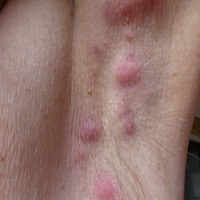
User login
Quantifying Itch: Measurement on the Way to Management
Itch is one of the most protean manifestations of skin disease and can take a substantial physical and emotional toll on patients. For physicians, it is a frequent—if often dreaded—patient concern with a rising incidence. Lack of specific itch therapies as well as associations with multiple dermatologic conditions, including xerosis, psoriasis, atopic dermatitis, cutaneous lymphoma, contact dermatitis, and internal malignancies, make management of these itchy patients challenging and deserving of our attention. Studies evaluating patients with chronic pruritus identified a considerable impact on health-related quality of life, including development of depression, inability to perform activities of daily living, and sleep difficulties. 1
How to Classify Itching
Itch, or pruritus, originally was defined as an unpleasant sensation that provokes the desire to scratch,2 but this definition likely limits our ability to assess itch.
Pain is another complex subjective symptom but is one that has been better studied. A previous intensity theory postulated that itch is a form of pain: low-intensity noxious stimuli are perceived as itch, while high-intensity stimuli are perceived as pain. Over time, our understanding of itch evolved, and it became clear that a specific neuronal pathway for itch also exists.3 However, the pathophysiology of itch and pain remain intertwined. Scratching may elicit pain, providing a change in sensation that replaces the itch, whereas opioid analgesics suppress pain but may worsen the itch.
We are gaining a better understanding of the biology and classification of itch, which will hopefully enable the development of new measures to accurately assess itch. Four main categories of itch currently exist: neurogenic, psychogenic, neuropathic, and pruritoceptive.4 Patients may have one or multiple types of itch, which can be differentiated clinically and biochemically. Neurogenic (also known as systemic) itch is transmitted via the central nervous system with possible involvement of itch-specific neurons in the spinal cord and encompasses itch associated with pruritus from other organ systems. As the term implies, psychogenic itch is associated with psychiatric disorders. Neuropathic itch is generated from the inappropriate firing of peripheral or central sensory neurons in the absence of pruritogenic stimuli, which can be seen in notalgia paresthetica, brachioradial pruritus, and postherpetic neuralgia. Pruritoceptive itch most commonly is encountered in dermatology and is associated with skin inflammation or other dermatoses.4
How to Assess Itch Quantitatively
There currently are 2 major questions about quantitative assessments of itch. First, how do we measure itch in studies that are designed to relieve a different skin disease that is associated with itch? Most clinical trials investigating therapeutic options for atopic dermatitis and psoriasis now include itch assessment and improvement as a secondary outcome. Second, how do we measure itch in studies that are designed with relief of itch as the primary end point? Both of these scenarios require a fundamental set of decisions. Itch clearly is a subjective experience, but it also is one that can be local, regional, generalized, or transitory. Just as with pain, an individual can be distracted from their itch to some extent and consequently experience it more acutely when there are fewer stimuli in their environment. Classically, patients will report that itching is worse at night, preventing them from sleeping. Sleep disruption previously has been demonstrated.5 Of course, the environment also can exacerbate itch, as dry air and in some cases humidity can flare the sensation.
Fundamentally, therefore, the questions that are asked to assess itch are incredibly relevant, and there is a matrix of possible avenues of inquiry. Should you measure the peak itch in one area or the peak itch overall? Is the duration, the frequency, or the persistence of the itching most relevant? What is the correct time frame in which to do an assessment: the last 24 hours, the last 48 hours, or the last week? Because these parameters have been so challenging, most investigators have used a visual analog scale, similar to what is used to assess pain, at a 24-hour interval to decrease recall bias. The most commonly employed tool is the itch numeric rating scale (NRS), which asks patients to rate their symptoms on a scale of 0 (no itch) to 10 (worst imaginable itch). Although the psychometric properties of the itch NRS have been validated, debate still exists as to whether the itch NRS is best administered at a specific time of day or if it should be updated to evaluate peak pruritus scores explicitly. Regardless, implementing these scales often is time consuming and burdensome in the clinical trial setting, as participants are asked to complete daily diaries at the same time each day using either paper forms or electronic tablets.
Once scores are collected, we then need to quantitate a meaningful difference in itch. For pain, there has been some acceptance of a 30% difference, or a 2-point reduction, as being clinically meaningful; however, there was substantial debate at the time of the approval of ixekizumab as to whether that was a similarly appropriate threshold for itch. Using data from ixekizumab phase 2 and phase 3 trials, a 4-point reduction in itch NRS was found to be optimal for evaluating clinically significant changes in moderate to severe psoriasis.6 A more recent study of the validity of the itch NRS in prurigo nodularis suggested a 1-point change was correlated with minimal clinical improvement.7 Thus, the interesting question of how assessment of itch varies across clinical trials and disease states needs to be raised. Psoriasis classically has been thought of as not particularly itchy, and atopic dermatitis and prurigo nodularis have been regarded as extraordinarily itchy, yet one study comparing baseline itch scores in psoriasis and atopic dermatitis suggested that the experience actually is somewhat similar.8
Final Thoughts
The subjective nature of itch makes NRSs our best option at this time, but the best disease severity assessment tools are objective, sensitive, and generalizable. Unfortunately, we do not have such tools available to us yet, but technology—smart devices to monitor nocturnal scratching and machine learning algorithms that use electromagnetic impact to capture motion associated with itching and scratching9—may offer new objective measures for itch that can be used to further validate the current itch NRS. Even if these technology-based approaches become the standard of measurement, they will certainly help us understand what we are measuring. And even better, the focus on how to develop meaningful end points around the improvement of itch will likely lead us to measure it more and drive the development of therapeutics that address the effect and consequences of this pernicious problem.
- Kini SP, DeLong LK, Veledar E, et al. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol. 2011;147:1153-1156. doi:10.1001/archdermatol.2011.178
- Savin JA. How should we define itching? J Am Acad Dermatol. 1998;39(2 pt 1):268-269. doi:10.1016/s0190-9622(98)70087-8
- Ikoma A, Rukwied R, Ständer S, et al. Neurophysiology of pruritus: interaction of itch and pain. Arch Dermatol. 2003;139:1475-1478. doi:10.1001/archderm.139.11.1475
- Garibyan L, Rheingold CG, Lerner EA. Understanding the pathophysiology of itch. Dermatol Ther. 2013;26:84-91. doi:10.1111/dth.12025
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161. doi:10.1016/j.jaad.2016.07.034
- Kimball AB, Naegeli AN, Edson-Heredia E, et al. Psychometric properties of the Itch Numeric Rating Scale in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:157-162. doi:10.1111/bjd.14464
- Kimel M, Zeidler C, Kwon P, et al. validation of psychometric properties of the itch numeric rating scale for pruritus associated with prurigo nodularis: a secondary analysis of a randomized clinical trial. JAMA Dermatol. 2020;156:1354-1358. doi:10.1001/jamadermatol.2020.3071
- Shahwan KT, Kimball AB. Itch intensity in moderate-to-severe plaque psoriasis versus atopic dermatitis: a meta-analysis. J Am Acad Dermatol. 2017;76:1198.el-1200.e1. doi:10.1016/j.jaad.2017.02.002
- Smith MP, Ly K, Thibodeaux Q, et al. Emerging methods to objectively assess pruritus in atopic dermatitis. Dermatol Ther (Heidelb). 2019;9:407-420. doi:10.1007/s13555-019-0312-3
Itch is one of the most protean manifestations of skin disease and can take a substantial physical and emotional toll on patients. For physicians, it is a frequent—if often dreaded—patient concern with a rising incidence. Lack of specific itch therapies as well as associations with multiple dermatologic conditions, including xerosis, psoriasis, atopic dermatitis, cutaneous lymphoma, contact dermatitis, and internal malignancies, make management of these itchy patients challenging and deserving of our attention. Studies evaluating patients with chronic pruritus identified a considerable impact on health-related quality of life, including development of depression, inability to perform activities of daily living, and sleep difficulties. 1
How to Classify Itching
Itch, or pruritus, originally was defined as an unpleasant sensation that provokes the desire to scratch,2 but this definition likely limits our ability to assess itch.
Pain is another complex subjective symptom but is one that has been better studied. A previous intensity theory postulated that itch is a form of pain: low-intensity noxious stimuli are perceived as itch, while high-intensity stimuli are perceived as pain. Over time, our understanding of itch evolved, and it became clear that a specific neuronal pathway for itch also exists.3 However, the pathophysiology of itch and pain remain intertwined. Scratching may elicit pain, providing a change in sensation that replaces the itch, whereas opioid analgesics suppress pain but may worsen the itch.
We are gaining a better understanding of the biology and classification of itch, which will hopefully enable the development of new measures to accurately assess itch. Four main categories of itch currently exist: neurogenic, psychogenic, neuropathic, and pruritoceptive.4 Patients may have one or multiple types of itch, which can be differentiated clinically and biochemically. Neurogenic (also known as systemic) itch is transmitted via the central nervous system with possible involvement of itch-specific neurons in the spinal cord and encompasses itch associated with pruritus from other organ systems. As the term implies, psychogenic itch is associated with psychiatric disorders. Neuropathic itch is generated from the inappropriate firing of peripheral or central sensory neurons in the absence of pruritogenic stimuli, which can be seen in notalgia paresthetica, brachioradial pruritus, and postherpetic neuralgia. Pruritoceptive itch most commonly is encountered in dermatology and is associated with skin inflammation or other dermatoses.4
How to Assess Itch Quantitatively
There currently are 2 major questions about quantitative assessments of itch. First, how do we measure itch in studies that are designed to relieve a different skin disease that is associated with itch? Most clinical trials investigating therapeutic options for atopic dermatitis and psoriasis now include itch assessment and improvement as a secondary outcome. Second, how do we measure itch in studies that are designed with relief of itch as the primary end point? Both of these scenarios require a fundamental set of decisions. Itch clearly is a subjective experience, but it also is one that can be local, regional, generalized, or transitory. Just as with pain, an individual can be distracted from their itch to some extent and consequently experience it more acutely when there are fewer stimuli in their environment. Classically, patients will report that itching is worse at night, preventing them from sleeping. Sleep disruption previously has been demonstrated.5 Of course, the environment also can exacerbate itch, as dry air and in some cases humidity can flare the sensation.
Fundamentally, therefore, the questions that are asked to assess itch are incredibly relevant, and there is a matrix of possible avenues of inquiry. Should you measure the peak itch in one area or the peak itch overall? Is the duration, the frequency, or the persistence of the itching most relevant? What is the correct time frame in which to do an assessment: the last 24 hours, the last 48 hours, or the last week? Because these parameters have been so challenging, most investigators have used a visual analog scale, similar to what is used to assess pain, at a 24-hour interval to decrease recall bias. The most commonly employed tool is the itch numeric rating scale (NRS), which asks patients to rate their symptoms on a scale of 0 (no itch) to 10 (worst imaginable itch). Although the psychometric properties of the itch NRS have been validated, debate still exists as to whether the itch NRS is best administered at a specific time of day or if it should be updated to evaluate peak pruritus scores explicitly. Regardless, implementing these scales often is time consuming and burdensome in the clinical trial setting, as participants are asked to complete daily diaries at the same time each day using either paper forms or electronic tablets.
Once scores are collected, we then need to quantitate a meaningful difference in itch. For pain, there has been some acceptance of a 30% difference, or a 2-point reduction, as being clinically meaningful; however, there was substantial debate at the time of the approval of ixekizumab as to whether that was a similarly appropriate threshold for itch. Using data from ixekizumab phase 2 and phase 3 trials, a 4-point reduction in itch NRS was found to be optimal for evaluating clinically significant changes in moderate to severe psoriasis.6 A more recent study of the validity of the itch NRS in prurigo nodularis suggested a 1-point change was correlated with minimal clinical improvement.7 Thus, the interesting question of how assessment of itch varies across clinical trials and disease states needs to be raised. Psoriasis classically has been thought of as not particularly itchy, and atopic dermatitis and prurigo nodularis have been regarded as extraordinarily itchy, yet one study comparing baseline itch scores in psoriasis and atopic dermatitis suggested that the experience actually is somewhat similar.8
Final Thoughts
The subjective nature of itch makes NRSs our best option at this time, but the best disease severity assessment tools are objective, sensitive, and generalizable. Unfortunately, we do not have such tools available to us yet, but technology—smart devices to monitor nocturnal scratching and machine learning algorithms that use electromagnetic impact to capture motion associated with itching and scratching9—may offer new objective measures for itch that can be used to further validate the current itch NRS. Even if these technology-based approaches become the standard of measurement, they will certainly help us understand what we are measuring. And even better, the focus on how to develop meaningful end points around the improvement of itch will likely lead us to measure it more and drive the development of therapeutics that address the effect and consequences of this pernicious problem.
Itch is one of the most protean manifestations of skin disease and can take a substantial physical and emotional toll on patients. For physicians, it is a frequent—if often dreaded—patient concern with a rising incidence. Lack of specific itch therapies as well as associations with multiple dermatologic conditions, including xerosis, psoriasis, atopic dermatitis, cutaneous lymphoma, contact dermatitis, and internal malignancies, make management of these itchy patients challenging and deserving of our attention. Studies evaluating patients with chronic pruritus identified a considerable impact on health-related quality of life, including development of depression, inability to perform activities of daily living, and sleep difficulties. 1
How to Classify Itching
Itch, or pruritus, originally was defined as an unpleasant sensation that provokes the desire to scratch,2 but this definition likely limits our ability to assess itch.
Pain is another complex subjective symptom but is one that has been better studied. A previous intensity theory postulated that itch is a form of pain: low-intensity noxious stimuli are perceived as itch, while high-intensity stimuli are perceived as pain. Over time, our understanding of itch evolved, and it became clear that a specific neuronal pathway for itch also exists.3 However, the pathophysiology of itch and pain remain intertwined. Scratching may elicit pain, providing a change in sensation that replaces the itch, whereas opioid analgesics suppress pain but may worsen the itch.
We are gaining a better understanding of the biology and classification of itch, which will hopefully enable the development of new measures to accurately assess itch. Four main categories of itch currently exist: neurogenic, psychogenic, neuropathic, and pruritoceptive.4 Patients may have one or multiple types of itch, which can be differentiated clinically and biochemically. Neurogenic (also known as systemic) itch is transmitted via the central nervous system with possible involvement of itch-specific neurons in the spinal cord and encompasses itch associated with pruritus from other organ systems. As the term implies, psychogenic itch is associated with psychiatric disorders. Neuropathic itch is generated from the inappropriate firing of peripheral or central sensory neurons in the absence of pruritogenic stimuli, which can be seen in notalgia paresthetica, brachioradial pruritus, and postherpetic neuralgia. Pruritoceptive itch most commonly is encountered in dermatology and is associated with skin inflammation or other dermatoses.4
How to Assess Itch Quantitatively
There currently are 2 major questions about quantitative assessments of itch. First, how do we measure itch in studies that are designed to relieve a different skin disease that is associated with itch? Most clinical trials investigating therapeutic options for atopic dermatitis and psoriasis now include itch assessment and improvement as a secondary outcome. Second, how do we measure itch in studies that are designed with relief of itch as the primary end point? Both of these scenarios require a fundamental set of decisions. Itch clearly is a subjective experience, but it also is one that can be local, regional, generalized, or transitory. Just as with pain, an individual can be distracted from their itch to some extent and consequently experience it more acutely when there are fewer stimuli in their environment. Classically, patients will report that itching is worse at night, preventing them from sleeping. Sleep disruption previously has been demonstrated.5 Of course, the environment also can exacerbate itch, as dry air and in some cases humidity can flare the sensation.
Fundamentally, therefore, the questions that are asked to assess itch are incredibly relevant, and there is a matrix of possible avenues of inquiry. Should you measure the peak itch in one area or the peak itch overall? Is the duration, the frequency, or the persistence of the itching most relevant? What is the correct time frame in which to do an assessment: the last 24 hours, the last 48 hours, or the last week? Because these parameters have been so challenging, most investigators have used a visual analog scale, similar to what is used to assess pain, at a 24-hour interval to decrease recall bias. The most commonly employed tool is the itch numeric rating scale (NRS), which asks patients to rate their symptoms on a scale of 0 (no itch) to 10 (worst imaginable itch). Although the psychometric properties of the itch NRS have been validated, debate still exists as to whether the itch NRS is best administered at a specific time of day or if it should be updated to evaluate peak pruritus scores explicitly. Regardless, implementing these scales often is time consuming and burdensome in the clinical trial setting, as participants are asked to complete daily diaries at the same time each day using either paper forms or electronic tablets.
Once scores are collected, we then need to quantitate a meaningful difference in itch. For pain, there has been some acceptance of a 30% difference, or a 2-point reduction, as being clinically meaningful; however, there was substantial debate at the time of the approval of ixekizumab as to whether that was a similarly appropriate threshold for itch. Using data from ixekizumab phase 2 and phase 3 trials, a 4-point reduction in itch NRS was found to be optimal for evaluating clinically significant changes in moderate to severe psoriasis.6 A more recent study of the validity of the itch NRS in prurigo nodularis suggested a 1-point change was correlated with minimal clinical improvement.7 Thus, the interesting question of how assessment of itch varies across clinical trials and disease states needs to be raised. Psoriasis classically has been thought of as not particularly itchy, and atopic dermatitis and prurigo nodularis have been regarded as extraordinarily itchy, yet one study comparing baseline itch scores in psoriasis and atopic dermatitis suggested that the experience actually is somewhat similar.8
Final Thoughts
The subjective nature of itch makes NRSs our best option at this time, but the best disease severity assessment tools are objective, sensitive, and generalizable. Unfortunately, we do not have such tools available to us yet, but technology—smart devices to monitor nocturnal scratching and machine learning algorithms that use electromagnetic impact to capture motion associated with itching and scratching9—may offer new objective measures for itch that can be used to further validate the current itch NRS. Even if these technology-based approaches become the standard of measurement, they will certainly help us understand what we are measuring. And even better, the focus on how to develop meaningful end points around the improvement of itch will likely lead us to measure it more and drive the development of therapeutics that address the effect and consequences of this pernicious problem.
- Kini SP, DeLong LK, Veledar E, et al. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol. 2011;147:1153-1156. doi:10.1001/archdermatol.2011.178
- Savin JA. How should we define itching? J Am Acad Dermatol. 1998;39(2 pt 1):268-269. doi:10.1016/s0190-9622(98)70087-8
- Ikoma A, Rukwied R, Ständer S, et al. Neurophysiology of pruritus: interaction of itch and pain. Arch Dermatol. 2003;139:1475-1478. doi:10.1001/archderm.139.11.1475
- Garibyan L, Rheingold CG, Lerner EA. Understanding the pathophysiology of itch. Dermatol Ther. 2013;26:84-91. doi:10.1111/dth.12025
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161. doi:10.1016/j.jaad.2016.07.034
- Kimball AB, Naegeli AN, Edson-Heredia E, et al. Psychometric properties of the Itch Numeric Rating Scale in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:157-162. doi:10.1111/bjd.14464
- Kimel M, Zeidler C, Kwon P, et al. validation of psychometric properties of the itch numeric rating scale for pruritus associated with prurigo nodularis: a secondary analysis of a randomized clinical trial. JAMA Dermatol. 2020;156:1354-1358. doi:10.1001/jamadermatol.2020.3071
- Shahwan KT, Kimball AB. Itch intensity in moderate-to-severe plaque psoriasis versus atopic dermatitis: a meta-analysis. J Am Acad Dermatol. 2017;76:1198.el-1200.e1. doi:10.1016/j.jaad.2017.02.002
- Smith MP, Ly K, Thibodeaux Q, et al. Emerging methods to objectively assess pruritus in atopic dermatitis. Dermatol Ther (Heidelb). 2019;9:407-420. doi:10.1007/s13555-019-0312-3
- Kini SP, DeLong LK, Veledar E, et al. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol. 2011;147:1153-1156. doi:10.1001/archdermatol.2011.178
- Savin JA. How should we define itching? J Am Acad Dermatol. 1998;39(2 pt 1):268-269. doi:10.1016/s0190-9622(98)70087-8
- Ikoma A, Rukwied R, Ständer S, et al. Neurophysiology of pruritus: interaction of itch and pain. Arch Dermatol. 2003;139:1475-1478. doi:10.1001/archderm.139.11.1475
- Garibyan L, Rheingold CG, Lerner EA. Understanding the pathophysiology of itch. Dermatol Ther. 2013;26:84-91. doi:10.1111/dth.12025
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161. doi:10.1016/j.jaad.2016.07.034
- Kimball AB, Naegeli AN, Edson-Heredia E, et al. Psychometric properties of the Itch Numeric Rating Scale in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:157-162. doi:10.1111/bjd.14464
- Kimel M, Zeidler C, Kwon P, et al. validation of psychometric properties of the itch numeric rating scale for pruritus associated with prurigo nodularis: a secondary analysis of a randomized clinical trial. JAMA Dermatol. 2020;156:1354-1358. doi:10.1001/jamadermatol.2020.3071
- Shahwan KT, Kimball AB. Itch intensity in moderate-to-severe plaque psoriasis versus atopic dermatitis: a meta-analysis. J Am Acad Dermatol. 2017;76:1198.el-1200.e1. doi:10.1016/j.jaad.2017.02.002
- Smith MP, Ly K, Thibodeaux Q, et al. Emerging methods to objectively assess pruritus in atopic dermatitis. Dermatol Ther (Heidelb). 2019;9:407-420. doi:10.1007/s13555-019-0312-3
Treatment of Psoriasis in Pregnancy
Historically, there have been limited data available on the management of psoriasis in pregnancy. The most comprehensive discussion of treatment guidelines is from 2012.1 In the interim, many biologics have been approved for treating psoriasis, with slow accumulation of pregnancy safety data. The 2019 American Academy of Dermatology–National Psoriasis Foundation guidelines on biologics for psoriasis contain updated information but also highlight the paucity of pregnancy safety data.2 This gap is in part a consequence of the exclusion and disenrollment of pregnant women from clinical trials.3 Additionally, lack of detection through registries contributes; pregnancy capture in registries is low compared to the expected number of pregnancies estimated from US Census data.4 Despite these shortcomings, psoriasis patients who are already pregnant or are considering becoming pregnant frequently are encountered in practice and may need treatment. This article reviews the evidence on commonly used treatments for psoriasis in pregnancy.
Background
For many patients, psoriasis improves during pregnancy5,6 and becomes worse postpartum. In a prospective study, most patients reported improvement in pregnancy corresponding to a significant decrease in
In addition to the maternal disease state, the issue of pregnancy outcomes is paramount. In the inflammatory bowel disease and rheumatology literature, it is established that uncontrolled disease is associated with poorer pregnancy outcomes.8-10 Guidelines vary among societies on the use of biologics in pregnancy generally (eTable 11,2,9,11-24), but some societies recommend systemic agents to achieve disease control during pregnancy.9,25
Assessing the potential interplay between disease severity and outcomes in pregnant women with psoriasis is further complicated by the slowly growing body of literature demonstrating that women with psoriasis have more comorbidities26 and worse pregnancy outcomes.27,28 Pregnant psoriasis patients are more likely to smoke, have depression, and be overweight or obese prior to pregnancy and are less likely to take prenatal vitamins.26 They also have an increased risk for cesarean birth, gestational diabetes, gestational hypertension, and preeclampsia.28 In contrast to these prior studies, a systematic review revealed no risk for adverse outcomes in pregnant women with psoriasis.29
Assessment of Treatments for Psoriasis in Pregnancy
In light of these issues, treatment of psoriasis during pregnancy should be assessed from several vantage points. Of note, the US Food and Drug Administration changed its classification scheme in 2015 to a more narrative format called the Pregnancy and Lactation Labeling Rule.30 Prior classifications, however, provide a reasonable starting point for categorizing the safety of drugs (Table31). Importantly, time of exposure to systemic agents also matters; first-trimester exposure is more likely to affect embryogenesis, whereas second- and third-trimester exposures are more prone to affect other aspects of fetal growth. eTable 2 provides data on the use of oral and topical medications to treat psoriasis in pregnancy.1,8,22,32-45

Topical Agents
Topical steroids are largely understood to be reasonable treatment options, though consideration of potency, formulation, area of application, and use of occlusion is important.1,46 Risk for orofacial cleft has been noted with first-trimester topical steroid exposure, though a 2015 Cochrane review update determined that the relative risk of this association was not significantly elevated.32
The impact of topical calcipotriene and salicylic acid has not been studied in human pregnancies,1 but systemic absorption can occur for both. There is potential for vitamin D toxicity with calcipotriene46; consequently, use during pregnancy is not recommended.1,46 Some authors recommend against topical salicylic acid in pregnancy; others report that limited exposure is permissible.47 In fact, as salicylic acid commonly is found in over-the-counter acne products, many women of childbearing potential likely have quotidian exposure.
Preterm delivery and low birthweight have been reported with oral tacrolimus; however, risk with topical tacrolimus is thought to be low1 because the molecular size likely prohibits notable absorption.47 Evidence for the use of anthralin and coal tar also is scarce. First-trimester coal tar use should be avoided; subsequent use in pregnancy should be restricted given concern for adverse outcomes.1
Phototherapy
Broadband or narrowband UVB therapy is recommended as second-line therapy in pregnancy. No cases of fetal risk or premature delivery associated with UVB therapy were found in our search.1 Phototherapy can exacerbate melasma47 and decrease folate levels48; as such, some authors recommend folate supplementation in females of childbearing age who are being treated with phototherapy.49 Psoralen, used in psoralen plus UVA therapy, is mutagenic and therefore contraindicated in pregnancy.1
Oral Medications
Both methotrexate, which is a teratogen, abortifacient, and mutagen,1 and systemic retinoids, which are teratogens, are contraindicated in pregnancy.1,47 Acitretin labeling recommends avoiding pregnancy for 3 years posttreatment50 because alcohol intake prolongs the medication’s half-life.22
Apremilast use is not documented in pregnant psoriasis patients51; an ongoing registry of the Organization of Tetralogy Information Specialists has not reported publicly to date.52 Animal studies of apremilast have documented dose-related decreased birthweight and fetal loss.22
Safety data for systemic steroids, used infrequently in psoriasis, are not well established. First-trimester prednisone exposure has been associated with prematurity, low birthweight, and congenital abnormalities.38 A separate evaluation of 1047 children exposed to betamethasone in utero failed to demonstrate significant change in birthweight or head circumference. However, repeat antenatal corticosteroid exposure was associated with attention problems at 2 years of age.39
Data regarding cyclosporine use, derived primarily from organ transplant recipients, suggest elevated risk for prematurity and low birthweight.53,54 A meta-analysis demonstrated that organ transplant recipients taking cyclosporine had a nonsignificantly elevated odds ratio for congenital malformations, prematurity, and low birthweight.42 Cyclosporine use for psoriasis in pregnancy is not well described; in a study, rates of prematurity and low birthweight were both 21%.43 Limited data are available for Janus kinase inhibitors, none of which are approved for psoriasis, though clinical trials in psoriasis and psoriatic arthritis are underway (ClinicalTrials.gov identifiers NCT04246372, NCT03104374, NCT03104400).
