05 were considered to indicate statistical significance. Descriptive statistics were calculated using Microsoft Excel (Redmond, WA), and GraphPad Prism (La Jolla, CA) was used for all statistical analyses.
Results
Initially, a total of 443 TKA patients and 111 THA patients were reviewed. Of these patients, 418 TKA patients and 100 THA patients met the inclusion criteria. Due to the retrospective design of this study, not all of the baseline characteristics were equal between groups (Table 1). Most notably, the number of patients who received the periarticular pain injection and the distribution of surgeons performing the procedures were different between groups in both the TKA and THA procedures.
Baseline Hgb levels were not found to be different between groups in either type of procedure; however, the baseline Hct levels of patients undergoing TKA who received tranexamic acid were found to be statistically higher when compared with those who did not receive tranexamic acid. Other baseline characteristics with statistically higher values included average weight and BMI in patients who received tranexamic acid and underwent THA and serum creatinine in patients who did not receive tranexamic acid and underwent TKA.
In the primary analysis (Tables 2 and 3), the mean estimated total blood loss in TKA patients was lower in patients who received tranexamic acid than it was in the control group (339.4 mL vs 457.4 mL, P < .001). Patients who underwent THA receiving tranexamic acid similarly had significantly less total blood loss than that of the control group (419.7 mL vs 585.7 mL, P < .001). Consistent with previous studies, patients undergoing TKA procedures in the treatment group when compared to the control group, respectively, were likely to have more blood loss postoperatively (275.9 mL vs 399.7 mL) than intraoperatively (63.5 mL vs 57.7 mL) regardless of tranexamic acid administration.6 On the other hand, patients who had undergone THA were more likely to experience more intraoperative blood loss (281.6 mL in treatment group vs 328.9 mL in control group) than postoperative blood loss (138.1 mL in treatment group vs 256.8 mL in control group) regardless of tranexamic acid administration.
In the secondary analysis, the change between preoperative and postoperative Hgb and Hct had results consistent with the total blood loss results. Patients receiving tranexamic acid in TKA procedures had a lower decrease in Hgb compared with the control group (3.3 mg/dL vs 4.0 mg/dL, P < .001). Similarly, patients undergoing TKA who received tranexamic acid had a smaller decrease in Hct than that of the control group (9.4% vs 11.1%, P < .001). Consistent with the TKA procedure results, patients undergoing THA who received tranexamic acid had a smaller decrease in Hgb (3.6 mg/dL vs 4.7 mg/dL, P < .001) and Hct (10.5% vs 13.0%, P = .0012) than that of the control group.
Patients who did not receive tranexamic acid were more likely to require at least 1 blood transfusion than were patients who received tranexamic acid in TKA (14 patients vs 0 patients, P = .0005) and THA procedures (8 patients vs 2 patients, P = .019). Despite the theoretically increased likelihood of clotting complications with tranexamic acid, no significant differences were observed between the treatment and control groups in either TKA (0 vs 4, P = .065) or THA (1 vs 0, P = .363) procedures.
Discussion
Patients undergoing total joint arthroplasty are at risk for significant blood loss with a potential need for postoperative blood transfusions.6,13 The use of tranexamic acid during these procedures offers a possible solution to decrease blood loss and minimize blood transfusions. This study retrospectively evaluated whether tranexamic acid safely decreased blood loss and the need for blood transfusions at SFVAHCS with the dosing strategy of 1 g IV at first incision and 1 g IV at incision closure.
Patients who received tranexamic acid in TKA and THA procedures had significantly less blood loss than did patients who did not receive tranexamic acid. Patients receiving tranexamic acid also had a significantly lower change from preoperative to postoperative Hgb and Hct levels than did patients who did not receive tranexamic acid in TKA and THA procedures. In addition to decreasing blood loss, the tranexamic acid groups had significantly fewer patients who required blood transfusions than that of the control groups.
This reduction in blood transfusions should be considered clinically significant.
Even though baseline Hct levels were found to be significantly different between the tranexamic acid group and the control group for patients who underwent TKA, medical record documentation indicated that the determination whether postoperative blood transfusions were needed was based primarily on Hgb levels. Baseline Hgb levels were found not to be significantly different between the tranexamic acid and control groups for either TKA or THA procedures. This suggests that at baseline the tranexamic acid and control groups had the same risk of reaching the Hgb threshold where blood transfusions would be required.
There was a significant difference in the proportion of patients receiving the periarticular pain injection of ropivacaine, ketorolac, epinephrine, and clonidine between the groups who received tranexamic acid and those who did not. Originally, this baseline characteristic was theorized to be a major confounder in the primary and secondary analyses because epinephrine is a vasoconstrictor and ketorolac is a reversible antiplatelet agent. However, Vendittoli and colleagues showed that patients receiving these periarticular pain injections during surgery actually had greater total blood loss than did patients who did not receive the injections, although this comparison did not reach statistical significance.14 The Vendittoli and colleagues results suggest that the pain injections did not confound the primary and secondary analyses by aiding in the process of reducing blood loss in these procedures.
