User login
For decades, tranexamic acid (TXA) has been used off-label to reduce perioperative blood loss in various surgical procedures, including orthopedic surgery, neurosurgery, urologic surgery, obstetrics and gynecology, and trauma surgery.1 TXA, a synthetic derivative of the amino acid lysine, produces antifibrinolytic activity by competitively inhibiting lysine-binding sites on plasminogen molecules—inhibiting the activation of plasmin and thus preserving the function of fibrin in clot formation. It is believed that, through this method, TXA retains blood clots more effectively, thereby reducing bleeding. Although intravenous delivery of TXA is generally accepted as safe, some studies have indicated that it may contribute to postoperative seizure activity as well as increased thromboembolic events.2,3 For these and other reasons, interest in topical (intra-articular) administration of TXA has increased.
Use of topical TXA in surgery has been expanding over the past several years, with reports of significant reductions in perioperative blood loss and transfusion requirements.4 Orthopedic surgeons specifically have explored the topical use of TXA, especially in total joint arthroplasty (TJA).5 The benefits are increased concentration at the operative site with reduced systemic exposure; cost reduction; and surgeon control.6,7 Several recent studies have yielded significant reductions in perioperative blood loss and transfusions with use of topical TXA.8-10 In the literature, effective dosing for topical TXA in TJA ranges from 250 mg to 3 g.11 The concentration of topical TXA is not consistently described but appears to fall between 15 and 100 mg/mL.12,13 Our institutions14 and several investigators15,16 have used topical TXA in TJA at a concentration of 100 mg/mL, so this was the initial TXA concentration we decided to study. We selected certain time points to allow for relatively early detection of cartilage damage and then followed it to 48 hours of exposure. In cases in which TXA is injected after capsular closure, it is unclear how rapidly the TXA diffuses out of the joint or to what degree it becomes diluted by bleeding or synovial fluid. Certainly, this varies from patient to patient. Clearly, TXA generally passes through the body unmodified when injected intravenously1 and therefore is unlikely to be chemically modified while in the joint.
Very little has been published on use of topical TXA in other orthopedic surgeries, such as intra-articular fracture fixation, ligament reconstruction, hemiarthroplasty, and unicompartmental arthroplasty. Unlike TJA, which removes all native cartilage, these procedures retain and depend on the viability of the native cartilage. Sitek and colleagues17 noted the effect of TXA on chondrocytes within the context of creating an extracellular fibrin matrix for chondrocyte transplant. There was no decrease in chondrocyte viability with TXA 10 mg/mL or 20 mg/mL. Use of fresh bovine cartilage explants as a model for the in vitro study of cartilage damage is well established, including chondrocyte viability and glycosaminoglycan (GAG) release as outcome measures.18,19 Human cartilage has also been studied in vitro using this model.20
In the present study, the primary goal was to test the hypothesis that TXA could be safely used in the presence of native cartilage. The secondary goal was to identify a safe concentration for intra-articular use if toxic characteristics were noted.
Materials and Methods
Young bovine stifle joints were obtained within 3 hours of slaughter at a local abattoir. The joint was disarticulated under sterile conditions, and the distal femoral articular surface was evaluated for any signs of damage or arthritis. All specimens contained healthy, undamaged articular surfaces. Full-thickness cartilage explants (excluding subchondral bone) were then immediately harvested—with use of a scalpel blade and a dermatologic biopsy punch 4 mm in diameter—from the distal, weight-bearing femur. The explants were placed in 24-well tissue-culture plates (USA Scientific), incubated in culture media (high-glucose Dulbecco’s Modified Eagle Medium, 10% fetal bovine serum, 1% penicillin/streptomycin, 1% fungizone; Life Technologies), and kept at 37°C and 5% CO2. Explants were allowed to rest in culture media for a minimum of 24 hours after harvest. The pH of the medium was not altered by the addition of TXA.
Bovine explants were randomly assigned to either TXA-exposure or control groups at several time points in replicates of 6. Culture medium was aspirated, and each explant was washed twice with sterile phosphate-buffered saline (PBS). Explants were then incubated at 37°C in culture medium as previously described, or in the same culture medium containing dissolved TXA at a concentration of 100 mg/mL. The explants were incubated at 37°C until harvest at 8, 24, or 48 hours after media addition. For harvest, the media were aspirated and stored at –20°C for GAG content analysis, and the explants were then harvested for LIVE/DEAD assay (Life Technologies) and GAG content analysis.
Explants were washed once in 75% ethanol and then digested in 0.5 mL papain digestion buffer (100 mM sodium phosphate, 10 mM EDTA, 10 mM L-cysteine, 0.125 mg/mL papain; Sigma-Aldrich) at 60°C for 24 hours. Digested samples were then diluted and subjected to GAG analysis.
Murine chondrocytes were harvested from the freshly harvested rib cages and sternums of mice (1-4 days old) as previously described.21 In brief, rib cages were washed twice in D-PBS and then incubated at 37°C for 60 min in 5 mL of 0.25% Trypsin-EDTA (Life Technologies). They were then washed in DMEM with 10% FBS, centrifuged at 1500 rpm for 5 min to remove the supernatant, and washed in sterile PBS. After removal of the PBS wash, the ribs were incubated in 2 mg/mL hyaluronidase in plain DMEM on a shaker at 37°C for 2 hours. Once soft tissue was removed, the rib cages were discarded, and the remaining soft tissue was incubated in a collagenase D/hyaluronidase digestion solution (collagenase D, 1 mg/mL; hyaluronidase, 1 mg/mL; BSA, 40 mg/mL in plain DMEM; Life Technologies) for 8 hours. The resultant cell suspension was filtered through a 40-µm cell strainer (BD Falcon). Isolated chondrocytes were then plated on culture slides (0.5×x106 cells; BD Falcon) and incubated in DMEM/F12 (1:1) complete media at 37°C and 5% CO2. Before experimental treatment, all cultures were visualized under phase microscopy to verify viability and morphology.
Murine chondrocytes were incubated in media (described above) containing TXA 0, 25, 50, or 100 mg/mL and were harvested 8, 24, or 48 hours after initial exposure. Cultures were maintained at 37°C and 5% CO2 until harvest. Culture medium was aspirated, and each sample was washed twice in sterile PBS before analysis with the LIVE/DEAD assay.
The amount of GAG released into the culture media was measured with a 1,9-dimethyl-methylene blue colorimetric assay (DMMB; 38.5 µM 1,9-dimethylmethylene blue, 40 mM glycine, 40.5 mM sodium chloride, 9.5 mM hydrochloric acid; Sigma-Aldrich) based on the method of Farndale and colleagues.22 In brief, 20 µL of media was mixed with the DMMB assay solution in a 96-well plate, and absorbance was read immediately at 530 nm on a microplate reader. Chondroitin 4-sulfate was used to produce a standard curve. Total GAG released into the media was then calculated based on the standard curve and calculated as a percentage of the total GAG content of each explant. Each sample time point and concentration had a replicate of 3.
