User login
To the Editor:
Cyclosporine is an immunomodulatory medication that impacts T-lymphocyte function through calcineurin inhibition and suppression of IL-2 expression. Oral cyclosporine at low doses (1–3 mg/kg/d) is one of the more common systemic treatment options for moderate to severe atopic dermatitis. At these doses it has been shown to have therapeutic benefit in several skin conditions, including chronic spontaneous urticaria,1 psoriasis,2 and atopic dermatitis.3 When used at higher doses for conditions such as glomerulonephritis or transplantation, adverse effects may be notable, and close monitoring of drug metabolism as well as end-organ function is required. In contrast, severe adverse effects are uncommon with the lower doses of cyclosporine used for cutaneous conditions, and monitoring serum drug levels is not routinely practiced.4
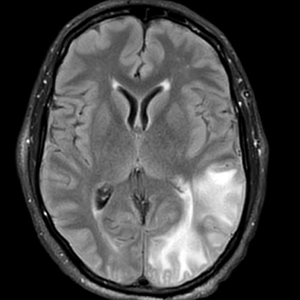
A 58-year-old man was referred to clinic with severe atopic dermatitis refractory to maximal topical therapy prescribed by an outside physician. He was started on cyclosporine as an anticipated bridge to dupilumab biologic therapy. He had no history of hypertension, renal disease, or hepatic insufficiency prior to starting therapy. He demonstrated notable clinical improvement at a cyclosporine dosage of 300 mg/d (equating to 3.7 mg/kg/d). Three months after initiation of therapy, the patient presented to a local emergency department with new-onset seizurelike activity, confusion, and agitation. He was normotensive with clinical concern for status epilepticus. An initial laboratory assessment included a complete blood cell count, serum electrolyte panel, and urine toxicology screen, which were unremarkable. Computed tomography of the head showed confluent white-matter hypodensities in the left parietal-temporal-occipital lobes. Magnetic resonance imaging (MRI) of the brain showed innumerable peripherally distributed foci of microhemorrhage and vasogenic edema within the left parietal-temporal-occipital lobes (Figure).
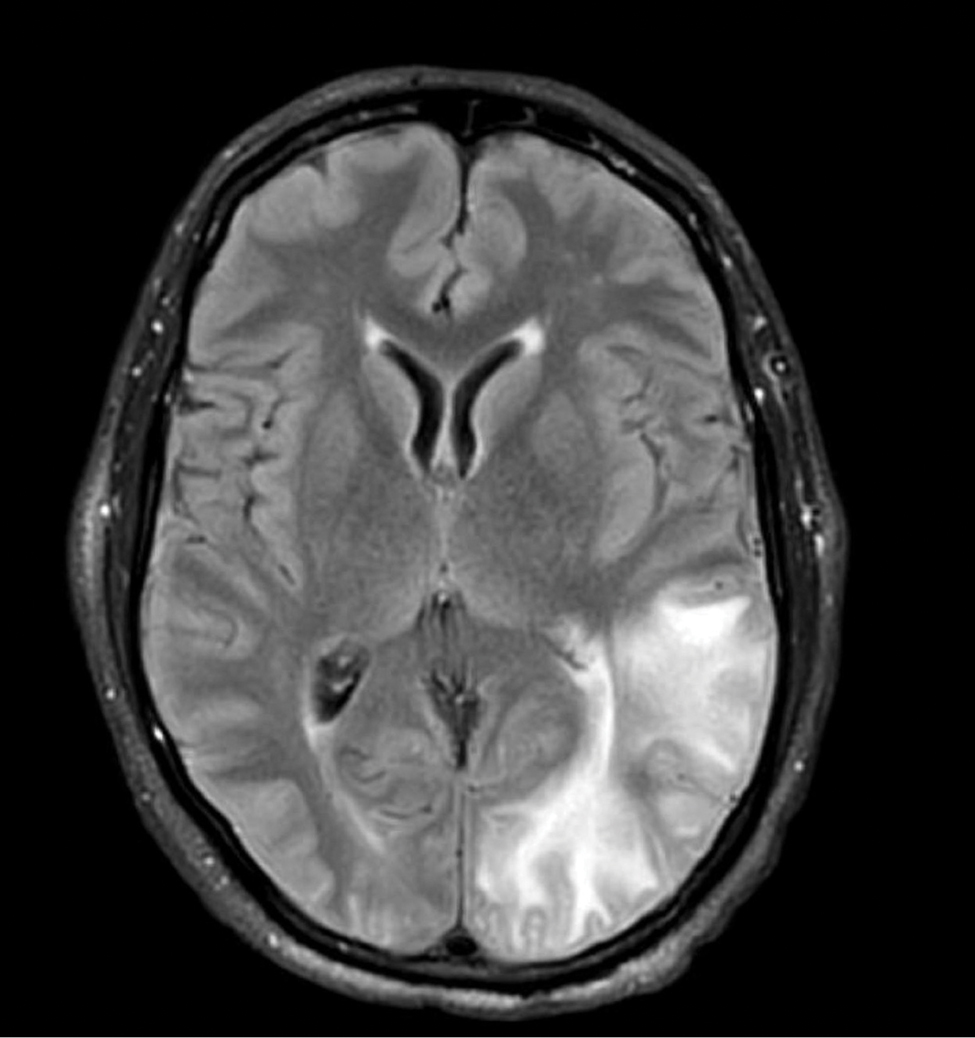