Biologics and Small-Molecule Inhibitors
Limited data on biologics in pregnancy exist25 (eTable 3). Placental transport of IgG antibodies, including biologics, increases throughout pregnancy, especially in the third trimester.82 Infants of mothers treated with a biologic with potential for placental transfer are therefore considered by some authors to be immunosuppressed during the first months of life.2
Looking globally across biologics used for psoriasis, limited safety data are encouraging. In a review of PSOLAR (Psoriasis Longitudinal Assessment and Registry), 83 pregnancies with biologic exposure resulted in 59 live births (71%); 18 spontaneous abortions (22%); 6 induced abortions (7%); no congenital abnormalities; and 7 reports of neonatal problems, including respiratory issues, ABO blood group mismatch, hospitalization, and opioid withdrawal.83
Use of tumor necrosis factor (TNF) inhibitors in pregnancy has the most data25 and is considered a reasonable treatment option. Historically, there was concern about the risk for VACTERL syndrome (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, limb abnormalities) with exposure to a TNF inhibitor,25,84-86 but further reports have alleviated these concerns. Active transplacental transport occurs for adalimumab, infliximab, and golimumab,87 but given structural differences, transport of certolizumab and etanercept is substantially less.88,89 In the CRIB study of placental transfer of certolizumab from mother to infant (N=14), pharmacokinetic data demonstrated no quantifiable certolizumab levels in 13 infants and minimal levels in 1 infant at birth.88 There are fewer data available on the use of other biologics in pregnancy, but for those in which active placental transport is relevant, similar concerns (ie, immunosuppression) might arise (eTable 3).
Concern over biologics largely involves risk for newborn immunosuppression. A case report detailed a Crohn disease patient treated with infliximab who gave birth to an infant who died of disseminated bacille Calmette-Guérin infection at 4.5 months after receiving the vaccine at 3 months.90 This case underscores the importance of delaying live vaccination in infants born to mothers who were treated with a biologic during pregnancy. Authors have provided various data on how long to avoid vaccination; some state as long as 1 year.91
In pregnant females with inflammatory bowel disease treated with a biologic, no correlation was observed among maternal, placental, and infant serum biologic levels and neonatal infection. However, an association between preterm birth and the level of the biologic in maternal and placental (but not infant) serum and preterm birth was observed.92
In another report from the same registry, combination therapy with a TNF inhibitor and another immunomodulator led to an increased risk for infection in infants at 12 months of age, compared to infants exposed to monotherapy89 or exposed to neither agent.93 A strategy to circumvent this potential problem is to avoid treatment with actively transported molecules in the third trimester.
Conclusion
Limited data exist to guide providers who are treating pregnant women with psoriasis. Our understanding of treatment of psoriasis in pregnancy is limited as a consequence of regulations surrounding clinical trials and inadequate detection of pregnancies in registries. Further efforts are necessary to better understand the relationship between psoriasis and pregnancy and how to manage pregnant women with psoriasis.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459-477.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Flood KS, Porter ML, Kimball AB. Use of biologics in pregnancy: limitations stemming from clinical trials and registry experience. J Eur Acad Dermatol Venereol. 2019;33:E276-E277.
- Horn EJ, Chambers CD, Menter A, et al. Pregnancy outcomes in psoriasis: why do we know so little? J Am Acad Dermatol. 2009;61:E5-E8.
- Raychaudhuri SP, Navare T, Gross J, et al. Clinical course of psoriasis during pregnancy. Int J Dermatol. 2003;42:518-520.
- Boyd AS, Morris LF, Phillips CM, et al. Psoriasis and pregnancy: hormone and immune system interaction. Int J Dermatol. 1996;35:169-172.
- Murase JE, Chan KK, Garite TJ, et al. Hormonal effect on psoriasis in pregnancy and post partum. Arch Dermatol. 2005;14:601-606.
- Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795-810.
- Nguyen GC, Seow CH, Maxwell C, et al. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology. 2016;150:734-757.
- Wise J. Rheumatic diseases should be actively treated in pregnancy, new guidelines say. BMJ. 2016;532:i312.
- Puig L, Carrascosa JM, Carretero G, et al. Spanish evidence-based guidelines on the treatment of psoriasis with biologic agents, 2013. part 1: on efficacy and choice of treatment. Actas Dermosifiliogr. 2013;104:694-709.
- Girolomoni G, Altomore G, Ayala F, et al. Differential management of mild-to-severe psoriasis with biologic drugs: an Italian Delphi consensus expert panel. J Dermatolog Treat. 2015;26:128-133.
- Yeung J, Gooderham MJ, Grewal P, et al. Management of plaque psoriasis with biologic therapies in women of child-bearing potential consensus paper. J Cutan Med Surg. 2020;24:3S-14S.
- Louthrenoo W, Kasitanon N, Kathamort W, et al. 2016 updated Thai Rheumatism Association Recommendations for the use of biologic and targeted synthetic disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis. Int J Rheum Dis. 2017;20:1166-1184.
- Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding—part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford). 2016;55:1693-1697.
- van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis. 2015;9:107-124.
- Orlando A, Armuzz A, Papi C, et al. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) Clinical Practice Guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis. 2011;43:1-20.
- Puchner A, Grochenig HP, Sautner J, et al. Immunosuppressives and biologics during pregnancy and lactation. Wien Klin Wochenschr. 2019;131:29-44.
- ACOG Committee opinion no. 776: immune modulating therapies in pregnancy and lactation. Obstet Gynecol. 2019;133:E287-E297.
- Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:S1-S106.
- Goëb V, Ardizzone M, Arnard L, et al. Recommendations for using TNF-α antagonists and French Clinical Practice Guidelines endorsed by the French National Authority for Health. Joint Bone Spine. 2013;80:574-581.
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. the Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100.
- Mahadevan U, Robinson C, Bernasko N, et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-1524.
- Mahadevan U, Cucchiara S, Hyam JS, et al. The London position statement of the World Congress of Gastroenterology on biological therapy for IBD with the European Crohn’s and Colitis Organisation: pregnancy and pediatrics. Am J Gastroenterol. 2011;106:214-223.
- Porter ML, Lockwood SJ, Kimball AB. Update on biologic safety for patients with psoriasis during pregnancy. Int J Womens Dermatol. 2017;3:21-25.
- Bandoli G, Johnson DL, Jones KL, et al. Potentially modifiable risk factors for adverse pregnancy outcomes in women with psoriasis. Br J Dermatol. 2010;163:334-339.
- Lima XT, Janakiraman V, Hughes MD, et al. The impact of psoriasis on pregnancy outcomes. J Invest Dermatol. 2012;132:85-91.
- Bröms G, Haerskjold A, Granath F, et al. Effect of maternal psoriasis on pregnancy and birth outcomes: a population-based cohort study from Denmark and Sweden. Acta Derm Venereol. 2018;98:728-734.
- Bobotsis R, Gulliver WP, Monaghan K, et al. Psoriasis and adverse pregnancy outcomes: a systematic review of observational studies. Br J Dermatol. 2016;175:464-472.
- Blattner CM, Danesh M, Safaee M, et al. Understanding the new FDA pregnancy and lactation labeling rules. Int J Womens Dermatol. 2016;2:5-7.
- Pernia S, DeMaagd G. The new pregnancy and lactation labeling rule. P T. 2016;4:713-715.
- Chi C-C, Wang S-H, Wojnarowska F, et al. Safety of topical corticosteroids in pregnancy. Cochrane Database Syst Rev. 2015:CD007346.
- Chi CC, Wang SH, Kirtschig G. Safety of topical corticosteroids in pregnancy. JAMA Dermatol. 2016;152:934-935.
- Dovonex (calcipotriene) Cream, 0.005% [package insert]. Dublin, Ireland: Leo Laboratories, Ltd; March 2015.
- Franssen ME, van der Wilt GJ, de Jong PC, et al. A retrospective study of the teratogenicity of dermatological coal tar products. Acta Derm Venereol. 1999;79:390-391.
- Garbis H, Elefant E, Bertolotti E, et al. Pregnancy outcome after periconceptional and first-trimester exposure to methoxsalen photochemotherapy. Arch Dermatol. 1995;131:492-493.
- Horizon Pharma USA. RAYOS (prednisone). https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf.
- Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385-392.
- Crowther CA, Doyle LW, Haslam RR, et al. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1179-1189.
- Palmsten K, Rolland M, Herbert MF, et al. Patterns of prednisone use during pregnancy in women with rheumatoid arthritis: daily and cumulative dose. Pharmacoepidemiol Drug Saf. 2018;27:430-438.
- Groth K, Brännström M, Mölne J, et al. Cyclosporine A exposure during pregnancy in mice: effects on reproductive performance in mothers and offspring. Hum Reprod. 2010;25:697-704.
- Bar Oz B, Hackman R, Einarson T, et al. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis. Transplantation. 2001;71:1051-1055.
- Paziana K, Del Monaco M, Cardonick E, et al. Ciclosporin use during pregnancy. Drug Saf. 2013;36:279-294.
- Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 pregnancy outcomes in transplant recipients treated with Sandimmun. Transplant Proc. 1997;29:2480.
- Otezla (apremilast) tablets, for oral use [package insert]. Summit, NJ: Celgene Corporation; June 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205437s006lbl.pdf. Accessed July 8, 2020.
- Kurizky PS, de Castro Ferreira C, Nogueira LSC, et al. Treatment of psoriasis and psoriatic arthritis during pregnancy and breastfeeding. An Bras Dermatol. 2015;90:367-375.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation. J Am Acad Dermatol. 2014;70:401.e1-401.e4.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Murase JE, Koo JY, Berger TG. Narrowband ultraviolet B phototherapy influences serum folate levels in patients with vitiligo. J Am Acad Dermatol. 2010;62:710-711.
- Soriatane (acitretin) capsules [package insert]. Morrisville, NC: Stiefel Laboratories, Inc; April 2011. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019821s018mg.pdf. Accessed July 8, 2020.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Help us better understand the effects of Otezla in pregnancy. MotherToBaby website. https://mothertobaby.org/ongoing-study/otezla/. Accessed July 8, 2020.
- Bangsgaard N, Rørbye C, Skov L. Treating psoriasis during pregnancy: safety and efficacy of treatments. Am J Clin Dermatol. 2015;16:389-398.
- Tyler K. Dermatologic therapy in pregnancy. Clin Obstet Gynecol. 2015;58:112-118.
- Luu M, Benzenine E, Doret M, et al. Continuous anti–TNF-α use throughout pregnancy: possible complications for the mother but not for the fetus. a retrospective cohort on the French National Health Insurance Database (EVASION). Am J Gastroenterol. 2018;113:1669-1677.
- Bröms G, Granath F, Ekbom A, et al. Low risk of birth defects for infants whose mothers are treated with anti-tumor necrosis factor agents during pregnancy. Clin Gastroenterol Hepatol. 2016;14:234-241.
- Mirdamadi K, Salinas T, Vali R, et al. Meta-analysis of pregnancy outcomes after exposure to TNF-α inhibitors during pregnancy for the treatment of arthritic diseases. J Popul Ther Clin Pharmacol. 2018;25:E53-E56.
- Shihab Z, Yeomans ND, De Cruz P. Anti-tumour necrosis factor α therapies and inflammatory bowel disease pregnancy outcomes: a meta-analysis. J Crohns Colitis. 2016;10:979-988.
- Bröms G, Kieler H, Ekbom A, et al. Anti-TNF treatment during pregnancy and birth outcomes: a population-based study from Denmark, Finland, and Sweden. Pharmacoepidemiol Drug Saf. 2020;29:316-327.
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, et al. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78-84.
- FDA determinations for pregnant and nursing women. National Psoriasis Foundation website. http://www.psoriasis.org/pregnancy/fda-determinations. Accessed July 8, 2020.
- Østensen M. Safety issues of biologics in pregnant patients with rheumatic diseases. Ann N Y Acad Sci. 2014;1317:32-38.
- Chambers CD, Johnson DL, Luo Y, et al. Pregnancy outcome in women treated with adalimumab for the treatment of rheumatoid arthritis: the OTIS Autoimmune Diseases in Pregnancy Project. Arthritis Rheum. 2012;64:2466.
- Clowse ME, Wolf DC, Forger F, et al. Pregnancy outcomes after exposure to certolizumab pegol: updated results from a pharmacovigilance safety database. Arthritis Rheumatol. 2018;70:1399-1407.
- Carman WJ, Accortt NA, Anthony MS, et al. Pregnancy and infant outcomes including major congenital malformations among women with chronic inflammatory arthritis or psoriasis, with and without etanercept use. Pharmacoepidemiol Drug Saf. 2017;26:1109-1118.
- Janssen. SIMPONI (golilumab). https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125289s0064lbl.pdf.
- Yurkon K, Guo CY, Harrison D, et al. Pregnancy outcomes in women with dermatologic conditions exposed to infliximab. J Am Acad Dermatol. 2014;70:AB179.
- Watson N, Wu K, Farr P, et al. Ustekinumab exposure during conception and pregnancy in patients with chronic plaque psoriasis: a case series of 10 pregnancies. Br J Dermatol. 2019;180:195-196.
- Naureckas S, Slater J, Gearhart N, et al. Pregnancy outcomes in women with psoriasis and psoriatic arthritis exposed to ustekinumab. J Am Acad Dermatol. 2016;74:AB264.
- Haycraft K, DiRuggiero D, Rozzo SJ, et al. Outcomes of pregnancies from tildrakizumab phases I to III clinical development program. J Clin Aesthet Dermatol. 2019;12:S27-S28.
- Tremfya (guselkumab) injection, for subcutaneous use [package insert]. Horsham, PA: Janssen Biotech, Inc; July 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761061s000lbl.pdf. Accessed Juy 8, 2020.
- Skyrizi (risankizumab-rzaa) injection, for subcutaneous use [package insert]. Northi Chicago, IL; April 2019. http://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761105s000lbl.pdf. Accessed July 8, 2020.
- Siliq (brodalumab) injection, for subcutaneous use [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America LLC; February 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf. Accessed July 8, 2020.
- Feldman S, Pangallo B, Xu W, et al. Ixekizumab and pregnancy outcome. J Am Acad Dermatol. 2017;76:AB419.
- Clarke DO, Hilbish KG, Waters DG, et al. Assessment of ixekizumab, an interleukin-17A monoclonal antibody, for potential effects on reproduction and development, including immune system function, in cynomolgus monkeys. Reprod Toxicol. 2015;58:160-173.
- Warren RB, Reich K, Langley RG, et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database. Br J Dermatol. 2018;179:1205-1207.
- Nardin C, Colas M, Curie V, et al. Pregnancy after tubal sterilization in a woman treated with biologics for severe psoriasis. Dermatol Ther (Heidelb). 2018;8:323-326.
- Xeljanz (tofacitinib) tablets for oral administration [package insert]. New York, NY: Pfizer; November 2012. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203214s000lbl.pdf. Accessed July 8, 2020.
- Pfizer. Xeljanz (tofacitinib). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf.
- Mahadevan U, Dubinsky M, Su C, et al. Outcomes of pregnancies with maternal/paternal exposure in the tofacitinib safety databases for ulcerative colitis. Inflamm Bowel Dis. 2018;24:2494-2500.
- Clowse ME, Feldman SR, Isaacs JD, et al. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf. 2016;39:755-762.
- Malek A, Sager R, Kuhn P, et al. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36:248-255.
- Kimball AB, Crow JA, Ridley K, et al. Pregnancy outcomes in women with moderate to severe psoriasis: the PSOLAR experience. J Am Acad Dermatol. 2014;70(suppl 1):AB179.
- Carter JD, Valeriano J, Vasey FB. Tumor necrosis factor-alpha inhibition and VATER association: a causal relationship. J Rheumatol. 2006;33:1014-1017.
- Carter JD, Ladhani A, Ricca LR, et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635-641.
- Koren G, Inoue M. Do tumor necrosis factor inhibitors cause malformations in humans? J Rheumatol. 2009;36:465-466.
- Johansen C, Jimenez-Solem E, Haerskjold A, et al. The use and safety of TNF inhibitors during pregnancy in women with psoriasis: a review. Int J Mol Sci. 2018;19:E1349.
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233.
- Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292.
- Cheent K, Nolan J, Sharig S, et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603-605.
- Julsgaard M, Christensen LA, Gibson PR, et al. Concentrations of adalimumab and infliximab in mothers and newborns, and effects on infection. Gastroenterology. 2016;151:110-119.
- Mahadevan U, Martin C, Kane SV, et al. Do infant serum levels of biologic agents at birth correlate with risk of adverse outcomes? results from the PIANO registry. Gastroenterology. 2016;150:S91-S92.
- Mahadevan U, Martin CF, Sandler RS, et al. PIANO: a 1000 patient prospective registry of pregnancy outcomes in women with IBD exposed to immunomodulators and biologic therapy [AGA abstract 865]. Gastroenterology. 2012;142:S-149.
Historically, there have been limited data available on the management of psoriasis in pregnancy. The most comprehensive discussion of treatment guidelines is from 2012.1 In the interim, many biologics have been approved for treating psoriasis, with slow accumulation of pregnancy safety data. The 2019 American Academy of Dermatology–National Psoriasis Foundation guidelines on biologics for psoriasis contain updated information but also highlight the paucity of pregnancy safety data.2 This gap is in part a consequence of the exclusion and disenrollment of pregnant women from clinical trials.3 Additionally, lack of detection through registries contributes; pregnancy capture in registries is low compared to the expected number of pregnancies estimated from US Census data.4 Despite these shortcomings, psoriasis patients who are already pregnant or are considering becoming pregnant frequently are encountered in practice and may need treatment. This article reviews the evidence on commonly used treatments for psoriasis in pregnancy.
Background
For many patients, psoriasis improves during pregnancy5,6 and becomes worse postpartum. In a prospective study, most patients reported improvement in pregnancy corresponding to a significant decrease in
In addition to the maternal disease state, the issue of pregnancy outcomes is paramount. In the inflammatory bowel disease and rheumatology literature, it is established that uncontrolled disease is associated with poorer pregnancy outcomes.8-10 Guidelines vary among societies on the use of biologics in pregnancy generally (eTable 11,2,9,11-24), but some societies recommend systemic agents to achieve disease control during pregnancy.9,25
Assessing the potential interplay between disease severity and outcomes in pregnant women with psoriasis is further complicated by the slowly growing body of literature demonstrating that women with psoriasis have more comorbidities26 and worse pregnancy outcomes.27,28 Pregnant psoriasis patients are more likely to smoke, have depression, and be overweight or obese prior to pregnancy and are less likely to take prenatal vitamins.26 They also have an increased risk for cesarean birth, gestational diabetes, gestational hypertension, and preeclampsia.28 In contrast to these prior studies, a systematic review revealed no risk for adverse outcomes in pregnant women with psoriasis.29
Assessment of Treatments for Psoriasis in Pregnancy
In light of these issues, treatment of psoriasis during pregnancy should be assessed from several vantage points. Of note, the US Food and Drug Administration changed its classification scheme in 2015 to a more narrative format called the Pregnancy and Lactation Labeling Rule.30 Prior classifications, however, provide a reasonable starting point for categorizing the safety of drugs (Table31). Importantly, time of exposure to systemic agents also matters; first-trimester exposure is more likely to affect embryogenesis, whereas second- and third-trimester exposures are more prone to affect other aspects of fetal growth. eTable 2 provides data on the use of oral and topical medications to treat psoriasis in pregnancy.1,8,22,32-45

Topical Agents
Topical steroids are largely understood to be reasonable treatment options, though consideration of potency, formulation, area of application, and use of occlusion is important.1,46 Risk for orofacial cleft has been noted with first-trimester topical steroid exposure, though a 2015 Cochrane review update determined that the relative risk of this association was not significantly elevated.32
The impact of topical calcipotriene and salicylic acid has not been studied in human pregnancies,1 but systemic absorption can occur for both. There is potential for vitamin D toxicity with calcipotriene46; consequently, use during pregnancy is not recommended.1,46 Some authors recommend against topical salicylic acid in pregnancy; others report that limited exposure is permissible.47 In fact, as salicylic acid commonly is found in over-the-counter acne products, many women of childbearing potential likely have quotidian exposure.
Preterm delivery and low birthweight have been reported with oral tacrolimus; however, risk with topical tacrolimus is thought to be low1 because the molecular size likely prohibits notable absorption.47 Evidence for the use of anthralin and coal tar also is scarce. First-trimester coal tar use should be avoided; subsequent use in pregnancy should be restricted given concern for adverse outcomes.1
Phototherapy
Broadband or narrowband UVB therapy is recommended as second-line therapy in pregnancy. No cases of fetal risk or premature delivery associated with UVB therapy were found in our search.1 Phototherapy can exacerbate melasma47 and decrease folate levels48; as such, some authors recommend folate supplementation in females of childbearing age who are being treated with phototherapy.49 Psoralen, used in psoralen plus UVA therapy, is mutagenic and therefore contraindicated in pregnancy.1
Oral Medications
Both methotrexate, which is a teratogen, abortifacient, and mutagen,1 and systemic retinoids, which are teratogens, are contraindicated in pregnancy.1,47 Acitretin labeling recommends avoiding pregnancy for 3 years posttreatment50 because alcohol intake prolongs the medication’s half-life.22
Apremilast use is not documented in pregnant psoriasis patients51; an ongoing registry of the Organization of Tetralogy Information Specialists has not reported publicly to date.52 Animal studies of apremilast have documented dose-related decreased birthweight and fetal loss.22
Safety data for systemic steroids, used infrequently in psoriasis, are not well established. First-trimester prednisone exposure has been associated with prematurity, low birthweight, and congenital abnormalities.38 A separate evaluation of 1047 children exposed to betamethasone in utero failed to demonstrate significant change in birthweight or head circumference. However, repeat antenatal corticosteroid exposure was associated with attention problems at 2 years of age.39
Data regarding cyclosporine use, derived primarily from organ transplant recipients, suggest elevated risk for prematurity and low birthweight.53,54 A meta-analysis demonstrated that organ transplant recipients taking cyclosporine had a nonsignificantly elevated odds ratio for congenital malformations, prematurity, and low birthweight.42 Cyclosporine use for psoriasis in pregnancy is not well described; in a study, rates of prematurity and low birthweight were both 21%.43 Limited data are available for Janus kinase inhibitors, none of which are approved for psoriasis, though clinical trials in psoriasis and psoriatic arthritis are underway (ClinicalTrials.gov identifiers NCT04246372, NCT03104374, NCT03104400).
Biologics and Small-Molecule Inhibitors
Limited data on biologics in pregnancy exist25 (eTable 3). Placental transport of IgG antibodies, including biologics, increases throughout pregnancy, especially in the third trimester.82 Infants of mothers treated with a biologic with potential for placental transfer are therefore considered by some authors to be immunosuppressed during the first months of life.2
Looking globally across biologics used for psoriasis, limited safety data are encouraging. In a review of PSOLAR (Psoriasis Longitudinal Assessment and Registry), 83 pregnancies with biologic exposure resulted in 59 live births (71%); 18 spontaneous abortions (22%); 6 induced abortions (7%); no congenital abnormalities; and 7 reports of neonatal problems, including respiratory issues, ABO blood group mismatch, hospitalization, and opioid withdrawal.83
Use of tumor necrosis factor (TNF) inhibitors in pregnancy has the most data25 and is considered a reasonable treatment option. Historically, there was concern about the risk for VACTERL syndrome (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, limb abnormalities) with exposure to a TNF inhibitor,25,84-86 but further reports have alleviated these concerns. Active transplacental transport occurs for adalimumab, infliximab, and golimumab,87 but given structural differences, transport of certolizumab and etanercept is substantially less.88,89 In the CRIB study of placental transfer of certolizumab from mother to infant (N=14), pharmacokinetic data demonstrated no quantifiable certolizumab levels in 13 infants and minimal levels in 1 infant at birth.88 There are fewer data available on the use of other biologics in pregnancy, but for those in which active placental transport is relevant, similar concerns (ie, immunosuppression) might arise (eTable 3).
Concern over biologics largely involves risk for newborn immunosuppression. A case report detailed a Crohn disease patient treated with infliximab who gave birth to an infant who died of disseminated bacille Calmette-Guérin infection at 4.5 months after receiving the vaccine at 3 months.90 This case underscores the importance of delaying live vaccination in infants born to mothers who were treated with a biologic during pregnancy. Authors have provided various data on how long to avoid vaccination; some state as long as 1 year.91
In pregnant females with inflammatory bowel disease treated with a biologic, no correlation was observed among maternal, placental, and infant serum biologic levels and neonatal infection. However, an association between preterm birth and the level of the biologic in maternal and placental (but not infant) serum and preterm birth was observed.92
In another report from the same registry, combination therapy with a TNF inhibitor and another immunomodulator led to an increased risk for infection in infants at 12 months of age, compared to infants exposed to monotherapy89 or exposed to neither agent.93 A strategy to circumvent this potential problem is to avoid treatment with actively transported molecules in the third trimester.
Conclusion
Limited data exist to guide providers who are treating pregnant women with psoriasis. Our understanding of treatment of psoriasis in pregnancy is limited as a consequence of regulations surrounding clinical trials and inadequate detection of pregnancies in registries. Further efforts are necessary to better understand the relationship between psoriasis and pregnancy and how to manage pregnant women with psoriasis.
