User login
To the Editor:
Infections caused by herpes simplex (HS) and herpes zoster (HZ) usually can be recognized by clinical findings; however, laboratory confirmation sometimes is required. Polymerase chain reaction (PCR) laboratory tests detect HS or HZ in a sensible and specific manner. New PCR systems such as real-time PCR (RT-PCR) give faster and more precise results. We report a case of recurrent concomitant HZ and HS diagnosed by RT-PCR.
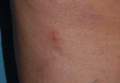
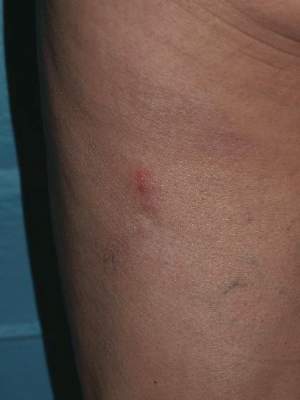
A 62-year-old woman presented with recurrent painful cutaneous lesions on the left buttock and thigh of 9 years’ duration. This eruption was preceded by a burning sensation of 1 week’s duration that extended toward the heel. Cutaneous lesions normally were sparse and persisted for a few days. She also had annular erythematous lesions of 3 years’ duration on the upper trunk and shoulders after sun exposure. On physical examination, an atrophic hypopigmented patch was seen with a few vesicles located on the thigh. Whitish atrophic patches also were found in a linear distribution on the left buttock and thigh (Figure).
Laboratory results included the following: antinuclear antibody, 1:400 on a nuclear dotted pattern; extractable nuclear antigens (anti-Ro60 and anti-Ro52) were positive (reference range, >15); and rheumatoid factor was 24.1 U/mL (reference range, 0–15 U/mL). The patient did not meet any other American College of Rheumatology criteria1,2 of systemic lupus erythematosus apart from photosensitivity. The rest of the analysis—complete blood cell count, liver enzymes, and biochemistry—was normal or negative. Human immunodeficiency virus, herpes simplex virus types 1 and 2 (HHV-1 and HHV-2), and varicella-zoster virus (VZV) IgM serologies were negative, whereas IgG VZV serology was positive.
The microbiological study via swab obtained from the roof and fluid from the vesicles showed an indeterminate result from the rapid direct antigen detection with immunofluorescent antibodies. Viral cultures were HHV-2 positive and VZV negative. Conventional PCR showed positive results, both for HHV-2 and VZV. A second analysis, performed with RT-PCR from a new sample taken 2 months later, showed the same results, which led to the diagnosis of recurrent concomitant HS and HZ with a recurrent HZ clinical pattern. The patient was started on valacyclovir 1 g daily, and the number and intensity of flares diminished in the months following treatment.
Concomitant HS and HZ on the same dermatome has been described in the literature.3,4 In a retrospective series of 20 immunocompetent patients, HZ was the main presumed diagnosis before laboratory confirmation of diagnosis, and only 1 case corresponded to recurrent HZ.3 Other cases of simultaneous HS and HZ have been described, but they did not occur on the same dermatome. Half of these reported cases were in immunosuppressed patients.5,6