Chondrocyte viability was assessed with use of the LIVE/DEAD Viability/Cytotoxicity Kit (Life Technologies) following the protocol. Cartilage explants were sectioned orthogonally to the articular surface at 100 µm per section. Four sections were obtained from each explant. Sections were then incubated in 60 µL of 1-µM calcein AM/1-µM ethidium homodimer-1 solution at room temperature in the dark for 30 minutes. Sections were then viewed with a fluorescent microscope, and 3 digital photographs (magnification ×4) were taken per sample with use of a fluorescein filter and a Texas red filter. The live and dead cells in an area were quantified with use of ImageJ, freely available image analysis software.23 This software was verified initially by blinded, manual count for accuracy. Each sample time point and concentration had a replicate of 3 or 4 explants.
Chondrocyte viability was assessed with the LIVE/DEAD Viability/Cytotoxicity Kit following the protocol. Slides were incubated in 200 µL of 1-µM calcein AM/1-µM ethidium homodimer-1 solution at 37°C in the dark for 30 minutes. Sections were then viewed with a fluorescent microscope, and 4 digital photographs (magnification ×4) were taken with use of a fluorescein filter and a Texas red filter. Live and dead cells in an area were quantified with use of ImageJ. Each sample time point and concentration had a replicate of 4 plates.
Statistical analyses were performed in the statistical environment R.24 Data were analyzed with a 2-tailed Student t test with Holm-Bonferroni correction made for multiple comparisons, and a family-wise error rate was set at α = 0.05.
Results
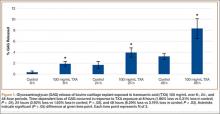
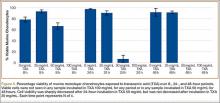
GAG release was notably higher in the explants exposed to TXA 100 mg/mL at all time points (Figure 1). Beginning 8 hours after initial incubation, there was a small but significant (P = .01) loss of GAG in TXA-treated explants (mean, 1.86%; SD, 0.44%) versus control explants (mean, 0.31%; SD, 0.24%). There was a trend of increasing loss with increasing time after initial incubation through 24 hours, GAG (mean, 3.92%; SD, 0.83%) versus control (mean, 1.63%; SD, 0.65%) (P = .02), reaching a peak at 48 hours, GAG (mean, 8.29%; SD, 1.82%) versus control (mean, 3.19%; SD, 0.53%) (P = .03).
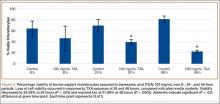
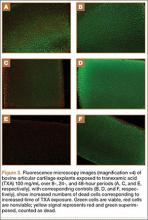
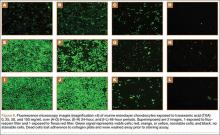
Cell viability was notably higher in the control groups 24 and 48 hours after initial incubation (Figure 2), with a visually observable (Figure 3) but variable and nonsignificant (P = .33) difference in viability at 8 hours, control (mean, 63.87%; SD, 13.63%) versus TXA (mean, 46.08%; SD, 22.51%). As incubation time increased from 8 hours, there were significant decreases in cell viability at 24 hours (mean, 39.28%; SD, 4.12%; P = .024) and 48 hours (mean, 21.98%; SD, 2.15%; P = .0005) relative to controls.
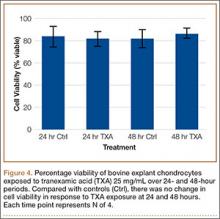
After results of exposing murine cells to TXA at different concentrations were obtained, bovine explants were exposed to TXA 25 mg/mL, and viability was recorded 24 and 48 hours after exposure (Figure 4). There was no significant difference in viability between samples.
Cell viability was similar between the TXA 25 mg/mL and control samples at all time points (Figure 5). The TXA 50 mg/mL sample dropped from 66.51% viability at 8 hours to 6.81% viability at 24 hours and complete cell death by 48 hours. The TXA 100 mg/mL samples had no observable viable cells at 8, 24, and 48 hours (Figure 6, confirmed with light microscopy). The TXA 0 mg/mL and 25 mg/mL samples remained largely unchanged: 78.28% and 92.99% viable at 8 hours, 97.29% and 90.22% viable at 24 hours, and 91.62% and 91.35% viable at 48 hours, respectively. See Figures 4 and 5 for viability at all time points and concentrations. Statistical analyses were not performed on these data because the zero values obtained for all samples incubated in TXA 100 mg/mL and the 48-hour TXA 50 mg/mL samples prevented accurate estimation of P values and thus meaningful comparisons of the treatment groups.
Discussion
The results of this study showed that TXA is cytotoxic to both bovine and murine chondrocytes at a concentration of 100 mg/mL. There is a time-dependent increase in GAG release as well as a decrease in chondrocyte viability in intact bovine cartilage. These data suggest that topical or intra-articular administration of TXA at this concentration in the setting of native cartilage may have unintended, detrimental effects.
Murine chondrocyte monolayer cultures exposed to TXA at lower concentrations did not exhibit a concentration-dependent curve with respect to viability. Chondrocytes exposed to TXA 25 mg/mL had no reduction in viability relative to control samples. When the concentration was doubled to 50 mg/mL, however, viability was reduced to 6.81% by 24 hours (Figure 5). These data suggest that, between 25 mg/mL and 50 mg/mL, there is a concentration at which TXA becomes cytotoxic to murine chondrocytes. It should be cautioned that, though TXA was cytotoxic to chondrocytes in this study, the effects are still unknown and indeed may be similar to effects on other types of cells that are present in a replaced joint—such as synovial cells, inflammatory cells, and osteoblasts.
The unaffected viability of murine chondrocytes with TXA 25 mg/mL indicated that this may be a cutoff concentration for safety in the presence of cartilage. To confirm these results, we exposed the bovine explants to TXA 25 mg/mL as well. Consistent with the prior study, chondrocyte viability was unaffected at 48 hours. Some clinical studies have effectively used topical TXA at this concentration, or at a lower concentration, to reduce blood loss in TJA,25 which suggests that 25 mg/mL may be a safe yet effective dose for clinical use of topical TXA.
As the methods used in this study did not distinguish between late-apoptotic and necrotic cell death, we could not determine which mechanism of death led to the viability loss observed. If apoptosis is occurring, how TXA initiates this sequence is unclear. There have been no studies directly linking TXA to apoptotic events, though some studies have indicated that TXA interacts with several molecules other than plasminogen, including GABA (γ-aminobutyric acid) receptors, glycine receptors, and tachykinin neurokinin 1 receptors.26-28 According to these studies, these interactions may be responsible for seizure activity and increased emesis caused by TXA use. In addition, TXA-containing compounds, such as trans-aminomethylcyclohexanecarbonyl-l-(O-picolyl)tyrosine-octylamide, have been shown to induce apoptosis.29
It appears that the extracellular matrix (ECM) of native cartilage explants has a protective effect on chondrocytes. With exposure to TXA 100 mg/mL, the explants retained 52% viability at 24 hours, whereas the monolayer cultures were nonviable at that point. The weak negative charge of the molecule may retard its penetration into the ECM, though there was an inconsistent presentation of cell death at explant superficial zones in treated samples (Figure 3). Consistent surface layer cell death would be expected if slowed penetration were the only protective mechanism. It is possible that the ECM acts as a buffer or solvent, effectively reducing the concentration of TXA directly interacting with the chondrocytes. Further exploration is needed to elucidate the significance of the ECM in protecting chondrocytes from TXA.
