User login
Acne is a chronic inflammatory condition of the pilosebaceous unit affecting approximately 79% to 95% of adolescents in the Western world.1 Treatment of acne depends on its severity. Topical tretinoin, adapalene, benzoyl peroxide, azelaic acid, and topical antibiotics generally are used in cases of noninflammatory or mild inflammatory disease. Isotretinoin is recommended for treatment of severe inflammatory acne (eg, nodulocystic or conglobata acne) and for cases of acne that have proven to be resistant to prior treatment with antibiotics or topical agents. Dosages of isotretinoin range from 0.5 to 2 mg/kg daily for 16 to 24 weeks.1 Isotretinoin reduces the activity and size of the sebaceous glands, normalizes keratinization of the sebaceous follicles, and decreases the number of Propionibacterium acnes. Isotretinoin also may cause clinical side effects and laboratory changes, the most important being teratogenicity. It also may cause mucocutaneous side effects including cracked lips, dryness of the skin and nasal mucosa, skin redness, eye dryness, and eye irritation.1 It also may cause blepharoconjuctivitis, photosensitivity, asteatotic dermatitis, pruritus, telogen effluvium, secondary bacterial colonization, nail fragility, periungual pyogenic granuloma, paronychia, myalgia, intracranial hypertension, nausea, headache, vomiting, depression, psychosis, suicide, constipation, and allergic reactions.2 Isotretinoin treatment may increase serum levels of liver enzymes, triglyc-erides (TGs), and low-density lipoprotein (LDL) cholesterol, and reduce the level of high-density lipoprotein (HDL) cholesterol.1 This retrospective study sought to evaluate the effect of isotretinoin on liver enzymes and lipids over 6 months.
Materials and Methods
Our retrospective study was conducted at the Hospital of Atatürk University in Erzurum, a city located in eastern Turkey. All patients who were treated in the department of dermatology and had received oral isotretinoin between June 2009 and June 2012 were included in the study. The study was based on an evaluation of the patients’ medical records. All patients received oral isotretinoin 0.5 to 1 mg/kg daily; the majority of patients received 30 to 40 mg daily. Patient medical records included age; gender; white blood cell (WBC) count; red blood cell (RBC) count; hemoglobin count; and aspartate aminotransferase (AST), alanine aminotransferase (ALT), TG, LDL, and HDL levels at the beginning of treatment. Aspartate aminotransferase, ALT, TG, LDL, and HDL levels also were measured at 3- and 6-month follow-up. Analysis of AST, ALT, TG, LDL, and HDL levels was based on the National Cholesterol Education Program guidelines.3 Aspartate aminotransferase and ALT levels were classified as normal (<40 U/L) and high (≥40 U/L). Triglyceride levels were classified as normal (<150 mg/dL), borderline high (150–199 mg/dL), high (200–499 mg/dL), and very high (≥500 mg/dL). Low-density lipoprotein levels were classified as optimal (<100 mg/dL), above optimal (100–129 mg/dL), borderline high (130–159 mg/dL), high (160–189 mg/dL), and very high (≥190 mg/dL). High-density lipoprotein levels were classified as low (<40 mg/dL), normal (40–59 mg/dL), and high (≥60 mg/dL). Normal WBC was defined as 3.5 to 12.5×103/mL. Normal hemoglobin count was defined as 11.5 to 15.0×106/mL for women and 13 to 17×106/mL for men. Normal RBC was defined as 4.0 to 5.2×106/mL for women and 4.5 to 5.9×106/mL for men. Statistical analysis was performed using SPSS version 17.0. Repeated measures analysis of variance was used to compare means between 3 groups (baseline, 3-month, and 6-month values). A paired sample t test was used to compare means between any 2 groups. Results with P<.05 were considered statistically significant.
Results
Treatment with oral isotretinoin was initiated in 349 patients at our institution from June 2009 to June 2012. Twenty-seven of these patients were excluded from the study because their medical records were not available. Medical records from 322 patients were obtained. The study population consisted of 226 (70.2%) women and 96 (29.8%) men. Patients ranged in age from 17 to 64 years, with a mean age of 23.9 years. The mean (standard deviation [SD]) age of the women was 23.9 (5.4) years and the mean (SD) age of the men was 23.8 (7.02) years. Most of the patients were on a regimen of 30 or 40 mg of isotretinoin daily. Differences between dosages and laboratory values were not analyzed because we assumed there would not be a significant difference, as most patients received the same dose. The mean (SD) WBC was 8.4 (3.5)×103/mL. The mean (SD) RBC was 4.9 (0.5)×106/mL. The mean (SD) hemoglobin count was 14.3 (1.7)×106/mL (women, 13.6 [1.5]×106/mL; men, 15.9 [1.1]×106/mL).
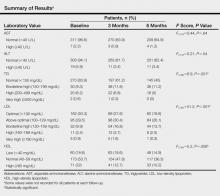
The study evaluated the effects of isotretinoin on liver enzymes (AST and ALT) and lipids (TGs, LDL, and HDL). Nearly all of the patients (>95%) had normal AST and ALT levels at baseline. The results are outlined in the Table. Some values were not recorded for all patients at each follow-up.
