User login
A 74-year-old Caucasian man presented to the hospital with intractable back and chest pain, a diffuse skin rash, and altered mental status. He said that 2 days ago, he’d gone to a different local hospital for treatment of back pain and a headache that had begun 3 days earlier. He was treated with intravenous hydromorphone and sent home with a prescription for meperidine. He said that several hours after being treated with the hydromorphone, the rash developed on his head and then spread to his trunk and upper extremities.
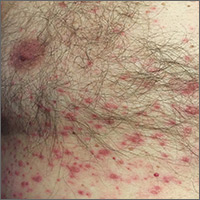
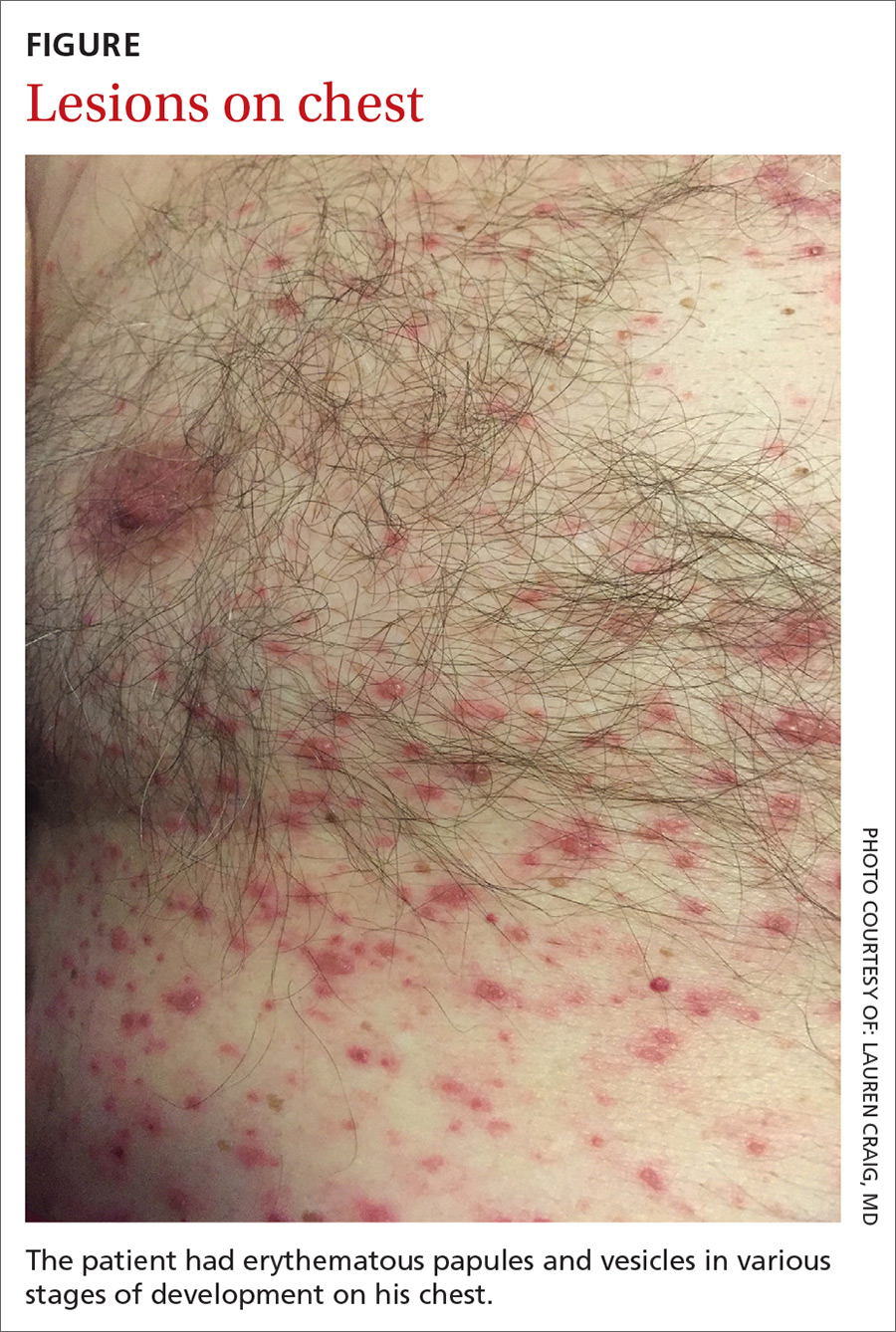
On physical examination, the patient was afebrile. He had numerous erythematous papules and vesicles in various stages of development on his scalp, face, neck, chest (FIGURE), abdomen, back, upper extremities, and groin. The lesions continued to spread and eventually involved his posterior oropharynx. The patient also developed conjunctivitis.