Although its findings were highly reproducible, the present study had several limitations, including its in vitro nature and its use of a bovine and murine model rather than a human cell and tissue platform. It may be prudent to expose chondrocytes to TXA for a shorter time to try to mimic what theoretically occurs in vivo. In vivo studies may be a reasonable direction for experimentation. Clarifying the mechanism of cell death is of experimental interest as well. As the first of its kind, the present study provides an important initial database for exploration.
This study is the first to show that TXA has a cytotoxic effect on chondrocytes and that it damages cartilage at clinically used concentrations. Although more studies are needed to verify a safe concentration of TXA for topical use with human cartilage, our data indicate that TXA 25 mg/mL may be an effective yet safe dose for intra-articular use in native joints.
1. McCormack PL. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs. 2012;72(5):585-617.
2. Murkin JM, Falter F, Granton J, Young B, Burt C, Chu M. High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg. 2010;110(2):350-353.
3. Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) study. Arch Surg. 2012;147(2):113-119.
4. Ker K, Prieto‐Merino D, Roberts I. Systematic review, meta‐analysis and meta‐regression of the effect of tranexamic acid on surgical blood loss. Br J Surg. 2013;100(10):1271-1279.
5. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309.
6. Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am. 2013;95(21):1961-1968.
7. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
8. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
9. Lee SH, Cho KY, Khurana S, Kim KI. Less blood loss under concomitant administration of tranexamic acid and indirect factor Xa inhibitor following total knee arthroplasty: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2611-2617.
10. Chimento GF, Huff T, Ochsner JL Jr, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
11. Aguilera-Roig X, Jordán-Sales M, Natera-Cisneros L, Monllau-García JC, Martínez-Zapata MJ. Tranexamic acid in orthopedic surgery [in Spanish]. Rev Esp Cir Ortop Traumatol. 2014;58(1):52-56.
12. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
13. Georgiadis AG, Muh SJ, Silverton CD, Weir RM, Laker MW. A prospective double-blind placebo controlled trial of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):78-82.
14. Tuttle JR, Ritterman SA, Cassidy DB, Anazonwu WA, Froehlich JA, Rubin LE. Cost benefit analysis of topical tranexamic acid in primary total hip and knee arthroplasty. J Arthroplasty. 2014;29(8):1512-1515.
15. Roy SP, Tanki UF, Dutta A, Jain SK, Nagi ON. Efficacy of intra-articular tranexamic acid in blood loss reduction following primary unilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2494-2501.
16. Ishida K, Tsumura N, Kitagawa A, et al. Intra-articular injection of tranexamic acid reduces not only blood loss but also knee joint swelling after total knee arthroplasty. Int Orthop. 2011;35(11):1639-1645.
17. Sitek P, Wysocka-Wycisk A, Kępski F, Król D, Bursig H, Dyląg S. PRP-fibrinogen gel-like chondrocyte carrier stabilized by TXA-preliminary study. Cell Tissue Bank. 2013;14(1):133-140.
18. Lo IK, Sciore P, Chung M, et al. Local anesthetics induce chondrocyte death in bovine articular cartilage disks in a dose- and duration-dependent manner. Arthroscopy. 2009;25(7):707-715.
19. Blumberg TJ, Natoli RM, Athanasiou KA. Effects of doxycycline on articular cartilage GAG release and mechanical properties following impact. Biotechnol Bioeng. 2008;100(3):506-515.
20. Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90(5):986-991.
21. Lefebvre V, Garofalo S, Zhou G, Metsäranta M, Vuorio E, De Crombrugghe B. Characterization of primary cultures of chondrocytes from type II collagen/beta-galactosidase transgenic mice. Matrix Biol. 1994;14(4):329-335.
22. Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;833(2):173-177.
23. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671-675.
24. R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008.
25. Sa-Ngasoongsong P, Channoom T, Kawinwonggowit V, et al. Postoperative blood loss reduction in computer-assisted surgery total knee replacement by low dose intra-articular tranexamic acid injection together with 2-hour clamp drain: a prospective triple-blinded randomized controlled trial. Orthop Rev. 2011;3(2):e12.
26. Lecker I, Wang DS, Romaschin AD, Peterson M, Mazer CD, Orser BA. Tranexamic acid concentrations associated with human seizures inhibit glycine receptors. J Clin Invest. 2012;122(12):4654-4666.
27. Kakiuchi H, Kawarai-Shimamura A, Kuwagata M, Orito K. Tranexamic acid induces kaolin intake stimulating a pathway involving tachykinin neurokinin 1 receptors in rats. Eur J Pharmacol. 2014;723:1-6.
28. Kratzer S, Irl H, Mattusch C, et al. Tranexamic acid impairs γ-aminobutyric acid receptor type A–mediated synaptic transmission in the murine amygdala: a potential mechanism for drug-induced seizures? Anesthesiology. 2014;120(3):639-649.
29. Lee E, Enomoto R, Takemura K, Tsuda Y, Okada Y. A selective plasmin inhibitor, trans-aminomethylcyclohexanecarbonyl-L-(O-picolyl)tyrosine-octylamide (YO-2), induces thymocyte apoptosis. Biochem Pharmacol. 2002;63(7):1315-1323.
For decades, tranexamic acid (TXA) has been used off-label to reduce perioperative blood loss in various surgical procedures, including orthopedic surgery, neurosurgery, urologic surgery, obstetrics and gynecology, and trauma surgery.1 TXA, a synthetic derivative of the amino acid lysine, produces antifibrinolytic activity by competitively inhibiting lysine-binding sites on plasminogen molecules—inhibiting the activation of plasmin and thus preserving the function of fibrin in clot formation. It is believed that, through this method, TXA retains blood clots more effectively, thereby reducing bleeding. Although intravenous delivery of TXA is generally accepted as safe, some studies have indicated that it may contribute to postoperative seizure activity as well as increased thromboembolic events.2,3 For these and other reasons, interest in topical (intra-articular) administration of TXA has increased.
Use of topical TXA in surgery has been expanding over the past several years, with reports of significant reductions in perioperative blood loss and transfusion requirements.4 Orthopedic surgeons specifically have explored the topical use of TXA, especially in total joint arthroplasty (TJA).5 The benefits are increased concentration at the operative site with reduced systemic exposure; cost reduction; and surgeon control.6,7 Several recent studies have yielded significant reductions in perioperative blood loss and transfusions with use of topical TXA.8-10 In the literature, effective dosing for topical TXA in TJA ranges from 250 mg to 3 g.11 The concentration of topical TXA is not consistently described but appears to fall between 15 and 100 mg/mL.12,13 Our institutions14 and several investigators15,16 have used topical TXA in TJA at a concentration of 100 mg/mL, so this was the initial TXA concentration we decided to study. We selected certain time points to allow for relatively early detection of cartilage damage and then followed it to 48 hours of exposure. In cases in which TXA is injected after capsular closure, it is unclear how rapidly the TXA diffuses out of the joint or to what degree it becomes diluted by bleeding or synovial fluid. Certainly, this varies from patient to patient. Clearly, TXA generally passes through the body unmodified when injected intravenously1 and therefore is unlikely to be chemically modified while in the joint.
