User login
Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide, and its incidence continues to increase. More than 5 million NMSCs are estimated to occur annually in the United States alone.1 There are more cases of basal cell carcinoma (BCC) than all other cancers combined, with squamous cell carcinoma (SCC) being the second most common cancer in the United States.1-3 The rising incidence of NMSCs highlights the importance of investigating additional treatment options with fewer side effects, better cosmetic outcomes, and better efficacy.1
Originally, treatment options for NMSCs largely relied on destructive and surgical methods. Basal cell carcinoma and SCC commonly are treated with cryosurgery; electrodesiccation and curettage; or more definitive surgical options, including excision and Mohs micrographic surgery (MMS). Over time, topical agents such as 5-fluorouracil, imiquimod, ingenol mebutate, and various forms of aminolevulinic acid (ALA) for photodynamic therapy (PDT) were included for superficial lesions as well as field treatment. The development of oral hedgehog (Hh) inhibitors, such as vismodegib, offered a promising alternative to patients with advanced disease. Each treatment has its own specific indications and side effects; thus, there is always room for novel therapeutic approaches. We review new and potential treatments from 2018 and beyond. Although only 5% of SCCs become locally advanced, recur, or metastasize, and 0.4% to 0.8% of BCCs progress to advanced disease, many of the newer studies target advanced NMSCs, given their life-threatening and debilitating nature.4,5 Similarly, the incidence of nevoid basal cell carcinoma (NBCC) syndrome is as low as 1 in 57,000 to 1 in 164,000 but continues to be studied because of its morbidity and the potential to contribute new treatment options for BCC in the general population.6
Topical Therapy
Sonidegib
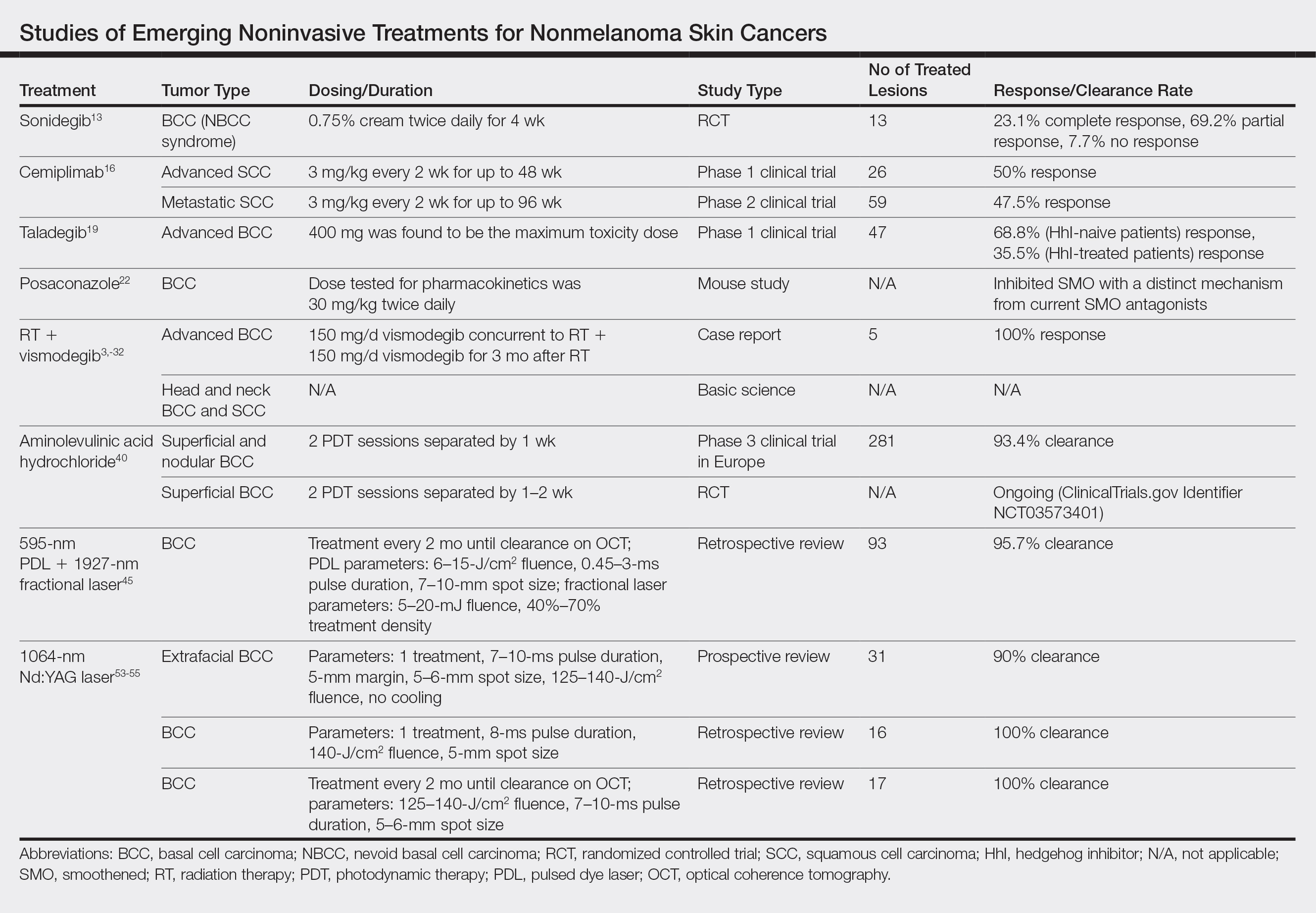
Basal cell carcinoma proliferation is a result of an unregulated Hh pathway that is initiated when the Hh ligand binds to the patched 1 protein (PTCH1).7-11 Patched 1 protein normally inhibits the smoothened (SMO) transmembrane receptor protein, decreasing the signaling cascade. In BCCs, there is a loss of PTCH1 function, effectively increasing the Hh pathway activity. Sonidegib is an Hh inhibitor that in turn prevents inhibition of PTCH1 in an attempt to reregulate the pathway.7-11 Although sonidegib is known for its 2015 approval by the US Food and Drug Administration (FDA) as a systemic therapy for locally advanced BCCs,12 one study investigated a topical formulation on 8 patients with NBCC syndrome.13 Patients were treated twice daily with sonidegib cream 0.75% for 4 weeks in a double-blind, randomized, vehicle-controlled study. A total of 27 BCCs were randomized and treated with either vehicle or sonidegib. A biopsy was taken at the end of the study of 1 sonidegib-treated and 1 vehicle-treated BCC lesion per patient. Of the 13 sonidegib-treated BCC lesions, 3 (23.1%) showed complete response, 9 (69.2%) showed partial response, and 1 (7.7%) showed no response vs 13 of 14 (92.8%) lesions that did not respond to the vehicle. Patients tolerated the treatment well without skin irritation or signs of local or systemic side effects.13 Topical sonidegib should be further investigated as an adjunct or in different vehicles given the successful regression of BCCs and its minimal side-effect profile.
Systemic Therapy
Cemiplimab
Cemiplimab is a human monoclonal antibody against programmed death receptor 1 (PD-1) that was FDA approved in September 2018 for the treatment of metastatic cutaneous SCC.14 Programmed death receptor 1 is found on T lymphocytes, B lymphocytes, and macrophages, which normally assist in the immune response to tumor cells. However, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) are found on tumor cells and bind to PD-1. Cemiplimab prevents PD-1 from binding to PD-L1 and PD-L2, allowing an appropriate immune response.14,15 A phase 1 clinical trial of cemiplimab showed a 50% (13/26) response rate.16 The phase 2 trial included patients with advanced SCC, but the primary analysis only considered patients with metastatic SCC. Phase 2 results showed a 47.5% (28/59) response rate. Patients received intravenous cemiplimab 3 mg/kg once every 2 weeks for up to 48 weeks in phase 1 and up to 96 weeks in phase 2. Both phases of the trial showed a response to treatment lasting longer than 6 months in more than 50% of patients. The most common adverse events were diarrhea, fatigue, nausea, constipation, and rash.16
Although immune-mediated adverse reactions are rare, they can occur given cemiplimab’s mechanism of action and may range from severe to fatal. Examples of immune-mediated adverse reactions that occurred during the study included pneumonitis, colitis, hepatitis, adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, type 1 diabetes mellitus, nephritis with renal dysfunction, and immune-mediated dermatologic reactions.14 It is important to monitor for immune-mediated adverse reactions and address them immediately once detected.
