User login
Emerging Noninvasive Treatments of Nonmelanoma Skin Cancers
Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide, and its incidence continues to increase. More than 5 million NMSCs are estimated to occur annually in the United States alone.1 There are more cases of basal cell carcinoma (BCC) than all other cancers combined, with squamous cell carcinoma (SCC) being the second most common cancer in the United States.1-3 The rising incidence of NMSCs highlights the importance of investigating additional treatment options with fewer side effects, better cosmetic outcomes, and better efficacy.1
Originally, treatment options for NMSCs largely relied on destructive and surgical methods. Basal cell carcinoma and SCC commonly are treated with cryosurgery; electrodesiccation and curettage; or more definitive surgical options, including excision and Mohs micrographic surgery (MMS). Over time, topical agents such as 5-fluorouracil, imiquimod, ingenol mebutate, and various forms of aminolevulinic acid (ALA) for photodynamic therapy (PDT) were included for superficial lesions as well as field treatment. The development of oral hedgehog (Hh) inhibitors, such as vismodegib, offered a promising alternative to patients with advanced disease. Each treatment has its own specific indications and side effects; thus, there is always room for novel therapeutic approaches. We review new and potential treatments from 2018 and beyond. Although only 5% of SCCs become locally advanced, recur, or metastasize, and 0.4% to 0.8% of BCCs progress to advanced disease, many of the newer studies target advanced NMSCs, given their life-threatening and debilitating nature.4,5 Similarly, the incidence of nevoid basal cell carcinoma (NBCC) syndrome is as low as 1 in 57,000 to 1 in 164,000 but continues to be studied because of its morbidity and the potential to contribute new treatment options for BCC in the general population.6
Topical Therapy
Sonidegib
Basal cell carcinoma proliferation is a result of an unregulated Hh pathway that is initiated when the Hh ligand binds to the patched 1 protein (PTCH1).7-11 Patched 1 protein normally inhibits the smoothened (SMO) transmembrane receptor protein, decreasing the signaling cascade. In BCCs, there is a loss of PTCH1 function, effectively increasing the Hh pathway activity. Sonidegib is an Hh inhibitor that in turn prevents inhibition of PTCH1 in an attempt to reregulate the pathway.7-11 Although sonidegib is known for its 2015 approval by the US Food and Drug Administration (FDA) as a systemic therapy for locally advanced BCCs,12 one study investigated a topical formulation on 8 patients with NBCC syndrome.13 Patients were treated twice daily with sonidegib cream 0.75% for 4 weeks in a double-blind, randomized, vehicle-controlled study. A total of 27 BCCs were randomized and treated with either vehicle or sonidegib. A biopsy was taken at the end of the study of 1 sonidegib-treated and 1 vehicle-treated BCC lesion per patient. Of the 13 sonidegib-treated BCC lesions, 3 (23.1%) showed complete response, 9 (69.2%) showed partial response, and 1 (7.7%) showed no response vs 13 of 14 (92.8%) lesions that did not respond to the vehicle. Patients tolerated the treatment well without skin irritation or signs of local or systemic side effects.13 Topical sonidegib should be further investigated as an adjunct or in different vehicles given the successful regression of BCCs and its minimal side-effect profile.
Systemic Therapy
Cemiplimab
Cemiplimab is a human monoclonal antibody against programmed death receptor 1 (PD-1) that was FDA approved in September 2018 for the treatment of metastatic cutaneous SCC.14 Programmed death receptor 1 is found on T lymphocytes, B lymphocytes, and macrophages, which normally assist in the immune response to tumor cells. However, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) are found on tumor cells and bind to PD-1. Cemiplimab prevents PD-1 from binding to PD-L1 and PD-L2, allowing an appropriate immune response.14,15 A phase 1 clinical trial of cemiplimab showed a 50% (13/26) response rate.16 The phase 2 trial included patients with advanced SCC, but the primary analysis only considered patients with metastatic SCC. Phase 2 results showed a 47.5% (28/59) response rate. Patients received intravenous cemiplimab 3 mg/kg once every 2 weeks for up to 48 weeks in phase 1 and up to 96 weeks in phase 2. Both phases of the trial showed a response to treatment lasting longer than 6 months in more than 50% of patients. The most common adverse events were diarrhea, fatigue, nausea, constipation, and rash.16
Although immune-mediated adverse reactions are rare, they can occur given cemiplimab’s mechanism of action and may range from severe to fatal. Examples of immune-mediated adverse reactions that occurred during the study included pneumonitis, colitis, hepatitis, adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, type 1 diabetes mellitus, nephritis with renal dysfunction, and immune-mediated dermatologic reactions.14 It is important to monitor for immune-mediated adverse reactions and address them immediately once detected.
Other PD-1 Inhibitors
Although PD-1 inhibitors have been studied in advanced SCCs, their clinical data are limited for BCCs.17 Prior to 2018, there was a small number of case reports of patients with BCC with partial to exceptional response to PD-1 inhibitors. Recently, 2 additional case reports were published with contrasting outcomes using 2 different PD-1 inhibitors. An elderly patient with metastatic non–small cell lung cancer was treated with nivolumab after failing chemotherapy. She subsequently developed a BCC on the nose that was resected but recurred 2 months later despite continuing nivolumab.17 Another case report detailed a patient with a history of BCC on the shoulder excised 5 years prior who presented with recurrence on the sternum and clavicle.18 One year later the patient was found to have BCC metastases to the lung. After progression of disease despite vismodegib and recurrence of BCC with taladegib, the patient was then placed on pembrolizumab. At 6 weeks and 12 months, computed tomography showed resolution of multiple lung lesions. Sixteen weeks after initiation of pembrolizumab treatment, spinal metastases were found, but the treatment was continued because of the improvement in the lung metastases.18
Taladegib
Taladegib is a SMO antagonist that has been through a phase 1 trial in patients with advanced cancer, including treatment-naive and previously treated BCCs.19 Eighty-four patients were treated to examine the safety profile and determine an appropriate phase 2 dose and administration schedule. The maximum tolerable dose was determined to be 400 mg because of dose-limiting toxicities. All clinical responses were in patients with BCCs (47/84 [55.9%] patients), with a response rate of 46.8%. Eleven of 16 (68.8%) Hh-treatment–naive patients and 11 of 31 (35.5%) patients previously treated with Hh responded to taladegib. Common adverse events were dysgeusia, fatigue, nausea, and muscle spasms.19 Although vismodegib is an FDA-approved SMO antagonist since 2012, treatment resistance and tolerability issues have been continuing concerns.20,21 Taladegib is a potential alternative that may be found to have improved pharmacodynamics and pharmacokinetics. Not only did in vitro studies show a preferable protein-binding profile with taladegib, but it also displayed dose proportionality, while vismodegib has been known to have nonlinear pharmacokinetics.19
Posaconazole
Posaconazole is a systemic antifungal agent that is a structural analogue to itraconazole.22 Itraconazole has been found to inhibit the Hh pathway as an SMO antagonist. In a study with mice, posaconazole was found to have strong activity against drug-resistant SMO mutants while inhibiting the growth of Hh-dependent BCCs in vivo. A marked decrease also was seen in the ciliary accumulation of SMO, suggesting a similar mechanism of action to itraconazole. Posaconazole’s use for BCCs currently is limited to basic science studies but may offer a potential alternative to itraconazole, which is known to have many drug-drug interactions and requires dose adjustments in renal and hepatic insufficiency. When used as an antifungal compared to itraconazole, posaconazole has a favorable long-term safety profile due to fewer drug-drug interactions and mild side effects; it also does not require dose adjustments in mild to moderate renal or hepatic insufficiency.22 Thus, posaconazole is a potentially safer alternative to itraconazole for the treatment of BCCs. Although phase 2 studies of itraconazole for BCCs have shown decreased cell proliferation, tumor size, and reduced GLI1 messenger RNA, side effects included fatigue and grade 4 heart failure.23,24
Radiation Therapy
Radiation therapies (RTs), such as superficial RT, have been long-established treatment options.25 However, there also are emerging methods of delivering RT, including electronic brachytherapy (EB). Although there is a low likelihood of residual tumor after RT given the number of sessions involved and the more aggressive nature of the treatment, these factors also can be a substantial burden on the patient. Furthermore, RT may result in subsequent scar tissue, which can hinder the use of other emerging technologies, such as noninvasive imaging devices, following RT.
Superficial RT
Superficial RT is a secondary option for the treatment of NMSC for use in special circumstances, such as when surgical intervention is contraindicated or refused, and after the benefits and risks of treatment alternatives have been discussed.26 However, depending on the tumor type and anatomical location, 6 to 18 treatments may be required, with treatment frequency ranging from 1 to 5 treatments per week.25 Patients may find this treatment regimen difficult to maintain given the length of time and frequency of treatments required. Side effects include radiation dermatitis and postinflammatory hypopigmentation or hyperpigmentation in patients with dark skin, and there is a risk for recurrence.25,27
Electronic Brachytherapy
Brachytherapy is a method of delivering RT via radioactive isotopes, whereas EB uses lower-energy photons that require less shielding.28 As a relatively new therapy, studies on the efficacy of EB on NMSC continue to grow but with limited data comparing EB with established treatments. Furthermore, there are limited long-term follow-up data, and future studies should expand the patient demographic to younger patients before treatment guidelines can be established.28
RT With Concurrent and Adjuvant Vismodegib
Vismodegib is an SMO inhibitor that was FDA approved in 2012 for the treatment of locally advanced BCC in patients who are not candidates for surgery or RT.29 Over time, studies have looked into other indications for vismodegib, such as a neoadjuvant to MMS or in patients with NBCC syndrome.11 Prior to 2018, there were only 2 known case reports of concurrent vismodegib and RT used for recurrent advanced BCC.30 Recently, vismodegib has been further examined in combination with RT in a case report,31 basic science study,32 and phase 2 trials (ClinicalTrials.gov Identifiers NCT02956889 and NCT01835626).
Prior studies showed low cure rates with vismodegib alone after RT (43%) as well as decreasing cure rates with primary RT alone as tumor size increased.33,34 In 2018, vismodegib was used concurrently and as an adjuvant to RT in a patient with advanced multifocal BCC.31 The patient had multiple large BCCs on the trunk that were painful and bleeding. The patient was started on RT and 150 mg/d vismodegib concurrently, which was then continued adjuvantly for 3 months until it was discontinued because of diarrhea. The patient had complete response in all lesions with resolution of symptoms.31 A separate basic science study further supported the potential role of vismodegib in radiation sensitization of both BCCs and head and neck SCCs.32 There presently are 2 phase 2 trials investigating the concurrent use of vismodegib and RT, which could help determine the efficacy of the combined approach for patients with advanced BCCs who are poor surgical candidates (NCT02956889 and NCT01835626).
Photodynamic Therapy
Photodynamic therapy has been in use since the 1970s when Dougherty et al35 performed one of the first studies on its use in skin cancer. Since then, PDT has been used for the treatment of actinic keratoses (AKs) and more recently BCCs. In PDT, a photosensitizer (PS) is applied and activated by a 400-nm blue light or 635-nm red light, depending on the PS used. The PS then produces highly reactive oxygen species, leading to apoptosis of the cancer cells.36 In Europe, red light PDT is licensed for the treatment of AKs as well as superficial and nodular BCCs, though approved indications vary between countries. In the United States, PDT is only FDA approved for the treatment of AKs.37
Aminolevulinic Acid Hydrochloride
Aminolevulinic acid hydrochloride is a red light PS used to treat AKs since 2011 and BCCs since 2017 in Europe in addition to AKs in the United States since 2016.38,39 A phase 3 noninferiority clinical trial in Europe of 281 patients compared the treatment of nonaggressive BCCs with ALA to methyl aminolevulinate (MAL) cream.40 The study found a complete response rate of 93.4% vs 91.8%. Superficial BCCs treated with ALA had a clearance rate of 94.7% vs 96.4% with MAL, while nodular BCCs treated with ALA had a clearance rate of 85.7% vs 76.2% with MAL. A 1-year clinical follow-up showed similar recurrence rates (8.4% for ALA vs 8.5% for MAL).40 The results of this study led to an expanded indication in Europe to include the treatment of BCCs.38 Aminolevulinic acid hydrochloride currently is undergoing phase 3 clinical trials in the United States for approval for the treatment of superficial BCCs (NCT03573401). If similar outcomes are achieved, US patients may have access to an alternative nonsurgical treatment of BCCs. The ongoing US trial is exclusively investigating the efficacy and safety for superficial BCCs, which may limit FDA approval to only superficial BCCs, accounting for only 8.4% to 24.1% of all BCCs.35,41,42
Laser Therapy
Ablative and nonablative lasers have been used to treat NMSCs in the literature. Ablative lasers destroy tumors through vaporization of tissue water, whereas nonablative lasers target the vasculature of tumors while preserving the surrounding tissue.43,44 Nonablative lasers include pulsed dye lasers (PDL) and Nd:YAG lasers. Examples of ablative lasers include CO2 and erbium:YAG lasers. Given the status of lasers as an emerging treatment method, there currently is no standardized laser setting for any of the laser therapies used to treat NMSCs. Although there is the potential for optimal cosmetic outcomes and a limited side-effect profile for nonablative laser therapies, there are limited data on long-term follow-up to study recurrence rates and establish a more standardized treatment protocol.
Pulsed Dye Lasers
Although there were no studies on PDL therapy alone in 2018, a study published in 2019 evaluated a combination laser treatment using a 595-nm PDL and 1927-nm fractional laser for the treatment of 93 BCCs, yielding a 95.7% (89/93) clearance rate and 4.5% (4/89) recurrence rate over a follow-up period of up to 6 years (range, 2.53 months to 6.03 years).45 Studies of PDL prior to 2018 had follow-ups ranging from 2 weeks to 6 months.46-51 Although the majority were biopsy-proven BCCs, reflectance confocal microscopy also was used for same-day diagnoses. Long-term follow-up included clinical examinations, dermoscopy, and optical coherence tomography.45 The clearance rate (95.7%) using noninvasive imaging in conjunction with the combination laser treatment was superior to both histologic and clinical clearance rates of prior PDL-only studies, which ranged from 25% to 95%.46-51 To have long-term follow-up data, the study used noninvasive imaging with clinical follow-up because histology would not be viable for long-term follow-up. This study was retrospective rather than prospective, which was a limitation.45
Nd:YAG Lasers
The majority of studies utilizing Nd:YAG lasers investigated their efficacy in treating BCCs, with the exception of 1 study of facial SCCs. This major study in 2009 of 627 BCCs showed a 2.5% recurrence rate after a follow-up time of 3 months to 5 years.52 Nd:YAG lasers continue to be investigated, including a more recent study of 31 extrafacial, biopsy-proven BCCs that were treated with the 1064-nm Nd:YAG laser, which showed a 90% histologic clearance on 1-month follow-up after a single treatment.53 In 2019, a retrospective review of 16 BCC lesions on the head, neck, trunk, and extremities showed 100% clearance after 1 treatment, with an average follow-up period of 9 months (range, 6–15 months).54 In a retrospective review, Markowitz and Psomadakis55 contributed data supporting the further investigation and use of the 1064-nm Nd:YAG laser for BCC treatment while leveraging noninvasive imaging to demonstrate a same-day management model. Seventeen BCC lesions on the face and body were diagnosed by reflectance confocal microscopy and treated with an Nd:YAG laser, and clearance was monitored clinically, dermoscopically, and by optical coherence tomography. There was 100% clearance of the lesions in the study, with 82.4% (14/17) clearing after 1 treatment; mean follow-up was 103 days (range, 48–371 days).55 These studies were limited by their short follow-up time; long-term data are needed to determine true rates of recurrence.
Ablative Lasers
Ablative lasers also have been used in the treatment of NMSCs. In addition to the potentially increased healing time compared to nonablative lasers, other limitations of ablative laser therapy include residual tumor burden or recurrence that may not be easily visualized in scarred tissue after nonablative management.44
Conclusion
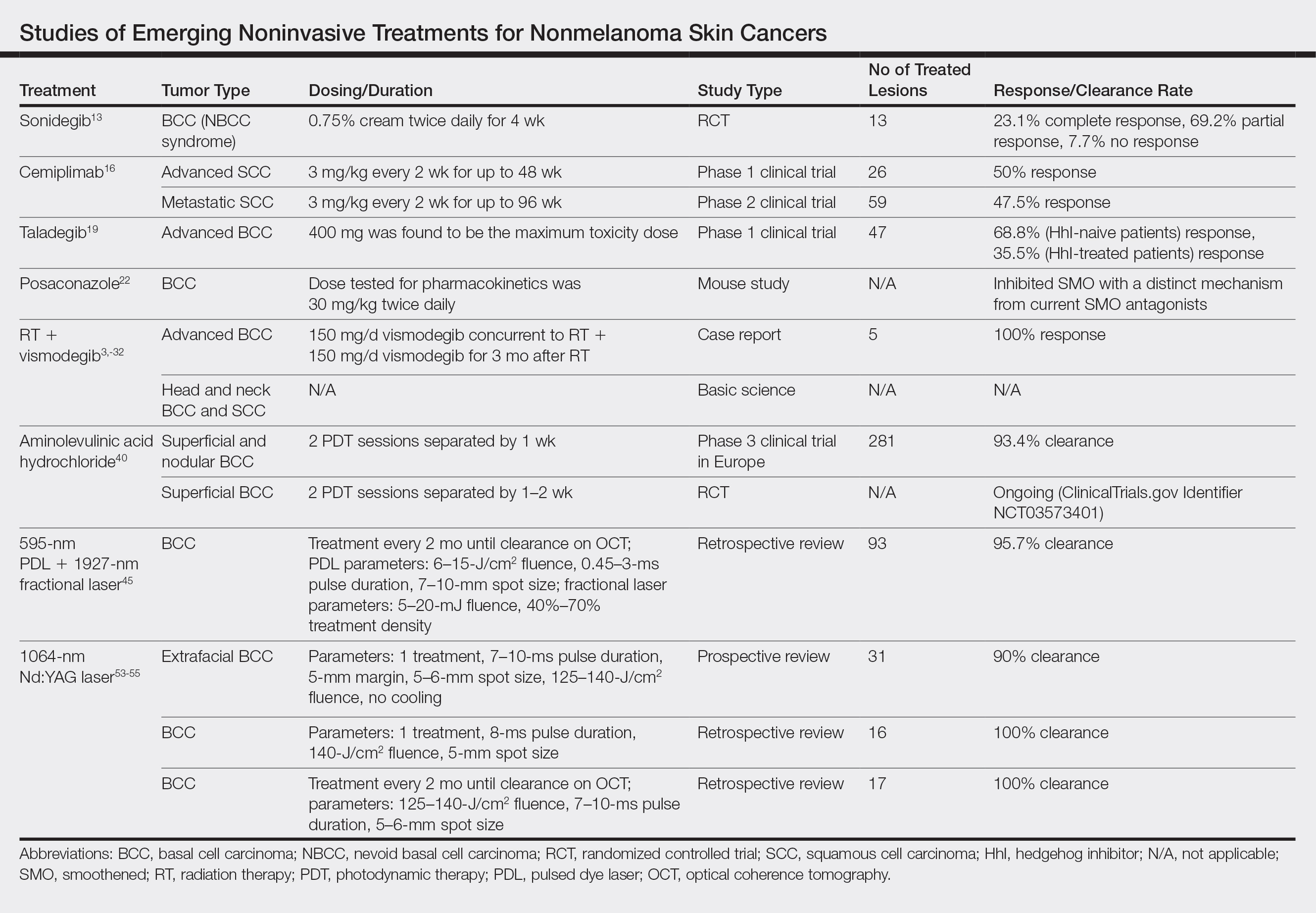
Although MMS remains the gold standard for invasive management of NMSCs, studies from 2018 and beyond (eTable) expanded not only on MMS topics such as increased patient access and improved techniques but also on the increasing potential of noninvasive treatments. Some of the noninvasive therapies were entirely new compounds, whereas others were already in use for a different disease indication. Furthering our knowledge and expanding our repertoire of management options will prepare us as the number of patients affected by NMSCs increases.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Kauvar AN, Arpey CJ, Hruza G, et al. Consensus for nonmelanoma skin cancer treatment, part II. Dermatol Surg. 2015;41:1214-1240.
- Ribero S, Stucci LS, Daniels GA, et al. Drug therapy of advanced cutaneous squamous cell carcinoma: is there any evidence? Curr Opin Oncol. 2017;29:129-135.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e2-966.e2.
- Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299-308.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Peris K, Licitra L, Ascierto PA, et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Futur Oncol. 2015;11:703-712.
- Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Ibrahim O, Gastman B, Zhang A. Advances in diagnosis and treatment of nonmelanoma skin cancer. Ann Plast Surg. 2014;73:615-619.
- Levine A, Siegel DM, Markowitz O. Update on noninvasive diagnostic imaging and management of nonmelanoma skin cancer. Curr Dermatol Rep. 2018;7:1-15.
- Casey D, Demko S, Shord S, et al. FDA approval summary: sonidegib for locally advanced basal cell carcinoma. Clin Cancer Res. 2017;23:2377-2381.
- Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131:1735-1744.
- Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841-1846.
- Chen L, Aria AB, Silapunt S, et al. Emerging nonsurgical therapies for locally advanced and metastatic nonmelanoma skin cancer. Dermatolog Surg. 2019;45:1-16.
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351.
- Sabbatino F, Marra A, Liguori L, et al. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature. J Immunother Cancer. 2018;6:126.
- Cannon JGD, Russell JS, Kim J, et al. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4:248-250.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111:1476-1481.
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14:633-641.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Nestor MS, Berman B, Goldberg D, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12:12-18.
