User login
ABSTRACT
Cryotherapy is the use of the anti-inflammatory and analgesic properties of ice to facilitate healing. Cryotherapy mediates these salutatory effects by reducing blood flow to the site of injury, down-regulating the production of inflammatory and pain-inducing prostaglandins, and diminishing the conductive ability of nerve endings. It is commonly used postoperatively in orthopedics to decrease analgesic requirements and blood loss as well as to increase range of motion, despite limited literature on its ability to produce such therapeutic effects in clinical practice. This article examines the available literature and the scientific evidence for the use and efficacy of cryotherapy in post-surgical orthopedic patients. It also reviews the potential pitfalls associated with improper use. Overall, this review seeks to provide insight into when, or whether, cryotherapy is appropriate for orthopedic patients during surgical recovery.
Continue to: Cold therapy has been a mainstay of medical treatment...
Cold therapy has been a mainstay of medical treatment since the days of Hippocrates. Initially used by ancient Egyptians to mitigate inflammation and by Hippocrates himself to treat hemorrhage, the therapeutic applications of ice evolved throughout history to become part of the treatment algorithm for a variety of health conditions.1 Ice made an ideal numbing agent for limb amputations and an anesthetic for certain cancers, but truly became ubiquitous when the first cold pack meant for medicinal use was patented in the early 1970s.1,2 Despite their armamentarium of advanced treatment modalities, physicians in the modern era continue to prescribe cryotherapy for their patients, particularly in the field of orthopedics. Most athletes know the “RICE” (Rest, Ice, Compression, Elevation) protocol and utilize it to minimize inflammation associated with soft tissue injuries.
Inflammation is a physiologic response to noxious stimuli. Cell damage results in the production of inflammatory mediators including prostaglandins, which play a crucial role in the vasodilation and pain associated with inflammation. Vasodilation and increased blood flow manifest as swelling, which can cause pain by putting pressure on nerve endings. The inflammatory prostaglandin E2 (PGE2) causes local increases in temperature and mediates pain.3,4 The application of cold therapy attenuates inflammatory microvascular and hemodynamic changes, reducing some of the deleterious effects of inflammation and minimizing pain. Animal models demonstrate that cryotherapy restores functional capillary density, reverses tumor necrosis factor-α (TNF-α)-induced microvasculature damage, and reduces the production of thrombogenic thromboxanes in injured soft tissue.5 Additionally, cold therapy after knee arthroscopy is associated with lower concentrations of PGE2 in the knee.3 Local cooling acts at the cellular level to decrease edema, reduce pain, and slow blood flow to the affected area, with the overall effect of alleviating inflammation.4,5
Cryotherapy is standard practice in postoperative orthopedic care, but there is limited literature demonstrating its efficacy in this setting. In addition, the advent of more advanced wearable cooling systems necessitates a thorough comparison of the various cryotherapy mechanisms both from healthcare and economic perspectives. The goal of this article is to examine the benefits of cryotherapy in the postoperative management of orthopedic surgical interventions and to review the effectiveness of differing types of cryotherapy. A secondary goal of this article is to review the literature on the adverse effects of cryotherapy in order to increase physician awareness of this issue and highlight the importance of patient education when utilizing cryotherapy postoperatively.
BENEFITS OF CRYOTHERAPY
Three standard types of cryotherapy are prescribed as postoperative therapy in orthopedics: compressive cryotherapy, continuous flow cryotherapy, and the application of ice. All aim to decrease the amount of inflammation of the surgical site, reduce patient pain, and aid in the recovery process. The application of ice or other cooling pack devices without compression is the most commonly used method, likely because it is the most economical and user-friendly cryotherapy option. Compressive cryotherapy is the application of ice or an ice pack secured to the site with a bandage or other device in a manner that also applies pressure to the site of injury. Finally, continuous flow cryotherapy systems are typically connected to a refrigeration control unit and apply compressive cooling through the uninterrupted flow of cold water or gas through a wrap around the injured site. Examples include the Game Ready® (CoolSystems, Inc.), Cryo/Cuff® IC Cooler (DJO Global), and Hilotherm Homecare (Hilotherm GmbH) systems, which are marketed as an improvement over traditional forms of cold therapy, as they are capable of cooling for hours at a time, allow for nighttime use, and provide the operator with temperature control.6-8
Postoperative cryotherapy is prescribed for a wide variety of orthopedic procedures, including anterior cruciate ligament (ACL) reconstruction surgery, rotator cuff surgery, and total knee arthroplasty (TKA). Current literature includes many studies monitoring postoperative outcomes in patients using cryotherapy as part of their treatment regimen, with the primary endpoints being visual analog scale (VAS) scores, analgesic consumption, and range of motion (ROM).9-16 As demonstrated by in Table 1, these studies do not provide conclusive evidence that cryotherapy significantly alters postoperative outcomes, despite its ubiquitous use by the orthopedics community. In fact, the literature reflects a seeming lack of consensus regarding the effect of cryotherapy on analgesic requirements, pain, and joint mobility following procedures. Interestingly, of the studies represented in Table 1, only half analyzed all 3 postoperative measures (analgesic consumption, pain, and ROM). Furthermore, solely Morsi13 concluded that cryotherapy resulted in significant improvements in all 3 outcome measures in a trial involving only 30 patients. Kullenberg and colleagues12 performed the largest study, but still included only 86 patients. In addition, all the studies focused on 1 joint or procedure. Thus, despite evidence that cryotherapy reduces inflammation at a molecular level, current literature does not unequivocally support the common belief that cryotherapy benefits patients in practice. More robust studies that include an analysis of analgesic consumption, VAS scores, and ROM (at minimum) and compare the relative efficacy of cryotherapy across joint types and procedures are necessary to determine whether postoperative cryotherapy in orthopedics is appropriate.
Table 1. Results from Studies that Compared Cryotherapy to Standard Care Within the First 2 Weeks Following Surgery
Author | Joint/Procedure Type | Number of Trial Participants | Cryotherapy Type | Analgesic Consumption | VAS Score | ROM |
Yu et al9 | Elbow arthrolysis | 59 | Continuous flow cryotherapy (Cryo/Cuff®; DJO Global) | No significant difference | Cryotherapy significantly decreased scores up to POD 7 (P < 0.05) | No significant difference |
Dambros et al10 | ACL reconstruction | 25 | Ice pack | Xa | No significant difference | No significant difference |
Leegwater et al11 | Hip arthroplasty | 30 | Continuous flow cryotherapy (Game Ready®; CoolSystems, Inc.) | Trend towards lower use (No significant difference) | No significant difference | Xa |
Kullenberg et al12 | Knee arthroplasty | 86 | Continuous flow cryotherapy (Cryo/Cuff®) | No significant difference | No significant difference | Significantly improved at POD 7 and POD 21 |
Morsi13 | Knee arthroplasty | 30 | Continuous flow cryotherapy | Significantly lower consumption (P < 0.01) | Cryotherapy significantly decreased scores (P < 0.001) | Significantly improved at POD 7; No significant difference 6 weeks postoperative |
Singh et al14 | Open vs arthroscopic shoulder procedures | 70 | Continuous flow cryotherapy (Breg Polar Care Glacier® Cold Therapy unit; Breg Inc.) | Xa | Cryotherapy significantly decreased scores at arthroscopic POD 14 (P = 0.043); No significant difference for open procedures | Xa |
Saito et al15 | Hip arthroplasty | 46 | Continuous flow cryotherapy (Icing System 2000; Nippon Sigmax Co., Ltd.) | Significantly lower epidural analgesic use (P < 0.001); no significant difference in adjunct analgesic consumption | Cryotherapy significantly decreased scores POD 1-4 (P < 0.05) | Xa |
Gibbons et al16 | Knee arthroplasty | 60 | Continuous flow cryotherapy (Cryo/Cuff®) | No significant difference | No significant difference | No significant difference |
Continue to: ADVANCED CRYOTHERAPY DEVICES...
ADVANCED CRYOTHERAPY DEVICES
Several recent studies explored the relative postoperative benefits of advanced cryotherapeutics in lieu of the traditional ice pack.6,7,17-21 As reflected in Table 2, these studies, much like the literature comparing cryotherapy to the control, do not reveal significant benefits of continuous flow cryotherapy after surgery. In fact, the only outcome measure that was found to differ significantly in more than 1 study was ROM. Though the makers of advanced cryotherapy systems market them as a vast improvement over traditional forms of cold therapy, there is insufficient evidence to support such claims. Even the most robust study that included 280 patients failed to show significant differences in the analgesic use and ROM after surgery.20 Of note, all but 1 study compared traditional and advanced cryotherapy following procedures on the knee. Additional research exploring outcomes after surgery on other joints is necessary before any conclusions can be made regarding postoperative benefits or risks within orthopedics more generally.