He was intubated and sedated with admission to the medical intensive care unit, where a random cyclosporine level drawn approximately 9 hours after the prior dose was noted to be 263 ng/mL. Although target therapeutic levels for cyclosporine vary based on indication, toxic supratherapeutic levels generally are considered to be greater than 400 ng/mL.5 He had no evidence of acute kidney injury, uremia, or hypertension throughout hospitalization. An electroencephalogram showed left parieto-occipital periodic epileptiform discharges with generalized slowing. Cyclosporine was discontinued, and he was started on levetiracetam. His clinical and neuroimaging findings improved over the course of the 1-week hospitalization without any further intervention. Four weeks after hospitalization, he had full neurologic, electroencephalogram, and imaging recovery. Based on the presenting symptoms, transient neuroimaging findings, and full recovery with discontinuation of cyclosporine, the patient was diagnosed with cyclosporine-induced posterior reversible encephalopathy syndrome (PRES).
The diagnosis of PRES requires evidence of acute neurologic symptoms and radiographic findings of cortical/subcortical white-matter changes on computed tomography or MRI consistent with edema. The pathophysiology is not fully understood but appears to be related to vasogenic edema, primarily impacting the posterior aspect of the brain. There have been many reported offending agents, and symptoms typically resolve following cessation of these medications. Cases of cyclosporine-induced PRES have been reported, but most occurred at higher doses within weeks of medication initiation. Two cases of cyclosporine-induced PRES treated with cutaneous dosing have been reported; neither patient was taking it for atopic dermatitis.6
Cyclosporine-induced PRES remains a pathophysiologic conundrum. However, there is evidence to support direct endothelial damage causing cellular apoptosis in the brain of mouse models that is medication specific and not necessarily related to the dosages used.7 Our case highlights a rare but important adverse event associated with even low-dose cyclosporine use that should be considered in patients currently taking cyclosporine who present with acute neurologic changes.
