User login
Circulating microRNAs may predict breast cancer treatment response
SAN ANTONIO – Circulating microRNAs may predict treatment response in HER2-positive breast cancer patients treated with neoadjuvant therapy, according to findings from the randomized phase III NeoALTTO trial.
Specifically, four circulating tumor microRNA signatures were found to predict pathologic complete response to specific treatments at specific time points in a “testing set” of patients from the study, Serena Di Cosimo, MD, of Istituto Nazionale dei Tumori, Milan, reported at the San Antonio Breast Cancer Symposium.
Overall results from the trial, which were reported in 2012 (The Lancet. 379[9816]:633-40) showed that the combination of lapatinib and trastuzumab significantly improved rates of pathologic complete response, compared with either drug alone in patients with HER2-positive early breast cancer, and the authors concluded that dual inhibition of HER2 might be a valid approach in such patients in the neoadjuvant treatment setting.
For the current analysis of NeoALTTO data, microRNA from plasma samples from 435 women – 141 treated with lapatinib, 151 treated with trastuzumab, and 143 treated with a combination of the two – were monitored longitudinally, as NeoALTTO “was done thinking about future translational studies, so blood samples were collected prospectively at baseline, after 2 weeks of treatment, at surgery, and at the time of relapse,” she noted.
Potential microRNAs associated with pathologic complete response were identified for each treatment arm and time point, and a multivariate model was used to identify specific signatures and their predictive capability.
A total of 30 microRNAs and 6 microRNA signatures were found to be predict pathologic complete response in a “training population,” and the predictive ability of 4 of those was confirmed in the testing set for lapatinib at baseline (area under the curve [AUC] = .86), lapatinib after 2 weeks (AUC = .71), trastuzumab after 2 weeks (AUC=.81), and lapatinib and trastuzumab after 2 weeks (AUC = .67), Dr. Di Cosimo said.
“Results obtained early post treatment are of special value,” she said, explaining that “women with unfavorable microRNA signatures can be expected to have poor response after just 2 weeks of treatment.”
The findings are of note because a significant proportion of breast cancer patients treated in the neoadjuvant setting do not achieve pathologic complete response and have an increased risk of relapse after surgery. Further, there is a lack of reliable predictors of response to help guide therapy in clinical practice, Dr. Ci Cosimo said.
“This is the first evidence of the potential of circulating microRNAs to discriminate between responsive and unresponsive HER2+ breast cancer patients,” she said.
At present, the signatures, overall, have been associated with pathologic complete response, but not event-free survival. However, one microRNA signature (140-5p) appeared to be associated with event-free survival after 2 weeks among patients treated with trastuzumab, she said, adding that functional studies are ongoing to investigate the biological role of microRNAs, and independent validation studies are planned.
Dr. Di Cosimo reported having no disclosures.
SAN ANTONIO – Circulating microRNAs may predict treatment response in HER2-positive breast cancer patients treated with neoadjuvant therapy, according to findings from the randomized phase III NeoALTTO trial.
Specifically, four circulating tumor microRNA signatures were found to predict pathologic complete response to specific treatments at specific time points in a “testing set” of patients from the study, Serena Di Cosimo, MD, of Istituto Nazionale dei Tumori, Milan, reported at the San Antonio Breast Cancer Symposium.
Overall results from the trial, which were reported in 2012 (The Lancet. 379[9816]:633-40) showed that the combination of lapatinib and trastuzumab significantly improved rates of pathologic complete response, compared with either drug alone in patients with HER2-positive early breast cancer, and the authors concluded that dual inhibition of HER2 might be a valid approach in such patients in the neoadjuvant treatment setting.
For the current analysis of NeoALTTO data, microRNA from plasma samples from 435 women – 141 treated with lapatinib, 151 treated with trastuzumab, and 143 treated with a combination of the two – were monitored longitudinally, as NeoALTTO “was done thinking about future translational studies, so blood samples were collected prospectively at baseline, after 2 weeks of treatment, at surgery, and at the time of relapse,” she noted.
Potential microRNAs associated with pathologic complete response were identified for each treatment arm and time point, and a multivariate model was used to identify specific signatures and their predictive capability.
A total of 30 microRNAs and 6 microRNA signatures were found to be predict pathologic complete response in a “training population,” and the predictive ability of 4 of those was confirmed in the testing set for lapatinib at baseline (area under the curve [AUC] = .86), lapatinib after 2 weeks (AUC = .71), trastuzumab after 2 weeks (AUC=.81), and lapatinib and trastuzumab after 2 weeks (AUC = .67), Dr. Di Cosimo said.
“Results obtained early post treatment are of special value,” she said, explaining that “women with unfavorable microRNA signatures can be expected to have poor response after just 2 weeks of treatment.”
The findings are of note because a significant proportion of breast cancer patients treated in the neoadjuvant setting do not achieve pathologic complete response and have an increased risk of relapse after surgery. Further, there is a lack of reliable predictors of response to help guide therapy in clinical practice, Dr. Ci Cosimo said.
“This is the first evidence of the potential of circulating microRNAs to discriminate between responsive and unresponsive HER2+ breast cancer patients,” she said.
At present, the signatures, overall, have been associated with pathologic complete response, but not event-free survival. However, one microRNA signature (140-5p) appeared to be associated with event-free survival after 2 weeks among patients treated with trastuzumab, she said, adding that functional studies are ongoing to investigate the biological role of microRNAs, and independent validation studies are planned.
Dr. Di Cosimo reported having no disclosures.
SAN ANTONIO – Circulating microRNAs may predict treatment response in HER2-positive breast cancer patients treated with neoadjuvant therapy, according to findings from the randomized phase III NeoALTTO trial.
Specifically, four circulating tumor microRNA signatures were found to predict pathologic complete response to specific treatments at specific time points in a “testing set” of patients from the study, Serena Di Cosimo, MD, of Istituto Nazionale dei Tumori, Milan, reported at the San Antonio Breast Cancer Symposium.
Overall results from the trial, which were reported in 2012 (The Lancet. 379[9816]:633-40) showed that the combination of lapatinib and trastuzumab significantly improved rates of pathologic complete response, compared with either drug alone in patients with HER2-positive early breast cancer, and the authors concluded that dual inhibition of HER2 might be a valid approach in such patients in the neoadjuvant treatment setting.
For the current analysis of NeoALTTO data, microRNA from plasma samples from 435 women – 141 treated with lapatinib, 151 treated with trastuzumab, and 143 treated with a combination of the two – were monitored longitudinally, as NeoALTTO “was done thinking about future translational studies, so blood samples were collected prospectively at baseline, after 2 weeks of treatment, at surgery, and at the time of relapse,” she noted.
Potential microRNAs associated with pathologic complete response were identified for each treatment arm and time point, and a multivariate model was used to identify specific signatures and their predictive capability.
A total of 30 microRNAs and 6 microRNA signatures were found to be predict pathologic complete response in a “training population,” and the predictive ability of 4 of those was confirmed in the testing set for lapatinib at baseline (area under the curve [AUC] = .86), lapatinib after 2 weeks (AUC = .71), trastuzumab after 2 weeks (AUC=.81), and lapatinib and trastuzumab after 2 weeks (AUC = .67), Dr. Di Cosimo said.
“Results obtained early post treatment are of special value,” she said, explaining that “women with unfavorable microRNA signatures can be expected to have poor response after just 2 weeks of treatment.”
The findings are of note because a significant proportion of breast cancer patients treated in the neoadjuvant setting do not achieve pathologic complete response and have an increased risk of relapse after surgery. Further, there is a lack of reliable predictors of response to help guide therapy in clinical practice, Dr. Ci Cosimo said.
“This is the first evidence of the potential of circulating microRNAs to discriminate between responsive and unresponsive HER2+ breast cancer patients,” she said.
At present, the signatures, overall, have been associated with pathologic complete response, but not event-free survival. However, one microRNA signature (140-5p) appeared to be associated with event-free survival after 2 weeks among patients treated with trastuzumab, she said, adding that functional studies are ongoing to investigate the biological role of microRNAs, and independent validation studies are planned.
Dr. Di Cosimo reported having no disclosures.
AT SABCS 2016
Key clinical point:
Major finding: A total of 30 microRNAs and 6 microRNA signatures were found to predict pathologic complete response in a “training population,” and the predictive ability of 4 of those was confirmed in the testing set.
Data source: An analysis of data from the randomized phase III NeoALTTO trial.
Disclosures: Dr. Di Cosimo reported having no disclosures.
CTCs help predict breast cancer outcomes in neoadjuvant setting
SAN ANTONIO – Circulating tumor cells are a useful prognostic biomarker in early breast cancer patients treated with neoadjuvant chemotherapy, according to findings from an international meta-analysis of individual patient data.
The cells (CTCs), which can be measured using a Food and Drug Administration–approved assay, are known to seed distant metastases and to be prognostic before and during therapy for patients with metastatic breast cancer, and prognostic before adjuvant therapy for patients with nonmetastatic breast cancer.
However, findings in the neoadjuvant setting have been variable, Francois-Clement Bidard, MD, of Institut Curie, Paris, reported at the San Antonio Breast Cancer Symposium.
In the study (the international meta-analysis of circulating tumor cell detection in early breast cancer patients treated by neoadjuvant chemotherapy, or IMENEO), CTCs were useful, independent of pathologic complete response, for predicting overall survival and distant disease-free survival in the neoadjuvant setting. Further, IMENEO showed for the first time that CTCs also predict locoregional relapse-free survival,
Based on the analysis of data from 2,156 patients from 21 studies and 16 centers in 10 countries, the CTC positivity rates using thresholds of one or more, two or more, and five or more, respectively, were 25%, 13%, and 6% in 1,574 patients tested at baseline, 17%, 6%, and 3% in 290 tested after neoadjuvant chemotherapy, 15%, 5%, and 1% in 1,200 tested before surgery, and 11%, 4%, and 1% in 285 tested after surgery, Dr. Bidard said.
Prior to neoadjuvant chemotherapy, at least one CTC was found in 19%, 22%, 24%, 29% and 41% of cT1, T2, T3, T4a-c, and T4d breast cancers, respectively, and this was marginally associated with hormone receptor negativity, he said, noting that later CTC detection rates were not associated with any patient baseline characteristics.
Nearly one in four patients (24%) achieved pathologic complete response, but this was not associated at any time point with CTC count.
For the primary study endpoint of overall survival, a significant association was found with the presence of at least two CTCs at baseline (hazard ratio, 2.6 for two CTCs; 3.84 for three to four CTCs; and 6.25 for five or more CTCs). Similar associations were found for distant disease-free survival (hazard ratios, 2.4, 3.4, and 5.0, respectively) and for locoregional relapse-free interval with two CTCs and five or more CTCs (hazard ratios, 2.4 and 4.2, respectively).