Historically, there have been limited data available on the management of psoriasis in pregnancy. The most comprehensive discussion of treatment guidelines is from 2012.1 In the interim, many biologics have been approved for treating psoriasis, with slow accumulation of pregnancy safety data. The 2019 American Academy of Dermatology–National Psoriasis Foundation guidelines on biologics for psoriasis contain updated information but also highlight the paucity of pregnancy safety data.2 This gap is in part a consequence of the exclusion and disenrollment of pregnant women from clinical trials.3 Additionally, lack of detection through registries contributes; pregnancy capture in registries is low compared to the expected number of pregnancies estimated from US Census data.4 Despite these shortcomings, psoriasis patients who are already pregnant or are considering becoming pregnant frequently are encountered in practice and may need treatment. This article reviews the evidence on commonly used treatments for psoriasis in pregnancy.
Background
For many patients, psoriasis improves during pregnancy5,6 and becomes worse postpartum. In a prospective study, most patients reported improvement in pregnancy corresponding to a significant decrease in
In addition to the maternal disease state, the issue of pregnancy outcomes is paramount. In the inflammatory bowel disease and rheumatology literature, it is established that uncontrolled disease is associated with poorer pregnancy outcomes.8-10 Guidelines vary among societies on the use of biologics in pregnancy generally (eTable 11,2,9,11-24), but some societies recommend systemic agents to achieve disease control during pregnancy.9,25
Assessing the potential interplay between disease severity and outcomes in pregnant women with psoriasis is further complicated by the slowly growing body of literature demonstrating that women with psoriasis have more comorbidities26 and worse pregnancy outcomes.27,28 Pregnant psoriasis patients are more likely to smoke, have depression, and be overweight or obese prior to pregnancy and are less likely to take prenatal vitamins.26 They also have an increased risk for cesarean birth, gestational diabetes, gestational hypertension, and preeclampsia.28 In contrast to these prior studies, a systematic review revealed no risk for adverse outcomes in pregnant women with psoriasis.29
Assessment of Treatments for Psoriasis in Pregnancy
In light of these issues, treatment of psoriasis during pregnancy should be assessed from several vantage points. Of note, the US Food and Drug Administration changed its classification scheme in 2015 to a more narrative format called the Pregnancy and Lactation Labeling Rule.30 Prior classifications, however, provide a reasonable starting point for categorizing the safety of drugs (Table31). Importantly, time of exposure to systemic agents also matters; first-trimester exposure is more likely to affect embryogenesis, whereas second- and third-trimester exposures are more prone to affect other aspects of fetal growth. eTable 2 provides data on the use of oral and topical medications to treat psoriasis in pregnancy.1,8,22,32-45

Topical Agents
Topical steroids are largely understood to be reasonable treatment options, though consideration of potency, formulation, area of application, and use of occlusion is important.1,46 Risk for orofacial cleft has been noted with first-trimester topical steroid exposure, though a 2015 Cochrane review update determined that the relative risk of this association was not significantly elevated.32
The impact of topical calcipotriene and salicylic acid has not been studied in human pregnancies,1 but systemic absorption can occur for both. There is potential for vitamin D toxicity with calcipotriene46; consequently, use during pregnancy is not recommended.1,46 Some authors recommend against topical salicylic acid in pregnancy; others report that limited exposure is permissible.47 In fact, as salicylic acid commonly is found in over-the-counter acne products, many women of childbearing potential likely have quotidian exposure.
Preterm delivery and low birthweight have been reported with oral tacrolimus; however, risk with topical tacrolimus is thought to be low1 because the molecular size likely prohibits notable absorption.47 Evidence for the use of anthralin and coal tar also is scarce. First-trimester coal tar use should be avoided; subsequent use in pregnancy should be restricted given concern for adverse outcomes.1
Phototherapy
Broadband or narrowband UVB therapy is recommended as second-line therapy in pregnancy. No cases of fetal risk or premature delivery associated with UVB therapy were found in our search.1 Phototherapy can exacerbate melasma47 and decrease folate levels48; as such, some authors recommend folate supplementation in females of childbearing age who are being treated with phototherapy.49 Psoralen, used in psoralen plus UVA therapy, is mutagenic and therefore contraindicated in pregnancy.1
Oral Medications
Both methotrexate, which is a teratogen, abortifacient, and mutagen,1 and systemic retinoids, which are teratogens, are contraindicated in pregnancy.1,47 Acitretin labeling recommends avoiding pregnancy for 3 years posttreatment50 because alcohol intake prolongs the medication’s half-life.22
Apremilast use is not documented in pregnant psoriasis patients51; an ongoing registry of the Organization of Tetralogy Information Specialists has not reported publicly to date.52 Animal studies of apremilast have documented dose-related decreased birthweight and fetal loss.22
Safety data for systemic steroids, used infrequently in psoriasis, are not well established. First-trimester prednisone exposure has been associated with prematurity, low birthweight, and congenital abnormalities.38 A separate evaluation of 1047 children exposed to betamethasone in utero failed to demonstrate significant change in birthweight or head circumference. However, repeat antenatal corticosteroid exposure was associated with attention problems at 2 years of age.39
Data regarding cyclosporine use, derived primarily from organ transplant recipients, suggest elevated risk for prematurity and low birthweight.53,54 A meta-analysis demonstrated that organ transplant recipients taking cyclosporine had a nonsignificantly elevated odds ratio for congenital malformations, prematurity, and low birthweight.42 Cyclosporine use for psoriasis in pregnancy is not well described; in a study, rates of prematurity and low birthweight were both 21%.43 Limited data are available for Janus kinase inhibitors, none of which are approved for psoriasis, though clinical trials in psoriasis and psoriatic arthritis are underway (ClinicalTrials.gov identifiers NCT04246372, NCT03104374, NCT03104400).
Biologics and Small-Molecule Inhibitors
Limited data on biologics in pregnancy exist25 (eTable 3). Placental transport of IgG antibodies, including biologics, increases throughout pregnancy, especially in the third trimester.82 Infants of mothers treated with a biologic with potential for placental transfer are therefore considered by some authors to be immunosuppressed during the first months of life.2
Looking globally across biologics used for psoriasis, limited safety data are encouraging. In a review of PSOLAR (Psoriasis Longitudinal Assessment and Registry), 83 pregnancies with biologic exposure resulted in 59 live births (71%); 18 spontaneous abortions (22%); 6 induced abortions (7%); no congenital abnormalities; and 7 reports of neonatal problems, including respiratory issues, ABO blood group mismatch, hospitalization, and opioid withdrawal.83
Use of tumor necrosis factor (TNF) inhibitors in pregnancy has the most data25 and is considered a reasonable treatment option. Historically, there was concern about the risk for VACTERL syndrome (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, limb abnormalities) with exposure to a TNF inhibitor,25,84-86 but further reports have alleviated these concerns. Active transplacental transport occurs for adalimumab, infliximab, and golimumab,87 but given structural differences, transport of certolizumab and etanercept is substantially less.88,89 In the CRIB study of placental transfer of certolizumab from mother to infant (N=14), pharmacokinetic data demonstrated no quantifiable certolizumab levels in 13 infants and minimal levels in 1 infant at birth.88 There are fewer data available on the use of other biologics in pregnancy, but for those in which active placental transport is relevant, similar concerns (ie, immunosuppression) might arise (eTable 3).
Concern over biologics largely involves risk for newborn immunosuppression. A case report detailed a Crohn disease patient treated with infliximab who gave birth to an infant who died of disseminated bacille Calmette-Guérin infection at 4.5 months after receiving the vaccine at 3 months.90 This case underscores the importance of delaying live vaccination in infants born to mothers who were treated with a biologic during pregnancy. Authors have provided various data on how long to avoid vaccination; some state as long as 1 year.91
In pregnant females with inflammatory bowel disease treated with a biologic, no correlation was observed among maternal, placental, and infant serum biologic levels and neonatal infection. However, an association between preterm birth and the level of the biologic in maternal and placental (but not infant) serum and preterm birth was observed.92
In another report from the same registry, combination therapy with a TNF inhibitor and another immunomodulator led to an increased risk for infection in infants at 12 months of age, compared to infants exposed to monotherapy89 or exposed to neither agent.93 A strategy to circumvent this potential problem is to avoid treatment with actively transported molecules in the third trimester.
Conclusion
Limited data exist to guide providers who are treating pregnant women with psoriasis. Our understanding of treatment of psoriasis in pregnancy is limited as a consequence of regulations surrounding clinical trials and inadequate detection of pregnancies in registries. Further efforts are necessary to better understand the relationship between psoriasis and pregnancy and how to manage pregnant women with psoriasis.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459-477.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Flood KS, Porter ML, Kimball AB. Use of biologics in pregnancy: limitations stemming from clinical trials and registry experience. J Eur Acad Dermatol Venereol. 2019;33:E276-E277.
- Horn EJ, Chambers CD, Menter A, et al. Pregnancy outcomes in psoriasis: why do we know so little? J Am Acad Dermatol. 2009;61:E5-E8.
- Raychaudhuri SP, Navare T, Gross J, et al. Clinical course of psoriasis during pregnancy. Int J Dermatol. 2003;42:518-520.
- Boyd AS, Morris LF, Phillips CM, et al. Psoriasis and pregnancy: hormone and immune system interaction. Int J Dermatol. 1996;35:169-172.
- Murase JE, Chan KK, Garite TJ, et al. Hormonal effect on psoriasis in pregnancy and post partum. Arch Dermatol. 2005;14:601-606.
- Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795-810.
- Nguyen GC, Seow CH, Maxwell C, et al. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology. 2016;150:734-757.
- Wise J. Rheumatic diseases should be actively treated in pregnancy, new guidelines say. BMJ. 2016;532:i312.
- Puig L, Carrascosa JM, Carretero G, et al. Spanish evidence-based guidelines on the treatment of psoriasis with biologic agents, 2013. part 1: on efficacy and choice of treatment. Actas Dermosifiliogr. 2013;104:694-709.
- Girolomoni G, Altomore G, Ayala F, et al. Differential management of mild-to-severe psoriasis with biologic drugs: an Italian Delphi consensus expert panel. J Dermatolog Treat. 2015;26:128-133.
- Yeung J, Gooderham MJ, Grewal P, et al. Management of plaque psoriasis with biologic therapies in women of child-bearing potential consensus paper. J Cutan Med Surg. 2020;24:3S-14S.
- Louthrenoo W, Kasitanon N, Kathamort W, et al. 2016 updated Thai Rheumatism Association Recommendations for the use of biologic and targeted synthetic disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis. Int J Rheum Dis. 2017;20:1166-1184.
- Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding—part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford). 2016;55:1693-1697.
- van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis. 2015;9:107-124.
- Orlando A, Armuzz A, Papi C, et al. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) Clinical Practice Guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis. 2011;43:1-20.
- Puchner A, Grochenig HP, Sautner J, et al. Immunosuppressives and biologics during pregnancy and lactation. Wien Klin Wochenschr. 2019;131:29-44.
- ACOG Committee opinion no. 776: immune modulating therapies in pregnancy and lactation. Obstet Gynecol. 2019;133:E287-E297.
- Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:S1-S106.
- Goëb V, Ardizzone M, Arnard L, et al. Recommendations for using TNF-α antagonists and French Clinical Practice Guidelines endorsed by the French National Authority for Health. Joint Bone Spine. 2013;80:574-581.
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. the Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100.
- Mahadevan U, Robinson C, Bernasko N, et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-1524.
- Mahadevan U, Cucchiara S, Hyam JS, et al. The London position statement of the World Congress of Gastroenterology on biological therapy for IBD with the European Crohn’s and Colitis Organisation: pregnancy and pediatrics. Am J Gastroenterol. 2011;106:214-223.
- Porter ML, Lockwood SJ, Kimball AB. Update on biologic safety for patients with psoriasis during pregnancy. Int J Womens Dermatol. 2017;3:21-25.
- Bandoli G, Johnson DL, Jones KL, et al. Potentially modifiable risk factors for adverse pregnancy outcomes in women with psoriasis. Br J Dermatol. 2010;163:334-339.
- Lima XT, Janakiraman V, Hughes MD, et al. The impact of psoriasis on pregnancy outcomes. J Invest Dermatol. 2012;132:85-91.
- Bröms G, Haerskjold A, Granath F, et al. Effect of maternal psoriasis on pregnancy and birth outcomes: a population-based cohort study from Denmark and Sweden. Acta Derm Venereol. 2018;98:728-734.
- Bobotsis R, Gulliver WP, Monaghan K, et al. Psoriasis and adverse pregnancy outcomes: a systematic review of observational studies. Br J Dermatol. 2016;175:464-472.
- Blattner CM, Danesh M, Safaee M, et al. Understanding the new FDA pregnancy and lactation labeling rules. Int J Womens Dermatol. 2016;2:5-7.
- Pernia S, DeMaagd G. The new pregnancy and lactation labeling rule. P T. 2016;4:713-715.
- Chi C-C, Wang S-H, Wojnarowska F, et al. Safety of topical corticosteroids in pregnancy. Cochrane Database Syst Rev. 2015:CD007346.
- Chi CC, Wang SH, Kirtschig G. Safety of topical corticosteroids in pregnancy. JAMA Dermatol. 2016;152:934-935.
- Dovonex (calcipotriene) Cream, 0.005% [package insert]. Dublin, Ireland: Leo Laboratories, Ltd; March 2015.
- Franssen ME, van der Wilt GJ, de Jong PC, et al. A retrospective study of the teratogenicity of dermatological coal tar products. Acta Derm Venereol. 1999;79:390-391.
- Garbis H, Elefant E, Bertolotti E, et al. Pregnancy outcome after periconceptional and first-trimester exposure to methoxsalen photochemotherapy. Arch Dermatol. 1995;131:492-493.
- Horizon Pharma USA. RAYOS (prednisone). https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf.
- Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385-392.
- Crowther CA, Doyle LW, Haslam RR, et al. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1179-1189.
- Palmsten K, Rolland M, Herbert MF, et al. Patterns of prednisone use during pregnancy in women with rheumatoid arthritis: daily and cumulative dose. Pharmacoepidemiol Drug Saf. 2018;27:430-438.
- Groth K, Brännström M, Mölne J, et al. Cyclosporine A exposure during pregnancy in mice: effects on reproductive performance in mothers and offspring. Hum Reprod. 2010;25:697-704.
- Bar Oz B, Hackman R, Einarson T, et al. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis. Transplantation. 2001;71:1051-1055.
- Paziana K, Del Monaco M, Cardonick E, et al. Ciclosporin use during pregnancy. Drug Saf. 2013;36:279-294.
- Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 pregnancy outcomes in transplant recipients treated with Sandimmun. Transplant Proc. 1997;29:2480.
- Otezla (apremilast) tablets, for oral use [package insert]. Summit, NJ: Celgene Corporation; June 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205437s006lbl.pdf. Accessed July 8, 2020.
- Kurizky PS, de Castro Ferreira C, Nogueira LSC, et al. Treatment of psoriasis and psoriatic arthritis during pregnancy and breastfeeding. An Bras Dermatol. 2015;90:367-375.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation. J Am Acad Dermatol. 2014;70:401.e1-401.e4.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Murase JE, Koo JY, Berger TG. Narrowband ultraviolet B phototherapy influences serum folate levels in patients with vitiligo. J Am Acad Dermatol. 2010;62:710-711.
- Soriatane (acitretin) capsules [package insert]. Morrisville, NC: Stiefel Laboratories, Inc; April 2011. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019821s018mg.pdf. Accessed July 8, 2020.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Help us better understand the effects of Otezla in pregnancy. MotherToBaby website. https://mothertobaby.org/ongoing-study/otezla/. Accessed July 8, 2020.
- Bangsgaard N, Rørbye C, Skov L. Treating psoriasis during pregnancy: safety and efficacy of treatments. Am J Clin Dermatol. 2015;16:389-398.
- Tyler K. Dermatologic therapy in pregnancy. Clin Obstet Gynecol. 2015;58:112-118.
- Luu M, Benzenine E, Doret M, et al. Continuous anti–TNF-α use throughout pregnancy: possible complications for the mother but not for the fetus. a retrospective cohort on the French National Health Insurance Database (EVASION). Am J Gastroenterol. 2018;113:1669-1677.
- Bröms G, Granath F, Ekbom A, et al. Low risk of birth defects for infants whose mothers are treated with anti-tumor necrosis factor agents during pregnancy. Clin Gastroenterol Hepatol. 2016;14:234-241.
- Mirdamadi K, Salinas T, Vali R, et al. Meta-analysis of pregnancy outcomes after exposure to TNF-α inhibitors during pregnancy for the treatment of arthritic diseases. J Popul Ther Clin Pharmacol. 2018;25:E53-E56.
- Shihab Z, Yeomans ND, De Cruz P. Anti-tumour necrosis factor α therapies and inflammatory bowel disease pregnancy outcomes: a meta-analysis. J Crohns Colitis. 2016;10:979-988.
- Bröms G, Kieler H, Ekbom A, et al. Anti-TNF treatment during pregnancy and birth outcomes: a population-based study from Denmark, Finland, and Sweden. Pharmacoepidemiol Drug Saf. 2020;29:316-327.
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, et al. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78-84.
- FDA determinations for pregnant and nursing women. National Psoriasis Foundation website. http://www.psoriasis.org/pregnancy/fda-determinations. Accessed July 8, 2020.
- Østensen M. Safety issues of biologics in pregnant patients with rheumatic diseases. Ann N Y Acad Sci. 2014;1317:32-38.
- Chambers CD, Johnson DL, Luo Y, et al. Pregnancy outcome in women treated with adalimumab for the treatment of rheumatoid arthritis: the OTIS Autoimmune Diseases in Pregnancy Project. Arthritis Rheum. 2012;64:2466.
- Clowse ME, Wolf DC, Forger F, et al. Pregnancy outcomes after exposure to certolizumab pegol: updated results from a pharmacovigilance safety database. Arthritis Rheumatol. 2018;70:1399-1407.
- Carman WJ, Accortt NA, Anthony MS, et al. Pregnancy and infant outcomes including major congenital malformations among women with chronic inflammatory arthritis or psoriasis, with and without etanercept use. Pharmacoepidemiol Drug Saf. 2017;26:1109-1118.
- Janssen. SIMPONI (golilumab). https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125289s0064lbl.pdf.
- Yurkon K, Guo CY, Harrison D, et al. Pregnancy outcomes in women with dermatologic conditions exposed to infliximab. J Am Acad Dermatol. 2014;70:AB179.
- Watson N, Wu K, Farr P, et al. Ustekinumab exposure during conception and pregnancy in patients with chronic plaque psoriasis: a case series of 10 pregnancies. Br J Dermatol. 2019;180:195-196.
- Naureckas S, Slater J, Gearhart N, et al. Pregnancy outcomes in women with psoriasis and psoriatic arthritis exposed to ustekinumab. J Am Acad Dermatol. 2016;74:AB264.
- Haycraft K, DiRuggiero D, Rozzo SJ, et al. Outcomes of pregnancies from tildrakizumab phases I to III clinical development program. J Clin Aesthet Dermatol. 2019;12:S27-S28.
- Tremfya (guselkumab) injection, for subcutaneous use [package insert]. Horsham, PA: Janssen Biotech, Inc; July 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761061s000lbl.pdf. Accessed Juy 8, 2020.
- Skyrizi (risankizumab-rzaa) injection, for subcutaneous use [package insert]. Northi Chicago, IL; April 2019. http://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761105s000lbl.pdf. Accessed July 8, 2020.
- Siliq (brodalumab) injection, for subcutaneous use [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America LLC; February 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf. Accessed July 8, 2020.
- Feldman S, Pangallo B, Xu W, et al. Ixekizumab and pregnancy outcome. J Am Acad Dermatol. 2017;76:AB419.
- Clarke DO, Hilbish KG, Waters DG, et al. Assessment of ixekizumab, an interleukin-17A monoclonal antibody, for potential effects on reproduction and development, including immune system function, in cynomolgus monkeys. Reprod Toxicol. 2015;58:160-173.
- Warren RB, Reich K, Langley RG, et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database. Br J Dermatol. 2018;179:1205-1207.
- Nardin C, Colas M, Curie V, et al. Pregnancy after tubal sterilization in a woman treated with biologics for severe psoriasis. Dermatol Ther (Heidelb). 2018;8:323-326.
- Xeljanz (tofacitinib) tablets for oral administration [package insert]. New York, NY: Pfizer; November 2012. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203214s000lbl.pdf. Accessed July 8, 2020.
- Pfizer. Xeljanz (tofacitinib). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf.
- Mahadevan U, Dubinsky M, Su C, et al. Outcomes of pregnancies with maternal/paternal exposure in the tofacitinib safety databases for ulcerative colitis. Inflamm Bowel Dis. 2018;24:2494-2500.
- Clowse ME, Feldman SR, Isaacs JD, et al. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf. 2016;39:755-762.
- Malek A, Sager R, Kuhn P, et al. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36:248-255.
- Kimball AB, Crow JA, Ridley K, et al. Pregnancy outcomes in women with moderate to severe psoriasis: the PSOLAR experience. J Am Acad Dermatol. 2014;70(suppl 1):AB179.
- Carter JD, Valeriano J, Vasey FB. Tumor necrosis factor-alpha inhibition and VATER association: a causal relationship. J Rheumatol. 2006;33:1014-1017.
- Carter JD, Ladhani A, Ricca LR, et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635-641.
- Koren G, Inoue M. Do tumor necrosis factor inhibitors cause malformations in humans? J Rheumatol. 2009;36:465-466.
- Johansen C, Jimenez-Solem E, Haerskjold A, et al. The use and safety of TNF inhibitors during pregnancy in women with psoriasis: a review. Int J Mol Sci. 2018;19:E1349.
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233.
- Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292.
- Cheent K, Nolan J, Sharig S, et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603-605.
- Julsgaard M, Christensen LA, Gibson PR, et al. Concentrations of adalimumab and infliximab in mothers and newborns, and effects on infection. Gastroenterology. 2016;151:110-119.
- Mahadevan U, Martin C, Kane SV, et al. Do infant serum levels of biologic agents at birth correlate with risk of adverse outcomes? results from the PIANO registry. Gastroenterology. 2016;150:S91-S92.
- Mahadevan U, Martin CF, Sandler RS, et al. PIANO: a 1000 patient prospective registry of pregnancy outcomes in women with IBD exposed to immunomodulators and biologic therapy [AGA abstract 865]. Gastroenterology. 2012;142:S-149.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459-477.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Flood KS, Porter ML, Kimball AB. Use of biologics in pregnancy: limitations stemming from clinical trials and registry experience. J Eur Acad Dermatol Venereol. 2019;33:E276-E277.
- Horn EJ, Chambers CD, Menter A, et al. Pregnancy outcomes in psoriasis: why do we know so little? J Am Acad Dermatol. 2009;61:E5-E8.
- Raychaudhuri SP, Navare T, Gross J, et al. Clinical course of psoriasis during pregnancy. Int J Dermatol. 2003;42:518-520.
- Boyd AS, Morris LF, Phillips CM, et al. Psoriasis and pregnancy: hormone and immune system interaction. Int J Dermatol. 1996;35:169-172.
- Murase JE, Chan KK, Garite TJ, et al. Hormonal effect on psoriasis in pregnancy and post partum. Arch Dermatol. 2005;14:601-606.
- Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795-810.
- Nguyen GC, Seow CH, Maxwell C, et al. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology. 2016;150:734-757.
- Wise J. Rheumatic diseases should be actively treated in pregnancy, new guidelines say. BMJ. 2016;532:i312.
- Puig L, Carrascosa JM, Carretero G, et al. Spanish evidence-based guidelines on the treatment of psoriasis with biologic agents, 2013. part 1: on efficacy and choice of treatment. Actas Dermosifiliogr. 2013;104:694-709.
- Girolomoni G, Altomore G, Ayala F, et al. Differential management of mild-to-severe psoriasis with biologic drugs: an Italian Delphi consensus expert panel. J Dermatolog Treat. 2015;26:128-133.
- Yeung J, Gooderham MJ, Grewal P, et al. Management of plaque psoriasis with biologic therapies in women of child-bearing potential consensus paper. J Cutan Med Surg. 2020;24:3S-14S.
- Louthrenoo W, Kasitanon N, Kathamort W, et al. 2016 updated Thai Rheumatism Association Recommendations for the use of biologic and targeted synthetic disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis. Int J Rheum Dis. 2017;20:1166-1184.
- Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding—part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford). 2016;55:1693-1697.
- van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis. 2015;9:107-124.
- Orlando A, Armuzz A, Papi C, et al. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) Clinical Practice Guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis. 2011;43:1-20.
- Puchner A, Grochenig HP, Sautner J, et al. Immunosuppressives and biologics during pregnancy and lactation. Wien Klin Wochenschr. 2019;131:29-44.
- ACOG Committee opinion no. 776: immune modulating therapies in pregnancy and lactation. Obstet Gynecol. 2019;133:E287-E297.
- Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:S1-S106.
- Goëb V, Ardizzone M, Arnard L, et al. Recommendations for using TNF-α antagonists and French Clinical Practice Guidelines endorsed by the French National Authority for Health. Joint Bone Spine. 2013;80:574-581.
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. the Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100.
- Mahadevan U, Robinson C, Bernasko N, et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019;156:1508-1524.
- Mahadevan U, Cucchiara S, Hyam JS, et al. The London position statement of the World Congress of Gastroenterology on biological therapy for IBD with the European Crohn’s and Colitis Organisation: pregnancy and pediatrics. Am J Gastroenterol. 2011;106:214-223.
- Porter ML, Lockwood SJ, Kimball AB. Update on biologic safety for patients with psoriasis during pregnancy. Int J Womens Dermatol. 2017;3:21-25.
- Bandoli G, Johnson DL, Jones KL, et al. Potentially modifiable risk factors for adverse pregnancy outcomes in women with psoriasis. Br J Dermatol. 2010;163:334-339.
- Lima XT, Janakiraman V, Hughes MD, et al. The impact of psoriasis on pregnancy outcomes. J Invest Dermatol. 2012;132:85-91.
- Bröms G, Haerskjold A, Granath F, et al. Effect of maternal psoriasis on pregnancy and birth outcomes: a population-based cohort study from Denmark and Sweden. Acta Derm Venereol. 2018;98:728-734.
- Bobotsis R, Gulliver WP, Monaghan K, et al. Psoriasis and adverse pregnancy outcomes: a systematic review of observational studies. Br J Dermatol. 2016;175:464-472.
- Blattner CM, Danesh M, Safaee M, et al. Understanding the new FDA pregnancy and lactation labeling rules. Int J Womens Dermatol. 2016;2:5-7.
- Pernia S, DeMaagd G. The new pregnancy and lactation labeling rule. P T. 2016;4:713-715.