Aspartate Aminotransferase Analysis
Aspartate aminotransferase levels were classified as normal and high. At baseline, mean (SD) AST levels were 20.2 (6.6) U/L, with normal levels in 311 (96.6%) patients and high in 7 (2.2%) patients. At 3-month follow-up, mean (SD) AST levels were 20.7 (5.2) U/L, with normal levels in 270 (83.9%) patients and high levels in 3 (0.9%) patients. At 6-month follow-up, mean (SD) AST levels were 21.3 (5.7) U/L, with normal levels in 209 (64.9%) patients and high levels in 4 (1.2%) patients. Aspartate aminotransferase levels increased at 3- and 6-month follow-up compared to baseline. Differences between AST levels were statistically significant (F2,416=4.2, P=.016). Differences between AST levels at baseline and 3-month follow-up were not statistically significant (P=.3). Differences between AST levels at 3- and 6-month follow-up were not statistically significant (P=.4). Differences between AST levels at baseline and 6-month follow-up were statistically significant (P=.07). Differences between AST classifications at the 3 time points were not statistically significant (F2,416=0.44, P=.64). Overall, the results indicated that AST levels increased over time in patients treated with isotretinoin, but the increase was not above the normal range and was not statistically significant.
Alanine Aminotransferase Analysis
Alanine aminotransferase levels were classified as normal or high. At baseline, mean (SD) ALT levels were 16.8 (11.2) U/L, with normal levels in 303 (94.1%) patients and high in 19 (5.9%) patients. At 3-month follow-up, mean (SD) ALT levels were 16.2 (9.3) U/L, with normal levels in 263 (81.7%) patients and high in 11 (3.4%) patients. At 6-month follow-up, mean (SD) ALT levels were 17.0 (11.3) U/L, with normal levels in 201 (62.4%) patients and high in 11 (3.4%) patients. Alanine aminotransferase levels at 3-month follow-up were lower than baseline but higher at 6-month follow-up compared to baseline and 3-month follow-up. Overall, ALT levels increased with time, but the differences between baseline and 3- and 6-month follow-up were not statistically significant (F2,416=0.32, P=.72). Differences between ALT classifications at each time point were not statistically significant (F2,418=0.21, P=.54). Overall, the results indicated that ALT levels increased over time in patients treated with isotretinoin, but the increase was not statistically significant.
Triglycerides Analysis
Triglyceride levels were classified as normal, borderline high, high, and very high. At baseline, mean (SD) TG levels were 107 (71) mg/dL, with normal levels in 270 (83.9%) patients, borderline high in 30 (9.3%) patients, high in 20 (6.2%) patients, and very high in 2 (0.6%) patients. At 3-month follow-up, mean (SD) TG levels were 117 (60) mg/dL, with normal levels in 197 (61.2%) patients, borderline high in 38 (11.8%) patients, high in 22 (6.8%) patients, and very high in 1 (0.3%) patient. At 6-month follow-up, mean (SD) TG levels were 122 (65) mg/dL, with normal levels in 145 (45%) patients, borderline high in 36 (11.2%) patients, high in 16 (5%) patients, and very high in 0 (0%) patients. Triglyceride levels increased and differences between TG levels at baseline and 3- and 6-month follow-up were statistically significant (F2,384=17, P<.001). Baseline TG levels compared to 3-month follow-up were statistically signif-icant (P<.001). Differences in TG levels at 6-month follow-up versus baseline were statistically significant (P<.001). However, changes in TG levels from 3- to 6-month follow-up were not statistically significant (P=.21). Differences between TG classifications at each time point were statistically significant (F2,386=6.9, P=.001). Overall, TG levels increased from baseline during isotretinoin treatment at 3- and 6-month follow-up, and these increases were above normal range; however, there was no statistically significant increase from 3- to 6-month follow-up.
Low-Density Lipoprotein Analysis
Low-density lipoprotein levels were classified as optimal, above optimal, borderline high, high, and very high. At baseline, mean (SD) LDL levels were 102 (28) mg/dL, with optimal levels in 162 (50.3%) patients, above optimal in 95 (29.5%) patients, borderline high in 32 (9.9%) patients, high in 11 (3.4%) patients, and very high in 3 (0.9%) patients. At 3-month follow-up, mean (SD) LDL levels were 113 (30) mg/dL, with optimal levels in 89 (27.6%) patients, above optimal in 98 (30.4%) patients, borderline high in 54 (16.8%) patients, high in 12 (3.7%) patients, and very high in 5 (1.6%) patients. At 6-month follow-up, mean (SD) LDL levels were 113 (27) mg/dL, with optimal levels in 60 (18.6%) patients, above optimal in 84 (26.1%) patients, borderline high in 44 (13.7%) patients, high in 8 (2.5%) patients, and very high in 1 (0.3%) patient. Overall, there were statistically significant increases in LDL levels at 3- and 6-month follow-up (F2,382<75, P<.001). Differences between baseline LDL levels and 3-month follow-up were statistically significant (P<.001). Differences between baseline LDL levels and 6-month follow-up were statistically significant (P<.001). However, differences in LDL levels at 3- and 6-month follow-up were not statistically significant (P=.74). Differences between LDL classifications at each time point were statistically significant (F2,382=51.2, P<.001). Overall, statistically significant increases in LDL levels from baseline were noted during isotretinoin treatment and this increase was above normal range; however, LDL levels did not significantly increase from 3- to 6-month follow-up.
High-Density Lipoprotein Analysis
High-density lipoprotein levels were classified as low, normal, and high. At baseline, mean (SD) HDL levels were 52.4 (16) mg/dL, with low levels in 60 (18.6%) patients, normal in 173 (53.7%) patients, and high in 71 (22%) patients. At 3-month follow-up, mean (SD) HDL levels were 48 (12) mg/dL, with low levels in 63 (19.6%) patients, normal in 154 (47.8%) patients, and high in 41 (12.7%) patients. At 6-month follow-up, mean (SD) HDL levels were 47.6 (12) mg/dL, with low levels in 48 (14.9%) patients, normal in 117 (36.3%) patients, and high in 33 (10.2%) patients. Overall, statistically significant decreases were noted in HDL levels (F2,384=19, P<.001). Differences between baseline HDL levels compared to 3-month follow-up were statistically significant (P<.001). Differences between baseline HDL levels compared to 6-month follow-up were statistically significant (P<.001). Differences in HDL levels at 3- and 6-month follow-up were statistically significant (P<.001). Differences between HDL classifications at each time point were statistically significant (F2,384=5.2, P=.006). Overall, there were statistically significant decreases in HDL levels during isotretinoin treatment from baseline and this decrease was above normal range; however, HDL levels did not decrease at 3- and 6-month follow-up.