Similar results were found using later time points, such as after the start of neoadjuvant chemotherapy or before surgery, he said.
On multivariate analysis, baseline CTC detection using any of the thresholds remained an independent predictor of overall and distant disease-free survival and locoregional relapse-free interval when considered together with pathologic complete response, cT, cN, and tumor subtype, suggesting that CTC measurement adds value to comprehensive prognostic models.
That is, they complement rather than duplicate usual prognostic factors and pathologic complete response rates to better predict outcomes in patients with early breast cancer in the neoadjuvant setting, Dr. Bidard said.
This study was supported by a research grant from Janssen Diagnostics. Dr. Bidard reported having no disclosures.
SAN ANTONIO – Circulating tumor cells are a useful prognostic biomarker in early breast cancer patients treated with neoadjuvant chemotherapy, according to findings from an international meta-analysis of individual patient data.
The cells (CTCs), which can be measured using a Food and Drug Administration–approved assay, are known to seed distant metastases and to be prognostic before and during therapy for patients with metastatic breast cancer, and prognostic before adjuvant therapy for patients with nonmetastatic breast cancer.
However, findings in the neoadjuvant setting have been variable, Francois-Clement Bidard, MD, of Institut Curie, Paris, reported at the San Antonio Breast Cancer Symposium.
In the study (the international meta-analysis of circulating tumor cell detection in early breast cancer patients treated by neoadjuvant chemotherapy, or IMENEO), CTCs were useful, independent of pathologic complete response, for predicting overall survival and distant disease-free survival in the neoadjuvant setting. Further, IMENEO showed for the first time that CTCs also predict locoregional relapse-free survival,
Based on the analysis of data from 2,156 patients from 21 studies and 16 centers in 10 countries, the CTC positivity rates using thresholds of one or more, two or more, and five or more, respectively, were 25%, 13%, and 6% in 1,574 patients tested at baseline, 17%, 6%, and 3% in 290 tested after neoadjuvant chemotherapy, 15%, 5%, and 1% in 1,200 tested before surgery, and 11%, 4%, and 1% in 285 tested after surgery, Dr. Bidard said.
Prior to neoadjuvant chemotherapy, at least one CTC was found in 19%, 22%, 24%, 29% and 41% of cT1, T2, T3, T4a-c, and T4d breast cancers, respectively, and this was marginally associated with hormone receptor negativity, he said, noting that later CTC detection rates were not associated with any patient baseline characteristics.
Nearly one in four patients (24%) achieved pathologic complete response, but this was not associated at any time point with CTC count.
For the primary study endpoint of overall survival, a significant association was found with the presence of at least two CTCs at baseline (hazard ratio, 2.6 for two CTCs; 3.84 for three to four CTCs; and 6.25 for five or more CTCs). Similar associations were found for distant disease-free survival (hazard ratios, 2.4, 3.4, and 5.0, respectively) and for locoregional relapse-free interval with two CTCs and five or more CTCs (hazard ratios, 2.4 and 4.2, respectively).
Similar results were found using later time points, such as after the start of neoadjuvant chemotherapy or before surgery, he said.
On multivariate analysis, baseline CTC detection using any of the thresholds remained an independent predictor of overall and distant disease-free survival and locoregional relapse-free interval when considered together with pathologic complete response, cT, cN, and tumor subtype, suggesting that CTC measurement adds value to comprehensive prognostic models.
That is, they complement rather than duplicate usual prognostic factors and pathologic complete response rates to better predict outcomes in patients with early breast cancer in the neoadjuvant setting, Dr. Bidard said.
This study was supported by a research grant from Janssen Diagnostics. Dr. Bidard reported having no disclosures.
SAN ANTONIO – Circulating tumor cells are a useful prognostic biomarker in early breast cancer patients treated with neoadjuvant chemotherapy, according to findings from an international meta-analysis of individual patient data.
The cells (CTCs), which can be measured using a Food and Drug Administration–approved assay, are known to seed distant metastases and to be prognostic before and during therapy for patients with metastatic breast cancer, and prognostic before adjuvant therapy for patients with nonmetastatic breast cancer.
However, findings in the neoadjuvant setting have been variable, Francois-Clement Bidard, MD, of Institut Curie, Paris, reported at the San Antonio Breast Cancer Symposium.
In the study (the international meta-analysis of circulating tumor cell detection in early breast cancer patients treated by neoadjuvant chemotherapy, or IMENEO), CTCs were useful, independent of pathologic complete response, for predicting overall survival and distant disease-free survival in the neoadjuvant setting. Further, IMENEO showed for the first time that CTCs also predict locoregional relapse-free survival,
Based on the analysis of data from 2,156 patients from 21 studies and 16 centers in 10 countries, the CTC positivity rates using thresholds of one or more, two or more, and five or more, respectively, were 25%, 13%, and 6% in 1,574 patients tested at baseline, 17%, 6%, and 3% in 290 tested after neoadjuvant chemotherapy, 15%, 5%, and 1% in 1,200 tested before surgery, and 11%, 4%, and 1% in 285 tested after surgery, Dr. Bidard said.
Prior to neoadjuvant chemotherapy, at least one CTC was found in 19%, 22%, 24%, 29% and 41% of cT1, T2, T3, T4a-c, and T4d breast cancers, respectively, and this was marginally associated with hormone receptor negativity, he said, noting that later CTC detection rates were not associated with any patient baseline characteristics.
Nearly one in four patients (24%) achieved pathologic complete response, but this was not associated at any time point with CTC count.
For the primary study endpoint of overall survival, a significant association was found with the presence of at least two CTCs at baseline (hazard ratio, 2.6 for two CTCs; 3.84 for three to four CTCs; and 6.25 for five or more CTCs). Similar associations were found for distant disease-free survival (hazard ratios, 2.4, 3.4, and 5.0, respectively) and for locoregional relapse-free interval with two CTCs and five or more CTCs (hazard ratios, 2.4 and 4.2, respectively).
Similar results were found using later time points, such as after the start of neoadjuvant chemotherapy or before surgery, he said.
On multivariate analysis, baseline CTC detection using any of the thresholds remained an independent predictor of overall and distant disease-free survival and locoregional relapse-free interval when considered together with pathologic complete response, cT, cN, and tumor subtype, suggesting that CTC measurement adds value to comprehensive prognostic models.
That is, they complement rather than duplicate usual prognostic factors and pathologic complete response rates to better predict outcomes in patients with early breast cancer in the neoadjuvant setting, Dr. Bidard said.
This study was supported by a research grant from Janssen Diagnostics. Dr. Bidard reported having no disclosures.
AT SABCS 2016
Key clinical point:
Major finding: Overall survival was associated with the presence of at least two CTCs at baseline (hazard ratio, 2.6 for two CTCs; 3.84 for three to four CTCs; and 6.25 for five or more CTCs).
Data source: A meta-analysis of data for 2,156 patients.
Disclosures: This study was supported by a research grant from Janssen Diagnostics. Dr. Bidard reported having no disclosures.
Gene, risk signatures could predict PARP inhibitor response in breast cancer
SAN ANTONIO – PARPi 7, BRCAness, and MammaPrint high1/(ultra)high2 signatures could help predict response to combination therapy with the poly ADP ribose polymerase (PARP) inhibitors veliparib and carboplatin among high-risk breast cancer patients and thereby improve patient care, according to findings from the I-SPY 2 clinical trial.
“I-SPY 2 is a phase II adaptively randomized neoadjuvant clinical trial with a shared control arm where patients receive standard neoadjuvant chemotherapy, and up to 4 simultaneous investigational arms. The primary endpoint of the trial is pathologic complete response, or pCR. The goal is to match therapies with the most responsive breast cancer subtypes,” Denise M. Wolf, PhD, of the University of California, San Francisco, explained at the San Antonio Breast Cancer Symposium.
The current analysis of I-Spy 2 data focuses on veliparib/carboplatin (VC), and the subtypes Dr. Wolf mentioned are defined by hormone receptor (HR) and HER2 expression, and by MammaPrint high1 or (ultra)high2 risk status, which, “roughly speaking, is a further stratification of the poor prognosis group into high- and extra-high-risk groups,” she said.
“This arm was open to HER2-negative patients, and graduated successfully in the triple-negative subset,” she noted, explaining that “agents or combinations graduate for efficacy if they reach 85% predicted probability of success in a subsequent phase III trial in the most responsive patient subset.”
The biomarker component of the trial aimed to evaluate biomarkers associated with the mechanisms of action of each investigational agent along with the predefined subsets.
Although the findings require verification in a larger trial, Dr. Wolf and her colleagues found that three biomarkers – the PARPi 7 gene signature, the 77-gene BRCAness signature (which both relate to DNA damage repair deficiency), and the MammaPrint high1 and (ultra)high2 risk categories – were each moderately correlated with treatment response in 72 patients receiving veliparib/carboplatin (VC), but not in 44 controls, and that the treatment-biomarker interactions retained statistical significance after adjusting for hormone receptor status.
“And so we asked the question, ‘Are these signatures identifying the same patients – and if not, might there be a way to combine them to identify a subset of patients who are especially likely to respond to VC?’” she said.
Further analysis showed that even though each of the biomarkers was a predictor of response, the biomarkers did not appear to identify exactly the same patients, therefore combining them might be of benefit.
“We did this using a simple voting scheme to combine pairs of biomarkers,” she said, adding that if the two paired biomarkers predicted resistance, the biomarker also predicted resistance. If only 1 predicted resistance, the combination predicted resistance, and only if both biomarkers predicted sensitivity did the combination predict sensitivity.
In the graduated triple-negative subset, for example, the 40% of patients who were PARPi 7–high and MammaPrint high2 (the two biomarkers most predictive of response) a dramatic separation was seen in the pCR probability curves, with an estimated pCR of 79% with VC treatment vs. 23% in the control arm.
“In contrast, triple-negative patients who were negative for one or more of the sensitivity markers had nearly overlapping probability response curves, from which we conclude that nearly all of the specific sensitivity to VC seen in the triple-negative patients is found in that subset who are positive for both sensitivity markers,” she said.
Additionally, although only 9% of HR-positive/HER2-negative patients were PARPi 7-high and MammaPrint high2, those patients also appeared to be more responsive to VC vs. the control arm (49% vs. 15%).
The results also demonstrate the value of an exploratory voting method for combining multiple biomarkers for the same treatment, Dr. Wolf noted.
However, the findings are limited by the small sample size and need to be evaluated in larger trials, and the biomarkers also need to be evaluated in carboplatin-only arms in order to “tease out whether the biomarkers are really specific to a PARP inhibitor with carboplatin or whether they might also apply to carboplatin alone.
