User login
Psoriasis is a chronic autoinflammatory disorder affecting approximately 2% to 4% of the Western population.1 While there is no absolute cure for psoriasis, novel therapies allow for substantial reduction in symptoms and considerable improvement in quality of life (QoL). In the past few years, multiple treatment guidelines (recommendations based on evidence-based literature reviews) and consensus statements (a set of declarations determined and voted on by a panel of experts in the field) have been developed to guide physicians worldwide in treating psoriasis in the clinical setting (eTable).2-10
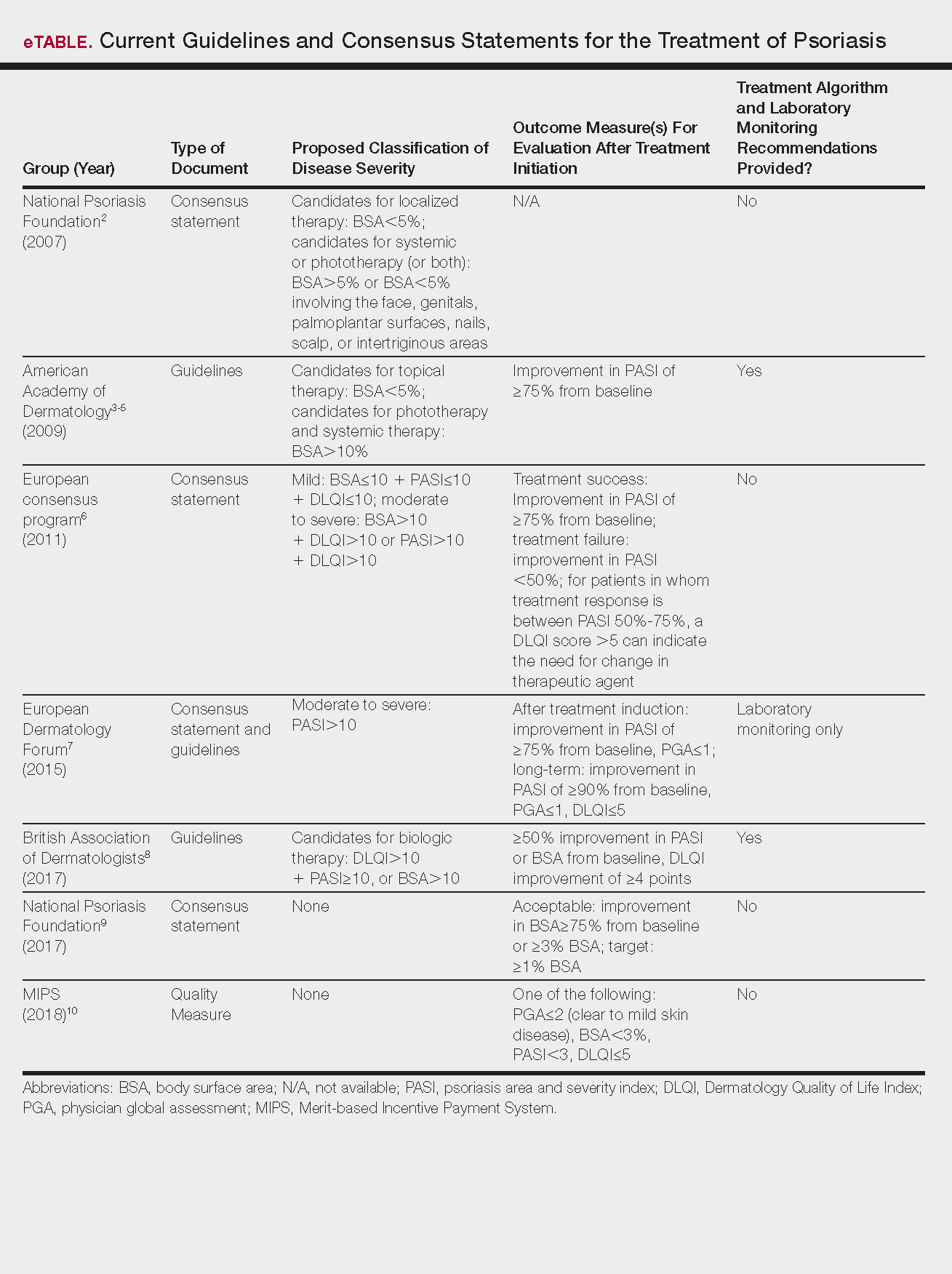
Because psoriasis is a complex disease with multiple comorbidities, applicability of these guidelines may be limited. Although some basic treatment algorithms exist, patient preference, disease severity, and other variables including comorbidities (eg, psoriatic arthritis [PsA], risk of major cardiac events, inflammatory bowel disease [IBD]), history of nonmelanoma skin cancer (NMSC), pregnancy and lactation, and specific contraindications to therapy (eg, renal failure, liver disease, active malignancy) should be considered. In this article, we summarize common themes across existing guidelines and consensus statements for the treatment of psoriasis and highlight areas where there is consistent agreement or lack of sufficient information.