Comment
Studies in the literature evaluating the effects of isotretinoin on liver enzymes and lipids suggested that oral isotretinoin may cause alterations in liver aminotransferases (AST and ALT), TGs, HDL, and LDL in various degrees.1 Zane et al4 studied 13,772 patients with acne undergoing oral isotretinoin therapy between March 1995 and September 2002. The investigators found increased liver transaminase and serum lipid levels. They suggested that these abnormalities were generally transient and reversible.4 Bershad et al5 reported an increase in LDL and TG but a decrease in HDL during isotretinoin therapy. These changes in the lipid profile also appeared to be transient and returned to baseline level 2 months following the end of treatment.5 In another study of 130 patients who were treated with isotretinoin, Vieira et al1 noted an increase in AST, ALT, and TG levels. Most of the studies in the literature that reported effects of isotretinoin on liver enzymes and lipids suggested that the effects were reversible.
Although many studies reported alterations in serum transaminase and lipid levels, other studies reported no effect. In one study of 150 participants, Brito et al2 found no statistically significant changes in liver transaminase, TG, HDL, or LDL levels following treatment with isotretinoin. In another study of 1292 participants by Alcalay et al,6 serum levels of liver enzymes were not elevated to a degree necessitating discontinuation of isotretinoin treatment. In another study of 30 participants, Baxter et al7 reported no significant changes in TG, LDL, or HDL levels measured at baseline or during treatment with isotretinoin.
Some studies suggest that routine laboratory tests are needed when treating patients with isotretinoin due to severe alterations in serum liver transaminase and lipid levels, while other studies conclude that the effects are minimal and laboratory tests are not needed. In the current study, we found that there were statistically significant increases in TG and LDL levels in patients who underwent treatment with isotretinoin. We also found statistically significant decreases in HDL levels. In our study, liver enzymes were less affected than lipids in patients who underwent treatment with isotretinoin. There were statistically significant increases in AST levels, but the clinical classification was not affected. There also were increases in ALT levels, but the changes were not statistically significant.
Overall, we advise dermatologists that isotretinoin can be administered with minimal concern regarding changes in serum transaminase and lipid levels; however, although severe laboratory alterations were not noted in our study, we advise physicians to use caution when administering isotretinoin in patients with a history of abnormal findings.
1. Vieira AS, Beijamini V, Melchiors AC. The effect of isotretinoin on triglyceride and liver aminotransferases. An Bras Dermatol. 2012;87:382-387.
2. Brito MFM, Pessoa IS, Galindo JCS, et al. Evaluation of clinical adverse effects and laboratory alterations in patients with acne vulgaris treated with oral isotretinoin [in English, Portuguese]. An Bras Dermatol. 2010;85:331-337.
3. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
4. Zane LT, Leyden WA, Marqueling AL, et al. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142:1016-1022.
5. Bershad S, Rubinstein A, Paterniti JR, et al. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med. 1985;313:981-985.
6. Alcalay J, Landau M, Zucker A. Analysis of laboratory data in acne patients treated with isotretinoin: is there really a need to perform routine laboratory tests? J Dermatolog Treat. 2001;12:9-12.
7. Baxter KF, Ling TC, Barth JH, et al. Retrospective survey of serum lipids in patients receiving more than three courses of isotretinoin. J Dermatolog Treat. 2003;14:216-218.
Acne is a chronic inflammatory condition of the pilosebaceous unit affecting approximately 79% to 95% of adolescents in the Western world.1 Treatment of acne depends on its severity. Topical tretinoin, adapalene, benzoyl peroxide, azelaic acid, and topical antibiotics generally are used in cases of noninflammatory or mild inflammatory disease. Isotretinoin is recommended for treatment of severe inflammatory acne (eg, nodulocystic or conglobata acne) and for cases of acne that have proven to be resistant to prior treatment with antibiotics or topical agents. Dosages of isotretinoin range from 0.5 to 2 mg/kg daily for 16 to 24 weeks.1 Isotretinoin reduces the activity and size of the sebaceous glands, normalizes keratinization of the sebaceous follicles, and decreases the number of Propionibacterium acnes. Isotretinoin also may cause clinical side effects and laboratory changes, the most important being teratogenicity. It also may cause mucocutaneous side effects including cracked lips, dryness of the skin and nasal mucosa, skin redness, eye dryness, and eye irritation.1 It also may cause blepharoconjuctivitis, photosensitivity, asteatotic dermatitis, pruritus, telogen effluvium, secondary bacterial colonization, nail fragility, periungual pyogenic granuloma, paronychia, myalgia, intracranial hypertension, nausea, headache, vomiting, depression, psychosis, suicide, constipation, and allergic reactions.2 Isotretinoin treatment may increase serum levels of liver enzymes, triglyc-erides (TGs), and low-density lipoprotein (LDL) cholesterol, and reduce the level of high-density lipoprotein (HDL) cholesterol.1 This retrospective study sought to evaluate the effect of isotretinoin on liver enzymes and lipids over 6 months.