Very little has been published on use of topical TXA in other orthopedic surgeries, such as intra-articular fracture fixation, ligament reconstruction, hemiarthroplasty, and unicompartmental arthroplasty. Unlike TJA, which removes all native cartilage, these procedures retain and depend on the viability of the native cartilage. Sitek and colleagues17 noted the effect of TXA on chondrocytes within the context of creating an extracellular fibrin matrix for chondrocyte transplant. There was no decrease in chondrocyte viability with TXA 10 mg/mL or 20 mg/mL. Use of fresh bovine cartilage explants as a model for the in vitro study of cartilage damage is well established, including chondrocyte viability and glycosaminoglycan (GAG) release as outcome measures.18,19 Human cartilage has also been studied in vitro using this model.20
In the present study, the primary goal was to test the hypothesis that TXA could be safely used in the presence of native cartilage. The secondary goal was to identify a safe concentration for intra-articular use if toxic characteristics were noted.
Materials and Methods
Young bovine stifle joints were obtained within 3 hours of slaughter at a local abattoir. The joint was disarticulated under sterile conditions, and the distal femoral articular surface was evaluated for any signs of damage or arthritis. All specimens contained healthy, undamaged articular surfaces. Full-thickness cartilage explants (excluding subchondral bone) were then immediately harvested—with use of a scalpel blade and a dermatologic biopsy punch 4 mm in diameter—from the distal, weight-bearing femur. The explants were placed in 24-well tissue-culture plates (USA Scientific), incubated in culture media (high-glucose Dulbecco’s Modified Eagle Medium, 10% fetal bovine serum, 1% penicillin/streptomycin, 1% fungizone; Life Technologies), and kept at 37°C and 5% CO2. Explants were allowed to rest in culture media for a minimum of 24 hours after harvest. The pH of the medium was not altered by the addition of TXA.
Bovine explants were randomly assigned to either TXA-exposure or control groups at several time points in replicates of 6. Culture medium was aspirated, and each explant was washed twice with sterile phosphate-buffered saline (PBS). Explants were then incubated at 37°C in culture medium as previously described, or in the same culture medium containing dissolved TXA at a concentration of 100 mg/mL. The explants were incubated at 37°C until harvest at 8, 24, or 48 hours after media addition. For harvest, the media were aspirated and stored at –20°C for GAG content analysis, and the explants were then harvested for LIVE/DEAD assay (Life Technologies) and GAG content analysis.
Explants were washed once in 75% ethanol and then digested in 0.5 mL papain digestion buffer (100 mM sodium phosphate, 10 mM EDTA, 10 mM L-cysteine, 0.125 mg/mL papain; Sigma-Aldrich) at 60°C for 24 hours. Digested samples were then diluted and subjected to GAG analysis.
Murine chondrocytes were harvested from the freshly harvested rib cages and sternums of mice (1-4 days old) as previously described.21 In brief, rib cages were washed twice in D-PBS and then incubated at 37°C for 60 min in 5 mL of 0.25% Trypsin-EDTA (Life Technologies). They were then washed in DMEM with 10% FBS, centrifuged at 1500 rpm for 5 min to remove the supernatant, and washed in sterile PBS. After removal of the PBS wash, the ribs were incubated in 2 mg/mL hyaluronidase in plain DMEM on a shaker at 37°C for 2 hours. Once soft tissue was removed, the rib cages were discarded, and the remaining soft tissue was incubated in a collagenase D/hyaluronidase digestion solution (collagenase D, 1 mg/mL; hyaluronidase, 1 mg/mL; BSA, 40 mg/mL in plain DMEM; Life Technologies) for 8 hours. The resultant cell suspension was filtered through a 40-µm cell strainer (BD Falcon). Isolated chondrocytes were then plated on culture slides (0.5×x106 cells; BD Falcon) and incubated in DMEM/F12 (1:1) complete media at 37°C and 5% CO2. Before experimental treatment, all cultures were visualized under phase microscopy to verify viability and morphology.
Murine chondrocytes were incubated in media (described above) containing TXA 0, 25, 50, or 100 mg/mL and were harvested 8, 24, or 48 hours after initial exposure. Cultures were maintained at 37°C and 5% CO2 until harvest. Culture medium was aspirated, and each sample was washed twice in sterile PBS before analysis with the LIVE/DEAD assay.
The amount of GAG released into the culture media was measured with a 1,9-dimethyl-methylene blue colorimetric assay (DMMB; 38.5 µM 1,9-dimethylmethylene blue, 40 mM glycine, 40.5 mM sodium chloride, 9.5 mM hydrochloric acid; Sigma-Aldrich) based on the method of Farndale and colleagues.22 In brief, 20 µL of media was mixed with the DMMB assay solution in a 96-well plate, and absorbance was read immediately at 530 nm on a microplate reader. Chondroitin 4-sulfate was used to produce a standard curve. Total GAG released into the media was then calculated based on the standard curve and calculated as a percentage of the total GAG content of each explant. Each sample time point and concentration had a replicate of 3.
Chondrocyte viability was assessed with use of the LIVE/DEAD Viability/Cytotoxicity Kit (Life Technologies) following the protocol. Cartilage explants were sectioned orthogonally to the articular surface at 100 µm per section. Four sections were obtained from each explant. Sections were then incubated in 60 µL of 1-µM calcein AM/1-µM ethidium homodimer-1 solution at room temperature in the dark for 30 minutes. Sections were then viewed with a fluorescent microscope, and 3 digital photographs (magnification ×4) were taken per sample with use of a fluorescein filter and a Texas red filter. The live and dead cells in an area were quantified with use of ImageJ, freely available image analysis software.23 This software was verified initially by blinded, manual count for accuracy. Each sample time point and concentration had a replicate of 3 or 4 explants.
Chondrocyte viability was assessed with the LIVE/DEAD Viability/Cytotoxicity Kit following the protocol. Slides were incubated in 200 µL of 1-µM calcein AM/1-µM ethidium homodimer-1 solution at 37°C in the dark for 30 minutes. Sections were then viewed with a fluorescent microscope, and 4 digital photographs (magnification ×4) were taken with use of a fluorescein filter and a Texas red filter. Live and dead cells in an area were quantified with use of ImageJ. Each sample time point and concentration had a replicate of 4 plates.
Statistical analyses were performed in the statistical environment R.24 Data were analyzed with a 2-tailed Student t test with Holm-Bonferroni correction made for multiple comparisons, and a family-wise error rate was set at α = 0.05.
Results
GAG release was notably higher in the explants exposed to TXA 100 mg/mL at all time points (Figure 1). Beginning 8 hours after initial incubation, there was a small but significant (P = .01) loss of GAG in TXA-treated explants (mean, 1.86%; SD, 0.44%) versus control explants (mean, 0.31%; SD, 0.24%). There was a trend of increasing loss with increasing time after initial incubation through 24 hours, GAG (mean, 3.92%; SD, 0.83%) versus control (mean, 1.63%; SD, 0.65%) (P = .02), reaching a peak at 48 hours, GAG (mean, 8.29%; SD, 1.82%) versus control (mean, 3.19%; SD, 0.53%) (P = .03).