Other PD-1 Inhibitors
Although PD-1 inhibitors have been studied in advanced SCCs, their clinical data are limited for BCCs.17 Prior to 2018, there was a small number of case reports of patients with BCC with partial to exceptional response to PD-1 inhibitors. Recently, 2 additional case reports were published with contrasting outcomes using 2 different PD-1 inhibitors. An elderly patient with metastatic non–small cell lung cancer was treated with nivolumab after failing chemotherapy. She subsequently developed a BCC on the nose that was resected but recurred 2 months later despite continuing nivolumab.17 Another case report detailed a patient with a history of BCC on the shoulder excised 5 years prior who presented with recurrence on the sternum and clavicle.18 One year later the patient was found to have BCC metastases to the lung. After progression of disease despite vismodegib and recurrence of BCC with taladegib, the patient was then placed on pembrolizumab. At 6 weeks and 12 months, computed tomography showed resolution of multiple lung lesions. Sixteen weeks after initiation of pembrolizumab treatment, spinal metastases were found, but the treatment was continued because of the improvement in the lung metastases.18
Taladegib
Taladegib is a SMO antagonist that has been through a phase 1 trial in patients with advanced cancer, including treatment-naive and previously treated BCCs.19 Eighty-four patients were treated to examine the safety profile and determine an appropriate phase 2 dose and administration schedule. The maximum tolerable dose was determined to be 400 mg because of dose-limiting toxicities. All clinical responses were in patients with BCCs (47/84 [55.9%] patients), with a response rate of 46.8%. Eleven of 16 (68.8%) Hh-treatment–naive patients and 11 of 31 (35.5%) patients previously treated with Hh responded to taladegib. Common adverse events were dysgeusia, fatigue, nausea, and muscle spasms.19 Although vismodegib is an FDA-approved SMO antagonist since 2012, treatment resistance and tolerability issues have been continuing concerns.20,21 Taladegib is a potential alternative that may be found to have improved pharmacodynamics and pharmacokinetics. Not only did in vitro studies show a preferable protein-binding profile with taladegib, but it also displayed dose proportionality, while vismodegib has been known to have nonlinear pharmacokinetics.19
Posaconazole
Posaconazole is a systemic antifungal agent that is a structural analogue to itraconazole.22 Itraconazole has been found to inhibit the Hh pathway as an SMO antagonist. In a study with mice, posaconazole was found to have strong activity against drug-resistant SMO mutants while inhibiting the growth of Hh-dependent BCCs in vivo. A marked decrease also was seen in the ciliary accumulation of SMO, suggesting a similar mechanism of action to itraconazole. Posaconazole’s use for BCCs currently is limited to basic science studies but may offer a potential alternative to itraconazole, which is known to have many drug-drug interactions and requires dose adjustments in renal and hepatic insufficiency. When used as an antifungal compared to itraconazole, posaconazole has a favorable long-term safety profile due to fewer drug-drug interactions and mild side effects; it also does not require dose adjustments in mild to moderate renal or hepatic insufficiency.22 Thus, posaconazole is a potentially safer alternative to itraconazole for the treatment of BCCs. Although phase 2 studies of itraconazole for BCCs have shown decreased cell proliferation, tumor size, and reduced GLI1 messenger RNA, side effects included fatigue and grade 4 heart failure.23,24
Radiation Therapy
Radiation therapies (RTs), such as superficial RT, have been long-established treatment options.25 However, there also are emerging methods of delivering RT, including electronic brachytherapy (EB). Although there is a low likelihood of residual tumor after RT given the number of sessions involved and the more aggressive nature of the treatment, these factors also can be a substantial burden on the patient. Furthermore, RT may result in subsequent scar tissue, which can hinder the use of other emerging technologies, such as noninvasive imaging devices, following RT.
Superficial RT
Superficial RT is a secondary option for the treatment of NMSC for use in special circumstances, such as when surgical intervention is contraindicated or refused, and after the benefits and risks of treatment alternatives have been discussed.26 However, depending on the tumor type and anatomical location, 6 to 18 treatments may be required, with treatment frequency ranging from 1 to 5 treatments per week.25 Patients may find this treatment regimen difficult to maintain given the length of time and frequency of treatments required. Side effects include radiation dermatitis and postinflammatory hypopigmentation or hyperpigmentation in patients with dark skin, and there is a risk for recurrence.25,27
Electronic Brachytherapy
Brachytherapy is a method of delivering RT via radioactive isotopes, whereas EB uses lower-energy photons that require less shielding.28 As a relatively new therapy, studies on the efficacy of EB on NMSC continue to grow but with limited data comparing EB with established treatments. Furthermore, there are limited long-term follow-up data, and future studies should expand the patient demographic to younger patients before treatment guidelines can be established.28
RT With Concurrent and Adjuvant Vismodegib
Vismodegib is an SMO inhibitor that was FDA approved in 2012 for the treatment of locally advanced BCC in patients who are not candidates for surgery or RT.29 Over time, studies have looked into other indications for vismodegib, such as a neoadjuvant to MMS or in patients with NBCC syndrome.11 Prior to 2018, there were only 2 known case reports of concurrent vismodegib and RT used for recurrent advanced BCC.30 Recently, vismodegib has been further examined in combination with RT in a case report,31 basic science study,32 and phase 2 trials (ClinicalTrials.gov Identifiers NCT02956889 and NCT01835626).
Prior studies showed low cure rates with vismodegib alone after RT (43%) as well as decreasing cure rates with primary RT alone as tumor size increased.33,34 In 2018, vismodegib was used concurrently and as an adjuvant to RT in a patient with advanced multifocal BCC.31 The patient had multiple large BCCs on the trunk that were painful and bleeding. The patient was started on RT and 150 mg/d vismodegib concurrently, which was then continued adjuvantly for 3 months until it was discontinued because of diarrhea. The patient had complete response in all lesions with resolution of symptoms.31 A separate basic science study further supported the potential role of vismodegib in radiation sensitization of both BCCs and head and neck SCCs.32 There presently are 2 phase 2 trials investigating the concurrent use of vismodegib and RT, which could help determine the efficacy of the combined approach for patients with advanced BCCs who are poor surgical candidates (NCT02956889 and NCT01835626).
Photodynamic Therapy
Photodynamic therapy has been in use since the 1970s when Dougherty et al35 performed one of the first studies on its use in skin cancer. Since then, PDT has been used for the treatment of actinic keratoses (AKs) and more recently BCCs. In PDT, a photosensitizer (PS) is applied and activated by a 400-nm blue light or 635-nm red light, depending on the PS used. The PS then produces highly reactive oxygen species, leading to apoptosis of the cancer cells.36 In Europe, red light PDT is licensed for the treatment of AKs as well as superficial and nodular BCCs, though approved indications vary between countries. In the United States, PDT is only FDA approved for the treatment of AKs.37
Aminolevulinic Acid Hydrochloride
Aminolevulinic acid hydrochloride is a red light PS used to treat AKs since 2011 and BCCs since 2017 in Europe in addition to AKs in the United States since 2016.38,39 A phase 3 noninferiority clinical trial in Europe of 281 patients compared the treatment of nonaggressive BCCs with ALA to methyl aminolevulinate (MAL) cream.40 The study found a complete response rate of 93.4% vs 91.8%. Superficial BCCs treated with ALA had a clearance rate of 94.7% vs 96.4% with MAL, while nodular BCCs treated with ALA had a clearance rate of 85.7% vs 76.2% with MAL. A 1-year clinical follow-up showed similar recurrence rates (8.4% for ALA vs 8.5% for MAL).40 The results of this study led to an expanded indication in Europe to include the treatment of BCCs.38 Aminolevulinic acid hydrochloride currently is undergoing phase 3 clinical trials in the United States for approval for the treatment of superficial BCCs (NCT03573401). If similar outcomes are achieved, US patients may have access to an alternative nonsurgical treatment of BCCs. The ongoing US trial is exclusively investigating the efficacy and safety for superficial BCCs, which may limit FDA approval to only superficial BCCs, accounting for only 8.4% to 24.1% of all BCCs.35,41,42
Laser Therapy
Ablative and nonablative lasers have been used to treat NMSCs in the literature. Ablative lasers destroy tumors through vaporization of tissue water, whereas nonablative lasers target the vasculature of tumors while preserving the surrounding tissue.43,44 Nonablative lasers include pulsed dye lasers (PDL) and Nd:YAG lasers. Examples of ablative lasers include CO2 and erbium:YAG lasers. Given the status of lasers as an emerging treatment method, there currently is no standardized laser setting for any of the laser therapies used to treat NMSCs. Although there is the potential for optimal cosmetic outcomes and a limited side-effect profile for nonablative laser therapies, there are limited data on long-term follow-up to study recurrence rates and establish a more standardized treatment protocol.