Materials and Methods
Our retrospective study was conducted at the Hospital of Atatürk University in Erzurum, a city located in eastern Turkey. All patients who were treated in the department of dermatology and had received oral isotretinoin between June 2009 and June 2012 were included in the study. The study was based on an evaluation of the patients’ medical records. All patients received oral isotretinoin 0.5 to 1 mg/kg daily; the majority of patients received 30 to 40 mg daily. Patient medical records included age; gender; white blood cell (WBC) count; red blood cell (RBC) count; hemoglobin count; and aspartate aminotransferase (AST), alanine aminotransferase (ALT), TG, LDL, and HDL levels at the beginning of treatment. Aspartate aminotransferase, ALT, TG, LDL, and HDL levels also were measured at 3- and 6-month follow-up. Analysis of AST, ALT, TG, LDL, and HDL levels was based on the National Cholesterol Education Program guidelines.3 Aspartate aminotransferase and ALT levels were classified as normal (<40 U/L) and high (≥40 U/L). Triglyceride levels were classified as normal (<150 mg/dL), borderline high (150–199 mg/dL), high (200–499 mg/dL), and very high (≥500 mg/dL). Low-density lipoprotein levels were classified as optimal (<100 mg/dL), above optimal (100–129 mg/dL), borderline high (130–159 mg/dL), high (160–189 mg/dL), and very high (≥190 mg/dL). High-density lipoprotein levels were classified as low (<40 mg/dL), normal (40–59 mg/dL), and high (≥60 mg/dL). Normal WBC was defined as 3.5 to 12.5×103/mL. Normal hemoglobin count was defined as 11.5 to 15.0×106/mL for women and 13 to 17×106/mL for men. Normal RBC was defined as 4.0 to 5.2×106/mL for women and 4.5 to 5.9×106/mL for men. Statistical analysis was performed using SPSS version 17.0. Repeated measures analysis of variance was used to compare means between 3 groups (baseline, 3-month, and 6-month values). A paired sample t test was used to compare means between any 2 groups. Results with P<.05 were considered statistically significant.
Results
Treatment with oral isotretinoin was initiated in 349 patients at our institution from June 2009 to June 2012. Twenty-seven of these patients were excluded from the study because their medical records were not available. Medical records from 322 patients were obtained. The study population consisted of 226 (70.2%) women and 96 (29.8%) men. Patients ranged in age from 17 to 64 years, with a mean age of 23.9 years. The mean (standard deviation [SD]) age of the women was 23.9 (5.4) years and the mean (SD) age of the men was 23.8 (7.02) years. Most of the patients were on a regimen of 30 or 40 mg of isotretinoin daily. Differences between dosages and laboratory values were not analyzed because we assumed there would not be a significant difference, as most patients received the same dose. The mean (SD) WBC was 8.4 (3.5)×103/mL. The mean (SD) RBC was 4.9 (0.5)×106/mL. The mean (SD) hemoglobin count was 14.3 (1.7)×106/mL (women, 13.6 [1.5]×106/mL; men, 15.9 [1.1]×106/mL).
The study evaluated the effects of isotretinoin on liver enzymes (AST and ALT) and lipids (TGs, LDL, and HDL). Nearly all of the patients (>95%) had normal AST and ALT levels at baseline. The results are outlined in the Table. Some values were not recorded for all patients at each follow-up.
Aspartate Aminotransferase Analysis
Aspartate aminotransferase levels were classified as normal and high. At baseline, mean (SD) AST levels were 20.2 (6.6) U/L, with normal levels in 311 (96.6%) patients and high in 7 (2.2%) patients. At 3-month follow-up, mean (SD) AST levels were 20.7 (5.2) U/L, with normal levels in 270 (83.9%) patients and high levels in 3 (0.9%) patients. At 6-month follow-up, mean (SD) AST levels were 21.3 (5.7) U/L, with normal levels in 209 (64.9%) patients and high levels in 4 (1.2%) patients. Aspartate aminotransferase levels increased at 3- and 6-month follow-up compared to baseline. Differences between AST levels were statistically significant (F2,416=4.2, P=.016). Differences between AST levels at baseline and 3-month follow-up were not statistically significant (P=.3). Differences between AST levels at 3- and 6-month follow-up were not statistically significant (P=.4). Differences between AST levels at baseline and 6-month follow-up were statistically significant (P=.07). Differences between AST classifications at the 3 time points were not statistically significant (F2,416=0.44, P=.64). Overall, the results indicated that AST levels increased over time in patients treated with isotretinoin, but the increase was not above the normal range and was not statistically significant.
Alanine Aminotransferase Analysis
Alanine aminotransferase levels were classified as normal or high. At baseline, mean (SD) ALT levels were 16.8 (11.2) U/L, with normal levels in 303 (94.1%) patients and high in 19 (5.9%) patients. At 3-month follow-up, mean (SD) ALT levels were 16.2 (9.3) U/L, with normal levels in 263 (81.7%) patients and high in 11 (3.4%) patients. At 6-month follow-up, mean (SD) ALT levels were 17.0 (11.3) U/L, with normal levels in 201 (62.4%) patients and high in 11 (3.4%) patients. Alanine aminotransferase levels at 3-month follow-up were lower than baseline but higher at 6-month follow-up compared to baseline and 3-month follow-up. Overall, ALT levels increased with time, but the differences between baseline and 3- and 6-month follow-up were not statistically significant (F2,416=0.32, P=.72). Differences between ALT classifications at each time point were not statistically significant (F2,418=0.21, P=.54). Overall, the results indicated that ALT levels increased over time in patients treated with isotretinoin, but the increase was not statistically significant.