“Our ongoing and future work is to develop predictive biomarkers for other I-Spy 2 agents,” she said.
Dr. Wolf reported having no disclosures.
SAN ANTONIO – PARPi 7, BRCAness, and MammaPrint high1/(ultra)high2 signatures could help predict response to combination therapy with the poly ADP ribose polymerase (PARP) inhibitors veliparib and carboplatin among high-risk breast cancer patients and thereby improve patient care, according to findings from the I-SPY 2 clinical trial.
“I-SPY 2 is a phase II adaptively randomized neoadjuvant clinical trial with a shared control arm where patients receive standard neoadjuvant chemotherapy, and up to 4 simultaneous investigational arms. The primary endpoint of the trial is pathologic complete response, or pCR. The goal is to match therapies with the most responsive breast cancer subtypes,” Denise M. Wolf, PhD, of the University of California, San Francisco, explained at the San Antonio Breast Cancer Symposium.
The current analysis of I-Spy 2 data focuses on veliparib/carboplatin (VC), and the subtypes Dr. Wolf mentioned are defined by hormone receptor (HR) and HER2 expression, and by MammaPrint high1 or (ultra)high2 risk status, which, “roughly speaking, is a further stratification of the poor prognosis group into high- and extra-high-risk groups,” she said.
“This arm was open to HER2-negative patients, and graduated successfully in the triple-negative subset,” she noted, explaining that “agents or combinations graduate for efficacy if they reach 85% predicted probability of success in a subsequent phase III trial in the most responsive patient subset.”
The biomarker component of the trial aimed to evaluate biomarkers associated with the mechanisms of action of each investigational agent along with the predefined subsets.
Although the findings require verification in a larger trial, Dr. Wolf and her colleagues found that three biomarkers – the PARPi 7 gene signature, the 77-gene BRCAness signature (which both relate to DNA damage repair deficiency), and the MammaPrint high1 and (ultra)high2 risk categories – were each moderately correlated with treatment response in 72 patients receiving veliparib/carboplatin (VC), but not in 44 controls, and that the treatment-biomarker interactions retained statistical significance after adjusting for hormone receptor status.
“And so we asked the question, ‘Are these signatures identifying the same patients – and if not, might there be a way to combine them to identify a subset of patients who are especially likely to respond to VC?’” she said.
Further analysis showed that even though each of the biomarkers was a predictor of response, the biomarkers did not appear to identify exactly the same patients, therefore combining them might be of benefit.
“We did this using a simple voting scheme to combine pairs of biomarkers,” she said, adding that if the two paired biomarkers predicted resistance, the biomarker also predicted resistance. If only 1 predicted resistance, the combination predicted resistance, and only if both biomarkers predicted sensitivity did the combination predict sensitivity.
In the graduated triple-negative subset, for example, the 40% of patients who were PARPi 7–high and MammaPrint high2 (the two biomarkers most predictive of response) a dramatic separation was seen in the pCR probability curves, with an estimated pCR of 79% with VC treatment vs. 23% in the control arm.
“In contrast, triple-negative patients who were negative for one or more of the sensitivity markers had nearly overlapping probability response curves, from which we conclude that nearly all of the specific sensitivity to VC seen in the triple-negative patients is found in that subset who are positive for both sensitivity markers,” she said.
Additionally, although only 9% of HR-positive/HER2-negative patients were PARPi 7-high and MammaPrint high2, those patients also appeared to be more responsive to VC vs. the control arm (49% vs. 15%).
The results also demonstrate the value of an exploratory voting method for combining multiple biomarkers for the same treatment, Dr. Wolf noted.
However, the findings are limited by the small sample size and need to be evaluated in larger trials, and the biomarkers also need to be evaluated in carboplatin-only arms in order to “tease out whether the biomarkers are really specific to a PARP inhibitor with carboplatin or whether they might also apply to carboplatin alone.
“Our ongoing and future work is to develop predictive biomarkers for other I-Spy 2 agents,” she said.
Dr. Wolf reported having no disclosures.
SAN ANTONIO – PARPi 7, BRCAness, and MammaPrint high1/(ultra)high2 signatures could help predict response to combination therapy with the poly ADP ribose polymerase (PARP) inhibitors veliparib and carboplatin among high-risk breast cancer patients and thereby improve patient care, according to findings from the I-SPY 2 clinical trial.
“I-SPY 2 is a phase II adaptively randomized neoadjuvant clinical trial with a shared control arm where patients receive standard neoadjuvant chemotherapy, and up to 4 simultaneous investigational arms. The primary endpoint of the trial is pathologic complete response, or pCR. The goal is to match therapies with the most responsive breast cancer subtypes,” Denise M. Wolf, PhD, of the University of California, San Francisco, explained at the San Antonio Breast Cancer Symposium.
The current analysis of I-Spy 2 data focuses on veliparib/carboplatin (VC), and the subtypes Dr. Wolf mentioned are defined by hormone receptor (HR) and HER2 expression, and by MammaPrint high1 or (ultra)high2 risk status, which, “roughly speaking, is a further stratification of the poor prognosis group into high- and extra-high-risk groups,” she said.
“This arm was open to HER2-negative patients, and graduated successfully in the triple-negative subset,” she noted, explaining that “agents or combinations graduate for efficacy if they reach 85% predicted probability of success in a subsequent phase III trial in the most responsive patient subset.”
The biomarker component of the trial aimed to evaluate biomarkers associated with the mechanisms of action of each investigational agent along with the predefined subsets.
Although the findings require verification in a larger trial, Dr. Wolf and her colleagues found that three biomarkers – the PARPi 7 gene signature, the 77-gene BRCAness signature (which both relate to DNA damage repair deficiency), and the MammaPrint high1 and (ultra)high2 risk categories – were each moderately correlated with treatment response in 72 patients receiving veliparib/carboplatin (VC), but not in 44 controls, and that the treatment-biomarker interactions retained statistical significance after adjusting for hormone receptor status.
“And so we asked the question, ‘Are these signatures identifying the same patients – and if not, might there be a way to combine them to identify a subset of patients who are especially likely to respond to VC?’” she said.
Further analysis showed that even though each of the biomarkers was a predictor of response, the biomarkers did not appear to identify exactly the same patients, therefore combining them might be of benefit.
“We did this using a simple voting scheme to combine pairs of biomarkers,” she said, adding that if the two paired biomarkers predicted resistance, the biomarker also predicted resistance. If only 1 predicted resistance, the combination predicted resistance, and only if both biomarkers predicted sensitivity did the combination predict sensitivity.
In the graduated triple-negative subset, for example, the 40% of patients who were PARPi 7–high and MammaPrint high2 (the two biomarkers most predictive of response) a dramatic separation was seen in the pCR probability curves, with an estimated pCR of 79% with VC treatment vs. 23% in the control arm.
“In contrast, triple-negative patients who were negative for one or more of the sensitivity markers had nearly overlapping probability response curves, from which we conclude that nearly all of the specific sensitivity to VC seen in the triple-negative patients is found in that subset who are positive for both sensitivity markers,” she said.
Additionally, although only 9% of HR-positive/HER2-negative patients were PARPi 7-high and MammaPrint high2, those patients also appeared to be more responsive to VC vs. the control arm (49% vs. 15%).
The results also demonstrate the value of an exploratory voting method for combining multiple biomarkers for the same treatment, Dr. Wolf noted.
However, the findings are limited by the small sample size and need to be evaluated in larger trials, and the biomarkers also need to be evaluated in carboplatin-only arms in order to “tease out whether the biomarkers are really specific to a PARP inhibitor with carboplatin or whether they might also apply to carboplatin alone.
“Our ongoing and future work is to develop predictive biomarkers for other I-Spy 2 agents,” she said.
Dr. Wolf reported having no disclosures.
AT SABCS 2016
Key clinical point:
Major finding: Estimated pCR in PARPi 7–high and MammaPrint 2 triple-negative patients was 79% vs. 23% for the VC arm vs. control arm.
Data source: The phase II adaptively randomized I-Spy 2 clinical trial of 116 subjects.
Disclosures: Dr. Wolf reported having no disclosures.
SLND after neoadjuvant chemo is feasible, but more study needed
SAN ANTONIO – Sentinel lymph node detection after neoadjuvant chemotherapy (NAC) is a safe and feasible strategy for preventing unnecessary systematic lymphadenectomy in patients with operable breast cancer and no clinical signs of cancer in the axillary lymph nodes prior to NAC, according to findings from the French prospective multicenter GANEA 2 trial.
However, further study is needed to assess the clinical impact of the 12% false negative rate associated with sentinel lymph node detection (SLND) in the current study, according to Jean-Marc Classe, MD, who reported the findings at the San Antonio Breast Cancer Symposium.
SLND was feasible in that it was achieved in 570 of 590 women (97%) with large operable breast tumors and negative findings on axillary sonography with fine needle cytology who were enrolled in the study, said Dr. Classe of Institut Cancerologie de l’Ouest Rene Gauducheau, Nantes, France.
Cancer cells were detected by SLND in 139 subjects after NAC and surgery, and all of those patients underwent axillary lymph node dissection. Another 418 had no sentinel node involvement after NAC and surgery, and had adequate follow-up; among those, overall 3-year survival was 97.8% and 3-year disease-free survival was 94.8%,
“In this group of patients ... we found only one axillary relapse,” he said.
These rates are comparable to historical survival rates among those without axillary involvement who undergo axillary lymph node dissection rather than sentinel lymph node detection, and the findings suggest that women with no clinical signs of axillary involvement could be spared systematic lymphadenectomy, he said.
“The standard surgical treatment after neoadjuvant chemotherapy is breast cancer surgery and lymphadenectomy level 1 and 2, but since the [National Surgical Adjuvant Breast and Bowel Project] B-27 trial, we all know that after neoadjuvant chemotherapy there are not any involved nodes in 50%-58% of patients,” he said, adding that about half of all lymphadenectomies in these patients are therefore unnecessary.
That percentage increases to more than 70% in “the very specific situation of patients treated for HER2+ breast cancer with cytologically proved axillary metastases after neoadjuvant chemotherapy,” he said.
“So we know that there is a place for sentinel lymph node biopsy after neoadjuvant chemotherapy in order to avoid unnecessary lymphadenectomy,” he said.
However, the high false negative rate associated with SLND in this and in prior studies, including the first GANEA trial, remains a concern. In fact, the most recent guidelines stated that the proof was too weak to strongly recommend sentinel lymph node biopsy after NAC, he noted.
The GANEA 2 trial was performed in response to a call in those guidelines for additional studies to assess the long-term risks of this strategy.