Laboratory findings included a white blood cell count of 4000/mcL (normal: 4500-11,000/mcL) with 65.9% segmented neutrophils (normal: 40%-60%), and 16.7% lymphocytes (normal: 20%-40%). Lab tests also revealed an aspartate aminotransferase level of 263 U/L (normal: 10-40 U/L), alanine aminotransferase of 236 U/L (normal: 7-56 U/L), and lactate dehydrogenase of 628 U/L (normal: 140-280 U/L).
The patient’s medical history was significant for hypertension, osteoarthritis, and IgG-kappa multiple myeloma, which had been treated with multiple chemotherapy regimens that included lenalidomide. Five years earlier, he’d undergone an autologous bone marrow transplant (BMT). At the time of presentation, the patient was being treated with daratumumab; he received his most recent treatment approximately one month earlier. Other medications included amlodipine, esomeprazole, and escitalopram.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Disseminated varicella-zoster virus infection
Because of the patient’s immunocompromised state, his presentation with altered mental status and diffuse rash was concerning. On hospital Day 2, a sample was taken from one of his skin lesions. Polymerase chain reaction (PCR) detected varicella-zoster virus (VZV), and we diagnosed disseminated VZV infection. On hospital Day 3, we performed a lumbar puncture because of worsening confusion and discovered that the cerebrospinal fluid was also positive for VZV.
Disseminated VZV is the most common cause of late infection in patients who have received an allogenic BMT; it is usually due to reactivation of the virus.1 In one study of 1186 patients who underwent BMT, 52% developed VZV infection within 5 years.2 Disseminated VZV may also involve visceral organs, causing pneumonitis, pancreatitis, hepatitis, or encephalitis. Mortality rates for disseminated VZV are as high as 50%.3 Because of this, physicians should be vigilant when patients who have received a BMT present with a rash and signs of systemic involvement.
Two reliable tests. Even when lesions are classic for VZV, the diagnosis must be confirmed by laboratory testing. Real-time PCR assay is a rapid and highly sensitive test for diagnosing VZV.4 Another rapid test that can be used to confirm the clinical diagnosis of VZV is a direct fluorescent antibody assay, which is becoming more widely available.
In contrast, the sensitivity of viral culture for VZV has been reported to be as low as 20%.5 Viral culture also takes much longer and has a significantly lower yield compared with newer methods.6 A biopsy of skin lesions will reveal multinucleated giant cells, but cannot differentiate between herpes simplex virus (HSV) and VZV.7
These lesions can be mimicked
When a rash develops following the use of intravenous hydromorphone, as occurred with our patient, a drug reaction must be ruled out. A drug reaction can cause almost any skin manifestation and may present as vesicles, a macular rash, a papular rash, or diffuse erythema. In this case, drug rash was ruled out by the positive VZV PCR.
Viral exanthems can also present in a variety of ways. They may cause a macular, papular, or vesicular rash.
Prompt management is crucial
Prompt treatment of VZV with acyclovir improves outcomes, but death may still occur, even with early diagnosis.3 Immunocompromised patients with VZV should be closely monitored for secondary infections, which may rapidly progress and become fatal.8 The Centers for Disease Control and Prevention recommends both airborne and contact precautions for patients with disseminated VZV until all lesions are dry and crusted.9
While the live zoster vaccine is approved for prevention of shingles in patients <60 years of age, it is contraindicated in patients with a history of primary or acquired immunodeficiency states including leukemia, lymphoma, or other malignant neoplasms affecting bone marrow.
Our patient. On admission, he was treated with intravenous (IV) acyclovir 10 mg/kg TID; IV vancomycin 15 mg/kg every 12 hours; and IV ceftriaxone 2 g/d. Slowly, his mental status returned to baseline, and his rash and conjunctivitis resolved. We discharged him on hospital Day 12. He was transitioned to oral valacyclovir 1000 mg TID. Including both inpatient and outpatient treatment, the patient received 3 weeks (total) of acyclovir/valacyclovir therapy.
CORRESPONDENCE
Caitlyn T. Reed, MD, School of Medicine, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; [email protected].
1. Locksley RM, Flournoy N, Sullivan KM, et al. Infection with varicella-zoster virus after marrow transplantation. J Infect Dis. 1985;152:1172-1181.