Disease Severity and Treatment Outcomes
There currently are no consensus definitions for mild, moderate, and severe psoriasis, but several consensus statements have attempted to standardize grading systems based on objective values, such as body surface area (BSA) and psoriasis area and severity index (PASI)(a scoring system used to grade the degree of redness, thickness, and scaling of psoriasis plaques), as well as subjective QoL measures.2,6 Although classification of disease severity varies, mild psoriasis generally is characterized as disease that can be managed with local and topical therapy, and moderate to severe psoriasis typically warrants consideration for escalated treatment with phototherapy or systemic agents.
Most definitions of disease severity in psoriasis reference 5% to 10% BSA involvement as a cutoff that should trigger consideration of systemic treatment; however, these criteria could result in undertreatment of patients with substantial disease. For example, patients who have limited BSA involvement but whose disease has a considerable impact on QoL, as well as those who have debilitating disease in localized areas (eg, palms, soles, scalp, nails) or substantial joint involvement may also be appropriate candidates for systemic treatment.5,8
Once therapy is initiated, patients should be evaluated for appropriate treatment response at dedicated intervals. While the time to maximum therapeutic benefit depends on the agent of choice, European guidelines recommend that patients be evaluated after an induction phase (typically 16–24 weeks) and define treatment success as either (1) at least 75% improvement in PASI or (2) at least 50% improvement in PASI and a Dermatology Quality of Life Index (DLQI) score of 5 or lower.6
Alternatively, the National Psoriasis Foundation (NPF) recommended BSA as the preferred outcome measure in a recent consensus statement and concluded that an outcome of 3% or less BSA involvement or improvement in BSA of 75% or more is considered a desirable treatment response.9 Additionally, the Medicare Merit-based Incentive Payment System (MIPS) guidelines for successful systemic treatment response include at least 1 of the following: (1) physician global assessment score of 2 or lower, (2) BSA involvement of less than 3%, (3) PASI score lower than 3, or (4) DLQI score of 5 or lower.10
Although an array of outcome measures have been utilized in clinical trials and proposed in psoriasis guidelines and consensus statements, BSA is typically a manageable measure of treatment response in a clinical setting; however, DLQI should also be assessed if possible, particularly in patients with debilitating localized disease.9
Treatment Options
Because topical treatment regimens can be arduous and typically do not result in sustained clearance, patient expectations should be ascertained prior to initiation of therapy. Topical corticosteroids often can be used as monotherapy in patients with mild psoriasis.3 Topical vitamin D analogues and retinoids also can be effective; however, combined use of these agents with topical steroids should be considered to increase efficacy, and combination formulations can be prescribed to simplify application and improve adherence.
Treatment with UVB or psoralen plus UVA phototherapy is recommended for patients with moderate to severe psoriasis as well as in those who have had minimal response to topical therapy.4 Targeted phototherapy with an excimer laser can be used in patients with BSA involvement of less than 10%.
Methotrexate (MTX), cyclosporine, and acitretin are the most commonly prescribed systemic medications for severe psoriasis in the United States.5 Despite the risk for hepatotoxicity, MTX appears to have the best combined safety and efficacy profile in terms of serious adverse events compared to other systemic agents.11 Guidelines for MTX monitoring, especially with regard to when to do a liver biopsy, have been substantially liberalized over time, and the recommended interval for biopsy has been extended by years; biopsy was previously recommended after a cumulative MTX dose of 1 to 1.5 g, but guidelines now suggest biopsy after 3.5 to 4 g in low-risk patients.5 While abnormally elevated liver function tests during treatment with MTX may necessitate liver biopsy, the use of transient elastography and a panel of serum biomarkers for liver function also can be used to monitor noninvasively for hepatotoxicity before biopsy is considered; these recommendations are likely to be incorporated into newer guidelines in development.12 Methotrexate has demonstrated safety and increased efficacy when used in combination with biologic agents such as adalimumab, etanercept, infliximab, and secukinumab7 and has been studied in combination with many biologics indicated for PsA.13
Due to a considerable risk of glomerulosclerosis, cyclosporine is approved for a maximum of 1 year of continuous treatment of psoriasis in the United States and2 years in Europe.5,7 Cyclosporine is best used as induction therapy in psoriasis patients with severe disease who are seeking faster abatement of symptoms.
Acitretin is another systemic treatment option, although efficacy of this agent is dose dependent. Higher dosing often is limited due to lower tolerability.5
Given that many insurance formularies primarily cover traditional systemic therapies and that MTX and phototherapy are generally well tolerated and cost effective, patients may need to be treated with traditional agents before escalating to biologics. Prior to starting treatment with any biologic, patients should typically be screened for tuberculosis (TB), human immunodeficiency virus infection, and immunization for, exposure to, and/or infection with hepatitis B and C virus, and any other active infections. In patients who do not demonstrate hepatitis immunity, the hepatitis B vaccine should be administered prior to starting treatment with a biologic.14 In psoriasis patients with latent TB, 2 months of treatment should be completed before initiating biologic therapy8; once a biologic has been initiated, all patients should be screened annually for TB.