Limitations
There are several limitations for extrapolating the results from this study to the general population. Due to the retrospective study design, there was no way to actively control potentially confounding variables during the TKA and THA procedures. Surgeons and surgery teams likely had slightly different techniques and protocols during and after surgery. Several baseline characteristics were not equal between patients who received tranexamic acid and those who did not. Therefore, it is unknown whether these baseline characteristics affected the results of this study. Postoperative anticoagulant use was not recorded and may have differed between study groups, depending on the patient’s risk of thromboembolic complications; however, the drains that collected blood loss were removed prior to the first dose of enoxaparin, which was administered the day after surgery.
Another limitation is that the method of measuring blood loss during and after the procedure was imprecise. Blood not suctioned through the suctioning device during surgery or not collected in the drain after surgery was not measured and may have increased the total blood loss. Hemoglobin and Hct levels also are sensitive to intravascular volume changes. If a patient required more IV fluids during or after a procedure, the fluids may have lowered the Hgb and/or Hct levels by dilution.
Conclusion
This study suggests that using tranexamic acid at a dose of 1 g IV at first incision and 1 g IV at incision closure safely and effectively reduced blood loss and the need for transfusions in patients undergoing TKA and THA procedures at SFVAHCS. Further prospective studies are needed to compare different tranexamic dosing strategies to minimize blood loss during these procedures. ˜
Acknowledgments
This study is the result of work supported with resources and the use of facilities at the Sioux Falls VA Health Care System in South Dakota.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Centers for Disease Control and Prevention. Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. https://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf. Published 2010. Accessed March 15, 2017.
3. Wei Z, Liu M. The effectiveness and safety of tranexamic acid in total hip or knee arthroplasty: a meta-analysis of 2720 cases. Transfus Med. 2015;25(3):151-162.
4. Glance LG, Dick AW, Mukamel DB, et al. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114(2):283-292.
5. Wu WC, Smith TS, Henderson WG, et al. Operative blood loss, blood transfusion, and 30-day mortality in older patients after major noncardiac surgery. Ann Surg. 2010;252(1):283-292.
6. Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86(4):561-565.
7. Gandhi R, Evans HMK, Mahomed SR, Mahomed NN. Tranexamic acid and the reduction of blood loss in total knee and hip arthroplasty: a meta-analysis. BMC Res Notes. 2013;6:184.
8. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
9. Yang ZG, Chen WP, Wu LE. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
10. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
11. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46.
12. Zhou XD, Tao LJ, Li J, Wu LD. Do we really need tranexamic acid in total hip arthroplasty? A meta-analysis of nineteen randomized controlled trials. Arch Orthop Trauma Surg. 2013;133(7):1017-1027.
13. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
14. Vendittoli PA, Makinen P, Drolet P, et al. A multimodal analgesia protocol for total knee arthroplasty: a randomized, controlled study. J Bone Joint Surg Am. 2006;88(2):282-289.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Centers for Disease Control and Prevention. Number of all-listed procedures for discharges from short-stay hospitals, by procedure category and age: United States, 2010. https://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf. Published 2010. Accessed March 15, 2017.
3. Wei Z, Liu M. The effectiveness and safety of tranexamic acid in total hip or knee arthroplasty: a meta-analysis of 2720 cases. Transfus Med. 2015;25(3):151-162.
4. Glance LG, Dick AW, Mukamel DB, et al. Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114(2):283-292.
5. Wu WC, Smith TS, Henderson WG, et al. Operative blood loss, blood transfusion, and 30-day mortality in older patients after major noncardiac surgery. Ann Surg. 2010;252(1):283-292.
6. Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86(4):561-565.
7. Gandhi R, Evans HMK, Mahomed SR, Mahomed NN. Tranexamic acid and the reduction of blood loss in total knee and hip arthroplasty: a meta-analysis. BMC Res Notes. 2013;6:184.
8. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585.
9. Yang ZG, Chen WP, Wu LE. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159.
10. Tan J, Chen H, Liu Q, Chen C, Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184(2):880-887.
11. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46.
12. Zhou XD, Tao LJ, Li J, Wu LD. Do we really need tranexamic acid in total hip arthroplasty? A meta-analysis of nineteen randomized controlled trials. Arch Orthop Trauma Surg. 2013;133(7):1017-1027.
13. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2-10.
14. Vendittoli PA, Makinen P, Drolet P, et al. A multimodal analgesia protocol for total knee arthroplasty: a randomized, controlled study. J Bone Joint Surg Am. 2006;88(2):282-289.