Triglycerides Analysis
Triglyceride levels were classified as normal, borderline high, high, and very high. At baseline, mean (SD) TG levels were 107 (71) mg/dL, with normal levels in 270 (83.9%) patients, borderline high in 30 (9.3%) patients, high in 20 (6.2%) patients, and very high in 2 (0.6%) patients. At 3-month follow-up, mean (SD) TG levels were 117 (60) mg/dL, with normal levels in 197 (61.2%) patients, borderline high in 38 (11.8%) patients, high in 22 (6.8%) patients, and very high in 1 (0.3%) patient. At 6-month follow-up, mean (SD) TG levels were 122 (65) mg/dL, with normal levels in 145 (45%) patients, borderline high in 36 (11.2%) patients, high in 16 (5%) patients, and very high in 0 (0%) patients. Triglyceride levels increased and differences between TG levels at baseline and 3- and 6-month follow-up were statistically significant (F2,384=17, P<.001). Baseline TG levels compared to 3-month follow-up were statistically signif-icant (P<.001). Differences in TG levels at 6-month follow-up versus baseline were statistically significant (P<.001). However, changes in TG levels from 3- to 6-month follow-up were not statistically significant (P=.21). Differences between TG classifications at each time point were statistically significant (F2,386=6.9, P=.001). Overall, TG levels increased from baseline during isotretinoin treatment at 3- and 6-month follow-up, and these increases were above normal range; however, there was no statistically significant increase from 3- to 6-month follow-up.
Low-Density Lipoprotein Analysis
Low-density lipoprotein levels were classified as optimal, above optimal, borderline high, high, and very high. At baseline, mean (SD) LDL levels were 102 (28) mg/dL, with optimal levels in 162 (50.3%) patients, above optimal in 95 (29.5%) patients, borderline high in 32 (9.9%) patients, high in 11 (3.4%) patients, and very high in 3 (0.9%) patients. At 3-month follow-up, mean (SD) LDL levels were 113 (30) mg/dL, with optimal levels in 89 (27.6%) patients, above optimal in 98 (30.4%) patients, borderline high in 54 (16.8%) patients, high in 12 (3.7%) patients, and very high in 5 (1.6%) patients. At 6-month follow-up, mean (SD) LDL levels were 113 (27) mg/dL, with optimal levels in 60 (18.6%) patients, above optimal in 84 (26.1%) patients, borderline high in 44 (13.7%) patients, high in 8 (2.5%) patients, and very high in 1 (0.3%) patient. Overall, there were statistically significant increases in LDL levels at 3- and 6-month follow-up (F2,382<75, P<.001). Differences between baseline LDL levels and 3-month follow-up were statistically significant (P<.001). Differences between baseline LDL levels and 6-month follow-up were statistically significant (P<.001). However, differences in LDL levels at 3- and 6-month follow-up were not statistically significant (P=.74). Differences between LDL classifications at each time point were statistically significant (F2,382=51.2, P<.001). Overall, statistically significant increases in LDL levels from baseline were noted during isotretinoin treatment and this increase was above normal range; however, LDL levels did not significantly increase from 3- to 6-month follow-up.
High-Density Lipoprotein Analysis
High-density lipoprotein levels were classified as low, normal, and high. At baseline, mean (SD) HDL levels were 52.4 (16) mg/dL, with low levels in 60 (18.6%) patients, normal in 173 (53.7%) patients, and high in 71 (22%) patients. At 3-month follow-up, mean (SD) HDL levels were 48 (12) mg/dL, with low levels in 63 (19.6%) patients, normal in 154 (47.8%) patients, and high in 41 (12.7%) patients. At 6-month follow-up, mean (SD) HDL levels were 47.6 (12) mg/dL, with low levels in 48 (14.9%) patients, normal in 117 (36.3%) patients, and high in 33 (10.2%) patients. Overall, statistically significant decreases were noted in HDL levels (F2,384=19, P<.001). Differences between baseline HDL levels compared to 3-month follow-up were statistically significant (P<.001). Differences between baseline HDL levels compared to 6-month follow-up were statistically significant (P<.001). Differences in HDL levels at 3- and 6-month follow-up were statistically significant (P<.001). Differences between HDL classifications at each time point were statistically significant (F2,384=5.2, P=.006). Overall, there were statistically significant decreases in HDL levels during isotretinoin treatment from baseline and this decrease was above normal range; however, HDL levels did not decrease at 3- and 6-month follow-up.
Comment
Studies in the literature evaluating the effects of isotretinoin on liver enzymes and lipids suggested that oral isotretinoin may cause alterations in liver aminotransferases (AST and ALT), TGs, HDL, and LDL in various degrees.1 Zane et al4 studied 13,772 patients with acne undergoing oral isotretinoin therapy between March 1995 and September 2002. The investigators found increased liver transaminase and serum lipid levels. They suggested that these abnormalities were generally transient and reversible.4 Bershad et al5 reported an increase in LDL and TG but a decrease in HDL during isotretinoin therapy. These changes in the lipid profile also appeared to be transient and returned to baseline level 2 months following the end of treatment.5 In another study of 130 patients who were treated with isotretinoin, Vieira et al1 noted an increase in AST, ALT, and TG levels. Most of the studies in the literature that reported effects of isotretinoin on liver enzymes and lipids suggested that the effects were reversible.
Although many studies reported alterations in serum transaminase and lipid levels, other studies reported no effect. In one study of 150 participants, Brito et al2 found no statistically significant changes in liver transaminase, TG, HDL, or LDL levels following treatment with isotretinoin. In another study of 1292 participants by Alcalay et al,6 serum levels of liver enzymes were not elevated to a degree necessitating discontinuation of isotretinoin treatment. In another study of 30 participants, Baxter et al7 reported no significant changes in TG, LDL, or HDL levels measured at baseline or during treatment with isotretinoin.