European guidelines for biologic treatment recommend that complete blood count and liver and renal function be evaluated at baseline, at months 1 and 3 of treatment, and then every 3 to 6 months thereafter while on the biologic agent.7 These recommendations are more stringent than those indicated in regulatory labeling and, based on the continual accumulation of data regarding the safety of these agents, some investigators have argued that laboratory testing might not be necessary at all.15
Treatment in Special Populations
Psoriasis patients often present with comorbidities or a complicated medical history, which can make it challenging to decide which therapy is most suitable. Patients with comorbid diseases (eg, PsA, risk of major cardiac event, IBD) or a history of NMSC and those who are pregnant or are lactating require special considerations to ensure treatment safety and efficacy.
Tumor necrosis factor α (TNF-α) and IL-17 inhibitors are used in the treatment of joint disorders and should be considered in patients with PsA. IL-23/IL-12 inhibition appears to have less benefit in patients with PsA, but studies on IL-23 inhibition (p19 antibodies) alone are ongoing.16 It has been reported that TNF-α inhibition may be beneficial in patients at risk for major cardiac events.8,17 In patients with IBD, IL-17 inhibitors should be avoided because they may exacerbate the condition; however, TNF-α and IL-23/IL-12 inhibition have shown to be safe in patients with IBD and many agents in these classes are approved by the US Food and Drug Administration for use in this population.18,19
Although biologics may increase the risk of developing NMSC20 and should generally be avoided in patients with any active malignancy, specific guidelines for screening and initiation of treatment in patients with a history of cancer are not clearly outlined. Prior to initiating systemic therapy in any patient, a careful medical history should be obtained. These agents often are not prescribed in patients with a history of cancer until remission has been established for at least 5 years, with the exception of patients with a history of treated NMSC.8 Annual skin monitoring for NMSC should be undertaken for psoriasis patients on most immunomodulating systemic therapies.
Recommendations for biologic treatment in psoriasis patients who are pregnant or lactating also are limited. European guidelines have noted pregnancy as an absolute contraindication to treatment with biologics,7but the regulatory guidance has recently changed for some agents, so this recommendation also may evolve.21 British8 and US5 guidelines do not consider pregnancy a contraindication for treatment with biologics.
Information on the safety of TNF-α antagonists during pregnancy comes primarily from use in patients with IBD and rheumatologic disease. To date, reports on the incidence of congenital malformations have been generally reassuring. Because IgG antibodies are actively transferred across the placenta in the late-second or the third trimesters, neonates born to mothers on biologic treatments may have high levels of some biologic drugs at birth. As a result, live vaccination should be avoided in neonates whose mothers were treated with IgG-based biologics.
Changing Treatment Agents
Patients may need to stop and change treatment agents due to ineffectiveness, personal preference, or worsening disease. When transitioning from any systemic or biologic agent to another (other than MTX), the British Association of Dermatologists recommends a washout period of at least 1 month before initiating a new therapy.8 Most guidelines do not define parameters for therapy escalation when patients fail multiple systemic agents, so physicians should use clinical judgment along with consideration of patient preference and comorbidity profile to ascertain which agent is most appropriate.
Conclusion
Keeping psoriasis treatment guidelines updated can be difficult, especially as new therapeutic options for psoriasis and treatment regimens rapidly evolve. Regulatory recommendations also vary worldwide, but most guidelines are reasonably consistent without being overly prescriptive, appropriately allowing for flexibility for application in clinical practice. Nonetheless, physicians should keep in mind new or changing guidelines while tailoring psoriasis treatment recommendations to best suit their individual patients.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTty (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence [published online September 27, 2012]. J Invest Dermatol. 2013;133:377-385.
- Pariser DM, Bagel J, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007;143:239-242.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1-10.
- Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris—update 2015—short version—EDF in cooperation with EADV and IPC [published online October 9, 2015]. J Eur Acad Dermatol Venereol. 2015;29:2277-2294.
- Smith CH, Jabbar-Lopez ZK, Yiu ZZ, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628-636.
- Armstrong AW, Siegel MP, Bagel J, et al. From the medical board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Quality ID #410: psoriasis: clinical response to oral systemic or biologic medications—national quality strategy domain: person and caregiver-centered experience and outcomes. Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Quality-Payment-Program/Resource-Library/2018-Resources.html. Accessed February 27, 2018.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Syst Rev. 2017;12:CD011535.
- Lynch M, Higgins E, McCormick PA, et al. The use of transient elastography and FibroTest for monitoring hepatotoxicity in patients receiving methotrexate for psoriasis. JAMA Dermatol. 2014;150:856-862.