Study subjects included patients with FIGO stage T1-T3 infiltrating breast cancer who were enrolled from 15 French institutions between July 2010 and February 2014. Those with inflammatory cancer, local relapse, contraindications for NAC, or interrupted NAC due to progressive disease were excluded.
Follow-up included a medical visit with clinical assessment every 6 months and annual mammography.
The findings suggest that in patients with no proof of node involvement before treatment, SLND “seems to be safe within the limits of the short-term follow-up of this study,” Dr. Classe said, noting that given the concerns about the false negative rate and the uncertainty about the clinical impact of that, this approach “is not proved to be a safe procedure outside of trials.”
The strategy will be further evaluated, with a focus on eliminating false negative results, in the GANEA 3 trial, he said.
Dr. Classe reported having no disclosures.
SAN ANTONIO – Sentinel lymph node detection after neoadjuvant chemotherapy (NAC) is a safe and feasible strategy for preventing unnecessary systematic lymphadenectomy in patients with operable breast cancer and no clinical signs of cancer in the axillary lymph nodes prior to NAC, according to findings from the French prospective multicenter GANEA 2 trial.
However, further study is needed to assess the clinical impact of the 12% false negative rate associated with sentinel lymph node detection (SLND) in the current study, according to Jean-Marc Classe, MD, who reported the findings at the San Antonio Breast Cancer Symposium.
SLND was feasible in that it was achieved in 570 of 590 women (97%) with large operable breast tumors and negative findings on axillary sonography with fine needle cytology who were enrolled in the study, said Dr. Classe of Institut Cancerologie de l’Ouest Rene Gauducheau, Nantes, France.
Cancer cells were detected by SLND in 139 subjects after NAC and surgery, and all of those patients underwent axillary lymph node dissection. Another 418 had no sentinel node involvement after NAC and surgery, and had adequate follow-up; among those, overall 3-year survival was 97.8% and 3-year disease-free survival was 94.8%,
“In this group of patients ... we found only one axillary relapse,” he said.
These rates are comparable to historical survival rates among those without axillary involvement who undergo axillary lymph node dissection rather than sentinel lymph node detection, and the findings suggest that women with no clinical signs of axillary involvement could be spared systematic lymphadenectomy, he said.
“The standard surgical treatment after neoadjuvant chemotherapy is breast cancer surgery and lymphadenectomy level 1 and 2, but since the [National Surgical Adjuvant Breast and Bowel Project] B-27 trial, we all know that after neoadjuvant chemotherapy there are not any involved nodes in 50%-58% of patients,” he said, adding that about half of all lymphadenectomies in these patients are therefore unnecessary.
That percentage increases to more than 70% in “the very specific situation of patients treated for HER2+ breast cancer with cytologically proved axillary metastases after neoadjuvant chemotherapy,” he said.
“So we know that there is a place for sentinel lymph node biopsy after neoadjuvant chemotherapy in order to avoid unnecessary lymphadenectomy,” he said.
However, the high false negative rate associated with SLND in this and in prior studies, including the first GANEA trial, remains a concern. In fact, the most recent guidelines stated that the proof was too weak to strongly recommend sentinel lymph node biopsy after NAC, he noted.
The GANEA 2 trial was performed in response to a call in those guidelines for additional studies to assess the long-term risks of this strategy.
Study subjects included patients with FIGO stage T1-T3 infiltrating breast cancer who were enrolled from 15 French institutions between July 2010 and February 2014. Those with inflammatory cancer, local relapse, contraindications for NAC, or interrupted NAC due to progressive disease were excluded.
Follow-up included a medical visit with clinical assessment every 6 months and annual mammography.
The findings suggest that in patients with no proof of node involvement before treatment, SLND “seems to be safe within the limits of the short-term follow-up of this study,” Dr. Classe said, noting that given the concerns about the false negative rate and the uncertainty about the clinical impact of that, this approach “is not proved to be a safe procedure outside of trials.”
The strategy will be further evaluated, with a focus on eliminating false negative results, in the GANEA 3 trial, he said.
Dr. Classe reported having no disclosures.
SAN ANTONIO – Sentinel lymph node detection after neoadjuvant chemotherapy (NAC) is a safe and feasible strategy for preventing unnecessary systematic lymphadenectomy in patients with operable breast cancer and no clinical signs of cancer in the axillary lymph nodes prior to NAC, according to findings from the French prospective multicenter GANEA 2 trial.
However, further study is needed to assess the clinical impact of the 12% false negative rate associated with sentinel lymph node detection (SLND) in the current study, according to Jean-Marc Classe, MD, who reported the findings at the San Antonio Breast Cancer Symposium.
SLND was feasible in that it was achieved in 570 of 590 women (97%) with large operable breast tumors and negative findings on axillary sonography with fine needle cytology who were enrolled in the study, said Dr. Classe of Institut Cancerologie de l’Ouest Rene Gauducheau, Nantes, France.
Cancer cells were detected by SLND in 139 subjects after NAC and surgery, and all of those patients underwent axillary lymph node dissection. Another 418 had no sentinel node involvement after NAC and surgery, and had adequate follow-up; among those, overall 3-year survival was 97.8% and 3-year disease-free survival was 94.8%,
“In this group of patients ... we found only one axillary relapse,” he said.
These rates are comparable to historical survival rates among those without axillary involvement who undergo axillary lymph node dissection rather than sentinel lymph node detection, and the findings suggest that women with no clinical signs of axillary involvement could be spared systematic lymphadenectomy, he said.
“The standard surgical treatment after neoadjuvant chemotherapy is breast cancer surgery and lymphadenectomy level 1 and 2, but since the [National Surgical Adjuvant Breast and Bowel Project] B-27 trial, we all know that after neoadjuvant chemotherapy there are not any involved nodes in 50%-58% of patients,” he said, adding that about half of all lymphadenectomies in these patients are therefore unnecessary.
That percentage increases to more than 70% in “the very specific situation of patients treated for HER2+ breast cancer with cytologically proved axillary metastases after neoadjuvant chemotherapy,” he said.
“So we know that there is a place for sentinel lymph node biopsy after neoadjuvant chemotherapy in order to avoid unnecessary lymphadenectomy,” he said.
However, the high false negative rate associated with SLND in this and in prior studies, including the first GANEA trial, remains a concern. In fact, the most recent guidelines stated that the proof was too weak to strongly recommend sentinel lymph node biopsy after NAC, he noted.
The GANEA 2 trial was performed in response to a call in those guidelines for additional studies to assess the long-term risks of this strategy.
Study subjects included patients with FIGO stage T1-T3 infiltrating breast cancer who were enrolled from 15 French institutions between July 2010 and February 2014. Those with inflammatory cancer, local relapse, contraindications for NAC, or interrupted NAC due to progressive disease were excluded.
Follow-up included a medical visit with clinical assessment every 6 months and annual mammography.
The findings suggest that in patients with no proof of node involvement before treatment, SLND “seems to be safe within the limits of the short-term follow-up of this study,” Dr. Classe said, noting that given the concerns about the false negative rate and the uncertainty about the clinical impact of that, this approach “is not proved to be a safe procedure outside of trials.”
The strategy will be further evaluated, with a focus on eliminating false negative results, in the GANEA 3 trial, he said.
Dr. Classe reported having no disclosures.
AT SABCS 2016
Key clinical point:
Major finding: Overall 3-year survival was 97.8% and 3-year disease-free survival was 94.8% in 418 breast cancer patients who had no sentinel node involvement after NAC and surgery.
Data source: The prospective multicenter GANEA 2 trial of 590 patients.
Disclosures: Dr. Classe reported having no disclosures.
VIDEO: Watson helps oncologists sleuth out best options in breast cancer
SAN ANTONIO – Every day, practicing oncologists sort through mountains of data from treatment guidelines, clinical trials, protocols, and algorithms to make the best possible therapeutic decisions for their patients.
At Manipal Hospitals in Bangalore, India, those decisions are informed with the aid of Watson for Oncology (WFO), a software platform developed by IBM in concert with clinicians and investigators at Memorial Sloan Kettering Cancer Center in New York City.
In this video interview at the San Antonio Breast Cancer Symposium, S.P. Somashekhar, MBBS, MS, MCH, chairman of the Manipal Comprehensive Cancer Center, discusses how the system is used by the members of the center’s multidisciplinary tumor board to inform decision making. He also describes results of a study he presented at the symposium that shows a relatively high degree of concordance between clinician and WFO choices in the treatment of patients with local breast cancer but less agreement when it comes to the management of metastatic disease, for which there is no universal standard of care.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Every day, practicing oncologists sort through mountains of data from treatment guidelines, clinical trials, protocols, and algorithms to make the best possible therapeutic decisions for their patients.
At Manipal Hospitals in Bangalore, India, those decisions are informed with the aid of Watson for Oncology (WFO), a software platform developed by IBM in concert with clinicians and investigators at Memorial Sloan Kettering Cancer Center in New York City.
In this video interview at the San Antonio Breast Cancer Symposium, S.P. Somashekhar, MBBS, MS, MCH, chairman of the Manipal Comprehensive Cancer Center, discusses how the system is used by the members of the center’s multidisciplinary tumor board to inform decision making. He also describes results of a study he presented at the symposium that shows a relatively high degree of concordance between clinician and WFO choices in the treatment of patients with local breast cancer but less agreement when it comes to the management of metastatic disease, for which there is no universal standard of care.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Every day, practicing oncologists sort through mountains of data from treatment guidelines, clinical trials, protocols, and algorithms to make the best possible therapeutic decisions for their patients.
At Manipal Hospitals in Bangalore, India, those decisions are informed with the aid of Watson for Oncology (WFO), a software platform developed by IBM in concert with clinicians and investigators at Memorial Sloan Kettering Cancer Center in New York City.
In this video interview at the San Antonio Breast Cancer Symposium, S.P. Somashekhar, MBBS, MS, MCH, chairman of the Manipal Comprehensive Cancer Center, discusses how the system is used by the members of the center’s multidisciplinary tumor board to inform decision making. He also describes results of a study he presented at the symposium that shows a relatively high degree of concordance between clinician and WFO choices in the treatment of patients with local breast cancer but less agreement when it comes to the management of metastatic disease, for which there is no universal standard of care.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SABCS 2016
PIK3 inhibitor gives slight PFS edge at high cost for HR+/HER2– advanced breast cancer
SAN ANTONIO – A combination of a PI3K inhibitor and selective estrogen receptor down-regulator (SERD) met its primary endpoint of 2.1 months better progression-free survival (PFS) in postmenopausal women with locally advanced or metastatic breast cancer who were quickly running out of other treatment options.