Pulsed Dye Lasers
Although there were no studies on PDL therapy alone in 2018, a study published in 2019 evaluated a combination laser treatment using a 595-nm PDL and 1927-nm fractional laser for the treatment of 93 BCCs, yielding a 95.7% (89/93) clearance rate and 4.5% (4/89) recurrence rate over a follow-up period of up to 6 years (range, 2.53 months to 6.03 years).45 Studies of PDL prior to 2018 had follow-ups ranging from 2 weeks to 6 months.46-51 Although the majority were biopsy-proven BCCs, reflectance confocal microscopy also was used for same-day diagnoses. Long-term follow-up included clinical examinations, dermoscopy, and optical coherence tomography.45 The clearance rate (95.7%) using noninvasive imaging in conjunction with the combination laser treatment was superior to both histologic and clinical clearance rates of prior PDL-only studies, which ranged from 25% to 95%.46-51 To have long-term follow-up data, the study used noninvasive imaging with clinical follow-up because histology would not be viable for long-term follow-up. This study was retrospective rather than prospective, which was a limitation.45
Nd:YAG Lasers
The majority of studies utilizing Nd:YAG lasers investigated their efficacy in treating BCCs, with the exception of 1 study of facial SCCs. This major study in 2009 of 627 BCCs showed a 2.5% recurrence rate after a follow-up time of 3 months to 5 years.52 Nd:YAG lasers continue to be investigated, including a more recent study of 31 extrafacial, biopsy-proven BCCs that were treated with the 1064-nm Nd:YAG laser, which showed a 90% histologic clearance on 1-month follow-up after a single treatment.53 In 2019, a retrospective review of 16 BCC lesions on the head, neck, trunk, and extremities showed 100% clearance after 1 treatment, with an average follow-up period of 9 months (range, 6–15 months).54 In a retrospective review, Markowitz and Psomadakis55 contributed data supporting the further investigation and use of the 1064-nm Nd:YAG laser for BCC treatment while leveraging noninvasive imaging to demonstrate a same-day management model. Seventeen BCC lesions on the face and body were diagnosed by reflectance confocal microscopy and treated with an Nd:YAG laser, and clearance was monitored clinically, dermoscopically, and by optical coherence tomography. There was 100% clearance of the lesions in the study, with 82.4% (14/17) clearing after 1 treatment; mean follow-up was 103 days (range, 48–371 days).55 These studies were limited by their short follow-up time; long-term data are needed to determine true rates of recurrence.
Ablative Lasers
Ablative lasers also have been used in the treatment of NMSCs. In addition to the potentially increased healing time compared to nonablative lasers, other limitations of ablative laser therapy include residual tumor burden or recurrence that may not be easily visualized in scarred tissue after nonablative management.44
Conclusion
Although MMS remains the gold standard for invasive management of NMSCs, studies from 2018 and beyond (eTable) expanded not only on MMS topics such as increased patient access and improved techniques but also on the increasing potential of noninvasive treatments. Some of the noninvasive therapies were entirely new compounds, whereas others were already in use for a different disease indication. Furthering our knowledge and expanding our repertoire of management options will prepare us as the number of patients affected by NMSCs increases.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Kauvar AN, Arpey CJ, Hruza G, et al. Consensus for nonmelanoma skin cancer treatment, part II. Dermatol Surg. 2015;41:1214-1240.
- Ribero S, Stucci LS, Daniels GA, et al. Drug therapy of advanced cutaneous squamous cell carcinoma: is there any evidence? Curr Opin Oncol. 2017;29:129-135.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e2-966.e2.
- Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299-308.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Peris K, Licitra L, Ascierto PA, et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Futur Oncol. 2015;11:703-712.
- Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Ibrahim O, Gastman B, Zhang A. Advances in diagnosis and treatment of nonmelanoma skin cancer. Ann Plast Surg. 2014;73:615-619.
- Levine A, Siegel DM, Markowitz O. Update on noninvasive diagnostic imaging and management of nonmelanoma skin cancer. Curr Dermatol Rep. 2018;7:1-15.
- Casey D, Demko S, Shord S, et al. FDA approval summary: sonidegib for locally advanced basal cell carcinoma. Clin Cancer Res. 2017;23:2377-2381.
- Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131:1735-1744.
- Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841-1846.
- Chen L, Aria AB, Silapunt S, et al. Emerging nonsurgical therapies for locally advanced and metastatic nonmelanoma skin cancer. Dermatolog Surg. 2019;45:1-16.
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351.
- Sabbatino F, Marra A, Liguori L, et al. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature. J Immunother Cancer. 2018;6:126.
- Cannon JGD, Russell JS, Kim J, et al. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4:248-250.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111:1476-1481.
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14:633-641.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Nestor MS, Berman B, Goldberg D, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12:12-18.
- American Academy of Dermatology and AAD Association. Position statement on superficial radiation therapy for basal cell carcinoma (BCC) and squamous cell carcinomas (SCC). https://server.aad.org/Forms/Policies/Uploads/PS/PS%20Superficial%20Radiation%20Therapy.pdf?. Updated August 9, 2014. Accessed February 26, 2020.
- Skiveren J, Mikkelsen MR, Daugbjerg H, et al. Skin reactions and quality of life after X-ray therapy of basal cell carcinoma. J Skin Cancer. 2012;2012:825095.
- Tom MC, Hepel JT, Patel R, et al. The American Brachytherapy Society consensus statement for electronic brachytherapy. Brachytherapy. 2019;18:292-298.
- Axelson M, Liu K, Jiang X, et al. US Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289-2293.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:E2485.
- Piccinno R, Benardon S, Gaiani FM, et al. Dermatologic radiotherapy in the treatment of extensive basal cell carcinomas: a retrospective study. J Dermatolog Treat. 2017;28:426-430.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol. 2001;51:748-755.
- Dougherty TJ, Kaufman JE, Goldfarb A, et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628-2635.
- Ding H, Yu H, Dong Y, et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276-280.
- Maytin EV, Kaw U, Ilyas M, et al. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: a bilaterally controlled comparison study. Photodiagnosis Photodyn Ther. 2018;22:7-13.
- European Medicines Agency. Ameluz 5-aminolevulinic acid hydrochloride. https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz. Updated May 13, 2019. Accessed February 25, 2020.
- Center for Drug Evaluation and Research. Approval package for Ameluz (aminolevulinic acid hydrochloride) gel, 10%. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208081Orig1s000Approv.pdf. Published May 10, 2016. Accessed February 25, 2020.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179:309-319.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Kamyab-Hesari K, Seirafi H, Naraghi ZS, et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014;28:250-253.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297, E1-E3.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O, Psomadakis CE. Patient-driven management using same-day noninvasive diagnosis and complete laser treatment of basal cell carcinomas: a pilot study. Cutis. 2019;104:345-348.
Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide, and its incidence continues to increase. More than 5 million NMSCs are estimated to occur annually in the United States alone.1 There are more cases of basal cell carcinoma (BCC) than all other cancers combined, with squamous cell carcinoma (SCC) being the second most common cancer in the United States.1-3 The rising incidence of NMSCs highlights the importance of investigating additional treatment options with fewer side effects, better cosmetic outcomes, and better efficacy.1
Originally, treatment options for NMSCs largely relied on destructive and surgical methods. Basal cell carcinoma and SCC commonly are treated with cryosurgery; electrodesiccation and curettage; or more definitive surgical options, including excision and Mohs micrographic surgery (MMS). Over time, topical agents such as 5-fluorouracil, imiquimod, ingenol mebutate, and various forms of aminolevulinic acid (ALA) for photodynamic therapy (PDT) were included for superficial lesions as well as field treatment. The development of oral hedgehog (Hh) inhibitors, such as vismodegib, offered a promising alternative to patients with advanced disease. Each treatment has its own specific indications and side effects; thus, there is always room for novel therapeutic approaches. We review new and potential treatments from 2018 and beyond. Although only 5% of SCCs become locally advanced, recur, or metastasize, and 0.4% to 0.8% of BCCs progress to advanced disease, many of the newer studies target advanced NMSCs, given their life-threatening and debilitating nature.4,5 Similarly, the incidence of nevoid basal cell carcinoma (NBCC) syndrome is as low as 1 in 57,000 to 1 in 164,000 but continues to be studied because of its morbidity and the potential to contribute new treatment options for BCC in the general population.6
Topical Therapy
Sonidegib
Basal cell carcinoma proliferation is a result of an unregulated Hh pathway that is initiated when the Hh ligand binds to the patched 1 protein (PTCH1).7-11 Patched 1 protein normally inhibits the smoothened (SMO) transmembrane receptor protein, decreasing the signaling cascade. In BCCs, there is a loss of PTCH1 function, effectively increasing the Hh pathway activity. Sonidegib is an Hh inhibitor that in turn prevents inhibition of PTCH1 in an attempt to reregulate the pathway.7-11 Although sonidegib is known for its 2015 approval by the US Food and Drug Administration (FDA) as a systemic therapy for locally advanced BCCs,12 one study investigated a topical formulation on 8 patients with NBCC syndrome.13 Patients were treated twice daily with sonidegib cream 0.75% for 4 weeks in a double-blind, randomized, vehicle-controlled study. A total of 27 BCCs were randomized and treated with either vehicle or sonidegib. A biopsy was taken at the end of the study of 1 sonidegib-treated and 1 vehicle-treated BCC lesion per patient. Of the 13 sonidegib-treated BCC lesions, 3 (23.1%) showed complete response, 9 (69.2%) showed partial response, and 1 (7.7%) showed no response vs 13 of 14 (92.8%) lesions that did not respond to the vehicle. Patients tolerated the treatment well without skin irritation or signs of local or systemic side effects.13 Topical sonidegib should be further investigated as an adjunct or in different vehicles given the successful regression of BCCs and its minimal side-effect profile.
Systemic Therapy
Cemiplimab
Cemiplimab is a human monoclonal antibody against programmed death receptor 1 (PD-1) that was FDA approved in September 2018 for the treatment of metastatic cutaneous SCC.14 Programmed death receptor 1 is found on T lymphocytes, B lymphocytes, and macrophages, which normally assist in the immune response to tumor cells. However, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) are found on tumor cells and bind to PD-1. Cemiplimab prevents PD-1 from binding to PD-L1 and PD-L2, allowing an appropriate immune response.14,15 A phase 1 clinical trial of cemiplimab showed a 50% (13/26) response rate.16 The phase 2 trial included patients with advanced SCC, but the primary analysis only considered patients with metastatic SCC. Phase 2 results showed a 47.5% (28/59) response rate. Patients received intravenous cemiplimab 3 mg/kg once every 2 weeks for up to 48 weeks in phase 1 and up to 96 weeks in phase 2. Both phases of the trial showed a response to treatment lasting longer than 6 months in more than 50% of patients. The most common adverse events were diarrhea, fatigue, nausea, constipation, and rash.16
Although immune-mediated adverse reactions are rare, they can occur given cemiplimab’s mechanism of action and may range from severe to fatal. Examples of immune-mediated adverse reactions that occurred during the study included pneumonitis, colitis, hepatitis, adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, type 1 diabetes mellitus, nephritis with renal dysfunction, and immune-mediated dermatologic reactions.14 It is important to monitor for immune-mediated adverse reactions and address them immediately once detected.
Other PD-1 Inhibitors
Although PD-1 inhibitors have been studied in advanced SCCs, their clinical data are limited for BCCs.17 Prior to 2018, there was a small number of case reports of patients with BCC with partial to exceptional response to PD-1 inhibitors. Recently, 2 additional case reports were published with contrasting outcomes using 2 different PD-1 inhibitors. An elderly patient with metastatic non–small cell lung cancer was treated with nivolumab after failing chemotherapy. She subsequently developed a BCC on the nose that was resected but recurred 2 months later despite continuing nivolumab.17 Another case report detailed a patient with a history of BCC on the shoulder excised 5 years prior who presented with recurrence on the sternum and clavicle.18 One year later the patient was found to have BCC metastases to the lung. After progression of disease despite vismodegib and recurrence of BCC with taladegib, the patient was then placed on pembrolizumab. At 6 weeks and 12 months, computed tomography showed resolution of multiple lung lesions. Sixteen weeks after initiation of pembrolizumab treatment, spinal metastases were found, but the treatment was continued because of the improvement in the lung metastases.18
Taladegib
Taladegib is a SMO antagonist that has been through a phase 1 trial in patients with advanced cancer, including treatment-naive and previously treated BCCs.19 Eighty-four patients were treated to examine the safety profile and determine an appropriate phase 2 dose and administration schedule. The maximum tolerable dose was determined to be 400 mg because of dose-limiting toxicities. All clinical responses were in patients with BCCs (47/84 [55.9%] patients), with a response rate of 46.8%. Eleven of 16 (68.8%) Hh-treatment–naive patients and 11 of 31 (35.5%) patients previously treated with Hh responded to taladegib. Common adverse events were dysgeusia, fatigue, nausea, and muscle spasms.19 Although vismodegib is an FDA-approved SMO antagonist since 2012, treatment resistance and tolerability issues have been continuing concerns.20,21 Taladegib is a potential alternative that may be found to have improved pharmacodynamics and pharmacokinetics. Not only did in vitro studies show a preferable protein-binding profile with taladegib, but it also displayed dose proportionality, while vismodegib has been known to have nonlinear pharmacokinetics.19
Posaconazole
Posaconazole is a systemic antifungal agent that is a structural analogue to itraconazole.22 Itraconazole has been found to inhibit the Hh pathway as an SMO antagonist. In a study with mice, posaconazole was found to have strong activity against drug-resistant SMO mutants while inhibiting the growth of Hh-dependent BCCs in vivo. A marked decrease also was seen in the ciliary accumulation of SMO, suggesting a similar mechanism of action to itraconazole. Posaconazole’s use for BCCs currently is limited to basic science studies but may offer a potential alternative to itraconazole, which is known to have many drug-drug interactions and requires dose adjustments in renal and hepatic insufficiency. When used as an antifungal compared to itraconazole, posaconazole has a favorable long-term safety profile due to fewer drug-drug interactions and mild side effects; it also does not require dose adjustments in mild to moderate renal or hepatic insufficiency.22 Thus, posaconazole is a potentially safer alternative to itraconazole for the treatment of BCCs. Although phase 2 studies of itraconazole for BCCs have shown decreased cell proliferation, tumor size, and reduced GLI1 messenger RNA, side effects included fatigue and grade 4 heart failure.23,24
Radiation Therapy
Radiation therapies (RTs), such as superficial RT, have been long-established treatment options.25 However, there also are emerging methods of delivering RT, including electronic brachytherapy (EB). Although there is a low likelihood of residual tumor after RT given the number of sessions involved and the more aggressive nature of the treatment, these factors also can be a substantial burden on the patient. Furthermore, RT may result in subsequent scar tissue, which can hinder the use of other emerging technologies, such as noninvasive imaging devices, following RT.