Cell viability was notably higher in the control groups 24 and 48 hours after initial incubation (Figure 2), with a visually observable (Figure 3) but variable and nonsignificant (P = .33) difference in viability at 8 hours, control (mean, 63.87%; SD, 13.63%) versus TXA (mean, 46.08%; SD, 22.51%). As incubation time increased from 8 hours, there were significant decreases in cell viability at 24 hours (mean, 39.28%; SD, 4.12%; P = .024) and 48 hours (mean, 21.98%; SD, 2.15%; P = .0005) relative to controls.
After results of exposing murine cells to TXA at different concentrations were obtained, bovine explants were exposed to TXA 25 mg/mL, and viability was recorded 24 and 48 hours after exposure (Figure 4). There was no significant difference in viability between samples.
Cell viability was similar between the TXA 25 mg/mL and control samples at all time points (Figure 5). The TXA 50 mg/mL sample dropped from 66.51% viability at 8 hours to 6.81% viability at 24 hours and complete cell death by 48 hours. The TXA 100 mg/mL samples had no observable viable cells at 8, 24, and 48 hours (Figure 6, confirmed with light microscopy). The TXA 0 mg/mL and 25 mg/mL samples remained largely unchanged: 78.28% and 92.99% viable at 8 hours, 97.29% and 90.22% viable at 24 hours, and 91.62% and 91.35% viable at 48 hours, respectively. See Figures 4 and 5 for viability at all time points and concentrations. Statistical analyses were not performed on these data because the zero values obtained for all samples incubated in TXA 100 mg/mL and the 48-hour TXA 50 mg/mL samples prevented accurate estimation of P values and thus meaningful comparisons of the treatment groups.
Discussion
The results of this study showed that TXA is cytotoxic to both bovine and murine chondrocytes at a concentration of 100 mg/mL. There is a time-dependent increase in GAG release as well as a decrease in chondrocyte viability in intact bovine cartilage. These data suggest that topical or intra-articular administration of TXA at this concentration in the setting of native cartilage may have unintended, detrimental effects.
Murine chondrocyte monolayer cultures exposed to TXA at lower concentrations did not exhibit a concentration-dependent curve with respect to viability. Chondrocytes exposed to TXA 25 mg/mL had no reduction in viability relative to control samples. When the concentration was doubled to 50 mg/mL, however, viability was reduced to 6.81% by 24 hours (Figure 5). These data suggest that, between 25 mg/mL and 50 mg/mL, there is a concentration at which TXA becomes cytotoxic to murine chondrocytes. It should be cautioned that, though TXA was cytotoxic to chondrocytes in this study, the effects are still unknown and indeed may be similar to effects on other types of cells that are present in a replaced joint—such as synovial cells, inflammatory cells, and osteoblasts.
The unaffected viability of murine chondrocytes with TXA 25 mg/mL indicated that this may be a cutoff concentration for safety in the presence of cartilage. To confirm these results, we exposed the bovine explants to TXA 25 mg/mL as well. Consistent with the prior study, chondrocyte viability was unaffected at 48 hours. Some clinical studies have effectively used topical TXA at this concentration, or at a lower concentration, to reduce blood loss in TJA,25 which suggests that 25 mg/mL may be a safe yet effective dose for clinical use of topical TXA.
As the methods used in this study did not distinguish between late-apoptotic and necrotic cell death, we could not determine which mechanism of death led to the viability loss observed. If apoptosis is occurring, how TXA initiates this sequence is unclear. There have been no studies directly linking TXA to apoptotic events, though some studies have indicated that TXA interacts with several molecules other than plasminogen, including GABA (γ-aminobutyric acid) receptors, glycine receptors, and tachykinin neurokinin 1 receptors.26-28 According to these studies, these interactions may be responsible for seizure activity and increased emesis caused by TXA use. In addition, TXA-containing compounds, such as trans-aminomethylcyclohexanecarbonyl-l-(O-picolyl)tyrosine-octylamide, have been shown to induce apoptosis.29
It appears that the extracellular matrix (ECM) of native cartilage explants has a protective effect on chondrocytes. With exposure to TXA 100 mg/mL, the explants retained 52% viability at 24 hours, whereas the monolayer cultures were nonviable at that point. The weak negative charge of the molecule may retard its penetration into the ECM, though there was an inconsistent presentation of cell death at explant superficial zones in treated samples (Figure 3). Consistent surface layer cell death would be expected if slowed penetration were the only protective mechanism. It is possible that the ECM acts as a buffer or solvent, effectively reducing the concentration of TXA directly interacting with the chondrocytes. Further exploration is needed to elucidate the significance of the ECM in protecting chondrocytes from TXA.
Although its findings were highly reproducible, the present study had several limitations, including its in vitro nature and its use of a bovine and murine model rather than a human cell and tissue platform. It may be prudent to expose chondrocytes to TXA for a shorter time to try to mimic what theoretically occurs in vivo. In vivo studies may be a reasonable direction for experimentation. Clarifying the mechanism of cell death is of experimental interest as well. As the first of its kind, the present study provides an important initial database for exploration.
This study is the first to show that TXA has a cytotoxic effect on chondrocytes and that it damages cartilage at clinically used concentrations. Although more studies are needed to verify a safe concentration of TXA for topical use with human cartilage, our data indicate that TXA 25 mg/mL may be an effective yet safe dose for intra-articular use in native joints.
For decades, tranexamic acid (TXA) has been used off-label to reduce perioperative blood loss in various surgical procedures, including orthopedic surgery, neurosurgery, urologic surgery, obstetrics and gynecology, and trauma surgery.1 TXA, a synthetic derivative of the amino acid lysine, produces antifibrinolytic activity by competitively inhibiting lysine-binding sites on plasminogen molecules—inhibiting the activation of plasmin and thus preserving the function of fibrin in clot formation. It is believed that, through this method, TXA retains blood clots more effectively, thereby reducing bleeding. Although intravenous delivery of TXA is generally accepted as safe, some studies have indicated that it may contribute to postoperative seizure activity as well as increased thromboembolic events.2,3 For these and other reasons, interest in topical (intra-articular) administration of TXA has increased.
Use of topical TXA in surgery has been expanding over the past several years, with reports of significant reductions in perioperative blood loss and transfusion requirements.4 Orthopedic surgeons specifically have explored the topical use of TXA, especially in total joint arthroplasty (TJA).5 The benefits are increased concentration at the operative site with reduced systemic exposure; cost reduction; and surgeon control.6,7 Several recent studies have yielded significant reductions in perioperative blood loss and transfusions with use of topical TXA.8-10 In the literature, effective dosing for topical TXA in TJA ranges from 250 mg to 3 g.11 The concentration of topical TXA is not consistently described but appears to fall between 15 and 100 mg/mL.12,13 Our institutions14 and several investigators15,16 have used topical TXA in TJA at a concentration of 100 mg/mL, so this was the initial TXA concentration we decided to study. We selected certain time points to allow for relatively early detection of cartilage damage and then followed it to 48 hours of exposure. In cases in which TXA is injected after capsular closure, it is unclear how rapidly the TXA diffuses out of the joint or to what degree it becomes diluted by bleeding or synovial fluid. Certainly, this varies from patient to patient. Clearly, TXA generally passes through the body unmodified when injected intravenously1 and therefore is unlikely to be chemically modified while in the joint.