Author | Joint / Procedure Type | Number of Trial Participants | Analgesic Consumption | VAS Score | ROM |
Kraeutler et al17 | Rotator cuff repair or subacromial decompression | 46 | No significant difference | No significant difference | Xa |
Thienpont18 | Knee arthroplasty | 116 | No significant difference | No significant difference | Significant reduction in active flexion with advanced cryotherapy (P = 0.02); No significant difference in other ROM tests |
Woolf et al19 | Knee arthroplasty | 53 | Decrease in night pain through POD 2 only | Xa | Xa |
Su et al20 | Knee arthroplasty | 280 | Significantly lower use with cryotherapy up to POD 14; No significant difference thereafter | Xa | No difference |
Barber21 | ACL reconstruction | 87 | Significantly lower use with cryotherapy POD 1 and 2 (P = 0.035) | Cryotherapy significantly decreased scores only POD 1 (P < 0.01) | Greater ROM with cryotherapy POD 7 (P < 0.03) |
Ruffilli et al6 | ACL reconstruction | 47 | No difference | Xa | Greater ROM with cryotherapy (P < 0.0001) |
Kuyucu et al7 | Knee arthroplasty | 60 | Xa | Cryotherapy significantly decreased scores (P < 0.05) | Greater ROM with cryotherapy (P < 0.05) |
RISKS AND ADVERSE EFFECTS OF CRYOTHERAPY
A rigorous analysis of the benefits of cryotherapy ought to incorporate other factors in addition to improvements in analgesic consumption, VAS score, and ROM. These include the financial and time investment involved in the use of continuous flow cryotherapy, which the majority of studies do not consider. Though many authors acknowledge that continuous flow cryotherapy is expensive, to our knowledge, none have yet performed a formal economic analysis of the cost of advanced cryotherapy to the patient as well as to the healthcare system at large.6,7,13,18,22-24 Dickinson and colleagues24 calculated the total cost of cryotherapy and rehabilitation following rotator cuff repair, but addressed only the up-front cost of the cold therapy system. For context, Table 3 summarizes the retail cost of the most popular cryotherapy devices on the market. Based on this information alone, it seems reasonable to conclude that these systems are associated with significantly more cost than traditional forms of cold therapy, and therefore would be an undesirable option for patients or hospital systems. Nevertheless, cost considerations are more nuanced than a simple comparison of price, necessitating more advanced economic analyses. Substantial savings may be on the table if future studies are able to prove postoperative cryotherapy shortens hospital stays, reduces medication costs, and results in fewer physical therapy sessions. Moreover, if all this is true, patients may experience quicker recovery and have overall greater post-procedure satisfaction.
Table 3. Cost of Most Popular Cryotherapy Units
System | Cost |
Cryo/Cuff® IC Cooler (DJO Global) | $125 |
DonJoy IceMan Classic (DJO Global) | $169 |
The Polar Care Kodiak (Breg, Inc.) | $180 |
Patient education required for optimal use of advanced cold therapy is another aspect of cryotherapy that is poorly represented in the literature. As Dickinson and colleagues24 point out, because it eliminates some dependency on the patient to remember to ice appropriately, continuous flow cryotherapy may have a positive impact on compliance and therefore yield improved outcomes.24 Hospital staff may be required to spend additional time with patients. However, this is necessary to ensure proper understanding on how to operate the system and avoid adverse outcomes. Patients may also find the large coolers inconvenient and may therefore be reluctant to use them, finding traditional ice more manageable. Future studies should consider gathering data on patient education, compliance, and overall reception/satisfaction to complete a more holistic investigation of the role of postoperative cryotherapy in orthopedics.
Cryotherapy is not without adverse outcomes, which have been documented primarily in the form of case study reports. Relevant case studies cited adverse outcomes including frostbite/skin loss, compartment syndrome, and perniosis as potential dangers of postoperative cryotherapy in orthopedics (Table 4).25-30 As an example, a patient recovering from patellar-tendon repair experienced bilateral frostbite and skin loss following 2 weeks of uninterrupted use of cryotherapy without any barrier between his skin and the system.29 A similar case study described 2 female patients, one recovering from a TKA and the other from a tibial revision of arthroplasty, who used cryotherapy systems without cessation and experienced frostbite and skin necrosis over the entirety of their knees.26 A third case study exploring 4 incidents of patellar frostbite and necrosis following knee arthroscopies proposed that poor patient understanding of proper cryotherapy use as well as poor recognition of the signs of frostbite contributed to these adverse outcomes. Furthermore, the cryotherapy brace used by all 4 patients included a feature designed to counteract patellar inflammation that also may have increased the likelihood of frostbite in this area due to poor tissue insulation. The authors noted that following the incidents, the makers of the brace removed patellar coverage to prevent future occurrences.30
Author | Adverse Effect | Procedure/Location |
Brown and Hahn25 | Frostbite | Bunionectomy; hallux valgus correction/feet |
Dundon et al26 | Skin necrosis | TKA/patella |
Khajavi et al27 | Compartment syndrome | Arthroscopic osteochondral autograft transfer/calf |
King et al28 | Perniosis | ACL reconstruction/knee |
Lee et al29 | Frostbite | Patellar-tendon repair/knees |
McGuire and Hendricks30 | Frostbite | Knee arthroscopy/patella |
Abbreviations: ACL, anterior cruciate ligament; TKA, total knee arthroplasty.
Frostbite linked to cryotherapy has also occurred following orthopedic procedures outside the knee. Brown and Hahn25 described 2 young females who developed skin necrosis following podiatric surgeries and constant cold therapy for roughly a week. Notably, 1 patient had cold sensitivity, which likely put her at an increased baseline risk of experiencing frostbite while using cryotherapy. Tissue necrosis is not the only danger of cold therapy discussed in this study. Surprisingly, 1 patient also developed compartment syndrome.25 Khajavi and colleagues27 also documented postoperative compartment syndrome in a patient following an arthroscopic osteochondral autograft transfer, which they attributed to reperfusion injury in the wake of first-degree frostbite. Hospital personnel also instructed this patient to use his cryotherapy system without interruption at the coldest temperature tolerable, contrary to manufacturer’s instructions.27
Continue to: King and colleagues...
King and colleagues28 described 2 cases of patients complaining of nodules, papules, and plaques soon after ACL reconstruction and the initiation of cryotherapy. A histological examination of their skin lesions demonstrated the presence of a perivascular and periadnexal superficial and deep lymphocytic infiltrate associated with perniosis. Dermatologists associated the perniosis with the cryotherapy cuff adhesive mechanisms, as their locations matched those of the lesions and symptoms subsided after cessation of cuff usage.28
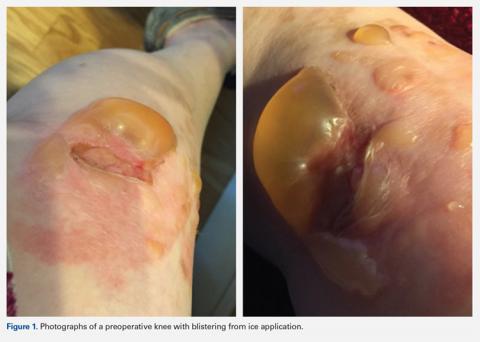
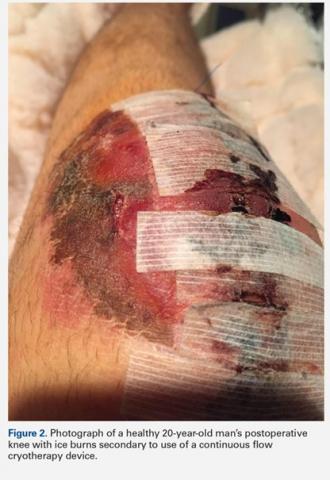
Cases of adverse effects with perioperative cryotherapy have also occurred at our own institution. The authors obtained informed written consent from the patients to print and publish their images. In 2 separate incidents, patients overdid icing and experienced rather extreme side effects including burns and blisters (Figures 1 and 2). In light of these adverse events, the physicians have questioned whether RICE ought to be part of their standard perioperative recommendations. These physicians are not alone in their uncertainty. Interestingly, even Mirkin,31 who coined the RICE mnemonic, now believes that consistent icing post-injury actually inhibits the body’s natural inflammatory healing response, delaying rather than speeding recovery, and suggests that icing ought to be used for pain control only.
DISCUSSION
Though there is ample literature supporting the common belief that cryotherapy minimizes inflammation at the cellular level, whether or not it results in meaningful improvements in post-surgical orthopedic outcomes remains unclear. Table 1 reflects a dearth of evidence to support the widespread current practice of cold therapy following orthopedic procedures, but few studies could demonstrate a significant difference in the analgesic use, VAS score, or ROM between cryotherapy and control groups. It is worth noting that these studies used different cryotherapy systems. Though in theory the continuous flow cryotherapy systems are similarly designed, there are potential differences among them that have not been controlled for in this analysis. All studies had <90 participants and focused on a single joint or procedure, making it difficult to draw large scale conclusions about the utility of cold therapy in the postoperative orthopedic population at large. Furthermore, researchers measured endpoints at a range of time intervals that were inconsistent across studies. In some cases, the significance of the impact of cryotherapy on recovery within a single study differed based on the time point at which researchers measured outcomes.12-14 This raises the question as to whether cryotherapy has no benefits, or whether they are simply time-dependent. Future studies should seek to ascertain whether there is a postoperative time window in which cryotherapy could potentially expedite the recovery process.
Similarly, Table 2 shows a lack of consensus regarding the effect of advanced cryotherapy when compared to traditional ice application on pain, analgesic use, and joint mobility after surgery. However, all but 1 of these studies focused on knee procedures. Therefore, our findings may not be applicable to orthopedic surgeries on other joints. Nevertheless, the use of advanced cryotherapy in postoperative orthopedic care may wane if researchers continue to show that it is no more beneficial than its far less expensive counterpart of ice and an ace bandage.
The case studies discussed in this review serve as cautionary tales of the dangers of cryotherapy when used improperly. Though frostbite and subsequent tissue necrosis seem most common, physicians should be made aware that compartment syndrome and perniosis are also possible consequences. Orthopedic patients perhaps have an increased risk of developing these side effects due to the nature of their injuries and the large cutaneous surface area to which cryotherapy is applied. These outcomes could seemingly be avoided with improved educational initiatives targeted at both healthcare personnel and patients. Orthopedic surgeons might consider adding a short, instructive video focusing on proper usage as well as signs of adverse events to their discharge protocol to limit occurrences of these pitfalls associated with cryotherapy.