The recurrent nature of HS is well known; however, recurrent cases of HZ are rare. Nevertheless, in a population-based cohort study of patients with a confirmed prior episode of HZ (N=1669), recurrences were found in 6% of patients.7 Recurrence was more common if the patient was immunosuppressed, was female and 50 years or older, and had pain for more than 30 days.7
The recurrence rate was high in our case, but no immunosuppressive factor could be found apart from probable subacute cutaneous lupus erythematosus. Systemic lupus erythematosus has been associated with a high risk for developing HZ secondary to cell-mediated immunosuppression. The annual incidence of HZ can reach 32 of 1000 patients with systemic lupus erythematosus, while in the general population the incidence is only 1.5 to 3 of 1000 patients.8-10
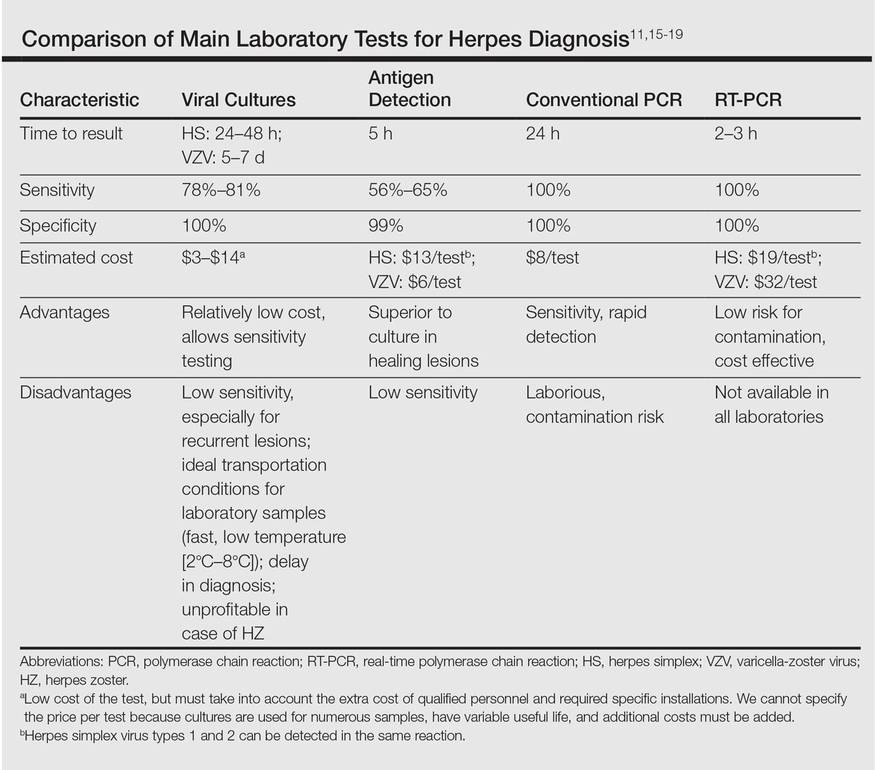
Direct detection of antigens of HS and HZ is a fast and inexpensive technique but lacks the sensitivity of viral cultures. Viral cultures used to be considered the gold standard; however, they are less sensitive than PCR.11 Furthermore, VZV detection is more difficult than HS, leading to a notable percentage of false-negative results.12 Polymerase chain reaction is a fast, reliable, and sensitive laboratory technique. Real-time PCR permits faster results than conventional PCR, specifically for HHV-1, HHV-2, and HZ detection. It also has minimal risk for contamination.13,14 In our opinion, PCR should be the gold standard instead of viral cultures. It has proven its superiority as a rapid method for detection, it is the most sensitive test, it is easier to perform, and it is cost effective (Table).11,15-19 However, viral cultures can allow sensitivity testing and are still an option for determination of susceptibility to antivirals.
In our case, a false-positive was excluded because no sign of possible contamination was found, repeated internal analysis from the same sample confirmed the results, and a new analysis from a new flare showed the same results 2 months later. However, we cannot rule out that the positivity for HZ of the second sample was due to the high sensitivity of the test and a virus latency in nerves.
We propose the use of PCR as a method of choice. Presumably more cases of recurrent HZ and concomitant HS and HZ will be seen with PCR use. In the case of a concomitant infection of HS and HZ, it is reasonable to use an antiviral dosage as in HZ treatment. No literature regarding outcomes from therapy could be found.
- Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271-1277.
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
- Giehl KA, Müller-Sander E, Rottenkolber M, et al. Identification and characterization of 20 immunocompetent patients with simultaneous varicella zoster and herpes simplex virus infection. JEADV. 2008;22:722-728.
