User login
SAN ANTONIO – About half of the women with stage 1 or 2 breast cancer who were treated using a scalp cooling device during chemotherapy retained their hair in the prospective, randomized Scalp Cooling Alopecia Prevention (SCALP) trial.
Of 229 women who were enrolled from seven U.S. sites between December 2013 and September 2016 and who planned to undergo at least four cycles of anthracycline- or taxane-based chemotherapy, 182 met eligibility criteria; 119 were randomized to undergo scalp cooling using the Orbis Paxman Hair Loss Prevention System (OPHLPS; Paxman Coolers) and 63 were assigned to a control group. Of those, 95 and 47, respectively, were evaluable and had completed four cycles of chemotherapy at the time of a preplanned interim analysis.
The study was stopped after the interim analysis because of the positive superiority in the treatment group, Dr. Nangia said.
Study subjects were women with early-stage breast cancer and planned neoadjuvant or adjuvant chemotherapy. Most (65%) received taxane-based chemotherapy and 35% received anthracycline-based chemotherapy; the latter has been shown to be associated with higher rates of hair loss.
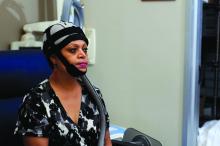
The OPHLPS system involves the use of a “cold cap” that is cooled using a refrigeration system and fitted to a patient’s head during chemotherapy treatment. For the current study, subjects underwent scalp cooling for 30 minutes before chemotherapy, during chemotherapy, and for 90 minutes after.
Most subjects (39%-52%) reported that they were reasonably comfortable during use of the device during treatment cycles 1-4. Only 2.4% reported being very uncomfortable, and that was only during cycle 2.
An analysis based on type of therapy showed that 65.1% of those receiving a taxane experienced hair preservation, compared with 21.9% of those receiving an anthracycline.
Scalp cooling, which reduces blood flow and chemoexposure to the hair follicles and thereby reduces hair loss, is widely used in Europe and, despite a great deal of interest in the technology in the United States, the Food and Drug Administration has been slow to get on board because of concerns over the potential for scalp metastasis. However, long-term outcomes data from Europe demonstrate that scalp metastasis is “exceedingly rare” and has occurred only in those with metastasis throughout the body, Dr. Nangia said.
Those data opened the door to the current study – the first prospective randomized trial of scalp-cooling.
In response to questions about the effects of scalp cooling across additional chemotherapy cycles (for example, those who undergo eight cycles), Dr. Nangia said that all patients completed four cycles, and data for those with eight cycles planned will be included in a final analysis.
“In other countries, they do have success rates with eight cycles, which is standard for some women,” she said. SCALP trial subjects will also be followed for 5 years to monitor overall survival, recurrence of cancer, and potential metastasis to the scalp, she said.
Based on the findings of the study, the maker of the device is seeking FDA clearance. If approved, the OPHLPS would compete with the DigniCap (Dignitana), which has already received approval.
“Scalp cooling devices are highly effective and should become available to women with breast cancer receiving chemotherapy ... Further study should be done exploring the technology for other types of tumors and with other chemotherapy regimens. More study looking at the impact of chemotherapy-induced alopecia on psyche and body image should be performed as well,” Dr. Nangia concluded, noting that tailored quality-of-life tools are needed to evaluate the impact of alopecia on quality of life.
Dr. Nangia reported receiving research funding from Paxman, the sponsor of the study, to her institution.
SAN ANTONIO – About half of the women with stage 1 or 2 breast cancer who were treated using a scalp cooling device during chemotherapy retained their hair in the prospective, randomized Scalp Cooling Alopecia Prevention (SCALP) trial.
Of 229 women who were enrolled from seven U.S. sites between December 2013 and September 2016 and who planned to undergo at least four cycles of anthracycline- or taxane-based chemotherapy, 182 met eligibility criteria; 119 were randomized to undergo scalp cooling using the Orbis Paxman Hair Loss Prevention System (OPHLPS; Paxman Coolers) and 63 were assigned to a control group. Of those, 95 and 47, respectively, were evaluable and had completed four cycles of chemotherapy at the time of a preplanned interim analysis.
The study was stopped after the interim analysis because of the positive superiority in the treatment group, Dr. Nangia said.
Study subjects were women with early-stage breast cancer and planned neoadjuvant or adjuvant chemotherapy. Most (65%) received taxane-based chemotherapy and 35% received anthracycline-based chemotherapy; the latter has been shown to be associated with higher rates of hair loss.
The OPHLPS system involves the use of a “cold cap” that is cooled using a refrigeration system and fitted to a patient’s head during chemotherapy treatment. For the current study, subjects underwent scalp cooling for 30 minutes before chemotherapy, during chemotherapy, and for 90 minutes after.
Most subjects (39%-52%) reported that they were reasonably comfortable during use of the device during treatment cycles 1-4. Only 2.4% reported being very uncomfortable, and that was only during cycle 2.
An analysis based on type of therapy showed that 65.1% of those receiving a taxane experienced hair preservation, compared with 21.9% of those receiving an anthracycline.