- American Academy of Dermatology and AAD Association. Position statement on superficial radiation therapy for basal cell carcinoma (BCC) and squamous cell carcinomas (SCC). https://server.aad.org/Forms/Policies/Uploads/PS/PS%20Superficial%20Radiation%20Therapy.pdf?. Updated August 9, 2014. Accessed February 26, 2020.
- Skiveren J, Mikkelsen MR, Daugbjerg H, et al. Skin reactions and quality of life after X-ray therapy of basal cell carcinoma. J Skin Cancer. 2012;2012:825095.
- Tom MC, Hepel JT, Patel R, et al. The American Brachytherapy Society consensus statement for electronic brachytherapy. Brachytherapy. 2019;18:292-298.
- Axelson M, Liu K, Jiang X, et al. US Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289-2293.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:E2485.
- Piccinno R, Benardon S, Gaiani FM, et al. Dermatologic radiotherapy in the treatment of extensive basal cell carcinomas: a retrospective study. J Dermatolog Treat. 2017;28:426-430.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol. 2001;51:748-755.
- Dougherty TJ, Kaufman JE, Goldfarb A, et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628-2635.
- Ding H, Yu H, Dong Y, et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276-280.
- Maytin EV, Kaw U, Ilyas M, et al. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: a bilaterally controlled comparison study. Photodiagnosis Photodyn Ther. 2018;22:7-13.
- European Medicines Agency. Ameluz 5-aminolevulinic acid hydrochloride. https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz. Updated May 13, 2019. Accessed February 25, 2020.
- Center for Drug Evaluation and Research. Approval package for Ameluz (aminolevulinic acid hydrochloride) gel, 10%. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208081Orig1s000Approv.pdf. Published May 10, 2016. Accessed February 25, 2020.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179:309-319.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Kamyab-Hesari K, Seirafi H, Naraghi ZS, et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014;28:250-253.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297, E1-E3.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O, Psomadakis CE. Patient-driven management using same-day noninvasive diagnosis and complete laser treatment of basal cell carcinomas: a pilot study. Cutis. 2019;104:345-348.
Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide, and its incidence continues to increase. More than 5 million NMSCs are estimated to occur annually in the United States alone.1 There are more cases of basal cell carcinoma (BCC) than all other cancers combined, with squamous cell carcinoma (SCC) being the second most common cancer in the United States.1-3 The rising incidence of NMSCs highlights the importance of investigating additional treatment options with fewer side effects, better cosmetic outcomes, and better efficacy.1
Originally, treatment options for NMSCs largely relied on destructive and surgical methods. Basal cell carcinoma and SCC commonly are treated with cryosurgery; electrodesiccation and curettage; or more definitive surgical options, including excision and Mohs micrographic surgery (MMS). Over time, topical agents such as 5-fluorouracil, imiquimod, ingenol mebutate, and various forms of aminolevulinic acid (ALA) for photodynamic therapy (PDT) were included for superficial lesions as well as field treatment. The development of oral hedgehog (Hh) inhibitors, such as vismodegib, offered a promising alternative to patients with advanced disease. Each treatment has its own specific indications and side effects; thus, there is always room for novel therapeutic approaches. We review new and potential treatments from 2018 and beyond. Although only 5% of SCCs become locally advanced, recur, or metastasize, and 0.4% to 0.8% of BCCs progress to advanced disease, many of the newer studies target advanced NMSCs, given their life-threatening and debilitating nature.4,5 Similarly, the incidence of nevoid basal cell carcinoma (NBCC) syndrome is as low as 1 in 57,000 to 1 in 164,000 but continues to be studied because of its morbidity and the potential to contribute new treatment options for BCC in the general population.6
Topical Therapy
Sonidegib
Basal cell carcinoma proliferation is a result of an unregulated Hh pathway that is initiated when the Hh ligand binds to the patched 1 protein (PTCH1).7-11 Patched 1 protein normally inhibits the smoothened (SMO) transmembrane receptor protein, decreasing the signaling cascade. In BCCs, there is a loss of PTCH1 function, effectively increasing the Hh pathway activity. Sonidegib is an Hh inhibitor that in turn prevents inhibition of PTCH1 in an attempt to reregulate the pathway.7-11 Although sonidegib is known for its 2015 approval by the US Food and Drug Administration (FDA) as a systemic therapy for locally advanced BCCs,12 one study investigated a topical formulation on 8 patients with NBCC syndrome.13 Patients were treated twice daily with sonidegib cream 0.75% for 4 weeks in a double-blind, randomized, vehicle-controlled study. A total of 27 BCCs were randomized and treated with either vehicle or sonidegib. A biopsy was taken at the end of the study of 1 sonidegib-treated and 1 vehicle-treated BCC lesion per patient. Of the 13 sonidegib-treated BCC lesions, 3 (23.1%) showed complete response, 9 (69.2%) showed partial response, and 1 (7.7%) showed no response vs 13 of 14 (92.8%) lesions that did not respond to the vehicle. Patients tolerated the treatment well without skin irritation or signs of local or systemic side effects.13 Topical sonidegib should be further investigated as an adjunct or in different vehicles given the successful regression of BCCs and its minimal side-effect profile.
Systemic Therapy
Cemiplimab
Cemiplimab is a human monoclonal antibody against programmed death receptor 1 (PD-1) that was FDA approved in September 2018 for the treatment of metastatic cutaneous SCC.14 Programmed death receptor 1 is found on T lymphocytes, B lymphocytes, and macrophages, which normally assist in the immune response to tumor cells. However, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) are found on tumor cells and bind to PD-1. Cemiplimab prevents PD-1 from binding to PD-L1 and PD-L2, allowing an appropriate immune response.14,15 A phase 1 clinical trial of cemiplimab showed a 50% (13/26) response rate.16 The phase 2 trial included patients with advanced SCC, but the primary analysis only considered patients with metastatic SCC. Phase 2 results showed a 47.5% (28/59) response rate. Patients received intravenous cemiplimab 3 mg/kg once every 2 weeks for up to 48 weeks in phase 1 and up to 96 weeks in phase 2. Both phases of the trial showed a response to treatment lasting longer than 6 months in more than 50% of patients. The most common adverse events were diarrhea, fatigue, nausea, constipation, and rash.16
Although immune-mediated adverse reactions are rare, they can occur given cemiplimab’s mechanism of action and may range from severe to fatal. Examples of immune-mediated adverse reactions that occurred during the study included pneumonitis, colitis, hepatitis, adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, type 1 diabetes mellitus, nephritis with renal dysfunction, and immune-mediated dermatologic reactions.14 It is important to monitor for immune-mediated adverse reactions and address them immediately once detected.
Other PD-1 Inhibitors
Although PD-1 inhibitors have been studied in advanced SCCs, their clinical data are limited for BCCs.17 Prior to 2018, there was a small number of case reports of patients with BCC with partial to exceptional response to PD-1 inhibitors. Recently, 2 additional case reports were published with contrasting outcomes using 2 different PD-1 inhibitors. An elderly patient with metastatic non–small cell lung cancer was treated with nivolumab after failing chemotherapy. She subsequently developed a BCC on the nose that was resected but recurred 2 months later despite continuing nivolumab.17 Another case report detailed a patient with a history of BCC on the shoulder excised 5 years prior who presented with recurrence on the sternum and clavicle.18 One year later the patient was found to have BCC metastases to the lung. After progression of disease despite vismodegib and recurrence of BCC with taladegib, the patient was then placed on pembrolizumab. At 6 weeks and 12 months, computed tomography showed resolution of multiple lung lesions. Sixteen weeks after initiation of pembrolizumab treatment, spinal metastases were found, but the treatment was continued because of the improvement in the lung metastases.18
Taladegib
Taladegib is a SMO antagonist that has been through a phase 1 trial in patients with advanced cancer, including treatment-naive and previously treated BCCs.19 Eighty-four patients were treated to examine the safety profile and determine an appropriate phase 2 dose and administration schedule. The maximum tolerable dose was determined to be 400 mg because of dose-limiting toxicities. All clinical responses were in patients with BCCs (47/84 [55.9%] patients), with a response rate of 46.8%. Eleven of 16 (68.8%) Hh-treatment–naive patients and 11 of 31 (35.5%) patients previously treated with Hh responded to taladegib. Common adverse events were dysgeusia, fatigue, nausea, and muscle spasms.19 Although vismodegib is an FDA-approved SMO antagonist since 2012, treatment resistance and tolerability issues have been continuing concerns.20,21 Taladegib is a potential alternative that may be found to have improved pharmacodynamics and pharmacokinetics. Not only did in vitro studies show a preferable protein-binding profile with taladegib, but it also displayed dose proportionality, while vismodegib has been known to have nonlinear pharmacokinetics.19
Posaconazole
Posaconazole is a systemic antifungal agent that is a structural analogue to itraconazole.22 Itraconazole has been found to inhibit the Hh pathway as an SMO antagonist. In a study with mice, posaconazole was found to have strong activity against drug-resistant SMO mutants while inhibiting the growth of Hh-dependent BCCs in vivo. A marked decrease also was seen in the ciliary accumulation of SMO, suggesting a similar mechanism of action to itraconazole. Posaconazole’s use for BCCs currently is limited to basic science studies but may offer a potential alternative to itraconazole, which is known to have many drug-drug interactions and requires dose adjustments in renal and hepatic insufficiency. When used as an antifungal compared to itraconazole, posaconazole has a favorable long-term safety profile due to fewer drug-drug interactions and mild side effects; it also does not require dose adjustments in mild to moderate renal or hepatic insufficiency.22 Thus, posaconazole is a potentially safer alternative to itraconazole for the treatment of BCCs. Although phase 2 studies of itraconazole for BCCs have shown decreased cell proliferation, tumor size, and reduced GLI1 messenger RNA, side effects included fatigue and grade 4 heart failure.23,24
Radiation Therapy
Radiation therapies (RTs), such as superficial RT, have been long-established treatment options.25 However, there also are emerging methods of delivering RT, including electronic brachytherapy (EB). Although there is a low likelihood of residual tumor after RT given the number of sessions involved and the more aggressive nature of the treatment, these factors also can be a substantial burden on the patient. Furthermore, RT may result in subsequent scar tissue, which can hinder the use of other emerging technologies, such as noninvasive imaging devices, following RT.
Superficial RT
Superficial RT is a secondary option for the treatment of NMSC for use in special circumstances, such as when surgical intervention is contraindicated or refused, and after the benefits and risks of treatment alternatives have been discussed.26 However, depending on the tumor type and anatomical location, 6 to 18 treatments may be required, with treatment frequency ranging from 1 to 5 treatments per week.25 Patients may find this treatment regimen difficult to maintain given the length of time and frequency of treatments required. Side effects include radiation dermatitis and postinflammatory hypopigmentation or hyperpigmentation in patients with dark skin, and there is a risk for recurrence.25,27
Electronic Brachytherapy
Brachytherapy is a method of delivering RT via radioactive isotopes, whereas EB uses lower-energy photons that require less shielding.28 As a relatively new therapy, studies on the efficacy of EB on NMSC continue to grow but with limited data comparing EB with established treatments. Furthermore, there are limited long-term follow-up data, and future studies should expand the patient demographic to younger patients before treatment guidelines can be established.28
RT With Concurrent and Adjuvant Vismodegib
Vismodegib is an SMO inhibitor that was FDA approved in 2012 for the treatment of locally advanced BCC in patients who are not candidates for surgery or RT.29 Over time, studies have looked into other indications for vismodegib, such as a neoadjuvant to MMS or in patients with NBCC syndrome.11 Prior to 2018, there were only 2 known case reports of concurrent vismodegib and RT used for recurrent advanced BCC.30 Recently, vismodegib has been further examined in combination with RT in a case report,31 basic science study,32 and phase 2 trials (ClinicalTrials.gov Identifiers NCT02956889 and NCT01835626).
Prior studies showed low cure rates with vismodegib alone after RT (43%) as well as decreasing cure rates with primary RT alone as tumor size increased.33,34 In 2018, vismodegib was used concurrently and as an adjuvant to RT in a patient with advanced multifocal BCC.31 The patient had multiple large BCCs on the trunk that were painful and bleeding. The patient was started on RT and 150 mg/d vismodegib concurrently, which was then continued adjuvantly for 3 months until it was discontinued because of diarrhea. The patient had complete response in all lesions with resolution of symptoms.31 A separate basic science study further supported the potential role of vismodegib in radiation sensitization of both BCCs and head and neck SCCs.32 There presently are 2 phase 2 trials investigating the concurrent use of vismodegib and RT, which could help determine the efficacy of the combined approach for patients with advanced BCCs who are poor surgical candidates (NCT02956889 and NCT01835626).
Photodynamic Therapy
Photodynamic therapy has been in use since the 1970s when Dougherty et al35 performed one of the first studies on its use in skin cancer. Since then, PDT has been used for the treatment of actinic keratoses (AKs) and more recently BCCs. In PDT, a photosensitizer (PS) is applied and activated by a 400-nm blue light or 635-nm red light, depending on the PS used. The PS then produces highly reactive oxygen species, leading to apoptosis of the cancer cells.36 In Europe, red light PDT is licensed for the treatment of AKs as well as superficial and nodular BCCs, though approved indications vary between countries. In the United States, PDT is only FDA approved for the treatment of AKs.37
Aminolevulinic Acid Hydrochloride
Aminolevulinic acid hydrochloride is a red light PS used to treat AKs since 2011 and BCCs since 2017 in Europe in addition to AKs in the United States since 2016.38,39 A phase 3 noninferiority clinical trial in Europe of 281 patients compared the treatment of nonaggressive BCCs with ALA to methyl aminolevulinate (MAL) cream.40 The study found a complete response rate of 93.4% vs 91.8%. Superficial BCCs treated with ALA had a clearance rate of 94.7% vs 96.4% with MAL, while nodular BCCs treated with ALA had a clearance rate of 85.7% vs 76.2% with MAL. A 1-year clinical follow-up showed similar recurrence rates (8.4% for ALA vs 8.5% for MAL).40 The results of this study led to an expanded indication in Europe to include the treatment of BCCs.38 Aminolevulinic acid hydrochloride currently is undergoing phase 3 clinical trials in the United States for approval for the treatment of superficial BCCs (NCT03573401). If similar outcomes are achieved, US patients may have access to an alternative nonsurgical treatment of BCCs. The ongoing US trial is exclusively investigating the efficacy and safety for superficial BCCs, which may limit FDA approval to only superficial BCCs, accounting for only 8.4% to 24.1% of all BCCs.35,41,42
Laser Therapy
Ablative and nonablative lasers have been used to treat NMSCs in the literature. Ablative lasers destroy tumors through vaporization of tissue water, whereas nonablative lasers target the vasculature of tumors while preserving the surrounding tissue.43,44 Nonablative lasers include pulsed dye lasers (PDL) and Nd:YAG lasers. Examples of ablative lasers include CO2 and erbium:YAG lasers. Given the status of lasers as an emerging treatment method, there currently is no standardized laser setting for any of the laser therapies used to treat NMSCs. Although there is the potential for optimal cosmetic outcomes and a limited side-effect profile for nonablative laser therapies, there are limited data on long-term follow-up to study recurrence rates and establish a more standardized treatment protocol.
Pulsed Dye Lasers
Although there were no studies on PDL therapy alone in 2018, a study published in 2019 evaluated a combination laser treatment using a 595-nm PDL and 1927-nm fractional laser for the treatment of 93 BCCs, yielding a 95.7% (89/93) clearance rate and 4.5% (4/89) recurrence rate over a follow-up period of up to 6 years (range, 2.53 months to 6.03 years).45 Studies of PDL prior to 2018 had follow-ups ranging from 2 weeks to 6 months.46-51 Although the majority were biopsy-proven BCCs, reflectance confocal microscopy also was used for same-day diagnoses. Long-term follow-up included clinical examinations, dermoscopy, and optical coherence tomography.45 The clearance rate (95.7%) using noninvasive imaging in conjunction with the combination laser treatment was superior to both histologic and clinical clearance rates of prior PDL-only studies, which ranged from 25% to 95%.46-51 To have long-term follow-up data, the study used noninvasive imaging with clinical follow-up because histology would not be viable for long-term follow-up. This study was retrospective rather than prospective, which was a limitation.45
Nd:YAG Lasers
The majority of studies utilizing Nd:YAG lasers investigated their efficacy in treating BCCs, with the exception of 1 study of facial SCCs. This major study in 2009 of 627 BCCs showed a 2.5% recurrence rate after a follow-up time of 3 months to 5 years.52 Nd:YAG lasers continue to be investigated, including a more recent study of 31 extrafacial, biopsy-proven BCCs that were treated with the 1064-nm Nd:YAG laser, which showed a 90% histologic clearance on 1-month follow-up after a single treatment.53 In 2019, a retrospective review of 16 BCC lesions on the head, neck, trunk, and extremities showed 100% clearance after 1 treatment, with an average follow-up period of 9 months (range, 6–15 months).54 In a retrospective review, Markowitz and Psomadakis55 contributed data supporting the further investigation and use of the 1064-nm Nd:YAG laser for BCC treatment while leveraging noninvasive imaging to demonstrate a same-day management model. Seventeen BCC lesions on the face and body were diagnosed by reflectance confocal microscopy and treated with an Nd:YAG laser, and clearance was monitored clinically, dermoscopically, and by optical coherence tomography. There was 100% clearance of the lesions in the study, with 82.4% (14/17) clearing after 1 treatment; mean follow-up was 103 days (range, 48–371 days).55 These studies were limited by their short follow-up time; long-term data are needed to determine true rates of recurrence.
Ablative Lasers
Ablative lasers also have been used in the treatment of NMSCs. In addition to the potentially increased healing time compared to nonablative lasers, other limitations of ablative laser therapy include residual tumor burden or recurrence that may not be easily visualized in scarred tissue after nonablative management.44
Conclusion
Although MMS remains the gold standard for invasive management of NMSCs, studies from 2018 and beyond (eTable) expanded not only on MMS topics such as increased patient access and improved techniques but also on the increasing potential of noninvasive treatments. Some of the noninvasive therapies were entirely new compounds, whereas others were already in use for a different disease indication. Furthering our knowledge and expanding our repertoire of management options will prepare us as the number of patients affected by NMSCs increases.

Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide, and its incidence continues to increase. More than 5 million NMSCs are estimated to occur annually in the United States alone.1 There are more cases of basal cell carcinoma (BCC) than all other cancers combined, with squamous cell carcinoma (SCC) being the second most common cancer in the United States.1-3 The rising incidence of NMSCs highlights the importance of investigating additional treatment options with fewer side effects, better cosmetic outcomes, and better efficacy.1
Originally, treatment options for NMSCs largely relied on destructive and surgical methods. Basal cell carcinoma and SCC commonly are treated with cryosurgery; electrodesiccation and curettage; or more definitive surgical options, including excision and Mohs micrographic surgery (MMS). Over time, topical agents such as 5-fluorouracil, imiquimod, ingenol mebutate, and various forms of aminolevulinic acid (ALA) for photodynamic therapy (PDT) were included for superficial lesions as well as field treatment. The development of oral hedgehog (Hh) inhibitors, such as vismodegib, offered a promising alternative to patients with advanced disease. Each treatment has its own specific indications and side effects; thus, there is always room for novel therapeutic approaches. We review new and potential treatments from 2018 and beyond. Although only 5% of SCCs become locally advanced, recur, or metastasize, and 0.4% to 0.8% of BCCs progress to advanced disease, many of the newer studies target advanced NMSCs, given their life-threatening and debilitating nature.4,5 Similarly, the incidence of nevoid basal cell carcinoma (NBCC) syndrome is as low as 1 in 57,000 to 1 in 164,000 but continues to be studied because of its morbidity and the potential to contribute new treatment options for BCC in the general population.6
Topical Therapy
Sonidegib
Basal cell carcinoma proliferation is a result of an unregulated Hh pathway that is initiated when the Hh ligand binds to the patched 1 protein (PTCH1).7-11 Patched 1 protein normally inhibits the smoothened (SMO) transmembrane receptor protein, decreasing the signaling cascade. In BCCs, there is a loss of PTCH1 function, effectively increasing the Hh pathway activity. Sonidegib is an Hh inhibitor that in turn prevents inhibition of PTCH1 in an attempt to reregulate the pathway.7-11 Although sonidegib is known for its 2015 approval by the US Food and Drug Administration (FDA) as a systemic therapy for locally advanced BCCs,12 one study investigated a topical formulation on 8 patients with NBCC syndrome.13 Patients were treated twice daily with sonidegib cream 0.75% for 4 weeks in a double-blind, randomized, vehicle-controlled study. A total of 27 BCCs were randomized and treated with either vehicle or sonidegib. A biopsy was taken at the end of the study of 1 sonidegib-treated and 1 vehicle-treated BCC lesion per patient. Of the 13 sonidegib-treated BCC lesions, 3 (23.1%) showed complete response, 9 (69.2%) showed partial response, and 1 (7.7%) showed no response vs 13 of 14 (92.8%) lesions that did not respond to the vehicle. Patients tolerated the treatment well without skin irritation or signs of local or systemic side effects.13 Topical sonidegib should be further investigated as an adjunct or in different vehicles given the successful regression of BCCs and its minimal side-effect profile.
Systemic Therapy
Cemiplimab
Cemiplimab is a human monoclonal antibody against programmed death receptor 1 (PD-1) that was FDA approved in September 2018 for the treatment of metastatic cutaneous SCC.14 Programmed death receptor 1 is found on T lymphocytes, B lymphocytes, and macrophages, which normally assist in the immune response to tumor cells. However, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) are found on tumor cells and bind to PD-1. Cemiplimab prevents PD-1 from binding to PD-L1 and PD-L2, allowing an appropriate immune response.14,15 A phase 1 clinical trial of cemiplimab showed a 50% (13/26) response rate.16 The phase 2 trial included patients with advanced SCC, but the primary analysis only considered patients with metastatic SCC. Phase 2 results showed a 47.5% (28/59) response rate. Patients received intravenous cemiplimab 3 mg/kg once every 2 weeks for up to 48 weeks in phase 1 and up to 96 weeks in phase 2. Both phases of the trial showed a response to treatment lasting longer than 6 months in more than 50% of patients. The most common adverse events were diarrhea, fatigue, nausea, constipation, and rash.16
Although immune-mediated adverse reactions are rare, they can occur given cemiplimab’s mechanism of action and may range from severe to fatal. Examples of immune-mediated adverse reactions that occurred during the study included pneumonitis, colitis, hepatitis, adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, type 1 diabetes mellitus, nephritis with renal dysfunction, and immune-mediated dermatologic reactions.14 It is important to monitor for immune-mediated adverse reactions and address them immediately once detected.
Other PD-1 Inhibitors
Although PD-1 inhibitors have been studied in advanced SCCs, their clinical data are limited for BCCs.17 Prior to 2018, there was a small number of case reports of patients with BCC with partial to exceptional response to PD-1 inhibitors. Recently, 2 additional case reports were published with contrasting outcomes using 2 different PD-1 inhibitors. An elderly patient with metastatic non–small cell lung cancer was treated with nivolumab after failing chemotherapy. She subsequently developed a BCC on the nose that was resected but recurred 2 months later despite continuing nivolumab.17 Another case report detailed a patient with a history of BCC on the shoulder excised 5 years prior who presented with recurrence on the sternum and clavicle.18 One year later the patient was found to have BCC metastases to the lung. After progression of disease despite vismodegib and recurrence of BCC with taladegib, the patient was then placed on pembrolizumab. At 6 weeks and 12 months, computed tomography showed resolution of multiple lung lesions. Sixteen weeks after initiation of pembrolizumab treatment, spinal metastases were found, but the treatment was continued because of the improvement in the lung metastases.18
Taladegib
Taladegib is a SMO antagonist that has been through a phase 1 trial in patients with advanced cancer, including treatment-naive and previously treated BCCs.19 Eighty-four patients were treated to examine the safety profile and determine an appropriate phase 2 dose and administration schedule. The maximum tolerable dose was determined to be 400 mg because of dose-limiting toxicities. All clinical responses were in patients with BCCs (47/84 [55.9%] patients), with a response rate of 46.8%. Eleven of 16 (68.8%) Hh-treatment–naive patients and 11 of 31 (35.5%) patients previously treated with Hh responded to taladegib. Common adverse events were dysgeusia, fatigue, nausea, and muscle spasms.19 Although vismodegib is an FDA-approved SMO antagonist since 2012, treatment resistance and tolerability issues have been continuing concerns.20,21 Taladegib is a potential alternative that may be found to have improved pharmacodynamics and pharmacokinetics. Not only did in vitro studies show a preferable protein-binding profile with taladegib, but it also displayed dose proportionality, while vismodegib has been known to have nonlinear pharmacokinetics.19
Posaconazole
Posaconazole is a systemic antifungal agent that is a structural analogue to itraconazole.22 Itraconazole has been found to inhibit the Hh pathway as an SMO antagonist. In a study with mice, posaconazole was found to have strong activity against drug-resistant SMO mutants while inhibiting the growth of Hh-dependent BCCs in vivo. A marked decrease also was seen in the ciliary accumulation of SMO, suggesting a similar mechanism of action to itraconazole. Posaconazole’s use for BCCs currently is limited to basic science studies but may offer a potential alternative to itraconazole, which is known to have many drug-drug interactions and requires dose adjustments in renal and hepatic insufficiency. When used as an antifungal compared to itraconazole, posaconazole has a favorable long-term safety profile due to fewer drug-drug interactions and mild side effects; it also does not require dose adjustments in mild to moderate renal or hepatic insufficiency.22 Thus, posaconazole is a potentially safer alternative to itraconazole for the treatment of BCCs. Although phase 2 studies of itraconazole for BCCs have shown decreased cell proliferation, tumor size, and reduced GLI1 messenger RNA, side effects included fatigue and grade 4 heart failure.23,24
Radiation Therapy
Radiation therapies (RTs), such as superficial RT, have been long-established treatment options.25 However, there also are emerging methods of delivering RT, including electronic brachytherapy (EB). Although there is a low likelihood of residual tumor after RT given the number of sessions involved and the more aggressive nature of the treatment, these factors also can be a substantial burden on the patient. Furthermore, RT may result in subsequent scar tissue, which can hinder the use of other emerging technologies, such as noninvasive imaging devices, following RT.
Superficial RT
Superficial RT is a secondary option for the treatment of NMSC for use in special circumstances, such as when surgical intervention is contraindicated or refused, and after the benefits and risks of treatment alternatives have been discussed.26 However, depending on the tumor type and anatomical location, 6 to 18 treatments may be required, with treatment frequency ranging from 1 to 5 treatments per week.25 Patients may find this treatment regimen difficult to maintain given the length of time and frequency of treatments required. Side effects include radiation dermatitis and postinflammatory hypopigmentation or hyperpigmentation in patients with dark skin, and there is a risk for recurrence.25,27
Electronic Brachytherapy
Brachytherapy is a method of delivering RT via radioactive isotopes, whereas EB uses lower-energy photons that require less shielding.28 As a relatively new therapy, studies on the efficacy of EB on NMSC continue to grow but with limited data comparing EB with established treatments. Furthermore, there are limited long-term follow-up data, and future studies should expand the patient demographic to younger patients before treatment guidelines can be established.28
RT With Concurrent and Adjuvant Vismodegib
Vismodegib is an SMO inhibitor that was FDA approved in 2012 for the treatment of locally advanced BCC in patients who are not candidates for surgery or RT.29 Over time, studies have looked into other indications for vismodegib, such as a neoadjuvant to MMS or in patients with NBCC syndrome.11 Prior to 2018, there were only 2 known case reports of concurrent vismodegib and RT used for recurrent advanced BCC.30 Recently, vismodegib has been further examined in combination with RT in a case report,31 basic science study,32 and phase 2 trials (ClinicalTrials.gov Identifiers NCT02956889 and NCT01835626).
Prior studies showed low cure rates with vismodegib alone after RT (43%) as well as decreasing cure rates with primary RT alone as tumor size increased.33,34 In 2018, vismodegib was used concurrently and as an adjuvant to RT in a patient with advanced multifocal BCC.31 The patient had multiple large BCCs on the trunk that were painful and bleeding. The patient was started on RT and 150 mg/d vismodegib concurrently, which was then continued adjuvantly for 3 months until it was discontinued because of diarrhea. The patient had complete response in all lesions with resolution of symptoms.31 A separate basic science study further supported the potential role of vismodegib in radiation sensitization of both BCCs and head and neck SCCs.32 There presently are 2 phase 2 trials investigating the concurrent use of vismodegib and RT, which could help determine the efficacy of the combined approach for patients with advanced BCCs who are poor surgical candidates (NCT02956889 and NCT01835626).
Photodynamic Therapy
Photodynamic therapy has been in use since the 1970s when Dougherty et al35 performed one of the first studies on its use in skin cancer. Since then, PDT has been used for the treatment of actinic keratoses (AKs) and more recently BCCs. In PDT, a photosensitizer (PS) is applied and activated by a 400-nm blue light or 635-nm red light, depending on the PS used. The PS then produces highly reactive oxygen species, leading to apoptosis of the cancer cells.36 In Europe, red light PDT is licensed for the treatment of AKs as well as superficial and nodular BCCs, though approved indications vary between countries. In the United States, PDT is only FDA approved for the treatment of AKs.37
Aminolevulinic Acid Hydrochloride
Aminolevulinic acid hydrochloride is a red light PS used to treat AKs since 2011 and BCCs since 2017 in Europe in addition to AKs in the United States since 2016.38,39 A phase 3 noninferiority clinical trial in Europe of 281 patients compared the treatment of nonaggressive BCCs with ALA to methyl aminolevulinate (MAL) cream.40 The study found a complete response rate of 93.4% vs 91.8%. Superficial BCCs treated with ALA had a clearance rate of 94.7% vs 96.4% with MAL, while nodular BCCs treated with ALA had a clearance rate of 85.7% vs 76.2% with MAL. A 1-year clinical follow-up showed similar recurrence rates (8.4% for ALA vs 8.5% for MAL).40 The results of this study led to an expanded indication in Europe to include the treatment of BCCs.38 Aminolevulinic acid hydrochloride currently is undergoing phase 3 clinical trials in the United States for approval for the treatment of superficial BCCs (NCT03573401). If similar outcomes are achieved, US patients may have access to an alternative nonsurgical treatment of BCCs. The ongoing US trial is exclusively investigating the efficacy and safety for superficial BCCs, which may limit FDA approval to only superficial BCCs, accounting for only 8.4% to 24.1% of all BCCs.35,41,42
Laser Therapy
Ablative and nonablative lasers have been used to treat NMSCs in the literature. Ablative lasers destroy tumors through vaporization of tissue water, whereas nonablative lasers target the vasculature of tumors while preserving the surrounding tissue.43,44 Nonablative lasers include pulsed dye lasers (PDL) and Nd:YAG lasers. Examples of ablative lasers include CO2 and erbium:YAG lasers. Given the status of lasers as an emerging treatment method, there currently is no standardized laser setting for any of the laser therapies used to treat NMSCs. Although there is the potential for optimal cosmetic outcomes and a limited side-effect profile for nonablative laser therapies, there are limited data on long-term follow-up to study recurrence rates and establish a more standardized treatment protocol.
Pulsed Dye Lasers
Although there were no studies on PDL therapy alone in 2018, a study published in 2019 evaluated a combination laser treatment using a 595-nm PDL and 1927-nm fractional laser for the treatment of 93 BCCs, yielding a 95.7% (89/93) clearance rate and 4.5% (4/89) recurrence rate over a follow-up period of up to 6 years (range, 2.53 months to 6.03 years).45 Studies of PDL prior to 2018 had follow-ups ranging from 2 weeks to 6 months.46-51 Although the majority were biopsy-proven BCCs, reflectance confocal microscopy also was used for same-day diagnoses. Long-term follow-up included clinical examinations, dermoscopy, and optical coherence tomography.45 The clearance rate (95.7%) using noninvasive imaging in conjunction with the combination laser treatment was superior to both histologic and clinical clearance rates of prior PDL-only studies, which ranged from 25% to 95%.46-51 To have long-term follow-up data, the study used noninvasive imaging with clinical follow-up because histology would not be viable for long-term follow-up. This study was retrospective rather than prospective, which was a limitation.45
Nd:YAG Lasers
The majority of studies utilizing Nd:YAG lasers investigated their efficacy in treating BCCs, with the exception of 1 study of facial SCCs. This major study in 2009 of 627 BCCs showed a 2.5% recurrence rate after a follow-up time of 3 months to 5 years.52 Nd:YAG lasers continue to be investigated, including a more recent study of 31 extrafacial, biopsy-proven BCCs that were treated with the 1064-nm Nd:YAG laser, which showed a 90% histologic clearance on 1-month follow-up after a single treatment.53 In 2019, a retrospective review of 16 BCC lesions on the head, neck, trunk, and extremities showed 100% clearance after 1 treatment, with an average follow-up period of 9 months (range, 6–15 months).54 In a retrospective review, Markowitz and Psomadakis55 contributed data supporting the further investigation and use of the 1064-nm Nd:YAG laser for BCC treatment while leveraging noninvasive imaging to demonstrate a same-day management model. Seventeen BCC lesions on the face and body were diagnosed by reflectance confocal microscopy and treated with an Nd:YAG laser, and clearance was monitored clinically, dermoscopically, and by optical coherence tomography. There was 100% clearance of the lesions in the study, with 82.4% (14/17) clearing after 1 treatment; mean follow-up was 103 days (range, 48–371 days).55 These studies were limited by their short follow-up time; long-term data are needed to determine true rates of recurrence.
Ablative Lasers
Ablative lasers also have been used in the treatment of NMSCs. In addition to the potentially increased healing time compared to nonablative lasers, other limitations of ablative laser therapy include residual tumor burden or recurrence that may not be easily visualized in scarred tissue after nonablative management.44
Conclusion
Although MMS remains the gold standard for invasive management of NMSCs, studies from 2018 and beyond (eTable) expanded not only on MMS topics such as increased patient access and improved techniques but also on the increasing potential of noninvasive treatments. Some of the noninvasive therapies were entirely new compounds, whereas others were already in use for a different disease indication. Furthering our knowledge and expanding our repertoire of management options will prepare us as the number of patients affected by NMSCs increases.

- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Kauvar AN, Arpey CJ, Hruza G, et al. Consensus for nonmelanoma skin cancer treatment, part II. Dermatol Surg. 2015;41:1214-1240.
- Ribero S, Stucci LS, Daniels GA, et al. Drug therapy of advanced cutaneous squamous cell carcinoma: is there any evidence? Curr Opin Oncol. 2017;29:129-135.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e2-966.e2.
- Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299-308.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Peris K, Licitra L, Ascierto PA, et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Futur Oncol. 2015;11:703-712.
- Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Ibrahim O, Gastman B, Zhang A. Advances in diagnosis and treatment of nonmelanoma skin cancer. Ann Plast Surg. 2014;73:615-619.
- Levine A, Siegel DM, Markowitz O. Update on noninvasive diagnostic imaging and management of nonmelanoma skin cancer. Curr Dermatol Rep. 2018;7:1-15.
- Casey D, Demko S, Shord S, et al. FDA approval summary: sonidegib for locally advanced basal cell carcinoma. Clin Cancer Res. 2017;23:2377-2381.
- Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131:1735-1744.
- Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841-1846.
- Chen L, Aria AB, Silapunt S, et al. Emerging nonsurgical therapies for locally advanced and metastatic nonmelanoma skin cancer. Dermatolog Surg. 2019;45:1-16.
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351.
- Sabbatino F, Marra A, Liguori L, et al. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature. J Immunother Cancer. 2018;6:126.
- Cannon JGD, Russell JS, Kim J, et al. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4:248-250.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111:1476-1481.
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14:633-641.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Nestor MS, Berman B, Goldberg D, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12:12-18.
- American Academy of Dermatology and AAD Association. Position statement on superficial radiation therapy for basal cell carcinoma (BCC) and squamous cell carcinomas (SCC). https://server.aad.org/Forms/Policies/Uploads/PS/PS%20Superficial%20Radiation%20Therapy.pdf?. Updated August 9, 2014. Accessed February 26, 2020.
- Skiveren J, Mikkelsen MR, Daugbjerg H, et al. Skin reactions and quality of life after X-ray therapy of basal cell carcinoma. J Skin Cancer. 2012;2012:825095.
- Tom MC, Hepel JT, Patel R, et al. The American Brachytherapy Society consensus statement for electronic brachytherapy. Brachytherapy. 2019;18:292-298.
- Axelson M, Liu K, Jiang X, et al. US Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289-2293.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:E2485.
- Piccinno R, Benardon S, Gaiani FM, et al. Dermatologic radiotherapy in the treatment of extensive basal cell carcinomas: a retrospective study. J Dermatolog Treat. 2017;28:426-430.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol. 2001;51:748-755.
- Dougherty TJ, Kaufman JE, Goldfarb A, et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628-2635.
- Ding H, Yu H, Dong Y, et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276-280.
- Maytin EV, Kaw U, Ilyas M, et al. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: a bilaterally controlled comparison study. Photodiagnosis Photodyn Ther. 2018;22:7-13.
- European Medicines Agency. Ameluz 5-aminolevulinic acid hydrochloride. https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz. Updated May 13, 2019. Accessed February 25, 2020.
- Center for Drug Evaluation and Research. Approval package for Ameluz (aminolevulinic acid hydrochloride) gel, 10%. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208081Orig1s000Approv.pdf. Published May 10, 2016. Accessed February 25, 2020.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179:309-319.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Kamyab-Hesari K, Seirafi H, Naraghi ZS, et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014;28:250-253.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297, E1-E3.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O, Psomadakis CE. Patient-driven management using same-day noninvasive diagnosis and complete laser treatment of basal cell carcinomas: a pilot study. Cutis. 2019;104:345-348.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Kauvar AN, Arpey CJ, Hruza G, et al. Consensus for nonmelanoma skin cancer treatment, part II. Dermatol Surg. 2015;41:1214-1240.
- Ribero S, Stucci LS, Daniels GA, et al. Drug therapy of advanced cutaneous squamous cell carcinoma: is there any evidence? Curr Opin Oncol. 2017;29:129-135.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e2-966.e2.
- Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299-308.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Peris K, Licitra L, Ascierto PA, et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Futur Oncol. 2015;11:703-712.
- Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Ibrahim O, Gastman B, Zhang A. Advances in diagnosis and treatment of nonmelanoma skin cancer. Ann Plast Surg. 2014;73:615-619.
- Levine A, Siegel DM, Markowitz O. Update on noninvasive diagnostic imaging and management of nonmelanoma skin cancer. Curr Dermatol Rep. 2018;7:1-15.
- Casey D, Demko S, Shord S, et al. FDA approval summary: sonidegib for locally advanced basal cell carcinoma. Clin Cancer Res. 2017;23:2377-2381.
- Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131:1735-1744.
- Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841-1846.
- Chen L, Aria AB, Silapunt S, et al. Emerging nonsurgical therapies for locally advanced and metastatic nonmelanoma skin cancer. Dermatolog Surg. 2019;45:1-16.
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351.
- Sabbatino F, Marra A, Liguori L, et al. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature. J Immunother Cancer. 2018;6:126.
- Cannon JGD, Russell JS, Kim J, et al. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4:248-250.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111:1476-1481.
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14:633-641.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Nestor MS, Berman B, Goldberg D, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12:12-18.
- American Academy of Dermatology and AAD Association. Position statement on superficial radiation therapy for basal cell carcinoma (BCC) and squamous cell carcinomas (SCC). https://server.aad.org/Forms/Policies/Uploads/PS/PS%20Superficial%20Radiation%20Therapy.pdf?. Updated August 9, 2014. Accessed February 26, 2020.
- Skiveren J, Mikkelsen MR, Daugbjerg H, et al. Skin reactions and quality of life after X-ray therapy of basal cell carcinoma. J Skin Cancer. 2012;2012:825095.
- Tom MC, Hepel JT, Patel R, et al. The American Brachytherapy Society consensus statement for electronic brachytherapy. Brachytherapy. 2019;18:292-298.
- Axelson M, Liu K, Jiang X, et al. US Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289-2293.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:E2485.
- Piccinno R, Benardon S, Gaiani FM, et al. Dermatologic radiotherapy in the treatment of extensive basal cell carcinomas: a retrospective study. J Dermatolog Treat. 2017;28:426-430.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol. 2001;51:748-755.
- Dougherty TJ, Kaufman JE, Goldfarb A, et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628-2635.
- Ding H, Yu H, Dong Y, et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276-280.
- Maytin EV, Kaw U, Ilyas M, et al. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: a bilaterally controlled comparison study. Photodiagnosis Photodyn Ther. 2018;22:7-13.
- European Medicines Agency. Ameluz 5-aminolevulinic acid hydrochloride. https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz. Updated May 13, 2019. Accessed February 25, 2020.
- Center for Drug Evaluation and Research. Approval package for Ameluz (aminolevulinic acid hydrochloride) gel, 10%. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208081Orig1s000Approv.pdf. Published May 10, 2016. Accessed February 25, 2020.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179:309-319.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Kamyab-Hesari K, Seirafi H, Naraghi ZS, et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014;28:250-253.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297, E1-E3.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O, Psomadakis CE. Patient-driven management using same-day noninvasive diagnosis and complete laser treatment of basal cell carcinomas: a pilot study. Cutis. 2019;104:345-348.
Practice Points
- As of 2018, there has been an increase in options for the noninvasive management of nonmelanoma skin cancers that should be considered.
- Recently, approved advances in treatment options have included not only advanced basal cell carcinoma but also advanced squamous cell carcinoma such as cemiplimab.
Optimal Cosmetic Outcomes for Basal Cell Carcinoma: A Retrospective Study of Nonablative Laser Management
Nonablative laser therapy is emerging as an effective noninvasive treatment option for basal cell carcinoma (BCC) with reduced adverse effects and good cosmetic outcomes compared to surgery. Vascular lasers, such as the pulsed dye laser (PDL), are thought to work by selectively targeting the tumor’s vascular network while preserving normal surrounding tissue.1,2 Although high energy and multiple passes might be required, adjunctive use of dynamic cooling reduces the risk for nonselective thermal injury vs ablative lasers, which destroy the tumor itself through vaporization of tissue water.2
With no established laser management guidelines for the treatment of BCC, earlier studies using a 595-nm PDL varied highly in their protocol.3-8 Pulsed dye laser parameters ranged from a spot size of 7 to 10 mm, fluence of 7.5 to 15 J/cm2, and pulse duration of 0.5 to 3 milliseconds. Follow-up ranged from 12 days to 25 months after the final laser treatment. The number of lesions in prior studies ranged from 7 to 100 BCCs, with the clinical clearance rate ranging from 71.4% to 75% for facial BCC and 78.6% to 95% for nonfacial BCC.3-8 Studies with histologic confirmation had a clearance rate of 66.6% for facial BCC and 25% to 92.3% for nonfacial BCC.3-5,7,8 Most studies examined BCCs on the trunk and extremities with few investigating facial BCC,3-8 which is especially important given that the head and neck are the most common and cosmetically sensitive anatomic locations.9-13
Noninvasive imaging devices, such as reflectance confocal microscopy (RCM) and optical coherence tomography (OCT) can assist with the diagnosis and treatment monitoring of BCC. These devices enable in vivo visualization of tissue in both cross-sectional and en face views and therefore can reduce the need for diagnostic biopsy. Reflectance confocal microscopy enables near-histologic visualization of the epidermis and superficial dermis with a resolution of 0.5 to 1 μm.14 Optical coherence tomography uses an infrared broadband light source that allows users to view skin architecture as deep as 1.5 to 2 mm with a resolution of 5 μm.15
When used synergistically, both devices can enhance the efficacy of nonablative laser treatment. With its increased depth and wider field of view, OCT is an optimal tool for repetitive evaluation of the same site over time and for following biopsy-confirmed tumors undergoing management.16 In addition to delineating tumor margins before treatment, imaging improves the detection of residual skin cancers, despite clearance on clinical and dermoscopic examination. Noninvasive imaging and nonsurgical management with laser therapy allow the physician to leave the skin intact and avoid scar tissue that might otherwise make it more difficult to detect and manage recurrence. The ability of OCT and RCM to monitor the efficacy of nonsurgical therapies for skin cancer has been demonstrated with imiquimod, photodynamic therapy, vismodegib, and ablative laser therapy.17-20
With limited data on nonablative laser management of BCC, several gaps in the literature exist. First, in previously published studies the number of treatments was either determined to be an arbitrary set number or based on clinical clearance, which has the potential to miss residual tumor. Second, many follow-ups were limited to shortly after the final treatment, which limits the accuracy of the clearance rate, given that inflammation and scars can hide residual tumor.21-23 Third, because many studies excised the treated area, long-term follow-up for recurrence was obscured. Last, only a few studies involved facial BCC, which is the most common and cosmetically concerning anatomic location.13
Our study attempted to address these gaps by evaluating the use of noninvasive imaging to guide management of primarily facial BCC. The objective was to perform a retrospective chart review on a subgroup of patients with BCC who were treated with combined nonablative PDL and fractional laser treatment with an extended follow-up period.
Methods
Study Design
We performed a retrospective chart review of 68 patients with 93 BCCs who had been treated with nonablative laser therapy as an alternative to surgery at the Mount Sinai Faculty Practice Associates between February 2011 and December 2018. Patients were followed throughout this period for assessment of clinical and subclinical recurrence. The Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects provided institutional review board approval.
Patients
Inclusion criteria included the following: (1) BCC diagnosed by biopsy (see eTable 1 for subtypes) and (2) treated with a nonablative laser due to patient preference and eligibility by the principal investigator (PI). As a retrospective study, lesions were included irrespective of tumor subtype or size. Although the risk for perineural invasion (PNI) is extremely low with BCC (<0.2%), none of the cases demonstrated PNI on diagnostic biopsy and none exhibited clinical evidence of PNI, such as paresthesia, pain, facial paralysis, or diplopia.24
Eligibility determined by the PI included limited clinical ulceration or bleeding, or both, and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). Patients who had Mohs micrographic surgery (MMS) or excision (or both) with recurrence at the treatment site were included. Detailed and thorough clinical and dermoscopic skin examination was critical in early detection of these cancers, allowing for treatment of less advanced tumors. The PI’s diagnostic approach utilized the published diagnostic color wheel algorithm,25 which encompasses both clinical and dermoscopic colors and patterns for early diagnosis (ie, ulceration, pink-white to white shiny areas, absence of pigmented network, leaflike structures, large blue-gray ovoid nests or globular structures, spoke wheel structures, a crystalline pattern, a singular vascular pattern of arborizing vessels), combined with OCT or RCM, when necessary.26 All lesions were imaged with OCT prior to laser treatment to confirm residual tumor following biopsy.
Although postsurgical patients were included, lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor (eg, vismodegib), were excluded.
Treatment Protocol
All patients received thorough information about the treatment, treatment alternatives, and potential adverse effects and complications. Lesions were selected based on clinical and dermoscopic findings and were biopsy confirmed. Clinical and dermoscopic photographs were taken at every visit. A camera was used for clinical photographs and a dermatoscope was attached for all contact polarized dermoscopic images. All lesions were imaged with OCT prior to laser therapy to delineate tumor margins and to confirm residual disease following biopsy to preclude biopsy-mediated regression.
Laser treatment consisted of a 595-nm PDL followed by fractional laser treatment with the 1927-nm setting. The range of PDL settings was similar to published studies of PDL for BCC (spot size, 7–10 mm; fluence, 6–15 J/cm2; pulse duration, 0.45–3 milliseconds).3-8 The fractional laser also was used at settings similar to earlier studies for actinic keratosis (fluence, 5–20 mJ; treatment density, 40%–70%).27 Laser treatment was performed by 1 of 5 medically trained providers who were fellows supervised by the PI.
All tumors received 1 to 7 treatments (average, 2.89) at 1- to 2-month intervals. Treatment end point (complete clearance) was judged on the absence of skin cancer clinically, dermoscopically on OCT, or histologically by biopsy, or a combination of these modalities. Recurrence was defined as a new histologically confirmed BCC occurring in an area that was previously documented as clear. Patients returned for follow-up 1 to 2 months after the final treatment to monitor tumor clearance and subsequently every 6 to 12 months for tumor recurrence. Posttreatment care included application of a thick emollient, such as a petrolatum-based product, until the area completely healed.
Data Collection
Clinical photographs, dermoscopic photographs, OCT scans, RCM scans, and biopsy reports were reviewed for each patient, as applicable. All patients were given an unidentifiable number; no protected health information was recorded. Data recorded for each patient included age, tumor subtype and location, tumor size, classification of the tumor as primary or a recurrence, number of treatments, treatment duration, lesion clearance, and length of follow-up.
Results
Patient and Lesion Characteristics
Sixty-eight patients with 93 BCCs (77 facial; 16 nonfacial) were included. The median age of patients was 70 years (range, 31–91 years). All 93 BCCs demonstrated residual tumor on OCT after diagnostic biopsy. Four BCCs had been treated earlier with MMS and were biopsy-proven recurrences. Most BCCs were of the nodular subtype; however, sclerosing, superficial, pigmented, morpheaform, and infiltrative subtypes also were included (eTable 1). Eight BCCs were obtained at outside institutions with no subtype provided. Facial BCCs had a mean (SD) clinical and dermoscopic diameter of 6.75 (4.71) mm (range, 2–24 mm). Patients were followed for 2.53 months to 6.03 years (mean follow-up, 2.43 years) and assessed for clinical and subclinical recurrence.
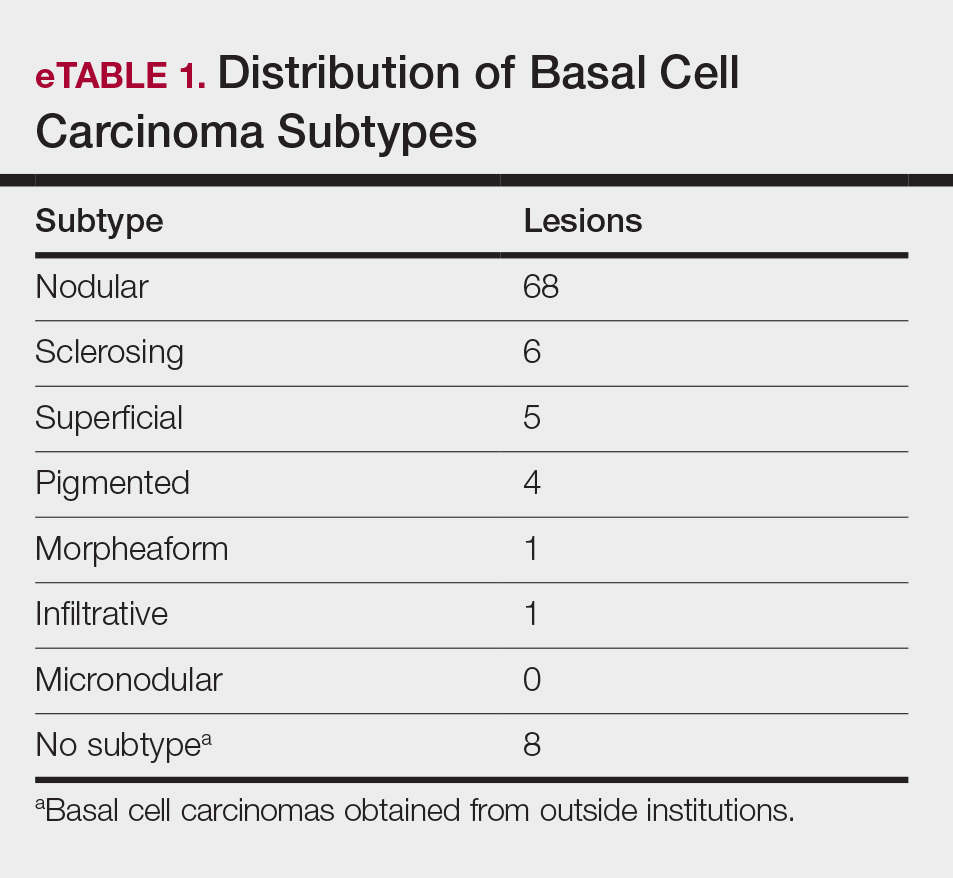
Tumor Clearance
Most lesions were effectively treated, with 89 of 93 BCCs (95.70%) demonstrating complete tumor clearance. Complete tumor clearance following laser therapy was reported in 74 of 77 facial BCCs (96.10%) and 15 of 16 nonfacial BCCs (93.75%)(eTable 2). Successfully treated BCCs underwent an average of 2.88 laser treatments over a mean duration of 3.54 months (range, 1 week to 1.92 years). Four incomplete responders underwent an average of 3.25 laser treatments over a mean duration of 3.44 months (range, 1.13–6.87 months). Of the 4 lesions that did not clear, 2 were nodular, 1 was pigmented, and 1 was sclerosing.
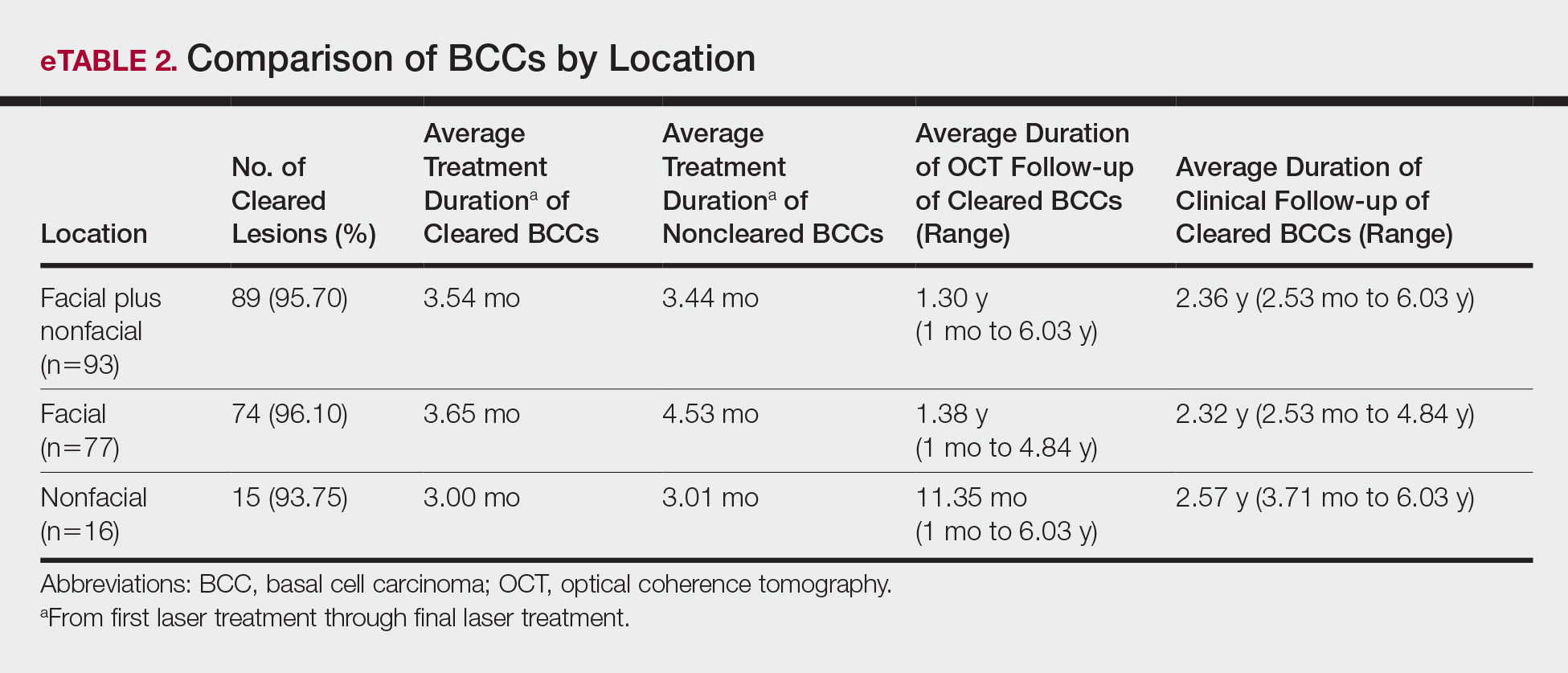
Number of Treatments
When the clearance rate is divided into lesions that received 3 or fewer laser treatments and those that received more than 3 laser treatments, the following results were determined:
• Lesions receiving 3 or fewer treatments had a clearance rate of 96.05% (73/76) for all BCCs, 96.72% (59/61) for facial BCCs, and 93.33% (14/15) for nonfacial BCCs.
• Lesions receiving more than 3 laser treatments had a clearance rate of 94.12% (16/17) for all BCCs, 93.75% (15/16) for facial BCCs, and 100% (1/1) for nonfacial BCCs.
The relationship between facial BCC tumor diameter and number of treatments required for clearance had a positive correlation coefficient (Pearson r=0.319), indicating that larger BCCs required more laser treatments (eTable 3).
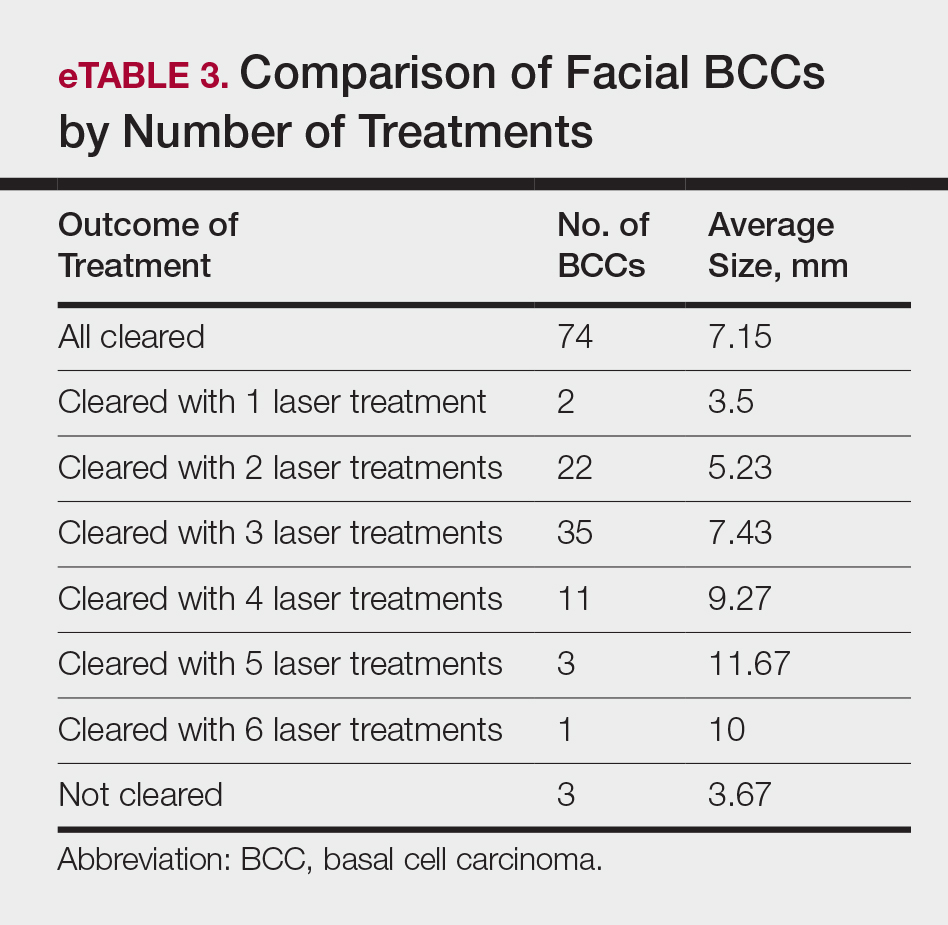
Tumor Recurrence
Four of 89 BCCs (4.49%)(4 of 74 facial BCCs [5.41%]) showed tumor recurrence following laser treatment, as assessed by OCT and dermoscopy. Of them, all were nodular BCCs. Prior to laser treatment, there were 4 additional patients each diagnosed with a recurrence from prior treatment with MMS; all were successfully treated with laser therapy without recurrence post–laser treatment (eFigure 1). Most of the recurrences from prior MMS required more than 3 laser treatments before clearing: 1 required 3 treatments, 2 required 4 treatments, and 1 required 6 treatments.
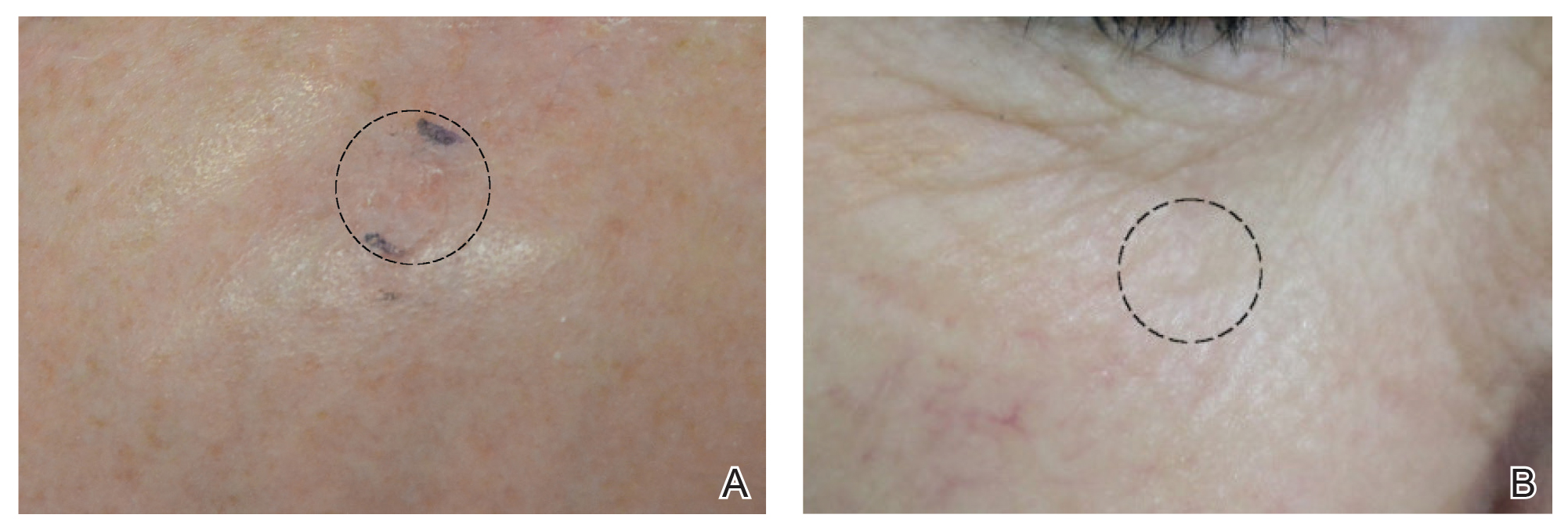
Of 93 lesions included in this study, 2 BCCs were deemed not clear on histologic analysis, which corresponded with residual tumor seen on OCT. Two additional lesions were determined to be not clear on OCT but were not confirmed as such on biopsy; both lesions were confirmed not clear, however, by histologic analysis on the first layer of MMS
Follow-up
All cleared lesions (89/93) showed complete clinical response to laser treatment for 6 months or more (median follow-up, 2–3 years; mode, 1–2 years; mean, 2.66 years)(eTable 4). Although 45% of patients (40/89) have been followed clinically and/or dermoscopically (as is done for MMS follow-ups) for 3 years to more than 5 years, only 20% of patients (18/89) were followed up with OCT in combination with clinical and/or dermoscopic examination between 3 years and more than 5 years. Follow-up took on a bimodal distribution, with a peak follow-up period at 1 to 2 years and again at 3 to 4 years. Half of the lesions (45/89) were followed up with OCT in combination with clinical and dermoscopic examination at 1 to 6 months (eTable 5). Of the 2 patients with 1-month OCT follow-up, 1 died from other medical causes and the other was unable to return for further follow-up scans.
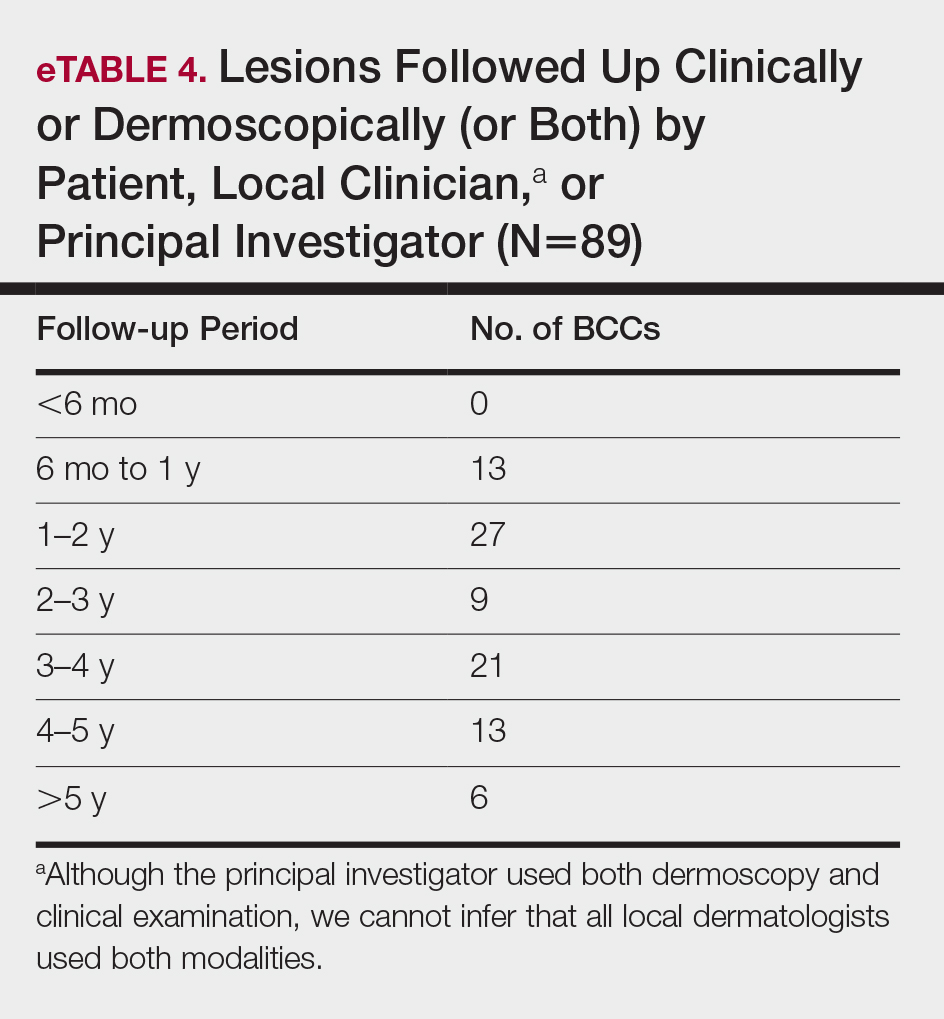