- De Vivo C, Bansal MG, Olarte M, et al. Concurrent herpes simplex type 1 and varicella-zoster in the V2 dermatome in an immunocompetent patient. Cutis. 2001;68:120-122.
- Hyun-Ho P, Mu-Hyoung L. Concurrent reactivation of varicella zoster virus and herpes simplex virus in an immunocompetent child. J Korean Med Sci. 2004;19:598-600.
- Godet C, Beby-Defaux A, Landron C, et al. Concomitant disseminated herpes simplex virus type 2 infection and varicella zoster virus primoinfection in a pregnant woman. Scand J Infect Dis. 2005;37:774-776.
- Yawn BP, Wollan PC, Kurland MJ, et al. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86:88-93.
- Borba EF, Ribeiro AC, Martin P, et al. Incidence, risk factors, and outcome or herpes systemic lupus erythematosus. JCR. 2010;16:119-122.
- Nagasawa K, Yamauchi Y, Tada Y, et al. High incidence of herpes zoster in patients with systemic lupus erythematosus: an immunological analysis. Ann Rheumatic Dis. 1990;49:630-633.
- Kang TY, Lee HS, Kim TH, et al. Clinical and genetic risk factors of herpes zoster in patients with systemic lupus erythematosus. Rheumatol Int. 2005;25:97-102.
- Slomka MJ, Emery L, Munday PE, et al. A comparison of PCR with virus isolation and direct antigen detection for diagnosis and typing of genital herpes. J Med Virol. 1998;55:177-183.
- Nahass GT, Goldstein BA, Zhu WY, et al. Comparison of Tzanck smear, viral culture, and DNA diagnostic methods in detection of herpes simplex and varicella-zoster infection. JAMA. 1992;268:2541-2544.
- Burrows J, Nitsche A, Bayly B, et al. Detection and subtyping of herpes simplex virus in clinical samples by LightCycler PCR, enzyme immunoassay and cell culture. BMC Microbiology. 2002;2:12.
- Bezold GD, Lange ME, Gall H, et al. Detection of cutaneous varicella zoster virus infections by immunofluorescence versus PCR. Eur J Dermatol. 2001;11:108-111.
- Ramaswamy M, McDonald C, Smith M, et al. Diagnosis of genital herpes by real time PCR in routine clinical practice. Sex Transm Infect. 2004;80:406-410.
- Wald A, Huang ML, Carrell D, et al. Polymerase chain reaction for detection of herpes simplex virus DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003;188:1345-1351.
- Marshall DS, Linfert DR, Draghi A, et al. Identification of herpes simplex virus genital infection: comparison of a multiplex PCR assay and traditional viral isolation techniques. Mod Pathol. 2001;14:152-156.
- Koening M, Reynolds KS, Aldous W, et al. Comparison of Light-Cycler PCR, enzyme immunoassay, and tissue culture for detection of herpes simplex virus. Diagn Microbiol Infect Dis. 2001;40:107-110.
- Coyle PV, Desai A, Wyatt D, et al. A comparison of virus isolation, indirect immunofluorescence and nested multiplex polymerase chain reaction for the diagnosis of primary and recurrent herpes simplex type 1 and type 2 infections. J Virol Methods. 1999;83:75-82.
To the Editor:
Infections caused by herpes simplex (HS) and herpes zoster (HZ) usually can be recognized by clinical findings; however, laboratory confirmation sometimes is required. Polymerase chain reaction (PCR) laboratory tests detect HS or HZ in a sensible and specific manner. New PCR systems such as real-time PCR (RT-PCR) give faster and more precise results. We report a case of recurrent concomitant HZ and HS diagnosed by RT-PCR.
A 62-year-old woman presented with recurrent painful cutaneous lesions on the left buttock and thigh of 9 years’ duration. This eruption was preceded by a burning sensation of 1 week’s duration that extended toward the heel. Cutaneous lesions normally were sparse and persisted for a few days. She also had annular erythematous lesions of 3 years’ duration on the upper trunk and shoulders after sun exposure. On physical examination, an atrophic hypopigmented patch was seen with a few vesicles located on the thigh. Whitish atrophic patches also were found in a linear distribution on the left buttock and thigh (Figure).