- Kulthanan K, Chaweekulrat P, Komoltri C, et al. Cyclosporine for chronic spontaneous urticaria: a meta-analysis and systematic review. J Allergy Clin Immunol Pract. 2018;6:586-599. doi:10.1016/j.jaip.2017.07.017
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323:1945-1960. doi:10.1001/jama.2020.4006
- Seger EW, Wechter T, Strowd L, et al. Relative efficacy of systemic treatments for atopic dermatitis [published online October 6, 2018]. J Am Acad Dermatol. 2019;80:411-416.e4. doi:10.1016/j.jaad.2018.09.053
- Blake SC, Murrell DF. Monitoring trough levels in cyclosporine for atopic dermatitis: a systematic review. Pediatr Dermatol. 2019;36:843-853. doi:10.1111/pde.13999
- Tapia C, Nessel TA, Zito PM. Cyclosporine. StatPearls Publishing: 2022. https://www.ncbi.nlm.nih.gov/books/NBK482450/
- Cosottini M, Lazzarotti G, Ceravolo R, et al. Cyclosporine‐related posterior reversible encephalopathy syndrome (PRES) in non‐transplant patient: a case report and literature review. Eur J Neurol. 2003;10:461-462. doi:10.1046/j.1468-1331.2003.00608_1.x
- Kochi S, Takanaga H, Matsuo H, et al. Induction of apoptosis in mouse brain capillary endothelial cells by cyclosporin A and tacrolimus. Life Sci. 2000;66:2255-2260. doi:10.1016/s0024-3205(00)00554-3
To the Editor:
Cyclosporine is an immunomodulatory medication that impacts T-lymphocyte function through calcineurin inhibition and suppression of IL-2 expression. Oral cyclosporine at low doses (1–3 mg/kg/d) is one of the more common systemic treatment options for moderate to severe atopic dermatitis. At these doses it has been shown to have therapeutic benefit in several skin conditions, including chronic spontaneous urticaria,1 psoriasis,2 and atopic dermatitis.3 When used at higher doses for conditions such as glomerulonephritis or transplantation, adverse effects may be notable, and close monitoring of drug metabolism as well as end-organ function is required. In contrast, severe adverse effects are uncommon with the lower doses of cyclosporine used for cutaneous conditions, and monitoring serum drug levels is not routinely practiced.4
A 58-year-old man was referred to clinic with severe atopic dermatitis refractory to maximal topical therapy prescribed by an outside physician. He was started on cyclosporine as an anticipated bridge to dupilumab biologic therapy. He had no history of hypertension, renal disease, or hepatic insufficiency prior to starting therapy. He demonstrated notable clinical improvement at a cyclosporine dosage of 300 mg/d (equating to 3.7 mg/kg/d). Three months after initiation of therapy, the patient presented to a local emergency department with new-onset seizurelike activity, confusion, and agitation. He was normotensive with clinical concern for status epilepticus. An initial laboratory assessment included a complete blood cell count, serum electrolyte panel, and urine toxicology screen, which were unremarkable. Computed tomography of the head showed confluent white-matter hypodensities in the left parietal-temporal-occipital lobes. Magnetic resonance imaging (MRI) of the brain showed innumerable peripherally distributed foci of microhemorrhage and vasogenic edema within the left parietal-temporal-occipital lobes (Figure).

He was intubated and sedated with admission to the medical intensive care unit, where a random cyclosporine level drawn approximately 9 hours after the prior dose was noted to be 263 ng/mL. Although target therapeutic levels for cyclosporine vary based on indication, toxic supratherapeutic levels generally are considered to be greater than 400 ng/mL.5 He had no evidence of acute kidney injury, uremia, or hypertension throughout hospitalization. An electroencephalogram showed left parieto-occipital periodic epileptiform discharges with generalized slowing. Cyclosporine was discontinued, and he was started on levetiracetam. His clinical and neuroimaging findings improved over the course of the 1-week hospitalization without any further intervention. Four weeks after hospitalization, he had full neurologic, electroencephalogram, and imaging recovery. Based on the presenting symptoms, transient neuroimaging findings, and full recovery with discontinuation of cyclosporine, the patient was diagnosed with cyclosporine-induced posterior reversible encephalopathy syndrome (PRES).