- Chi C-C, Wang S-H, Wojnarowska F, et al. Safety of topical corticosteroids in pregnancy. Cochrane Database Syst Rev. 2015:CD007346.
- Chi CC, Wang SH, Kirtschig G. Safety of topical corticosteroids in pregnancy. JAMA Dermatol. 2016;152:934-935.
- Dovonex (calcipotriene) Cream, 0.005% [package insert]. Dublin, Ireland: Leo Laboratories, Ltd; March 2015.
- Franssen ME, van der Wilt GJ, de Jong PC, et al. A retrospective study of the teratogenicity of dermatological coal tar products. Acta Derm Venereol. 1999;79:390-391.
- Garbis H, Elefant E, Bertolotti E, et al. Pregnancy outcome after periconceptional and first-trimester exposure to methoxsalen photochemotherapy. Arch Dermatol. 1995;131:492-493.
- Horizon Pharma USA. RAYOS (prednisone). https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202020s000lbl.pdf.
- Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385-392.
- Crowther CA, Doyle LW, Haslam RR, et al. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1179-1189.
- Palmsten K, Rolland M, Herbert MF, et al. Patterns of prednisone use during pregnancy in women with rheumatoid arthritis: daily and cumulative dose. Pharmacoepidemiol Drug Saf. 2018;27:430-438.
- Groth K, Brännström M, Mölne J, et al. Cyclosporine A exposure during pregnancy in mice: effects on reproductive performance in mothers and offspring. Hum Reprod. 2010;25:697-704.
- Bar Oz B, Hackman R, Einarson T, et al. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis. Transplantation. 2001;71:1051-1055.
- Paziana K, Del Monaco M, Cardonick E, et al. Ciclosporin use during pregnancy. Drug Saf. 2013;36:279-294.
- Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 pregnancy outcomes in transplant recipients treated with Sandimmun. Transplant Proc. 1997;29:2480.
- Otezla (apremilast) tablets, for oral use [package insert]. Summit, NJ: Celgene Corporation; June 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205437s006lbl.pdf. Accessed July 8, 2020.
- Kurizky PS, de Castro Ferreira C, Nogueira LSC, et al. Treatment of psoriasis and psoriatic arthritis during pregnancy and breastfeeding. An Bras Dermatol. 2015;90:367-375.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation. J Am Acad Dermatol. 2014;70:401.e1-401.e4.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Murase JE, Koo JY, Berger TG. Narrowband ultraviolet B phototherapy influences serum folate levels in patients with vitiligo. J Am Acad Dermatol. 2010;62:710-711.
- Soriatane (acitretin) capsules [package insert]. Morrisville, NC: Stiefel Laboratories, Inc; April 2011. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019821s018mg.pdf. Accessed July 8, 2020.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Help us better understand the effects of Otezla in pregnancy. MotherToBaby website. https://mothertobaby.org/ongoing-study/otezla/. Accessed July 8, 2020.
- Bangsgaard N, Rørbye C, Skov L. Treating psoriasis during pregnancy: safety and efficacy of treatments. Am J Clin Dermatol. 2015;16:389-398.
- Tyler K. Dermatologic therapy in pregnancy. Clin Obstet Gynecol. 2015;58:112-118.
- Luu M, Benzenine E, Doret M, et al. Continuous anti–TNF-α use throughout pregnancy: possible complications for the mother but not for the fetus. a retrospective cohort on the French National Health Insurance Database (EVASION). Am J Gastroenterol. 2018;113:1669-1677.
- Bröms G, Granath F, Ekbom A, et al. Low risk of birth defects for infants whose mothers are treated with anti-tumor necrosis factor agents during pregnancy. Clin Gastroenterol Hepatol. 2016;14:234-241.
- Mirdamadi K, Salinas T, Vali R, et al. Meta-analysis of pregnancy outcomes after exposure to TNF-α inhibitors during pregnancy for the treatment of arthritic diseases. J Popul Ther Clin Pharmacol. 2018;25:E53-E56.
- Shihab Z, Yeomans ND, De Cruz P. Anti-tumour necrosis factor α therapies and inflammatory bowel disease pregnancy outcomes: a meta-analysis. J Crohns Colitis. 2016;10:979-988.
- Bröms G, Kieler H, Ekbom A, et al. Anti-TNF treatment during pregnancy and birth outcomes: a population-based study from Denmark, Finland, and Sweden. Pharmacoepidemiol Drug Saf. 2020;29:316-327.
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, et al. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78-84.
- FDA determinations for pregnant and nursing women. National Psoriasis Foundation website. http://www.psoriasis.org/pregnancy/fda-determinations. Accessed July 8, 2020.
- Østensen M. Safety issues of biologics in pregnant patients with rheumatic diseases. Ann N Y Acad Sci. 2014;1317:32-38.
- Chambers CD, Johnson DL, Luo Y, et al. Pregnancy outcome in women treated with adalimumab for the treatment of rheumatoid arthritis: the OTIS Autoimmune Diseases in Pregnancy Project. Arthritis Rheum. 2012;64:2466.
- Clowse ME, Wolf DC, Forger F, et al. Pregnancy outcomes after exposure to certolizumab pegol: updated results from a pharmacovigilance safety database. Arthritis Rheumatol. 2018;70:1399-1407.
- Carman WJ, Accortt NA, Anthony MS, et al. Pregnancy and infant outcomes including major congenital malformations among women with chronic inflammatory arthritis or psoriasis, with and without etanercept use. Pharmacoepidemiol Drug Saf. 2017;26:1109-1118.
- Janssen. SIMPONI (golilumab). https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125289s0064lbl.pdf.
- Yurkon K, Guo CY, Harrison D, et al. Pregnancy outcomes in women with dermatologic conditions exposed to infliximab. J Am Acad Dermatol. 2014;70:AB179.
- Watson N, Wu K, Farr P, et al. Ustekinumab exposure during conception and pregnancy in patients with chronic plaque psoriasis: a case series of 10 pregnancies. Br J Dermatol. 2019;180:195-196.
- Naureckas S, Slater J, Gearhart N, et al. Pregnancy outcomes in women with psoriasis and psoriatic arthritis exposed to ustekinumab. J Am Acad Dermatol. 2016;74:AB264.
- Haycraft K, DiRuggiero D, Rozzo SJ, et al. Outcomes of pregnancies from tildrakizumab phases I to III clinical development program. J Clin Aesthet Dermatol. 2019;12:S27-S28.
- Tremfya (guselkumab) injection, for subcutaneous use [package insert]. Horsham, PA: Janssen Biotech, Inc; July 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761061s000lbl.pdf. Accessed Juy 8, 2020.
- Skyrizi (risankizumab-rzaa) injection, for subcutaneous use [package insert]. Northi Chicago, IL; April 2019. http://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761105s000lbl.pdf. Accessed July 8, 2020.
- Siliq (brodalumab) injection, for subcutaneous use [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals North America LLC; February 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf. Accessed July 8, 2020.
- Feldman S, Pangallo B, Xu W, et al. Ixekizumab and pregnancy outcome. J Am Acad Dermatol. 2017;76:AB419.
- Clarke DO, Hilbish KG, Waters DG, et al. Assessment of ixekizumab, an interleukin-17A monoclonal antibody, for potential effects on reproduction and development, including immune system function, in cynomolgus monkeys. Reprod Toxicol. 2015;58:160-173.
- Warren RB, Reich K, Langley RG, et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database. Br J Dermatol. 2018;179:1205-1207.
- Nardin C, Colas M, Curie V, et al. Pregnancy after tubal sterilization in a woman treated with biologics for severe psoriasis. Dermatol Ther (Heidelb). 2018;8:323-326.
- Xeljanz (tofacitinib) tablets for oral administration [package insert]. New York, NY: Pfizer; November 2012. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203214s000lbl.pdf. Accessed July 8, 2020.
- Pfizer. Xeljanz (tofacitinib). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf.
- Mahadevan U, Dubinsky M, Su C, et al. Outcomes of pregnancies with maternal/paternal exposure in the tofacitinib safety databases for ulcerative colitis. Inflamm Bowel Dis. 2018;24:2494-2500.
- Clowse ME, Feldman SR, Isaacs JD, et al. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf. 2016;39:755-762.
- Malek A, Sager R, Kuhn P, et al. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36:248-255.
- Kimball AB, Crow JA, Ridley K, et al. Pregnancy outcomes in women with moderate to severe psoriasis: the PSOLAR experience. J Am Acad Dermatol. 2014;70(suppl 1):AB179.
- Carter JD, Valeriano J, Vasey FB. Tumor necrosis factor-alpha inhibition and VATER association: a causal relationship. J Rheumatol. 2006;33:1014-1017.
- Carter JD, Ladhani A, Ricca LR, et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635-641.
- Koren G, Inoue M. Do tumor necrosis factor inhibitors cause malformations in humans? J Rheumatol. 2009;36:465-466.
- Johansen C, Jimenez-Solem E, Haerskjold A, et al. The use and safety of TNF inhibitors during pregnancy in women with psoriasis: a review. Int J Mol Sci. 2018;19:E1349.
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233.
- Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292.
- Cheent K, Nolan J, Sharig S, et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603-605.
- Julsgaard M, Christensen LA, Gibson PR, et al. Concentrations of adalimumab and infliximab in mothers and newborns, and effects on infection. Gastroenterology. 2016;151:110-119.
- Mahadevan U, Martin C, Kane SV, et al. Do infant serum levels of biologic agents at birth correlate with risk of adverse outcomes? results from the PIANO registry. Gastroenterology. 2016;150:S91-S92.
- Mahadevan U, Martin CF, Sandler RS, et al. PIANO: a 1000 patient prospective registry of pregnancy outcomes in women with IBD exposed to immunomodulators and biologic therapy [AGA abstract 865]. Gastroenterology. 2012;142:S-149.
Practice Points
- Robust safety data often are lacking for the use of topical and systemic agents to treat psoriasis in pregnancy.
- Professional society guidelines on the use of systemic agents in pregnancy vary among dermatology, gastroenterology, and rheumatology organizations.
Hidradenitis Suppurativa for the Dermatologic Hospitalist
Hidradenitis suppurativa (HS) is a common chronic inflammatory skin disease characterized by purulent subcutaneous nodules, papules, abscesses, and fistula tracts that lead to scarring and fibrosis. Lesions develop primarily in the axilla, groin, and other intertriginous and hair-bearing areas.
The natural history of the disease is characterized by periods of disease flare, followed by periods of disease quiescence. Patients might have weeks or months of low disease activity but frequently develop multiple exacerbating episodes over the course of weeks or months. The condition primarily presents in adolescent and peripubescent years, continuing throughout adulthood. Some evidence suggests a bimodal disease distribution, with a second peak of incidence in middle-aged adults. Women and men are affected equally; however, the disease can be phenotypically different in men and women.
Patients frequently present in emergency and inpatient settings for evaluation because of the pain and severity of HS flares as well as associated systemic symptoms. Inpatient and emergency department (ED) care are unique opportunities for dermatologic hospitalist and dermatologic consultative services to educate other physicians about the condition and initiate aggressive treatments that are frequently necessary to control HS flares. This article aims to address best methods for treating HS in these settings.
Pathophysiology
Although the exact pathophysiology of the condition is unknown, HS is thought to begin with follicular occlusion with downstream inflammation mediating neutrophilic activity and scarring. Hyperplasia of the infundibular epithelium is observed on histology, and the resulting occlusion, contained keratin, and follicular rupture initiate robust downstream inflammation.1,2 Follicular occlusion might be initially androgen mediated3 or might occur in combination with friction4 and genetic or acquired factors involving Notch signaling. Although HS characteristically presents in areas of high apocrine density, apocrine glands are not thought to be the primary mediator of disease activity.5 IL-17, IL-23, tumor necrosis factor α, and IL-1β are implicated in the pathogenesis of HS, but it is unknown if these cytokines are the driving pathologic factor in HS or if they are merely secondary sequelae.6
Demographics and Prevalence in Hospitalized Patients
Although increasing treatment availability has brought more attention to HS, true prevalence is unknown. A prevalence of 1% has been reported in many European countries.7 Global prevalence has been more difficult to determine, with variable data suggesting a prevalence of 0.03% to 8%, depending on the population included.8 Most patients studied in a US-based claims database were aged 30 to 64 years, and the overall prevalence was 0.05%.9 Despite prevalence similar to psoriasis, utilization of high-cost emergency and inpatient admissions is notably higher among patients with HS. Recent claims data suggest that HS patients utilize the ED at a rate 3 times higher than psoriasis patients and are admitted as inpatients at a rate 5 times higher.10 Similar data suggest an associated increased cost of care for patients with HS vs other conditions, such as psoriasis, due to frequent ED and inpatient stays.11 Although HS frequently presents in the inpatient and emergency settings, there is little literature on best methods for managing patients in these settings.
Pearls for Inpatient and Emergency Evaluation and Management
Initial Evaluation
When dermatologic consultative services are asked to evaluate patients with HS, preliminary evaluation should reflect the acuity of the patient. Vital signs and toxicity should be reviewed to ensure that there is no evidence of severe infection necessitating critical or acute care.
History
History-taking should reflect assessment of the patient’s baseline disease, including date of initial onset; exacerbating factors, such as friction, smoking, pregnancy, and menses; and the current history of the patient’s flare. A history of antibiotics, immunosuppression, topical therapy, antiandrogen therapy, and vitamin A analog therapy also should be reviewed. If an initial diagnosis is made in the ED or inpatient setting, a family and personal history should focus on specific risk factors and disease associations, including inflammatory bowel disease,12 pilonidal cysts,13 polycystic ovary syndrome,14 and metabolic syndrome.15
Physical Examination
As with all dermatologic consultations, a full-body skin examination, with special attention to the axilla, inframammary skin, groin, buttocks, and perineum, should be undertaken. In addition to these common areas of disease progression, examination should focus on atypical sites for disease manifestation, including the posterior auricular scalp, skin folds in the pannus and back, and the beard area in men. Evaluation of axillary and gluteal hair should note features of folliculitis and hair removal, which can exacerbate HS. Examination also should include an investigation of cutaneous manifestations of comorbid conditions, including acanthosis nigricans, contiguous or metastatic cutaneous Crohn disease, erythema nodosum, and pilonidal cysts. Caution should be exercised when diagnosing pilonidal cysts, as isolated or evolving HS in the gluteal cleft often is misdiagnosed as a pilonidal cyst.
Laboratory Evaluation
Testing often is misleading in patients with HS, especially in the acute setting, because the condition is a chronic inflammatory process. The C-reactive protein level as well as the absolute white blood cell and neutrophil counts often are elevated, even in the absence of acute infection.16 In fact, although patients often are treated with intravenous antibiotics by inpatient and emergency teams in the setting of these 3 laboratory abnormalities, these findings often reflect disease activity, not frank infection. Fever, especially low-grade fever, also can reflect ongoing disease activity. Thrombocytosis and anemia also are anecdotally common, though these findings have not been reported specifically in the literature.
Bacterial Cultures
The role of lesional and perilesional bacterial cultures is controversial in HS. Prior studies have demonstrated that biofilm formation may be associated with the chronic inflammation seen in HS.17 However, most data to date suggest that infection is not the primary driver of HS disease flares, as demonstrated by the frequency of sterile cultures and the variable response of the disease to penicillin and related antibiotics.18
Imaging
Ultrasonography and magnetic resonance imaging can be conducted if there is concern about deeper abscesses that are not apparent on examination. When interpreted by nondermatologic practitioners, however, the findings of these modalities can result in unnecessary surgical intervention, given the concern for development of infectious abscess.19
Diagnosis
Many patients with HS experience a notable delay in time to diagnosis, living with symptoms for 7 years on average prior to being given a name for their condition.20 Often, patients seek ED care at initial presentation because lesions can present quickly and are associated with remarkable pain. Inpatient dermatologic evaluation can provide patients with definitive diagnosis, appropriate counseling that provides an overview of the natural history of the disease, lifestyle recommendations, and expedited connection to outpatient longitudinal care.
Diagnosis is made clinically by assessment for typical lesions, such as painful or tender papules, nodules, or abscesses in the axillae, inframammary region, groin, thighs, and perineal and perianal regions. Cordlike scarring often is seen in the absence of active inflammatory lesions.21 Double-headed open comedones and prominent follicular occlusion are seen in some phenotypes but are not required for diagnosis.22
Multiple scoring modalities are in use23; the Hurley staging system, initially developed for surgical staging, has become a commonly used method in the clinical setting24:
• Hurley stage I: isolated nodules or abscesses;
• Hurley stage II: widely separate lesions and sinus tracts or scarring are suggestive; and
• Hurley stage III: multiple lesions with near-diffuse involvement and formation of sinus tracts and scarring.
Other scoring modalities, such as the Hidradenitis Suppurativa Clinical Response (HiSCR), are more commonly used in the clinical trial setting and quantitatively capture lesion count improvement while the patient is being treated.25
Treatment
Evaluation in the ED might necessitate recommendations for inpatient admission. Dermatologic consultation can be helpful in providing ED physicians with context for interpretation of laboratory results and clinical findings. Specifically, dermatologic evaluation can help differentiate presentations consistent with a primary infection from a more common presentation of HS flaring and associated bacterial colonization. Indications for inpatient admission are severe pain; concern for systemic infection, including high fever or sepsis; and need for surgical intervention. Patients with severe disease who do not have a longitudinal care plan or who lack the ability to care for lesions at home also are candidates for inpatient admission, where they can receive more intensive nursing and wound care as well as outpatient logistical management.
Acute care should be aimed at treatments that work quickly and aggressively and have both anti-inflammatory and antimicrobial effects. Severe flares require aggressive initial treatment to ensure more long-term remission. Adalimumab, maintained at 40 mg/wk after a loading dose, is the mainstay of evidence-based treatment for moderate to severe HS in patients 12 years or older; however, this treatment might not be easy to initiate in the inpatient setting because of its cost and availability and the fact that it is not as fast acting as other therapies.26 For patients with severe disease flares, prednisone,27 infliximab,28 or cyclosporine29 can be used in combination with antimicrobial therapy in the inpatient setting to quickly control active flaring. Intravenous antimicrobial therapy might be necessary in severe disease and should include coverage of gram-positive30 and anaerobic organisms.31
Although management of acute flares is critical, especially for hospitalized patients, initiating longitudinal treatment modalities while the patient is an inpatient will help prevent future readmissions, facilitate better outcomes, and enable longer periods of disease-free progression. Specific treatments, stratified by disease severity, are listed in the Table.
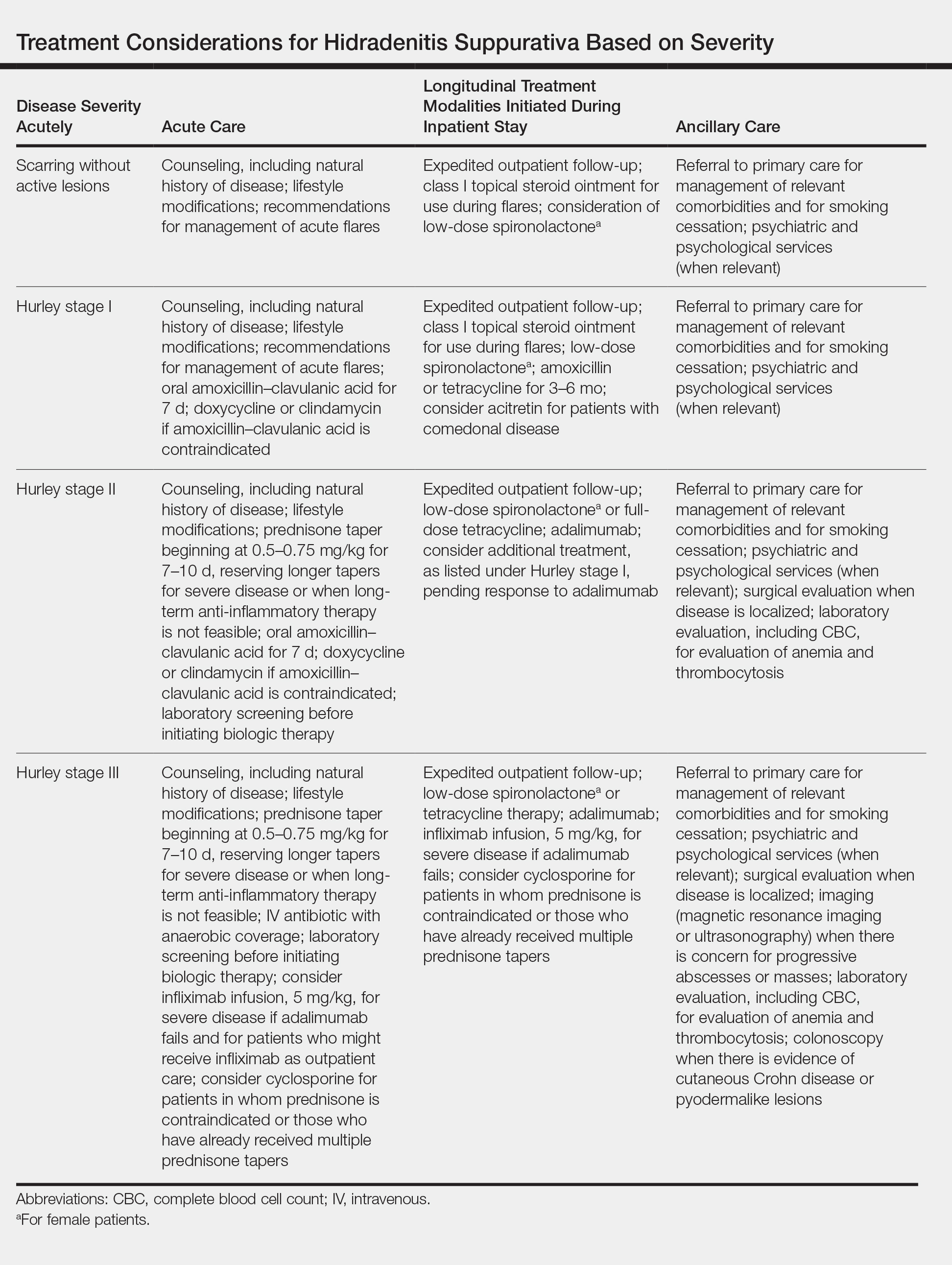
Postdischarge Lifestyle Modification
All disease management should include recommendations for lifestyle modification, including counseling on terminal hair removal (ie, avoid shaving, plucking, and waxing) and recommendations for daily and weekly decolonization with chlorhexidine or other antimicrobial soap, a weekly vinegar bath, and antiperspirant use in the groin and axilla. Avoiding tight clothes and humidity might also be helpful.
Other beneficial postdischarge strategies include smoking cessation and weight loss, which often are beneficial but difficult for many patients to achieve on their own; connecting patients with a primary care provider, which can facilitate better long-term outcomes; informing patients of the natural history of the disease and providing strategies for them to implement for acute flares to help avoid readmission and ED visits; and writing a “pill-in-pocket” prescription for a course of an antibiotic that provides good staphylococcal and anaerobic coverage, which can be helpful for patients who are prone to infrequent flares.
Lastly, appropriate postdischarge maintenance therapy also can be initiated during the inpatient stay, including maintenance antibiotic therapy, spironolactone32 for female patients, and acitretin33 for comedonal-predominant patients.
Final Thoughts
Hidradenitis suppurativa is a common dermatologic condition that frequently presents in emergency and inpatient settings, given its association with painful and acutely indurated lesions that often appear concerning for infection. Elevated inflammatory markers and fever are common in HS and are not necessarily suggestive of infection. As such, while antibiotics may be part of acute management of HS, care also should address the inflammatory component of the disease. Longitudinal outpatient care coordination with a dermatologist and primary care physician is imperative for limiting ED and inpatient care utilization.
- Jemec GB, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol. 1996;34:994-999.
- Prens E, Deckers I. Pathophysiology of hidradenitis suppurativa: an update. J Am Acad Dermatol. 2015;73(suppl 1):S8-S11.
- Barth JH, Kealey T. Androgen metabolism by isolated human axillary apocrine glands in hidradenitis suppurativa. Br J Dermatol. 1991;125:304-308.
- de Winter K, van der Zee HH, Prens EP. Is mechanical stress an important pathogenic factor in hidradenitis suppurativa? Exp Dermatol. 2012;21:176-177.
- Yu CC, Cook MG. Hidradenitis suppurativa: a disease of follicular epithelium, rather than apocrine glands. Br J Dermatol. 1990;122:763-769.
- Deckers IE, van der Zee HH, Prens EP. Epidemiology of hidradenitis suppurativa: prevalence, pathogenesis, and factors associated with the development of HS. Curr Dermatol Rep. 2014;3:54-60.
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: Results from two case-control studies. J Am Acad Dermatol. 2008;59:596-601.
- Jemec GE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(suppl 1):S4-S7.
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419.
- Khalsa A, Liu G, Kirby JS. Increased utilization of emergency department and inpatient care by patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73:609-614.
- Kirby JS, Miller JJ, Adams DR, et al. Health care utilization patterns and costs for patients with hidradenitis suppurativa. JAMA Dermatol. 2014;150:937-944.
- Deckers IE, Benhadou F, Koldijk MJ, et al. Inflammatory bowel disease is associated with hidradenitis suppurativa: results from a multicenter cross-sectional study. J Am Acad Dermatol. 2017;76:49-53.
- Benhadou F, Van der Zee HH, Pascual JC, et al. Pilonidal sinus disease: an intergluteal localization of hidradenitis suppurativa/acne inversa: a cross-sectional study among 2465 patients [published online March 27, 2019]. Br J Dermatol. doi:10.1111/bjd.17927.
- Garg A, Neuren E, Strunk A. Hidradenitis suppurativa is associated with polycystic ovary syndrome: a population-based analysis in the United States. J Invest Dermatol. 2018;138:1288-1292.
- Porter ML, Kimball AB. Comorbidities of hidradenitis suppurativa. Semin Cutan Med Surg. 2017;36:55-57.
- Hessam S, Sand M, Gambichler T, et al. Correlation of inflammatory serum markers with disease severity in patients with hidradenitis suppurativa (HS). J Am Acad Dermatol. 2015;73:998-1005.
- Ring HC, Bay L, Nilsson M, et al. Bacterial biofilm in chronic lesions of hidradenitis suppurativa. Br J Dermatol. 2017;176:993-1000.
- Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123.