Very little has been published on use of topical TXA in other orthopedic surgeries, such as intra-articular fracture fixation, ligament reconstruction, hemiarthroplasty, and unicompartmental arthroplasty. Unlike TJA, which removes all native cartilage, these procedures retain and depend on the viability of the native cartilage. Sitek and colleagues17 noted the effect of TXA on chondrocytes within the context of creating an extracellular fibrin matrix for chondrocyte transplant. There was no decrease in chondrocyte viability with TXA 10 mg/mL or 20 mg/mL. Use of fresh bovine cartilage explants as a model for the in vitro study of cartilage damage is well established, including chondrocyte viability and glycosaminoglycan (GAG) release as outcome measures.18,19 Human cartilage has also been studied in vitro using this model.20
In the present study, the primary goal was to test the hypothesis that TXA could be safely used in the presence of native cartilage. The secondary goal was to identify a safe concentration for intra-articular use if toxic characteristics were noted.
Materials and Methods
Young bovine stifle joints were obtained within 3 hours of slaughter at a local abattoir. The joint was disarticulated under sterile conditions, and the distal femoral articular surface was evaluated for any signs of damage or arthritis. All specimens contained healthy, undamaged articular surfaces. Full-thickness cartilage explants (excluding subchondral bone) were then immediately harvested—with use of a scalpel blade and a dermatologic biopsy punch 4 mm in diameter—from the distal, weight-bearing femur. The explants were placed in 24-well tissue-culture plates (USA Scientific), incubated in culture media (high-glucose Dulbecco’s Modified Eagle Medium, 10% fetal bovine serum, 1% penicillin/streptomycin, 1% fungizone; Life Technologies), and kept at 37°C and 5% CO2. Explants were allowed to rest in culture media for a minimum of 24 hours after harvest. The pH of the medium was not altered by the addition of TXA.
Bovine explants were randomly assigned to either TXA-exposure or control groups at several time points in replicates of 6. Culture medium was aspirated, and each explant was washed twice with sterile phosphate-buffered saline (PBS). Explants were then incubated at 37°C in culture medium as previously described, or in the same culture medium containing dissolved TXA at a concentration of 100 mg/mL. The explants were incubated at 37°C until harvest at 8, 24, or 48 hours after media addition. For harvest, the media were aspirated and stored at –20°C for GAG content analysis, and the explants were then harvested for LIVE/DEAD assay (Life Technologies) and GAG content analysis.
Explants were washed once in 75% ethanol and then digested in 0.5 mL papain digestion buffer (100 mM sodium phosphate, 10 mM EDTA, 10 mM L-cysteine, 0.125 mg/mL papain; Sigma-Aldrich) at 60°C for 24 hours. Digested samples were then diluted and subjected to GAG analysis.
Murine chondrocytes were harvested from the freshly harvested rib cages and sternums of mice (1-4 days old) as previously described.21 In brief, rib cages were washed twice in D-PBS and then incubated at 37°C for 60 min in 5 mL of 0.25% Trypsin-EDTA (Life Technologies). They were then washed in DMEM with 10% FBS, centrifuged at 1500 rpm for 5 min to remove the supernatant, and washed in sterile PBS. After removal of the PBS wash, the ribs were incubated in 2 mg/mL hyaluronidase in plain DMEM on a shaker at 37°C for 2 hours. Once soft tissue was removed, the rib cages were discarded, and the remaining soft tissue was incubated in a collagenase D/hyaluronidase digestion solution (collagenase D, 1 mg/mL; hyaluronidase, 1 mg/mL; BSA, 40 mg/mL in plain DMEM; Life Technologies) for 8 hours. The resultant cell suspension was filtered through a 40-µm cell strainer (BD Falcon). Isolated chondrocytes were then plated on culture slides (0.5×x106 cells; BD Falcon) and incubated in DMEM/F12 (1:1) complete media at 37°C and 5% CO2. Before experimental treatment, all cultures were visualized under phase microscopy to verify viability and morphology.
Murine chondrocytes were incubated in media (described above) containing TXA 0, 25, 50, or 100 mg/mL and were harvested 8, 24, or 48 hours after initial exposure. Cultures were maintained at 37°C and 5% CO2 until harvest. Culture medium was aspirated, and each sample was washed twice in sterile PBS before analysis with the LIVE/DEAD assay.
The amount of GAG released into the culture media was measured with a 1,9-dimethyl-methylene blue colorimetric assay (DMMB; 38.5 µM 1,9-dimethylmethylene blue, 40 mM glycine, 40.5 mM sodium chloride, 9.5 mM hydrochloric acid; Sigma-Aldrich) based on the method of Farndale and colleagues.22 In brief, 20 µL of media was mixed with the DMMB assay solution in a 96-well plate, and absorbance was read immediately at 530 nm on a microplate reader. Chondroitin 4-sulfate was used to produce a standard curve. Total GAG released into the media was then calculated based on the standard curve and calculated as a percentage of the total GAG content of each explant. Each sample time point and concentration had a replicate of 3.
Chondrocyte viability was assessed with use of the LIVE/DEAD Viability/Cytotoxicity Kit (Life Technologies) following the protocol. Cartilage explants were sectioned orthogonally to the articular surface at 100 µm per section. Four sections were obtained from each explant. Sections were then incubated in 60 µL of 1-µM calcein AM/1-µM ethidium homodimer-1 solution at room temperature in the dark for 30 minutes. Sections were then viewed with a fluorescent microscope, and 3 digital photographs (magnification ×4) were taken per sample with use of a fluorescein filter and a Texas red filter. The live and dead cells in an area were quantified with use of ImageJ, freely available image analysis software.23 This software was verified initially by blinded, manual count for accuracy. Each sample time point and concentration had a replicate of 3 or 4 explants.
Chondrocyte viability was assessed with the LIVE/DEAD Viability/Cytotoxicity Kit following the protocol. Slides were incubated in 200 µL of 1-µM calcein AM/1-µM ethidium homodimer-1 solution at 37°C in the dark for 30 minutes. Sections were then viewed with a fluorescent microscope, and 4 digital photographs (magnification ×4) were taken with use of a fluorescein filter and a Texas red filter. Live and dead cells in an area were quantified with use of ImageJ. Each sample time point and concentration had a replicate of 4 plates.
Statistical analyses were performed in the statistical environment R.24 Data were analyzed with a 2-tailed Student t test with Holm-Bonferroni correction made for multiple comparisons, and a family-wise error rate was set at α = 0.05.
Results
GAG release was notably higher in the explants exposed to TXA 100 mg/mL at all time points (Figure 1). Beginning 8 hours after initial incubation, there was a small but significant (P = .01) loss of GAG in TXA-treated explants (mean, 1.86%; SD, 0.44%) versus control explants (mean, 0.31%; SD, 0.24%). There was a trend of increasing loss with increasing time after initial incubation through 24 hours, GAG (mean, 3.92%; SD, 0.83%) versus control (mean, 1.63%; SD, 0.65%) (P = .02), reaching a peak at 48 hours, GAG (mean, 8.29%; SD, 1.82%) versus control (mean, 3.19%; SD, 0.53%) (P = .03).