Yet the small gain in PFS came at a very high price in terms of toxicities, including mood disorders that may have led to patient suicide attempts, according to investigators.
The BELLE-3 trial looked at the combination of the SERD fulvestrant (Faslodex) and an experimental inhibitor of the PI3 kinase, buparlisib, in postmenopausal women with hormone receptor–positive, human epidermal growth factor receptor–2 (HER2)-negative breast cancer treated with an aromatase inhibitor (AI) who experienced disease progression either on or after receiving therapy with an inhibitor of the mammalian target of rapamycin complex 1 (mTORC1).
The combination of fulvestrant and buparlisib was associated with a median PFS of 3.9 months, compared with 1.8 months for fulvestrant and placebo (P less than .001), Angelo Di Leo, MD, of Ospedale Misericordia e Dolce in Prato, Italy, reported at the San Antonio Breast Cancer Symposium,
Objective response rates (ORR) were low, at 7.8% in the combination arm, and 2.1% in the fulvestrant-plus-placebo arm.
Although the PFS difference was statistically significant, “the higher rate of toxicity in patients receiving buparlisib and fulvestrant, including transaminase elevations and mood disorders, may represent a clinically relevant challenge for future development of this compound in this particular group of patients,” Dr. Di Leo said.
Blocks AKT pathway
The preclinical rationale for the use of a P13K inhibitor after disease progression on mTORC1 inhibitor is that current mTOR inhibitors such as everolimus have a feedback mechanism that activates the AKT pathway, and that the use of P13K inhibitors can “abrogate or attenuate this activation, potentially blocking that pathway,” explained coinvestigator Ruth O’Regan, MD, head of the division of hematology and oncology at the University of Wisconsin–Madison School of Medicine and Public Health. Dr. O’Regan discussed the BELLE-3 findings in a briefing prior to Dr. Di Leo’s presentation of the data in general session.
In BELLE-3, 432 postmenopausal women with HR+/HER2-, AI-pretreated, locally advanced or metastatic breast cancer that had progressed on or after treatment with an mTOR inhibitor as the last line of therapy were enrolled. The patients were stratified by the presence or absence of visceral disease and then randomized on a 2:1 basis to fulvestrant 500 mg daily plus either buparlisib 100 mg/day (289 patients), or placebo (143).
As noted, the primary endpoint of investigator-assessed PFS favored the addition of buparlisib, with a hazard ratio for progression of 0.67 (P less than .001). PFS results by independent central review were similar (HR 0.57, P less than .001).
The ORR for the buparlisib/fulvestrant combination, 7.6%, consisted of 0.3% complete responses, and 7.3% partial responses. The ORR for placebo/fulvestrant, 2.1%, was composed entirely of partial responses. The respective clinical benefit rates, defined as a combination of complete and partial responses and stable disease, were 24.6% and 15.4.
The benefit of buparlisib was evidently entirely among patients with visceral disease, with a PFS of 3.1 vs. 1.5 months. In contrast, PFS among patients with no visceral disease was 4.2 vs. 4.1 months, respectively, and was not significant.
In addition, the P13K inhibitor seemed to benefit patients with PIK3CA mutations detected in either the primary tumor or in circulating DNA samples, but not patients with wild-type PIK3CA.
Depression, anxiety with combination
Patients assigned to buparlisib/fulvestrant had substantially higher proportions of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) elevations compared with patients on placebo fulvestrant, as well as more reported depression and anxiety. Three patients in the buparlisib arm attempted suicide. There were no reported suicide attempts in the placebo arm.
Dr. O’Regan said at the briefing that mood disorders are known adverse events associated with buparlisib, and that patients with psychiatric disorders were excluded from the trial.
Carlos Arteaga, MD, co-leader of the Breast Cancer Research Program at Vanderbilt-Ingram Cancer Center in Nashville, Tenn., who moderated the briefing, said in an interview that PI kinase mutations do not appear to be a good target, and that a pan-PIK3 inhibitor such as buparlisib may be hitting too many targets at once.
Inhibiting all of the PIK3 isoforms – alpha, beta, gamma, and delta – “may be a little too tough,” he said.
The trial should serve as impetus for developing agents that inhibit only the alpha isoform of PIK3 which mutates and appears to be the driver of some types of breast cancer, he said.
Following Dr. Di Leo’s presentation, perennial SABCS gadfly Steven Vogl, MD, New York, told the speaker that “you presented this very nicely, it’s very interesting biology. I don’t think you’d want to do this again to a human, right? Three month prolongation in PFS, miserable, with diarrhea, depression – you don’t want to do this again, is that correct?”
“I think buparlisib is probably not the best compound to be used in this particular setting of patients,” Dr. Di Leo said, but added that a better-tolerated PI3K inhibitor might be more effective.
“Actually, the study is raising an important question: Should we use the PI3K inhibitor in place of the mTOR inhibitor, or perhaps, as the study suggests, should we use the P13K inhibitors sequentially, after the mTOR inhibitor. This is an open question,” he replied.
Novartis sponsored the study. Dr. Di Leo disclosed consulting and lecture fees from the company, and Dr. O’Regan disclosed contracted research support. Dr. Arteaga reported no disclosures relevant to the study.
SAN ANTONIO – A combination of a PI3K inhibitor and selective estrogen receptor down-regulator (SERD) met its primary endpoint of 2.1 months better progression-free survival (PFS) in postmenopausal women with locally advanced or metastatic breast cancer who were quickly running out of other treatment options.
Yet the small gain in PFS came at a very high price in terms of toxicities, including mood disorders that may have led to patient suicide attempts, according to investigators.
The BELLE-3 trial looked at the combination of the SERD fulvestrant (Faslodex) and an experimental inhibitor of the PI3 kinase, buparlisib, in postmenopausal women with hormone receptor–positive, human epidermal growth factor receptor–2 (HER2)-negative breast cancer treated with an aromatase inhibitor (AI) who experienced disease progression either on or after receiving therapy with an inhibitor of the mammalian target of rapamycin complex 1 (mTORC1).
The combination of fulvestrant and buparlisib was associated with a median PFS of 3.9 months, compared with 1.8 months for fulvestrant and placebo (P less than .001), Angelo Di Leo, MD, of Ospedale Misericordia e Dolce in Prato, Italy, reported at the San Antonio Breast Cancer Symposium,
Objective response rates (ORR) were low, at 7.8% in the combination arm, and 2.1% in the fulvestrant-plus-placebo arm.
Although the PFS difference was statistically significant, “the higher rate of toxicity in patients receiving buparlisib and fulvestrant, including transaminase elevations and mood disorders, may represent a clinically relevant challenge for future development of this compound in this particular group of patients,” Dr. Di Leo said.
Blocks AKT pathway
The preclinical rationale for the use of a P13K inhibitor after disease progression on mTORC1 inhibitor is that current mTOR inhibitors such as everolimus have a feedback mechanism that activates the AKT pathway, and that the use of P13K inhibitors can “abrogate or attenuate this activation, potentially blocking that pathway,” explained coinvestigator Ruth O’Regan, MD, head of the division of hematology and oncology at the University of Wisconsin–Madison School of Medicine and Public Health. Dr. O’Regan discussed the BELLE-3 findings in a briefing prior to Dr. Di Leo’s presentation of the data in general session.
In BELLE-3, 432 postmenopausal women with HR+/HER2-, AI-pretreated, locally advanced or metastatic breast cancer that had progressed on or after treatment with an mTOR inhibitor as the last line of therapy were enrolled. The patients were stratified by the presence or absence of visceral disease and then randomized on a 2:1 basis to fulvestrant 500 mg daily plus either buparlisib 100 mg/day (289 patients), or placebo (143).
As noted, the primary endpoint of investigator-assessed PFS favored the addition of buparlisib, with a hazard ratio for progression of 0.67 (P less than .001). PFS results by independent central review were similar (HR 0.57, P less than .001).
The ORR for the buparlisib/fulvestrant combination, 7.6%, consisted of 0.3% complete responses, and 7.3% partial responses. The ORR for placebo/fulvestrant, 2.1%, was composed entirely of partial responses. The respective clinical benefit rates, defined as a combination of complete and partial responses and stable disease, were 24.6% and 15.4.
The benefit of buparlisib was evidently entirely among patients with visceral disease, with a PFS of 3.1 vs. 1.5 months. In contrast, PFS among patients with no visceral disease was 4.2 vs. 4.1 months, respectively, and was not significant.
In addition, the P13K inhibitor seemed to benefit patients with PIK3CA mutations detected in either the primary tumor or in circulating DNA samples, but not patients with wild-type PIK3CA.
Depression, anxiety with combination
Patients assigned to buparlisib/fulvestrant had substantially higher proportions of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) elevations compared with patients on placebo fulvestrant, as well as more reported depression and anxiety. Three patients in the buparlisib arm attempted suicide. There were no reported suicide attempts in the placebo arm.
Dr. O’Regan said at the briefing that mood disorders are known adverse events associated with buparlisib, and that patients with psychiatric disorders were excluded from the trial.
Carlos Arteaga, MD, co-leader of the Breast Cancer Research Program at Vanderbilt-Ingram Cancer Center in Nashville, Tenn., who moderated the briefing, said in an interview that PI kinase mutations do not appear to be a good target, and that a pan-PIK3 inhibitor such as buparlisib may be hitting too many targets at once.
Inhibiting all of the PIK3 isoforms – alpha, beta, gamma, and delta – “may be a little too tough,” he said.
The trial should serve as impetus for developing agents that inhibit only the alpha isoform of PIK3 which mutates and appears to be the driver of some types of breast cancer, he said.
Following Dr. Di Leo’s presentation, perennial SABCS gadfly Steven Vogl, MD, New York, told the speaker that “you presented this very nicely, it’s very interesting biology. I don’t think you’d want to do this again to a human, right? Three month prolongation in PFS, miserable, with diarrhea, depression – you don’t want to do this again, is that correct?”
“I think buparlisib is probably not the best compound to be used in this particular setting of patients,” Dr. Di Leo said, but added that a better-tolerated PI3K inhibitor might be more effective.
“Actually, the study is raising an important question: Should we use the PI3K inhibitor in place of the mTOR inhibitor, or perhaps, as the study suggests, should we use the P13K inhibitors sequentially, after the mTOR inhibitor. This is an open question,” he replied.
Novartis sponsored the study. Dr. Di Leo disclosed consulting and lecture fees from the company, and Dr. O’Regan disclosed contracted research support. Dr. Arteaga reported no disclosures relevant to the study.
SAN ANTONIO – A combination of a PI3K inhibitor and selective estrogen receptor down-regulator (SERD) met its primary endpoint of 2.1 months better progression-free survival (PFS) in postmenopausal women with locally advanced or metastatic breast cancer who were quickly running out of other treatment options.