- Wortsman X. Imaging of hidradenitis suppurativa. Dermatol Clin. 2016;34:59-68.
- Saunte DM, Boer J, Stratigos A, et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol. 2015;173:1546-1549.
- Revuz JE, Jemec GB. Diagnosing hidradenitis suppurativa. Dermatol Clin. 2016;34:1-5.
- Canoui-Poitrine F, Le Thuaut A, Revuz JE, et al. Identification of three hidradenitis suppurativa phenotypes: latent class analysis of a cross-sectional study. J Invest Dermatol. 2013;133:1506-1511.
- Porter ML, Kimball AB. Hidradenitis suppurativa scoring systems: can we choose just one? Cutis. 2017;99:156-157.
- Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH, Jr, eds. Dermatologic Surgery: Principles and Practice. New York, NY: Marcel Dekker, Inc; 1989:732-738.
- Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol. 2016;30:989-994.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Wong D, Walsh S, Alhusayen R. Low-dose systemic corticosteroid treatment for recalcitrant hidradenitis suppurativa. J Am Acad Dermatol. 2016;75:1059-1062.
- Sullivan TP, Welsh E, Kerdel FA. Infliximab for hidradenitis suppurativa. Br J Dermatol. 2003;149:1046-1049.
- Anderson MD, Zauli S, Bettoli V, et al. Cyclosporine treatment of severe hidradenitis suppurativa—a case series. J Dermatolog Treat. 2016;27:247-250.
- Ring HC, Riis Mikkelsen P, Miller IM, et al. The bacteriology of hidradenitis suppurativa: a systematic review. Exp Dermatol. 2015;24:727-731.
- Guet-Revillet H, Coignard-Biehler H, Jais JP, et al. Bacterial pathogens associated with hidradenitis suppurativa, France. Emerg Infect Dis. 2014;20:1990-1998.
- Golbari NM, Porter ML, Kimball AB. Antiandrogen therapy with spironolactone for the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2019;80:114-119.
- Matusiak L, Bieniek A, Szepietowski JC. Acitretin treatment for hidradenitis suppurativa: a prospective series of 17 patients. Br J Dermatol. 2014;171:170-174.
Hidradenitis suppurativa (HS) is a common chronic inflammatory skin disease characterized by purulent subcutaneous nodules, papules, abscesses, and fistula tracts that lead to scarring and fibrosis. Lesions develop primarily in the axilla, groin, and other intertriginous and hair-bearing areas.
The natural history of the disease is characterized by periods of disease flare, followed by periods of disease quiescence. Patients might have weeks or months of low disease activity but frequently develop multiple exacerbating episodes over the course of weeks or months. The condition primarily presents in adolescent and peripubescent years, continuing throughout adulthood. Some evidence suggests a bimodal disease distribution, with a second peak of incidence in middle-aged adults. Women and men are affected equally; however, the disease can be phenotypically different in men and women.
Patients frequently present in emergency and inpatient settings for evaluation because of the pain and severity of HS flares as well as associated systemic symptoms. Inpatient and emergency department (ED) care are unique opportunities for dermatologic hospitalist and dermatologic consultative services to educate other physicians about the condition and initiate aggressive treatments that are frequently necessary to control HS flares. This article aims to address best methods for treating HS in these settings.
Pathophysiology
Although the exact pathophysiology of the condition is unknown, HS is thought to begin with follicular occlusion with downstream inflammation mediating neutrophilic activity and scarring. Hyperplasia of the infundibular epithelium is observed on histology, and the resulting occlusion, contained keratin, and follicular rupture initiate robust downstream inflammation.1,2 Follicular occlusion might be initially androgen mediated3 or might occur in combination with friction4 and genetic or acquired factors involving Notch signaling. Although HS characteristically presents in areas of high apocrine density, apocrine glands are not thought to be the primary mediator of disease activity.5 IL-17, IL-23, tumor necrosis factor α, and IL-1β are implicated in the pathogenesis of HS, but it is unknown if these cytokines are the driving pathologic factor in HS or if they are merely secondary sequelae.6
Demographics and Prevalence in Hospitalized Patients
Although increasing treatment availability has brought more attention to HS, true prevalence is unknown. A prevalence of 1% has been reported in many European countries.7 Global prevalence has been more difficult to determine, with variable data suggesting a prevalence of 0.03% to 8%, depending on the population included.8 Most patients studied in a US-based claims database were aged 30 to 64 years, and the overall prevalence was 0.05%.9 Despite prevalence similar to psoriasis, utilization of high-cost emergency and inpatient admissions is notably higher among patients with HS. Recent claims data suggest that HS patients utilize the ED at a rate 3 times higher than psoriasis patients and are admitted as inpatients at a rate 5 times higher.10 Similar data suggest an associated increased cost of care for patients with HS vs other conditions, such as psoriasis, due to frequent ED and inpatient stays.11 Although HS frequently presents in the inpatient and emergency settings, there is little literature on best methods for managing patients in these settings.
Pearls for Inpatient and Emergency Evaluation and Management
Initial Evaluation
When dermatologic consultative services are asked to evaluate patients with HS, preliminary evaluation should reflect the acuity of the patient. Vital signs and toxicity should be reviewed to ensure that there is no evidence of severe infection necessitating critical or acute care.
History
History-taking should reflect assessment of the patient’s baseline disease, including date of initial onset; exacerbating factors, such as friction, smoking, pregnancy, and menses; and the current history of the patient’s flare. A history of antibiotics, immunosuppression, topical therapy, antiandrogen therapy, and vitamin A analog therapy also should be reviewed. If an initial diagnosis is made in the ED or inpatient setting, a family and personal history should focus on specific risk factors and disease associations, including inflammatory bowel disease,12 pilonidal cysts,13 polycystic ovary syndrome,14 and metabolic syndrome.15
Physical Examination
As with all dermatologic consultations, a full-body skin examination, with special attention to the axilla, inframammary skin, groin, buttocks, and perineum, should be undertaken. In addition to these common areas of disease progression, examination should focus on atypical sites for disease manifestation, including the posterior auricular scalp, skin folds in the pannus and back, and the beard area in men. Evaluation of axillary and gluteal hair should note features of folliculitis and hair removal, which can exacerbate HS. Examination also should include an investigation of cutaneous manifestations of comorbid conditions, including acanthosis nigricans, contiguous or metastatic cutaneous Crohn disease, erythema nodosum, and pilonidal cysts. Caution should be exercised when diagnosing pilonidal cysts, as isolated or evolving HS in the gluteal cleft often is misdiagnosed as a pilonidal cyst.
Laboratory Evaluation
Testing often is misleading in patients with HS, especially in the acute setting, because the condition is a chronic inflammatory process. The C-reactive protein level as well as the absolute white blood cell and neutrophil counts often are elevated, even in the absence of acute infection.16 In fact, although patients often are treated with intravenous antibiotics by inpatient and emergency teams in the setting of these 3 laboratory abnormalities, these findings often reflect disease activity, not frank infection. Fever, especially low-grade fever, also can reflect ongoing disease activity. Thrombocytosis and anemia also are anecdotally common, though these findings have not been reported specifically in the literature.
Bacterial Cultures
The role of lesional and perilesional bacterial cultures is controversial in HS. Prior studies have demonstrated that biofilm formation may be associated with the chronic inflammation seen in HS.17 However, most data to date suggest that infection is not the primary driver of HS disease flares, as demonstrated by the frequency of sterile cultures and the variable response of the disease to penicillin and related antibiotics.18
Imaging
Ultrasonography and magnetic resonance imaging can be conducted if there is concern about deeper abscesses that are not apparent on examination. When interpreted by nondermatologic practitioners, however, the findings of these modalities can result in unnecessary surgical intervention, given the concern for development of infectious abscess.19
Diagnosis
Many patients with HS experience a notable delay in time to diagnosis, living with symptoms for 7 years on average prior to being given a name for their condition.20 Often, patients seek ED care at initial presentation because lesions can present quickly and are associated with remarkable pain. Inpatient dermatologic evaluation can provide patients with definitive diagnosis, appropriate counseling that provides an overview of the natural history of the disease, lifestyle recommendations, and expedited connection to outpatient longitudinal care.
Diagnosis is made clinically by assessment for typical lesions, such as painful or tender papules, nodules, or abscesses in the axillae, inframammary region, groin, thighs, and perineal and perianal regions. Cordlike scarring often is seen in the absence of active inflammatory lesions.21 Double-headed open comedones and prominent follicular occlusion are seen in some phenotypes but are not required for diagnosis.22
Multiple scoring modalities are in use23; the Hurley staging system, initially developed for surgical staging, has become a commonly used method in the clinical setting24:
• Hurley stage I: isolated nodules or abscesses;
• Hurley stage II: widely separate lesions and sinus tracts or scarring are suggestive; and
• Hurley stage III: multiple lesions with near-diffuse involvement and formation of sinus tracts and scarring.
Other scoring modalities, such as the Hidradenitis Suppurativa Clinical Response (HiSCR), are more commonly used in the clinical trial setting and quantitatively capture lesion count improvement while the patient is being treated.25
Treatment
Evaluation in the ED might necessitate recommendations for inpatient admission. Dermatologic consultation can be helpful in providing ED physicians with context for interpretation of laboratory results and clinical findings. Specifically, dermatologic evaluation can help differentiate presentations consistent with a primary infection from a more common presentation of HS flaring and associated bacterial colonization. Indications for inpatient admission are severe pain; concern for systemic infection, including high fever or sepsis; and need for surgical intervention. Patients with severe disease who do not have a longitudinal care plan or who lack the ability to care for lesions at home also are candidates for inpatient admission, where they can receive more intensive nursing and wound care as well as outpatient logistical management.
Acute care should be aimed at treatments that work quickly and aggressively and have both anti-inflammatory and antimicrobial effects. Severe flares require aggressive initial treatment to ensure more long-term remission. Adalimumab, maintained at 40 mg/wk after a loading dose, is the mainstay of evidence-based treatment for moderate to severe HS in patients 12 years or older; however, this treatment might not be easy to initiate in the inpatient setting because of its cost and availability and the fact that it is not as fast acting as other therapies.26 For patients with severe disease flares, prednisone,27 infliximab,28 or cyclosporine29 can be used in combination with antimicrobial therapy in the inpatient setting to quickly control active flaring. Intravenous antimicrobial therapy might be necessary in severe disease and should include coverage of gram-positive30 and anaerobic organisms.31
Although management of acute flares is critical, especially for hospitalized patients, initiating longitudinal treatment modalities while the patient is an inpatient will help prevent future readmissions, facilitate better outcomes, and enable longer periods of disease-free progression. Specific treatments, stratified by disease severity, are listed in the Table.

Postdischarge Lifestyle Modification
All disease management should include recommendations for lifestyle modification, including counseling on terminal hair removal (ie, avoid shaving, plucking, and waxing) and recommendations for daily and weekly decolonization with chlorhexidine or other antimicrobial soap, a weekly vinegar bath, and antiperspirant use in the groin and axilla. Avoiding tight clothes and humidity might also be helpful.
Other beneficial postdischarge strategies include smoking cessation and weight loss, which often are beneficial but difficult for many patients to achieve on their own; connecting patients with a primary care provider, which can facilitate better long-term outcomes; informing patients of the natural history of the disease and providing strategies for them to implement for acute flares to help avoid readmission and ED visits; and writing a “pill-in-pocket” prescription for a course of an antibiotic that provides good staphylococcal and anaerobic coverage, which can be helpful for patients who are prone to infrequent flares.
Lastly, appropriate postdischarge maintenance therapy also can be initiated during the inpatient stay, including maintenance antibiotic therapy, spironolactone32 for female patients, and acitretin33 for comedonal-predominant patients.
Final Thoughts
Hidradenitis suppurativa is a common dermatologic condition that frequently presents in emergency and inpatient settings, given its association with painful and acutely indurated lesions that often appear concerning for infection. Elevated inflammatory markers and fever are common in HS and are not necessarily suggestive of infection. As such, while antibiotics may be part of acute management of HS, care also should address the inflammatory component of the disease. Longitudinal outpatient care coordination with a dermatologist and primary care physician is imperative for limiting ED and inpatient care utilization.
Hidradenitis suppurativa (HS) is a common chronic inflammatory skin disease characterized by purulent subcutaneous nodules, papules, abscesses, and fistula tracts that lead to scarring and fibrosis. Lesions develop primarily in the axilla, groin, and other intertriginous and hair-bearing areas.
The natural history of the disease is characterized by periods of disease flare, followed by periods of disease quiescence. Patients might have weeks or months of low disease activity but frequently develop multiple exacerbating episodes over the course of weeks or months. The condition primarily presents in adolescent and peripubescent years, continuing throughout adulthood. Some evidence suggests a bimodal disease distribution, with a second peak of incidence in middle-aged adults. Women and men are affected equally; however, the disease can be phenotypically different in men and women.
Patients frequently present in emergency and inpatient settings for evaluation because of the pain and severity of HS flares as well as associated systemic symptoms. Inpatient and emergency department (ED) care are unique opportunities for dermatologic hospitalist and dermatologic consultative services to educate other physicians about the condition and initiate aggressive treatments that are frequently necessary to control HS flares. This article aims to address best methods for treating HS in these settings.
Pathophysiology
Although the exact pathophysiology of the condition is unknown, HS is thought to begin with follicular occlusion with downstream inflammation mediating neutrophilic activity and scarring. Hyperplasia of the infundibular epithelium is observed on histology, and the resulting occlusion, contained keratin, and follicular rupture initiate robust downstream inflammation.1,2 Follicular occlusion might be initially androgen mediated3 or might occur in combination with friction4 and genetic or acquired factors involving Notch signaling. Although HS characteristically presents in areas of high apocrine density, apocrine glands are not thought to be the primary mediator of disease activity.5 IL-17, IL-23, tumor necrosis factor α, and IL-1β are implicated in the pathogenesis of HS, but it is unknown if these cytokines are the driving pathologic factor in HS or if they are merely secondary sequelae.6
Demographics and Prevalence in Hospitalized Patients
Although increasing treatment availability has brought more attention to HS, true prevalence is unknown. A prevalence of 1% has been reported in many European countries.7 Global prevalence has been more difficult to determine, with variable data suggesting a prevalence of 0.03% to 8%, depending on the population included.8 Most patients studied in a US-based claims database were aged 30 to 64 years, and the overall prevalence was 0.05%.9 Despite prevalence similar to psoriasis, utilization of high-cost emergency and inpatient admissions is notably higher among patients with HS. Recent claims data suggest that HS patients utilize the ED at a rate 3 times higher than psoriasis patients and are admitted as inpatients at a rate 5 times higher.10 Similar data suggest an associated increased cost of care for patients with HS vs other conditions, such as psoriasis, due to frequent ED and inpatient stays.11 Although HS frequently presents in the inpatient and emergency settings, there is little literature on best methods for managing patients in these settings.
Pearls for Inpatient and Emergency Evaluation and Management
Initial Evaluation
When dermatologic consultative services are asked to evaluate patients with HS, preliminary evaluation should reflect the acuity of the patient. Vital signs and toxicity should be reviewed to ensure that there is no evidence of severe infection necessitating critical or acute care.
History
History-taking should reflect assessment of the patient’s baseline disease, including date of initial onset; exacerbating factors, such as friction, smoking, pregnancy, and menses; and the current history of the patient’s flare. A history of antibiotics, immunosuppression, topical therapy, antiandrogen therapy, and vitamin A analog therapy also should be reviewed. If an initial diagnosis is made in the ED or inpatient setting, a family and personal history should focus on specific risk factors and disease associations, including inflammatory bowel disease,12 pilonidal cysts,13 polycystic ovary syndrome,14 and metabolic syndrome.15
Physical Examination
As with all dermatologic consultations, a full-body skin examination, with special attention to the axilla, inframammary skin, groin, buttocks, and perineum, should be undertaken. In addition to these common areas of disease progression, examination should focus on atypical sites for disease manifestation, including the posterior auricular scalp, skin folds in the pannus and back, and the beard area in men. Evaluation of axillary and gluteal hair should note features of folliculitis and hair removal, which can exacerbate HS. Examination also should include an investigation of cutaneous manifestations of comorbid conditions, including acanthosis nigricans, contiguous or metastatic cutaneous Crohn disease, erythema nodosum, and pilonidal cysts. Caution should be exercised when diagnosing pilonidal cysts, as isolated or evolving HS in the gluteal cleft often is misdiagnosed as a pilonidal cyst.
Laboratory Evaluation
Testing often is misleading in patients with HS, especially in the acute setting, because the condition is a chronic inflammatory process. The C-reactive protein level as well as the absolute white blood cell and neutrophil counts often are elevated, even in the absence of acute infection.16 In fact, although patients often are treated with intravenous antibiotics by inpatient and emergency teams in the setting of these 3 laboratory abnormalities, these findings often reflect disease activity, not frank infection. Fever, especially low-grade fever, also can reflect ongoing disease activity. Thrombocytosis and anemia also are anecdotally common, though these findings have not been reported specifically in the literature.
Bacterial Cultures
The role of lesional and perilesional bacterial cultures is controversial in HS. Prior studies have demonstrated that biofilm formation may be associated with the chronic inflammation seen in HS.17 However, most data to date suggest that infection is not the primary driver of HS disease flares, as demonstrated by the frequency of sterile cultures and the variable response of the disease to penicillin and related antibiotics.18
Imaging
Ultrasonography and magnetic resonance imaging can be conducted if there is concern about deeper abscesses that are not apparent on examination. When interpreted by nondermatologic practitioners, however, the findings of these modalities can result in unnecessary surgical intervention, given the concern for development of infectious abscess.19
Diagnosis
Many patients with HS experience a notable delay in time to diagnosis, living with symptoms for 7 years on average prior to being given a name for their condition.20 Often, patients seek ED care at initial presentation because lesions can present quickly and are associated with remarkable pain. Inpatient dermatologic evaluation can provide patients with definitive diagnosis, appropriate counseling that provides an overview of the natural history of the disease, lifestyle recommendations, and expedited connection to outpatient longitudinal care.
Diagnosis is made clinically by assessment for typical lesions, such as painful or tender papules, nodules, or abscesses in the axillae, inframammary region, groin, thighs, and perineal and perianal regions. Cordlike scarring often is seen in the absence of active inflammatory lesions.21 Double-headed open comedones and prominent follicular occlusion are seen in some phenotypes but are not required for diagnosis.22
Multiple scoring modalities are in use23; the Hurley staging system, initially developed for surgical staging, has become a commonly used method in the clinical setting24:
• Hurley stage I: isolated nodules or abscesses;
• Hurley stage II: widely separate lesions and sinus tracts or scarring are suggestive; and
• Hurley stage III: multiple lesions with near-diffuse involvement and formation of sinus tracts and scarring.
Other scoring modalities, such as the Hidradenitis Suppurativa Clinical Response (HiSCR), are more commonly used in the clinical trial setting and quantitatively capture lesion count improvement while the patient is being treated.25
Treatment
Evaluation in the ED might necessitate recommendations for inpatient admission. Dermatologic consultation can be helpful in providing ED physicians with context for interpretation of laboratory results and clinical findings. Specifically, dermatologic evaluation can help differentiate presentations consistent with a primary infection from a more common presentation of HS flaring and associated bacterial colonization. Indications for inpatient admission are severe pain; concern for systemic infection, including high fever or sepsis; and need for surgical intervention. Patients with severe disease who do not have a longitudinal care plan or who lack the ability to care for lesions at home also are candidates for inpatient admission, where they can receive more intensive nursing and wound care as well as outpatient logistical management.
Acute care should be aimed at treatments that work quickly and aggressively and have both anti-inflammatory and antimicrobial effects. Severe flares require aggressive initial treatment to ensure more long-term remission. Adalimumab, maintained at 40 mg/wk after a loading dose, is the mainstay of evidence-based treatment for moderate to severe HS in patients 12 years or older; however, this treatment might not be easy to initiate in the inpatient setting because of its cost and availability and the fact that it is not as fast acting as other therapies.26 For patients with severe disease flares, prednisone,27 infliximab,28 or cyclosporine29 can be used in combination with antimicrobial therapy in the inpatient setting to quickly control active flaring. Intravenous antimicrobial therapy might be necessary in severe disease and should include coverage of gram-positive30 and anaerobic organisms.31
Although management of acute flares is critical, especially for hospitalized patients, initiating longitudinal treatment modalities while the patient is an inpatient will help prevent future readmissions, facilitate better outcomes, and enable longer periods of disease-free progression. Specific treatments, stratified by disease severity, are listed in the Table.

Postdischarge Lifestyle Modification
All disease management should include recommendations for lifestyle modification, including counseling on terminal hair removal (ie, avoid shaving, plucking, and waxing) and recommendations for daily and weekly decolonization with chlorhexidine or other antimicrobial soap, a weekly vinegar bath, and antiperspirant use in the groin and axilla. Avoiding tight clothes and humidity might also be helpful.
Other beneficial postdischarge strategies include smoking cessation and weight loss, which often are beneficial but difficult for many patients to achieve on their own; connecting patients with a primary care provider, which can facilitate better long-term outcomes; informing patients of the natural history of the disease and providing strategies for them to implement for acute flares to help avoid readmission and ED visits; and writing a “pill-in-pocket” prescription for a course of an antibiotic that provides good staphylococcal and anaerobic coverage, which can be helpful for patients who are prone to infrequent flares.
Lastly, appropriate postdischarge maintenance therapy also can be initiated during the inpatient stay, including maintenance antibiotic therapy, spironolactone32 for female patients, and acitretin33 for comedonal-predominant patients.
Final Thoughts
Hidradenitis suppurativa is a common dermatologic condition that frequently presents in emergency and inpatient settings, given its association with painful and acutely indurated lesions that often appear concerning for infection. Elevated inflammatory markers and fever are common in HS and are not necessarily suggestive of infection. As such, while antibiotics may be part of acute management of HS, care also should address the inflammatory component of the disease. Longitudinal outpatient care coordination with a dermatologist and primary care physician is imperative for limiting ED and inpatient care utilization.
- Jemec GB, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol. 1996;34:994-999.
- Prens E, Deckers I. Pathophysiology of hidradenitis suppurativa: an update. J Am Acad Dermatol. 2015;73(suppl 1):S8-S11.
- Barth JH, Kealey T. Androgen metabolism by isolated human axillary apocrine glands in hidradenitis suppurativa. Br J Dermatol. 1991;125:304-308.
- de Winter K, van der Zee HH, Prens EP. Is mechanical stress an important pathogenic factor in hidradenitis suppurativa? Exp Dermatol. 2012;21:176-177.
- Yu CC, Cook MG. Hidradenitis suppurativa: a disease of follicular epithelium, rather than apocrine glands. Br J Dermatol. 1990;122:763-769.
- Deckers IE, van der Zee HH, Prens EP. Epidemiology of hidradenitis suppurativa: prevalence, pathogenesis, and factors associated with the development of HS. Curr Dermatol Rep. 2014;3:54-60.
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: Results from two case-control studies. J Am Acad Dermatol. 2008;59:596-601.
- Jemec GE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(suppl 1):S4-S7.
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419.
- Khalsa A, Liu G, Kirby JS. Increased utilization of emergency department and inpatient care by patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73:609-614.
- Kirby JS, Miller JJ, Adams DR, et al. Health care utilization patterns and costs for patients with hidradenitis suppurativa. JAMA Dermatol. 2014;150:937-944.
- Deckers IE, Benhadou F, Koldijk MJ, et al. Inflammatory bowel disease is associated with hidradenitis suppurativa: results from a multicenter cross-sectional study. J Am Acad Dermatol. 2017;76:49-53.
- Benhadou F, Van der Zee HH, Pascual JC, et al. Pilonidal sinus disease: an intergluteal localization of hidradenitis suppurativa/acne inversa: a cross-sectional study among 2465 patients [published online March 27, 2019]. Br J Dermatol. doi:10.1111/bjd.17927.
- Garg A, Neuren E, Strunk A. Hidradenitis suppurativa is associated with polycystic ovary syndrome: a population-based analysis in the United States. J Invest Dermatol. 2018;138:1288-1292.
- Porter ML, Kimball AB. Comorbidities of hidradenitis suppurativa. Semin Cutan Med Surg. 2017;36:55-57.
- Hessam S, Sand M, Gambichler T, et al. Correlation of inflammatory serum markers with disease severity in patients with hidradenitis suppurativa (HS). J Am Acad Dermatol. 2015;73:998-1005.
- Ring HC, Bay L, Nilsson M, et al. Bacterial biofilm in chronic lesions of hidradenitis suppurativa. Br J Dermatol. 2017;176:993-1000.
- Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123.
- Wortsman X. Imaging of hidradenitis suppurativa. Dermatol Clin. 2016;34:59-68.
- Saunte DM, Boer J, Stratigos A, et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol. 2015;173:1546-1549.
- Revuz JE, Jemec GB. Diagnosing hidradenitis suppurativa. Dermatol Clin. 2016;34:1-5.
- Canoui-Poitrine F, Le Thuaut A, Revuz JE, et al. Identification of three hidradenitis suppurativa phenotypes: latent class analysis of a cross-sectional study. J Invest Dermatol. 2013;133:1506-1511.
- Porter ML, Kimball AB. Hidradenitis suppurativa scoring systems: can we choose just one? Cutis. 2017;99:156-157.
- Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH, Jr, eds. Dermatologic Surgery: Principles and Practice. New York, NY: Marcel Dekker, Inc; 1989:732-738.
- Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol. 2016;30:989-994.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Wong D, Walsh S, Alhusayen R. Low-dose systemic corticosteroid treatment for recalcitrant hidradenitis suppurativa. J Am Acad Dermatol. 2016;75:1059-1062.
- Sullivan TP, Welsh E, Kerdel FA. Infliximab for hidradenitis suppurativa. Br J Dermatol. 2003;149:1046-1049.
- Anderson MD, Zauli S, Bettoli V, et al. Cyclosporine treatment of severe hidradenitis suppurativa—a case series. J Dermatolog Treat. 2016;27:247-250.
- Ring HC, Riis Mikkelsen P, Miller IM, et al. The bacteriology of hidradenitis suppurativa: a systematic review. Exp Dermatol. 2015;24:727-731.
- Guet-Revillet H, Coignard-Biehler H, Jais JP, et al. Bacterial pathogens associated with hidradenitis suppurativa, France. Emerg Infect Dis. 2014;20:1990-1998.
- Golbari NM, Porter ML, Kimball AB. Antiandrogen therapy with spironolactone for the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2019;80:114-119.