Some studies suggest that routine laboratory tests are needed when treating patients with isotretinoin due to severe alterations in serum liver transaminase and lipid levels, while other studies conclude that the effects are minimal and laboratory tests are not needed. In the current study, we found that there were statistically significant increases in TG and LDL levels in patients who underwent treatment with isotretinoin. We also found statistically significant decreases in HDL levels. In our study, liver enzymes were less affected than lipids in patients who underwent treatment with isotretinoin. There were statistically significant increases in AST levels, but the clinical classification was not affected. There also were increases in ALT levels, but the changes were not statistically significant.
Overall, we advise dermatologists that isotretinoin can be administered with minimal concern regarding changes in serum transaminase and lipid levels; however, although severe laboratory alterations were not noted in our study, we advise physicians to use caution when administering isotretinoin in patients with a history of abnormal findings.
Acne is a chronic inflammatory condition of the pilosebaceous unit affecting approximately 79% to 95% of adolescents in the Western world.1 Treatment of acne depends on its severity. Topical tretinoin, adapalene, benzoyl peroxide, azelaic acid, and topical antibiotics generally are used in cases of noninflammatory or mild inflammatory disease. Isotretinoin is recommended for treatment of severe inflammatory acne (eg, nodulocystic or conglobata acne) and for cases of acne that have proven to be resistant to prior treatment with antibiotics or topical agents. Dosages of isotretinoin range from 0.5 to 2 mg/kg daily for 16 to 24 weeks.1 Isotretinoin reduces the activity and size of the sebaceous glands, normalizes keratinization of the sebaceous follicles, and decreases the number of Propionibacterium acnes. Isotretinoin also may cause clinical side effects and laboratory changes, the most important being teratogenicity. It also may cause mucocutaneous side effects including cracked lips, dryness of the skin and nasal mucosa, skin redness, eye dryness, and eye irritation.1 It also may cause blepharoconjuctivitis, photosensitivity, asteatotic dermatitis, pruritus, telogen effluvium, secondary bacterial colonization, nail fragility, periungual pyogenic granuloma, paronychia, myalgia, intracranial hypertension, nausea, headache, vomiting, depression, psychosis, suicide, constipation, and allergic reactions.2 Isotretinoin treatment may increase serum levels of liver enzymes, triglyc-erides (TGs), and low-density lipoprotein (LDL) cholesterol, and reduce the level of high-density lipoprotein (HDL) cholesterol.1 This retrospective study sought to evaluate the effect of isotretinoin on liver enzymes and lipids over 6 months.
Materials and Methods
Our retrospective study was conducted at the Hospital of Atatürk University in Erzurum, a city located in eastern Turkey. All patients who were treated in the department of dermatology and had received oral isotretinoin between June 2009 and June 2012 were included in the study. The study was based on an evaluation of the patients’ medical records. All patients received oral isotretinoin 0.5 to 1 mg/kg daily; the majority of patients received 30 to 40 mg daily. Patient medical records included age; gender; white blood cell (WBC) count; red blood cell (RBC) count; hemoglobin count; and aspartate aminotransferase (AST), alanine aminotransferase (ALT), TG, LDL, and HDL levels at the beginning of treatment. Aspartate aminotransferase, ALT, TG, LDL, and HDL levels also were measured at 3- and 6-month follow-up. Analysis of AST, ALT, TG, LDL, and HDL levels was based on the National Cholesterol Education Program guidelines.3 Aspartate aminotransferase and ALT levels were classified as normal (<40 U/L) and high (≥40 U/L). Triglyceride levels were classified as normal (<150 mg/dL), borderline high (150–199 mg/dL), high (200–499 mg/dL), and very high (≥500 mg/dL). Low-density lipoprotein levels were classified as optimal (<100 mg/dL), above optimal (100–129 mg/dL), borderline high (130–159 mg/dL), high (160–189 mg/dL), and very high (≥190 mg/dL). High-density lipoprotein levels were classified as low (<40 mg/dL), normal (40–59 mg/dL), and high (≥60 mg/dL). Normal WBC was defined as 3.5 to 12.5×103/mL. Normal hemoglobin count was defined as 11.5 to 15.0×106/mL for women and 13 to 17×106/mL for men. Normal RBC was defined as 4.0 to 5.2×106/mL for women and 4.5 to 5.9×106/mL for men. Statistical analysis was performed using SPSS version 17.0. Repeated measures analysis of variance was used to compare means between 3 groups (baseline, 3-month, and 6-month values). A paired sample t test was used to compare means between any 2 groups. Results with P<.05 were considered statistically significant.
Results
Treatment with oral isotretinoin was initiated in 349 patients at our institution from June 2009 to June 2012. Twenty-seven of these patients were excluded from the study because their medical records were not available. Medical records from 322 patients were obtained. The study population consisted of 226 (70.2%) women and 96 (29.8%) men. Patients ranged in age from 17 to 64 years, with a mean age of 23.9 years. The mean (standard deviation [SD]) age of the women was 23.9 (5.4) years and the mean (SD) age of the men was 23.8 (7.02) years. Most of the patients were on a regimen of 30 or 40 mg of isotretinoin daily. Differences between dosages and laboratory values were not analyzed because we assumed there would not be a significant difference, as most patients received the same dose. The mean (SD) WBC was 8.4 (3.5)×103/mL. The mean (SD) RBC was 4.9 (0.5)×106/mL. The mean (SD) hemoglobin count was 14.3 (1.7)×106/mL (women, 13.6 [1.5]×106/mL; men, 15.9 [1.1]×106/mL).
The study evaluated the effects of isotretinoin on liver enzymes (AST and ALT) and lipids (TGs, LDL, and HDL). Nearly all of the patients (>95%) had normal AST and ALT levels at baseline. The results are outlined in the Table. Some values were not recorded for all patients at each follow-up.