Laboratory results included the following: antinuclear antibody, 1:400 on a nuclear dotted pattern; extractable nuclear antigens (anti-Ro60 and anti-Ro52) were positive (reference range, >15); and rheumatoid factor was 24.1 U/mL (reference range, 0–15 U/mL). The patient did not meet any other American College of Rheumatology criteria1,2 of systemic lupus erythematosus apart from photosensitivity. The rest of the analysis—complete blood cell count, liver enzymes, and biochemistry—was normal or negative. Human immunodeficiency virus, herpes simplex virus types 1 and 2 (HHV-1 and HHV-2), and varicella-zoster virus (VZV) IgM serologies were negative, whereas IgG VZV serology was positive.
The microbiological study via swab obtained from the roof and fluid from the vesicles showed an indeterminate result from the rapid direct antigen detection with immunofluorescent antibodies. Viral cultures were HHV-2 positive and VZV negative. Conventional PCR showed positive results, both for HHV-2 and VZV. A second analysis, performed with RT-PCR from a new sample taken 2 months later, showed the same results, which led to the diagnosis of recurrent concomitant HS and HZ with a recurrent HZ clinical pattern. The patient was started on valacyclovir 1 g daily, and the number and intensity of flares diminished in the months following treatment.
Concomitant HS and HZ on the same dermatome has been described in the literature.3,4 In a retrospective series of 20 immunocompetent patients, HZ was the main presumed diagnosis before laboratory confirmation of diagnosis, and only 1 case corresponded to recurrent HZ.3 Other cases of simultaneous HS and HZ have been described, but they did not occur on the same dermatome. Half of these reported cases were in immunosuppressed patients.5,6
The recurrent nature of HS is well known; however, recurrent cases of HZ are rare. Nevertheless, in a population-based cohort study of patients with a confirmed prior episode of HZ (N=1669), recurrences were found in 6% of patients.7 Recurrence was more common if the patient was immunosuppressed, was female and 50 years or older, and had pain for more than 30 days.7
The recurrence rate was high in our case, but no immunosuppressive factor could be found apart from probable subacute cutaneous lupus erythematosus. Systemic lupus erythematosus has been associated with a high risk for developing HZ secondary to cell-mediated immunosuppression. The annual incidence of HZ can reach 32 of 1000 patients with systemic lupus erythematosus, while in the general population the incidence is only 1.5 to 3 of 1000 patients.8-10
Direct detection of antigens of HS and HZ is a fast and inexpensive technique but lacks the sensitivity of viral cultures. Viral cultures used to be considered the gold standard; however, they are less sensitive than PCR.11 Furthermore, VZV detection is more difficult than HS, leading to a notable percentage of false-negative results.12 Polymerase chain reaction is a fast, reliable, and sensitive laboratory technique. Real-time PCR permits faster results than conventional PCR, specifically for HHV-1, HHV-2, and HZ detection. It also has minimal risk for contamination.13,14 In our opinion, PCR should be the gold standard instead of viral cultures. It has proven its superiority as a rapid method for detection, it is the most sensitive test, it is easier to perform, and it is cost effective (Table).11,15-19 However, viral cultures can allow sensitivity testing and are still an option for determination of susceptibility to antivirals.

In our case, a false-positive was excluded because no sign of possible contamination was found, repeated internal analysis from the same sample confirmed the results, and a new analysis from a new flare showed the same results 2 months later. However, we cannot rule out that the positivity for HZ of the second sample was due to the high sensitivity of the test and a virus latency in nerves.
We propose the use of PCR as a method of choice. Presumably more cases of recurrent HZ and concomitant HS and HZ will be seen with PCR use. In the case of a concomitant infection of HS and HZ, it is reasonable to use an antiviral dosage as in HZ treatment. No literature regarding outcomes from therapy could be found.