Scalp cooling, which reduces blood flow and chemoexposure to the hair follicles and thereby reduces hair loss, is widely used in Europe and, despite a great deal of interest in the technology in the United States, the Food and Drug Administration has been slow to get on board because of concerns over the potential for scalp metastasis. However, long-term outcomes data from Europe demonstrate that scalp metastasis is “exceedingly rare” and has occurred only in those with metastasis throughout the body, Dr. Nangia said.
Those data opened the door to the current study – the first prospective randomized trial of scalp-cooling.
In response to questions about the effects of scalp cooling across additional chemotherapy cycles (for example, those who undergo eight cycles), Dr. Nangia said that all patients completed four cycles, and data for those with eight cycles planned will be included in a final analysis.
“In other countries, they do have success rates with eight cycles, which is standard for some women,” she said. SCALP trial subjects will also be followed for 5 years to monitor overall survival, recurrence of cancer, and potential metastasis to the scalp, she said.
Based on the findings of the study, the maker of the device is seeking FDA clearance. If approved, the OPHLPS would compete with the DigniCap (Dignitana), which has already received approval.
“Scalp cooling devices are highly effective and should become available to women with breast cancer receiving chemotherapy ... Further study should be done exploring the technology for other types of tumors and with other chemotherapy regimens. More study looking at the impact of chemotherapy-induced alopecia on psyche and body image should be performed as well,” Dr. Nangia concluded, noting that tailored quality-of-life tools are needed to evaluate the impact of alopecia on quality of life.
Dr. Nangia reported receiving research funding from Paxman, the sponsor of the study, to her institution.
SAN ANTONIO – About half of the women with stage 1 or 2 breast cancer who were treated using a scalp cooling device during chemotherapy retained their hair in the prospective, randomized Scalp Cooling Alopecia Prevention (SCALP) trial.
Of 229 women who were enrolled from seven U.S. sites between December 2013 and September 2016 and who planned to undergo at least four cycles of anthracycline- or taxane-based chemotherapy, 182 met eligibility criteria; 119 were randomized to undergo scalp cooling using the Orbis Paxman Hair Loss Prevention System (OPHLPS; Paxman Coolers) and 63 were assigned to a control group. Of those, 95 and 47, respectively, were evaluable and had completed four cycles of chemotherapy at the time of a preplanned interim analysis.
The study was stopped after the interim analysis because of the positive superiority in the treatment group, Dr. Nangia said.
Study subjects were women with early-stage breast cancer and planned neoadjuvant or adjuvant chemotherapy. Most (65%) received taxane-based chemotherapy and 35% received anthracycline-based chemotherapy; the latter has been shown to be associated with higher rates of hair loss.
The OPHLPS system involves the use of a “cold cap” that is cooled using a refrigeration system and fitted to a patient’s head during chemotherapy treatment. For the current study, subjects underwent scalp cooling for 30 minutes before chemotherapy, during chemotherapy, and for 90 minutes after.
Most subjects (39%-52%) reported that they were reasonably comfortable during use of the device during treatment cycles 1-4. Only 2.4% reported being very uncomfortable, and that was only during cycle 2.
An analysis based on type of therapy showed that 65.1% of those receiving a taxane experienced hair preservation, compared with 21.9% of those receiving an anthracycline.
Scalp cooling, which reduces blood flow and chemoexposure to the hair follicles and thereby reduces hair loss, is widely used in Europe and, despite a great deal of interest in the technology in the United States, the Food and Drug Administration has been slow to get on board because of concerns over the potential for scalp metastasis. However, long-term outcomes data from Europe demonstrate that scalp metastasis is “exceedingly rare” and has occurred only in those with metastasis throughout the body, Dr. Nangia said.
Those data opened the door to the current study – the first prospective randomized trial of scalp-cooling.
In response to questions about the effects of scalp cooling across additional chemotherapy cycles (for example, those who undergo eight cycles), Dr. Nangia said that all patients completed four cycles, and data for those with eight cycles planned will be included in a final analysis.
“In other countries, they do have success rates with eight cycles, which is standard for some women,” she said. SCALP trial subjects will also be followed for 5 years to monitor overall survival, recurrence of cancer, and potential metastasis to the scalp, she said.
Based on the findings of the study, the maker of the device is seeking FDA clearance. If approved, the OPHLPS would compete with the DigniCap (Dignitana), which has already received approval.
“Scalp cooling devices are highly effective and should become available to women with breast cancer receiving chemotherapy ... Further study should be done exploring the technology for other types of tumors and with other chemotherapy regimens. More study looking at the impact of chemotherapy-induced alopecia on psyche and body image should be performed as well,” Dr. Nangia concluded, noting that tailored quality-of-life tools are needed to evaluate the impact of alopecia on quality of life.
Dr. Nangia reported receiving research funding from Paxman, the sponsor of the study, to her institution.
AT SABCS 2016
Key clinical point:
Major finding: 50.5% of treated patients experienced no more than grade 1 alopecia (less than 50% hair loss), compared with 0% of control subjects.
Data source: The prospective, randomized SCALP trial involving 182 women.
Disclosures: Dr. Nangia reported receiving research funding from Paxman, sponsor of the study, to her institution.