Aspartate Aminotransferase Analysis
Aspartate aminotransferase levels were classified as normal and high. At baseline, mean (SD) AST levels were 20.2 (6.6) U/L, with normal levels in 311 (96.6%) patients and high in 7 (2.2%) patients. At 3-month follow-up, mean (SD) AST levels were 20.7 (5.2) U/L, with normal levels in 270 (83.9%) patients and high levels in 3 (0.9%) patients. At 6-month follow-up, mean (SD) AST levels were 21.3 (5.7) U/L, with normal levels in 209 (64.9%) patients and high levels in 4 (1.2%) patients. Aspartate aminotransferase levels increased at 3- and 6-month follow-up compared to baseline. Differences between AST levels were statistically significant (F2,416=4.2, P=.016). Differences between AST levels at baseline and 3-month follow-up were not statistically significant (P=.3). Differences between AST levels at 3- and 6-month follow-up were not statistically significant (P=.4). Differences between AST levels at baseline and 6-month follow-up were statistically significant (P=.07). Differences between AST classifications at the 3 time points were not statistically significant (F2,416=0.44, P=.64). Overall, the results indicated that AST levels increased over time in patients treated with isotretinoin, but the increase was not above the normal range and was not statistically significant.
Alanine Aminotransferase Analysis
Alanine aminotransferase levels were classified as normal or high. At baseline, mean (SD) ALT levels were 16.8 (11.2) U/L, with normal levels in 303 (94.1%) patients and high in 19 (5.9%) patients. At 3-month follow-up, mean (SD) ALT levels were 16.2 (9.3) U/L, with normal levels in 263 (81.7%) patients and high in 11 (3.4%) patients. At 6-month follow-up, mean (SD) ALT levels were 17.0 (11.3) U/L, with normal levels in 201 (62.4%) patients and high in 11 (3.4%) patients. Alanine aminotransferase levels at 3-month follow-up were lower than baseline but higher at 6-month follow-up compared to baseline and 3-month follow-up. Overall, ALT levels increased with time, but the differences between baseline and 3- and 6-month follow-up were not statistically significant (F2,416=0.32, P=.72). Differences between ALT classifications at each time point were not statistically significant (F2,418=0.21, P=.54). Overall, the results indicated that ALT levels increased over time in patients treated with isotretinoin, but the increase was not statistically significant.
Triglycerides Analysis
Triglyceride levels were classified as normal, borderline high, high, and very high. At baseline, mean (SD) TG levels were 107 (71) mg/dL, with normal levels in 270 (83.9%) patients, borderline high in 30 (9.3%) patients, high in 20 (6.2%) patients, and very high in 2 (0.6%) patients. At 3-month follow-up, mean (SD) TG levels were 117 (60) mg/dL, with normal levels in 197 (61.2%) patients, borderline high in 38 (11.8%) patients, high in 22 (6.8%) patients, and very high in 1 (0.3%) patient. At 6-month follow-up, mean (SD) TG levels were 122 (65) mg/dL, with normal levels in 145 (45%) patients, borderline high in 36 (11.2%) patients, high in 16 (5%) patients, and very high in 0 (0%) patients. Triglyceride levels increased and differences between TG levels at baseline and 3- and 6-month follow-up were statistically significant (F2,384=17, P<.001). Baseline TG levels compared to 3-month follow-up were statistically signif-icant (P<.001). Differences in TG levels at 6-month follow-up versus baseline were statistically significant (P<.001). However, changes in TG levels from 3- to 6-month follow-up were not statistically significant (P=.21). Differences between TG classifications at each time point were statistically significant (F2,386=6.9, P=.001). Overall, TG levels increased from baseline during isotretinoin treatment at 3- and 6-month follow-up, and these increases were above normal range; however, there was no statistically significant increase from 3- to 6-month follow-up.
Low-Density Lipoprotein Analysis
Low-density lipoprotein levels were classified as optimal, above optimal, borderline high, high, and very high. At baseline, mean (SD) LDL levels were 102 (28) mg/dL, with optimal levels in 162 (50.3%) patients, above optimal in 95 (29.5%) patients, borderline high in 32 (9.9%) patients, high in 11 (3.4%) patients, and very high in 3 (0.9%) patients. At 3-month follow-up, mean (SD) LDL levels were 113 (30) mg/dL, with optimal levels in 89 (27.6%) patients, above optimal in 98 (30.4%) patients, borderline high in 54 (16.8%) patients, high in 12 (3.7%) patients, and very high in 5 (1.6%) patients. At 6-month follow-up, mean (SD) LDL levels were 113 (27) mg/dL, with optimal levels in 60 (18.6%) patients, above optimal in 84 (26.1%) patients, borderline high in 44 (13.7%) patients, high in 8 (2.5%) patients, and very high in 1 (0.3%) patient. Overall, there were statistically significant increases in LDL levels at 3- and 6-month follow-up (F2,382<75, P<.001). Differences between baseline LDL levels and 3-month follow-up were statistically significant (P<.001). Differences between baseline LDL levels and 6-month follow-up were statistically significant (P<.001). However, differences in LDL levels at 3- and 6-month follow-up were not statistically significant (P=.74). Differences between LDL classifications at each time point were statistically significant (F2,382=51.2, P<.001). Overall, statistically significant increases in LDL levels from baseline were noted during isotretinoin treatment and this increase was above normal range; however, LDL levels did not significantly increase from 3- to 6-month follow-up.