To the Editor:
Infections caused by herpes simplex (HS) and herpes zoster (HZ) usually can be recognized by clinical findings; however, laboratory confirmation sometimes is required. Polymerase chain reaction (PCR) laboratory tests detect HS or HZ in a sensible and specific manner. New PCR systems such as real-time PCR (RT-PCR) give faster and more precise results. We report a case of recurrent concomitant HZ and HS diagnosed by RT-PCR.
A 62-year-old woman presented with recurrent painful cutaneous lesions on the left buttock and thigh of 9 years’ duration. This eruption was preceded by a burning sensation of 1 week’s duration that extended toward the heel. Cutaneous lesions normally were sparse and persisted for a few days. She also had annular erythematous lesions of 3 years’ duration on the upper trunk and shoulders after sun exposure. On physical examination, an atrophic hypopigmented patch was seen with a few vesicles located on the thigh. Whitish atrophic patches also were found in a linear distribution on the left buttock and thigh (Figure).

Laboratory results included the following: antinuclear antibody, 1:400 on a nuclear dotted pattern; extractable nuclear antigens (anti-Ro60 and anti-Ro52) were positive (reference range, >15); and rheumatoid factor was 24.1 U/mL (reference range, 0–15 U/mL). The patient did not meet any other American College of Rheumatology criteria1,2 of systemic lupus erythematosus apart from photosensitivity. The rest of the analysis—complete blood cell count, liver enzymes, and biochemistry—was normal or negative. Human immunodeficiency virus, herpes simplex virus types 1 and 2 (HHV-1 and HHV-2), and varicella-zoster virus (VZV) IgM serologies were negative, whereas IgG VZV serology was positive.
The microbiological study via swab obtained from the roof and fluid from the vesicles showed an indeterminate result from the rapid direct antigen detection with immunofluorescent antibodies. Viral cultures were HHV-2 positive and VZV negative. Conventional PCR showed positive results, both for HHV-2 and VZV. A second analysis, performed with RT-PCR from a new sample taken 2 months later, showed the same results, which led to the diagnosis of recurrent concomitant HS and HZ with a recurrent HZ clinical pattern. The patient was started on valacyclovir 1 g daily, and the number and intensity of flares diminished in the months following treatment.
Concomitant HS and HZ on the same dermatome has been described in the literature.3,4 In a retrospective series of 20 immunocompetent patients, HZ was the main presumed diagnosis before laboratory confirmation of diagnosis, and only 1 case corresponded to recurrent HZ.3 Other cases of simultaneous HS and HZ have been described, but they did not occur on the same dermatome. Half of these reported cases were in immunosuppressed patients.5,6
The recurrent nature of HS is well known; however, recurrent cases of HZ are rare. Nevertheless, in a population-based cohort study of patients with a confirmed prior episode of HZ (N=1669), recurrences were found in 6% of patients.7 Recurrence was more common if the patient was immunosuppressed, was female and 50 years or older, and had pain for more than 30 days.7
The recurrence rate was high in our case, but no immunosuppressive factor could be found apart from probable subacute cutaneous lupus erythematosus. Systemic lupus erythematosus has been associated with a high risk for developing HZ secondary to cell-mediated immunosuppression. The annual incidence of HZ can reach 32 of 1000 patients with systemic lupus erythematosus, while in the general population the incidence is only 1.5 to 3 of 1000 patients.8-10
Direct detection of antigens of HS and HZ is a fast and inexpensive technique but lacks the sensitivity of viral cultures. Viral cultures used to be considered the gold standard; however, they are less sensitive than PCR.11 Furthermore, VZV detection is more difficult than HS, leading to a notable percentage of false-negative results.12 Polymerase chain reaction is a fast, reliable, and sensitive laboratory technique. Real-time PCR permits faster results than conventional PCR, specifically for HHV-1, HHV-2, and HZ detection. It also has minimal risk for contamination.13,14 In our opinion, PCR should be the gold standard instead of viral cultures. It has proven its superiority as a rapid method for detection, it is the most sensitive test, it is easier to perform, and it is cost effective (Table).11,15-19 However, viral cultures can allow sensitivity testing and are still an option for determination of susceptibility to antivirals.