CONCLUSION
There is inadequate literature to support the of use postoperative cryotherapy of any kind in the field of orthopedics at this time. More robust, standardized studies, and a formidable economic analysis of advanced cold therapy systems are necessary before physicians prescribing cryotherapy can be confident that they are augmenting patient recovery. Nevertheless, as new developments in medicinal cryotherapy occur, it may be possible for the orthopedic community to wield its salutatory effects to limit complications and improve post-surgical outcomes.
1. Freiman N, Bouganim N. History of cryotherapy. Dermatol Online J. 2005;11(2):9.
2. Spencer JH, inventor; Nortech Lab Inc, assignee. Device for use as a hot and cold compress. US patent US3780537A. December 25, 1973.
3. Stålman A, Berglund L, Dungnerc E, Arner P, Felländer-Tsai L. Temperature-sensitive release of prostaglandin E₂ and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2106/JBJS.J.01790.
4. Kawabata A. Prostaglandin E2 and pain--an update. Biol Pharm Bull. 2011;34(8):1170-1173. doi:10.1248/bpb.34.1170.
5. Schaser KD, Stover JF, Melcher I, et al. Local cooling restores microcirculatory hemodynamics after closed soft-tissue trauma in rats. J Trauma. 2006;61(3):642-649. doi:10.1097/01.ta.0000174922.08781.2f.
6. Ruffilli A, Buda R, Castagnini F, et al. Temperature-controlled continuous cold flow device versus traditional icing regimen following anterior cruciate ligament reconstruction: a prospective randomized comparative trial. Arch Orthop Trauma Surg. 2015;135(10):1405-1410. doi:10.1007/s00402-015-2273-z.
7. Kuyucu E, Bülbül M, Kara A, Koçyiğit F, Erdil M. Is cold therapy really efficient after knee arthroplasty? Ann Med Surg. 2015;4(4):475-478. doi:10.1016/j.amsu.2015.10.019.
8. Martin SS, Spindler KP, Tarter JW, Detwiler K, Petersen HA. Cryotherapy: an effective modality for decreasing intraarticular temperature after knee arthroscopy. Am J Sports Med. 2001;29(3):288-291. doi:10.1177/03635465010290030501.
9. Yu SY, Chen S, Yan HD, Fan CY. Effect of cryotherapy after elbow arthrolysis: A prospective, single-blinded, randomized controlled study. Arch Phys Med Rehabil. 2015;96(1):1-6. doi:10.1016/j.apmr.2014.08.011.
10. Dambros C, Martimbianco ALC, Polachini LO, Lahoz GL, Chamlian TR, Cohen M. Effectiveness of cryotherapy after anterior cruciate ligament reconstruction. Acta Ortop Bras. 2012;20(5):285-290. doi:10.1590/S1413-78522012000500008.
11. Leegwater NC, Nolte PA, de Korte N, et al. The efficacy of continuous-flow cryo and cyclic compression therapy after hip fracture surgery on postoperative pain: design of a prospective, open-label, parallel, multicenter, randomized controlled, clinical trial. BMC Musculoskelet Disord. 2016;17(1):153. doi:10.1186/s12891-016-1000-4.
12. Kullenberg B, Ylipää S, Söderlund K, Resch S. Postoperative cryotherapy after total knee arthroplasty: a prospective study of 86 patients. J Arthroplasty. 2006;21(8):1175-1179. doi:10.1016/j.arth.2006.02.159.
13. Morsi E. Continuous-flow cold therapy after total knee arthroplasty. J Arthroplasty. 2002;17(6):718-722. doi:10.1054/arth.2002.33562.
14. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: A prospective, randomized investigation. J Shoulder Elb Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
15. Saito N, Horiuchi H, Kobayashi S, Nawata M, Takaoka K. Continuous local cooling for pain relief following total hip arthroplasty. J Arthroplasty. 2004;19(3):334-337. doi:10.1016/j.arth.2003.10.011.
16. Gibbons C, Solan M, Ricketts D, Patterson M. Cryotherapy compared with Robert Jones bandage after total knee replacement: A prospective randomized trial. Int Orthop. 2001;25(4):250-252. doi:10.1007/s002640100227.
17. Kraeutler MJ, Reynolds KA, Long C, McCarty EC. Compressive cryotherapy versus ice-a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elb Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
18. Thienpont E. Does Advanced Cryotherapy Reduce Pain and Narcotic Consumption After Knee Arthroplasty? Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
19. Woolf SK, Barfield WR, Merrill KD, McBryde AM Jr. Comparison of a continuous temperature-controlled cryotherapy device to a simple icing regimen following outpatient knee arthroscopy. J Knee Surg. 2008;21(1):15-19.
20. Su EP, Perna M, Boettner F, et al. A prospective, multi-center, randomised trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620X.94B11.30832.
21. Barber F. A comparison of crushed ice and continuous flow cold therapy. Am J Knee Surg. 2000;13(2):97-101.
22. Demoulin C, Brouwers M, Darot S, Gillet P, Crielaard JM, Vanderthommen M. Comparison of gaseous cryotherapy with more traditional forms of cryotherapy following total knee arthroplasty. Ann Phys Rehabil Med. 2012;55(4):229-240. doi:10.1016/j.rehab.2012.03.004.
23. Mumith A, Pavlou P, Barrett M, Thurston B, Garrett S. Enhancing postoperative rehabilitation following knee arthroplasty using a new cryotherapy product: a prospective study. Geriatr Orthop Surg Rehabil. 2015;6(4):316-321. doi:10.1177/2151458515609722.
24. Dickinson RN, Kuhn JE, Bergner JL, Rizzone KH. A systematic review of cost-effective treatment of postoperative rotator cuff repairs. J Shoulder Elb Surg. 2017;26(5):915-922. doi:10.1016/j.jse.2017.02.009.
25. Brown WC, Hahn DB. Frostbite of the Feet After Cryotherapy: A Report of Two Cases. J Foot Ankle Surg. 2009;48(5):577-580. doi:10.1053/j.jfas.2009.06.003.
26. Dundon JM, Rymer MC, Johnson RM. Total patellar skin loss from cryotherapy after total knee arthroplasty. J Arthroplasty. 2013;28(2):376.e5-e7. doi:10.1016/j.arth.2012.05.024.
27. Khajavi K, Pavelko T, Mishra A. Compartment syndrome arising from use of an electronic cooling pad. Am J Sports Med. 2004;32(6):1538-1541. doi:10.1177/0363546503262191.
28. King J, Plotner A, Adams B. Perniosis induced by a cold therapy system. Arch Dermatol. 2012;148(9):1101-1102.
29. Lee CK, Pardun J, Buntic R, Kiehn M, Brooks D, Buncke HJ. Severe frostbite of the knees after cryotherapy. Orthopedics. 2007;30(1):63-64.
30. McGuire DA, Hendricks SD. Incidences of frostbite in arthroscopic knee surgery postoperative cryotherapy rehabilitation. Arthroscopy. 2006;22(10):1141.e1-e6. doi:10.1016/j.arthro.2005.06.027.
31. Mirkin G. Why Ice Delays Recovery. http://www.drmirkin.com/fitness/why-ice-delays-recovery.html. Published September 16, 2015. Accessed July 17, 2017.
ABSTRACT
Cryotherapy is the use of the anti-inflammatory and analgesic properties of ice to facilitate healing. Cryotherapy mediates these salutatory effects by reducing blood flow to the site of injury, down-regulating the production of inflammatory and pain-inducing prostaglandins, and diminishing the conductive ability of nerve endings. It is commonly used postoperatively in orthopedics to decrease analgesic requirements and blood loss as well as to increase range of motion, despite limited literature on its ability to produce such therapeutic effects in clinical practice. This article examines the available literature and the scientific evidence for the use and efficacy of cryotherapy in post-surgical orthopedic patients. It also reviews the potential pitfalls associated with improper use. Overall, this review seeks to provide insight into when, or whether, cryotherapy is appropriate for orthopedic patients during surgical recovery.
Continue to: Cold therapy has been a mainstay of medical treatment...
Cold therapy has been a mainstay of medical treatment since the days of Hippocrates. Initially used by ancient Egyptians to mitigate inflammation and by Hippocrates himself to treat hemorrhage, the therapeutic applications of ice evolved throughout history to become part of the treatment algorithm for a variety of health conditions.1 Ice made an ideal numbing agent for limb amputations and an anesthetic for certain cancers, but truly became ubiquitous when the first cold pack meant for medicinal use was patented in the early 1970s.1,2 Despite their armamentarium of advanced treatment modalities, physicians in the modern era continue to prescribe cryotherapy for their patients, particularly in the field of orthopedics. Most athletes know the “RICE” (Rest, Ice, Compression, Elevation) protocol and utilize it to minimize inflammation associated with soft tissue injuries.
Inflammation is a physiologic response to noxious stimuli. Cell damage results in the production of inflammatory mediators including prostaglandins, which play a crucial role in the vasodilation and pain associated with inflammation. Vasodilation and increased blood flow manifest as swelling, which can cause pain by putting pressure on nerve endings. The inflammatory prostaglandin E2 (PGE2) causes local increases in temperature and mediates pain.3,4 The application of cold therapy attenuates inflammatory microvascular and hemodynamic changes, reducing some of the deleterious effects of inflammation and minimizing pain. Animal models demonstrate that cryotherapy restores functional capillary density, reverses tumor necrosis factor-α (TNF-α)-induced microvasculature damage, and reduces the production of thrombogenic thromboxanes in injured soft tissue.5 Additionally, cold therapy after knee arthroscopy is associated with lower concentrations of PGE2 in the knee.3 Local cooling acts at the cellular level to decrease edema, reduce pain, and slow blood flow to the affected area, with the overall effect of alleviating inflammation.4,5
Cryotherapy is standard practice in postoperative orthopedic care, but there is limited literature demonstrating its efficacy in this setting. In addition, the advent of more advanced wearable cooling systems necessitates a thorough comparison of the various cryotherapy mechanisms both from healthcare and economic perspectives. The goal of this article is to examine the benefits of cryotherapy in the postoperative management of orthopedic surgical interventions and to review the effectiveness of differing types of cryotherapy. A secondary goal of this article is to review the literature on the adverse effects of cryotherapy in order to increase physician awareness of this issue and highlight the importance of patient education when utilizing cryotherapy postoperatively.