Comment
High Tumor Clearance Rates With OCT
This study yielded a clearance rate of 95.70% for all BCCs, 96.10% for facial BCCs, and 93.75% for nonfacial BCCs. This rate is higher than the clinical or histologic clearance rate (or both) of earlier studies on facial and nonfacial BCCs, which ranged from 25% to 95%.8-11 In this study, we were able to utilize OCT and histology to confirm clearance. Optical coherence tomography, which has been shown to have a high sensitivity ranging from 86% to 95.7%, is therefore optimally used in treatment monitoring.19,26,28 Optical coherence tomography has a broader specificity range of 75.3% to 98% and was not utilized for diagnostic purposes in this study. Combining OCT with a color wheel dermoscopic approach was helpful in confirming treatment efficacy of nonsurgical therapies and is significantly more accurate than clinical analysis alone (P<.01).19,26,28
We suspect that the higher clearance rates observed in our study were due to the OCT-guided treatment protocol. Optical coherence tomography was used for margination while providing a modality for tailored treatment through visualization of residual tumor on clinically and dermoscopically clear follow-ups, given that several studies found residual tumor at the lateral edge of the tumor margin on histopathologic analysis.5 Utilizing noninvasive imaging technology to delineate tumor margins before treatment can improve efficacy and limit unnecessary treatment to the surrounding normal skin (eFigure 2).29
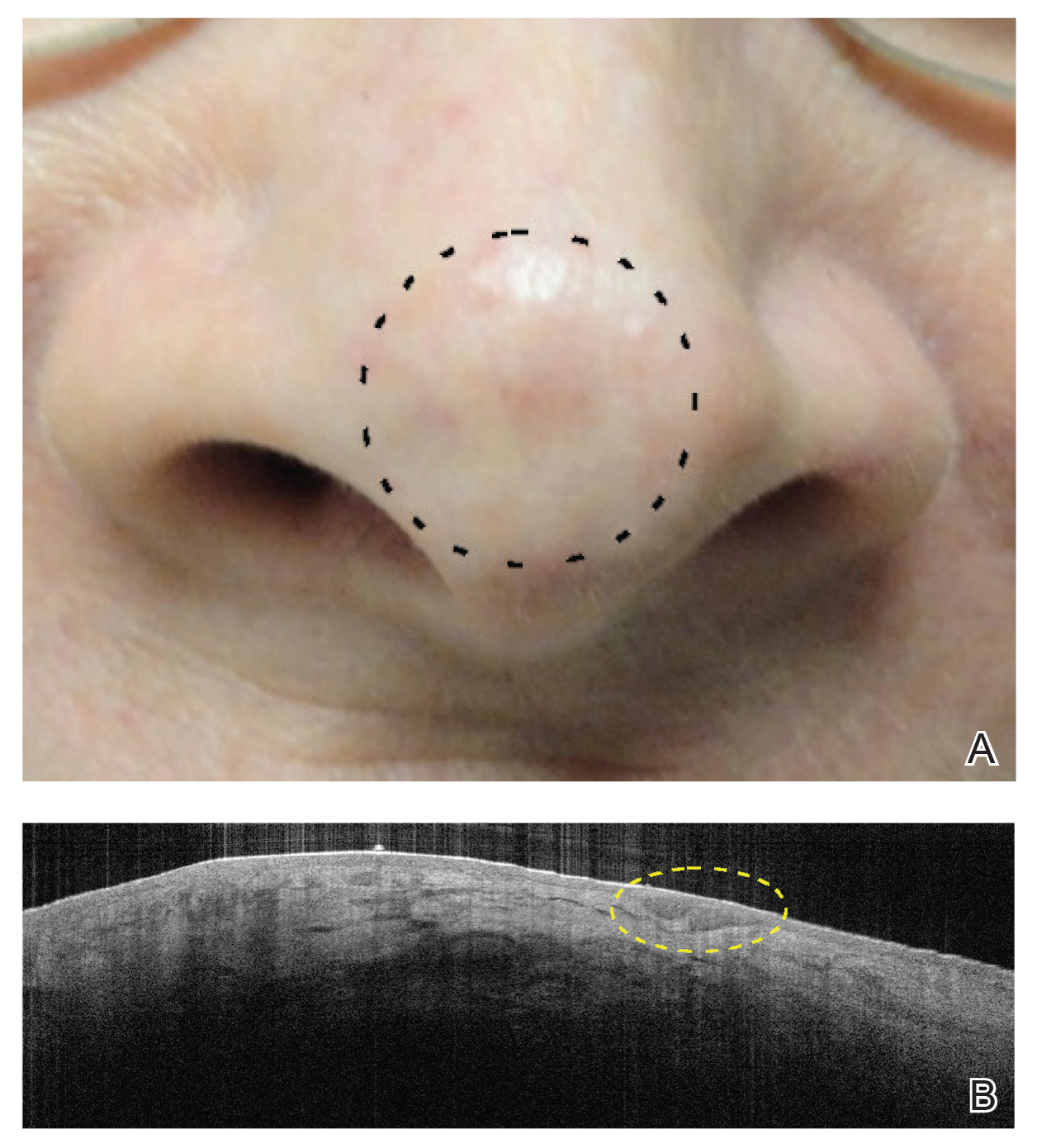
After grouping lesions by number of laser treatments, the clearance rate remained similar among facial BCCs with 3 or fewer treatments (59/61 [96.72%]), but there was a slightly decreased clearance rate for facial BCCs with more than 3 treatments (15/16 [93.75%]), which may be explained by the need for more laser treatments for larger BCCs (eTable 3). The relationship between facial BCC size and number of laser treatments was found to correlate positively (Pearson r=0.319). The largest lesion (24 mm) was successfully treated with 5 treatments (Figure). The number of nonfacial lesions was limited in this study and was not statistically significant.
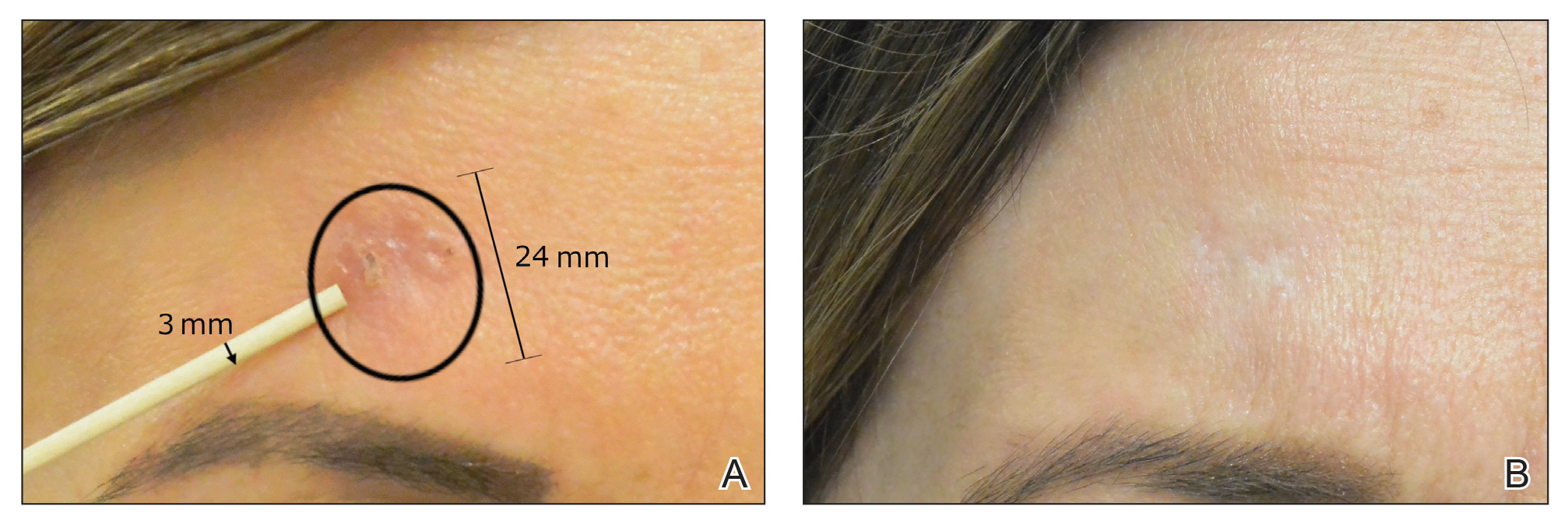
there was no clinical evidence of residual BCC.
Cosmetic Outcome
Adverse effects, including erythema, purpura, blistering, and crusting, were short-term and well tolerated. Few patients had subsequent hypopigmentation in the initial months after treatment, which we consider an optimal cosmetic outcome. For example, the patient shown in the Figure would have required extensive reconstruction of the defect using bilateral rotation flaps with incisions along the hairline, grafting, or second-intention healing with partial closure to avoid brow-lifting.30 Given the relatively young age of this patient (a 45-year-old woman) and therefore limited skin laxity, secondary intention or even attempting to match grafted tissue could have resulted in a less than optimal cosmetic outcome. None of the patients experienced clinical or dermoscopic evidence of scarring from the laser treatment.
A few lesions were found to have subclinical inflammation on OCT, which might have obscured residual tumor on the 1-month follow-up scan. This condition may be similar to how pre-MMS diagnostic biopsy scars mask skin cancer during surgery, making it necessary to obtain additional layers beyond the biopsy scar tissue. This scar tissue would otherwise obscure tumor on histology during MMS, similar to subclinical inflammation obscuring residual tumor on OCT.21-23,31 Invasive and noninvasive management of skin cancers will have different healing times and therefore different optimal times to confirm clearance by histology compared to noninvasive imaging. All of the lesions in which inflammation was obscured on OCT 1-month posttreatment remained cleared. However, 1 lesion was found to be clear at a 4-week clearance scan after only 2 nonablative laser treatments and was confirmed as scar tissue on histology. Scar tissue on histology might have obscured any residual tumor. The patient appeared clinically and dermoscopically to have a milia in the same location only 5 months later; however, on OCT and histology, the lesion was confirmed to be a BCC.
Treatment Intervals
Several other studies either used a set number of treatments or determined the number of treatments based on clinical clearance.3-8 When determining the best treatment interval, we considered the period for patients to be clinically and dermoscopically healed to be 1 month. Patients came for their final follow-up scan an additional month after the final treatment in case there was any obscuring inflammation on OCT at 1 month. Given that patients responded well to nonablative laser treatment once skin clinically healed and most patients required 3 treatments, the PI began recommending a total of 3 treatments performed 4 to 6 weeks apart in clinical practice, followed by a final clearance scan 2 months after the third treatment. A period of 2 months was considered ideal for the final clearance scan because no inflammation was seen at the 2-month follow-up in the group of patients who had inflammation at the 1-month follow-up on OCT in our study. Some patients had an extended treatment duration because of noncompliance with the 4- to 6-week follow-up regimen. Although this extension of treatment duration potentially skews the clearance rate, we still included these patients, given the retrospective design of this study.
Lesions That Did Not Clear
Four BCCs did not clear, 3 of which were facial BCCs. All 4 lesions demonstrated residual tumor on OCT. Of the 3 facial lesions that did not clear:
• One was the patient who had obscuring inflammation at the 1-month follow-up and only scar tissue on histologic confirmation.
• Another was a pigmented BCC on the right cheek of a patient with Fitzpatrick skin type IV. This patient received 3 treatments without a response clinically or on OCT. (Most patients who showed complete clearance also showed reduction in tumor size after the first laser treatment. Of note, there were other patients who had lighter skin types with pigmented BCCs and all of these patients had complete response to this treatment regimen; therefore, we do not think that a pigmented BCC is an exclusion to this therapy.)
• The third was a BCC on the nose of a nonadherent patient, which may have contributed to the lack of clearance. We defined nonadherent patients as those who did not follow-up within the appropriate periods and who therefore ran the risk for tumor growth in between treatments.
The nonfacial BCC that did not clear had histologic features of focal sclerosing BCC, a more aggressive subtype of basal cell skin cancer.
Tumor Recurrence
Only 4 of 89 BCCs (4.49%) recurred, with a 5.41% (4/74) recurrence rate among facial BCCs. All recurrences lacked clinical and dermoscopic evidence of BCC but were found on follow-up OCT scan and confirmed with RCM. All recurrences were found 1.5 to 3.9 years posttreatment.
Recurrent tumors following MMS required, on average, more laser treatments than primary tumors to achieve successful tumor clearance, which we attribute to scar tissue from prior therapy obscuring recurrence, resulting in delayed diagnosis, and to inflammation and fibrosis masking residual tumors (eFigure 1). An added benefit of laser treatment is that all 4 recurrent tumors demonstrated improved cosmetic appearance of the original MMS scar.
The benefit of using OCT scans to check for recurrences is that OCT can find residual skin cancers despite the area looking clinically clear, which is especially important during clinical evaluation of a healed postsurgical scar for recurrence because OCT imaging allows us to look as deep as 2 mm under the skin. Nonsurgical treatments also enable us to leave skin intact and avoid creating scar tissue, which makes it easier to detect and manage recurrence.
Limitations
There were several important limitations of this retrospective study:
• Patients were treated by 1 of 5 medically trained fellows. Although the fellows worked under the supervision of the PI, variation in their work from one to another might have led to different end points.
• All patients who appeared clinically clear were offered biopsy to confirm clearance on histology. Some patients agreed to biopsy, but many did not because they were pleased with the cosmetic outcome, which is similar to other studies exhibiting only clinical clearance rates without providing histologic clearance following nonsurgical therapy.6 We believe that imaging with OCT circumvents this problem and offers more accurate confirmation than clinical or dermoscopic correlation alone, or the combination of the 2 modalities.
• Lack of treatment standardization and short length of follow-up can result in underestimation of the recurrence rate. In particular, most patients were followed up with OCT in less than 6 months. These are unavoidable features in a retrospective study and we are currently addressing this problem in a new prospective study.
Extended Follow-up
Although this study is not a prospective design, it does provide recurrence data over extended follow-up for the nonablative laser management of BCCs (eTables 4 and 5). Studies have demonstrated that MMS has a 5-year cure rate as high as 99% for BCC.32 Given the limited follow-up period of prior nonablative laser management studies, recurrences might not have been fully evaluated. Our study had a 4.49% recurrence rate for all BCCs and a 5.41% recurrence rate for facial BCCs but was not detectable by clinical examination combined with dermoscopic findings alone. All recurrences required the utilization of OCT or RCM or a combination of these modalities to be diagnosed. In 1 patient with recurrence, we were able to see residual tumor on both OCT and RCM without any inflammation obscuring the scan, given that 3 years had passed. Although 2 months is an optimal follow-up time for OCT, we have not found an optimal follow-up time for RCM, which is another reason why OCT might be preferable to other imaging modalities, such as RCM and high-definition OCT, that have higher resolution but provide less depth on imaging. Although only 40 of 89 patients (4.49%) had follow-up ranging from 3 years to greater than 5 years, long-term follow-up to date has been limited in prior studies.
We believe the high clearance rates and limited recurrence are secondary to the utilization of noninvasive imaging, as the majority of these recurrences would not have been diagnosed based on clinical and/or dermoscopic information alone. Additionally, the 4 biopsy-proven post-MMS recurrence patients that were treated in this study also may not have been diagnosed this early without the use of additional noninvasive imaging. In our opinion, although laser management can be used without noninvasive imaging guidance—dermoscopy, OCT, and/or RCM—this technology is critical not only for early detection but also for proper management of patients.
Conclusion
This study showed a 95.70% clearance rate for all BCCs and a 96.10% clearance rate for facial BCCs. Although we had a zero clinical recurrence rate, 4.49% of all BCCs and 5.41% of facial BCCs had recurred on subsequent monitoring with noninvasive imaging. Given the large size of the study and extended follow-up, we found nonablative laser management to be a reliable treatment alternative with improved cosmetic outcome (Figure) and minimal short-term adverse effects compared to surgery.
Tailored care for the individual patient is based on a variety of options and patient preference, including ease of compliance, number of follow-up visits, invasive vs noninvasive diagnosis and monitoring, and downtime for healing. The use of noninvasive imaging also allowed us to find a more standardized treatment regimen using this nonablative laser combination. We found that 3 or fewer and more than 3 treatments had similar efficacy in tumor clearance. We recommend a standard laser protocol of 3 treatments every 4 to 6 weeks with follow-up 2 months after the final treatment to assess for clearance with OCT.
Larger BCCs might require additional treatments; therefore, we caution against laser therapy without concomitant use of OCT imaging to visualize residual tumor. Utilizing other noninvasive modalities, such as dermoscopy, in combination with thorough skin examination also is critical in the early detection of skin cancers to improve the efficacy of this less-aggressive, nonablative, and cosmetically optimal treatment protocol.
Acknowledgement—We would like to acknowledge Dimitrios Karponis, BSc, from the Impirial College London, England, for his assistance with a portion of the statistical analysis.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas. part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Silverman MK, Kopf AW, Grin CM, et al. Recurrence rates of treated basal cell carcinomas. part 2: curettage-electrodesiccation. J Dermatol Surg Oncol. 1991;17:720-726.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Subramaniam P, Olsen CM, Thompson BS, et al. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol. 2017;153:175-182.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast.J Invest Dermatol. 1995;104:946-952.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Sattler E, Kästle R, Welzel J. Optical coherence tomography in dermatology. J Biomed Opt. 2013;18:061224.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Segura S, Puig S, Carrera C, et al. Non-invasive management of non-melanoma skin cancer in patients with cancer predisposition genodermatosis: a role for confocal microscopy and photodynamic therapy. J Eur Acad Dermatol Venereol. 2011;25:819-827.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:43-52.
- Couzan C, Cinotti E, Labeille B, et al. Reflectance confocal microscopy identification of subclinical basal cell carcinomas during and after vismodegib treatment. J Eur Acad Dermatol Venereol. 2018;32:763-767.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Wagner RF Jr, Cottel WI. Multifocal recurrent basal cell carcinoma following primary tumor treatment by electrodesiccation and curettage. J Am Acad Dermatol. 1987;17:1047-1049.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Lewin JM, Carucci JA. Advances in the management of basal cell carcinoma. F1000Prime Rep. 2015;7:53.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Olsen J, Themstrup L, De Carvalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
- Levine A, Siegel D, Markowitz O. Imaging in cutaneous surgery. Future Oncol. 2017;13:2329-2340.
- Gross K, Steinman H, Rapini R. Mohs Surgery: Fundamentals and Techniques. St. Louis, MO: Mosby; 1998.
- Suzuki HS, Serafini SZ, Sato MS. Utility of dermoscopy for demarcation of surgical margins in Mohs micrographic surgery. An Bras Dermatol. 2014;89:38-43.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431
Nonablative laser therapy is emerging as an effective noninvasive treatment option for basal cell carcinoma (BCC) with reduced adverse effects and good cosmetic outcomes compared to surgery. Vascular lasers, such as the pulsed dye laser (PDL), are thought to work by selectively targeting the tumor’s vascular network while preserving normal surrounding tissue.1,2 Although high energy and multiple passes might be required, adjunctive use of dynamic cooling reduces the risk for nonselective thermal injury vs ablative lasers, which destroy the tumor itself through vaporization of tissue water.2
With no established laser management guidelines for the treatment of BCC, earlier studies using a 595-nm PDL varied highly in their protocol.3-8 Pulsed dye laser parameters ranged from a spot size of 7 to 10 mm, fluence of 7.5 to 15 J/cm2, and pulse duration of 0.5 to 3 milliseconds. Follow-up ranged from 12 days to 25 months after the final laser treatment. The number of lesions in prior studies ranged from 7 to 100 BCCs, with the clinical clearance rate ranging from 71.4% to 75% for facial BCC and 78.6% to 95% for nonfacial BCC.3-8 Studies with histologic confirmation had a clearance rate of 66.6% for facial BCC and 25% to 92.3% for nonfacial BCC.3-5,7,8 Most studies examined BCCs on the trunk and extremities with few investigating facial BCC,3-8 which is especially important given that the head and neck are the most common and cosmetically sensitive anatomic locations.9-13
Noninvasive imaging devices, such as reflectance confocal microscopy (RCM) and optical coherence tomography (OCT) can assist with the diagnosis and treatment monitoring of BCC. These devices enable in vivo visualization of tissue in both cross-sectional and en face views and therefore can reduce the need for diagnostic biopsy. Reflectance confocal microscopy enables near-histologic visualization of the epidermis and superficial dermis with a resolution of 0.5 to 1 μm.14 Optical coherence tomography uses an infrared broadband light source that allows users to view skin architecture as deep as 1.5 to 2 mm with a resolution of 5 μm.15
When used synergistically, both devices can enhance the efficacy of nonablative laser treatment. With its increased depth and wider field of view, OCT is an optimal tool for repetitive evaluation of the same site over time and for following biopsy-confirmed tumors undergoing management.16 In addition to delineating tumor margins before treatment, imaging improves the detection of residual skin cancers, despite clearance on clinical and dermoscopic examination. Noninvasive imaging and nonsurgical management with laser therapy allow the physician to leave the skin intact and avoid scar tissue that might otherwise make it more difficult to detect and manage recurrence. The ability of OCT and RCM to monitor the efficacy of nonsurgical therapies for skin cancer has been demonstrated with imiquimod, photodynamic therapy, vismodegib, and ablative laser therapy.17-20
With limited data on nonablative laser management of BCC, several gaps in the literature exist. First, in previously published studies the number of treatments was either determined to be an arbitrary set number or based on clinical clearance, which has the potential to miss residual tumor. Second, many follow-ups were limited to shortly after the final treatment, which limits the accuracy of the clearance rate, given that inflammation and scars can hide residual tumor.21-23 Third, because many studies excised the treated area, long-term follow-up for recurrence was obscured. Last, only a few studies involved facial BCC, which is the most common and cosmetically concerning anatomic location.13
Our study attempted to address these gaps by evaluating the use of noninvasive imaging to guide management of primarily facial BCC. The objective was to perform a retrospective chart review on a subgroup of patients with BCC who were treated with combined nonablative PDL and fractional laser treatment with an extended follow-up period.
Methods
Study Design
We performed a retrospective chart review of 68 patients with 93 BCCs who had been treated with nonablative laser therapy as an alternative to surgery at the Mount Sinai Faculty Practice Associates between February 2011 and December 2018. Patients were followed throughout this period for assessment of clinical and subclinical recurrence. The Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects provided institutional review board approval.
Patients
Inclusion criteria included the following: (1) BCC diagnosed by biopsy (see eTable 1 for subtypes) and (2) treated with a nonablative laser due to patient preference and eligibility by the principal investigator (PI). As a retrospective study, lesions were included irrespective of tumor subtype or size. Although the risk for perineural invasion (PNI) is extremely low with BCC (<0.2%), none of the cases demonstrated PNI on diagnostic biopsy and none exhibited clinical evidence of PNI, such as paresthesia, pain, facial paralysis, or diplopia.24
Eligibility determined by the PI included limited clinical ulceration or bleeding, or both, and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). Patients who had Mohs micrographic surgery (MMS) or excision (or both) with recurrence at the treatment site were included. Detailed and thorough clinical and dermoscopic skin examination was critical in early detection of these cancers, allowing for treatment of less advanced tumors. The PI’s diagnostic approach utilized the published diagnostic color wheel algorithm,25 which encompasses both clinical and dermoscopic colors and patterns for early diagnosis (ie, ulceration, pink-white to white shiny areas, absence of pigmented network, leaflike structures, large blue-gray ovoid nests or globular structures, spoke wheel structures, a crystalline pattern, a singular vascular pattern of arborizing vessels), combined with OCT or RCM, when necessary.26 All lesions were imaged with OCT prior to laser treatment to confirm residual tumor following biopsy.
Although postsurgical patients were included, lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor (eg, vismodegib), were excluded.
Treatment Protocol
All patients received thorough information about the treatment, treatment alternatives, and potential adverse effects and complications. Lesions were selected based on clinical and dermoscopic findings and were biopsy confirmed. Clinical and dermoscopic photographs were taken at every visit. A camera was used for clinical photographs and a dermatoscope was attached for all contact polarized dermoscopic images. All lesions were imaged with OCT prior to laser therapy to delineate tumor margins and to confirm residual disease following biopsy to preclude biopsy-mediated regression.
Laser treatment consisted of a 595-nm PDL followed by fractional laser treatment with the 1927-nm setting. The range of PDL settings was similar to published studies of PDL for BCC (spot size, 7–10 mm; fluence, 6–15 J/cm2; pulse duration, 0.45–3 milliseconds).3-8 The fractional laser also was used at settings similar to earlier studies for actinic keratosis (fluence, 5–20 mJ; treatment density, 40%–70%).27 Laser treatment was performed by 1 of 5 medically trained providers who were fellows supervised by the PI.
All tumors received 1 to 7 treatments (average, 2.89) at 1- to 2-month intervals. Treatment end point (complete clearance) was judged on the absence of skin cancer clinically, dermoscopically on OCT, or histologically by biopsy, or a combination of these modalities. Recurrence was defined as a new histologically confirmed BCC occurring in an area that was previously documented as clear. Patients returned for follow-up 1 to 2 months after the final treatment to monitor tumor clearance and subsequently every 6 to 12 months for tumor recurrence. Posttreatment care included application of a thick emollient, such as a petrolatum-based product, until the area completely healed.
Data Collection
Clinical photographs, dermoscopic photographs, OCT scans, RCM scans, and biopsy reports were reviewed for each patient, as applicable. All patients were given an unidentifiable number; no protected health information was recorded. Data recorded for each patient included age, tumor subtype and location, tumor size, classification of the tumor as primary or a recurrence, number of treatments, treatment duration, lesion clearance, and length of follow-up.
Results
Patient and Lesion Characteristics
Sixty-eight patients with 93 BCCs (77 facial; 16 nonfacial) were included. The median age of patients was 70 years (range, 31–91 years). All 93 BCCs demonstrated residual tumor on OCT after diagnostic biopsy. Four BCCs had been treated earlier with MMS and were biopsy-proven recurrences. Most BCCs were of the nodular subtype; however, sclerosing, superficial, pigmented, morpheaform, and infiltrative subtypes also were included (eTable 1). Eight BCCs were obtained at outside institutions with no subtype provided. Facial BCCs had a mean (SD) clinical and dermoscopic diameter of 6.75 (4.71) mm (range, 2–24 mm). Patients were followed for 2.53 months to 6.03 years (mean follow-up, 2.43 years) and assessed for clinical and subclinical recurrence.