2. Han CS, Miller W, Haake R, et al. Varicella zoster infection after bone marrow transplantation: incidence, risk factors and complications. Bone Marrow Transplant. 1994;13:277-283.
3. David DS, Tegtmeier BR, O’Donnell MR, at el. Visceral varicella-zoster after bone marrow transplantation: report of a case series and review of the literature. Am J Gastroenterol. 1998;93:810-813.
4. Harbecke R, Oxman MN, Arnold BA, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310-1322.
5. Sauerbrei A, Eichhorn U, Schacke M, et al. Laboratory diagnosis of herpes zoster. J Clin Virol. 1999;14:31-36.
6. Gnann JW Jr, Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med. 2002;347;340-346.
7. Mendoza N, Madkan V, Sra K, et al. Human herpesviruses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd edition. China: Elsevier Limited; 2012:1321-1343.
8. Woznowski M, Quack I, Bölke E, et al. Fulminant staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 2010;15:410-414.
9. Centers for Disease Control and Prevention. Preventing varicella in healthcare settings. Available at: http://www.cdc.gov/chickenpox/hcp/healthcare-setting.html. Accessed October 6,2017.
A 74-year-old Caucasian man presented to the hospital with intractable back and chest pain, a diffuse skin rash, and altered mental status. He said that 2 days ago, he’d gone to a different local hospital for treatment of back pain and a headache that had begun 3 days earlier. He was treated with intravenous hydromorphone and sent home with a prescription for meperidine. He said that several hours after being treated with the hydromorphone, the rash developed on his head and then spread to his trunk and upper extremities.
On physical examination, the patient was afebrile. He had numerous erythematous papules and vesicles in various stages of development on his scalp, face, neck, chest (FIGURE), abdomen, back, upper extremities, and groin. The lesions continued to spread and eventually involved his posterior oropharynx. The patient also developed conjunctivitis.

Laboratory findings included a white blood cell count of 4000/mcL (normal: 4500-11,000/mcL) with 65.9% segmented neutrophils (normal: 40%-60%), and 16.7% lymphocytes (normal: 20%-40%). Lab tests also revealed an aspartate aminotransferase level of 263 U/L (normal: 10-40 U/L), alanine aminotransferase of 236 U/L (normal: 7-56 U/L), and lactate dehydrogenase of 628 U/L (normal: 140-280 U/L).
The patient’s medical history was significant for hypertension, osteoarthritis, and IgG-kappa multiple myeloma, which had been treated with multiple chemotherapy regimens that included lenalidomide. Five years earlier, he’d undergone an autologous bone marrow transplant (BMT). At the time of presentation, the patient was being treated with daratumumab; he received his most recent treatment approximately one month earlier. Other medications included amlodipine, esomeprazole, and escitalopram.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Disseminated varicella-zoster virus infection
Because of the patient’s immunocompromised state, his presentation with altered mental status and diffuse rash was concerning. On hospital Day 2, a sample was taken from one of his skin lesions. Polymerase chain reaction (PCR) detected varicella-zoster virus (VZV), and we diagnosed disseminated VZV infection. On hospital Day 3, we performed a lumbar puncture because of worsening confusion and discovered that the cerebrospinal fluid was also positive for VZV.
Disseminated VZV is the most common cause of late infection in patients who have received an allogenic BMT; it is usually due to reactivation of the virus.1 In one study of 1186 patients who underwent BMT, 52% developed VZV infection within 5 years.2 Disseminated VZV may also involve visceral organs, causing pneumonitis, pancreatitis, hepatitis, or encephalitis. Mortality rates for disseminated VZV are as high as 50%.3 Because of this, physicians should be vigilant when patients who have received a BMT present with a rash and signs of systemic involvement.
Two reliable tests. Even when lesions are classic for VZV, the diagnosis must be confirmed by laboratory testing. Real-time PCR assay is a rapid and highly sensitive test for diagnosing VZV.4 Another rapid test that can be used to confirm the clinical diagnosis of VZV is a direct fluorescent antibody assay, which is becoming more widely available.
In contrast, the sensitivity of viral culture for VZV has been reported to be as low as 20%.5 Viral culture also takes much longer and has a significantly lower yield compared with newer methods.6 A biopsy of skin lesions will reveal multinucleated giant cells, but cannot differentiate between herpes simplex virus (HSV) and VZV.7
These lesions can be mimicked
When a rash develops following the use of intravenous hydromorphone, as occurred with our patient, a drug reaction must be ruled out. A drug reaction can cause almost any skin manifestation and may present as vesicles, a macular rash, a papular rash, or diffuse erythema. In this case, drug rash was ruled out by the positive VZV PCR.