Yet the small gain in PFS came at a very high price in terms of toxicities, including mood disorders that may have led to patient suicide attempts, according to investigators.
The BELLE-3 trial looked at the combination of the SERD fulvestrant (Faslodex) and an experimental inhibitor of the PI3 kinase, buparlisib, in postmenopausal women with hormone receptor–positive, human epidermal growth factor receptor–2 (HER2)-negative breast cancer treated with an aromatase inhibitor (AI) who experienced disease progression either on or after receiving therapy with an inhibitor of the mammalian target of rapamycin complex 1 (mTORC1).
The combination of fulvestrant and buparlisib was associated with a median PFS of 3.9 months, compared with 1.8 months for fulvestrant and placebo (P less than .001), Angelo Di Leo, MD, of Ospedale Misericordia e Dolce in Prato, Italy, reported at the San Antonio Breast Cancer Symposium,
Objective response rates (ORR) were low, at 7.8% in the combination arm, and 2.1% in the fulvestrant-plus-placebo arm.
Although the PFS difference was statistically significant, “the higher rate of toxicity in patients receiving buparlisib and fulvestrant, including transaminase elevations and mood disorders, may represent a clinically relevant challenge for future development of this compound in this particular group of patients,” Dr. Di Leo said.
Blocks AKT pathway
The preclinical rationale for the use of a P13K inhibitor after disease progression on mTORC1 inhibitor is that current mTOR inhibitors such as everolimus have a feedback mechanism that activates the AKT pathway, and that the use of P13K inhibitors can “abrogate or attenuate this activation, potentially blocking that pathway,” explained coinvestigator Ruth O’Regan, MD, head of the division of hematology and oncology at the University of Wisconsin–Madison School of Medicine and Public Health. Dr. O’Regan discussed the BELLE-3 findings in a briefing prior to Dr. Di Leo’s presentation of the data in general session.
In BELLE-3, 432 postmenopausal women with HR+/HER2-, AI-pretreated, locally advanced or metastatic breast cancer that had progressed on or after treatment with an mTOR inhibitor as the last line of therapy were enrolled. The patients were stratified by the presence or absence of visceral disease and then randomized on a 2:1 basis to fulvestrant 500 mg daily plus either buparlisib 100 mg/day (289 patients), or placebo (143).
As noted, the primary endpoint of investigator-assessed PFS favored the addition of buparlisib, with a hazard ratio for progression of 0.67 (P less than .001). PFS results by independent central review were similar (HR 0.57, P less than .001).
The ORR for the buparlisib/fulvestrant combination, 7.6%, consisted of 0.3% complete responses, and 7.3% partial responses. The ORR for placebo/fulvestrant, 2.1%, was composed entirely of partial responses. The respective clinical benefit rates, defined as a combination of complete and partial responses and stable disease, were 24.6% and 15.4.
The benefit of buparlisib was evidently entirely among patients with visceral disease, with a PFS of 3.1 vs. 1.5 months. In contrast, PFS among patients with no visceral disease was 4.2 vs. 4.1 months, respectively, and was not significant.
In addition, the P13K inhibitor seemed to benefit patients with PIK3CA mutations detected in either the primary tumor or in circulating DNA samples, but not patients with wild-type PIK3CA.
Depression, anxiety with combination
Patients assigned to buparlisib/fulvestrant had substantially higher proportions of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) elevations compared with patients on placebo fulvestrant, as well as more reported depression and anxiety. Three patients in the buparlisib arm attempted suicide. There were no reported suicide attempts in the placebo arm.
Dr. O’Regan said at the briefing that mood disorders are known adverse events associated with buparlisib, and that patients with psychiatric disorders were excluded from the trial.
Carlos Arteaga, MD, co-leader of the Breast Cancer Research Program at Vanderbilt-Ingram Cancer Center in Nashville, Tenn., who moderated the briefing, said in an interview that PI kinase mutations do not appear to be a good target, and that a pan-PIK3 inhibitor such as buparlisib may be hitting too many targets at once.
Inhibiting all of the PIK3 isoforms – alpha, beta, gamma, and delta – “may be a little too tough,” he said.
The trial should serve as impetus for developing agents that inhibit only the alpha isoform of PIK3 which mutates and appears to be the driver of some types of breast cancer, he said.
Following Dr. Di Leo’s presentation, perennial SABCS gadfly Steven Vogl, MD, New York, told the speaker that “you presented this very nicely, it’s very interesting biology. I don’t think you’d want to do this again to a human, right? Three month prolongation in PFS, miserable, with diarrhea, depression – you don’t want to do this again, is that correct?”
“I think buparlisib is probably not the best compound to be used in this particular setting of patients,” Dr. Di Leo said, but added that a better-tolerated PI3K inhibitor might be more effective.
“Actually, the study is raising an important question: Should we use the PI3K inhibitor in place of the mTOR inhibitor, or perhaps, as the study suggests, should we use the P13K inhibitors sequentially, after the mTOR inhibitor. This is an open question,” he replied.
Novartis sponsored the study. Dr. Di Leo disclosed consulting and lecture fees from the company, and Dr. O’Regan disclosed contracted research support. Dr. Arteaga reported no disclosures relevant to the study.
AT SABCS 2016
Key clinical point: The PI3K inhibitor buparlisib plus fulvestrant slightly prolonged progression-free survival of HR+/HER2– breast cancer pretreated with an aromatase inhibitor and mTOR inhibitor.
Major finding: The combination met its primary endpoint of better PFS than fulvestrant/placebo, but with high liver toxicity and mood disorders.
Data source: Randomized phase III trial of 432 women with hormone receptor–positive, HER2-negative, AI-pretreated breast cancer that progressed on or after mTOR inhibitor therapy.
Disclosures: Novartis sponsored the study. Dr. Di Leo disclosed consulting and lecture fees from the company, and Dr. O’Regan disclosed contracted research support. Dr. Arteaga reported no disclosures relevant to the study.
Cooling device reduces breast cancer–related alopecia during chemotherapy
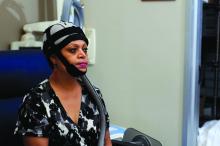
SAN ANTONIO – About half of the women with stage 1 or 2 breast cancer who were treated using a scalp cooling device during chemotherapy retained their hair in the prospective, randomized Scalp Cooling Alopecia Prevention (SCALP) trial.
Of 229 women who were enrolled from seven U.S. sites between December 2013 and September 2016 and who planned to undergo at least four cycles of anthracycline- or taxane-based chemotherapy, 182 met eligibility criteria; 119 were randomized to undergo scalp cooling using the Orbis Paxman Hair Loss Prevention System (OPHLPS; Paxman Coolers) and 63 were assigned to a control group. Of those, 95 and 47, respectively, were evaluable and had completed four cycles of chemotherapy at the time of a preplanned interim analysis.
The study was stopped after the interim analysis because of the positive superiority in the treatment group, Dr. Nangia said.
Study subjects were women with early-stage breast cancer and planned neoadjuvant or adjuvant chemotherapy. Most (65%) received taxane-based chemotherapy and 35% received anthracycline-based chemotherapy; the latter has been shown to be associated with higher rates of hair loss.
The OPHLPS system involves the use of a “cold cap” that is cooled using a refrigeration system and fitted to a patient’s head during chemotherapy treatment. For the current study, subjects underwent scalp cooling for 30 minutes before chemotherapy, during chemotherapy, and for 90 minutes after.
Most subjects (39%-52%) reported that they were reasonably comfortable during use of the device during treatment cycles 1-4. Only 2.4% reported being very uncomfortable, and that was only during cycle 2.
An analysis based on type of therapy showed that 65.1% of those receiving a taxane experienced hair preservation, compared with 21.9% of those receiving an anthracycline.
Scalp cooling, which reduces blood flow and chemoexposure to the hair follicles and thereby reduces hair loss, is widely used in Europe and, despite a great deal of interest in the technology in the United States, the Food and Drug Administration has been slow to get on board because of concerns over the potential for scalp metastasis. However, long-term outcomes data from Europe demonstrate that scalp metastasis is “exceedingly rare” and has occurred only in those with metastasis throughout the body, Dr. Nangia said.
Those data opened the door to the current study – the first prospective randomized trial of scalp-cooling.
In response to questions about the effects of scalp cooling across additional chemotherapy cycles (for example, those who undergo eight cycles), Dr. Nangia said that all patients completed four cycles, and data for those with eight cycles planned will be included in a final analysis.
“In other countries, they do have success rates with eight cycles, which is standard for some women,” she said. SCALP trial subjects will also be followed for 5 years to monitor overall survival, recurrence of cancer, and potential metastasis to the scalp, she said.
Based on the findings of the study, the maker of the device is seeking FDA clearance. If approved, the OPHLPS would compete with the DigniCap (Dignitana), which has already received approval.
“Scalp cooling devices are highly effective and should become available to women with breast cancer receiving chemotherapy ... Further study should be done exploring the technology for other types of tumors and with other chemotherapy regimens. More study looking at the impact of chemotherapy-induced alopecia on psyche and body image should be performed as well,” Dr. Nangia concluded, noting that tailored quality-of-life tools are needed to evaluate the impact of alopecia on quality of life.
Dr. Nangia reported receiving research funding from Paxman, the sponsor of the study, to her institution.
SAN ANTONIO – About half of the women with stage 1 or 2 breast cancer who were treated using a scalp cooling device during chemotherapy retained their hair in the prospective, randomized Scalp Cooling Alopecia Prevention (SCALP) trial.
Of 229 women who were enrolled from seven U.S. sites between December 2013 and September 2016 and who planned to undergo at least four cycles of anthracycline- or taxane-based chemotherapy, 182 met eligibility criteria; 119 were randomized to undergo scalp cooling using the Orbis Paxman Hair Loss Prevention System (OPHLPS; Paxman Coolers) and 63 were assigned to a control group. Of those, 95 and 47, respectively, were evaluable and had completed four cycles of chemotherapy at the time of a preplanned interim analysis.
The study was stopped after the interim analysis because of the positive superiority in the treatment group, Dr. Nangia said.
Study subjects were women with early-stage breast cancer and planned neoadjuvant or adjuvant chemotherapy. Most (65%) received taxane-based chemotherapy and 35% received anthracycline-based chemotherapy; the latter has been shown to be associated with higher rates of hair loss.
The OPHLPS system involves the use of a “cold cap” that is cooled using a refrigeration system and fitted to a patient’s head during chemotherapy treatment. For the current study, subjects underwent scalp cooling for 30 minutes before chemotherapy, during chemotherapy, and for 90 minutes after.