Tumor Clearance
Most lesions were effectively treated, with 89 of 93 BCCs (95.70%) demonstrating complete tumor clearance. Complete tumor clearance following laser therapy was reported in 74 of 77 facial BCCs (96.10%) and 15 of 16 nonfacial BCCs (93.75%)(eTable 2). Successfully treated BCCs underwent an average of 2.88 laser treatments over a mean duration of 3.54 months (range, 1 week to 1.92 years). Four incomplete responders underwent an average of 3.25 laser treatments over a mean duration of 3.44 months (range, 1.13–6.87 months). Of the 4 lesions that did not clear, 2 were nodular, 1 was pigmented, and 1 was sclerosing.

Number of Treatments
When the clearance rate is divided into lesions that received 3 or fewer laser treatments and those that received more than 3 laser treatments, the following results were determined:
• Lesions receiving 3 or fewer treatments had a clearance rate of 96.05% (73/76) for all BCCs, 96.72% (59/61) for facial BCCs, and 93.33% (14/15) for nonfacial BCCs.
• Lesions receiving more than 3 laser treatments had a clearance rate of 94.12% (16/17) for all BCCs, 93.75% (15/16) for facial BCCs, and 100% (1/1) for nonfacial BCCs.
The relationship between facial BCC tumor diameter and number of treatments required for clearance had a positive correlation coefficient (Pearson r=0.319), indicating that larger BCCs required more laser treatments (eTable 3).

Tumor Recurrence
Four of 89 BCCs (4.49%)(4 of 74 facial BCCs [5.41%]) showed tumor recurrence following laser treatment, as assessed by OCT and dermoscopy. Of them, all were nodular BCCs. Prior to laser treatment, there were 4 additional patients each diagnosed with a recurrence from prior treatment with MMS; all were successfully treated with laser therapy without recurrence post–laser treatment (eFigure 1). Most of the recurrences from prior MMS required more than 3 laser treatments before clearing: 1 required 3 treatments, 2 required 4 treatments, and 1 required 6 treatments.

Of 93 lesions included in this study, 2 BCCs were deemed not clear on histologic analysis, which corresponded with residual tumor seen on OCT. Two additional lesions were determined to be not clear on OCT but were not confirmed as such on biopsy; both lesions were confirmed not clear, however, by histologic analysis on the first layer of MMS
Follow-up
All cleared lesions (89/93) showed complete clinical response to laser treatment for 6 months or more (median follow-up, 2–3 years; mode, 1–2 years; mean, 2.66 years)(eTable 4). Although 45% of patients (40/89) have been followed clinically and/or dermoscopically (as is done for MMS follow-ups) for 3 years to more than 5 years, only 20% of patients (18/89) were followed up with OCT in combination with clinical and/or dermoscopic examination between 3 years and more than 5 years. Follow-up took on a bimodal distribution, with a peak follow-up period at 1 to 2 years and again at 3 to 4 years. Half of the lesions (45/89) were followed up with OCT in combination with clinical and dermoscopic examination at 1 to 6 months (eTable 5). Of the 2 patients with 1-month OCT follow-up, 1 died from other medical causes and the other was unable to return for further follow-up scans.


Comment
High Tumor Clearance Rates With OCT
This study yielded a clearance rate of 95.70% for all BCCs, 96.10% for facial BCCs, and 93.75% for nonfacial BCCs. This rate is higher than the clinical or histologic clearance rate (or both) of earlier studies on facial and nonfacial BCCs, which ranged from 25% to 95%.8-11 In this study, we were able to utilize OCT and histology to confirm clearance. Optical coherence tomography, which has been shown to have a high sensitivity ranging from 86% to 95.7%, is therefore optimally used in treatment monitoring.19,26,28 Optical coherence tomography has a broader specificity range of 75.3% to 98% and was not utilized for diagnostic purposes in this study. Combining OCT with a color wheel dermoscopic approach was helpful in confirming treatment efficacy of nonsurgical therapies and is significantly more accurate than clinical analysis alone (P<.01).19,26,28
We suspect that the higher clearance rates observed in our study were due to the OCT-guided treatment protocol. Optical coherence tomography was used for margination while providing a modality for tailored treatment through visualization of residual tumor on clinically and dermoscopically clear follow-ups, given that several studies found residual tumor at the lateral edge of the tumor margin on histopathologic analysis.5 Utilizing noninvasive imaging technology to delineate tumor margins before treatment can improve efficacy and limit unnecessary treatment to the surrounding normal skin (eFigure 2).29

After grouping lesions by number of laser treatments, the clearance rate remained similar among facial BCCs with 3 or fewer treatments (59/61 [96.72%]), but there was a slightly decreased clearance rate for facial BCCs with more than 3 treatments (15/16 [93.75%]), which may be explained by the need for more laser treatments for larger BCCs (eTable 3). The relationship between facial BCC size and number of laser treatments was found to correlate positively (Pearson r=0.319). The largest lesion (24 mm) was successfully treated with 5 treatments (Figure). The number of nonfacial lesions was limited in this study and was not statistically significant.

there was no clinical evidence of residual BCC.
Cosmetic Outcome
Adverse effects, including erythema, purpura, blistering, and crusting, were short-term and well tolerated. Few patients had subsequent hypopigmentation in the initial months after treatment, which we consider an optimal cosmetic outcome. For example, the patient shown in the Figure would have required extensive reconstruction of the defect using bilateral rotation flaps with incisions along the hairline, grafting, or second-intention healing with partial closure to avoid brow-lifting.30 Given the relatively young age of this patient (a 45-year-old woman) and therefore limited skin laxity, secondary intention or even attempting to match grafted tissue could have resulted in a less than optimal cosmetic outcome. None of the patients experienced clinical or dermoscopic evidence of scarring from the laser treatment.
A few lesions were found to have subclinical inflammation on OCT, which might have obscured residual tumor on the 1-month follow-up scan. This condition may be similar to how pre-MMS diagnostic biopsy scars mask skin cancer during surgery, making it necessary to obtain additional layers beyond the biopsy scar tissue. This scar tissue would otherwise obscure tumor on histology during MMS, similar to subclinical inflammation obscuring residual tumor on OCT.21-23,31 Invasive and noninvasive management of skin cancers will have different healing times and therefore different optimal times to confirm clearance by histology compared to noninvasive imaging. All of the lesions in which inflammation was obscured on OCT 1-month posttreatment remained cleared. However, 1 lesion was found to be clear at a 4-week clearance scan after only 2 nonablative laser treatments and was confirmed as scar tissue on histology. Scar tissue on histology might have obscured any residual tumor. The patient appeared clinically and dermoscopically to have a milia in the same location only 5 months later; however, on OCT and histology, the lesion was confirmed to be a BCC.
Treatment Intervals
Several other studies either used a set number of treatments or determined the number of treatments based on clinical clearance.3-8 When determining the best treatment interval, we considered the period for patients to be clinically and dermoscopically healed to be 1 month. Patients came for their final follow-up scan an additional month after the final treatment in case there was any obscuring inflammation on OCT at 1 month. Given that patients responded well to nonablative laser treatment once skin clinically healed and most patients required 3 treatments, the PI began recommending a total of 3 treatments performed 4 to 6 weeks apart in clinical practice, followed by a final clearance scan 2 months after the third treatment. A period of 2 months was considered ideal for the final clearance scan because no inflammation was seen at the 2-month follow-up in the group of patients who had inflammation at the 1-month follow-up on OCT in our study. Some patients had an extended treatment duration because of noncompliance with the 4- to 6-week follow-up regimen. Although this extension of treatment duration potentially skews the clearance rate, we still included these patients, given the retrospective design of this study.
Lesions That Did Not Clear
Four BCCs did not clear, 3 of which were facial BCCs. All 4 lesions demonstrated residual tumor on OCT. Of the 3 facial lesions that did not clear:
• One was the patient who had obscuring inflammation at the 1-month follow-up and only scar tissue on histologic confirmation.
• Another was a pigmented BCC on the right cheek of a patient with Fitzpatrick skin type IV. This patient received 3 treatments without a response clinically or on OCT. (Most patients who showed complete clearance also showed reduction in tumor size after the first laser treatment. Of note, there were other patients who had lighter skin types with pigmented BCCs and all of these patients had complete response to this treatment regimen; therefore, we do not think that a pigmented BCC is an exclusion to this therapy.)
• The third was a BCC on the nose of a nonadherent patient, which may have contributed to the lack of clearance. We defined nonadherent patients as those who did not follow-up within the appropriate periods and who therefore ran the risk for tumor growth in between treatments.
The nonfacial BCC that did not clear had histologic features of focal sclerosing BCC, a more aggressive subtype of basal cell skin cancer.
Tumor Recurrence
Only 4 of 89 BCCs (4.49%) recurred, with a 5.41% (4/74) recurrence rate among facial BCCs. All recurrences lacked clinical and dermoscopic evidence of BCC but were found on follow-up OCT scan and confirmed with RCM. All recurrences were found 1.5 to 3.9 years posttreatment.
Recurrent tumors following MMS required, on average, more laser treatments than primary tumors to achieve successful tumor clearance, which we attribute to scar tissue from prior therapy obscuring recurrence, resulting in delayed diagnosis, and to inflammation and fibrosis masking residual tumors (eFigure 1). An added benefit of laser treatment is that all 4 recurrent tumors demonstrated improved cosmetic appearance of the original MMS scar.
The benefit of using OCT scans to check for recurrences is that OCT can find residual skin cancers despite the area looking clinically clear, which is especially important during clinical evaluation of a healed postsurgical scar for recurrence because OCT imaging allows us to look as deep as 2 mm under the skin. Nonsurgical treatments also enable us to leave skin intact and avoid creating scar tissue, which makes it easier to detect and manage recurrence.
Limitations
There were several important limitations of this retrospective study:
• Patients were treated by 1 of 5 medically trained fellows. Although the fellows worked under the supervision of the PI, variation in their work from one to another might have led to different end points.
• All patients who appeared clinically clear were offered biopsy to confirm clearance on histology. Some patients agreed to biopsy, but many did not because they were pleased with the cosmetic outcome, which is similar to other studies exhibiting only clinical clearance rates without providing histologic clearance following nonsurgical therapy.6 We believe that imaging with OCT circumvents this problem and offers more accurate confirmation than clinical or dermoscopic correlation alone, or the combination of the 2 modalities.
• Lack of treatment standardization and short length of follow-up can result in underestimation of the recurrence rate. In particular, most patients were followed up with OCT in less than 6 months. These are unavoidable features in a retrospective study and we are currently addressing this problem in a new prospective study.
Extended Follow-up
Although this study is not a prospective design, it does provide recurrence data over extended follow-up for the nonablative laser management of BCCs (eTables 4 and 5). Studies have demonstrated that MMS has a 5-year cure rate as high as 99% for BCC.32 Given the limited follow-up period of prior nonablative laser management studies, recurrences might not have been fully evaluated. Our study had a 4.49% recurrence rate for all BCCs and a 5.41% recurrence rate for facial BCCs but was not detectable by clinical examination combined with dermoscopic findings alone. All recurrences required the utilization of OCT or RCM or a combination of these modalities to be diagnosed. In 1 patient with recurrence, we were able to see residual tumor on both OCT and RCM without any inflammation obscuring the scan, given that 3 years had passed. Although 2 months is an optimal follow-up time for OCT, we have not found an optimal follow-up time for RCM, which is another reason why OCT might be preferable to other imaging modalities, such as RCM and high-definition OCT, that have higher resolution but provide less depth on imaging. Although only 40 of 89 patients (4.49%) had follow-up ranging from 3 years to greater than 5 years, long-term follow-up to date has been limited in prior studies.
We believe the high clearance rates and limited recurrence are secondary to the utilization of noninvasive imaging, as the majority of these recurrences would not have been diagnosed based on clinical and/or dermoscopic information alone. Additionally, the 4 biopsy-proven post-MMS recurrence patients that were treated in this study also may not have been diagnosed this early without the use of additional noninvasive imaging. In our opinion, although laser management can be used without noninvasive imaging guidance—dermoscopy, OCT, and/or RCM—this technology is critical not only for early detection but also for proper management of patients.
Conclusion
This study showed a 95.70% clearance rate for all BCCs and a 96.10% clearance rate for facial BCCs. Although we had a zero clinical recurrence rate, 4.49% of all BCCs and 5.41% of facial BCCs had recurred on subsequent monitoring with noninvasive imaging. Given the large size of the study and extended follow-up, we found nonablative laser management to be a reliable treatment alternative with improved cosmetic outcome (Figure) and minimal short-term adverse effects compared to surgery.
Tailored care for the individual patient is based on a variety of options and patient preference, including ease of compliance, number of follow-up visits, invasive vs noninvasive diagnosis and monitoring, and downtime for healing. The use of noninvasive imaging also allowed us to find a more standardized treatment regimen using this nonablative laser combination. We found that 3 or fewer and more than 3 treatments had similar efficacy in tumor clearance. We recommend a standard laser protocol of 3 treatments every 4 to 6 weeks with follow-up 2 months after the final treatment to assess for clearance with OCT.
Larger BCCs might require additional treatments; therefore, we caution against laser therapy without concomitant use of OCT imaging to visualize residual tumor. Utilizing other noninvasive modalities, such as dermoscopy, in combination with thorough skin examination also is critical in the early detection of skin cancers to improve the efficacy of this less-aggressive, nonablative, and cosmetically optimal treatment protocol.
Acknowledgement—We would like to acknowledge Dimitrios Karponis, BSc, from the Impirial College London, England, for his assistance with a portion of the statistical analysis.
Nonablative laser therapy is emerging as an effective noninvasive treatment option for basal cell carcinoma (BCC) with reduced adverse effects and good cosmetic outcomes compared to surgery. Vascular lasers, such as the pulsed dye laser (PDL), are thought to work by selectively targeting the tumor’s vascular network while preserving normal surrounding tissue.1,2 Although high energy and multiple passes might be required, adjunctive use of dynamic cooling reduces the risk for nonselective thermal injury vs ablative lasers, which destroy the tumor itself through vaporization of tissue water.2
With no established laser management guidelines for the treatment of BCC, earlier studies using a 595-nm PDL varied highly in their protocol.3-8 Pulsed dye laser parameters ranged from a spot size of 7 to 10 mm, fluence of 7.5 to 15 J/cm2, and pulse duration of 0.5 to 3 milliseconds. Follow-up ranged from 12 days to 25 months after the final laser treatment. The number of lesions in prior studies ranged from 7 to 100 BCCs, with the clinical clearance rate ranging from 71.4% to 75% for facial BCC and 78.6% to 95% for nonfacial BCC.3-8 Studies with histologic confirmation had a clearance rate of 66.6% for facial BCC and 25% to 92.3% for nonfacial BCC.3-5,7,8 Most studies examined BCCs on the trunk and extremities with few investigating facial BCC,3-8 which is especially important given that the head and neck are the most common and cosmetically sensitive anatomic locations.9-13
Noninvasive imaging devices, such as reflectance confocal microscopy (RCM) and optical coherence tomography (OCT) can assist with the diagnosis and treatment monitoring of BCC. These devices enable in vivo visualization of tissue in both cross-sectional and en face views and therefore can reduce the need for diagnostic biopsy. Reflectance confocal microscopy enables near-histologic visualization of the epidermis and superficial dermis with a resolution of 0.5 to 1 μm.14 Optical coherence tomography uses an infrared broadband light source that allows users to view skin architecture as deep as 1.5 to 2 mm with a resolution of 5 μm.15
When used synergistically, both devices can enhance the efficacy of nonablative laser treatment. With its increased depth and wider field of view, OCT is an optimal tool for repetitive evaluation of the same site over time and for following biopsy-confirmed tumors undergoing management.16 In addition to delineating tumor margins before treatment, imaging improves the detection of residual skin cancers, despite clearance on clinical and dermoscopic examination. Noninvasive imaging and nonsurgical management with laser therapy allow the physician to leave the skin intact and avoid scar tissue that might otherwise make it more difficult to detect and manage recurrence. The ability of OCT and RCM to monitor the efficacy of nonsurgical therapies for skin cancer has been demonstrated with imiquimod, photodynamic therapy, vismodegib, and ablative laser therapy.17-20
With limited data on nonablative laser management of BCC, several gaps in the literature exist. First, in previously published studies the number of treatments was either determined to be an arbitrary set number or based on clinical clearance, which has the potential to miss residual tumor. Second, many follow-ups were limited to shortly after the final treatment, which limits the accuracy of the clearance rate, given that inflammation and scars can hide residual tumor.21-23 Third, because many studies excised the treated area, long-term follow-up for recurrence was obscured. Last, only a few studies involved facial BCC, which is the most common and cosmetically concerning anatomic location.13
Our study attempted to address these gaps by evaluating the use of noninvasive imaging to guide management of primarily facial BCC. The objective was to perform a retrospective chart review on a subgroup of patients with BCC who were treated with combined nonablative PDL and fractional laser treatment with an extended follow-up period.
Methods
Study Design
We performed a retrospective chart review of 68 patients with 93 BCCs who had been treated with nonablative laser therapy as an alternative to surgery at the Mount Sinai Faculty Practice Associates between February 2011 and December 2018. Patients were followed throughout this period for assessment of clinical and subclinical recurrence. The Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects provided institutional review board approval.
Patients
Inclusion criteria included the following: (1) BCC diagnosed by biopsy (see eTable 1 for subtypes) and (2) treated with a nonablative laser due to patient preference and eligibility by the principal investigator (PI). As a retrospective study, lesions were included irrespective of tumor subtype or size. Although the risk for perineural invasion (PNI) is extremely low with BCC (<0.2%), none of the cases demonstrated PNI on diagnostic biopsy and none exhibited clinical evidence of PNI, such as paresthesia, pain, facial paralysis, or diplopia.24
Eligibility determined by the PI included limited clinical ulceration or bleeding, or both, and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). Patients who had Mohs micrographic surgery (MMS) or excision (or both) with recurrence at the treatment site were included. Detailed and thorough clinical and dermoscopic skin examination was critical in early detection of these cancers, allowing for treatment of less advanced tumors. The PI’s diagnostic approach utilized the published diagnostic color wheel algorithm,25 which encompasses both clinical and dermoscopic colors and patterns for early diagnosis (ie, ulceration, pink-white to white shiny areas, absence of pigmented network, leaflike structures, large blue-gray ovoid nests or globular structures, spoke wheel structures, a crystalline pattern, a singular vascular pattern of arborizing vessels), combined with OCT or RCM, when necessary.26 All lesions were imaged with OCT prior to laser treatment to confirm residual tumor following biopsy.
Although postsurgical patients were included, lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor (eg, vismodegib), were excluded.
Treatment Protocol
All patients received thorough information about the treatment, treatment alternatives, and potential adverse effects and complications. Lesions were selected based on clinical and dermoscopic findings and were biopsy confirmed. Clinical and dermoscopic photographs were taken at every visit. A camera was used for clinical photographs and a dermatoscope was attached for all contact polarized dermoscopic images. All lesions were imaged with OCT prior to laser therapy to delineate tumor margins and to confirm residual disease following biopsy to preclude biopsy-mediated regression.
Laser treatment consisted of a 595-nm PDL followed by fractional laser treatment with the 1927-nm setting. The range of PDL settings was similar to published studies of PDL for BCC (spot size, 7–10 mm; fluence, 6–15 J/cm2; pulse duration, 0.45–3 milliseconds).3-8 The fractional laser also was used at settings similar to earlier studies for actinic keratosis (fluence, 5–20 mJ; treatment density, 40%–70%).27 Laser treatment was performed by 1 of 5 medically trained providers who were fellows supervised by the PI.
All tumors received 1 to 7 treatments (average, 2.89) at 1- to 2-month intervals. Treatment end point (complete clearance) was judged on the absence of skin cancer clinically, dermoscopically on OCT, or histologically by biopsy, or a combination of these modalities. Recurrence was defined as a new histologically confirmed BCC occurring in an area that was previously documented as clear. Patients returned for follow-up 1 to 2 months after the final treatment to monitor tumor clearance and subsequently every 6 to 12 months for tumor recurrence. Posttreatment care included application of a thick emollient, such as a petrolatum-based product, until the area completely healed.
Data Collection
Clinical photographs, dermoscopic photographs, OCT scans, RCM scans, and biopsy reports were reviewed for each patient, as applicable. All patients were given an unidentifiable number; no protected health information was recorded. Data recorded for each patient included age, tumor subtype and location, tumor size, classification of the tumor as primary or a recurrence, number of treatments, treatment duration, lesion clearance, and length of follow-up.
Results
Patient and Lesion Characteristics
Sixty-eight patients with 93 BCCs (77 facial; 16 nonfacial) were included. The median age of patients was 70 years (range, 31–91 years). All 93 BCCs demonstrated residual tumor on OCT after diagnostic biopsy. Four BCCs had been treated earlier with MMS and were biopsy-proven recurrences. Most BCCs were of the nodular subtype; however, sclerosing, superficial, pigmented, morpheaform, and infiltrative subtypes also were included (eTable 1). Eight BCCs were obtained at outside institutions with no subtype provided. Facial BCCs had a mean (SD) clinical and dermoscopic diameter of 6.75 (4.71) mm (range, 2–24 mm). Patients were followed for 2.53 months to 6.03 years (mean follow-up, 2.43 years) and assessed for clinical and subclinical recurrence.