Viral exanthems can also present in a variety of ways. They may cause a macular, papular, or vesicular rash.
Prompt management is crucial
Prompt treatment of VZV with acyclovir improves outcomes, but death may still occur, even with early diagnosis.3 Immunocompromised patients with VZV should be closely monitored for secondary infections, which may rapidly progress and become fatal.8 The Centers for Disease Control and Prevention recommends both airborne and contact precautions for patients with disseminated VZV until all lesions are dry and crusted.9
While the live zoster vaccine is approved for prevention of shingles in patients <60 years of age, it is contraindicated in patients with a history of primary or acquired immunodeficiency states including leukemia, lymphoma, or other malignant neoplasms affecting bone marrow.
Our patient. On admission, he was treated with intravenous (IV) acyclovir 10 mg/kg TID; IV vancomycin 15 mg/kg every 12 hours; and IV ceftriaxone 2 g/d. Slowly, his mental status returned to baseline, and his rash and conjunctivitis resolved. We discharged him on hospital Day 12. He was transitioned to oral valacyclovir 1000 mg TID. Including both inpatient and outpatient treatment, the patient received 3 weeks (total) of acyclovir/valacyclovir therapy.
CORRESPONDENCE
Caitlyn T. Reed, MD, School of Medicine, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; [email protected].
A 74-year-old Caucasian man presented to the hospital with intractable back and chest pain, a diffuse skin rash, and altered mental status. He said that 2 days ago, he’d gone to a different local hospital for treatment of back pain and a headache that had begun 3 days earlier. He was treated with intravenous hydromorphone and sent home with a prescription for meperidine. He said that several hours after being treated with the hydromorphone, the rash developed on his head and then spread to his trunk and upper extremities.
On physical examination, the patient was afebrile. He had numerous erythematous papules and vesicles in various stages of development on his scalp, face, neck, chest (FIGURE), abdomen, back, upper extremities, and groin. The lesions continued to spread and eventually involved his posterior oropharynx. The patient also developed conjunctivitis.

Laboratory findings included a white blood cell count of 4000/mcL (normal: 4500-11,000/mcL) with 65.9% segmented neutrophils (normal: 40%-60%), and 16.7% lymphocytes (normal: 20%-40%). Lab tests also revealed an aspartate aminotransferase level of 263 U/L (normal: 10-40 U/L), alanine aminotransferase of 236 U/L (normal: 7-56 U/L), and lactate dehydrogenase of 628 U/L (normal: 140-280 U/L).
The patient’s medical history was significant for hypertension, osteoarthritis, and IgG-kappa multiple myeloma, which had been treated with multiple chemotherapy regimens that included lenalidomide. Five years earlier, he’d undergone an autologous bone marrow transplant (BMT). At the time of presentation, the patient was being treated with daratumumab; he received his most recent treatment approximately one month earlier. Other medications included amlodipine, esomeprazole, and escitalopram.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Disseminated varicella-zoster virus infection
Because of the patient’s immunocompromised state, his presentation with altered mental status and diffuse rash was concerning. On hospital Day 2, a sample was taken from one of his skin lesions. Polymerase chain reaction (PCR) detected varicella-zoster virus (VZV), and we diagnosed disseminated VZV infection. On hospital Day 3, we performed a lumbar puncture because of worsening confusion and discovered that the cerebrospinal fluid was also positive for VZV.
Disseminated VZV is the most common cause of late infection in patients who have received an allogenic BMT; it is usually due to reactivation of the virus.1 In one study of 1186 patients who underwent BMT, 52% developed VZV infection within 5 years.2 Disseminated VZV may also involve visceral organs, causing pneumonitis, pancreatitis, hepatitis, or encephalitis. Mortality rates for disseminated VZV are as high as 50%.3 Because of this, physicians should be vigilant when patients who have received a BMT present with a rash and signs of systemic involvement.
Two reliable tests. Even when lesions are classic for VZV, the diagnosis must be confirmed by laboratory testing. Real-time PCR assay is a rapid and highly sensitive test for diagnosing VZV.4 Another rapid test that can be used to confirm the clinical diagnosis of VZV is a direct fluorescent antibody assay, which is becoming more widely available.