- Behrens F, Canete J, Olivieri I, et al. Tumor necrosis factor inhibitor monotherapy versus combination with MTX in the treatment of PsA: a systemic review of the literature. Rheumatology. 2015;54:915-926.
- Karadağ Ö, Kaşifoğlu T, Özer B, et al. Viral hepatitis screening guideline before biological drug use in rheumatic patients. Eur J Rheumatol. 2016;3:25-28.
- Ahn CS, Dothard EH, Garner ML, et al. To test or not to test? an updated evidence-based assessment of the value of screening and monitoring tests when using systemic biologic agents to treat psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2015;73:420-428.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Humira [package insert]. North Chicago, IL: Abbott Laboratories; 2011.
- Stelara [package insert]. Bloomington, IN: Janssen Biotech, Inc; 2016.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Cimzia [package insert]. UCB, Inc: Smyrna, GA; 2016.
Psoriasis is a chronic autoinflammatory disorder affecting approximately 2% to 4% of the Western population.1 While there is no absolute cure for psoriasis, novel therapies allow for substantial reduction in symptoms and considerable improvement in quality of life (QoL). In the past few years, multiple treatment guidelines (recommendations based on evidence-based literature reviews) and consensus statements (a set of declarations determined and voted on by a panel of experts in the field) have been developed to guide physicians worldwide in treating psoriasis in the clinical setting (eTable).2-10

Because psoriasis is a complex disease with multiple comorbidities, applicability of these guidelines may be limited. Although some basic treatment algorithms exist, patient preference, disease severity, and other variables including comorbidities (eg, psoriatic arthritis [PsA], risk of major cardiac events, inflammatory bowel disease [IBD]), history of nonmelanoma skin cancer (NMSC), pregnancy and lactation, and specific contraindications to therapy (eg, renal failure, liver disease, active malignancy) should be considered. In this article, we summarize common themes across existing guidelines and consensus statements for the treatment of psoriasis and highlight areas where there is consistent agreement or lack of sufficient information.
Disease Severity and Treatment Outcomes
There currently are no consensus definitions for mild, moderate, and severe psoriasis, but several consensus statements have attempted to standardize grading systems based on objective values, such as body surface area (BSA) and psoriasis area and severity index (PASI)(a scoring system used to grade the degree of redness, thickness, and scaling of psoriasis plaques), as well as subjective QoL measures.2,6 Although classification of disease severity varies, mild psoriasis generally is characterized as disease that can be managed with local and topical therapy, and moderate to severe psoriasis typically warrants consideration for escalated treatment with phototherapy or systemic agents.
Most definitions of disease severity in psoriasis reference 5% to 10% BSA involvement as a cutoff that should trigger consideration of systemic treatment; however, these criteria could result in undertreatment of patients with substantial disease. For example, patients who have limited BSA involvement but whose disease has a considerable impact on QoL, as well as those who have debilitating disease in localized areas (eg, palms, soles, scalp, nails) or substantial joint involvement may also be appropriate candidates for systemic treatment.5,8
Once therapy is initiated, patients should be evaluated for appropriate treatment response at dedicated intervals. While the time to maximum therapeutic benefit depends on the agent of choice, European guidelines recommend that patients be evaluated after an induction phase (typically 16–24 weeks) and define treatment success as either (1) at least 75% improvement in PASI or (2) at least 50% improvement in PASI and a Dermatology Quality of Life Index (DLQI) score of 5 or lower.6
Alternatively, the National Psoriasis Foundation (NPF) recommended BSA as the preferred outcome measure in a recent consensus statement and concluded that an outcome of 3% or less BSA involvement or improvement in BSA of 75% or more is considered a desirable treatment response.9 Additionally, the Medicare Merit-based Incentive Payment System (MIPS) guidelines for successful systemic treatment response include at least 1 of the following: (1) physician global assessment score of 2 or lower, (2) BSA involvement of less than 3%, (3) PASI score lower than 3, or (4) DLQI score of 5 or lower.10
Although an array of outcome measures have been utilized in clinical trials and proposed in psoriasis guidelines and consensus statements, BSA is typically a manageable measure of treatment response in a clinical setting; however, DLQI should also be assessed if possible, particularly in patients with debilitating localized disease.9
Treatment Options
Because topical treatment regimens can be arduous and typically do not result in sustained clearance, patient expectations should be ascertained prior to initiation of therapy. Topical corticosteroids often can be used as monotherapy in patients with mild psoriasis.3 Topical vitamin D analogues and retinoids also can be effective; however, combined use of these agents with topical steroids should be considered to increase efficacy, and combination formulations can be prescribed to simplify application and improve adherence.
Treatment with UVB or psoralen plus UVA phototherapy is recommended for patients with moderate to severe psoriasis as well as in those who have had minimal response to topical therapy.4 Targeted phototherapy with an excimer laser can be used in patients with BSA involvement of less than 10%.