The diagnosis of PRES requires evidence of acute neurologic symptoms and radiographic findings of cortical/subcortical white-matter changes on computed tomography or MRI consistent with edema. The pathophysiology is not fully understood but appears to be related to vasogenic edema, primarily impacting the posterior aspect of the brain. There have been many reported offending agents, and symptoms typically resolve following cessation of these medications. Cases of cyclosporine-induced PRES have been reported, but most occurred at higher doses within weeks of medication initiation. Two cases of cyclosporine-induced PRES treated with cutaneous dosing have been reported; neither patient was taking it for atopic dermatitis.6
Cyclosporine-induced PRES remains a pathophysiologic conundrum. However, there is evidence to support direct endothelial damage causing cellular apoptosis in the brain of mouse models that is medication specific and not necessarily related to the dosages used.7 Our case highlights a rare but important adverse event associated with even low-dose cyclosporine use that should be considered in patients currently taking cyclosporine who present with acute neurologic changes.
To the Editor:
Cyclosporine is an immunomodulatory medication that impacts T-lymphocyte function through calcineurin inhibition and suppression of IL-2 expression. Oral cyclosporine at low doses (1–3 mg/kg/d) is one of the more common systemic treatment options for moderate to severe atopic dermatitis. At these doses it has been shown to have therapeutic benefit in several skin conditions, including chronic spontaneous urticaria,1 psoriasis,2 and atopic dermatitis.3 When used at higher doses for conditions such as glomerulonephritis or transplantation, adverse effects may be notable, and close monitoring of drug metabolism as well as end-organ function is required. In contrast, severe adverse effects are uncommon with the lower doses of cyclosporine used for cutaneous conditions, and monitoring serum drug levels is not routinely practiced.4
A 58-year-old man was referred to clinic with severe atopic dermatitis refractory to maximal topical therapy prescribed by an outside physician. He was started on cyclosporine as an anticipated bridge to dupilumab biologic therapy. He had no history of hypertension, renal disease, or hepatic insufficiency prior to starting therapy. He demonstrated notable clinical improvement at a cyclosporine dosage of 300 mg/d (equating to 3.7 mg/kg/d). Three months after initiation of therapy, the patient presented to a local emergency department with new-onset seizurelike activity, confusion, and agitation. He was normotensive with clinical concern for status epilepticus. An initial laboratory assessment included a complete blood cell count, serum electrolyte panel, and urine toxicology screen, which were unremarkable. Computed tomography of the head showed confluent white-matter hypodensities in the left parietal-temporal-occipital lobes. Magnetic resonance imaging (MRI) of the brain showed innumerable peripherally distributed foci of microhemorrhage and vasogenic edema within the left parietal-temporal-occipital lobes (Figure).

He was intubated and sedated with admission to the medical intensive care unit, where a random cyclosporine level drawn approximately 9 hours after the prior dose was noted to be 263 ng/mL. Although target therapeutic levels for cyclosporine vary based on indication, toxic supratherapeutic levels generally are considered to be greater than 400 ng/mL.5 He had no evidence of acute kidney injury, uremia, or hypertension throughout hospitalization. An electroencephalogram showed left parieto-occipital periodic epileptiform discharges with generalized slowing. Cyclosporine was discontinued, and he was started on levetiracetam. His clinical and neuroimaging findings improved over the course of the 1-week hospitalization without any further intervention. Four weeks after hospitalization, he had full neurologic, electroencephalogram, and imaging recovery. Based on the presenting symptoms, transient neuroimaging findings, and full recovery with discontinuation of cyclosporine, the patient was diagnosed with cyclosporine-induced posterior reversible encephalopathy syndrome (PRES).
The diagnosis of PRES requires evidence of acute neurologic symptoms and radiographic findings of cortical/subcortical white-matter changes on computed tomography or MRI consistent with edema. The pathophysiology is not fully understood but appears to be related to vasogenic edema, primarily impacting the posterior aspect of the brain. There have been many reported offending agents, and symptoms typically resolve following cessation of these medications. Cases of cyclosporine-induced PRES have been reported, but most occurred at higher doses within weeks of medication initiation. Two cases of cyclosporine-induced PRES treated with cutaneous dosing have been reported; neither patient was taking it for atopic dermatitis.6
Cyclosporine-induced PRES remains a pathophysiologic conundrum. However, there is evidence to support direct endothelial damage causing cellular apoptosis in the brain of mouse models that is medication specific and not necessarily related to the dosages used.7 Our case highlights a rare but important adverse event associated with even low-dose cyclosporine use that should be considered in patients currently taking cyclosporine who present with acute neurologic changes.