In our case, a false-positive was excluded because no sign of possible contamination was found, repeated internal analysis from the same sample confirmed the results, and a new analysis from a new flare showed the same results 2 months later. However, we cannot rule out that the positivity for HZ of the second sample was due to the high sensitivity of the test and a virus latency in nerves.
We propose the use of PCR as a method of choice. Presumably more cases of recurrent HZ and concomitant HS and HZ will be seen with PCR use. In the case of a concomitant infection of HS and HZ, it is reasonable to use an antiviral dosage as in HZ treatment. No literature regarding outcomes from therapy could be found.
- Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271-1277.
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
- Giehl KA, Müller-Sander E, Rottenkolber M, et al. Identification and characterization of 20 immunocompetent patients with simultaneous varicella zoster and herpes simplex virus infection. JEADV. 2008;22:722-728.
- De Vivo C, Bansal MG, Olarte M, et al. Concurrent herpes simplex type 1 and varicella-zoster in the V2 dermatome in an immunocompetent patient. Cutis. 2001;68:120-122.
- Hyun-Ho P, Mu-Hyoung L. Concurrent reactivation of varicella zoster virus and herpes simplex virus in an immunocompetent child. J Korean Med Sci. 2004;19:598-600.
- Godet C, Beby-Defaux A, Landron C, et al. Concomitant disseminated herpes simplex virus type 2 infection and varicella zoster virus primoinfection in a pregnant woman. Scand J Infect Dis. 2005;37:774-776.
- Yawn BP, Wollan PC, Kurland MJ, et al. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86:88-93.
- Borba EF, Ribeiro AC, Martin P, et al. Incidence, risk factors, and outcome or herpes systemic lupus erythematosus. JCR. 2010;16:119-122.
- Nagasawa K, Yamauchi Y, Tada Y, et al. High incidence of herpes zoster in patients with systemic lupus erythematosus: an immunological analysis. Ann Rheumatic Dis. 1990;49:630-633.
- Kang TY, Lee HS, Kim TH, et al. Clinical and genetic risk factors of herpes zoster in patients with systemic lupus erythematosus. Rheumatol Int. 2005;25:97-102.
- Slomka MJ, Emery L, Munday PE, et al. A comparison of PCR with virus isolation and direct antigen detection for diagnosis and typing of genital herpes. J Med Virol. 1998;55:177-183.
- Nahass GT, Goldstein BA, Zhu WY, et al. Comparison of Tzanck smear, viral culture, and DNA diagnostic methods in detection of herpes simplex and varicella-zoster infection. JAMA. 1992;268:2541-2544.
- Burrows J, Nitsche A, Bayly B, et al. Detection and subtyping of herpes simplex virus in clinical samples by LightCycler PCR, enzyme immunoassay and cell culture. BMC Microbiology. 2002;2:12.
- Bezold GD, Lange ME, Gall H, et al. Detection of cutaneous varicella zoster virus infections by immunofluorescence versus PCR. Eur J Dermatol. 2001;11:108-111.
- Ramaswamy M, McDonald C, Smith M, et al. Diagnosis of genital herpes by real time PCR in routine clinical practice. Sex Transm Infect. 2004;80:406-410.
- Wald A, Huang ML, Carrell D, et al. Polymerase chain reaction for detection of herpes simplex virus DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003;188:1345-1351.
- Marshall DS, Linfert DR, Draghi A, et al. Identification of herpes simplex virus genital infection: comparison of a multiplex PCR assay and traditional viral isolation techniques. Mod Pathol. 2001;14:152-156.