BENEFITS OF CRYOTHERAPY
Three standard types of cryotherapy are prescribed as postoperative therapy in orthopedics: compressive cryotherapy, continuous flow cryotherapy, and the application of ice. All aim to decrease the amount of inflammation of the surgical site, reduce patient pain, and aid in the recovery process. The application of ice or other cooling pack devices without compression is the most commonly used method, likely because it is the most economical and user-friendly cryotherapy option. Compressive cryotherapy is the application of ice or an ice pack secured to the site with a bandage or other device in a manner that also applies pressure to the site of injury. Finally, continuous flow cryotherapy systems are typically connected to a refrigeration control unit and apply compressive cooling through the uninterrupted flow of cold water or gas through a wrap around the injured site. Examples include the Game Ready® (CoolSystems, Inc.), Cryo/Cuff® IC Cooler (DJO Global), and Hilotherm Homecare (Hilotherm GmbH) systems, which are marketed as an improvement over traditional forms of cold therapy, as they are capable of cooling for hours at a time, allow for nighttime use, and provide the operator with temperature control.6-8
Postoperative cryotherapy is prescribed for a wide variety of orthopedic procedures, including anterior cruciate ligament (ACL) reconstruction surgery, rotator cuff surgery, and total knee arthroplasty (TKA). Current literature includes many studies monitoring postoperative outcomes in patients using cryotherapy as part of their treatment regimen, with the primary endpoints being visual analog scale (VAS) scores, analgesic consumption, and range of motion (ROM).9-16 As demonstrated by in Table 1, these studies do not provide conclusive evidence that cryotherapy significantly alters postoperative outcomes, despite its ubiquitous use by the orthopedics community. In fact, the literature reflects a seeming lack of consensus regarding the effect of cryotherapy on analgesic requirements, pain, and joint mobility following procedures. Interestingly, of the studies represented in Table 1, only half analyzed all 3 postoperative measures (analgesic consumption, pain, and ROM). Furthermore, solely Morsi13 concluded that cryotherapy resulted in significant improvements in all 3 outcome measures in a trial involving only 30 patients. Kullenberg and colleagues12 performed the largest study, but still included only 86 patients. In addition, all the studies focused on 1 joint or procedure. Thus, despite evidence that cryotherapy reduces inflammation at a molecular level, current literature does not unequivocally support the common belief that cryotherapy benefits patients in practice. More robust studies that include an analysis of analgesic consumption, VAS scores, and ROM (at minimum) and compare the relative efficacy of cryotherapy across joint types and procedures are necessary to determine whether postoperative cryotherapy in orthopedics is appropriate.
Table 1. Results from Studies that Compared Cryotherapy to Standard Care Within the First 2 Weeks Following Surgery
Author | Joint/Procedure Type | Number of Trial Participants | Cryotherapy Type | Analgesic Consumption | VAS Score | ROM |
Yu et al9 | Elbow arthrolysis | 59 | Continuous flow cryotherapy (Cryo/Cuff®; DJO Global) | No significant difference | Cryotherapy significantly decreased scores up to POD 7 (P < 0.05) | No significant difference |
Dambros et al10 | ACL reconstruction | 25 | Ice pack | Xa | No significant difference | No significant difference |
Leegwater et al11 | Hip arthroplasty | 30 | Continuous flow cryotherapy (Game Ready®; CoolSystems, Inc.) | Trend towards lower use (No significant difference) | No significant difference | Xa |
Kullenberg et al12 | Knee arthroplasty | 86 | Continuous flow cryotherapy (Cryo/Cuff®) | No significant difference | No significant difference | Significantly improved at POD 7 and POD 21 |
Morsi13 | Knee arthroplasty | 30 | Continuous flow cryotherapy | Significantly lower consumption (P < 0.01) | Cryotherapy significantly decreased scores (P < 0.001) | Significantly improved at POD 7; No significant difference 6 weeks postoperative |
Singh et al14 | Open vs arthroscopic shoulder procedures | 70 | Continuous flow cryotherapy (Breg Polar Care Glacier® Cold Therapy unit; Breg Inc.) | Xa | Cryotherapy significantly decreased scores at arthroscopic POD 14 (P = 0.043); No significant difference for open procedures | Xa |
Saito et al15 | Hip arthroplasty | 46 | Continuous flow cryotherapy (Icing System 2000; Nippon Sigmax Co., Ltd.) | Significantly lower epidural analgesic use (P < 0.001); no significant difference in adjunct analgesic consumption | Cryotherapy significantly decreased scores POD 1-4 (P < 0.05) | Xa |
Gibbons et al16 | Knee arthroplasty | 60 | Continuous flow cryotherapy (Cryo/Cuff®) | No significant difference | No significant difference | No significant difference |
Continue to: ADVANCED CRYOTHERAPY DEVICES...
ADVANCED CRYOTHERAPY DEVICES
Several recent studies explored the relative postoperative benefits of advanced cryotherapeutics in lieu of the traditional ice pack.6,7,17-21 As reflected in Table 2, these studies, much like the literature comparing cryotherapy to the control, do not reveal significant benefits of continuous flow cryotherapy after surgery. In fact, the only outcome measure that was found to differ significantly in more than 1 study was ROM. Though the makers of advanced cryotherapy systems market them as a vast improvement over traditional forms of cold therapy, there is insufficient evidence to support such claims. Even the most robust study that included 280 patients failed to show significant differences in the analgesic use and ROM after surgery.20 Of note, all but 1 study compared traditional and advanced cryotherapy following procedures on the knee. Additional research exploring outcomes after surgery on other joints is necessary before any conclusions can be made regarding postoperative benefits or risks within orthopedics more generally.
Author | Joint / Procedure Type | Number of Trial Participants | Analgesic Consumption | VAS Score | ROM |
Kraeutler et al17 | Rotator cuff repair or subacromial decompression | 46 | No significant difference | No significant difference | Xa |
Thienpont18 | Knee arthroplasty | 116 | No significant difference | No significant difference | Significant reduction in active flexion with advanced cryotherapy (P = 0.02); No significant difference in other ROM tests |
Woolf et al19 | Knee arthroplasty | 53 | Decrease in night pain through POD 2 only | Xa | Xa |
Su et al20 | Knee arthroplasty | 280 | Significantly lower use with cryotherapy up to POD 14; No significant difference thereafter | Xa | No difference |
Barber21 | ACL reconstruction | 87 | Significantly lower use with cryotherapy POD 1 and 2 (P = 0.035) | Cryotherapy significantly decreased scores only POD 1 (P < 0.01) | Greater ROM with cryotherapy POD 7 (P < 0.03) |
Ruffilli et al6 | ACL reconstruction | 47 | No difference | Xa | Greater ROM with cryotherapy (P < 0.0001) |
Kuyucu et al7 | Knee arthroplasty | 60 | Xa | Cryotherapy significantly decreased scores (P < 0.05) | Greater ROM with cryotherapy (P < 0.05) |
RISKS AND ADVERSE EFFECTS OF CRYOTHERAPY
A rigorous analysis of the benefits of cryotherapy ought to incorporate other factors in addition to improvements in analgesic consumption, VAS score, and ROM. These include the financial and time investment involved in the use of continuous flow cryotherapy, which the majority of studies do not consider. Though many authors acknowledge that continuous flow cryotherapy is expensive, to our knowledge, none have yet performed a formal economic analysis of the cost of advanced cryotherapy to the patient as well as to the healthcare system at large.6,7,13,18,22-24 Dickinson and colleagues24 calculated the total cost of cryotherapy and rehabilitation following rotator cuff repair, but addressed only the up-front cost of the cold therapy system. For context, Table 3 summarizes the retail cost of the most popular cryotherapy devices on the market. Based on this information alone, it seems reasonable to conclude that these systems are associated with significantly more cost than traditional forms of cold therapy, and therefore would be an undesirable option for patients or hospital systems. Nevertheless, cost considerations are more nuanced than a simple comparison of price, necessitating more advanced economic analyses. Substantial savings may be on the table if future studies are able to prove postoperative cryotherapy shortens hospital stays, reduces medication costs, and results in fewer physical therapy sessions. Moreover, if all this is true, patients may experience quicker recovery and have overall greater post-procedure satisfaction.
Table 3. Cost of Most Popular Cryotherapy Units
System | Cost |
Cryo/Cuff® IC Cooler (DJO Global) | $125 |
DonJoy IceMan Classic (DJO Global) | $169 |
The Polar Care Kodiak (Breg, Inc.) | $180 |
Patient education required for optimal use of advanced cold therapy is another aspect of cryotherapy that is poorly represented in the literature. As Dickinson and colleagues24 point out, because it eliminates some dependency on the patient to remember to ice appropriately, continuous flow cryotherapy may have a positive impact on compliance and therefore yield improved outcomes.24 Hospital staff may be required to spend additional time with patients. However, this is necessary to ensure proper understanding on how to operate the system and avoid adverse outcomes. Patients may also find the large coolers inconvenient and may therefore be reluctant to use them, finding traditional ice more manageable. Future studies should consider gathering data on patient education, compliance, and overall reception/satisfaction to complete a more holistic investigation of the role of postoperative cryotherapy in orthopedics.