Superficial RT
Superficial RT is a secondary option for the treatment of NMSC for use in special circumstances, such as when surgical intervention is contraindicated or refused, and after the benefits and risks of treatment alternatives have been discussed.26 However, depending on the tumor type and anatomical location, 6 to 18 treatments may be required, with treatment frequency ranging from 1 to 5 treatments per week.25 Patients may find this treatment regimen difficult to maintain given the length of time and frequency of treatments required. Side effects include radiation dermatitis and postinflammatory hypopigmentation or hyperpigmentation in patients with dark skin, and there is a risk for recurrence.25,27
Electronic Brachytherapy
Brachytherapy is a method of delivering RT via radioactive isotopes, whereas EB uses lower-energy photons that require less shielding.28 As a relatively new therapy, studies on the efficacy of EB on NMSC continue to grow but with limited data comparing EB with established treatments. Furthermore, there are limited long-term follow-up data, and future studies should expand the patient demographic to younger patients before treatment guidelines can be established.28
RT With Concurrent and Adjuvant Vismodegib
Vismodegib is an SMO inhibitor that was FDA approved in 2012 for the treatment of locally advanced BCC in patients who are not candidates for surgery or RT.29 Over time, studies have looked into other indications for vismodegib, such as a neoadjuvant to MMS or in patients with NBCC syndrome.11 Prior to 2018, there were only 2 known case reports of concurrent vismodegib and RT used for recurrent advanced BCC.30 Recently, vismodegib has been further examined in combination with RT in a case report,31 basic science study,32 and phase 2 trials (ClinicalTrials.gov Identifiers NCT02956889 and NCT01835626).
Prior studies showed low cure rates with vismodegib alone after RT (43%) as well as decreasing cure rates with primary RT alone as tumor size increased.33,34 In 2018, vismodegib was used concurrently and as an adjuvant to RT in a patient with advanced multifocal BCC.31 The patient had multiple large BCCs on the trunk that were painful and bleeding. The patient was started on RT and 150 mg/d vismodegib concurrently, which was then continued adjuvantly for 3 months until it was discontinued because of diarrhea. The patient had complete response in all lesions with resolution of symptoms.31 A separate basic science study further supported the potential role of vismodegib in radiation sensitization of both BCCs and head and neck SCCs.32 There presently are 2 phase 2 trials investigating the concurrent use of vismodegib and RT, which could help determine the efficacy of the combined approach for patients with advanced BCCs who are poor surgical candidates (NCT02956889 and NCT01835626).
Photodynamic Therapy
Photodynamic therapy has been in use since the 1970s when Dougherty et al35 performed one of the first studies on its use in skin cancer. Since then, PDT has been used for the treatment of actinic keratoses (AKs) and more recently BCCs. In PDT, a photosensitizer (PS) is applied and activated by a 400-nm blue light or 635-nm red light, depending on the PS used. The PS then produces highly reactive oxygen species, leading to apoptosis of the cancer cells.36 In Europe, red light PDT is licensed for the treatment of AKs as well as superficial and nodular BCCs, though approved indications vary between countries. In the United States, PDT is only FDA approved for the treatment of AKs.37
Aminolevulinic Acid Hydrochloride
Aminolevulinic acid hydrochloride is a red light PS used to treat AKs since 2011 and BCCs since 2017 in Europe in addition to AKs in the United States since 2016.38,39 A phase 3 noninferiority clinical trial in Europe of 281 patients compared the treatment of nonaggressive BCCs with ALA to methyl aminolevulinate (MAL) cream.40 The study found a complete response rate of 93.4% vs 91.8%. Superficial BCCs treated with ALA had a clearance rate of 94.7% vs 96.4% with MAL, while nodular BCCs treated with ALA had a clearance rate of 85.7% vs 76.2% with MAL. A 1-year clinical follow-up showed similar recurrence rates (8.4% for ALA vs 8.5% for MAL).40 The results of this study led to an expanded indication in Europe to include the treatment of BCCs.38 Aminolevulinic acid hydrochloride currently is undergoing phase 3 clinical trials in the United States for approval for the treatment of superficial BCCs (NCT03573401). If similar outcomes are achieved, US patients may have access to an alternative nonsurgical treatment of BCCs. The ongoing US trial is exclusively investigating the efficacy and safety for superficial BCCs, which may limit FDA approval to only superficial BCCs, accounting for only 8.4% to 24.1% of all BCCs.35,41,42
Laser Therapy
Ablative and nonablative lasers have been used to treat NMSCs in the literature. Ablative lasers destroy tumors through vaporization of tissue water, whereas nonablative lasers target the vasculature of tumors while preserving the surrounding tissue.43,44 Nonablative lasers include pulsed dye lasers (PDL) and Nd:YAG lasers. Examples of ablative lasers include CO2 and erbium:YAG lasers. Given the status of lasers as an emerging treatment method, there currently is no standardized laser setting for any of the laser therapies used to treat NMSCs. Although there is the potential for optimal cosmetic outcomes and a limited side-effect profile for nonablative laser therapies, there are limited data on long-term follow-up to study recurrence rates and establish a more standardized treatment protocol.
Pulsed Dye Lasers
Although there were no studies on PDL therapy alone in 2018, a study published in 2019 evaluated a combination laser treatment using a 595-nm PDL and 1927-nm fractional laser for the treatment of 93 BCCs, yielding a 95.7% (89/93) clearance rate and 4.5% (4/89) recurrence rate over a follow-up period of up to 6 years (range, 2.53 months to 6.03 years).45 Studies of PDL prior to 2018 had follow-ups ranging from 2 weeks to 6 months.46-51 Although the majority were biopsy-proven BCCs, reflectance confocal microscopy also was used for same-day diagnoses. Long-term follow-up included clinical examinations, dermoscopy, and optical coherence tomography.45 The clearance rate (95.7%) using noninvasive imaging in conjunction with the combination laser treatment was superior to both histologic and clinical clearance rates of prior PDL-only studies, which ranged from 25% to 95%.46-51 To have long-term follow-up data, the study used noninvasive imaging with clinical follow-up because histology would not be viable for long-term follow-up. This study was retrospective rather than prospective, which was a limitation.45
Nd:YAG Lasers
The majority of studies utilizing Nd:YAG lasers investigated their efficacy in treating BCCs, with the exception of 1 study of facial SCCs. This major study in 2009 of 627 BCCs showed a 2.5% recurrence rate after a follow-up time of 3 months to 5 years.52 Nd:YAG lasers continue to be investigated, including a more recent study of 31 extrafacial, biopsy-proven BCCs that were treated with the 1064-nm Nd:YAG laser, which showed a 90% histologic clearance on 1-month follow-up after a single treatment.53 In 2019, a retrospective review of 16 BCC lesions on the head, neck, trunk, and extremities showed 100% clearance after 1 treatment, with an average follow-up period of 9 months (range, 6–15 months).54 In a retrospective review, Markowitz and Psomadakis55 contributed data supporting the further investigation and use of the 1064-nm Nd:YAG laser for BCC treatment while leveraging noninvasive imaging to demonstrate a same-day management model. Seventeen BCC lesions on the face and body were diagnosed by reflectance confocal microscopy and treated with an Nd:YAG laser, and clearance was monitored clinically, dermoscopically, and by optical coherence tomography. There was 100% clearance of the lesions in the study, with 82.4% (14/17) clearing after 1 treatment; mean follow-up was 103 days (range, 48–371 days).55 These studies were limited by their short follow-up time; long-term data are needed to determine true rates of recurrence.
Ablative Lasers
Ablative lasers also have been used in the treatment of NMSCs. In addition to the potentially increased healing time compared to nonablative lasers, other limitations of ablative laser therapy include residual tumor burden or recurrence that may not be easily visualized in scarred tissue after nonablative management.44
Conclusion
Although MMS remains the gold standard for invasive management of NMSCs, studies from 2018 and beyond (eTable) expanded not only on MMS topics such as increased patient access and improved techniques but also on the increasing potential of noninvasive treatments. Some of the noninvasive therapies were entirely new compounds, whereas others were already in use for a different disease indication. Furthering our knowledge and expanding our repertoire of management options will prepare us as the number of patients affected by NMSCs increases.

Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide, and its incidence continues to increase. More than 5 million NMSCs are estimated to occur annually in the United States alone.1 There are more cases of basal cell carcinoma (BCC) than all other cancers combined, with squamous cell carcinoma (SCC) being the second most common cancer in the United States.1-3 The rising incidence of NMSCs highlights the importance of investigating additional treatment options with fewer side effects, better cosmetic outcomes, and better efficacy.1
Originally, treatment options for NMSCs largely relied on destructive and surgical methods. Basal cell carcinoma and SCC commonly are treated with cryosurgery; electrodesiccation and curettage; or more definitive surgical options, including excision and Mohs micrographic surgery (MMS). Over time, topical agents such as 5-fluorouracil, imiquimod, ingenol mebutate, and various forms of aminolevulinic acid (ALA) for photodynamic therapy (PDT) were included for superficial lesions as well as field treatment. The development of oral hedgehog (Hh) inhibitors, such as vismodegib, offered a promising alternative to patients with advanced disease. Each treatment has its own specific indications and side effects; thus, there is always room for novel therapeutic approaches. We review new and potential treatments from 2018 and beyond. Although only 5% of SCCs become locally advanced, recur, or metastasize, and 0.4% to 0.8% of BCCs progress to advanced disease, many of the newer studies target advanced NMSCs, given their life-threatening and debilitating nature.4,5 Similarly, the incidence of nevoid basal cell carcinoma (NBCC) syndrome is as low as 1 in 57,000 to 1 in 164,000 but continues to be studied because of its morbidity and the potential to contribute new treatment options for BCC in the general population.6
Topical Therapy
Sonidegib
Basal cell carcinoma proliferation is a result of an unregulated Hh pathway that is initiated when the Hh ligand binds to the patched 1 protein (PTCH1).7-11 Patched 1 protein normally inhibits the smoothened (SMO) transmembrane receptor protein, decreasing the signaling cascade. In BCCs, there is a loss of PTCH1 function, effectively increasing the Hh pathway activity. Sonidegib is an Hh inhibitor that in turn prevents inhibition of PTCH1 in an attempt to reregulate the pathway.7-11 Although sonidegib is known for its 2015 approval by the US Food and Drug Administration (FDA) as a systemic therapy for locally advanced BCCs,12 one study investigated a topical formulation on 8 patients with NBCC syndrome.13 Patients were treated twice daily with sonidegib cream 0.75% for 4 weeks in a double-blind, randomized, vehicle-controlled study. A total of 27 BCCs were randomized and treated with either vehicle or sonidegib. A biopsy was taken at the end of the study of 1 sonidegib-treated and 1 vehicle-treated BCC lesion per patient. Of the 13 sonidegib-treated BCC lesions, 3 (23.1%) showed complete response, 9 (69.2%) showed partial response, and 1 (7.7%) showed no response vs 13 of 14 (92.8%) lesions that did not respond to the vehicle. Patients tolerated the treatment well without skin irritation or signs of local or systemic side effects.13 Topical sonidegib should be further investigated as an adjunct or in different vehicles given the successful regression of BCCs and its minimal side-effect profile.
Systemic Therapy
Cemiplimab
Cemiplimab is a human monoclonal antibody against programmed death receptor 1 (PD-1) that was FDA approved in September 2018 for the treatment of metastatic cutaneous SCC.14 Programmed death receptor 1 is found on T lymphocytes, B lymphocytes, and macrophages, which normally assist in the immune response to tumor cells. However, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) are found on tumor cells and bind to PD-1. Cemiplimab prevents PD-1 from binding to PD-L1 and PD-L2, allowing an appropriate immune response.14,15 A phase 1 clinical trial of cemiplimab showed a 50% (13/26) response rate.16 The phase 2 trial included patients with advanced SCC, but the primary analysis only considered patients with metastatic SCC. Phase 2 results showed a 47.5% (28/59) response rate. Patients received intravenous cemiplimab 3 mg/kg once every 2 weeks for up to 48 weeks in phase 1 and up to 96 weeks in phase 2. Both phases of the trial showed a response to treatment lasting longer than 6 months in more than 50% of patients. The most common adverse events were diarrhea, fatigue, nausea, constipation, and rash.16
Although immune-mediated adverse reactions are rare, they can occur given cemiplimab’s mechanism of action and may range from severe to fatal. Examples of immune-mediated adverse reactions that occurred during the study included pneumonitis, colitis, hepatitis, adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, type 1 diabetes mellitus, nephritis with renal dysfunction, and immune-mediated dermatologic reactions.14 It is important to monitor for immune-mediated adverse reactions and address them immediately once detected.
Other PD-1 Inhibitors
Although PD-1 inhibitors have been studied in advanced SCCs, their clinical data are limited for BCCs.17 Prior to 2018, there was a small number of case reports of patients with BCC with partial to exceptional response to PD-1 inhibitors. Recently, 2 additional case reports were published with contrasting outcomes using 2 different PD-1 inhibitors. An elderly patient with metastatic non–small cell lung cancer was treated with nivolumab after failing chemotherapy. She subsequently developed a BCC on the nose that was resected but recurred 2 months later despite continuing nivolumab.17 Another case report detailed a patient with a history of BCC on the shoulder excised 5 years prior who presented with recurrence on the sternum and clavicle.18 One year later the patient was found to have BCC metastases to the lung. After progression of disease despite vismodegib and recurrence of BCC with taladegib, the patient was then placed on pembrolizumab. At 6 weeks and 12 months, computed tomography showed resolution of multiple lung lesions. Sixteen weeks after initiation of pembrolizumab treatment, spinal metastases were found, but the treatment was continued because of the improvement in the lung metastases.18
Taladegib
Taladegib is a SMO antagonist that has been through a phase 1 trial in patients with advanced cancer, including treatment-naive and previously treated BCCs.19 Eighty-four patients were treated to examine the safety profile and determine an appropriate phase 2 dose and administration schedule. The maximum tolerable dose was determined to be 400 mg because of dose-limiting toxicities. All clinical responses were in patients with BCCs (47/84 [55.9%] patients), with a response rate of 46.8%. Eleven of 16 (68.8%) Hh-treatment–naive patients and 11 of 31 (35.5%) patients previously treated with Hh responded to taladegib. Common adverse events were dysgeusia, fatigue, nausea, and muscle spasms.19 Although vismodegib is an FDA-approved SMO antagonist since 2012, treatment resistance and tolerability issues have been continuing concerns.20,21 Taladegib is a potential alternative that may be found to have improved pharmacodynamics and pharmacokinetics. Not only did in vitro studies show a preferable protein-binding profile with taladegib, but it also displayed dose proportionality, while vismodegib has been known to have nonlinear pharmacokinetics.19
Posaconazole
Posaconazole is a systemic antifungal agent that is a structural analogue to itraconazole.22 Itraconazole has been found to inhibit the Hh pathway as an SMO antagonist. In a study with mice, posaconazole was found to have strong activity against drug-resistant SMO mutants while inhibiting the growth of Hh-dependent BCCs in vivo. A marked decrease also was seen in the ciliary accumulation of SMO, suggesting a similar mechanism of action to itraconazole. Posaconazole’s use for BCCs currently is limited to basic science studies but may offer a potential alternative to itraconazole, which is known to have many drug-drug interactions and requires dose adjustments in renal and hepatic insufficiency. When used as an antifungal compared to itraconazole, posaconazole has a favorable long-term safety profile due to fewer drug-drug interactions and mild side effects; it also does not require dose adjustments in mild to moderate renal or hepatic insufficiency.22 Thus, posaconazole is a potentially safer alternative to itraconazole for the treatment of BCCs. Although phase 2 studies of itraconazole for BCCs have shown decreased cell proliferation, tumor size, and reduced GLI1 messenger RNA, side effects included fatigue and grade 4 heart failure.23,24
Radiation Therapy
Radiation therapies (RTs), such as superficial RT, have been long-established treatment options.25 However, there also are emerging methods of delivering RT, including electronic brachytherapy (EB). Although there is a low likelihood of residual tumor after RT given the number of sessions involved and the more aggressive nature of the treatment, these factors also can be a substantial burden on the patient. Furthermore, RT may result in subsequent scar tissue, which can hinder the use of other emerging technologies, such as noninvasive imaging devices, following RT.