- Matusiak L, Bieniek A, Szepietowski JC. Acitretin treatment for hidradenitis suppurativa: a prospective series of 17 patients. Br J Dermatol. 2014;171:170-174.
- Jemec GB, Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol. 1996;34:994-999.
- Prens E, Deckers I. Pathophysiology of hidradenitis suppurativa: an update. J Am Acad Dermatol. 2015;73(suppl 1):S8-S11.
- Barth JH, Kealey T. Androgen metabolism by isolated human axillary apocrine glands in hidradenitis suppurativa. Br J Dermatol. 1991;125:304-308.
- de Winter K, van der Zee HH, Prens EP. Is mechanical stress an important pathogenic factor in hidradenitis suppurativa? Exp Dermatol. 2012;21:176-177.
- Yu CC, Cook MG. Hidradenitis suppurativa: a disease of follicular epithelium, rather than apocrine glands. Br J Dermatol. 1990;122:763-769.
- Deckers IE, van der Zee HH, Prens EP. Epidemiology of hidradenitis suppurativa: prevalence, pathogenesis, and factors associated with the development of HS. Curr Dermatol Rep. 2014;3:54-60.
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: Results from two case-control studies. J Am Acad Dermatol. 2008;59:596-601.
- Jemec GE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(suppl 1):S4-S7.
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419.
- Khalsa A, Liu G, Kirby JS. Increased utilization of emergency department and inpatient care by patients with hidradenitis suppurativa. J Am Acad Dermatol. 2015;73:609-614.
- Kirby JS, Miller JJ, Adams DR, et al. Health care utilization patterns and costs for patients with hidradenitis suppurativa. JAMA Dermatol. 2014;150:937-944.
- Deckers IE, Benhadou F, Koldijk MJ, et al. Inflammatory bowel disease is associated with hidradenitis suppurativa: results from a multicenter cross-sectional study. J Am Acad Dermatol. 2017;76:49-53.
- Benhadou F, Van der Zee HH, Pascual JC, et al. Pilonidal sinus disease: an intergluteal localization of hidradenitis suppurativa/acne inversa: a cross-sectional study among 2465 patients [published online March 27, 2019]. Br J Dermatol. doi:10.1111/bjd.17927.
- Garg A, Neuren E, Strunk A. Hidradenitis suppurativa is associated with polycystic ovary syndrome: a population-based analysis in the United States. J Invest Dermatol. 2018;138:1288-1292.
- Porter ML, Kimball AB. Comorbidities of hidradenitis suppurativa. Semin Cutan Med Surg. 2017;36:55-57.
- Hessam S, Sand M, Gambichler T, et al. Correlation of inflammatory serum markers with disease severity in patients with hidradenitis suppurativa (HS). J Am Acad Dermatol. 2015;73:998-1005.
- Ring HC, Bay L, Nilsson M, et al. Bacterial biofilm in chronic lesions of hidradenitis suppurativa. Br J Dermatol. 2017;176:993-1000.
- Yazdanyar S, Jemec GB. Hidradenitis suppurativa: a review of cause and treatment. Curr Opin Infect Dis. 2011;24:118-123.
- Wortsman X. Imaging of hidradenitis suppurativa. Dermatol Clin. 2016;34:59-68.
- Saunte DM, Boer J, Stratigos A, et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol. 2015;173:1546-1549.
- Revuz JE, Jemec GB. Diagnosing hidradenitis suppurativa. Dermatol Clin. 2016;34:1-5.
- Canoui-Poitrine F, Le Thuaut A, Revuz JE, et al. Identification of three hidradenitis suppurativa phenotypes: latent class analysis of a cross-sectional study. J Invest Dermatol. 2013;133:1506-1511.
- Porter ML, Kimball AB. Hidradenitis suppurativa scoring systems: can we choose just one? Cutis. 2017;99:156-157.
- Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH, Jr, eds. Dermatologic Surgery: Principles and Practice. New York, NY: Marcel Dekker, Inc; 1989:732-738.
- Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (Hidradenitis Suppurativa Clinical Response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study. J Eur Acad Dermatol Venereol. 2016;30:989-994.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434.
- Wong D, Walsh S, Alhusayen R. Low-dose systemic corticosteroid treatment for recalcitrant hidradenitis suppurativa. J Am Acad Dermatol. 2016;75:1059-1062.
- Sullivan TP, Welsh E, Kerdel FA. Infliximab for hidradenitis suppurativa. Br J Dermatol. 2003;149:1046-1049.
- Anderson MD, Zauli S, Bettoli V, et al. Cyclosporine treatment of severe hidradenitis suppurativa—a case series. J Dermatolog Treat. 2016;27:247-250.
- Ring HC, Riis Mikkelsen P, Miller IM, et al. The bacteriology of hidradenitis suppurativa: a systematic review. Exp Dermatol. 2015;24:727-731.
- Guet-Revillet H, Coignard-Biehler H, Jais JP, et al. Bacterial pathogens associated with hidradenitis suppurativa, France. Emerg Infect Dis. 2014;20:1990-1998.
- Golbari NM, Porter ML, Kimball AB. Antiandrogen therapy with spironolactone for the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 2019;80:114-119.
- Matusiak L, Bieniek A, Szepietowski JC. Acitretin treatment for hidradenitis suppurativa: a prospective series of 17 patients. Br J Dermatol. 2014;171:170-174.
Practice Points
- Hidradenitis suppurativa (HS) is a common dermatologic condition that frequently presents in emergency and inpatient settings.
- Anemia, leukocytosis, neutrophilia, an elevated erythrocyte sedimentation rate, and an elevated C-reactive protein level are common markers of chronic inflammation in HS patients and might not signify infection.
- Acute management of HS should focus on anti-inflammatory and antibiotic regimens, with increasing severity dictating the need for more aggressive therapy.
- Longitudinal outpatient care coordination with a dermatologist and primary care physician is imperative for limiting emergency department and inpatient care utilization.
Current Guidelines for Psoriasis Treatment: A Work in Progress
Psoriasis is a chronic autoinflammatory disorder affecting approximately 2% to 4% of the Western population.1 While there is no absolute cure for psoriasis, novel therapies allow for substantial reduction in symptoms and considerable improvement in quality of life (QoL). In the past few years, multiple treatment guidelines (recommendations based on evidence-based literature reviews) and consensus statements (a set of declarations determined and voted on by a panel of experts in the field) have been developed to guide physicians worldwide in treating psoriasis in the clinical setting (eTable).2-10
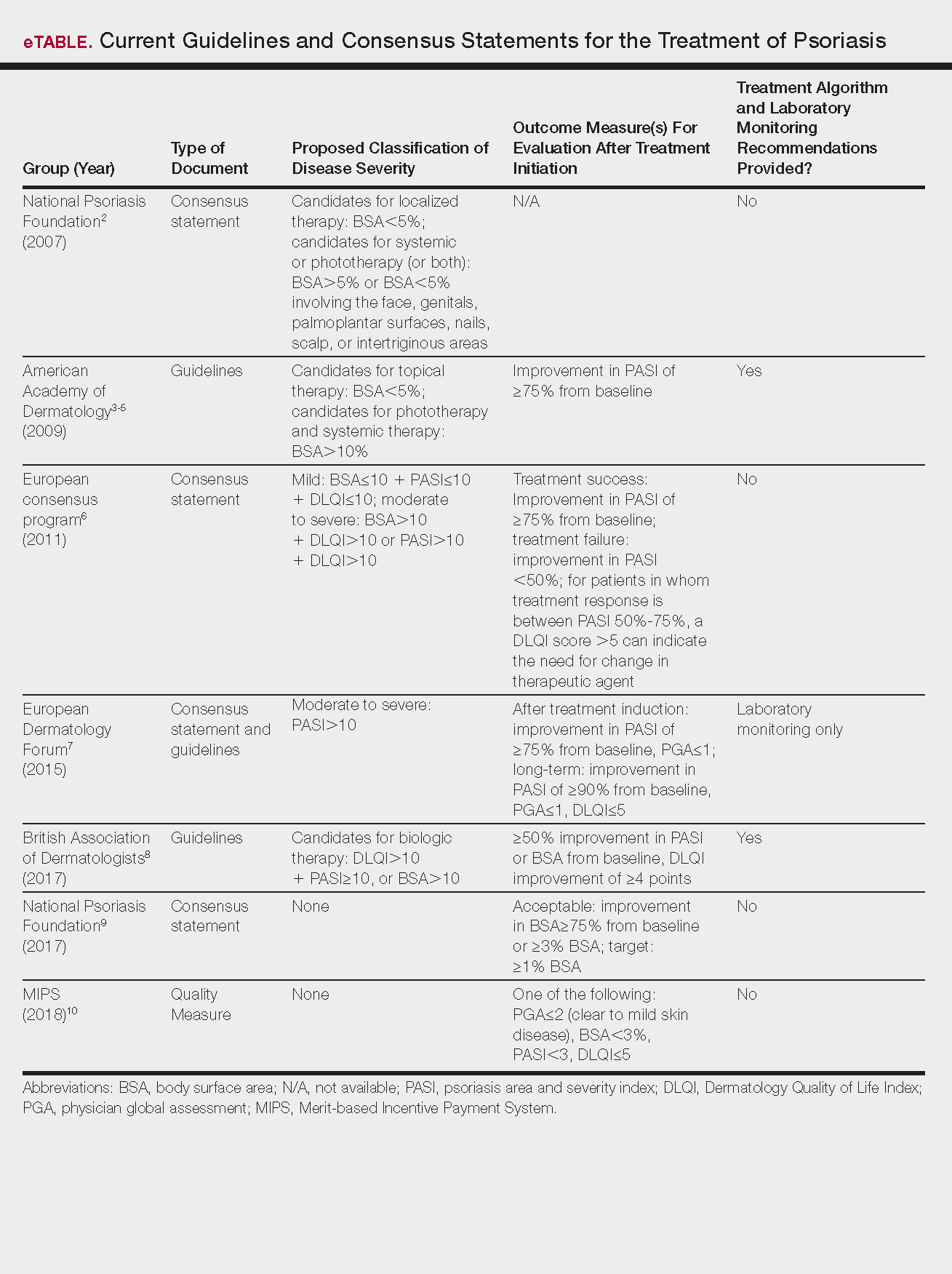
Because psoriasis is a complex disease with multiple comorbidities, applicability of these guidelines may be limited. Although some basic treatment algorithms exist, patient preference, disease severity, and other variables including comorbidities (eg, psoriatic arthritis [PsA], risk of major cardiac events, inflammatory bowel disease [IBD]), history of nonmelanoma skin cancer (NMSC), pregnancy and lactation, and specific contraindications to therapy (eg, renal failure, liver disease, active malignancy) should be considered. In this article, we summarize common themes across existing guidelines and consensus statements for the treatment of psoriasis and highlight areas where there is consistent agreement or lack of sufficient information.
Disease Severity and Treatment Outcomes
There currently are no consensus definitions for mild, moderate, and severe psoriasis, but several consensus statements have attempted to standardize grading systems based on objective values, such as body surface area (BSA) and psoriasis area and severity index (PASI)(a scoring system used to grade the degree of redness, thickness, and scaling of psoriasis plaques), as well as subjective QoL measures.2,6 Although classification of disease severity varies, mild psoriasis generally is characterized as disease that can be managed with local and topical therapy, and moderate to severe psoriasis typically warrants consideration for escalated treatment with phototherapy or systemic agents.
Most definitions of disease severity in psoriasis reference 5% to 10% BSA involvement as a cutoff that should trigger consideration of systemic treatment; however, these criteria could result in undertreatment of patients with substantial disease. For example, patients who have limited BSA involvement but whose disease has a considerable impact on QoL, as well as those who have debilitating disease in localized areas (eg, palms, soles, scalp, nails) or substantial joint involvement may also be appropriate candidates for systemic treatment.5,8
Once therapy is initiated, patients should be evaluated for appropriate treatment response at dedicated intervals. While the time to maximum therapeutic benefit depends on the agent of choice, European guidelines recommend that patients be evaluated after an induction phase (typically 16–24 weeks) and define treatment success as either (1) at least 75% improvement in PASI or (2) at least 50% improvement in PASI and a Dermatology Quality of Life Index (DLQI) score of 5 or lower.6
Alternatively, the National Psoriasis Foundation (NPF) recommended BSA as the preferred outcome measure in a recent consensus statement and concluded that an outcome of 3% or less BSA involvement or improvement in BSA of 75% or more is considered a desirable treatment response.9 Additionally, the Medicare Merit-based Incentive Payment System (MIPS) guidelines for successful systemic treatment response include at least 1 of the following: (1) physician global assessment score of 2 or lower, (2) BSA involvement of less than 3%, (3) PASI score lower than 3, or (4) DLQI score of 5 or lower.10
Although an array of outcome measures have been utilized in clinical trials and proposed in psoriasis guidelines and consensus statements, BSA is typically a manageable measure of treatment response in a clinical setting; however, DLQI should also be assessed if possible, particularly in patients with debilitating localized disease.9
Treatment Options
Because topical treatment regimens can be arduous and typically do not result in sustained clearance, patient expectations should be ascertained prior to initiation of therapy. Topical corticosteroids often can be used as monotherapy in patients with mild psoriasis.3 Topical vitamin D analogues and retinoids also can be effective; however, combined use of these agents with topical steroids should be considered to increase efficacy, and combination formulations can be prescribed to simplify application and improve adherence.
Treatment with UVB or psoralen plus UVA phototherapy is recommended for patients with moderate to severe psoriasis as well as in those who have had minimal response to topical therapy.4 Targeted phototherapy with an excimer laser can be used in patients with BSA involvement of less than 10%.
Methotrexate (MTX), cyclosporine, and acitretin are the most commonly prescribed systemic medications for severe psoriasis in the United States.5 Despite the risk for hepatotoxicity, MTX appears to have the best combined safety and efficacy profile in terms of serious adverse events compared to other systemic agents.11 Guidelines for MTX monitoring, especially with regard to when to do a liver biopsy, have been substantially liberalized over time, and the recommended interval for biopsy has been extended by years; biopsy was previously recommended after a cumulative MTX dose of 1 to 1.5 g, but guidelines now suggest biopsy after 3.5 to 4 g in low-risk patients.5 While abnormally elevated liver function tests during treatment with MTX may necessitate liver biopsy, the use of transient elastography and a panel of serum biomarkers for liver function also can be used to monitor noninvasively for hepatotoxicity before biopsy is considered; these recommendations are likely to be incorporated into newer guidelines in development.12 Methotrexate has demonstrated safety and increased efficacy when used in combination with biologic agents such as adalimumab, etanercept, infliximab, and secukinumab7 and has been studied in combination with many biologics indicated for PsA.13
Due to a considerable risk of glomerulosclerosis, cyclosporine is approved for a maximum of 1 year of continuous treatment of psoriasis in the United States and2 years in Europe.5,7 Cyclosporine is best used as induction therapy in psoriasis patients with severe disease who are seeking faster abatement of symptoms.
Acitretin is another systemic treatment option, although efficacy of this agent is dose dependent. Higher dosing often is limited due to lower tolerability.5
Given that many insurance formularies primarily cover traditional systemic therapies and that MTX and phototherapy are generally well tolerated and cost effective, patients may need to be treated with traditional agents before escalating to biologics. Prior to starting treatment with any biologic, patients should typically be screened for tuberculosis (TB), human immunodeficiency virus infection, and immunization for, exposure to, and/or infection with hepatitis B and C virus, and any other active infections. In patients who do not demonstrate hepatitis immunity, the hepatitis B vaccine should be administered prior to starting treatment with a biologic.14 In psoriasis patients with latent TB, 2 months of treatment should be completed before initiating biologic therapy8; once a biologic has been initiated, all patients should be screened annually for TB.
European guidelines for biologic treatment recommend that complete blood count and liver and renal function be evaluated at baseline, at months 1 and 3 of treatment, and then every 3 to 6 months thereafter while on the biologic agent.7 These recommendations are more stringent than those indicated in regulatory labeling and, based on the continual accumulation of data regarding the safety of these agents, some investigators have argued that laboratory testing might not be necessary at all.15
Treatment in Special Populations
Psoriasis patients often present with comorbidities or a complicated medical history, which can make it challenging to decide which therapy is most suitable. Patients with comorbid diseases (eg, PsA, risk of major cardiac event, IBD) or a history of NMSC and those who are pregnant or are lactating require special considerations to ensure treatment safety and efficacy.
Tumor necrosis factor α (TNF-α) and IL-17 inhibitors are used in the treatment of joint disorders and should be considered in patients with PsA. IL-23/IL-12 inhibition appears to have less benefit in patients with PsA, but studies on IL-23 inhibition (p19 antibodies) alone are ongoing.16 It has been reported that TNF-α inhibition may be beneficial in patients at risk for major cardiac events.8,17 In patients with IBD, IL-17 inhibitors should be avoided because they may exacerbate the condition; however, TNF-α and IL-23/IL-12 inhibition have shown to be safe in patients with IBD and many agents in these classes are approved by the US Food and Drug Administration for use in this population.18,19
Although biologics may increase the risk of developing NMSC20 and should generally be avoided in patients with any active malignancy, specific guidelines for screening and initiation of treatment in patients with a history of cancer are not clearly outlined. Prior to initiating systemic therapy in any patient, a careful medical history should be obtained. These agents often are not prescribed in patients with a history of cancer until remission has been established for at least 5 years, with the exception of patients with a history of treated NMSC.8 Annual skin monitoring for NMSC should be undertaken for psoriasis patients on most immunomodulating systemic therapies.
Recommendations for biologic treatment in psoriasis patients who are pregnant or lactating also are limited. European guidelines have noted pregnancy as an absolute contraindication to treatment with biologics,7but the regulatory guidance has recently changed for some agents, so this recommendation also may evolve.21 British8 and US5 guidelines do not consider pregnancy a contraindication for treatment with biologics.
Information on the safety of TNF-α antagonists during pregnancy comes primarily from use in patients with IBD and rheumatologic disease. To date, reports on the incidence of congenital malformations have been generally reassuring. Because IgG antibodies are actively transferred across the placenta in the late-second or the third trimesters, neonates born to mothers on biologic treatments may have high levels of some biologic drugs at birth. As a result, live vaccination should be avoided in neonates whose mothers were treated with IgG-based biologics.
Changing Treatment Agents
Patients may need to stop and change treatment agents due to ineffectiveness, personal preference, or worsening disease. When transitioning from any systemic or biologic agent to another (other than MTX), the British Association of Dermatologists recommends a washout period of at least 1 month before initiating a new therapy.8 Most guidelines do not define parameters for therapy escalation when patients fail multiple systemic agents, so physicians should use clinical judgment along with consideration of patient preference and comorbidity profile to ascertain which agent is most appropriate.
Conclusion
Keeping psoriasis treatment guidelines updated can be difficult, especially as new therapeutic options for psoriasis and treatment regimens rapidly evolve. Regulatory recommendations also vary worldwide, but most guidelines are reasonably consistent without being overly prescriptive, appropriately allowing for flexibility for application in clinical practice. Nonetheless, physicians should keep in mind new or changing guidelines while tailoring psoriasis treatment recommendations to best suit their individual patients.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTty (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence [published online September 27, 2012]. J Invest Dermatol. 2013;133:377-385.
- Pariser DM, Bagel J, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007;143:239-242.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1-10.
- Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris—update 2015—short version—EDF in cooperation with EADV and IPC [published online October 9, 2015]. J Eur Acad Dermatol Venereol. 2015;29:2277-2294.
- Smith CH, Jabbar-Lopez ZK, Yiu ZZ, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628-636.
- Armstrong AW, Siegel MP, Bagel J, et al. From the medical board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Quality ID #410: psoriasis: clinical response to oral systemic or biologic medications—national quality strategy domain: person and caregiver-centered experience and outcomes. Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Quality-Payment-Program/Resource-Library/2018-Resources.html. Accessed February 27, 2018.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Syst Rev. 2017;12:CD011535.
- Lynch M, Higgins E, McCormick PA, et al. The use of transient elastography and FibroTest for monitoring hepatotoxicity in patients receiving methotrexate for psoriasis. JAMA Dermatol. 2014;150:856-862.
- Behrens F, Canete J, Olivieri I, et al. Tumor necrosis factor inhibitor monotherapy versus combination with MTX in the treatment of PsA: a systemic review of the literature. Rheumatology. 2015;54:915-926.
- Karadağ Ö, Kaşifoğlu T, Özer B, et al. Viral hepatitis screening guideline before biological drug use in rheumatic patients. Eur J Rheumatol. 2016;3:25-28.
- Ahn CS, Dothard EH, Garner ML, et al. To test or not to test? an updated evidence-based assessment of the value of screening and monitoring tests when using systemic biologic agents to treat psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2015;73:420-428.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Humira [package insert]. North Chicago, IL: Abbott Laboratories; 2011.
- Stelara [package insert]. Bloomington, IN: Janssen Biotech, Inc; 2016.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Cimzia [package insert]. UCB, Inc: Smyrna, GA; 2016.
Psoriasis is a chronic autoinflammatory disorder affecting approximately 2% to 4% of the Western population.1 While there is no absolute cure for psoriasis, novel therapies allow for substantial reduction in symptoms and considerable improvement in quality of life (QoL). In the past few years, multiple treatment guidelines (recommendations based on evidence-based literature reviews) and consensus statements (a set of declarations determined and voted on by a panel of experts in the field) have been developed to guide physicians worldwide in treating psoriasis in the clinical setting (eTable).2-10

Because psoriasis is a complex disease with multiple comorbidities, applicability of these guidelines may be limited. Although some basic treatment algorithms exist, patient preference, disease severity, and other variables including comorbidities (eg, psoriatic arthritis [PsA], risk of major cardiac events, inflammatory bowel disease [IBD]), history of nonmelanoma skin cancer (NMSC), pregnancy and lactation, and specific contraindications to therapy (eg, renal failure, liver disease, active malignancy) should be considered. In this article, we summarize common themes across existing guidelines and consensus statements for the treatment of psoriasis and highlight areas where there is consistent agreement or lack of sufficient information.
Disease Severity and Treatment Outcomes
There currently are no consensus definitions for mild, moderate, and severe psoriasis, but several consensus statements have attempted to standardize grading systems based on objective values, such as body surface area (BSA) and psoriasis area and severity index (PASI)(a scoring system used to grade the degree of redness, thickness, and scaling of psoriasis plaques), as well as subjective QoL measures.2,6 Although classification of disease severity varies, mild psoriasis generally is characterized as disease that can be managed with local and topical therapy, and moderate to severe psoriasis typically warrants consideration for escalated treatment with phototherapy or systemic agents.
Most definitions of disease severity in psoriasis reference 5% to 10% BSA involvement as a cutoff that should trigger consideration of systemic treatment; however, these criteria could result in undertreatment of patients with substantial disease. For example, patients who have limited BSA involvement but whose disease has a considerable impact on QoL, as well as those who have debilitating disease in localized areas (eg, palms, soles, scalp, nails) or substantial joint involvement may also be appropriate candidates for systemic treatment.5,8
Once therapy is initiated, patients should be evaluated for appropriate treatment response at dedicated intervals. While the time to maximum therapeutic benefit depends on the agent of choice, European guidelines recommend that patients be evaluated after an induction phase (typically 16–24 weeks) and define treatment success as either (1) at least 75% improvement in PASI or (2) at least 50% improvement in PASI and a Dermatology Quality of Life Index (DLQI) score of 5 or lower.6
Alternatively, the National Psoriasis Foundation (NPF) recommended BSA as the preferred outcome measure in a recent consensus statement and concluded that an outcome of 3% or less BSA involvement or improvement in BSA of 75% or more is considered a desirable treatment response.9 Additionally, the Medicare Merit-based Incentive Payment System (MIPS) guidelines for successful systemic treatment response include at least 1 of the following: (1) physician global assessment score of 2 or lower, (2) BSA involvement of less than 3%, (3) PASI score lower than 3, or (4) DLQI score of 5 or lower.10
Although an array of outcome measures have been utilized in clinical trials and proposed in psoriasis guidelines and consensus statements, BSA is typically a manageable measure of treatment response in a clinical setting; however, DLQI should also be assessed if possible, particularly in patients with debilitating localized disease.9
Treatment Options
Because topical treatment regimens can be arduous and typically do not result in sustained clearance, patient expectations should be ascertained prior to initiation of therapy. Topical corticosteroids often can be used as monotherapy in patients with mild psoriasis.3 Topical vitamin D analogues and retinoids also can be effective; however, combined use of these agents with topical steroids should be considered to increase efficacy, and combination formulations can be prescribed to simplify application and improve adherence.
Treatment with UVB or psoralen plus UVA phototherapy is recommended for patients with moderate to severe psoriasis as well as in those who have had minimal response to topical therapy.4 Targeted phototherapy with an excimer laser can be used in patients with BSA involvement of less than 10%.
Methotrexate (MTX), cyclosporine, and acitretin are the most commonly prescribed systemic medications for severe psoriasis in the United States.5 Despite the risk for hepatotoxicity, MTX appears to have the best combined safety and efficacy profile in terms of serious adverse events compared to other systemic agents.11 Guidelines for MTX monitoring, especially with regard to when to do a liver biopsy, have been substantially liberalized over time, and the recommended interval for biopsy has been extended by years; biopsy was previously recommended after a cumulative MTX dose of 1 to 1.5 g, but guidelines now suggest biopsy after 3.5 to 4 g in low-risk patients.5 While abnormally elevated liver function tests during treatment with MTX may necessitate liver biopsy, the use of transient elastography and a panel of serum biomarkers for liver function also can be used to monitor noninvasively for hepatotoxicity before biopsy is considered; these recommendations are likely to be incorporated into newer guidelines in development.12 Methotrexate has demonstrated safety and increased efficacy when used in combination with biologic agents such as adalimumab, etanercept, infliximab, and secukinumab7 and has been studied in combination with many biologics indicated for PsA.13
Due to a considerable risk of glomerulosclerosis, cyclosporine is approved for a maximum of 1 year of continuous treatment of psoriasis in the United States and2 years in Europe.5,7 Cyclosporine is best used as induction therapy in psoriasis patients with severe disease who are seeking faster abatement of symptoms.