Cryotherapy is not without adverse outcomes, which have been documented primarily in the form of case study reports. Relevant case studies cited adverse outcomes including frostbite/skin loss, compartment syndrome, and perniosis as potential dangers of postoperative cryotherapy in orthopedics (Table 4).25-30 As an example, a patient recovering from patellar-tendon repair experienced bilateral frostbite and skin loss following 2 weeks of uninterrupted use of cryotherapy without any barrier between his skin and the system.29 A similar case study described 2 female patients, one recovering from a TKA and the other from a tibial revision of arthroplasty, who used cryotherapy systems without cessation and experienced frostbite and skin necrosis over the entirety of their knees.26 A third case study exploring 4 incidents of patellar frostbite and necrosis following knee arthroscopies proposed that poor patient understanding of proper cryotherapy use as well as poor recognition of the signs of frostbite contributed to these adverse outcomes. Furthermore, the cryotherapy brace used by all 4 patients included a feature designed to counteract patellar inflammation that also may have increased the likelihood of frostbite in this area due to poor tissue insulation. The authors noted that following the incidents, the makers of the brace removed patellar coverage to prevent future occurrences.30
Author | Adverse Effect | Procedure/Location |
Brown and Hahn25 | Frostbite | Bunionectomy; hallux valgus correction/feet |
Dundon et al26 | Skin necrosis | TKA/patella |
Khajavi et al27 | Compartment syndrome | Arthroscopic osteochondral autograft transfer/calf |
King et al28 | Perniosis | ACL reconstruction/knee |
Lee et al29 | Frostbite | Patellar-tendon repair/knees |
McGuire and Hendricks30 | Frostbite | Knee arthroscopy/patella |
Abbreviations: ACL, anterior cruciate ligament; TKA, total knee arthroplasty.
Frostbite linked to cryotherapy has also occurred following orthopedic procedures outside the knee. Brown and Hahn25 described 2 young females who developed skin necrosis following podiatric surgeries and constant cold therapy for roughly a week. Notably, 1 patient had cold sensitivity, which likely put her at an increased baseline risk of experiencing frostbite while using cryotherapy. Tissue necrosis is not the only danger of cold therapy discussed in this study. Surprisingly, 1 patient also developed compartment syndrome.25 Khajavi and colleagues27 also documented postoperative compartment syndrome in a patient following an arthroscopic osteochondral autograft transfer, which they attributed to reperfusion injury in the wake of first-degree frostbite. Hospital personnel also instructed this patient to use his cryotherapy system without interruption at the coldest temperature tolerable, contrary to manufacturer’s instructions.27
Continue to: King and colleagues...
King and colleagues28 described 2 cases of patients complaining of nodules, papules, and plaques soon after ACL reconstruction and the initiation of cryotherapy. A histological examination of their skin lesions demonstrated the presence of a perivascular and periadnexal superficial and deep lymphocytic infiltrate associated with perniosis. Dermatologists associated the perniosis with the cryotherapy cuff adhesive mechanisms, as their locations matched those of the lesions and symptoms subsided after cessation of cuff usage.28
Cases of adverse effects with perioperative cryotherapy have also occurred at our own institution. The authors obtained informed written consent from the patients to print and publish their images. In 2 separate incidents, patients overdid icing and experienced rather extreme side effects including burns and blisters (Figures 1 and 2). In light of these adverse events, the physicians have questioned whether RICE ought to be part of their standard perioperative recommendations. These physicians are not alone in their uncertainty. Interestingly, even Mirkin,31 who coined the RICE mnemonic, now believes that consistent icing post-injury actually inhibits the body’s natural inflammatory healing response, delaying rather than speeding recovery, and suggests that icing ought to be used for pain control only.
DISCUSSION
Though there is ample literature supporting the common belief that cryotherapy minimizes inflammation at the cellular level, whether or not it results in meaningful improvements in post-surgical orthopedic outcomes remains unclear. Table 1 reflects a dearth of evidence to support the widespread current practice of cold therapy following orthopedic procedures, but few studies could demonstrate a significant difference in the analgesic use, VAS score, or ROM between cryotherapy and control groups. It is worth noting that these studies used different cryotherapy systems. Though in theory the continuous flow cryotherapy systems are similarly designed, there are potential differences among them that have not been controlled for in this analysis. All studies had <90 participants and focused on a single joint or procedure, making it difficult to draw large scale conclusions about the utility of cold therapy in the postoperative orthopedic population at large. Furthermore, researchers measured endpoints at a range of time intervals that were inconsistent across studies. In some cases, the significance of the impact of cryotherapy on recovery within a single study differed based on the time point at which researchers measured outcomes.12-14 This raises the question as to whether cryotherapy has no benefits, or whether they are simply time-dependent. Future studies should seek to ascertain whether there is a postoperative time window in which cryotherapy could potentially expedite the recovery process.
Similarly, Table 2 shows a lack of consensus regarding the effect of advanced cryotherapy when compared to traditional ice application on pain, analgesic use, and joint mobility after surgery. However, all but 1 of these studies focused on knee procedures. Therefore, our findings may not be applicable to orthopedic surgeries on other joints. Nevertheless, the use of advanced cryotherapy in postoperative orthopedic care may wane if researchers continue to show that it is no more beneficial than its far less expensive counterpart of ice and an ace bandage.
The case studies discussed in this review serve as cautionary tales of the dangers of cryotherapy when used improperly. Though frostbite and subsequent tissue necrosis seem most common, physicians should be made aware that compartment syndrome and perniosis are also possible consequences. Orthopedic patients perhaps have an increased risk of developing these side effects due to the nature of their injuries and the large cutaneous surface area to which cryotherapy is applied. These outcomes could seemingly be avoided with improved educational initiatives targeted at both healthcare personnel and patients. Orthopedic surgeons might consider adding a short, instructive video focusing on proper usage as well as signs of adverse events to their discharge protocol to limit occurrences of these pitfalls associated with cryotherapy.
CONCLUSION
There is inadequate literature to support the of use postoperative cryotherapy of any kind in the field of orthopedics at this time. More robust, standardized studies, and a formidable economic analysis of advanced cold therapy systems are necessary before physicians prescribing cryotherapy can be confident that they are augmenting patient recovery. Nevertheless, as new developments in medicinal cryotherapy occur, it may be possible for the orthopedic community to wield its salutatory effects to limit complications and improve post-surgical outcomes.
ABSTRACT
Cryotherapy is the use of the anti-inflammatory and analgesic properties of ice to facilitate healing. Cryotherapy mediates these salutatory effects by reducing blood flow to the site of injury, down-regulating the production of inflammatory and pain-inducing prostaglandins, and diminishing the conductive ability of nerve endings. It is commonly used postoperatively in orthopedics to decrease analgesic requirements and blood loss as well as to increase range of motion, despite limited literature on its ability to produce such therapeutic effects in clinical practice. This article examines the available literature and the scientific evidence for the use and efficacy of cryotherapy in post-surgical orthopedic patients. It also reviews the potential pitfalls associated with improper use. Overall, this review seeks to provide insight into when, or whether, cryotherapy is appropriate for orthopedic patients during surgical recovery.
Continue to: Cold therapy has been a mainstay of medical treatment...
Cold therapy has been a mainstay of medical treatment since the days of Hippocrates. Initially used by ancient Egyptians to mitigate inflammation and by Hippocrates himself to treat hemorrhage, the therapeutic applications of ice evolved throughout history to become part of the treatment algorithm for a variety of health conditions.1 Ice made an ideal numbing agent for limb amputations and an anesthetic for certain cancers, but truly became ubiquitous when the first cold pack meant for medicinal use was patented in the early 1970s.1,2 Despite their armamentarium of advanced treatment modalities, physicians in the modern era continue to prescribe cryotherapy for their patients, particularly in the field of orthopedics. Most athletes know the “RICE” (Rest, Ice, Compression, Elevation) protocol and utilize it to minimize inflammation associated with soft tissue injuries.
Inflammation is a physiologic response to noxious stimuli. Cell damage results in the production of inflammatory mediators including prostaglandins, which play a crucial role in the vasodilation and pain associated with inflammation. Vasodilation and increased blood flow manifest as swelling, which can cause pain by putting pressure on nerve endings. The inflammatory prostaglandin E2 (PGE2) causes local increases in temperature and mediates pain.3,4 The application of cold therapy attenuates inflammatory microvascular and hemodynamic changes, reducing some of the deleterious effects of inflammation and minimizing pain. Animal models demonstrate that cryotherapy restores functional capillary density, reverses tumor necrosis factor-α (TNF-α)-induced microvasculature damage, and reduces the production of thrombogenic thromboxanes in injured soft tissue.5 Additionally, cold therapy after knee arthroscopy is associated with lower concentrations of PGE2 in the knee.3 Local cooling acts at the cellular level to decrease edema, reduce pain, and slow blood flow to the affected area, with the overall effect of alleviating inflammation.4,5
Cryotherapy is standard practice in postoperative orthopedic care, but there is limited literature demonstrating its efficacy in this setting. In addition, the advent of more advanced wearable cooling systems necessitates a thorough comparison of the various cryotherapy mechanisms both from healthcare and economic perspectives. The goal of this article is to examine the benefits of cryotherapy in the postoperative management of orthopedic surgical interventions and to review the effectiveness of differing types of cryotherapy. A secondary goal of this article is to review the literature on the adverse effects of cryotherapy in order to increase physician awareness of this issue and highlight the importance of patient education when utilizing cryotherapy postoperatively.