High-Density Lipoprotein Analysis
High-density lipoprotein levels were classified as low, normal, and high. At baseline, mean (SD) HDL levels were 52.4 (16) mg/dL, with low levels in 60 (18.6%) patients, normal in 173 (53.7%) patients, and high in 71 (22%) patients. At 3-month follow-up, mean (SD) HDL levels were 48 (12) mg/dL, with low levels in 63 (19.6%) patients, normal in 154 (47.8%) patients, and high in 41 (12.7%) patients. At 6-month follow-up, mean (SD) HDL levels were 47.6 (12) mg/dL, with low levels in 48 (14.9%) patients, normal in 117 (36.3%) patients, and high in 33 (10.2%) patients. Overall, statistically significant decreases were noted in HDL levels (F2,384=19, P<.001). Differences between baseline HDL levels compared to 3-month follow-up were statistically significant (P<.001). Differences between baseline HDL levels compared to 6-month follow-up were statistically significant (P<.001). Differences in HDL levels at 3- and 6-month follow-up were statistically significant (P<.001). Differences between HDL classifications at each time point were statistically significant (F2,384=5.2, P=.006). Overall, there were statistically significant decreases in HDL levels during isotretinoin treatment from baseline and this decrease was above normal range; however, HDL levels did not decrease at 3- and 6-month follow-up.
Comment
Studies in the literature evaluating the effects of isotretinoin on liver enzymes and lipids suggested that oral isotretinoin may cause alterations in liver aminotransferases (AST and ALT), TGs, HDL, and LDL in various degrees.1 Zane et al4 studied 13,772 patients with acne undergoing oral isotretinoin therapy between March 1995 and September 2002. The investigators found increased liver transaminase and serum lipid levels. They suggested that these abnormalities were generally transient and reversible.4 Bershad et al5 reported an increase in LDL and TG but a decrease in HDL during isotretinoin therapy. These changes in the lipid profile also appeared to be transient and returned to baseline level 2 months following the end of treatment.5 In another study of 130 patients who were treated with isotretinoin, Vieira et al1 noted an increase in AST, ALT, and TG levels. Most of the studies in the literature that reported effects of isotretinoin on liver enzymes and lipids suggested that the effects were reversible.
Although many studies reported alterations in serum transaminase and lipid levels, other studies reported no effect. In one study of 150 participants, Brito et al2 found no statistically significant changes in liver transaminase, TG, HDL, or LDL levels following treatment with isotretinoin. In another study of 1292 participants by Alcalay et al,6 serum levels of liver enzymes were not elevated to a degree necessitating discontinuation of isotretinoin treatment. In another study of 30 participants, Baxter et al7 reported no significant changes in TG, LDL, or HDL levels measured at baseline or during treatment with isotretinoin.
Some studies suggest that routine laboratory tests are needed when treating patients with isotretinoin due to severe alterations in serum liver transaminase and lipid levels, while other studies conclude that the effects are minimal and laboratory tests are not needed. In the current study, we found that there were statistically significant increases in TG and LDL levels in patients who underwent treatment with isotretinoin. We also found statistically significant decreases in HDL levels. In our study, liver enzymes were less affected than lipids in patients who underwent treatment with isotretinoin. There were statistically significant increases in AST levels, but the clinical classification was not affected. There also were increases in ALT levels, but the changes were not statistically significant.
Overall, we advise dermatologists that isotretinoin can be administered with minimal concern regarding changes in serum transaminase and lipid levels; however, although severe laboratory alterations were not noted in our study, we advise physicians to use caution when administering isotretinoin in patients with a history of abnormal findings.
1. Vieira AS, Beijamini V, Melchiors AC. The effect of isotretinoin on triglyceride and liver aminotransferases. An Bras Dermatol. 2012;87:382-387.
2. Brito MFM, Pessoa IS, Galindo JCS, et al. Evaluation of clinical adverse effects and laboratory alterations in patients with acne vulgaris treated with oral isotretinoin [in English, Portuguese]. An Bras Dermatol. 2010;85:331-337.
3. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
4. Zane LT, Leyden WA, Marqueling AL, et al. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142:1016-1022.
5. Bershad S, Rubinstein A, Paterniti JR, et al. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med. 1985;313:981-985.
6. Alcalay J, Landau M, Zucker A. Analysis of laboratory data in acne patients treated with isotretinoin: is there really a need to perform routine laboratory tests? J Dermatolog Treat. 2001;12:9-12.
7. Baxter KF, Ling TC, Barth JH, et al. Retrospective survey of serum lipids in patients receiving more than three courses of isotretinoin. J Dermatolog Treat. 2003;14:216-218.
1. Vieira AS, Beijamini V, Melchiors AC. The effect of isotretinoin on triglyceride and liver aminotransferases. An Bras Dermatol. 2012;87:382-387.
2. Brito MFM, Pessoa IS, Galindo JCS, et al. Evaluation of clinical adverse effects and laboratory alterations in patients with acne vulgaris treated with oral isotretinoin [in English, Portuguese]. An Bras Dermatol. 2010;85:331-337.
3. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
4. Zane LT, Leyden WA, Marqueling AL, et al. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142:1016-1022.
5. Bershad S, Rubinstein A, Paterniti JR, et al. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med. 1985;313:981-985.
6. Alcalay J, Landau M, Zucker A. Analysis of laboratory data in acne patients treated with isotretinoin: is there really a need to perform routine laboratory tests? J Dermatolog Treat. 2001;12:9-12.
7. Baxter KF, Ling TC, Barth JH, et al. Retrospective survey of serum lipids in patients receiving more than three courses of isotretinoin. J Dermatolog Treat. 2003;14:216-218.
Practice Points
- Isotretinoin is recommended for treatment of severe inflammatory acne and for cases resistant to prior treatment with antibiotics or topical agents; however, it may cause alterations in lipids and liver enzymes.
- In our study, liver enzymes were less affected than lipids in patients who were treated with isotretinoin.
- Use caution when administering isotretinoin in patients with a history of abnormal findings.