Superficial RT
Superficial RT is a secondary option for the treatment of NMSC for use in special circumstances, such as when surgical intervention is contraindicated or refused, and after the benefits and risks of treatment alternatives have been discussed.26 However, depending on the tumor type and anatomical location, 6 to 18 treatments may be required, with treatment frequency ranging from 1 to 5 treatments per week.25 Patients may find this treatment regimen difficult to maintain given the length of time and frequency of treatments required. Side effects include radiation dermatitis and postinflammatory hypopigmentation or hyperpigmentation in patients with dark skin, and there is a risk for recurrence.25,27
Electronic Brachytherapy
Brachytherapy is a method of delivering RT via radioactive isotopes, whereas EB uses lower-energy photons that require less shielding.28 As a relatively new therapy, studies on the efficacy of EB on NMSC continue to grow but with limited data comparing EB with established treatments. Furthermore, there are limited long-term follow-up data, and future studies should expand the patient demographic to younger patients before treatment guidelines can be established.28
RT With Concurrent and Adjuvant Vismodegib
Vismodegib is an SMO inhibitor that was FDA approved in 2012 for the treatment of locally advanced BCC in patients who are not candidates for surgery or RT.29 Over time, studies have looked into other indications for vismodegib, such as a neoadjuvant to MMS or in patients with NBCC syndrome.11 Prior to 2018, there were only 2 known case reports of concurrent vismodegib and RT used for recurrent advanced BCC.30 Recently, vismodegib has been further examined in combination with RT in a case report,31 basic science study,32 and phase 2 trials (ClinicalTrials.gov Identifiers NCT02956889 and NCT01835626).
Prior studies showed low cure rates with vismodegib alone after RT (43%) as well as decreasing cure rates with primary RT alone as tumor size increased.33,34 In 2018, vismodegib was used concurrently and as an adjuvant to RT in a patient with advanced multifocal BCC.31 The patient had multiple large BCCs on the trunk that were painful and bleeding. The patient was started on RT and 150 mg/d vismodegib concurrently, which was then continued adjuvantly for 3 months until it was discontinued because of diarrhea. The patient had complete response in all lesions with resolution of symptoms.31 A separate basic science study further supported the potential role of vismodegib in radiation sensitization of both BCCs and head and neck SCCs.32 There presently are 2 phase 2 trials investigating the concurrent use of vismodegib and RT, which could help determine the efficacy of the combined approach for patients with advanced BCCs who are poor surgical candidates (NCT02956889 and NCT01835626).
Photodynamic Therapy
Photodynamic therapy has been in use since the 1970s when Dougherty et al35 performed one of the first studies on its use in skin cancer. Since then, PDT has been used for the treatment of actinic keratoses (AKs) and more recently BCCs. In PDT, a photosensitizer (PS) is applied and activated by a 400-nm blue light or 635-nm red light, depending on the PS used. The PS then produces highly reactive oxygen species, leading to apoptosis of the cancer cells.36 In Europe, red light PDT is licensed for the treatment of AKs as well as superficial and nodular BCCs, though approved indications vary between countries. In the United States, PDT is only FDA approved for the treatment of AKs.37
Aminolevulinic Acid Hydrochloride
Aminolevulinic acid hydrochloride is a red light PS used to treat AKs since 2011 and BCCs since 2017 in Europe in addition to AKs in the United States since 2016.38,39 A phase 3 noninferiority clinical trial in Europe of 281 patients compared the treatment of nonaggressive BCCs with ALA to methyl aminolevulinate (MAL) cream.40 The study found a complete response rate of 93.4% vs 91.8%. Superficial BCCs treated with ALA had a clearance rate of 94.7% vs 96.4% with MAL, while nodular BCCs treated with ALA had a clearance rate of 85.7% vs 76.2% with MAL. A 1-year clinical follow-up showed similar recurrence rates (8.4% for ALA vs 8.5% for MAL).40 The results of this study led to an expanded indication in Europe to include the treatment of BCCs.38 Aminolevulinic acid hydrochloride currently is undergoing phase 3 clinical trials in the United States for approval for the treatment of superficial BCCs (NCT03573401). If similar outcomes are achieved, US patients may have access to an alternative nonsurgical treatment of BCCs. The ongoing US trial is exclusively investigating the efficacy and safety for superficial BCCs, which may limit FDA approval to only superficial BCCs, accounting for only 8.4% to 24.1% of all BCCs.35,41,42
Laser Therapy
Ablative and nonablative lasers have been used to treat NMSCs in the literature. Ablative lasers destroy tumors through vaporization of tissue water, whereas nonablative lasers target the vasculature of tumors while preserving the surrounding tissue.43,44 Nonablative lasers include pulsed dye lasers (PDL) and Nd:YAG lasers. Examples of ablative lasers include CO2 and erbium:YAG lasers. Given the status of lasers as an emerging treatment method, there currently is no standardized laser setting for any of the laser therapies used to treat NMSCs. Although there is the potential for optimal cosmetic outcomes and a limited side-effect profile for nonablative laser therapies, there are limited data on long-term follow-up to study recurrence rates and establish a more standardized treatment protocol.
Pulsed Dye Lasers
Although there were no studies on PDL therapy alone in 2018, a study published in 2019 evaluated a combination laser treatment using a 595-nm PDL and 1927-nm fractional laser for the treatment of 93 BCCs, yielding a 95.7% (89/93) clearance rate and 4.5% (4/89) recurrence rate over a follow-up period of up to 6 years (range, 2.53 months to 6.03 years).45 Studies of PDL prior to 2018 had follow-ups ranging from 2 weeks to 6 months.46-51 Although the majority were biopsy-proven BCCs, reflectance confocal microscopy also was used for same-day diagnoses. Long-term follow-up included clinical examinations, dermoscopy, and optical coherence tomography.45 The clearance rate (95.7%) using noninvasive imaging in conjunction with the combination laser treatment was superior to both histologic and clinical clearance rates of prior PDL-only studies, which ranged from 25% to 95%.46-51 To have long-term follow-up data, the study used noninvasive imaging with clinical follow-up because histology would not be viable for long-term follow-up. This study was retrospective rather than prospective, which was a limitation.45
Nd:YAG Lasers
The majority of studies utilizing Nd:YAG lasers investigated their efficacy in treating BCCs, with the exception of 1 study of facial SCCs. This major study in 2009 of 627 BCCs showed a 2.5% recurrence rate after a follow-up time of 3 months to 5 years.52 Nd:YAG lasers continue to be investigated, including a more recent study of 31 extrafacial, biopsy-proven BCCs that were treated with the 1064-nm Nd:YAG laser, which showed a 90% histologic clearance on 1-month follow-up after a single treatment.53 In 2019, a retrospective review of 16 BCC lesions on the head, neck, trunk, and extremities showed 100% clearance after 1 treatment, with an average follow-up period of 9 months (range, 6–15 months).54 In a retrospective review, Markowitz and Psomadakis55 contributed data supporting the further investigation and use of the 1064-nm Nd:YAG laser for BCC treatment while leveraging noninvasive imaging to demonstrate a same-day management model. Seventeen BCC lesions on the face and body were diagnosed by reflectance confocal microscopy and treated with an Nd:YAG laser, and clearance was monitored clinically, dermoscopically, and by optical coherence tomography. There was 100% clearance of the lesions in the study, with 82.4% (14/17) clearing after 1 treatment; mean follow-up was 103 days (range, 48–371 days).55 These studies were limited by their short follow-up time; long-term data are needed to determine true rates of recurrence.