BENEFITS OF CRYOTHERAPY
Three standard types of cryotherapy are prescribed as postoperative therapy in orthopedics: compressive cryotherapy, continuous flow cryotherapy, and the application of ice. All aim to decrease the amount of inflammation of the surgical site, reduce patient pain, and aid in the recovery process. The application of ice or other cooling pack devices without compression is the most commonly used method, likely because it is the most economical and user-friendly cryotherapy option. Compressive cryotherapy is the application of ice or an ice pack secured to the site with a bandage or other device in a manner that also applies pressure to the site of injury. Finally, continuous flow cryotherapy systems are typically connected to a refrigeration control unit and apply compressive cooling through the uninterrupted flow of cold water or gas through a wrap around the injured site. Examples include the Game Ready® (CoolSystems, Inc.), Cryo/Cuff® IC Cooler (DJO Global), and Hilotherm Homecare (Hilotherm GmbH) systems, which are marketed as an improvement over traditional forms of cold therapy, as they are capable of cooling for hours at a time, allow for nighttime use, and provide the operator with temperature control.6-8
Postoperative cryotherapy is prescribed for a wide variety of orthopedic procedures, including anterior cruciate ligament (ACL) reconstruction surgery, rotator cuff surgery, and total knee arthroplasty (TKA). Current literature includes many studies monitoring postoperative outcomes in patients using cryotherapy as part of their treatment regimen, with the primary endpoints being visual analog scale (VAS) scores, analgesic consumption, and range of motion (ROM).9-16 As demonstrated by in Table 1, these studies do not provide conclusive evidence that cryotherapy significantly alters postoperative outcomes, despite its ubiquitous use by the orthopedics community. In fact, the literature reflects a seeming lack of consensus regarding the effect of cryotherapy on analgesic requirements, pain, and joint mobility following procedures. Interestingly, of the studies represented in Table 1, only half analyzed all 3 postoperative measures (analgesic consumption, pain, and ROM). Furthermore, solely Morsi13 concluded that cryotherapy resulted in significant improvements in all 3 outcome measures in a trial involving only 30 patients. Kullenberg and colleagues12 performed the largest study, but still included only 86 patients. In addition, all the studies focused on 1 joint or procedure. Thus, despite evidence that cryotherapy reduces inflammation at a molecular level, current literature does not unequivocally support the common belief that cryotherapy benefits patients in practice. More robust studies that include an analysis of analgesic consumption, VAS scores, and ROM (at minimum) and compare the relative efficacy of cryotherapy across joint types and procedures are necessary to determine whether postoperative cryotherapy in orthopedics is appropriate.
Table 1. Results from Studies that Compared Cryotherapy to Standard Care Within the First 2 Weeks Following Surgery
Author | Joint/Procedure Type | Number of Trial Participants | Cryotherapy Type | Analgesic Consumption | VAS Score | ROM |
Yu et al9 | Elbow arthrolysis | 59 | Continuous flow cryotherapy (Cryo/Cuff®; DJO Global) | No significant difference | Cryotherapy significantly decreased scores up to POD 7 (P < 0.05) | No significant difference |
Dambros et al10 | ACL reconstruction | 25 | Ice pack | Xa | No significant difference | No significant difference |
Leegwater et al11 | Hip arthroplasty | 30 | Continuous flow cryotherapy (Game Ready®; CoolSystems, Inc.) | Trend towards lower use (No significant difference) | No significant difference | Xa |
Kullenberg et al12 | Knee arthroplasty | 86 | Continuous flow cryotherapy (Cryo/Cuff®) | No significant difference | No significant difference | Significantly improved at POD 7 and POD 21 |
Morsi13 | Knee arthroplasty | 30 | Continuous flow cryotherapy | Significantly lower consumption (P < 0.01) | Cryotherapy significantly decreased scores (P < 0.001) | Significantly improved at POD 7; No significant difference 6 weeks postoperative |
Singh et al14 | Open vs arthroscopic shoulder procedures | 70 | Continuous flow cryotherapy (Breg Polar Care Glacier® Cold Therapy unit; Breg Inc.) | Xa | Cryotherapy significantly decreased scores at arthroscopic POD 14 (P = 0.043); No significant difference for open procedures | Xa |
Saito et al15 | Hip arthroplasty | 46 | Continuous flow cryotherapy (Icing System 2000; Nippon Sigmax Co., Ltd.) | Significantly lower epidural analgesic use (P < 0.001); no significant difference in adjunct analgesic consumption | Cryotherapy significantly decreased scores POD 1-4 (P < 0.05) | Xa |
Gibbons et al16 | Knee arthroplasty | 60 | Continuous flow cryotherapy (Cryo/Cuff®) | No significant difference | No significant difference | No significant difference |
Continue to: ADVANCED CRYOTHERAPY DEVICES...
ADVANCED CRYOTHERAPY DEVICES
Several recent studies explored the relative postoperative benefits of advanced cryotherapeutics in lieu of the traditional ice pack.6,7,17-21 As reflected in Table 2, these studies, much like the literature comparing cryotherapy to the control, do not reveal significant benefits of continuous flow cryotherapy after surgery. In fact, the only outcome measure that was found to differ significantly in more than 1 study was ROM. Though the makers of advanced cryotherapy systems market them as a vast improvement over traditional forms of cold therapy, there is insufficient evidence to support such claims. Even the most robust study that included 280 patients failed to show significant differences in the analgesic use and ROM after surgery.20 Of note, all but 1 study compared traditional and advanced cryotherapy following procedures on the knee. Additional research exploring outcomes after surgery on other joints is necessary before any conclusions can be made regarding postoperative benefits or risks within orthopedics more generally.
Author | Joint / Procedure Type | Number of Trial Participants | Analgesic Consumption | VAS Score | ROM |
Kraeutler et al17 | Rotator cuff repair or subacromial decompression | 46 | No significant difference | No significant difference | Xa |
Thienpont18 | Knee arthroplasty | 116 | No significant difference | No significant difference | Significant reduction in active flexion with advanced cryotherapy (P = 0.02); No significant difference in other ROM tests |
Woolf et al19 | Knee arthroplasty | 53 | Decrease in night pain through POD 2 only | Xa | Xa |
Su et al20 | Knee arthroplasty | 280 | Significantly lower use with cryotherapy up to POD 14; No significant difference thereafter | Xa | No difference |
Barber21 | ACL reconstruction | 87 | Significantly lower use with cryotherapy POD 1 and 2 (P = 0.035) | Cryotherapy significantly decreased scores only POD 1 (P < 0.01) | Greater ROM with cryotherapy POD 7 (P < 0.03) |
Ruffilli et al6 | ACL reconstruction | 47 | No difference | Xa | Greater ROM with cryotherapy (P < 0.0001) |
Kuyucu et al7 | Knee arthroplasty | 60 | Xa | Cryotherapy significantly decreased scores (P < 0.05) | Greater ROM with cryotherapy (P < 0.05) |
RISKS AND ADVERSE EFFECTS OF CRYOTHERAPY
A rigorous analysis of the benefits of cryotherapy ought to incorporate other factors in addition to improvements in analgesic consumption, VAS score, and ROM. These include the financial and time investment involved in the use of continuous flow cryotherapy, which the majority of studies do not consider. Though many authors acknowledge that continuous flow cryotherapy is expensive, to our knowledge, none have yet performed a formal economic analysis of the cost of advanced cryotherapy to the patient as well as to the healthcare system at large.6,7,13,18,22-24 Dickinson and colleagues24 calculated the total cost of cryotherapy and rehabilitation following rotator cuff repair, but addressed only the up-front cost of the cold therapy system. For context, Table 3 summarizes the retail cost of the most popular cryotherapy devices on the market. Based on this information alone, it seems reasonable to conclude that these systems are associated with significantly more cost than traditional forms of cold therapy, and therefore would be an undesirable option for patients or hospital systems. Nevertheless, cost considerations are more nuanced than a simple comparison of price, necessitating more advanced economic analyses. Substantial savings may be on the table if future studies are able to prove postoperative cryotherapy shortens hospital stays, reduces medication costs, and results in fewer physical therapy sessions. Moreover, if all this is true, patients may experience quicker recovery and have overall greater post-procedure satisfaction.
Table 3. Cost of Most Popular Cryotherapy Units
System | Cost |
Cryo/Cuff® IC Cooler (DJO Global) | $125 |
DonJoy IceMan Classic (DJO Global) | $169 |
The Polar Care Kodiak (Breg, Inc.) | $180 |
Patient education required for optimal use of advanced cold therapy is another aspect of cryotherapy that is poorly represented in the literature. As Dickinson and colleagues24 point out, because it eliminates some dependency on the patient to remember to ice appropriately, continuous flow cryotherapy may have a positive impact on compliance and therefore yield improved outcomes.24 Hospital staff may be required to spend additional time with patients. However, this is necessary to ensure proper understanding on how to operate the system and avoid adverse outcomes. Patients may also find the large coolers inconvenient and may therefore be reluctant to use them, finding traditional ice more manageable. Future studies should consider gathering data on patient education, compliance, and overall reception/satisfaction to complete a more holistic investigation of the role of postoperative cryotherapy in orthopedics.
Cryotherapy is not without adverse outcomes, which have been documented primarily in the form of case study reports. Relevant case studies cited adverse outcomes including frostbite/skin loss, compartment syndrome, and perniosis as potential dangers of postoperative cryotherapy in orthopedics (Table 4).25-30 As an example, a patient recovering from patellar-tendon repair experienced bilateral frostbite and skin loss following 2 weeks of uninterrupted use of cryotherapy without any barrier between his skin and the system.29 A similar case study described 2 female patients, one recovering from a TKA and the other from a tibial revision of arthroplasty, who used cryotherapy systems without cessation and experienced frostbite and skin necrosis over the entirety of their knees.26 A third case study exploring 4 incidents of patellar frostbite and necrosis following knee arthroscopies proposed that poor patient understanding of proper cryotherapy use as well as poor recognition of the signs of frostbite contributed to these adverse outcomes. Furthermore, the cryotherapy brace used by all 4 patients included a feature designed to counteract patellar inflammation that also may have increased the likelihood of frostbite in this area due to poor tissue insulation. The authors noted that following the incidents, the makers of the brace removed patellar coverage to prevent future occurrences.30
Author | Adverse Effect | Procedure/Location |
Brown and Hahn25 | Frostbite | Bunionectomy; hallux valgus correction/feet |
Dundon et al26 | Skin necrosis | TKA/patella |
Khajavi et al27 | Compartment syndrome | Arthroscopic osteochondral autograft transfer/calf |
King et al28 | Perniosis | ACL reconstruction/knee |
Lee et al29 | Frostbite | Patellar-tendon repair/knees |
McGuire and Hendricks30 | Frostbite | Knee arthroscopy/patella |
Abbreviations: ACL, anterior cruciate ligament; TKA, total knee arthroplasty.