Most subjects (39%-52%) reported that they were reasonably comfortable during use of the device during treatment cycles 1-4. Only 2.4% reported being very uncomfortable, and that was only during cycle 2.
An analysis based on type of therapy showed that 65.1% of those receiving a taxane experienced hair preservation, compared with 21.9% of those receiving an anthracycline.
Scalp cooling, which reduces blood flow and chemoexposure to the hair follicles and thereby reduces hair loss, is widely used in Europe and, despite a great deal of interest in the technology in the United States, the Food and Drug Administration has been slow to get on board because of concerns over the potential for scalp metastasis. However, long-term outcomes data from Europe demonstrate that scalp metastasis is “exceedingly rare” and has occurred only in those with metastasis throughout the body, Dr. Nangia said.
Those data opened the door to the current study – the first prospective randomized trial of scalp-cooling.
In response to questions about the effects of scalp cooling across additional chemotherapy cycles (for example, those who undergo eight cycles), Dr. Nangia said that all patients completed four cycles, and data for those with eight cycles planned will be included in a final analysis.
“In other countries, they do have success rates with eight cycles, which is standard for some women,” she said. SCALP trial subjects will also be followed for 5 years to monitor overall survival, recurrence of cancer, and potential metastasis to the scalp, she said.
Based on the findings of the study, the maker of the device is seeking FDA clearance. If approved, the OPHLPS would compete with the DigniCap (Dignitana), which has already received approval.
“Scalp cooling devices are highly effective and should become available to women with breast cancer receiving chemotherapy ... Further study should be done exploring the technology for other types of tumors and with other chemotherapy regimens. More study looking at the impact of chemotherapy-induced alopecia on psyche and body image should be performed as well,” Dr. Nangia concluded, noting that tailored quality-of-life tools are needed to evaluate the impact of alopecia on quality of life.
Dr. Nangia reported receiving research funding from Paxman, the sponsor of the study, to her institution.
SAN ANTONIO – About half of the women with stage 1 or 2 breast cancer who were treated using a scalp cooling device during chemotherapy retained their hair in the prospective, randomized Scalp Cooling Alopecia Prevention (SCALP) trial.
Of 229 women who were enrolled from seven U.S. sites between December 2013 and September 2016 and who planned to undergo at least four cycles of anthracycline- or taxane-based chemotherapy, 182 met eligibility criteria; 119 were randomized to undergo scalp cooling using the Orbis Paxman Hair Loss Prevention System (OPHLPS; Paxman Coolers) and 63 were assigned to a control group. Of those, 95 and 47, respectively, were evaluable and had completed four cycles of chemotherapy at the time of a preplanned interim analysis.
The study was stopped after the interim analysis because of the positive superiority in the treatment group, Dr. Nangia said.
Study subjects were women with early-stage breast cancer and planned neoadjuvant or adjuvant chemotherapy. Most (65%) received taxane-based chemotherapy and 35% received anthracycline-based chemotherapy; the latter has been shown to be associated with higher rates of hair loss.
The OPHLPS system involves the use of a “cold cap” that is cooled using a refrigeration system and fitted to a patient’s head during chemotherapy treatment. For the current study, subjects underwent scalp cooling for 30 minutes before chemotherapy, during chemotherapy, and for 90 minutes after.
Most subjects (39%-52%) reported that they were reasonably comfortable during use of the device during treatment cycles 1-4. Only 2.4% reported being very uncomfortable, and that was only during cycle 2.
An analysis based on type of therapy showed that 65.1% of those receiving a taxane experienced hair preservation, compared with 21.9% of those receiving an anthracycline.
Scalp cooling, which reduces blood flow and chemoexposure to the hair follicles and thereby reduces hair loss, is widely used in Europe and, despite a great deal of interest in the technology in the United States, the Food and Drug Administration has been slow to get on board because of concerns over the potential for scalp metastasis. However, long-term outcomes data from Europe demonstrate that scalp metastasis is “exceedingly rare” and has occurred only in those with metastasis throughout the body, Dr. Nangia said.
Those data opened the door to the current study – the first prospective randomized trial of scalp-cooling.
In response to questions about the effects of scalp cooling across additional chemotherapy cycles (for example, those who undergo eight cycles), Dr. Nangia said that all patients completed four cycles, and data for those with eight cycles planned will be included in a final analysis.
“In other countries, they do have success rates with eight cycles, which is standard for some women,” she said. SCALP trial subjects will also be followed for 5 years to monitor overall survival, recurrence of cancer, and potential metastasis to the scalp, she said.
Based on the findings of the study, the maker of the device is seeking FDA clearance. If approved, the OPHLPS would compete with the DigniCap (Dignitana), which has already received approval.
“Scalp cooling devices are highly effective and should become available to women with breast cancer receiving chemotherapy ... Further study should be done exploring the technology for other types of tumors and with other chemotherapy regimens. More study looking at the impact of chemotherapy-induced alopecia on psyche and body image should be performed as well,” Dr. Nangia concluded, noting that tailored quality-of-life tools are needed to evaluate the impact of alopecia on quality of life.
Dr. Nangia reported receiving research funding from Paxman, the sponsor of the study, to her institution.
AT SABCS 2016
Key clinical point:
Major finding: 50.5% of treated patients experienced no more than grade 1 alopecia (less than 50% hair loss), compared with 0% of control subjects.
Data source: The prospective, randomized SCALP trial involving 182 women.
Disclosures: Dr. Nangia reported receiving research funding from Paxman, sponsor of the study, to her institution.
VIDEO: AIs associated with endothelial dysfunction, a predictor of CVD
SAN ANTONIO – Aromatase inhibitors (AIs) appear to be associated with declines in vascular endothelial function, which in turn have been associated with the development of cardiovascular disease, suggests a small study presented at the San Antonio Breast Cancer Symposium.
In this video interview, Anne Blaes, MD, from the Masonic Cancer Center at the University of Minnesota in Minneapolis, talks about the study, which compared endothelial function between postmenopausal women with hormone receptor–positive breast cancers treated with AIs and healthy postmenopausal controls.
Those treated with AIs, independent of the duration of therapy, had significantly worse endothelial function than healthy postmenopausal controls, as measured by the EndoPAT ratio.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Aromatase inhibitors (AIs) appear to be associated with declines in vascular endothelial function, which in turn have been associated with the development of cardiovascular disease, suggests a small study presented at the San Antonio Breast Cancer Symposium.
In this video interview, Anne Blaes, MD, from the Masonic Cancer Center at the University of Minnesota in Minneapolis, talks about the study, which compared endothelial function between postmenopausal women with hormone receptor–positive breast cancers treated with AIs and healthy postmenopausal controls.
Those treated with AIs, independent of the duration of therapy, had significantly worse endothelial function than healthy postmenopausal controls, as measured by the EndoPAT ratio.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Aromatase inhibitors (AIs) appear to be associated with declines in vascular endothelial function, which in turn have been associated with the development of cardiovascular disease, suggests a small study presented at the San Antonio Breast Cancer Symposium.
In this video interview, Anne Blaes, MD, from the Masonic Cancer Center at the University of Minnesota in Minneapolis, talks about the study, which compared endothelial function between postmenopausal women with hormone receptor–positive breast cancers treated with AIs and healthy postmenopausal controls.
Those treated with AIs, independent of the duration of therapy, had significantly worse endothelial function than healthy postmenopausal controls, as measured by the EndoPAT ratio.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SABCS 2016
It’s elementary: Watson aids in breast cancer decisions
SAN ANTONIO – When oncologists at Manipal Hospitals in Bangalore, India, need help with a breast cancer conundrum, they can make the electronic equivalent of the famous cry for help “Watson, come here, I want you!”
In this case, Watson is not the real-life sidekick of Alexander Graham Bell or the fictional companion of Sherlock Holmes, but Watson for Oncology (WFO), an IBM-created artificial intelligence platform being developed in the United States and used in clinical practice in India to help guide clinical decision making but not to replace clinicians’ judgment, explained S.P. Somashekhar, MBBS, MS, MCH, FRCS, chairman of the Manipal Comprehensive Cancer Center.
This is no game
WFO is the clinical cousin of the artificial intelligence platform, also named Watson, that beat all-time champion Ken Jennings on the television game show “Jeopardy!” The system was named after Thomas J. Watson, IBM’s first chief executive officer.
The version of Watson used in India was developed by physicians and investigators at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and by IBM. Watson for Oncology is fed national treatment guidelines, more than 1,000 training cases, MSKCC internal guidelines, and medical literature curated by MSKCC; it then chews over the data and spits out evidence-based recommendations.
At Manipal Hospitals, the electronic medical record contains an “Ask Watson” button that allows clinicians to interrogate the artificial entity’s vast stores of medical data to offer recommendations that clinicians can then use, modify, or reject at their discretion.
The system analyzes more than 100 patient attributes and then offers options with a green label for “recommended,” amber label “for consideration,” or a red label for “not recommended.”
Concordance study
At SABCS, Dr. Somashekhar presented results of a study evaluating the concordance of treatment recommendations between WFO and the members of the Manipal Multidisciplinary Tumor Board (MTB).
They looked at data on 638 patients treated in their hospital system over the three prior years, assessing the MTBs initial, best joint decision at the time of the original treatment decision (T1), and compared it with WFO’s recommendation made in 2016 (T2) and with a blinded MTB re-review of nonconcordant cases, also made in 2016.
Evaluating concordance by stage, they found that, for 514 cases of nonmetastatic disease, there was 79% concordance between the original MTB decisions and WFO’s recommendations for therapies in the combined “for consideration” and “recommended” categories.
For 124 cases of metastatic disease, however, Watson and its human counterparts were more frequently at odds, with only a 46% concordance.
In a subset analysis by receptor status, the investigators found that Watson was best – that is, most highly concordant – in triple-negative disease, with a 67.9% concordance in regard to MTB choices. In contrast, for patients with metastatic disease negative for the human epidermal growth factor receptor-2 (HER2), the concordance rate was a low 35%.
Dr. Somashekhar commented that part of the explanation for the discordant concordance rates by tumor subtype can be attributed to the fact that patients with triple-negative breast cancers have fewer treatment options than patients with HER2 negative–only tumors, who have a much broader array of possibilities.
One area where WFO has its two-legged colleagues beaten hands down, however, is in the time it takes to capture and analyze data: humans took a mean of 20 minutes, Watson a median of 40 seconds.
The investigators acknowledged that WFO represents a further step toward personalized medicine, but emphasized that software will never replace the doctor-patient relationship or override the treating physician’s decisions.
‘This one would actually help’
At the briefing, moderator C. Kent Osborne, MD, from Baylor College of Medicine, Houston, commented, “I guess this not going to put us out of business as physicians – at least I hope not.”