Acitretin is another systemic treatment option, although efficacy of this agent is dose dependent. Higher dosing often is limited due to lower tolerability.5
Given that many insurance formularies primarily cover traditional systemic therapies and that MTX and phototherapy are generally well tolerated and cost effective, patients may need to be treated with traditional agents before escalating to biologics. Prior to starting treatment with any biologic, patients should typically be screened for tuberculosis (TB), human immunodeficiency virus infection, and immunization for, exposure to, and/or infection with hepatitis B and C virus, and any other active infections. In patients who do not demonstrate hepatitis immunity, the hepatitis B vaccine should be administered prior to starting treatment with a biologic.14 In psoriasis patients with latent TB, 2 months of treatment should be completed before initiating biologic therapy8; once a biologic has been initiated, all patients should be screened annually for TB.
European guidelines for biologic treatment recommend that complete blood count and liver and renal function be evaluated at baseline, at months 1 and 3 of treatment, and then every 3 to 6 months thereafter while on the biologic agent.7 These recommendations are more stringent than those indicated in regulatory labeling and, based on the continual accumulation of data regarding the safety of these agents, some investigators have argued that laboratory testing might not be necessary at all.15
Treatment in Special Populations
Psoriasis patients often present with comorbidities or a complicated medical history, which can make it challenging to decide which therapy is most suitable. Patients with comorbid diseases (eg, PsA, risk of major cardiac event, IBD) or a history of NMSC and those who are pregnant or are lactating require special considerations to ensure treatment safety and efficacy.
Tumor necrosis factor α (TNF-α) and IL-17 inhibitors are used in the treatment of joint disorders and should be considered in patients with PsA. IL-23/IL-12 inhibition appears to have less benefit in patients with PsA, but studies on IL-23 inhibition (p19 antibodies) alone are ongoing.16 It has been reported that TNF-α inhibition may be beneficial in patients at risk for major cardiac events.8,17 In patients with IBD, IL-17 inhibitors should be avoided because they may exacerbate the condition; however, TNF-α and IL-23/IL-12 inhibition have shown to be safe in patients with IBD and many agents in these classes are approved by the US Food and Drug Administration for use in this population.18,19
Although biologics may increase the risk of developing NMSC20 and should generally be avoided in patients with any active malignancy, specific guidelines for screening and initiation of treatment in patients with a history of cancer are not clearly outlined. Prior to initiating systemic therapy in any patient, a careful medical history should be obtained. These agents often are not prescribed in patients with a history of cancer until remission has been established for at least 5 years, with the exception of patients with a history of treated NMSC.8 Annual skin monitoring for NMSC should be undertaken for psoriasis patients on most immunomodulating systemic therapies.
Recommendations for biologic treatment in psoriasis patients who are pregnant or lactating also are limited. European guidelines have noted pregnancy as an absolute contraindication to treatment with biologics,7but the regulatory guidance has recently changed for some agents, so this recommendation also may evolve.21 British8 and US5 guidelines do not consider pregnancy a contraindication for treatment with biologics.
Information on the safety of TNF-α antagonists during pregnancy comes primarily from use in patients with IBD and rheumatologic disease. To date, reports on the incidence of congenital malformations have been generally reassuring. Because IgG antibodies are actively transferred across the placenta in the late-second or the third trimesters, neonates born to mothers on biologic treatments may have high levels of some biologic drugs at birth. As a result, live vaccination should be avoided in neonates whose mothers were treated with IgG-based biologics.
Changing Treatment Agents
Patients may need to stop and change treatment agents due to ineffectiveness, personal preference, or worsening disease. When transitioning from any systemic or biologic agent to another (other than MTX), the British Association of Dermatologists recommends a washout period of at least 1 month before initiating a new therapy.8 Most guidelines do not define parameters for therapy escalation when patients fail multiple systemic agents, so physicians should use clinical judgment along with consideration of patient preference and comorbidity profile to ascertain which agent is most appropriate.
Conclusion
Keeping psoriasis treatment guidelines updated can be difficult, especially as new therapeutic options for psoriasis and treatment regimens rapidly evolve. Regulatory recommendations also vary worldwide, but most guidelines are reasonably consistent without being overly prescriptive, appropriately allowing for flexibility for application in clinical practice. Nonetheless, physicians should keep in mind new or changing guidelines while tailoring psoriasis treatment recommendations to best suit their individual patients.
Psoriasis is a chronic autoinflammatory disorder affecting approximately 2% to 4% of the Western population.1 While there is no absolute cure for psoriasis, novel therapies allow for substantial reduction in symptoms and considerable improvement in quality of life (QoL). In the past few years, multiple treatment guidelines (recommendations based on evidence-based literature reviews) and consensus statements (a set of declarations determined and voted on by a panel of experts in the field) have been developed to guide physicians worldwide in treating psoriasis in the clinical setting (eTable).2-10

Because psoriasis is a complex disease with multiple comorbidities, applicability of these guidelines may be limited. Although some basic treatment algorithms exist, patient preference, disease severity, and other variables including comorbidities (eg, psoriatic arthritis [PsA], risk of major cardiac events, inflammatory bowel disease [IBD]), history of nonmelanoma skin cancer (NMSC), pregnancy and lactation, and specific contraindications to therapy (eg, renal failure, liver disease, active malignancy) should be considered. In this article, we summarize common themes across existing guidelines and consensus statements for the treatment of psoriasis and highlight areas where there is consistent agreement or lack of sufficient information.
Disease Severity and Treatment Outcomes
There currently are no consensus definitions for mild, moderate, and severe psoriasis, but several consensus statements have attempted to standardize grading systems based on objective values, such as body surface area (BSA) and psoriasis area and severity index (PASI)(a scoring system used to grade the degree of redness, thickness, and scaling of psoriasis plaques), as well as subjective QoL measures.2,6 Although classification of disease severity varies, mild psoriasis generally is characterized as disease that can be managed with local and topical therapy, and moderate to severe psoriasis typically warrants consideration for escalated treatment with phototherapy or systemic agents.
Most definitions of disease severity in psoriasis reference 5% to 10% BSA involvement as a cutoff that should trigger consideration of systemic treatment; however, these criteria could result in undertreatment of patients with substantial disease. For example, patients who have limited BSA involvement but whose disease has a considerable impact on QoL, as well as those who have debilitating disease in localized areas (eg, palms, soles, scalp, nails) or substantial joint involvement may also be appropriate candidates for systemic treatment.5,8
Once therapy is initiated, patients should be evaluated for appropriate treatment response at dedicated intervals. While the time to maximum therapeutic benefit depends on the agent of choice, European guidelines recommend that patients be evaluated after an induction phase (typically 16–24 weeks) and define treatment success as either (1) at least 75% improvement in PASI or (2) at least 50% improvement in PASI and a Dermatology Quality of Life Index (DLQI) score of 5 or lower.6
Alternatively, the National Psoriasis Foundation (NPF) recommended BSA as the preferred outcome measure in a recent consensus statement and concluded that an outcome of 3% or less BSA involvement or improvement in BSA of 75% or more is considered a desirable treatment response.9 Additionally, the Medicare Merit-based Incentive Payment System (MIPS) guidelines for successful systemic treatment response include at least 1 of the following: (1) physician global assessment score of 2 or lower, (2) BSA involvement of less than 3%, (3) PASI score lower than 3, or (4) DLQI score of 5 or lower.10
Although an array of outcome measures have been utilized in clinical trials and proposed in psoriasis guidelines and consensus statements, BSA is typically a manageable measure of treatment response in a clinical setting; however, DLQI should also be assessed if possible, particularly in patients with debilitating localized disease.9
Treatment Options
Because topical treatment regimens can be arduous and typically do not result in sustained clearance, patient expectations should be ascertained prior to initiation of therapy. Topical corticosteroids often can be used as monotherapy in patients with mild psoriasis.3 Topical vitamin D analogues and retinoids also can be effective; however, combined use of these agents with topical steroids should be considered to increase efficacy, and combination formulations can be prescribed to simplify application and improve adherence.
Treatment with UVB or psoralen plus UVA phototherapy is recommended for patients with moderate to severe psoriasis as well as in those who have had minimal response to topical therapy.4 Targeted phototherapy with an excimer laser can be used in patients with BSA involvement of less than 10%.
Methotrexate (MTX), cyclosporine, and acitretin are the most commonly prescribed systemic medications for severe psoriasis in the United States.5 Despite the risk for hepatotoxicity, MTX appears to have the best combined safety and efficacy profile in terms of serious adverse events compared to other systemic agents.11 Guidelines for MTX monitoring, especially with regard to when to do a liver biopsy, have been substantially liberalized over time, and the recommended interval for biopsy has been extended by years; biopsy was previously recommended after a cumulative MTX dose of 1 to 1.5 g, but guidelines now suggest biopsy after 3.5 to 4 g in low-risk patients.5 While abnormally elevated liver function tests during treatment with MTX may necessitate liver biopsy, the use of transient elastography and a panel of serum biomarkers for liver function also can be used to monitor noninvasively for hepatotoxicity before biopsy is considered; these recommendations are likely to be incorporated into newer guidelines in development.12 Methotrexate has demonstrated safety and increased efficacy when used in combination with biologic agents such as adalimumab, etanercept, infliximab, and secukinumab7 and has been studied in combination with many biologics indicated for PsA.13
Due to a considerable risk of glomerulosclerosis, cyclosporine is approved for a maximum of 1 year of continuous treatment of psoriasis in the United States and2 years in Europe.5,7 Cyclosporine is best used as induction therapy in psoriasis patients with severe disease who are seeking faster abatement of symptoms.
Acitretin is another systemic treatment option, although efficacy of this agent is dose dependent. Higher dosing often is limited due to lower tolerability.5
Given that many insurance formularies primarily cover traditional systemic therapies and that MTX and phototherapy are generally well tolerated and cost effective, patients may need to be treated with traditional agents before escalating to biologics. Prior to starting treatment with any biologic, patients should typically be screened for tuberculosis (TB), human immunodeficiency virus infection, and immunization for, exposure to, and/or infection with hepatitis B and C virus, and any other active infections. In patients who do not demonstrate hepatitis immunity, the hepatitis B vaccine should be administered prior to starting treatment with a biologic.14 In psoriasis patients with latent TB, 2 months of treatment should be completed before initiating biologic therapy8; once a biologic has been initiated, all patients should be screened annually for TB.
European guidelines for biologic treatment recommend that complete blood count and liver and renal function be evaluated at baseline, at months 1 and 3 of treatment, and then every 3 to 6 months thereafter while on the biologic agent.7 These recommendations are more stringent than those indicated in regulatory labeling and, based on the continual accumulation of data regarding the safety of these agents, some investigators have argued that laboratory testing might not be necessary at all.15
Treatment in Special Populations
Psoriasis patients often present with comorbidities or a complicated medical history, which can make it challenging to decide which therapy is most suitable. Patients with comorbid diseases (eg, PsA, risk of major cardiac event, IBD) or a history of NMSC and those who are pregnant or are lactating require special considerations to ensure treatment safety and efficacy.
Tumor necrosis factor α (TNF-α) and IL-17 inhibitors are used in the treatment of joint disorders and should be considered in patients with PsA. IL-23/IL-12 inhibition appears to have less benefit in patients with PsA, but studies on IL-23 inhibition (p19 antibodies) alone are ongoing.16 It has been reported that TNF-α inhibition may be beneficial in patients at risk for major cardiac events.8,17 In patients with IBD, IL-17 inhibitors should be avoided because they may exacerbate the condition; however, TNF-α and IL-23/IL-12 inhibition have shown to be safe in patients with IBD and many agents in these classes are approved by the US Food and Drug Administration for use in this population.18,19
Although biologics may increase the risk of developing NMSC20 and should generally be avoided in patients with any active malignancy, specific guidelines for screening and initiation of treatment in patients with a history of cancer are not clearly outlined. Prior to initiating systemic therapy in any patient, a careful medical history should be obtained. These agents often are not prescribed in patients with a history of cancer until remission has been established for at least 5 years, with the exception of patients with a history of treated NMSC.8 Annual skin monitoring for NMSC should be undertaken for psoriasis patients on most immunomodulating systemic therapies.
Recommendations for biologic treatment in psoriasis patients who are pregnant or lactating also are limited. European guidelines have noted pregnancy as an absolute contraindication to treatment with biologics,7but the regulatory guidance has recently changed for some agents, so this recommendation also may evolve.21 British8 and US5 guidelines do not consider pregnancy a contraindication for treatment with biologics.
Information on the safety of TNF-α antagonists during pregnancy comes primarily from use in patients with IBD and rheumatologic disease. To date, reports on the incidence of congenital malformations have been generally reassuring. Because IgG antibodies are actively transferred across the placenta in the late-second or the third trimesters, neonates born to mothers on biologic treatments may have high levels of some biologic drugs at birth. As a result, live vaccination should be avoided in neonates whose mothers were treated with IgG-based biologics.
Changing Treatment Agents
Patients may need to stop and change treatment agents due to ineffectiveness, personal preference, or worsening disease. When transitioning from any systemic or biologic agent to another (other than MTX), the British Association of Dermatologists recommends a washout period of at least 1 month before initiating a new therapy.8 Most guidelines do not define parameters for therapy escalation when patients fail multiple systemic agents, so physicians should use clinical judgment along with consideration of patient preference and comorbidity profile to ascertain which agent is most appropriate.
Conclusion
Keeping psoriasis treatment guidelines updated can be difficult, especially as new therapeutic options for psoriasis and treatment regimens rapidly evolve. Regulatory recommendations also vary worldwide, but most guidelines are reasonably consistent without being overly prescriptive, appropriately allowing for flexibility for application in clinical practice. Nonetheless, physicians should keep in mind new or changing guidelines while tailoring psoriasis treatment recommendations to best suit their individual patients.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTty (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence [published online September 27, 2012]. J Invest Dermatol. 2013;133:377-385.
- Pariser DM, Bagel J, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007;143:239-242.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1-10.
- Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris—update 2015—short version—EDF in cooperation with EADV and IPC [published online October 9, 2015]. J Eur Acad Dermatol Venereol. 2015;29:2277-2294.
- Smith CH, Jabbar-Lopez ZK, Yiu ZZ, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628-636.
- Armstrong AW, Siegel MP, Bagel J, et al. From the medical board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Quality ID #410: psoriasis: clinical response to oral systemic or biologic medications—national quality strategy domain: person and caregiver-centered experience and outcomes. Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Quality-Payment-Program/Resource-Library/2018-Resources.html. Accessed February 27, 2018.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Syst Rev. 2017;12:CD011535.
- Lynch M, Higgins E, McCormick PA, et al. The use of transient elastography and FibroTest for monitoring hepatotoxicity in patients receiving methotrexate for psoriasis. JAMA Dermatol. 2014;150:856-862.
- Behrens F, Canete J, Olivieri I, et al. Tumor necrosis factor inhibitor monotherapy versus combination with MTX in the treatment of PsA: a systemic review of the literature. Rheumatology. 2015;54:915-926.
- Karadağ Ö, Kaşifoğlu T, Özer B, et al. Viral hepatitis screening guideline before biological drug use in rheumatic patients. Eur J Rheumatol. 2016;3:25-28.
- Ahn CS, Dothard EH, Garner ML, et al. To test or not to test? an updated evidence-based assessment of the value of screening and monitoring tests when using systemic biologic agents to treat psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2015;73:420-428.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Humira [package insert]. North Chicago, IL: Abbott Laboratories; 2011.
- Stelara [package insert]. Bloomington, IN: Janssen Biotech, Inc; 2016.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Cimzia [package insert]. UCB, Inc: Smyrna, GA; 2016.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTty (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence [published online September 27, 2012]. J Invest Dermatol. 2013;133:377-385.
- Pariser DM, Bagel J, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007;143:239-242.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1-10.
- Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris—update 2015—short version—EDF in cooperation with EADV and IPC [published online October 9, 2015]. J Eur Acad Dermatol Venereol. 2015;29:2277-2294.
- Smith CH, Jabbar-Lopez ZK, Yiu ZZ, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628-636.
- Armstrong AW, Siegel MP, Bagel J, et al. From the medical board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Quality ID #410: psoriasis: clinical response to oral systemic or biologic medications—national quality strategy domain: person and caregiver-centered experience and outcomes. Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Quality-Payment-Program/Resource-Library/2018-Resources.html. Accessed February 27, 2018.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database of Syst Rev. 2017;12:CD011535.
- Lynch M, Higgins E, McCormick PA, et al. The use of transient elastography and FibroTest for monitoring hepatotoxicity in patients receiving methotrexate for psoriasis. JAMA Dermatol. 2014;150:856-862.
- Behrens F, Canete J, Olivieri I, et al. Tumor necrosis factor inhibitor monotherapy versus combination with MTX in the treatment of PsA: a systemic review of the literature. Rheumatology. 2015;54:915-926.
- Karadağ Ö, Kaşifoğlu T, Özer B, et al. Viral hepatitis screening guideline before biological drug use in rheumatic patients. Eur J Rheumatol. 2016;3:25-28.
- Ahn CS, Dothard EH, Garner ML, et al. To test or not to test? an updated evidence-based assessment of the value of screening and monitoring tests when using systemic biologic agents to treat psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2015;73:420-428.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator–controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Humira [package insert]. North Chicago, IL: Abbott Laboratories; 2011.
- Stelara [package insert]. Bloomington, IN: Janssen Biotech, Inc; 2016.
- Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886-2895.
- Cimzia [package insert]. UCB, Inc: Smyrna, GA; 2016.
Practice Points
- Guidelines and consensus statements for psoriasis treatment are generally but not always consistent.
- As guidelines evolve, individual patient preferences, disease severity, and comorbid conditions remain important considerations when selecting treatment agents for psoriasis.
- More frequent updates to psoriasis treatment guidelines are becoming increasingly important given the rapid changes in the field.
Hidradenitis Suppurativa Scoring Systems: Can We Choose Just One?
Interest in hidradenitis suppurativa (HS) has exploded in the last few years. A PubMed search of articles indexed for MEDLINE using the MeSH term hidradenitis suppurativa yielded more than 900 articles on HS since 1947, with a sharp increase in publications over the last few years and 119 articles published in 2015 alone. In addition to publications, we recently saw adalimumab become the first and only US Food and Drug Administration–approved treatment of moderate to severe HS.
With new treatment options and enthusiasm for HS, further attention needs to be paid to the scoring systems or outcome measures that clinicians use to grade HS severity and disease. Utilization of validated outcome measures allows for comparability between treatment effects, which is essential for clinical trials, meta-analyses, and monitoring of treatment response in daily clinical practice. Designing a scoring scale for any dermatologic disease is challenging; however, as we move forward with value-based reimbursement models, we likely will encounter quality reporting guidelines that mandate providers demonstrate the positive impact of treatment. Thus, scoring systems for HS, particularly ones that accurately assess this impact of treatment, are essential. For psoriasis, the physician global assessment (PGA) and psoriasis area and severity index are standard outcome measures of disease severity in clinical trials. The PGA also can be used in a clinical setting to longitudinally track patient treatment outcomes.1 Both the psoriasis area and severity index and PGA were cited as acceptable scoring tools for Medicare’s Physician Quality Reporting System quality metrics reporting (Measure #410: Psoriasis: Clinical Response to Oral Systemic or Biologic Medications). Unfortunately, no such outcome measures consensus currently exists for scoring systems in HS.
Many scoring systems have been proposed for HS. The most well known is the Hurley staging system. Developed in 1989 for surgical approaches, it is a straightforward tool to categorize disease severity but does not emphasize the inflammatory component of HS. Recently, a refined Hurley stage classification system was proposed. This 3-step algorithm expanded the Hurley stage classification to incorporate disease extensiveness, degree of inflammation, and presence of sinus tracts.2 The modified Sartorius score (also known as the modified HS score) is a more detailed scoring system for assessing disease activity that requires measurements and precise counting of lesions.3 The HS-PGA is an ordinal scale specific to HS that categorizes patients into clear, minimal, mild, moderate, severe, or very severe disease, and it was used successfully in a phase 2 interventional clinical trial.4 The HS clinical response (HiSCR) score is an HS-specific, binary scoring system for patients with 3 or more abscesses or inflammatory nodules. It was engineered using raw data and outcomes from a large clinical trial, and subsequently was employed as the primary end point in 2 randomized controlled trials.5,6 It is the only HS scoring system to undergo an extensive validation process of both physician- and patient-reported measures for assessment of therapeutic response in controlling the inflammatory manifestations of HS.
Designing a scoring system for clinical trials can be complicated. Sample sizes are dependent on the delta, or change, in efficacy or variation in response, and the design of the score will affect how easy it is to detect a statistically meaningful difference. These choices are a critical part of the design of small studies, particularly if obtaining enough statistical power can be challenging. Additionally, it is easier to detect change in more homogenous populations where we expect a more consistent response. Hidradenitis suppurativa is not a particularly homogenous disease, which furthers the risk of designing a trial that cannot detect important differences. The PGA often is required by the US Food and Drug Administration and has the major advantage that it is easy to understand, but the categories can sometimes be too broad to detect change easily, and more granular data can provide the basis for more in-depth analyses. An ideal outcome measure is a simplified scoring system that assesses disease severity and responsiveness to treatment while accurately serving as a surrogate for patient-reported outcomes, such as the dermatology life quality index, visual analog scale for HS skin pain, the work productivity and activity impairment questionnaire (specific health problem), or the patient global assessment. Validation processes for outcome measures, such as the one that HiSCR underwent, are essential to ensure that the proposed scoring system has clinical meaningfulness to both the physician and patient.
A 2016 Cochrane review of interventions for HS included 12 randomized controlled trials that employed a total of 30 different outcome measures instruments. Because use of multiple scoring systems makes it difficult to compare analyses of treatment, the authors concluded that there was a need for improved validation of HS outcome measures for future clinical trials.7 Schmitt et al8 recognized that atopic dermatitis also was in a similar predicament; they noted that more than 20 outcome measures were employed to assess disease severity in clinical trials. The authors called this situation “a significant threat to evidence-based health care” and outlined the Harmonizing Outcome Measures for Eczema (HOME) research initiative’s methodology for creation of core outcome sets for any dermatologic disease. Their consensus process involved first identifying what to measure, termed outcome domains, followed by developing how to measure these domains through outcome measures instruments, which would be assessed for validity, reliability, sensitivity to change, and feasibility.8
Using the framework set forth by the HOME initiative and data from the 2016 Cochrane review,7 a recent review of all outcome measures instruments currently employed in HS found that 90% (27/30) were not validated.9 Even those that were validated still could not be fully recommended by the authors. The authors identified 10 potential outcome domains for measurement, including quality of life, pain, lesion count, PGA, patient global self-assessment, recurrence rate, overall satisfaction with treatment, impairment of function, cosmesis, and duration of recovery. They recommended a further consensus process to better define these outcomes.9
Measuring all of these variables seems daunting, but as the speed of HS research rapidly progresses, we would greatly benefit from employing a standard validated scoring system that captures both disease severity and activity. Several groups are working to improve our current tools, but we will need to move quickly to a common approach so we can better compare treatment effects and build an evidence base for treatment decisions. For now, the HiSCR is the most validated clinical trials instrument, but it may not be ideal for the clinical setting. In our practice, we grade all patients each visit with Hurley staging, the validated HS-PGA scoring system to track improvement in inflammatory lesions, and a 10-point pain scale to monitor disease activity and severity. We have found these tools to be quick and effective for measuring treatment response and would recommend employment of these scoring systems as a standard measure in clinical practice until further consensus is reached.
- Pascoe VL, Enamandram M, Corey KC, et al. Using the Physician Global Assessment in a clinical setting to measure and track patient outcomes. JAMA Dermatol. 2015;151:375-381.
- Horváth B, Janse IC, Blok JL, et al. Hurley staging refined: a proposal by the Dutch Hidradenitis Suppurativa Expert Group [published online August 18, 2016]. Acta Derm Venereol. doi:10.2340/00015555-2513.
- Revuz J. Modifications to the Sartorius score and instructions for evaluating the severity of suppurative hidradenitis [in French]. Ann Dermatol Venereol. 2007;134:173-174.
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846-855.
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59.
- Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (hidradenitis suppurativa clinical response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study [published online July 22, 2015]. J Eur Acad Dermatol Venereol. 2016;30:989-994.
- Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality [published online March 30, 2016]. Br J Dermatol. 2016;174:970-978.
- Schmitt J, Apfelbacher C, Spuls PI, et al. The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology [published online September 4, 2014]. J Invest Dermatol. 2015;135:24-30.
- Ingram JR, Hadjieconomou S, Piguet V. Development of core outcome sets in hidradenitis suppurativa: systematic review of outcome measure instruments to inform the process [published online May 2, 2016]. Br J Dermatol. 2016;175:263-272.
Interest in hidradenitis suppurativa (HS) has exploded in the last few years. A PubMed search of articles indexed for MEDLINE using the MeSH term hidradenitis suppurativa yielded more than 900 articles on HS since 1947, with a sharp increase in publications over the last few years and 119 articles published in 2015 alone. In addition to publications, we recently saw adalimumab become the first and only US Food and Drug Administration–approved treatment of moderate to severe HS.
With new treatment options and enthusiasm for HS, further attention needs to be paid to the scoring systems or outcome measures that clinicians use to grade HS severity and disease. Utilization of validated outcome measures allows for comparability between treatment effects, which is essential for clinical trials, meta-analyses, and monitoring of treatment response in daily clinical practice. Designing a scoring scale for any dermatologic disease is challenging; however, as we move forward with value-based reimbursement models, we likely will encounter quality reporting guidelines that mandate providers demonstrate the positive impact of treatment. Thus, scoring systems for HS, particularly ones that accurately assess this impact of treatment, are essential. For psoriasis, the physician global assessment (PGA) and psoriasis area and severity index are standard outcome measures of disease severity in clinical trials. The PGA also can be used in a clinical setting to longitudinally track patient treatment outcomes.1 Both the psoriasis area and severity index and PGA were cited as acceptable scoring tools for Medicare’s Physician Quality Reporting System quality metrics reporting (Measure #410: Psoriasis: Clinical Response to Oral Systemic or Biologic Medications). Unfortunately, no such outcome measures consensus currently exists for scoring systems in HS.