Cell viability was notably higher in the control groups 24 and 48 hours after initial incubation (Figure 2), with a visually observable (Figure 3) but variable and nonsignificant (P = .33) difference in viability at 8 hours, control (mean, 63.87%; SD, 13.63%) versus TXA (mean, 46.08%; SD, 22.51%). As incubation time increased from 8 hours, there were significant decreases in cell viability at 24 hours (mean, 39.28%; SD, 4.12%; P = .024) and 48 hours (mean, 21.98%; SD, 2.15%; P = .0005) relative to controls.
After results of exposing murine cells to TXA at different concentrations were obtained, bovine explants were exposed to TXA 25 mg/mL, and viability was recorded 24 and 48 hours after exposure (Figure 4). There was no significant difference in viability between samples.
Cell viability was similar between the TXA 25 mg/mL and control samples at all time points (Figure 5). The TXA 50 mg/mL sample dropped from 66.51% viability at 8 hours to 6.81% viability at 24 hours and complete cell death by 48 hours. The TXA 100 mg/mL samples had no observable viable cells at 8, 24, and 48 hours (Figure 6, confirmed with light microscopy). The TXA 0 mg/mL and 25 mg/mL samples remained largely unchanged: 78.28% and 92.99% viable at 8 hours, 97.29% and 90.22% viable at 24 hours, and 91.62% and 91.35% viable at 48 hours, respectively. See Figures 4 and 5 for viability at all time points and concentrations. Statistical analyses were not performed on these data because the zero values obtained for all samples incubated in TXA 100 mg/mL and the 48-hour TXA 50 mg/mL samples prevented accurate estimation of P values and thus meaningful comparisons of the treatment groups.
Discussion
The results of this study showed that TXA is cytotoxic to both bovine and murine chondrocytes at a concentration of 100 mg/mL. There is a time-dependent increase in GAG release as well as a decrease in chondrocyte viability in intact bovine cartilage. These data suggest that topical or intra-articular administration of TXA at this concentration in the setting of native cartilage may have unintended, detrimental effects.
Murine chondrocyte monolayer cultures exposed to TXA at lower concentrations did not exhibit a concentration-dependent curve with respect to viability. Chondrocytes exposed to TXA 25 mg/mL had no reduction in viability relative to control samples. When the concentration was doubled to 50 mg/mL, however, viability was reduced to 6.81% by 24 hours (Figure 5). These data suggest that, between 25 mg/mL and 50 mg/mL, there is a concentration at which TXA becomes cytotoxic to murine chondrocytes. It should be cautioned that, though TXA was cytotoxic to chondrocytes in this study, the effects are still unknown and indeed may be similar to effects on other types of cells that are present in a replaced joint—such as synovial cells, inflammatory cells, and osteoblasts.
The unaffected viability of murine chondrocytes with TXA 25 mg/mL indicated that this may be a cutoff concentration for safety in the presence of cartilage. To confirm these results, we exposed the bovine explants to TXA 25 mg/mL as well. Consistent with the prior study, chondrocyte viability was unaffected at 48 hours. Some clinical studies have effectively used topical TXA at this concentration, or at a lower concentration, to reduce blood loss in TJA,25 which suggests that 25 mg/mL may be a safe yet effective dose for clinical use of topical TXA.
As the methods used in this study did not distinguish between late-apoptotic and necrotic cell death, we could not determine which mechanism of death led to the viability loss observed. If apoptosis is occurring, how TXA initiates this sequence is unclear. There have been no studies directly linking TXA to apoptotic events, though some studies have indicated that TXA interacts with several molecules other than plasminogen, including GABA (γ-aminobutyric acid) receptors, glycine receptors, and tachykinin neurokinin 1 receptors.26-28 According to these studies, these interactions may be responsible for seizure activity and increased emesis caused by TXA use. In addition, TXA-containing compounds, such as trans-aminomethylcyclohexanecarbonyl-l-(O-picolyl)tyrosine-octylamide, have been shown to induce apoptosis.29
It appears that the extracellular matrix (ECM) of native cartilage explants has a protective effect on chondrocytes. With exposure to TXA 100 mg/mL, the explants retained 52% viability at 24 hours, whereas the monolayer cultures were nonviable at that point. The weak negative charge of the molecule may retard its penetration into the ECM, though there was an inconsistent presentation of cell death at explant superficial zones in treated samples (Figure 3). Consistent surface layer cell death would be expected if slowed penetration were the only protective mechanism. It is possible that the ECM acts as a buffer or solvent, effectively reducing the concentration of TXA directly interacting with the chondrocytes. Further exploration is needed to elucidate the significance of the ECM in protecting chondrocytes from TXA.
Although its findings were highly reproducible, the present study had several limitations, including its in vitro nature and its use of a bovine and murine model rather than a human cell and tissue platform. It may be prudent to expose chondrocytes to TXA for a shorter time to try to mimic what theoretically occurs in vivo. In vivo studies may be a reasonable direction for experimentation. Clarifying the mechanism of cell death is of experimental interest as well. As the first of its kind, the present study provides an important initial database for exploration.
This study is the first to show that TXA has a cytotoxic effect on chondrocytes and that it damages cartilage at clinically used concentrations. Although more studies are needed to verify a safe concentration of TXA for topical use with human cartilage, our data indicate that TXA 25 mg/mL may be an effective yet safe dose for intra-articular use in native joints.
1. McCormack PL. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs. 2012;72(5):585-617.
2. Murkin JM, Falter F, Granton J, Young B, Burt C, Chu M. High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg. 2010;110(2):350-353.
3. Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) study. Arch Surg. 2012;147(2):113-119.
4. Ker K, Prieto‐Merino D, Roberts I. Systematic review, meta‐analysis and meta‐regression of the effect of tranexamic acid on surgical blood loss. Br J Surg. 2013;100(10):1271-1279.
5. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309.
6. Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am. 2013;95(21):1961-1968.
7. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
8. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
9. Lee SH, Cho KY, Khurana S, Kim KI. Less blood loss under concomitant administration of tranexamic acid and indirect factor Xa inhibitor following total knee arthroplasty: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2611-2617.
10. Chimento GF, Huff T, Ochsner JL Jr, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
11. Aguilera-Roig X, Jordán-Sales M, Natera-Cisneros L, Monllau-García JC, Martínez-Zapata MJ. Tranexamic acid in orthopedic surgery [in Spanish]. Rev Esp Cir Ortop Traumatol. 2014;58(1):52-56.
12. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
13. Georgiadis AG, Muh SJ, Silverton CD, Weir RM, Laker MW. A prospective double-blind placebo controlled trial of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):78-82.
14. Tuttle JR, Ritterman SA, Cassidy DB, Anazonwu WA, Froehlich JA, Rubin LE. Cost benefit analysis of topical tranexamic acid in primary total hip and knee arthroplasty. J Arthroplasty. 2014;29(8):1512-1515.
15. Roy SP, Tanki UF, Dutta A, Jain SK, Nagi ON. Efficacy of intra-articular tranexamic acid in blood loss reduction following primary unilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2494-2501.