Asked whether WFO could be a useful clinical tool or just another nuisance task added to an already overcrowded clinic schedule, Dr. Osborne responded, “I think this one would actually help.”
This study was investigative and received no external funding. Dr. Somashekhar, Dr. Osborne, and Dr. Blaes reported no conflicts of interest.
SAN ANTONIO – When oncologists at Manipal Hospitals in Bangalore, India, need help with a breast cancer conundrum, they can make the electronic equivalent of the famous cry for help “Watson, come here, I want you!”
In this case, Watson is not the real-life sidekick of Alexander Graham Bell or the fictional companion of Sherlock Holmes, but Watson for Oncology (WFO), an IBM-created artificial intelligence platform being developed in the United States and used in clinical practice in India to help guide clinical decision making but not to replace clinicians’ judgment, explained S.P. Somashekhar, MBBS, MS, MCH, FRCS, chairman of the Manipal Comprehensive Cancer Center.
This is no game
WFO is the clinical cousin of the artificial intelligence platform, also named Watson, that beat all-time champion Ken Jennings on the television game show “Jeopardy!” The system was named after Thomas J. Watson, IBM’s first chief executive officer.
The version of Watson used in India was developed by physicians and investigators at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and by IBM. Watson for Oncology is fed national treatment guidelines, more than 1,000 training cases, MSKCC internal guidelines, and medical literature curated by MSKCC; it then chews over the data and spits out evidence-based recommendations.
At Manipal Hospitals, the electronic medical record contains an “Ask Watson” button that allows clinicians to interrogate the artificial entity’s vast stores of medical data to offer recommendations that clinicians can then use, modify, or reject at their discretion.
The system analyzes more than 100 patient attributes and then offers options with a green label for “recommended,” amber label “for consideration,” or a red label for “not recommended.”
Concordance study
At SABCS, Dr. Somashekhar presented results of a study evaluating the concordance of treatment recommendations between WFO and the members of the Manipal Multidisciplinary Tumor Board (MTB).
They looked at data on 638 patients treated in their hospital system over the three prior years, assessing the MTBs initial, best joint decision at the time of the original treatment decision (T1), and compared it with WFO’s recommendation made in 2016 (T2) and with a blinded MTB re-review of nonconcordant cases, also made in 2016.
Evaluating concordance by stage, they found that, for 514 cases of nonmetastatic disease, there was 79% concordance between the original MTB decisions and WFO’s recommendations for therapies in the combined “for consideration” and “recommended” categories.
For 124 cases of metastatic disease, however, Watson and its human counterparts were more frequently at odds, with only a 46% concordance.
In a subset analysis by receptor status, the investigators found that Watson was best – that is, most highly concordant – in triple-negative disease, with a 67.9% concordance in regard to MTB choices. In contrast, for patients with metastatic disease negative for the human epidermal growth factor receptor-2 (HER2), the concordance rate was a low 35%.
Dr. Somashekhar commented that part of the explanation for the discordant concordance rates by tumor subtype can be attributed to the fact that patients with triple-negative breast cancers have fewer treatment options than patients with HER2 negative–only tumors, who have a much broader array of possibilities.
One area where WFO has its two-legged colleagues beaten hands down, however, is in the time it takes to capture and analyze data: humans took a mean of 20 minutes, Watson a median of 40 seconds.
The investigators acknowledged that WFO represents a further step toward personalized medicine, but emphasized that software will never replace the doctor-patient relationship or override the treating physician’s decisions.
‘This one would actually help’
At the briefing, moderator C. Kent Osborne, MD, from Baylor College of Medicine, Houston, commented, “I guess this not going to put us out of business as physicians – at least I hope not.”
Asked whether WFO could be a useful clinical tool or just another nuisance task added to an already overcrowded clinic schedule, Dr. Osborne responded, “I think this one would actually help.”
This study was investigative and received no external funding. Dr. Somashekhar, Dr. Osborne, and Dr. Blaes reported no conflicts of interest.
SAN ANTONIO – When oncologists at Manipal Hospitals in Bangalore, India, need help with a breast cancer conundrum, they can make the electronic equivalent of the famous cry for help “Watson, come here, I want you!”
In this case, Watson is not the real-life sidekick of Alexander Graham Bell or the fictional companion of Sherlock Holmes, but Watson for Oncology (WFO), an IBM-created artificial intelligence platform being developed in the United States and used in clinical practice in India to help guide clinical decision making but not to replace clinicians’ judgment, explained S.P. Somashekhar, MBBS, MS, MCH, FRCS, chairman of the Manipal Comprehensive Cancer Center.
This is no game
WFO is the clinical cousin of the artificial intelligence platform, also named Watson, that beat all-time champion Ken Jennings on the television game show “Jeopardy!” The system was named after Thomas J. Watson, IBM’s first chief executive officer.
The version of Watson used in India was developed by physicians and investigators at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and by IBM. Watson for Oncology is fed national treatment guidelines, more than 1,000 training cases, MSKCC internal guidelines, and medical literature curated by MSKCC; it then chews over the data and spits out evidence-based recommendations.
At Manipal Hospitals, the electronic medical record contains an “Ask Watson” button that allows clinicians to interrogate the artificial entity’s vast stores of medical data to offer recommendations that clinicians can then use, modify, or reject at their discretion.
The system analyzes more than 100 patient attributes and then offers options with a green label for “recommended,” amber label “for consideration,” or a red label for “not recommended.”
Concordance study
At SABCS, Dr. Somashekhar presented results of a study evaluating the concordance of treatment recommendations between WFO and the members of the Manipal Multidisciplinary Tumor Board (MTB).
They looked at data on 638 patients treated in their hospital system over the three prior years, assessing the MTBs initial, best joint decision at the time of the original treatment decision (T1), and compared it with WFO’s recommendation made in 2016 (T2) and with a blinded MTB re-review of nonconcordant cases, also made in 2016.
Evaluating concordance by stage, they found that, for 514 cases of nonmetastatic disease, there was 79% concordance between the original MTB decisions and WFO’s recommendations for therapies in the combined “for consideration” and “recommended” categories.
For 124 cases of metastatic disease, however, Watson and its human counterparts were more frequently at odds, with only a 46% concordance.
In a subset analysis by receptor status, the investigators found that Watson was best – that is, most highly concordant – in triple-negative disease, with a 67.9% concordance in regard to MTB choices. In contrast, for patients with metastatic disease negative for the human epidermal growth factor receptor-2 (HER2), the concordance rate was a low 35%.
Dr. Somashekhar commented that part of the explanation for the discordant concordance rates by tumor subtype can be attributed to the fact that patients with triple-negative breast cancers have fewer treatment options than patients with HER2 negative–only tumors, who have a much broader array of possibilities.
One area where WFO has its two-legged colleagues beaten hands down, however, is in the time it takes to capture and analyze data: humans took a mean of 20 minutes, Watson a median of 40 seconds.
The investigators acknowledged that WFO represents a further step toward personalized medicine, but emphasized that software will never replace the doctor-patient relationship or override the treating physician’s decisions.
‘This one would actually help’
At the briefing, moderator C. Kent Osborne, MD, from Baylor College of Medicine, Houston, commented, “I guess this not going to put us out of business as physicians – at least I hope not.”
Asked whether WFO could be a useful clinical tool or just another nuisance task added to an already overcrowded clinic schedule, Dr. Osborne responded, “I think this one would actually help.”
This study was investigative and received no external funding. Dr. Somashekhar, Dr. Osborne, and Dr. Blaes reported no conflicts of interest.
AT SABCS 2016
Key clinical point: There was good, if imperfect, concordance in breast cancer treatment decisions between the software platform Watson for Oncology and human tumor board members.
Major finding: Concordance on treatment decisions for nonmetastatic breast cancers was 79%; concordance for metastatic cancers was 46%.
Data source: Single-institution study comparing treatment-decision concordance rates between a computerized decision tool and human reviewers.
Disclosures: This study was investigative and received no external funding. Dr. Somashekhar, Dr. Osborne, and Dr. Blaes reported no conflicts of interest.
VIDEO: Watson for Oncology offers electronic curbside consults in breast cancer
SAN ANTONIO – Watson for Oncology or WFO, is the clinical cousin of the IBM-created cognitive computing system best known as the machine that defeated all-time champions on the TV quiz show “Jeopardy!”
WFO, however, has the much more important function of providing oncologists with evidence-based support for clinical decisions. Data being presented here at the San Antonio Breast Cancer Symposium show that in Bangalore, India, where WFO is used in a large hospital system, there is good concordance between tumor board recommendations and WFO’s recommendations about the treatment of breast cancer, although much more work needs to be done. The investigators emphasize that WFO is a highly useful tool that can augment but will not replace clinical judgment, and can never replace the physician-patient relationship.
In this video interview, Andrew Norden, MD, deputy chief health officer for the IBM Watson project, based in Cambridge, Mass., describes how Watson for Oncology works, and how different versions of the Watson platform are being used in medicine throughout the world.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Watson for Oncology or WFO, is the clinical cousin of the IBM-created cognitive computing system best known as the machine that defeated all-time champions on the TV quiz show “Jeopardy!”
WFO, however, has the much more important function of providing oncologists with evidence-based support for clinical decisions. Data being presented here at the San Antonio Breast Cancer Symposium show that in Bangalore, India, where WFO is used in a large hospital system, there is good concordance between tumor board recommendations and WFO’s recommendations about the treatment of breast cancer, although much more work needs to be done. The investigators emphasize that WFO is a highly useful tool that can augment but will not replace clinical judgment, and can never replace the physician-patient relationship.
In this video interview, Andrew Norden, MD, deputy chief health officer for the IBM Watson project, based in Cambridge, Mass., describes how Watson for Oncology works, and how different versions of the Watson platform are being used in medicine throughout the world.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Watson for Oncology or WFO, is the clinical cousin of the IBM-created cognitive computing system best known as the machine that defeated all-time champions on the TV quiz show “Jeopardy!”
WFO, however, has the much more important function of providing oncologists with evidence-based support for clinical decisions. Data being presented here at the San Antonio Breast Cancer Symposium show that in Bangalore, India, where WFO is used in a large hospital system, there is good concordance between tumor board recommendations and WFO’s recommendations about the treatment of breast cancer, although much more work needs to be done. The investigators emphasize that WFO is a highly useful tool that can augment but will not replace clinical judgment, and can never replace the physician-patient relationship.
In this video interview, Andrew Norden, MD, deputy chief health officer for the IBM Watson project, based in Cambridge, Mass., describes how Watson for Oncology works, and how different versions of the Watson platform are being used in medicine throughout the world.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SABCS 2016