- Kulthanan K, Chaweekulrat P, Komoltri C, et al. Cyclosporine for chronic spontaneous urticaria: a meta-analysis and systematic review. J Allergy Clin Immunol Pract. 2018;6:586-599. doi:10.1016/j.jaip.2017.07.017
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323:1945-1960. doi:10.1001/jama.2020.4006
- Seger EW, Wechter T, Strowd L, et al. Relative efficacy of systemic treatments for atopic dermatitis [published online October 6, 2018]. J Am Acad Dermatol. 2019;80:411-416.e4. doi:10.1016/j.jaad.2018.09.053
- Blake SC, Murrell DF. Monitoring trough levels in cyclosporine for atopic dermatitis: a systematic review. Pediatr Dermatol. 2019;36:843-853. doi:10.1111/pde.13999
- Tapia C, Nessel TA, Zito PM. Cyclosporine. StatPearls Publishing: 2022. https://www.ncbi.nlm.nih.gov/books/NBK482450/
- Cosottini M, Lazzarotti G, Ceravolo R, et al. Cyclosporine‐related posterior reversible encephalopathy syndrome (PRES) in non‐transplant patient: a case report and literature review. Eur J Neurol. 2003;10:461-462. doi:10.1046/j.1468-1331.2003.00608_1.x
- Kochi S, Takanaga H, Matsuo H, et al. Induction of apoptosis in mouse brain capillary endothelial cells by cyclosporin A and tacrolimus. Life Sci. 2000;66:2255-2260. doi:10.1016/s0024-3205(00)00554-3
- Kulthanan K, Chaweekulrat P, Komoltri C, et al. Cyclosporine for chronic spontaneous urticaria: a meta-analysis and systematic review. J Allergy Clin Immunol Pract. 2018;6:586-599. doi:10.1016/j.jaip.2017.07.017
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323:1945-1960. doi:10.1001/jama.2020.4006
- Seger EW, Wechter T, Strowd L, et al. Relative efficacy of systemic treatments for atopic dermatitis [published online October 6, 2018]. J Am Acad Dermatol. 2019;80:411-416.e4. doi:10.1016/j.jaad.2018.09.053
- Blake SC, Murrell DF. Monitoring trough levels in cyclosporine for atopic dermatitis: a systematic review. Pediatr Dermatol. 2019;36:843-853. doi:10.1111/pde.13999
- Tapia C, Nessel TA, Zito PM. Cyclosporine. StatPearls Publishing: 2022. https://www.ncbi.nlm.nih.gov/books/NBK482450/
- Cosottini M, Lazzarotti G, Ceravolo R, et al. Cyclosporine‐related posterior reversible encephalopathy syndrome (PRES) in non‐transplant patient: a case report and literature review. Eur J Neurol. 2003;10:461-462. doi:10.1046/j.1468-1331.2003.00608_1.x
- Kochi S, Takanaga H, Matsuo H, et al. Induction of apoptosis in mouse brain capillary endothelial cells by cyclosporin A and tacrolimus. Life Sci. 2000;66:2255-2260. doi:10.1016/s0024-3205(00)00554-3
Practice Points
- Cyclosporine is an immunomodulatory therapeutic utilized for several indications in dermatology practice, most commonly in low doses.
- Posterior reversible encephalopathy syndrome (PRES) is a known but rare adverse effect of cyclosporine presenting with acute encephalopathic changes and radiographic findings on central imaging.
- Knowledge of this association is critical, as symptoms are reversible with prompt recognition, appropriate inpatient supportive care, and discontinuation of offending medications.