In contrast, the sensitivity of viral culture for VZV has been reported to be as low as 20%.5 Viral culture also takes much longer and has a significantly lower yield compared with newer methods.6 A biopsy of skin lesions will reveal multinucleated giant cells, but cannot differentiate between herpes simplex virus (HSV) and VZV.7
These lesions can be mimicked
When a rash develops following the use of intravenous hydromorphone, as occurred with our patient, a drug reaction must be ruled out. A drug reaction can cause almost any skin manifestation and may present as vesicles, a macular rash, a papular rash, or diffuse erythema. In this case, drug rash was ruled out by the positive VZV PCR.
Viral exanthems can also present in a variety of ways. They may cause a macular, papular, or vesicular rash.
Prompt management is crucial
Prompt treatment of VZV with acyclovir improves outcomes, but death may still occur, even with early diagnosis.3 Immunocompromised patients with VZV should be closely monitored for secondary infections, which may rapidly progress and become fatal.8 The Centers for Disease Control and Prevention recommends both airborne and contact precautions for patients with disseminated VZV until all lesions are dry and crusted.9
While the live zoster vaccine is approved for prevention of shingles in patients <60 years of age, it is contraindicated in patients with a history of primary or acquired immunodeficiency states including leukemia, lymphoma, or other malignant neoplasms affecting bone marrow.
Our patient. On admission, he was treated with intravenous (IV) acyclovir 10 mg/kg TID; IV vancomycin 15 mg/kg every 12 hours; and IV ceftriaxone 2 g/d. Slowly, his mental status returned to baseline, and his rash and conjunctivitis resolved. We discharged him on hospital Day 12. He was transitioned to oral valacyclovir 1000 mg TID. Including both inpatient and outpatient treatment, the patient received 3 weeks (total) of acyclovir/valacyclovir therapy.
CORRESPONDENCE
Caitlyn T. Reed, MD, School of Medicine, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; [email protected].
1. Locksley RM, Flournoy N, Sullivan KM, et al. Infection with varicella-zoster virus after marrow transplantation. J Infect Dis. 1985;152:1172-1181.
2. Han CS, Miller W, Haake R, et al. Varicella zoster infection after bone marrow transplantation: incidence, risk factors and complications. Bone Marrow Transplant. 1994;13:277-283.
3. David DS, Tegtmeier BR, O’Donnell MR, at el. Visceral varicella-zoster after bone marrow transplantation: report of a case series and review of the literature. Am J Gastroenterol. 1998;93:810-813.
4. Harbecke R, Oxman MN, Arnold BA, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310-1322.
5. Sauerbrei A, Eichhorn U, Schacke M, et al. Laboratory diagnosis of herpes zoster. J Clin Virol. 1999;14:31-36.
6. Gnann JW Jr, Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med. 2002;347;340-346.
7. Mendoza N, Madkan V, Sra K, et al. Human herpesviruses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd edition. China: Elsevier Limited; 2012:1321-1343.
8. Woznowski M, Quack I, Bölke E, et al. Fulminant staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 2010;15:410-414.
9. Centers for Disease Control and Prevention. Preventing varicella in healthcare settings. Available at: http://www.cdc.gov/chickenpox/hcp/healthcare-setting.html. Accessed October 6,2017.
1. Locksley RM, Flournoy N, Sullivan KM, et al. Infection with varicella-zoster virus after marrow transplantation. J Infect Dis. 1985;152:1172-1181.
2. Han CS, Miller W, Haake R, et al. Varicella zoster infection after bone marrow transplantation: incidence, risk factors and complications. Bone Marrow Transplant. 1994;13:277-283.
3. David DS, Tegtmeier BR, O’Donnell MR, at el. Visceral varicella-zoster after bone marrow transplantation: report of a case series and review of the literature. Am J Gastroenterol. 1998;93:810-813.
4. Harbecke R, Oxman MN, Arnold BA, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310-1322.
5. Sauerbrei A, Eichhorn U, Schacke M, et al. Laboratory diagnosis of herpes zoster. J Clin Virol. 1999;14:31-36.
6. Gnann JW Jr, Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med. 2002;347;340-346.
7. Mendoza N, Madkan V, Sra K, et al. Human herpesviruses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd edition. China: Elsevier Limited; 2012:1321-1343.
8. Woznowski M, Quack I, Bölke E, et al. Fulminant staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 2010;15:410-414.
9. Centers for Disease Control and Prevention. Preventing varicella in healthcare settings. Available at: http://www.cdc.gov/chickenpox/hcp/healthcare-setting.html. Accessed October 6,2017.