Frostbite linked to cryotherapy has also occurred following orthopedic procedures outside the knee. Brown and Hahn25 described 2 young females who developed skin necrosis following podiatric surgeries and constant cold therapy for roughly a week. Notably, 1 patient had cold sensitivity, which likely put her at an increased baseline risk of experiencing frostbite while using cryotherapy. Tissue necrosis is not the only danger of cold therapy discussed in this study. Surprisingly, 1 patient also developed compartment syndrome.25 Khajavi and colleagues27 also documented postoperative compartment syndrome in a patient following an arthroscopic osteochondral autograft transfer, which they attributed to reperfusion injury in the wake of first-degree frostbite. Hospital personnel also instructed this patient to use his cryotherapy system without interruption at the coldest temperature tolerable, contrary to manufacturer’s instructions.27
Continue to: King and colleagues...
King and colleagues28 described 2 cases of patients complaining of nodules, papules, and plaques soon after ACL reconstruction and the initiation of cryotherapy. A histological examination of their skin lesions demonstrated the presence of a perivascular and periadnexal superficial and deep lymphocytic infiltrate associated with perniosis. Dermatologists associated the perniosis with the cryotherapy cuff adhesive mechanisms, as their locations matched those of the lesions and symptoms subsided after cessation of cuff usage.28
Cases of adverse effects with perioperative cryotherapy have also occurred at our own institution. The authors obtained informed written consent from the patients to print and publish their images. In 2 separate incidents, patients overdid icing and experienced rather extreme side effects including burns and blisters (Figures 1 and 2). In light of these adverse events, the physicians have questioned whether RICE ought to be part of their standard perioperative recommendations. These physicians are not alone in their uncertainty. Interestingly, even Mirkin,31 who coined the RICE mnemonic, now believes that consistent icing post-injury actually inhibits the body’s natural inflammatory healing response, delaying rather than speeding recovery, and suggests that icing ought to be used for pain control only.
DISCUSSION
Though there is ample literature supporting the common belief that cryotherapy minimizes inflammation at the cellular level, whether or not it results in meaningful improvements in post-surgical orthopedic outcomes remains unclear. Table 1 reflects a dearth of evidence to support the widespread current practice of cold therapy following orthopedic procedures, but few studies could demonstrate a significant difference in the analgesic use, VAS score, or ROM between cryotherapy and control groups. It is worth noting that these studies used different cryotherapy systems. Though in theory the continuous flow cryotherapy systems are similarly designed, there are potential differences among them that have not been controlled for in this analysis. All studies had <90 participants and focused on a single joint or procedure, making it difficult to draw large scale conclusions about the utility of cold therapy in the postoperative orthopedic population at large. Furthermore, researchers measured endpoints at a range of time intervals that were inconsistent across studies. In some cases, the significance of the impact of cryotherapy on recovery within a single study differed based on the time point at which researchers measured outcomes.12-14 This raises the question as to whether cryotherapy has no benefits, or whether they are simply time-dependent. Future studies should seek to ascertain whether there is a postoperative time window in which cryotherapy could potentially expedite the recovery process.
Similarly, Table 2 shows a lack of consensus regarding the effect of advanced cryotherapy when compared to traditional ice application on pain, analgesic use, and joint mobility after surgery. However, all but 1 of these studies focused on knee procedures. Therefore, our findings may not be applicable to orthopedic surgeries on other joints. Nevertheless, the use of advanced cryotherapy in postoperative orthopedic care may wane if researchers continue to show that it is no more beneficial than its far less expensive counterpart of ice and an ace bandage.
The case studies discussed in this review serve as cautionary tales of the dangers of cryotherapy when used improperly. Though frostbite and subsequent tissue necrosis seem most common, physicians should be made aware that compartment syndrome and perniosis are also possible consequences. Orthopedic patients perhaps have an increased risk of developing these side effects due to the nature of their injuries and the large cutaneous surface area to which cryotherapy is applied. These outcomes could seemingly be avoided with improved educational initiatives targeted at both healthcare personnel and patients. Orthopedic surgeons might consider adding a short, instructive video focusing on proper usage as well as signs of adverse events to their discharge protocol to limit occurrences of these pitfalls associated with cryotherapy.
CONCLUSION
There is inadequate literature to support the of use postoperative cryotherapy of any kind in the field of orthopedics at this time. More robust, standardized studies, and a formidable economic analysis of advanced cold therapy systems are necessary before physicians prescribing cryotherapy can be confident that they are augmenting patient recovery. Nevertheless, as new developments in medicinal cryotherapy occur, it may be possible for the orthopedic community to wield its salutatory effects to limit complications and improve post-surgical outcomes.
1. Freiman N, Bouganim N. History of cryotherapy. Dermatol Online J. 2005;11(2):9.
2. Spencer JH, inventor; Nortech Lab Inc, assignee. Device for use as a hot and cold compress. US patent US3780537A. December 25, 1973.
3. Stålman A, Berglund L, Dungnerc E, Arner P, Felländer-Tsai L. Temperature-sensitive release of prostaglandin E₂ and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2106/JBJS.J.01790.
4. Kawabata A. Prostaglandin E2 and pain--an update. Biol Pharm Bull. 2011;34(8):1170-1173. doi:10.1248/bpb.34.1170.
5. Schaser KD, Stover JF, Melcher I, et al. Local cooling restores microcirculatory hemodynamics after closed soft-tissue trauma in rats. J Trauma. 2006;61(3):642-649. doi:10.1097/01.ta.0000174922.08781.2f.
6. Ruffilli A, Buda R, Castagnini F, et al. Temperature-controlled continuous cold flow device versus traditional icing regimen following anterior cruciate ligament reconstruction: a prospective randomized comparative trial. Arch Orthop Trauma Surg. 2015;135(10):1405-1410. doi:10.1007/s00402-015-2273-z.
7. Kuyucu E, Bülbül M, Kara A, Koçyiğit F, Erdil M. Is cold therapy really efficient after knee arthroplasty? Ann Med Surg. 2015;4(4):475-478. doi:10.1016/j.amsu.2015.10.019.
8. Martin SS, Spindler KP, Tarter JW, Detwiler K, Petersen HA. Cryotherapy: an effective modality for decreasing intraarticular temperature after knee arthroscopy. Am J Sports Med. 2001;29(3):288-291. doi:10.1177/03635465010290030501.
9. Yu SY, Chen S, Yan HD, Fan CY. Effect of cryotherapy after elbow arthrolysis: A prospective, single-blinded, randomized controlled study. Arch Phys Med Rehabil. 2015;96(1):1-6. doi:10.1016/j.apmr.2014.08.011.
10. Dambros C, Martimbianco ALC, Polachini LO, Lahoz GL, Chamlian TR, Cohen M. Effectiveness of cryotherapy after anterior cruciate ligament reconstruction. Acta Ortop Bras. 2012;20(5):285-290. doi:10.1590/S1413-78522012000500008.
11. Leegwater NC, Nolte PA, de Korte N, et al. The efficacy of continuous-flow cryo and cyclic compression therapy after hip fracture surgery on postoperative pain: design of a prospective, open-label, parallel, multicenter, randomized controlled, clinical trial. BMC Musculoskelet Disord. 2016;17(1):153. doi:10.1186/s12891-016-1000-4.
12. Kullenberg B, Ylipää S, Söderlund K, Resch S. Postoperative cryotherapy after total knee arthroplasty: a prospective study of 86 patients. J Arthroplasty. 2006;21(8):1175-1179. doi:10.1016/j.arth.2006.02.159.
13. Morsi E. Continuous-flow cold therapy after total knee arthroplasty. J Arthroplasty. 2002;17(6):718-722. doi:10.1054/arth.2002.33562.
14. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: A prospective, randomized investigation. J Shoulder Elb Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
15. Saito N, Horiuchi H, Kobayashi S, Nawata M, Takaoka K. Continuous local cooling for pain relief following total hip arthroplasty. J Arthroplasty. 2004;19(3):334-337. doi:10.1016/j.arth.2003.10.011.
16. Gibbons C, Solan M, Ricketts D, Patterson M. Cryotherapy compared with Robert Jones bandage after total knee replacement: A prospective randomized trial. Int Orthop. 2001;25(4):250-252. doi:10.1007/s002640100227.
17. Kraeutler MJ, Reynolds KA, Long C, McCarty EC. Compressive cryotherapy versus ice-a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elb Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
18. Thienpont E. Does Advanced Cryotherapy Reduce Pain and Narcotic Consumption After Knee Arthroplasty? Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
19. Woolf SK, Barfield WR, Merrill KD, McBryde AM Jr. Comparison of a continuous temperature-controlled cryotherapy device to a simple icing regimen following outpatient knee arthroscopy. J Knee Surg. 2008;21(1):15-19.
20. Su EP, Perna M, Boettner F, et al. A prospective, multi-center, randomised trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620X.94B11.30832.