16. Ishida K, Tsumura N, Kitagawa A, et al. Intra-articular injection of tranexamic acid reduces not only blood loss but also knee joint swelling after total knee arthroplasty. Int Orthop. 2011;35(11):1639-1645.
17. Sitek P, Wysocka-Wycisk A, Kępski F, Król D, Bursig H, Dyląg S. PRP-fibrinogen gel-like chondrocyte carrier stabilized by TXA-preliminary study. Cell Tissue Bank. 2013;14(1):133-140.
18. Lo IK, Sciore P, Chung M, et al. Local anesthetics induce chondrocyte death in bovine articular cartilage disks in a dose- and duration-dependent manner. Arthroscopy. 2009;25(7):707-715.
19. Blumberg TJ, Natoli RM, Athanasiou KA. Effects of doxycycline on articular cartilage GAG release and mechanical properties following impact. Biotechnol Bioeng. 2008;100(3):506-515.
20. Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90(5):986-991.
21. Lefebvre V, Garofalo S, Zhou G, Metsäranta M, Vuorio E, De Crombrugghe B. Characterization of primary cultures of chondrocytes from type II collagen/beta-galactosidase transgenic mice. Matrix Biol. 1994;14(4):329-335.
22. Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;833(2):173-177.
23. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671-675.
24. R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008.
25. Sa-Ngasoongsong P, Channoom T, Kawinwonggowit V, et al. Postoperative blood loss reduction in computer-assisted surgery total knee replacement by low dose intra-articular tranexamic acid injection together with 2-hour clamp drain: a prospective triple-blinded randomized controlled trial. Orthop Rev. 2011;3(2):e12.
26. Lecker I, Wang DS, Romaschin AD, Peterson M, Mazer CD, Orser BA. Tranexamic acid concentrations associated with human seizures inhibit glycine receptors. J Clin Invest. 2012;122(12):4654-4666.
27. Kakiuchi H, Kawarai-Shimamura A, Kuwagata M, Orito K. Tranexamic acid induces kaolin intake stimulating a pathway involving tachykinin neurokinin 1 receptors in rats. Eur J Pharmacol. 2014;723:1-6.
28. Kratzer S, Irl H, Mattusch C, et al. Tranexamic acid impairs γ-aminobutyric acid receptor type A–mediated synaptic transmission in the murine amygdala: a potential mechanism for drug-induced seizures? Anesthesiology. 2014;120(3):639-649.
29. Lee E, Enomoto R, Takemura K, Tsuda Y, Okada Y. A selective plasmin inhibitor, trans-aminomethylcyclohexanecarbonyl-L-(O-picolyl)tyrosine-octylamide (YO-2), induces thymocyte apoptosis. Biochem Pharmacol. 2002;63(7):1315-1323.
1. McCormack PL. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs. 2012;72(5):585-617.
2. Murkin JM, Falter F, Granton J, Young B, Burt C, Chu M. High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg. 2010;110(2):350-353.
3. Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) study. Arch Surg. 2012;147(2):113-119.
4. Ker K, Prieto‐Merino D, Roberts I. Systematic review, meta‐analysis and meta‐regression of the effect of tranexamic acid on surgical blood loss. Br J Surg. 2013;100(10):1271-1279.
5. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309.
6. Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am. 2013;95(21):1961-1968.
7. Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969-1974.
8. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28(9):1473-1476.
9. Lee SH, Cho KY, Khurana S, Kim KI. Less blood loss under concomitant administration of tranexamic acid and indirect factor Xa inhibitor following total knee arthroplasty: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2611-2617.
10. Chimento GF, Huff T, Ochsner JL Jr, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):74-77.
11. Aguilera-Roig X, Jordán-Sales M, Natera-Cisneros L, Monllau-García JC, Martínez-Zapata MJ. Tranexamic acid in orthopedic surgery [in Spanish]. Rev Esp Cir Ortop Traumatol. 2014;58(1):52-56.
12. Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92(15):2503-2513.
13. Georgiadis AG, Muh SJ, Silverton CD, Weir RM, Laker MW. A prospective double-blind placebo controlled trial of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):78-82.
14. Tuttle JR, Ritterman SA, Cassidy DB, Anazonwu WA, Froehlich JA, Rubin LE. Cost benefit analysis of topical tranexamic acid in primary total hip and knee arthroplasty. J Arthroplasty. 2014;29(8):1512-1515.
15. Roy SP, Tanki UF, Dutta A, Jain SK, Nagi ON. Efficacy of intra-articular tranexamic acid in blood loss reduction following primary unilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2494-2501.
16. Ishida K, Tsumura N, Kitagawa A, et al. Intra-articular injection of tranexamic acid reduces not only blood loss but also knee joint swelling after total knee arthroplasty. Int Orthop. 2011;35(11):1639-1645.
17. Sitek P, Wysocka-Wycisk A, Kępski F, Król D, Bursig H, Dyląg S. PRP-fibrinogen gel-like chondrocyte carrier stabilized by TXA-preliminary study. Cell Tissue Bank. 2013;14(1):133-140.
18. Lo IK, Sciore P, Chung M, et al. Local anesthetics induce chondrocyte death in bovine articular cartilage disks in a dose- and duration-dependent manner. Arthroscopy. 2009;25(7):707-715.
19. Blumberg TJ, Natoli RM, Athanasiou KA. Effects of doxycycline on articular cartilage GAG release and mechanical properties following impact. Biotechnol Bioeng. 2008;100(3):506-515.
20. Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90(5):986-991.
21. Lefebvre V, Garofalo S, Zhou G, Metsäranta M, Vuorio E, De Crombrugghe B. Characterization of primary cultures of chondrocytes from type II collagen/beta-galactosidase transgenic mice. Matrix Biol. 1994;14(4):329-335.
22. Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;833(2):173-177.
23. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671-675.
24. R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008.
25. Sa-Ngasoongsong P, Channoom T, Kawinwonggowit V, et al. Postoperative blood loss reduction in computer-assisted surgery total knee replacement by low dose intra-articular tranexamic acid injection together with 2-hour clamp drain: a prospective triple-blinded randomized controlled trial. Orthop Rev. 2011;3(2):e12.
26. Lecker I, Wang DS, Romaschin AD, Peterson M, Mazer CD, Orser BA. Tranexamic acid concentrations associated with human seizures inhibit glycine receptors. J Clin Invest. 2012;122(12):4654-4666.
27. Kakiuchi H, Kawarai-Shimamura A, Kuwagata M, Orito K. Tranexamic acid induces kaolin intake stimulating a pathway involving tachykinin neurokinin 1 receptors in rats. Eur J Pharmacol. 2014;723:1-6.
28. Kratzer S, Irl H, Mattusch C, et al. Tranexamic acid impairs γ-aminobutyric acid receptor type A–mediated synaptic transmission in the murine amygdala: a potential mechanism for drug-induced seizures? Anesthesiology. 2014;120(3):639-649.
29. Lee E, Enomoto R, Takemura K, Tsuda Y, Okada Y. A selective plasmin inhibitor, trans-aminomethylcyclohexanecarbonyl-L-(O-picolyl)tyrosine-octylamide (YO-2), induces thymocyte apoptosis. Biochem Pharmacol. 2002;63(7):1315-1323.