Methotrexate (MTX), cyclosporine, and acitretin are the most commonly prescribed systemic medications for severe psoriasis in the United States.5 Despite the risk for hepatotoxicity, MTX appears to have the best combined safety and efficacy profile in terms of serious adverse events compared to other systemic agents.11 Guidelines for MTX monitoring, especially with regard to when to do a liver biopsy, have been substantially liberalized over time, and the recommended interval for biopsy has been extended by years; biopsy was previously recommended after a cumulative MTX dose of 1 to 1.5 g, but guidelines now suggest biopsy after 3.5 to 4 g in low-risk patients.5 While abnormally elevated liver function tests during treatment with MTX may necessitate liver biopsy, the use of transient elastography and a panel of serum biomarkers for liver function also can be used to monitor noninvasively for hepatotoxicity before biopsy is considered; these recommendations are likely to be incorporated into newer guidelines in development.12 Methotrexate has demonstrated safety and increased efficacy when used in combination with biologic agents such as adalimumab, etanercept, infliximab, and secukinumab7 and has been studied in combination with many biologics indicated for PsA.13
Due to a considerable risk of glomerulosclerosis, cyclosporine is approved for a maximum of 1 year of continuous treatment of psoriasis in the United States and2 years in Europe.5,7 Cyclosporine is best used as induction therapy in psoriasis patients with severe disease who are seeking faster abatement of symptoms.
Acitretin is another systemic treatment option, although efficacy of this agent is dose dependent. Higher dosing often is limited due to lower tolerability.5
Given that many insurance formularies primarily cover traditional systemic therapies and that MTX and phototherapy are generally well tolerated and cost effective, patients may need to be treated with traditional agents before escalating to biologics. Prior to starting treatment with any biologic, patients should typically be screened for tuberculosis (TB), human immunodeficiency virus infection, and immunization for, exposure to, and/or infection with hepatitis B and C virus, and any other active infections. In patients who do not demonstrate hepatitis immunity, the hepatitis B vaccine should be administered prior to starting treatment with a biologic.14 In psoriasis patients with latent TB, 2 months of treatment should be completed before initiating biologic therapy8; once a biologic has been initiated, all patients should be screened annually for TB.
European guidelines for biologic treatment recommend that complete blood count and liver and renal function be evaluated at baseline, at months 1 and 3 of treatment, and then every 3 to 6 months thereafter while on the biologic agent.7 These recommendations are more stringent than those indicated in regulatory labeling and, based on the continual accumulation of data regarding the safety of these agents, some investigators have argued that laboratory testing might not be necessary at all.15
Treatment in Special Populations
Psoriasis patients often present with comorbidities or a complicated medical history, which can make it challenging to decide which therapy is most suitable. Patients with comorbid diseases (eg, PsA, risk of major cardiac event, IBD) or a history of NMSC and those who are pregnant or are lactating require special considerations to ensure treatment safety and efficacy.
Tumor necrosis factor α (TNF-α) and IL-17 inhibitors are used in the treatment of joint disorders and should be considered in patients with PsA. IL-23/IL-12 inhibition appears to have less benefit in patients with PsA, but studies on IL-23 inhibition (p19 antibodies) alone are ongoing.16 It has been reported that TNF-α inhibition may be beneficial in patients at risk for major cardiac events.8,17 In patients with IBD, IL-17 inhibitors should be avoided because they may exacerbate the condition; however, TNF-α and IL-23/IL-12 inhibition have shown to be safe in patients with IBD and many agents in these classes are approved by the US Food and Drug Administration for use in this population.18,19
Although biologics may increase the risk of developing NMSC20 and should generally be avoided in patients with any active malignancy, specific guidelines for screening and initiation of treatment in patients with a history of cancer are not clearly outlined. Prior to initiating systemic therapy in any patient, a careful medical history should be obtained. These agents often are not prescribed in patients with a history of cancer until remission has been established for at least 5 years, with the exception of patients with a history of treated NMSC.8 Annual skin monitoring for NMSC should be undertaken for psoriasis patients on most immunomodulating systemic therapies.
Recommendations for biologic treatment in psoriasis patients who are pregnant or lactating also are limited. European guidelines have noted pregnancy as an absolute contraindication to treatment with biologics,7but the regulatory guidance has recently changed for some agents, so this recommendation also may evolve.21 British8 and US5 guidelines do not consider pregnancy a contraindication for treatment with biologics.
Information on the safety of TNF-α antagonists during pregnancy comes primarily from use in patients with IBD and rheumatologic disease. To date, reports on the incidence of congenital malformations have been generally reassuring. Because IgG antibodies are actively transferred across the placenta in the late-second or the third trimesters, neonates born to mothers on biologic treatments may have high levels of some biologic drugs at birth. As a result, live vaccination should be avoided in neonates whose mothers were treated with IgG-based biologics.