21. Barber F. A comparison of crushed ice and continuous flow cold therapy. Am J Knee Surg. 2000;13(2):97-101.
22. Demoulin C, Brouwers M, Darot S, Gillet P, Crielaard JM, Vanderthommen M. Comparison of gaseous cryotherapy with more traditional forms of cryotherapy following total knee arthroplasty. Ann Phys Rehabil Med. 2012;55(4):229-240. doi:10.1016/j.rehab.2012.03.004.
23. Mumith A, Pavlou P, Barrett M, Thurston B, Garrett S. Enhancing postoperative rehabilitation following knee arthroplasty using a new cryotherapy product: a prospective study. Geriatr Orthop Surg Rehabil. 2015;6(4):316-321. doi:10.1177/2151458515609722.
24. Dickinson RN, Kuhn JE, Bergner JL, Rizzone KH. A systematic review of cost-effective treatment of postoperative rotator cuff repairs. J Shoulder Elb Surg. 2017;26(5):915-922. doi:10.1016/j.jse.2017.02.009.
25. Brown WC, Hahn DB. Frostbite of the Feet After Cryotherapy: A Report of Two Cases. J Foot Ankle Surg. 2009;48(5):577-580. doi:10.1053/j.jfas.2009.06.003.
26. Dundon JM, Rymer MC, Johnson RM. Total patellar skin loss from cryotherapy after total knee arthroplasty. J Arthroplasty. 2013;28(2):376.e5-e7. doi:10.1016/j.arth.2012.05.024.
27. Khajavi K, Pavelko T, Mishra A. Compartment syndrome arising from use of an electronic cooling pad. Am J Sports Med. 2004;32(6):1538-1541. doi:10.1177/0363546503262191.
28. King J, Plotner A, Adams B. Perniosis induced by a cold therapy system. Arch Dermatol. 2012;148(9):1101-1102.
29. Lee CK, Pardun J, Buntic R, Kiehn M, Brooks D, Buncke HJ. Severe frostbite of the knees after cryotherapy. Orthopedics. 2007;30(1):63-64.
30. McGuire DA, Hendricks SD. Incidences of frostbite in arthroscopic knee surgery postoperative cryotherapy rehabilitation. Arthroscopy. 2006;22(10):1141.e1-e6. doi:10.1016/j.arthro.2005.06.027.
31. Mirkin G. Why Ice Delays Recovery. http://www.drmirkin.com/fitness/why-ice-delays-recovery.html. Published September 16, 2015. Accessed July 17, 2017.
1. Freiman N, Bouganim N. History of cryotherapy. Dermatol Online J. 2005;11(2):9.
2. Spencer JH, inventor; Nortech Lab Inc, assignee. Device for use as a hot and cold compress. US patent US3780537A. December 25, 1973.
3. Stålman A, Berglund L, Dungnerc E, Arner P, Felländer-Tsai L. Temperature-sensitive release of prostaglandin E₂ and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2106/JBJS.J.01790.
4. Kawabata A. Prostaglandin E2 and pain--an update. Biol Pharm Bull. 2011;34(8):1170-1173. doi:10.1248/bpb.34.1170.
5. Schaser KD, Stover JF, Melcher I, et al. Local cooling restores microcirculatory hemodynamics after closed soft-tissue trauma in rats. J Trauma. 2006;61(3):642-649. doi:10.1097/01.ta.0000174922.08781.2f.
6. Ruffilli A, Buda R, Castagnini F, et al. Temperature-controlled continuous cold flow device versus traditional icing regimen following anterior cruciate ligament reconstruction: a prospective randomized comparative trial. Arch Orthop Trauma Surg. 2015;135(10):1405-1410. doi:10.1007/s00402-015-2273-z.
7. Kuyucu E, Bülbül M, Kara A, Koçyiğit F, Erdil M. Is cold therapy really efficient after knee arthroplasty? Ann Med Surg. 2015;4(4):475-478. doi:10.1016/j.amsu.2015.10.019.
8. Martin SS, Spindler KP, Tarter JW, Detwiler K, Petersen HA. Cryotherapy: an effective modality for decreasing intraarticular temperature after knee arthroscopy. Am J Sports Med. 2001;29(3):288-291. doi:10.1177/03635465010290030501.
9. Yu SY, Chen S, Yan HD, Fan CY. Effect of cryotherapy after elbow arthrolysis: A prospective, single-blinded, randomized controlled study. Arch Phys Med Rehabil. 2015;96(1):1-6. doi:10.1016/j.apmr.2014.08.011.
10. Dambros C, Martimbianco ALC, Polachini LO, Lahoz GL, Chamlian TR, Cohen M. Effectiveness of cryotherapy after anterior cruciate ligament reconstruction. Acta Ortop Bras. 2012;20(5):285-290. doi:10.1590/S1413-78522012000500008.
11. Leegwater NC, Nolte PA, de Korte N, et al. The efficacy of continuous-flow cryo and cyclic compression therapy after hip fracture surgery on postoperative pain: design of a prospective, open-label, parallel, multicenter, randomized controlled, clinical trial. BMC Musculoskelet Disord. 2016;17(1):153. doi:10.1186/s12891-016-1000-4.
12. Kullenberg B, Ylipää S, Söderlund K, Resch S. Postoperative cryotherapy after total knee arthroplasty: a prospective study of 86 patients. J Arthroplasty. 2006;21(8):1175-1179. doi:10.1016/j.arth.2006.02.159.
13. Morsi E. Continuous-flow cold therapy after total knee arthroplasty. J Arthroplasty. 2002;17(6):718-722. doi:10.1054/arth.2002.33562.
14. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: A prospective, randomized investigation. J Shoulder Elb Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
15. Saito N, Horiuchi H, Kobayashi S, Nawata M, Takaoka K. Continuous local cooling for pain relief following total hip arthroplasty. J Arthroplasty. 2004;19(3):334-337. doi:10.1016/j.arth.2003.10.011.
16. Gibbons C, Solan M, Ricketts D, Patterson M. Cryotherapy compared with Robert Jones bandage after total knee replacement: A prospective randomized trial. Int Orthop. 2001;25(4):250-252. doi:10.1007/s002640100227.
17. Kraeutler MJ, Reynolds KA, Long C, McCarty EC. Compressive cryotherapy versus ice-a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elb Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
18. Thienpont E. Does Advanced Cryotherapy Reduce Pain and Narcotic Consumption After Knee Arthroplasty? Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
19. Woolf SK, Barfield WR, Merrill KD, McBryde AM Jr. Comparison of a continuous temperature-controlled cryotherapy device to a simple icing regimen following outpatient knee arthroscopy. J Knee Surg. 2008;21(1):15-19.
20. Su EP, Perna M, Boettner F, et al. A prospective, multi-center, randomised trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620X.94B11.30832.
21. Barber F. A comparison of crushed ice and continuous flow cold therapy. Am J Knee Surg. 2000;13(2):97-101.
22. Demoulin C, Brouwers M, Darot S, Gillet P, Crielaard JM, Vanderthommen M. Comparison of gaseous cryotherapy with more traditional forms of cryotherapy following total knee arthroplasty. Ann Phys Rehabil Med. 2012;55(4):229-240. doi:10.1016/j.rehab.2012.03.004.
23. Mumith A, Pavlou P, Barrett M, Thurston B, Garrett S. Enhancing postoperative rehabilitation following knee arthroplasty using a new cryotherapy product: a prospective study. Geriatr Orthop Surg Rehabil. 2015;6(4):316-321. doi:10.1177/2151458515609722.
24. Dickinson RN, Kuhn JE, Bergner JL, Rizzone KH. A systematic review of cost-effective treatment of postoperative rotator cuff repairs. J Shoulder Elb Surg. 2017;26(5):915-922. doi:10.1016/j.jse.2017.02.009.
25. Brown WC, Hahn DB. Frostbite of the Feet After Cryotherapy: A Report of Two Cases. J Foot Ankle Surg. 2009;48(5):577-580. doi:10.1053/j.jfas.2009.06.003.
26. Dundon JM, Rymer MC, Johnson RM. Total patellar skin loss from cryotherapy after total knee arthroplasty. J Arthroplasty. 2013;28(2):376.e5-e7. doi:10.1016/j.arth.2012.05.024.
27. Khajavi K, Pavelko T, Mishra A. Compartment syndrome arising from use of an electronic cooling pad. Am J Sports Med. 2004;32(6):1538-1541. doi:10.1177/0363546503262191.
28. King J, Plotner A, Adams B. Perniosis induced by a cold therapy system. Arch Dermatol. 2012;148(9):1101-1102.
29. Lee CK, Pardun J, Buntic R, Kiehn M, Brooks D, Buncke HJ. Severe frostbite of the knees after cryotherapy. Orthopedics. 2007;30(1):63-64.
30. McGuire DA, Hendricks SD. Incidences of frostbite in arthroscopic knee surgery postoperative cryotherapy rehabilitation. Arthroscopy. 2006;22(10):1141.e1-e6. doi:10.1016/j.arthro.2005.06.027.
31. Mirkin G. Why Ice Delays Recovery. http://www.drmirkin.com/fitness/why-ice-delays-recovery.html. Published September 16, 2015. Accessed July 17, 2017.
TAKE-HOME POINTS
- Cryotherapy is often used in postoperative orthopedic care but there is limited literature demonstrating its efficacy.
- Postoperative cryotherapy has been used to reduce visual analog scale pain scores, analgesic consumption, and to increase range of motion.
- There is no consensus on the advantages of postoperative cryotherapy vs traditional ice application.
- Adverse outcomes from postoperative cryotherapy use include frostbite/skin loss, compartment syndrome, and perniosis.
- Future studies, including a formidable economic analysis of advanced cold therapy systems are necessary before physicians prescribing cryotherapy can be confident that they are augmenting patient recovery.