Tumor Clearance
Most lesions were effectively treated, with 89 of 93 BCCs (95.70%) demonstrating complete tumor clearance. Complete tumor clearance following laser therapy was reported in 74 of 77 facial BCCs (96.10%) and 15 of 16 nonfacial BCCs (93.75%)(eTable 2). Successfully treated BCCs underwent an average of 2.88 laser treatments over a mean duration of 3.54 months (range, 1 week to 1.92 years). Four incomplete responders underwent an average of 3.25 laser treatments over a mean duration of 3.44 months (range, 1.13–6.87 months). Of the 4 lesions that did not clear, 2 were nodular, 1 was pigmented, and 1 was sclerosing.

Number of Treatments
When the clearance rate is divided into lesions that received 3 or fewer laser treatments and those that received more than 3 laser treatments, the following results were determined:
• Lesions receiving 3 or fewer treatments had a clearance rate of 96.05% (73/76) for all BCCs, 96.72% (59/61) for facial BCCs, and 93.33% (14/15) for nonfacial BCCs.
• Lesions receiving more than 3 laser treatments had a clearance rate of 94.12% (16/17) for all BCCs, 93.75% (15/16) for facial BCCs, and 100% (1/1) for nonfacial BCCs.
The relationship between facial BCC tumor diameter and number of treatments required for clearance had a positive correlation coefficient (Pearson r=0.319), indicating that larger BCCs required more laser treatments (eTable 3).

Tumor Recurrence
Four of 89 BCCs (4.49%)(4 of 74 facial BCCs [5.41%]) showed tumor recurrence following laser treatment, as assessed by OCT and dermoscopy. Of them, all were nodular BCCs. Prior to laser treatment, there were 4 additional patients each diagnosed with a recurrence from prior treatment with MMS; all were successfully treated with laser therapy without recurrence post–laser treatment (eFigure 1). Most of the recurrences from prior MMS required more than 3 laser treatments before clearing: 1 required 3 treatments, 2 required 4 treatments, and 1 required 6 treatments.

Of 93 lesions included in this study, 2 BCCs were deemed not clear on histologic analysis, which corresponded with residual tumor seen on OCT. Two additional lesions were determined to be not clear on OCT but were not confirmed as such on biopsy; both lesions were confirmed not clear, however, by histologic analysis on the first layer of MMS
Follow-up
All cleared lesions (89/93) showed complete clinical response to laser treatment for 6 months or more (median follow-up, 2–3 years; mode, 1–2 years; mean, 2.66 years)(eTable 4). Although 45% of patients (40/89) have been followed clinically and/or dermoscopically (as is done for MMS follow-ups) for 3 years to more than 5 years, only 20% of patients (18/89) were followed up with OCT in combination with clinical and/or dermoscopic examination between 3 years and more than 5 years. Follow-up took on a bimodal distribution, with a peak follow-up period at 1 to 2 years and again at 3 to 4 years. Half of the lesions (45/89) were followed up with OCT in combination with clinical and dermoscopic examination at 1 to 6 months (eTable 5). Of the 2 patients with 1-month OCT follow-up, 1 died from other medical causes and the other was unable to return for further follow-up scans.


Comment
High Tumor Clearance Rates With OCT
This study yielded a clearance rate of 95.70% for all BCCs, 96.10% for facial BCCs, and 93.75% for nonfacial BCCs. This rate is higher than the clinical or histologic clearance rate (or both) of earlier studies on facial and nonfacial BCCs, which ranged from 25% to 95%.8-11 In this study, we were able to utilize OCT and histology to confirm clearance. Optical coherence tomography, which has been shown to have a high sensitivity ranging from 86% to 95.7%, is therefore optimally used in treatment monitoring.19,26,28 Optical coherence tomography has a broader specificity range of 75.3% to 98% and was not utilized for diagnostic purposes in this study. Combining OCT with a color wheel dermoscopic approach was helpful in confirming treatment efficacy of nonsurgical therapies and is significantly more accurate than clinical analysis alone (P<.01).19,26,28
We suspect that the higher clearance rates observed in our study were due to the OCT-guided treatment protocol. Optical coherence tomography was used for margination while providing a modality for tailored treatment through visualization of residual tumor on clinically and dermoscopically clear follow-ups, given that several studies found residual tumor at the lateral edge of the tumor margin on histopathologic analysis.5 Utilizing noninvasive imaging technology to delineate tumor margins before treatment can improve efficacy and limit unnecessary treatment to the surrounding normal skin (eFigure 2).29

After grouping lesions by number of laser treatments, the clearance rate remained similar among facial BCCs with 3 or fewer treatments (59/61 [96.72%]), but there was a slightly decreased clearance rate for facial BCCs with more than 3 treatments (15/16 [93.75%]), which may be explained by the need for more laser treatments for larger BCCs (eTable 3). The relationship between facial BCC size and number of laser treatments was found to correlate positively (Pearson r=0.319). The largest lesion (24 mm) was successfully treated with 5 treatments (Figure). The number of nonfacial lesions was limited in this study and was not statistically significant.