Many scoring systems have been proposed for HS. The most well known is the Hurley staging system. Developed in 1989 for surgical approaches, it is a straightforward tool to categorize disease severity but does not emphasize the inflammatory component of HS. Recently, a refined Hurley stage classification system was proposed. This 3-step algorithm expanded the Hurley stage classification to incorporate disease extensiveness, degree of inflammation, and presence of sinus tracts.2 The modified Sartorius score (also known as the modified HS score) is a more detailed scoring system for assessing disease activity that requires measurements and precise counting of lesions.3 The HS-PGA is an ordinal scale specific to HS that categorizes patients into clear, minimal, mild, moderate, severe, or very severe disease, and it was used successfully in a phase 2 interventional clinical trial.4 The HS clinical response (HiSCR) score is an HS-specific, binary scoring system for patients with 3 or more abscesses or inflammatory nodules. It was engineered using raw data and outcomes from a large clinical trial, and subsequently was employed as the primary end point in 2 randomized controlled trials.5,6 It is the only HS scoring system to undergo an extensive validation process of both physician- and patient-reported measures for assessment of therapeutic response in controlling the inflammatory manifestations of HS.
Designing a scoring system for clinical trials can be complicated. Sample sizes are dependent on the delta, or change, in efficacy or variation in response, and the design of the score will affect how easy it is to detect a statistically meaningful difference. These choices are a critical part of the design of small studies, particularly if obtaining enough statistical power can be challenging. Additionally, it is easier to detect change in more homogenous populations where we expect a more consistent response. Hidradenitis suppurativa is not a particularly homogenous disease, which furthers the risk of designing a trial that cannot detect important differences. The PGA often is required by the US Food and Drug Administration and has the major advantage that it is easy to understand, but the categories can sometimes be too broad to detect change easily, and more granular data can provide the basis for more in-depth analyses. An ideal outcome measure is a simplified scoring system that assesses disease severity and responsiveness to treatment while accurately serving as a surrogate for patient-reported outcomes, such as the dermatology life quality index, visual analog scale for HS skin pain, the work productivity and activity impairment questionnaire (specific health problem), or the patient global assessment. Validation processes for outcome measures, such as the one that HiSCR underwent, are essential to ensure that the proposed scoring system has clinical meaningfulness to both the physician and patient.
A 2016 Cochrane review of interventions for HS included 12 randomized controlled trials that employed a total of 30 different outcome measures instruments. Because use of multiple scoring systems makes it difficult to compare analyses of treatment, the authors concluded that there was a need for improved validation of HS outcome measures for future clinical trials.7 Schmitt et al8 recognized that atopic dermatitis also was in a similar predicament; they noted that more than 20 outcome measures were employed to assess disease severity in clinical trials. The authors called this situation “a significant threat to evidence-based health care” and outlined the Harmonizing Outcome Measures for Eczema (HOME) research initiative’s methodology for creation of core outcome sets for any dermatologic disease. Their consensus process involved first identifying what to measure, termed outcome domains, followed by developing how to measure these domains through outcome measures instruments, which would be assessed for validity, reliability, sensitivity to change, and feasibility.8
Using the framework set forth by the HOME initiative and data from the 2016 Cochrane review,7 a recent review of all outcome measures instruments currently employed in HS found that 90% (27/30) were not validated.9 Even those that were validated still could not be fully recommended by the authors. The authors identified 10 potential outcome domains for measurement, including quality of life, pain, lesion count, PGA, patient global self-assessment, recurrence rate, overall satisfaction with treatment, impairment of function, cosmesis, and duration of recovery. They recommended a further consensus process to better define these outcomes.9
Measuring all of these variables seems daunting, but as the speed of HS research rapidly progresses, we would greatly benefit from employing a standard validated scoring system that captures both disease severity and activity. Several groups are working to improve our current tools, but we will need to move quickly to a common approach so we can better compare treatment effects and build an evidence base for treatment decisions. For now, the HiSCR is the most validated clinical trials instrument, but it may not be ideal for the clinical setting. In our practice, we grade all patients each visit with Hurley staging, the validated HS-PGA scoring system to track improvement in inflammatory lesions, and a 10-point pain scale to monitor disease activity and severity. We have found these tools to be quick and effective for measuring treatment response and would recommend employment of these scoring systems as a standard measure in clinical practice until further consensus is reached.
Interest in hidradenitis suppurativa (HS) has exploded in the last few years. A PubMed search of articles indexed for MEDLINE using the MeSH term hidradenitis suppurativa yielded more than 900 articles on HS since 1947, with a sharp increase in publications over the last few years and 119 articles published in 2015 alone. In addition to publications, we recently saw adalimumab become the first and only US Food and Drug Administration–approved treatment of moderate to severe HS.
With new treatment options and enthusiasm for HS, further attention needs to be paid to the scoring systems or outcome measures that clinicians use to grade HS severity and disease. Utilization of validated outcome measures allows for comparability between treatment effects, which is essential for clinical trials, meta-analyses, and monitoring of treatment response in daily clinical practice. Designing a scoring scale for any dermatologic disease is challenging; however, as we move forward with value-based reimbursement models, we likely will encounter quality reporting guidelines that mandate providers demonstrate the positive impact of treatment. Thus, scoring systems for HS, particularly ones that accurately assess this impact of treatment, are essential. For psoriasis, the physician global assessment (PGA) and psoriasis area and severity index are standard outcome measures of disease severity in clinical trials. The PGA also can be used in a clinical setting to longitudinally track patient treatment outcomes.1 Both the psoriasis area and severity index and PGA were cited as acceptable scoring tools for Medicare’s Physician Quality Reporting System quality metrics reporting (Measure #410: Psoriasis: Clinical Response to Oral Systemic or Biologic Medications). Unfortunately, no such outcome measures consensus currently exists for scoring systems in HS.
Many scoring systems have been proposed for HS. The most well known is the Hurley staging system. Developed in 1989 for surgical approaches, it is a straightforward tool to categorize disease severity but does not emphasize the inflammatory component of HS. Recently, a refined Hurley stage classification system was proposed. This 3-step algorithm expanded the Hurley stage classification to incorporate disease extensiveness, degree of inflammation, and presence of sinus tracts.2 The modified Sartorius score (also known as the modified HS score) is a more detailed scoring system for assessing disease activity that requires measurements and precise counting of lesions.3 The HS-PGA is an ordinal scale specific to HS that categorizes patients into clear, minimal, mild, moderate, severe, or very severe disease, and it was used successfully in a phase 2 interventional clinical trial.4 The HS clinical response (HiSCR) score is an HS-specific, binary scoring system for patients with 3 or more abscesses or inflammatory nodules. It was engineered using raw data and outcomes from a large clinical trial, and subsequently was employed as the primary end point in 2 randomized controlled trials.5,6 It is the only HS scoring system to undergo an extensive validation process of both physician- and patient-reported measures for assessment of therapeutic response in controlling the inflammatory manifestations of HS.
Designing a scoring system for clinical trials can be complicated. Sample sizes are dependent on the delta, or change, in efficacy or variation in response, and the design of the score will affect how easy it is to detect a statistically meaningful difference. These choices are a critical part of the design of small studies, particularly if obtaining enough statistical power can be challenging. Additionally, it is easier to detect change in more homogenous populations where we expect a more consistent response. Hidradenitis suppurativa is not a particularly homogenous disease, which furthers the risk of designing a trial that cannot detect important differences. The PGA often is required by the US Food and Drug Administration and has the major advantage that it is easy to understand, but the categories can sometimes be too broad to detect change easily, and more granular data can provide the basis for more in-depth analyses. An ideal outcome measure is a simplified scoring system that assesses disease severity and responsiveness to treatment while accurately serving as a surrogate for patient-reported outcomes, such as the dermatology life quality index, visual analog scale for HS skin pain, the work productivity and activity impairment questionnaire (specific health problem), or the patient global assessment. Validation processes for outcome measures, such as the one that HiSCR underwent, are essential to ensure that the proposed scoring system has clinical meaningfulness to both the physician and patient.
A 2016 Cochrane review of interventions for HS included 12 randomized controlled trials that employed a total of 30 different outcome measures instruments. Because use of multiple scoring systems makes it difficult to compare analyses of treatment, the authors concluded that there was a need for improved validation of HS outcome measures for future clinical trials.7 Schmitt et al8 recognized that atopic dermatitis also was in a similar predicament; they noted that more than 20 outcome measures were employed to assess disease severity in clinical trials. The authors called this situation “a significant threat to evidence-based health care” and outlined the Harmonizing Outcome Measures for Eczema (HOME) research initiative’s methodology for creation of core outcome sets for any dermatologic disease. Their consensus process involved first identifying what to measure, termed outcome domains, followed by developing how to measure these domains through outcome measures instruments, which would be assessed for validity, reliability, sensitivity to change, and feasibility.8
Using the framework set forth by the HOME initiative and data from the 2016 Cochrane review,7 a recent review of all outcome measures instruments currently employed in HS found that 90% (27/30) were not validated.9 Even those that were validated still could not be fully recommended by the authors. The authors identified 10 potential outcome domains for measurement, including quality of life, pain, lesion count, PGA, patient global self-assessment, recurrence rate, overall satisfaction with treatment, impairment of function, cosmesis, and duration of recovery. They recommended a further consensus process to better define these outcomes.9
Measuring all of these variables seems daunting, but as the speed of HS research rapidly progresses, we would greatly benefit from employing a standard validated scoring system that captures both disease severity and activity. Several groups are working to improve our current tools, but we will need to move quickly to a common approach so we can better compare treatment effects and build an evidence base for treatment decisions. For now, the HiSCR is the most validated clinical trials instrument, but it may not be ideal for the clinical setting. In our practice, we grade all patients each visit with Hurley staging, the validated HS-PGA scoring system to track improvement in inflammatory lesions, and a 10-point pain scale to monitor disease activity and severity. We have found these tools to be quick and effective for measuring treatment response and would recommend employment of these scoring systems as a standard measure in clinical practice until further consensus is reached.
- Pascoe VL, Enamandram M, Corey KC, et al. Using the Physician Global Assessment in a clinical setting to measure and track patient outcomes. JAMA Dermatol. 2015;151:375-381.
- Horváth B, Janse IC, Blok JL, et al. Hurley staging refined: a proposal by the Dutch Hidradenitis Suppurativa Expert Group [published online August 18, 2016]. Acta Derm Venereol. doi:10.2340/00015555-2513.
- Revuz J. Modifications to the Sartorius score and instructions for evaluating the severity of suppurative hidradenitis [in French]. Ann Dermatol Venereol. 2007;134:173-174.
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846-855.
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59.
- Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (hidradenitis suppurativa clinical response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study [published online July 22, 2015]. J Eur Acad Dermatol Venereol. 2016;30:989-994.
- Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality [published online March 30, 2016]. Br J Dermatol. 2016;174:970-978.
- Schmitt J, Apfelbacher C, Spuls PI, et al. The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology [published online September 4, 2014]. J Invest Dermatol. 2015;135:24-30.
- Ingram JR, Hadjieconomou S, Piguet V. Development of core outcome sets in hidradenitis suppurativa: systematic review of outcome measure instruments to inform the process [published online May 2, 2016]. Br J Dermatol. 2016;175:263-272.
- Pascoe VL, Enamandram M, Corey KC, et al. Using the Physician Global Assessment in a clinical setting to measure and track patient outcomes. JAMA Dermatol. 2015;151:375-381.
- Horváth B, Janse IC, Blok JL, et al. Hurley staging refined: a proposal by the Dutch Hidradenitis Suppurativa Expert Group [published online August 18, 2016]. Acta Derm Venereol. doi:10.2340/00015555-2513.
- Revuz J. Modifications to the Sartorius score and instructions for evaluating the severity of suppurative hidradenitis [in French]. Ann Dermatol Venereol. 2007;134:173-174.
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846-855.
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59.
- Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (hidradenitis suppurativa clinical response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study [published online July 22, 2015]. J Eur Acad Dermatol Venereol. 2016;30:989-994.
- Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality [published online March 30, 2016]. Br J Dermatol. 2016;174:970-978.
- Schmitt J, Apfelbacher C, Spuls PI, et al. The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology [published online September 4, 2014]. J Invest Dermatol. 2015;135:24-30.
- Ingram JR, Hadjieconomou S, Piguet V. Development of core outcome sets in hidradenitis suppurativa: systematic review of outcome measure instruments to inform the process [published online May 2, 2016]. Br J Dermatol. 2016;175:263-272.
Remission of Psoriasis 13 Years After Autologous Stem Cell Transplant
To the Editor:
Remission of psoriasis after bone marrow transplant has been reported1; however, follow-up typically has been limited. We describe a case of a remote recurrence of psoriasis 13 years after autologous stem cell transplant.
In September 1997, a 48-year-old woman with a 20-year history of moderate to severe plaque psoriasis, previously managed with oral methotrexate (up to 30–40 mg weekly), presented to her primary care physician with a persistent cough and newly pervasive arthralgia and myalgia. A complete blood cell count showed a white blood cell count of 6.9 mg/dL (reference range, 3.8–10.8 mg/dL), hemoglobin level of 11.6 mg/dL (reference range, 11.7–15.5 mg/dL), and hematocrit level of 34% (reference range, 35.0%–45.0%). Bone survey demonstrated a 4×2-cm lytic expansile lesion in the occipital bone, and a bone marrow aspirate and biopsy revealed 40% plasma cells. She was subsequently diagnosed with stage IIIA IgA λ and free λ light chain multiple myeloma with an IgA level of 62.1 g/L (reference range, 1.24–2.17 g/L).
Therapy was initiated with dexamethasone in combination with pamidronate and she was transitioned to cyclophosphamide chemotherapy. A peripheral autologous blood stem cell transplant followed. Throughout her initial treatment, the psoriasis continued to flare and she remained on methotrexate to help maintain control of the skin disease.
In November 1998, the patient underwent an autologous CD34+ stem cell transplant. There were no complications following the transplant and the multiple myeloma has remained in complete remission to date. Following the stem cell transplant, the patient remained free of psoriatic disease for just over 13 years without any intervention. Recurrent erythematous plaques with thick scale on the bilateral lower legs prompted her return to dermatology in August 2011. To date, she continues to note intermittent though mild flares of her psoriasis. She has responded well to methotrexate 7.5 mg weekly and clobetasol propionate ointment 0.05% as needed with satisfactory control of her disease.
The development of autoimmune disease following an allogenic bone marrow transplant such as psoriasis, thyroiditis, myasthenia gravis, insulin-dependent diabetes mellitus, and vitiligo has been well described.2-4 The causal relationship between allogenic hematopoetic stem cell transplant (HSCT) and subsequent autoimmune disease has been attributed to T cells and passive transfer of autoimmune disease from the donor to the recipient.1
In the case of autologous HSCT, the remission of autoimmune disease thereafter has been attributed to myeloablative conditioning, whereby high-dose chemotherapy eliminates self-reactive lymphocytes, resulting in ablation of peripheral reactive and alloreactive T cells with depletion of thymus and reactive B cells.1
In 1997, Cooley et al5 described 4 patients with autoimmune disease who experienced complete but temporary (up to 26 months) remission following autologous bone marrow or stem cell transplant. A comprehensive review of psoriasis cases that resolved after HSCT published by Kaffenberger et al6 in 2013 cited 6 cases of autologous and 13 cases of allogenic transplant that resulted in resolution of psoriatic disease. Recurrence of disease was witnessed in 3 of 13 allogenic HSCT patients and 5 of 6 autologous HSCT patients. Of note, all 5 of these patients developed recurrent disease within 24 months following autologous HSCT, albeit with more benign disease.6
Similar to those cases presented by Kaffenberger et al,6 our patient’s psoriatic disease was notably mild compared to her disease prior to HSCT. Although an unintended but welcome outcome of multiple myeloma treatment, the nearly quiescent nature of her skin disease following autologous HSCT has led to a dramatic improvement in her quality of life.
We highlight a unique case in which psoriatic skin disease recurred more than 1 decade after autologous HSCT.
1. Braiteh F, Hymes SR, Giralt SA, et al. Complete remission of psoriasis after autologous hematopoietic stem-cell transplantation for multiple myeloma. J Clin Oncol. 2008;26:4511-4513.
2. Kishimoto Y, Yamamoto Y, Matsumoto N, et al. Transfer of autoimmune thyroiditis and resolution of palmoplantar pustular psoriasis following allogeneic bone marrow transplantation. Bone Marrow Transpl. 1997;19:1041-1043.
3. Wahie S, Alexandroff A, Reynolds NY, et al. Psoriasis occurring after myeloablative therapy and autologous stem cell transplantation. Br J Dermatol. 2006;154:177-204.
4. Snowden JA, Heaton DC. Development of psoriasis after syngenic bone marrow transplant from psoriatic donor: further evidence for adoptive autoimmunity. Br J Dermatol. 1997;137:130-132.
5. Cooley HM, Snowden JA, Grigg AP, et al. Outcome of rheumatoid arthritis and psoriasis following autologous stem cell transplantation for hematologic malignancy. Arthritis Rheum. 1997;40:1712-1715.
6. Kaffenberger BH, Wong HK, Jarjour W, et al. Remission of psoriasis after allogenic but not autologous, hematopoietic stem-cell transplantation. J Am Acad Dermatol. 2013;68:489-492.
To the Editor:
Remission of psoriasis after bone marrow transplant has been reported1; however, follow-up typically has been limited. We describe a case of a remote recurrence of psoriasis 13 years after autologous stem cell transplant.
In September 1997, a 48-year-old woman with a 20-year history of moderate to severe plaque psoriasis, previously managed with oral methotrexate (up to 30–40 mg weekly), presented to her primary care physician with a persistent cough and newly pervasive arthralgia and myalgia. A complete blood cell count showed a white blood cell count of 6.9 mg/dL (reference range, 3.8–10.8 mg/dL), hemoglobin level of 11.6 mg/dL (reference range, 11.7–15.5 mg/dL), and hematocrit level of 34% (reference range, 35.0%–45.0%). Bone survey demonstrated a 4×2-cm lytic expansile lesion in the occipital bone, and a bone marrow aspirate and biopsy revealed 40% plasma cells. She was subsequently diagnosed with stage IIIA IgA λ and free λ light chain multiple myeloma with an IgA level of 62.1 g/L (reference range, 1.24–2.17 g/L).
Therapy was initiated with dexamethasone in combination with pamidronate and she was transitioned to cyclophosphamide chemotherapy. A peripheral autologous blood stem cell transplant followed. Throughout her initial treatment, the psoriasis continued to flare and she remained on methotrexate to help maintain control of the skin disease.
In November 1998, the patient underwent an autologous CD34+ stem cell transplant. There were no complications following the transplant and the multiple myeloma has remained in complete remission to date. Following the stem cell transplant, the patient remained free of psoriatic disease for just over 13 years without any intervention. Recurrent erythematous plaques with thick scale on the bilateral lower legs prompted her return to dermatology in August 2011. To date, she continues to note intermittent though mild flares of her psoriasis. She has responded well to methotrexate 7.5 mg weekly and clobetasol propionate ointment 0.05% as needed with satisfactory control of her disease.
The development of autoimmune disease following an allogenic bone marrow transplant such as psoriasis, thyroiditis, myasthenia gravis, insulin-dependent diabetes mellitus, and vitiligo has been well described.2-4 The causal relationship between allogenic hematopoetic stem cell transplant (HSCT) and subsequent autoimmune disease has been attributed to T cells and passive transfer of autoimmune disease from the donor to the recipient.1
In the case of autologous HSCT, the remission of autoimmune disease thereafter has been attributed to myeloablative conditioning, whereby high-dose chemotherapy eliminates self-reactive lymphocytes, resulting in ablation of peripheral reactive and alloreactive T cells with depletion of thymus and reactive B cells.1
In 1997, Cooley et al5 described 4 patients with autoimmune disease who experienced complete but temporary (up to 26 months) remission following autologous bone marrow or stem cell transplant. A comprehensive review of psoriasis cases that resolved after HSCT published by Kaffenberger et al6 in 2013 cited 6 cases of autologous and 13 cases of allogenic transplant that resulted in resolution of psoriatic disease. Recurrence of disease was witnessed in 3 of 13 allogenic HSCT patients and 5 of 6 autologous HSCT patients. Of note, all 5 of these patients developed recurrent disease within 24 months following autologous HSCT, albeit with more benign disease.6
Similar to those cases presented by Kaffenberger et al,6 our patient’s psoriatic disease was notably mild compared to her disease prior to HSCT. Although an unintended but welcome outcome of multiple myeloma treatment, the nearly quiescent nature of her skin disease following autologous HSCT has led to a dramatic improvement in her quality of life.
We highlight a unique case in which psoriatic skin disease recurred more than 1 decade after autologous HSCT.
To the Editor:
Remission of psoriasis after bone marrow transplant has been reported1; however, follow-up typically has been limited. We describe a case of a remote recurrence of psoriasis 13 years after autologous stem cell transplant.
In September 1997, a 48-year-old woman with a 20-year history of moderate to severe plaque psoriasis, previously managed with oral methotrexate (up to 30–40 mg weekly), presented to her primary care physician with a persistent cough and newly pervasive arthralgia and myalgia. A complete blood cell count showed a white blood cell count of 6.9 mg/dL (reference range, 3.8–10.8 mg/dL), hemoglobin level of 11.6 mg/dL (reference range, 11.7–15.5 mg/dL), and hematocrit level of 34% (reference range, 35.0%–45.0%). Bone survey demonstrated a 4×2-cm lytic expansile lesion in the occipital bone, and a bone marrow aspirate and biopsy revealed 40% plasma cells. She was subsequently diagnosed with stage IIIA IgA λ and free λ light chain multiple myeloma with an IgA level of 62.1 g/L (reference range, 1.24–2.17 g/L).
Therapy was initiated with dexamethasone in combination with pamidronate and she was transitioned to cyclophosphamide chemotherapy. A peripheral autologous blood stem cell transplant followed. Throughout her initial treatment, the psoriasis continued to flare and she remained on methotrexate to help maintain control of the skin disease.
In November 1998, the patient underwent an autologous CD34+ stem cell transplant. There were no complications following the transplant and the multiple myeloma has remained in complete remission to date. Following the stem cell transplant, the patient remained free of psoriatic disease for just over 13 years without any intervention. Recurrent erythematous plaques with thick scale on the bilateral lower legs prompted her return to dermatology in August 2011. To date, she continues to note intermittent though mild flares of her psoriasis. She has responded well to methotrexate 7.5 mg weekly and clobetasol propionate ointment 0.05% as needed with satisfactory control of her disease.
The development of autoimmune disease following an allogenic bone marrow transplant such as psoriasis, thyroiditis, myasthenia gravis, insulin-dependent diabetes mellitus, and vitiligo has been well described.2-4 The causal relationship between allogenic hematopoetic stem cell transplant (HSCT) and subsequent autoimmune disease has been attributed to T cells and passive transfer of autoimmune disease from the donor to the recipient.1
In the case of autologous HSCT, the remission of autoimmune disease thereafter has been attributed to myeloablative conditioning, whereby high-dose chemotherapy eliminates self-reactive lymphocytes, resulting in ablation of peripheral reactive and alloreactive T cells with depletion of thymus and reactive B cells.1
In 1997, Cooley et al5 described 4 patients with autoimmune disease who experienced complete but temporary (up to 26 months) remission following autologous bone marrow or stem cell transplant. A comprehensive review of psoriasis cases that resolved after HSCT published by Kaffenberger et al6 in 2013 cited 6 cases of autologous and 13 cases of allogenic transplant that resulted in resolution of psoriatic disease. Recurrence of disease was witnessed in 3 of 13 allogenic HSCT patients and 5 of 6 autologous HSCT patients. Of note, all 5 of these patients developed recurrent disease within 24 months following autologous HSCT, albeit with more benign disease.6
Similar to those cases presented by Kaffenberger et al,6 our patient’s psoriatic disease was notably mild compared to her disease prior to HSCT. Although an unintended but welcome outcome of multiple myeloma treatment, the nearly quiescent nature of her skin disease following autologous HSCT has led to a dramatic improvement in her quality of life.
We highlight a unique case in which psoriatic skin disease recurred more than 1 decade after autologous HSCT.
1. Braiteh F, Hymes SR, Giralt SA, et al. Complete remission of psoriasis after autologous hematopoietic stem-cell transplantation for multiple myeloma. J Clin Oncol. 2008;26:4511-4513.
2. Kishimoto Y, Yamamoto Y, Matsumoto N, et al. Transfer of autoimmune thyroiditis and resolution of palmoplantar pustular psoriasis following allogeneic bone marrow transplantation. Bone Marrow Transpl. 1997;19:1041-1043.
3. Wahie S, Alexandroff A, Reynolds NY, et al. Psoriasis occurring after myeloablative therapy and autologous stem cell transplantation. Br J Dermatol. 2006;154:177-204.
4. Snowden JA, Heaton DC. Development of psoriasis after syngenic bone marrow transplant from psoriatic donor: further evidence for adoptive autoimmunity. Br J Dermatol. 1997;137:130-132.
5. Cooley HM, Snowden JA, Grigg AP, et al. Outcome of rheumatoid arthritis and psoriasis following autologous stem cell transplantation for hematologic malignancy. Arthritis Rheum. 1997;40:1712-1715.
6. Kaffenberger BH, Wong HK, Jarjour W, et al. Remission of psoriasis after allogenic but not autologous, hematopoietic stem-cell transplantation. J Am Acad Dermatol. 2013;68:489-492.
1. Braiteh F, Hymes SR, Giralt SA, et al. Complete remission of psoriasis after autologous hematopoietic stem-cell transplantation for multiple myeloma. J Clin Oncol. 2008;26:4511-4513.
2. Kishimoto Y, Yamamoto Y, Matsumoto N, et al. Transfer of autoimmune thyroiditis and resolution of palmoplantar pustular psoriasis following allogeneic bone marrow transplantation. Bone Marrow Transpl. 1997;19:1041-1043.
3. Wahie S, Alexandroff A, Reynolds NY, et al. Psoriasis occurring after myeloablative therapy and autologous stem cell transplantation. Br J Dermatol. 2006;154:177-204.
4. Snowden JA, Heaton DC. Development of psoriasis after syngenic bone marrow transplant from psoriatic donor: further evidence for adoptive autoimmunity. Br J Dermatol. 1997;137:130-132.
5. Cooley HM, Snowden JA, Grigg AP, et al. Outcome of rheumatoid arthritis and psoriasis following autologous stem cell transplantation for hematologic malignancy. Arthritis Rheum. 1997;40:1712-1715.
6. Kaffenberger BH, Wong HK, Jarjour W, et al. Remission of psoriasis after allogenic but not autologous, hematopoietic stem-cell transplantation. J Am Acad Dermatol. 2013;68:489-492.