Ablative Lasers
Ablative lasers also have been used in the treatment of NMSCs. In addition to the potentially increased healing time compared to nonablative lasers, other limitations of ablative laser therapy include residual tumor burden or recurrence that may not be easily visualized in scarred tissue after nonablative management.44
Conclusion
Although MMS remains the gold standard for invasive management of NMSCs, studies from 2018 and beyond (eTable) expanded not only on MMS topics such as increased patient access and improved techniques but also on the increasing potential of noninvasive treatments. Some of the noninvasive therapies were entirely new compounds, whereas others were already in use for a different disease indication. Furthering our knowledge and expanding our repertoire of management options will prepare us as the number of patients affected by NMSCs increases.

- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Kauvar AN, Arpey CJ, Hruza G, et al. Consensus for nonmelanoma skin cancer treatment, part II. Dermatol Surg. 2015;41:1214-1240.
- Ribero S, Stucci LS, Daniels GA, et al. Drug therapy of advanced cutaneous squamous cell carcinoma: is there any evidence? Curr Opin Oncol. 2017;29:129-135.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e2-966.e2.
- Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299-308.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Peris K, Licitra L, Ascierto PA, et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Futur Oncol. 2015;11:703-712.
- Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Ibrahim O, Gastman B, Zhang A. Advances in diagnosis and treatment of nonmelanoma skin cancer. Ann Plast Surg. 2014;73:615-619.
- Levine A, Siegel DM, Markowitz O. Update on noninvasive diagnostic imaging and management of nonmelanoma skin cancer. Curr Dermatol Rep. 2018;7:1-15.
- Casey D, Demko S, Shord S, et al. FDA approval summary: sonidegib for locally advanced basal cell carcinoma. Clin Cancer Res. 2017;23:2377-2381.
- Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131:1735-1744.
- Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841-1846.
- Chen L, Aria AB, Silapunt S, et al. Emerging nonsurgical therapies for locally advanced and metastatic nonmelanoma skin cancer. Dermatolog Surg. 2019;45:1-16.
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351.
- Sabbatino F, Marra A, Liguori L, et al. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature. J Immunother Cancer. 2018;6:126.
- Cannon JGD, Russell JS, Kim J, et al. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4:248-250.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111:1476-1481.
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14:633-641.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Nestor MS, Berman B, Goldberg D, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12:12-18.
- American Academy of Dermatology and AAD Association. Position statement on superficial radiation therapy for basal cell carcinoma (BCC) and squamous cell carcinomas (SCC). https://server.aad.org/Forms/Policies/Uploads/PS/PS%20Superficial%20Radiation%20Therapy.pdf?. Updated August 9, 2014. Accessed February 26, 2020.
- Skiveren J, Mikkelsen MR, Daugbjerg H, et al. Skin reactions and quality of life after X-ray therapy of basal cell carcinoma. J Skin Cancer. 2012;2012:825095.
- Tom MC, Hepel JT, Patel R, et al. The American Brachytherapy Society consensus statement for electronic brachytherapy. Brachytherapy. 2019;18:292-298.
- Axelson M, Liu K, Jiang X, et al. US Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289-2293.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:E2485.
- Piccinno R, Benardon S, Gaiani FM, et al. Dermatologic radiotherapy in the treatment of extensive basal cell carcinomas: a retrospective study. J Dermatolog Treat. 2017;28:426-430.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol. 2001;51:748-755.
- Dougherty TJ, Kaufman JE, Goldfarb A, et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628-2635.
- Ding H, Yu H, Dong Y, et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276-280.
- Maytin EV, Kaw U, Ilyas M, et al. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: a bilaterally controlled comparison study. Photodiagnosis Photodyn Ther. 2018;22:7-13.
- European Medicines Agency. Ameluz 5-aminolevulinic acid hydrochloride. https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz. Updated May 13, 2019. Accessed February 25, 2020.
- Center for Drug Evaluation and Research. Approval package for Ameluz (aminolevulinic acid hydrochloride) gel, 10%. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208081Orig1s000Approv.pdf. Published May 10, 2016. Accessed February 25, 2020.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179:309-319.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Kamyab-Hesari K, Seirafi H, Naraghi ZS, et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014;28:250-253.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297, E1-E3.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O, Psomadakis CE. Patient-driven management using same-day noninvasive diagnosis and complete laser treatment of basal cell carcinomas: a pilot study. Cutis. 2019;104:345-348.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Kauvar AN, Arpey CJ, Hruza G, et al. Consensus for nonmelanoma skin cancer treatment, part II. Dermatol Surg. 2015;41:1214-1240.
- Ribero S, Stucci LS, Daniels GA, et al. Drug therapy of advanced cutaneous squamous cell carcinoma: is there any evidence? Curr Opin Oncol. 2017;29:129-135.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e2-966.e2.
- Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299-308.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Peris K, Licitra L, Ascierto PA, et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Futur Oncol. 2015;11:703-712.
- Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Ibrahim O, Gastman B, Zhang A. Advances in diagnosis and treatment of nonmelanoma skin cancer. Ann Plast Surg. 2014;73:615-619.
- Levine A, Siegel DM, Markowitz O. Update on noninvasive diagnostic imaging and management of nonmelanoma skin cancer. Curr Dermatol Rep. 2018;7:1-15.
- Casey D, Demko S, Shord S, et al. FDA approval summary: sonidegib for locally advanced basal cell carcinoma. Clin Cancer Res. 2017;23:2377-2381.
- Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131:1735-1744.
- Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841-1846.
- Chen L, Aria AB, Silapunt S, et al. Emerging nonsurgical therapies for locally advanced and metastatic nonmelanoma skin cancer. Dermatolog Surg. 2019;45:1-16.
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351.
- Sabbatino F, Marra A, Liguori L, et al. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature. J Immunother Cancer. 2018;6:126.
- Cannon JGD, Russell JS, Kim J, et al. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4:248-250.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111:1476-1481.
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14:633-641.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Nestor MS, Berman B, Goldberg D, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12:12-18.
- American Academy of Dermatology and AAD Association. Position statement on superficial radiation therapy for basal cell carcinoma (BCC) and squamous cell carcinomas (SCC). https://server.aad.org/Forms/Policies/Uploads/PS/PS%20Superficial%20Radiation%20Therapy.pdf?. Updated August 9, 2014. Accessed February 26, 2020.
- Skiveren J, Mikkelsen MR, Daugbjerg H, et al. Skin reactions and quality of life after X-ray therapy of basal cell carcinoma. J Skin Cancer. 2012;2012:825095.
- Tom MC, Hepel JT, Patel R, et al. The American Brachytherapy Society consensus statement for electronic brachytherapy. Brachytherapy. 2019;18:292-298.
- Axelson M, Liu K, Jiang X, et al. US Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289-2293.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:E2485.
- Piccinno R, Benardon S, Gaiani FM, et al. Dermatologic radiotherapy in the treatment of extensive basal cell carcinomas: a retrospective study. J Dermatolog Treat. 2017;28:426-430.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol. 2001;51:748-755.
- Dougherty TJ, Kaufman JE, Goldfarb A, et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628-2635.
- Ding H, Yu H, Dong Y, et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276-280.
- Maytin EV, Kaw U, Ilyas M, et al. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: a bilaterally controlled comparison study. Photodiagnosis Photodyn Ther. 2018;22:7-13.
- European Medicines Agency. Ameluz 5-aminolevulinic acid hydrochloride. https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz. Updated May 13, 2019. Accessed February 25, 2020.
- Center for Drug Evaluation and Research. Approval package for Ameluz (aminolevulinic acid hydrochloride) gel, 10%. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208081Orig1s000Approv.pdf. Published May 10, 2016. Accessed February 25, 2020.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179:309-319.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Kamyab-Hesari K, Seirafi H, Naraghi ZS, et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014;28:250-253.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297, E1-E3.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O, Psomadakis CE. Patient-driven management using same-day noninvasive diagnosis and complete laser treatment of basal cell carcinomas: a pilot study. Cutis. 2019;104:345-348.
Practice Points
- As of 2018, there has been an increase in options for the noninvasive management of nonmelanoma skin cancers that should be considered.
- Recently, approved advances in treatment options have included not only advanced basal cell carcinoma but also advanced squamous cell carcinoma such as cemiplimab.