Changing Treatment Agents
Patients may need to stop and change treatment agents due to ineffectiveness, personal preference, or worsening disease. When transitioning from any systemic or biologic agent to another (other than MTX), the British Association of Dermatologists recommends a washout period of at least 1 month before initiating a new therapy.8 Most guidelines do not define parameters for therapy escalation when patients fail multiple systemic agents, so physicians should use clinical judgment along with consideration of patient preference and comorbidity profile to ascertain which agent is most appropriate.
Conclusion
Keeping psoriasis treatment guidelines updated can be difficult, especially as new therapeutic options for psoriasis and treatment regimens rapidly evolve. Regulatory recommendations also vary worldwide, but most guidelines are reasonably consistent without being overly prescriptive, appropriately allowing for flexibility for application in clinical practice. Nonetheless, physicians should keep in mind new or changing guidelines while tailoring psoriasis treatment recommendations to best suit their individual patients.
Psoriasis is a chronic autoinflammatory disorder affecting approximately 2% to 4% of the Western population.1 While there is no absolute cure for psoriasis, novel therapies allow for substantial reduction in symptoms and considerable improvement in quality of life (QoL). In the past few years, multiple treatment guidelines (recommendations based on evidence-based literature reviews) and consensus statements (a set of declarations determined and voted on by a panel of experts in the field) have been developed to guide physicians worldwide in treating psoriasis in the clinical setting (eTable).2-10

Because psoriasis is a complex disease with multiple comorbidities, applicability of these guidelines may be limited. Although some basic treatment algorithms exist, patient preference, disease severity, and other variables including comorbidities (eg, psoriatic arthritis [PsA], risk of major cardiac events, inflammatory bowel disease [IBD]), history of nonmelanoma skin cancer (NMSC), pregnancy and lactation, and specific contraindications to therapy (eg, renal failure, liver disease, active malignancy) should be considered. In this article, we summarize common themes across existing guidelines and consensus statements for the treatment of psoriasis and highlight areas where there is consistent agreement or lack of sufficient information.
Disease Severity and Treatment Outcomes
There currently are no consensus definitions for mild, moderate, and severe psoriasis, but several consensus statements have attempted to standardize grading systems based on objective values, such as body surface area (BSA) and psoriasis area and severity index (PASI)(a scoring system used to grade the degree of redness, thickness, and scaling of psoriasis plaques), as well as subjective QoL measures.2,6 Although classification of disease severity varies, mild psoriasis generally is characterized as disease that can be managed with local and topical therapy, and moderate to severe psoriasis typically warrants consideration for escalated treatment with phototherapy or systemic agents.
Most definitions of disease severity in psoriasis reference 5% to 10% BSA involvement as a cutoff that should trigger consideration of systemic treatment; however, these criteria could result in undertreatment of patients with substantial disease. For example, patients who have limited BSA involvement but whose disease has a considerable impact on QoL, as well as those who have debilitating disease in localized areas (eg, palms, soles, scalp, nails) or substantial joint involvement may also be appropriate candidates for systemic treatment.5,8
Once therapy is initiated, patients should be evaluated for appropriate treatment response at dedicated intervals. While the time to maximum therapeutic benefit depends on the agent of choice, European guidelines recommend that patients be evaluated after an induction phase (typically 16–24 weeks) and define treatment success as either (1) at least 75% improvement in PASI or (2) at least 50% improvement in PASI and a Dermatology Quality of Life Index (DLQI) score of 5 or lower.6
Alternatively, the National Psoriasis Foundation (NPF) recommended BSA as the preferred outcome measure in a recent consensus statement and concluded that an outcome of 3% or less BSA involvement or improvement in BSA of 75% or more is considered a desirable treatment response.9 Additionally, the Medicare Merit-based Incentive Payment System (MIPS) guidelines for successful systemic treatment response include at least 1 of the following: (1) physician global assessment score of 2 or lower, (2) BSA involvement of less than 3%, (3) PASI score lower than 3, or (4) DLQI score of 5 or lower.10
Although an array of outcome measures have been utilized in clinical trials and proposed in psoriasis guidelines and consensus statements, BSA is typically a manageable measure of treatment response in a clinical setting; however, DLQI should also be assessed if possible, particularly in patients with debilitating localized disease.9
Treatment Options
Because topical treatment regimens can be arduous and typically do not result in sustained clearance, patient expectations should be ascertained prior to initiation of therapy. Topical corticosteroids often can be used as monotherapy in patients with mild psoriasis.3 Topical vitamin D analogues and retinoids also can be effective; however, combined use of these agents with topical steroids should be considered to increase efficacy, and combination formulations can be prescribed to simplify application and improve adherence.
Treatment with UVB or psoralen plus UVA phototherapy is recommended for patients with moderate to severe psoriasis as well as in those who have had minimal response to topical therapy.4 Targeted phototherapy with an excimer laser can be used in patients with BSA involvement of less than 10%.