- Koening M, Reynolds KS, Aldous W, et al. Comparison of Light-Cycler PCR, enzyme immunoassay, and tissue culture for detection of herpes simplex virus. Diagn Microbiol Infect Dis. 2001;40:107-110.
- Coyle PV, Desai A, Wyatt D, et al. A comparison of virus isolation, indirect immunofluorescence and nested multiplex polymerase chain reaction for the diagnosis of primary and recurrent herpes simplex type 1 and type 2 infections. J Virol Methods. 1999;83:75-82.
- Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271-1277.
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
- Giehl KA, Müller-Sander E, Rottenkolber M, et al. Identification and characterization of 20 immunocompetent patients with simultaneous varicella zoster and herpes simplex virus infection. JEADV. 2008;22:722-728.
- De Vivo C, Bansal MG, Olarte M, et al. Concurrent herpes simplex type 1 and varicella-zoster in the V2 dermatome in an immunocompetent patient. Cutis. 2001;68:120-122.
- Hyun-Ho P, Mu-Hyoung L. Concurrent reactivation of varicella zoster virus and herpes simplex virus in an immunocompetent child. J Korean Med Sci. 2004;19:598-600.
- Godet C, Beby-Defaux A, Landron C, et al. Concomitant disseminated herpes simplex virus type 2 infection and varicella zoster virus primoinfection in a pregnant woman. Scand J Infect Dis. 2005;37:774-776.
- Yawn BP, Wollan PC, Kurland MJ, et al. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86:88-93.
- Borba EF, Ribeiro AC, Martin P, et al. Incidence, risk factors, and outcome or herpes systemic lupus erythematosus. JCR. 2010;16:119-122.
- Nagasawa K, Yamauchi Y, Tada Y, et al. High incidence of herpes zoster in patients with systemic lupus erythematosus: an immunological analysis. Ann Rheumatic Dis. 1990;49:630-633.
- Kang TY, Lee HS, Kim TH, et al. Clinical and genetic risk factors of herpes zoster in patients with systemic lupus erythematosus. Rheumatol Int. 2005;25:97-102.
- Slomka MJ, Emery L, Munday PE, et al. A comparison of PCR with virus isolation and direct antigen detection for diagnosis and typing of genital herpes. J Med Virol. 1998;55:177-183.
- Nahass GT, Goldstein BA, Zhu WY, et al. Comparison of Tzanck smear, viral culture, and DNA diagnostic methods in detection of herpes simplex and varicella-zoster infection. JAMA. 1992;268:2541-2544.
- Burrows J, Nitsche A, Bayly B, et al. Detection and subtyping of herpes simplex virus in clinical samples by LightCycler PCR, enzyme immunoassay and cell culture. BMC Microbiology. 2002;2:12.
- Bezold GD, Lange ME, Gall H, et al. Detection of cutaneous varicella zoster virus infections by immunofluorescence versus PCR. Eur J Dermatol. 2001;11:108-111.
- Ramaswamy M, McDonald C, Smith M, et al. Diagnosis of genital herpes by real time PCR in routine clinical practice. Sex Transm Infect. 2004;80:406-410.
- Wald A, Huang ML, Carrell D, et al. Polymerase chain reaction for detection of herpes simplex virus DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003;188:1345-1351.
- Marshall DS, Linfert DR, Draghi A, et al. Identification of herpes simplex virus genital infection: comparison of a multiplex PCR assay and traditional viral isolation techniques. Mod Pathol. 2001;14:152-156.
- Koening M, Reynolds KS, Aldous W, et al. Comparison of Light-Cycler PCR, enzyme immunoassay, and tissue culture for detection of herpes simplex virus. Diagn Microbiol Infect Dis. 2001;40:107-110.
- Coyle PV, Desai A, Wyatt D, et al. A comparison of virus isolation, indirect immunofluorescence and nested multiplex polymerase chain reaction for the diagnosis of primary and recurrent herpes simplex type 1 and type 2 infections. J Virol Methods. 1999;83:75-82.