Methotrexate (MTX), cyclosporine, and acitretin are the most commonly prescribed systemic medications for severe psoriasis in the United States.5 Despite the risk for hepatotoxicity, MTX appears to have the best combined safety and efficacy profile in terms of serious adverse events compared to other systemic agents.11 Guidelines for MTX monitoring, especially with regard to when to do a liver biopsy, have been substantially liberalized over time, and the recommended interval for biopsy has been extended by years; biopsy was previously recommended after a cumulative MTX dose of 1 to 1.5 g, but guidelines now suggest biopsy after 3.5 to 4 g in low-risk patients.5 While abnormally elevated liver function tests during treatment with MTX may necessitate liver biopsy, the use of transient elastography and a panel of serum biomarkers for liver function also can be used to monitor noninvasively for hepatotoxicity before biopsy is considered; these recommendations are likely to be incorporated into newer guidelines in development.12 Methotrexate has demonstrated safety and increased efficacy when used in combination with biologic agents such as adalimumab, etanercept, infliximab, and secukinumab7 and has been studied in combination with many biologics indicated for PsA.13
Due to a considerable risk of glomerulosclerosis, cyclosporine is approved for a maximum of 1 year of continuous treatment of psoriasis in the United States and2 years in Europe.5,7 Cyclosporine is best used as induction therapy in psoriasis patients with severe disease who are seeking faster abatement of symptoms.
Acitretin is another systemic treatment option, although efficacy of this agent is dose dependent. Higher dosing often is limited due to lower tolerability.5
Given that many insurance formularies primarily cover traditional systemic therapies and that MTX and phototherapy are generally well tolerated and cost effective, patients may need to be treated with traditional agents before escalating to biologics. Prior to starting treatment with any biologic, patients should typically be screened for tuberculosis (TB), human immunodeficiency virus infection, and immunization for, exposure to, and/or infection with hepatitis B and C virus, and any other active infections. In patients who do not demonstrate hepatitis immunity, the hepatitis B vaccine should be administered prior to starting treatment with a biologic.14 In psoriasis patients with latent TB, 2 months of treatment should be completed before initiating biologic therapy8; once a biologic has been initiated, all patients should be screened annually for TB.
European guidelines for biologic treatment recommend that complete blood count and liver and renal function be evaluated at baseline, at months 1 and 3 of treatment, and then every 3 to 6 months thereafter while on the biologic agent.7 These recommendations are more stringent than those indicated in regulatory labeling and, based on the continual accumulation of data regarding the safety of these agents, some investigators have argued that laboratory testing might not be necessary at all.15
Treatment in Special Populations
Psoriasis patients often present with comorbidities or a complicated medical history, which can make it challenging to decide which therapy is most suitable. Patients with comorbid diseases (eg, PsA, risk of major cardiac event, IBD) or a history of NMSC and those who are pregnant or are lactating require special considerations to ensure treatment safety and efficacy.
Tumor necrosis factor α (TNF-α) and IL-17 inhibitors are used in the treatment of joint disorders and should be considered in patients with PsA. IL-23/IL-12 inhibition appears to have less benefit in patients with PsA, but studies on IL-23 inhibition (p19 antibodies) alone are ongoing.16 It has been reported that TNF-α inhibition may be beneficial in patients at risk for major cardiac events.8,17 In patients with IBD, IL-17 inhibitors should be avoided because they may exacerbate the condition; however, TNF-α and IL-23/IL-12 inhibition have shown to be safe in patients with IBD and many agents in these classes are approved by the US Food and Drug Administration for use in this population.18,19
Although biologics may increase the risk of developing NMSC20 and should generally be avoided in patients with any active malignancy, specific guidelines for screening and initiation of treatment in patients with a history of cancer are not clearly outlined. Prior to initiating systemic therapy in any patient, a careful medical history should be obtained. These agents often are not prescribed in patients with a history of cancer until remission has been established for at least 5 years, with the exception of patients with a history of treated NMSC.8 Annual skin monitoring for NMSC should be undertaken for psoriasis patients on most immunomodulating systemic therapies.
Recommendations for biologic treatment in psoriasis patients who are pregnant or lactating also are limited. European guidelines have noted pregnancy as an absolute contraindication to treatment with biologics,7but the regulatory guidance has recently changed for some agents, so this recommendation also may evolve.21 British8 and US5 guidelines do not consider pregnancy a contraindication for treatment with biologics.
Information on the safety of TNF-α antagonists during pregnancy comes primarily from use in patients with IBD and rheumatologic disease. To date, reports on the incidence of congenital malformations have been generally reassuring. Because IgG antibodies are actively transferred across the placenta in the late-second or the third trimesters, neonates born to mothers on biologic treatments may have high levels of some biologic drugs at birth. As a result, live vaccination should be avoided in neonates whose mothers were treated with IgG-based biologics.
Changing Treatment Agents
Patients may need to stop and change treatment agents due to ineffectiveness, personal preference, or worsening disease. When transitioning from any systemic or biologic agent to another (other than MTX), the British Association of Dermatologists recommends a washout period of at least 1 month before initiating a new therapy.8 Most guidelines do not define parameters for therapy escalation when patients fail multiple systemic agents, so physicians should use clinical judgment along with consideration of patient preference and comorbidity profile to ascertain which agent is most appropriate.
Conclusion
Keeping psoriasis treatment guidelines updated can be difficult, especially as new therapeutic options for psoriasis and treatment regimens rapidly evolve. Regulatory recommendations also vary worldwide, but most guidelines are reasonably consistent without being overly prescriptive, appropriately allowing for flexibility for application in clinical practice. Nonetheless, physicians should keep in mind new or changing guidelines while tailoring psoriasis treatment recommendations to best suit their individual patients.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTty (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence [published online September 27, 2012]. J Invest Dermatol. 2013;133:377-385.
- Pariser DM, Bagel J, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007;143:239-242.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1-10.
- Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris—update 2015—short version—EDF in cooperation with EADV and IPC [published online October 9, 2015]. J Eur Acad Dermatol Venereol. 2015;29:2277-2294.
- Smith CH, Jabbar-Lopez ZK, Yiu ZZ, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628-636.
- Armstrong AW, Siegel MP, Bagel J, et al. From the medical board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Quality ID #410: psoriasis: clinical response to oral systemic or biologic medications—national quality strategy domain: person and caregiver-centered experience and outcomes. Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Quality-Payment-Program/Resource-Library/2018-Resources.html. Accessed February 27, 2018.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Syst Rev. 2017;12:CD011535.
- Lynch M, Higgins E, McCormick PA, et al. The use of transient elastography and FibroTest for monitoring hepatotoxicity in patients receiving methotrexate for psoriasis. JAMA Dermatol. 2014;150:856-862.
- Behrens F, Canete J, Olivieri I, et al. Tumor necrosis factor inhibitor monotherapy versus combination with MTX in the treatment of PsA: a systemic review of the literature. Rheumatology. 2015;54:915-926.
- Karadağ Ö, Kaşifoğlu T, Özer B, et al. Viral hepatitis screening guideline before biological drug use in rheumatic patients. Eur J Rheumatol. 2016;3:25-28.
- Ahn CS, Dothard EH, Garner ML, et al. To test or not to test? an updated evidence-based assessment of the value of screening and monitoring tests when using systemic biologic agents to treat psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2015;73:420-428.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Humira [package insert]. North Chicago, IL: Abbott Laboratories; 2011.
- Stelara [package insert]. Bloomington, IN: Janssen Biotech, Inc; 2016.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Cimzia [package insert]. UCB, Inc: Smyrna, GA; 2016.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTty (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence [published online September 27, 2012]. J Invest Dermatol. 2013;133:377-385.
- Pariser DM, Bagel J, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007;143:239-242.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1-10.
- Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris—update 2015—short version—EDF in cooperation with EADV and IPC [published online October 9, 2015]. J Eur Acad Dermatol Venereol. 2015;29:2277-2294.
- Smith CH, Jabbar-Lopez ZK, Yiu ZZ, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628-636.
- Armstrong AW, Siegel MP, Bagel J, et al. From the medical board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Quality ID #410: psoriasis: clinical response to oral systemic or biologic medications—national quality strategy domain: person and caregiver-centered experience and outcomes. Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Quality-Payment-Program/Resource-Library/2018-Resources.html. Accessed February 27, 2018.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Syst Rev. 2017;12:CD011535.
- Lynch M, Higgins E, McCormick PA, et al. The use of transient elastography and FibroTest for monitoring hepatotoxicity in patients receiving methotrexate for psoriasis. JAMA Dermatol. 2014;150:856-862.
- Behrens F, Canete J, Olivieri I, et al. Tumor necrosis factor inhibitor monotherapy versus combination with MTX in the treatment of PsA: a systemic review of the literature. Rheumatology. 2015;54:915-926.
- Karadağ Ö, Kaşifoğlu T, Özer B, et al. Viral hepatitis screening guideline before biological drug use in rheumatic patients. Eur J Rheumatol. 2016;3:25-28.
- Ahn CS, Dothard EH, Garner ML, et al. To test or not to test? an updated evidence-based assessment of the value of screening and monitoring tests when using systemic biologic agents to treat psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2015;73:420-428.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Humira [package insert]. North Chicago, IL: Abbott Laboratories; 2011.
- Stelara [package insert]. Bloomington, IN: Janssen Biotech, Inc; 2016.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Cimzia [package insert]. UCB, Inc: Smyrna, GA; 2016.
Practice Points
- Guidelines and consensus statements for psoriasis treatment are generally but not always consistent.
- As guidelines evolve, individual patient preferences, disease severity, and comorbid conditions remain important considerations when selecting treatment agents for psoriasis.
- More frequent updates to psoriasis treatment guidelines are becoming increasingly important given the rapid changes in the field.