there was no clinical evidence of residual BCC.
Cosmetic Outcome
Adverse effects, including erythema, purpura, blistering, and crusting, were short-term and well tolerated. Few patients had subsequent hypopigmentation in the initial months after treatment, which we consider an optimal cosmetic outcome. For example, the patient shown in the Figure would have required extensive reconstruction of the defect using bilateral rotation flaps with incisions along the hairline, grafting, or second-intention healing with partial closure to avoid brow-lifting.30 Given the relatively young age of this patient (a 45-year-old woman) and therefore limited skin laxity, secondary intention or even attempting to match grafted tissue could have resulted in a less than optimal cosmetic outcome. None of the patients experienced clinical or dermoscopic evidence of scarring from the laser treatment.
A few lesions were found to have subclinical inflammation on OCT, which might have obscured residual tumor on the 1-month follow-up scan. This condition may be similar to how pre-MMS diagnostic biopsy scars mask skin cancer during surgery, making it necessary to obtain additional layers beyond the biopsy scar tissue. This scar tissue would otherwise obscure tumor on histology during MMS, similar to subclinical inflammation obscuring residual tumor on OCT.21-23,31 Invasive and noninvasive management of skin cancers will have different healing times and therefore different optimal times to confirm clearance by histology compared to noninvasive imaging. All of the lesions in which inflammation was obscured on OCT 1-month posttreatment remained cleared. However, 1 lesion was found to be clear at a 4-week clearance scan after only 2 nonablative laser treatments and was confirmed as scar tissue on histology. Scar tissue on histology might have obscured any residual tumor. The patient appeared clinically and dermoscopically to have a milia in the same location only 5 months later; however, on OCT and histology, the lesion was confirmed to be a BCC.
Treatment Intervals
Several other studies either used a set number of treatments or determined the number of treatments based on clinical clearance.3-8 When determining the best treatment interval, we considered the period for patients to be clinically and dermoscopically healed to be 1 month. Patients came for their final follow-up scan an additional month after the final treatment in case there was any obscuring inflammation on OCT at 1 month. Given that patients responded well to nonablative laser treatment once skin clinically healed and most patients required 3 treatments, the PI began recommending a total of 3 treatments performed 4 to 6 weeks apart in clinical practice, followed by a final clearance scan 2 months after the third treatment. A period of 2 months was considered ideal for the final clearance scan because no inflammation was seen at the 2-month follow-up in the group of patients who had inflammation at the 1-month follow-up on OCT in our study. Some patients had an extended treatment duration because of noncompliance with the 4- to 6-week follow-up regimen. Although this extension of treatment duration potentially skews the clearance rate, we still included these patients, given the retrospective design of this study.
Lesions That Did Not Clear
Four BCCs did not clear, 3 of which were facial BCCs. All 4 lesions demonstrated residual tumor on OCT. Of the 3 facial lesions that did not clear:
• One was the patient who had obscuring inflammation at the 1-month follow-up and only scar tissue on histologic confirmation.
• Another was a pigmented BCC on the right cheek of a patient with Fitzpatrick skin type IV. This patient received 3 treatments without a response clinically or on OCT. (Most patients who showed complete clearance also showed reduction in tumor size after the first laser treatment. Of note, there were other patients who had lighter skin types with pigmented BCCs and all of these patients had complete response to this treatment regimen; therefore, we do not think that a pigmented BCC is an exclusion to this therapy.)
• The third was a BCC on the nose of a nonadherent patient, which may have contributed to the lack of clearance. We defined nonadherent patients as those who did not follow-up within the appropriate periods and who therefore ran the risk for tumor growth in between treatments.
The nonfacial BCC that did not clear had histologic features of focal sclerosing BCC, a more aggressive subtype of basal cell skin cancer.
Tumor Recurrence
Only 4 of 89 BCCs (4.49%) recurred, with a 5.41% (4/74) recurrence rate among facial BCCs. All recurrences lacked clinical and dermoscopic evidence of BCC but were found on follow-up OCT scan and confirmed with RCM. All recurrences were found 1.5 to 3.9 years posttreatment.
Recurrent tumors following MMS required, on average, more laser treatments than primary tumors to achieve successful tumor clearance, which we attribute to scar tissue from prior therapy obscuring recurrence, resulting in delayed diagnosis, and to inflammation and fibrosis masking residual tumors (eFigure 1). An added benefit of laser treatment is that all 4 recurrent tumors demonstrated improved cosmetic appearance of the original MMS scar.
The benefit of using OCT scans to check for recurrences is that OCT can find residual skin cancers despite the area looking clinically clear, which is especially important during clinical evaluation of a healed postsurgical scar for recurrence because OCT imaging allows us to look as deep as 2 mm under the skin. Nonsurgical treatments also enable us to leave skin intact and avoid creating scar tissue, which makes it easier to detect and manage recurrence.
Limitations
There were several important limitations of this retrospective study:
• Patients were treated by 1 of 5 medically trained fellows. Although the fellows worked under the supervision of the PI, variation in their work from one to another might have led to different end points.
• All patients who appeared clinically clear were offered biopsy to confirm clearance on histology. Some patients agreed to biopsy, but many did not because they were pleased with the cosmetic outcome, which is similar to other studies exhibiting only clinical clearance rates without providing histologic clearance following nonsurgical therapy.6 We believe that imaging with OCT circumvents this problem and offers more accurate confirmation than clinical or dermoscopic correlation alone, or the combination of the 2 modalities.
• Lack of treatment standardization and short length of follow-up can result in underestimation of the recurrence rate. In particular, most patients were followed up with OCT in less than 6 months. These are unavoidable features in a retrospective study and we are currently addressing this problem in a new prospective study.
Extended Follow-up
Although this study is not a prospective design, it does provide recurrence data over extended follow-up for the nonablative laser management of BCCs (eTables 4 and 5). Studies have demonstrated that MMS has a 5-year cure rate as high as 99% for BCC.32 Given the limited follow-up period of prior nonablative laser management studies, recurrences might not have been fully evaluated. Our study had a 4.49% recurrence rate for all BCCs and a 5.41% recurrence rate for facial BCCs but was not detectable by clinical examination combined with dermoscopic findings alone. All recurrences required the utilization of OCT or RCM or a combination of these modalities to be diagnosed. In 1 patient with recurrence, we were able to see residual tumor on both OCT and RCM without any inflammation obscuring the scan, given that 3 years had passed. Although 2 months is an optimal follow-up time for OCT, we have not found an optimal follow-up time for RCM, which is another reason why OCT might be preferable to other imaging modalities, such as RCM and high-definition OCT, that have higher resolution but provide less depth on imaging. Although only 40 of 89 patients (4.49%) had follow-up ranging from 3 years to greater than 5 years, long-term follow-up to date has been limited in prior studies.
We believe the high clearance rates and limited recurrence are secondary to the utilization of noninvasive imaging, as the majority of these recurrences would not have been diagnosed based on clinical and/or dermoscopic information alone. Additionally, the 4 biopsy-proven post-MMS recurrence patients that were treated in this study also may not have been diagnosed this early without the use of additional noninvasive imaging. In our opinion, although laser management can be used without noninvasive imaging guidance—dermoscopy, OCT, and/or RCM—this technology is critical not only for early detection but also for proper management of patients.
Conclusion
This study showed a 95.70% clearance rate for all BCCs and a 96.10% clearance rate for facial BCCs. Although we had a zero clinical recurrence rate, 4.49% of all BCCs and 5.41% of facial BCCs had recurred on subsequent monitoring with noninvasive imaging. Given the large size of the study and extended follow-up, we found nonablative laser management to be a reliable treatment alternative with improved cosmetic outcome (Figure) and minimal short-term adverse effects compared to surgery.
Tailored care for the individual patient is based on a variety of options and patient preference, including ease of compliance, number of follow-up visits, invasive vs noninvasive diagnosis and monitoring, and downtime for healing. The use of noninvasive imaging also allowed us to find a more standardized treatment regimen using this nonablative laser combination. We found that 3 or fewer and more than 3 treatments had similar efficacy in tumor clearance. We recommend a standard laser protocol of 3 treatments every 4 to 6 weeks with follow-up 2 months after the final treatment to assess for clearance with OCT.
Larger BCCs might require additional treatments; therefore, we caution against laser therapy without concomitant use of OCT imaging to visualize residual tumor. Utilizing other noninvasive modalities, such as dermoscopy, in combination with thorough skin examination also is critical in the early detection of skin cancers to improve the efficacy of this less-aggressive, nonablative, and cosmetically optimal treatment protocol.
Acknowledgement—We would like to acknowledge Dimitrios Karponis, BSc, from the Impirial College London, England, for his assistance with a portion of the statistical analysis.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas. part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Silverman MK, Kopf AW, Grin CM, et al. Recurrence rates of treated basal cell carcinomas. part 2: curettage-electrodesiccation. J Dermatol Surg Oncol. 1991;17:720-726.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Subramaniam P, Olsen CM, Thompson BS, et al. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol. 2017;153:175-182.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast.J Invest Dermatol. 1995;104:946-952.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Sattler E, Kästle R, Welzel J. Optical coherence tomography in dermatology. J Biomed Opt. 2013;18:061224.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Segura S, Puig S, Carrera C, et al. Non-invasive management of non-melanoma skin cancer in patients with cancer predisposition genodermatosis: a role for confocal microscopy and photodynamic therapy. J Eur Acad Dermatol Venereol. 2011;25:819-827.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:43-52.
- Couzan C, Cinotti E, Labeille B, et al. Reflectance confocal microscopy identification of subclinical basal cell carcinomas during and after vismodegib treatment. J Eur Acad Dermatol Venereol. 2018;32:763-767.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Wagner RF Jr, Cottel WI. Multifocal recurrent basal cell carcinoma following primary tumor treatment by electrodesiccation and curettage. J Am Acad Dermatol. 1987;17:1047-1049.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Lewin JM, Carucci JA. Advances in the management of basal cell carcinoma. F1000Prime Rep. 2015;7:53.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Olsen J, Themstrup L, De Carvalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
- Levine A, Siegel D, Markowitz O. Imaging in cutaneous surgery. Future Oncol. 2017;13:2329-2340.
- Gross K, Steinman H, Rapini R. Mohs Surgery: Fundamentals and Techniques. St. Louis, MO: Mosby; 1998.
- Suzuki HS, Serafini SZ, Sato MS. Utility of dermoscopy for demarcation of surgical margins in Mohs micrographic surgery. An Bras Dermatol. 2014;89:38-43.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas. part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Silverman MK, Kopf AW, Grin CM, et al. Recurrence rates of treated basal cell carcinomas. part 2: curettage-electrodesiccation. J Dermatol Surg Oncol. 1991;17:720-726.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Subramaniam P, Olsen CM, Thompson BS, et al. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol. 2017;153:175-182.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast.J Invest Dermatol. 1995;104:946-952.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Sattler E, Kästle R, Welzel J. Optical coherence tomography in dermatology. J Biomed Opt. 2013;18:061224.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Segura S, Puig S, Carrera C, et al. Non-invasive management of non-melanoma skin cancer in patients with cancer predisposition genodermatosis: a role for confocal microscopy and photodynamic therapy. J Eur Acad Dermatol Venereol. 2011;25:819-827.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:43-52.
- Couzan C, Cinotti E, Labeille B, et al. Reflectance confocal microscopy identification of subclinical basal cell carcinomas during and after vismodegib treatment. J Eur Acad Dermatol Venereol. 2018;32:763-767.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Wagner RF Jr, Cottel WI. Multifocal recurrent basal cell carcinoma following primary tumor treatment by electrodesiccation and curettage. J Am Acad Dermatol. 1987;17:1047-1049.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Lewin JM, Carucci JA. Advances in the management of basal cell carcinoma. F1000Prime Rep. 2015;7:53.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Olsen J, Themstrup L, De Carvalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
- Levine A, Siegel D, Markowitz O. Imaging in cutaneous surgery. Future Oncol. 2017;13:2329-2340.
- Gross K, Steinman H, Rapini R. Mohs Surgery: Fundamentals and Techniques. St. Louis, MO: Mosby; 1998.
- Suzuki HS, Serafini SZ, Sato MS. Utility of dermoscopy for demarcation of surgical margins in Mohs micrographic surgery. An Bras Dermatol. 2014;89:38-43.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431
Practice Points
- A major benefit of nonablative laser therapy over more invasive options in the management of basal cell carcinoma (BCC) is minimal scarring.
- When patients are managed with nonablative laser therapy, follow-up with clinical, dermoscopic, and/or noninvasive imaging is more efficient during treatment as well as when assessing for recurrences.
- Optical coherence tomography in combination with nonablative laser therapy allows for detection of residual skin cancers that would not be evident on clinical and/or dermoscopic examination.
- Utilizing early detection techniques, such as a color wheel dermoscopic approach, along with other noninvasive imaging modalities facilitates the use of less invasive treatment options for primary and/or recurrent BCCs.
New Diagnostic Procedure Codes and Reimbursement
As the US population continues to grow and patients become more aware of their health needs, payers are beginning to recognize the benefits of more efficient and cost-effective health care. With the implementation of the new Medicare Physician Fee Schedule on January 1, 2019, some old billing codes were revalued while others were replaced entirely with new codes.1 The restructuring of the standard biopsy codes now takes the complexity of different sampling techniques into consideration. Furthermore, Current Procedural Terminology (CPT) Category III tracking codes for some imaging devices (eg, optical coherence tomography) added in 2017 require more data before obtaining a Category I reimbursable code, while codes for other imaging devices such as reflectance confocal microscopy (RCM) remain relatively the same.2-4 Notably, the majority of the new 2019 telemedicine codes are applicable to dermatology.2,3 In this article, we discuss the new CPT codes for reporting diagnostic procedures, including biopsy, noninvasive imaging, and telemedicine services. We also provide a summary of the national average reimbursement rates for these procedures.
Background on Reimbursement
To better understand how reimbursement works, it is important to know that all billing codes are provided a relative value unit (RVU), a number representing the value of the work involved and cost of providing a service relative to other services.5 The total RVU consists of the work RVU (wRVU), practice expense RVU (peRVU), and malpractice expense RVU (mRVU). The wRVU represents the time, effort, and complexity involved in performing the service. The peRVU reflects the direct cost of supplies, personnel, and durable equipment involved in providing the service, excluding typical office overhead costs such as rent, utilities, and administrative staff. The mRVU is to cover the cost of malpractice insurance.5 The peRVU can be further specified as facility versus nonfacility services depending on where the service is performed.6 A facility peRVU is for services completed in a facility such as a hospital, outpatient hospital setting, or nursing home. The facility provides some of the involved supplies, personnel, and equipment for which they can recapture costs by separate reporting, resulting in a lower total RVU for the provider charges compared with nonfacility locations where the physician must provide these items.6 Many physicians may not be aware of how critical their role is in determining their own reimbursement rates by understanding RVUs and properly filling out Relative Value Scale Update Committee (RUC) surveys. If surveys sent to practitioners are accurately completed, RVUs have the potential to be fairly valued; however, if respondents are unaware of all of the components that are inherent to a procedure, they may end up minimizing the effort or time involved, which would skew the results and hurt those who perform the procedure. Rather than inputting appropriate preoperative and postoperative service times, many respondents often put 0s and 1s throughout the survey, which misrepresents the amount of time involved for a procedure. For example, inputting a preoperative time as 0 or 1 minute may severely underestimate the work involved for a procedure if the true preoperative time is 5 minutes. Such survey responses affect whether or not RVUs are valued appropriately.
The billing codes and their RVUs as well as Medicare payment values in your area can be found on the Centers for Medicare & Medicaid Services website.2,3 Table 1 provides a comparison of the old and new biopsy codes, and Table 2 shows the new RCM codes.
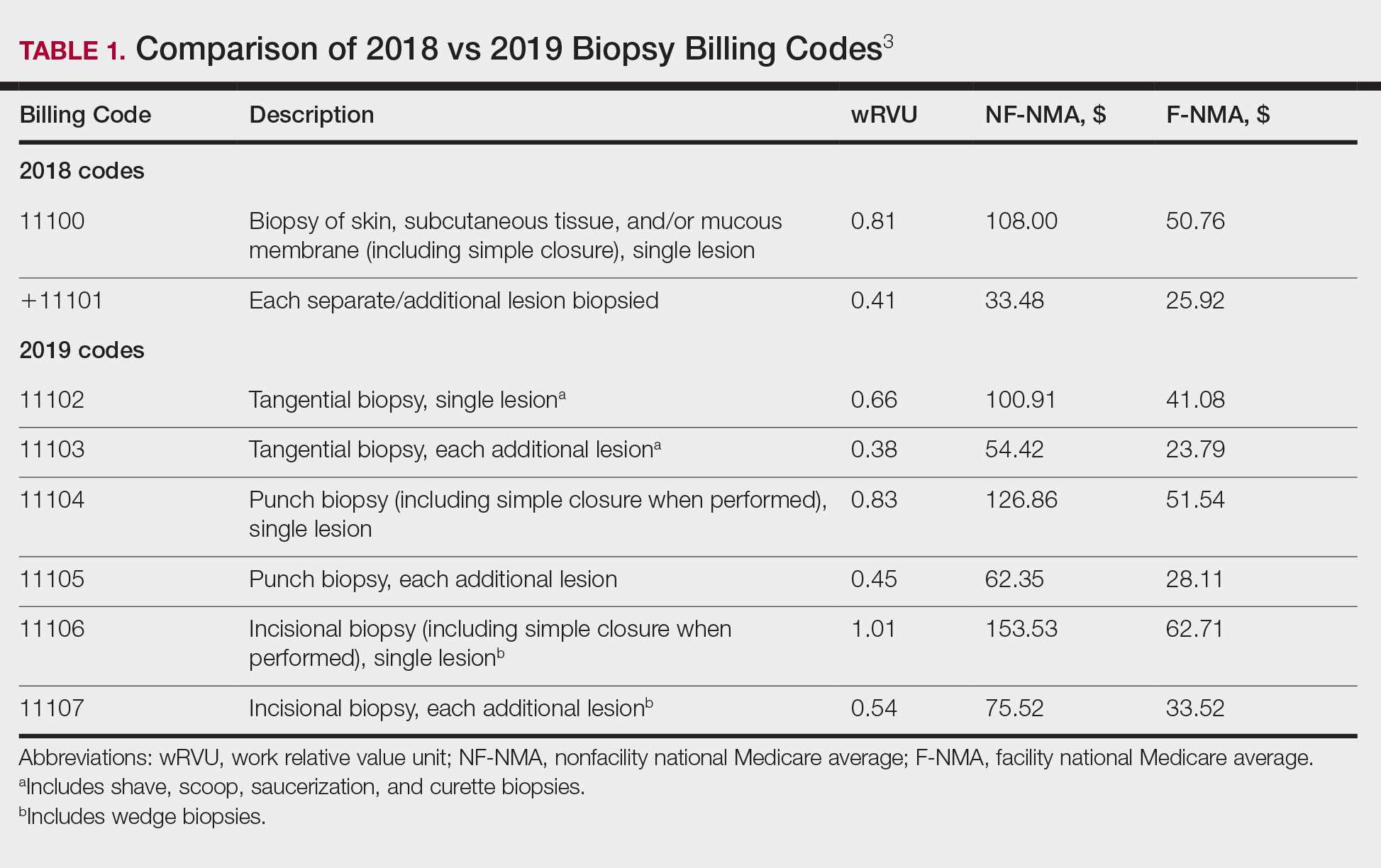
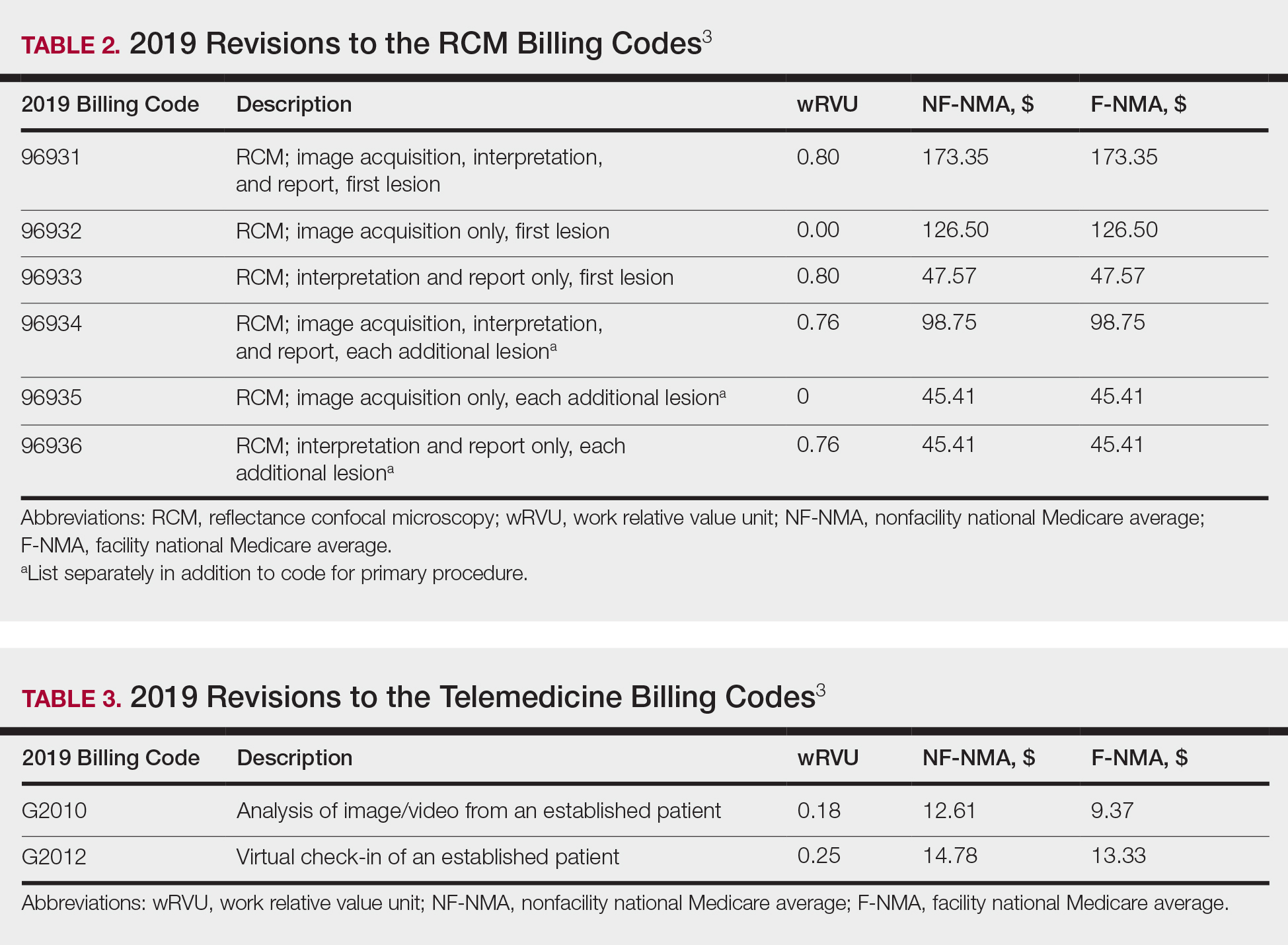
Biopsy Codes
Prior to 2019, biopsies were reimbursed using CPT code 11100 for the initial biopsy and 11101 for each additional biopsy.2 Called up for refinement in the RUC process, initial data from the Physician Practice Expense Information Survey pointed to the likelihood of different sampling techniques having different amounts of work being supplied by different techniques.1 Imaging modalities such as dermoscopy or RCM could help minimize the need for surgical biopsies. Dermoscopy, which has been proven to allow for more efficient and accurate diagnoses in dermatology, is reimbursed in Europe but not in the United States.7-9 In 2016, CPT codes 96931 through 96936 were created for RCM and are covered by most insurances.10 Optical coherence tomography, another noninvasive imaging technology, currently is not reimbursed but did receive Category III codes (0470T-0471T), also known as a tracking codes, in 2017.4 Category III codes are used for emerging technologies that have future potential but do not have enough US-based evidence to support receiving Category I CPT codes. The use of Category III codes allows for data collection on emerging technologies and services, with the potential to convert the Category III codes to Category I codes once certain criteria are met.11
Beginning in 2019, the standard biopsy codes 11100 and 11101 were replaced with 6 new codes to represent primary (11102, 11104, 11106) and add-on biopsies (11103, 11105, 11107) based on the sampling technique utilized and the thickness of the sample (Table 1). Previously, the biopsy codes did not reflect the complexity of the different biopsy techniques, whereas the new codes provide differentiation of the method of removal (ie, tangential, punch, incisional).2,3 The base code is dependent on whichever biopsy performed has the highest complexity, with incisional biopsy--a partial excision--being considered the most complex.3 Punch biopsy is considered the next level of complexity, followed by tangential biopsy. Each of the 6 new biopsy codes also received a new wRVU, which determines reimbursement under Medicare and most other insurers when combined with direct peRVU and mRVU. Additional biopsies, reported using the add-on codes, are reimbursed at a lower level than the base codes because of removal of duplicate inputs for preservice and postservice care.3
Telehealth Codes
Telemedicine services offer another form of imaging that providers can use to communicate remotely with patients through a live interactive video stream (with audio), a store-and-forward system with photographs or videos shared asynchronously, or remote patient monitoring.12 Although live video streaming uses a webcam, store-and-forward services involve sending photographs or videos electronically for later evaluation.12,13 Remote patient monitoring allows the collection of health-related data and transmission to a physician without the need for an office visit.13 Most states require physicians to have a license in the state in which the patient is located at the time of the encounter. Given the difficulty of applying for licensure in multiple states, several states started creating their own special licenses to allow out-of-state providers to offer services through telemedicine.14 The Federation of State Medical Boards then created the Interstate Medical Licensure Compact (IMLC) for an expedited process to apply for medical licensure in other states. The IMLC was formed to increase access to health care in underserved or rural areas including but not limited to the use of telemedicine.15 To qualify for IMLC, a physician must have a medical license in a state registered with the IMLC (ie, state of principal license) and have at least one of the following in their state of principal license: primary residence, 25% of their medical practice, a current employer, or US federal income taxes filed.15 The remaining states that do not have a licensing process for telemedicine allow practice in contiguous states or may provide temporary licenses dependent on the situation.14
Since 2017, billing codes for telemedicine have been the same as those used for in-person evaluation and management services with modifiers -95 or GQ added to the end of the code. Modifier -95 has been used for real-time telemedicine services, while modifier GQ has been used for store-and-forward services.16 For example, the code 99201, which is used to bill for new patients at outpatient visits, would become 99201-95 if performed using a live audio and video feed or 99201-GQ if information was sent electronically for later analysis. To receive reimbursement from Medicare, modifier -95 requires real-time communication using both audio and video; however, modifier GQ is only reimbursable in federal telemedicine demonstration programs in Alaska or Hawaii.12 Note that reimbursement is up to the discretion of private providers, and even Medicare reimbursement can vary from state to state.
In 2019, new Healthcare Common Procedure Coding System telemedicine codes were introduced to include virtual check-ins (G2012) and evaluation of patient-transmitted images and videos (G2010). G2010 is the first store-and-forward code that has the potential to be reimbursed outside of Alaska or Hawaii.3,12 G2012 allows providers to monitor the patients' well-being outside of the office setting, a cost-effective alternative if patients do not require a full visit. More detailed descriptions of the new codes can be found in Table 3.1
Final Thoughts
As insurance providers continue to better monitor health care costs, it is of utmost importance that physicians become more involved in accurately assessing their services and procedures, given that the changes in RVUs mirror the Centers for Medicare & Medicaid Services' utilization of the RUC's interpretation of our survey responses.1 The current billing codes attempt to better represent the work involved for each service, one example being the modification to more specific biopsy codes in 2019.
With the growth of technology, CPT and Healthcare Common Procedure Coding System codes also reflect a push toward more efficient health care delivery and broader coverage for provider services, as demonstrated by the introduction of new telemedicine codes as well as recent additions of noninvasive imaging codes. Although technology makes health care more cost-effective for patients, clinicians can still maintain their overall reimbursements by efficiently seeing an increasing number of patients; for example, a patient diagnosed noninvasively using RCM can then receive same-day care, which impacts patients' quality of life by minimizing travel time, number of office visits, and time taken off from work, while allowing providers to manage a higher patient volume more productively. The new CPT codes discussed here reflect the growth of medical technology potential, which increases our diagnostic capability, making it even more critical for physicians to engage with these developments.
- Centers for Medicare & Medicaid Services. Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; Quality Payment Program; Medicaid Promoting Interoperability Program; Quality Payment Program--Extreme and Uncontrollable Circumstance Policy for the 2019 MIPS Payment Year; Provisions From the Medicare Shared Savings Program-- Accountable Care Organizations--Pathways to Success; and Expanding the Use of Telehealth Services for the Treatment of Opioid Use Disorder Under the Substance Use-Disorder Prevention That Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act. Fed Registr. 2018;83(226):59452-60303. To be codified at 42 CFR §405, 410, 411, 414, 415, 425, and 495.
- Centers for Medicare & Medicaid Services. CY 2018 PFS Final Rule Addenda. https://www.cms.gov/Medicare/Medicare-Fee-for-Service Payment/PhysicianFeeSched/Downloads/CY2018-PFS-FR-Addenda.zip. Published 2018. Accessed March 28, 2019.
- Overview: Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed March 28, 2019.
- Medicare Learning Network. July 2017 update of the hospital outpatient prospective payment system (OPPS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM10122.pdf. Published 2017. Accessed March 21, 2019.
- Medicare Learning Network. Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/medcrephysfeeschedfctsht.pdf. Published February 2017. Accessed March 19, 2019.
- Medicare Learning Network. How to use the searchable Medicare Physician Fee Schedule (MPFS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/How_to_MPFS_Booklet_ICN901344.pdf. Published September 2017. Accessed March 19, 2019.
- Fox GN. Dermoscopy: an invaluable tool for evaluating skin lesions. Am Fam Physician. 2008;78:704, 706.
- Soyer HP, Argenziano G, Talamini R, et al. Is dermoscopy useful for the diagnosis of melanoma? Arch Dermatol. 2001;137:1361-1363.
- Kornek T, Schäfer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists' perspective. Dermatology. 2012;225:289-293.
- American Academy of Dermatology Association. New CPT coding updates for 2016. Derm Coding Consult. 2015;19:1-2. https://www.aad.org/File Library/Main navigation/Member resources and programs/Publications/DCC/DCC_Winter_2015.pdf. Published 2014. Accessed March 21, 2019.
- American Medical Association. CPT Category III codes. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/physicians/cpt/cpt-category3-codes-long-descriptors.pdf. Updated July 26, 2018. Accessed March 21, 2019.
- Medicare Learning Network. Telehealth services. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/TelehealthSrvcsfctsht.pdf. Accessed March 19, 2019.
- Final policy, payment, and quality provisions in the Medicare Physician Fee Schedule for calendar year 2018. Centers for Medicare & Medicaid Services website. https://www.cms.gov/newsroom/fact-sheets/final-policy-payment-and-quality-provisions-medicare-physician-fee-schedule-calendar-year-2018. Published November 2, 2017. Accessed March 19, 2019.
- State Telehealth Laws & Reimbursement Policies. Sacramento, CA: Center for Connected Health Policy; 2018. https://www.cchpca.org/sites/default/files/2018-10/CCHP_50_State_Report_Fall_2018.pdf. Accessed March 19, 2019.
- The IMLC. Interstate Medical Licensure Compact website. https://imlcc.org/. Accessed March 19, 2019.
- Current Procedural Terminology 2018, Professional Edition. Chicago, IL: American Medical Association; 2018.
As the US population continues to grow and patients become more aware of their health needs, payers are beginning to recognize the benefits of more efficient and cost-effective health care. With the implementation of the new Medicare Physician Fee Schedule on January 1, 2019, some old billing codes were revalued while others were replaced entirely with new codes.1 The restructuring of the standard biopsy codes now takes the complexity of different sampling techniques into consideration. Furthermore, Current Procedural Terminology (CPT) Category III tracking codes for some imaging devices (eg, optical coherence tomography) added in 2017 require more data before obtaining a Category I reimbursable code, while codes for other imaging devices such as reflectance confocal microscopy (RCM) remain relatively the same.2-4 Notably, the majority of the new 2019 telemedicine codes are applicable to dermatology.2,3 In this article, we discuss the new CPT codes for reporting diagnostic procedures, including biopsy, noninvasive imaging, and telemedicine services. We also provide a summary of the national average reimbursement rates for these procedures.
Background on Reimbursement
To better understand how reimbursement works, it is important to know that all billing codes are provided a relative value unit (RVU), a number representing the value of the work involved and cost of providing a service relative to other services.5 The total RVU consists of the work RVU (wRVU), practice expense RVU (peRVU), and malpractice expense RVU (mRVU). The wRVU represents the time, effort, and complexity involved in performing the service. The peRVU reflects the direct cost of supplies, personnel, and durable equipment involved in providing the service, excluding typical office overhead costs such as rent, utilities, and administrative staff. The mRVU is to cover the cost of malpractice insurance.5 The peRVU can be further specified as facility versus nonfacility services depending on where the service is performed.6 A facility peRVU is for services completed in a facility such as a hospital, outpatient hospital setting, or nursing home. The facility provides some of the involved supplies, personnel, and equipment for which they can recapture costs by separate reporting, resulting in a lower total RVU for the provider charges compared with nonfacility locations where the physician must provide these items.6 Many physicians may not be aware of how critical their role is in determining their own reimbursement rates by understanding RVUs and properly filling out Relative Value Scale Update Committee (RUC) surveys. If surveys sent to practitioners are accurately completed, RVUs have the potential to be fairly valued; however, if respondents are unaware of all of the components that are inherent to a procedure, they may end up minimizing the effort or time involved, which would skew the results and hurt those who perform the procedure. Rather than inputting appropriate preoperative and postoperative service times, many respondents often put 0s and 1s throughout the survey, which misrepresents the amount of time involved for a procedure. For example, inputting a preoperative time as 0 or 1 minute may severely underestimate the work involved for a procedure if the true preoperative time is 5 minutes. Such survey responses affect whether or not RVUs are valued appropriately.
The billing codes and their RVUs as well as Medicare payment values in your area can be found on the Centers for Medicare & Medicaid Services website.2,3 Table 1 provides a comparison of the old and new biopsy codes, and Table 2 shows the new RCM codes.


Biopsy Codes
Prior to 2019, biopsies were reimbursed using CPT code 11100 for the initial biopsy and 11101 for each additional biopsy.2 Called up for refinement in the RUC process, initial data from the Physician Practice Expense Information Survey pointed to the likelihood of different sampling techniques having different amounts of work being supplied by different techniques.1 Imaging modalities such as dermoscopy or RCM could help minimize the need for surgical biopsies. Dermoscopy, which has been proven to allow for more efficient and accurate diagnoses in dermatology, is reimbursed in Europe but not in the United States.7-9 In 2016, CPT codes 96931 through 96936 were created for RCM and are covered by most insurances.10 Optical coherence tomography, another noninvasive imaging technology, currently is not reimbursed but did receive Category III codes (0470T-0471T), also known as a tracking codes, in 2017.4 Category III codes are used for emerging technologies that have future potential but do not have enough US-based evidence to support receiving Category I CPT codes. The use of Category III codes allows for data collection on emerging technologies and services, with the potential to convert the Category III codes to Category I codes once certain criteria are met.11
Beginning in 2019, the standard biopsy codes 11100 and 11101 were replaced with 6 new codes to represent primary (11102, 11104, 11106) and add-on biopsies (11103, 11105, 11107) based on the sampling technique utilized and the thickness of the sample (Table 1). Previously, the biopsy codes did not reflect the complexity of the different biopsy techniques, whereas the new codes provide differentiation of the method of removal (ie, tangential, punch, incisional).2,3 The base code is dependent on whichever biopsy performed has the highest complexity, with incisional biopsy--a partial excision--being considered the most complex.3 Punch biopsy is considered the next level of complexity, followed by tangential biopsy. Each of the 6 new biopsy codes also received a new wRVU, which determines reimbursement under Medicare and most other insurers when combined with direct peRVU and mRVU. Additional biopsies, reported using the add-on codes, are reimbursed at a lower level than the base codes because of removal of duplicate inputs for preservice and postservice care.3
Telehealth Codes
Telemedicine services offer another form of imaging that providers can use to communicate remotely with patients through a live interactive video stream (with audio), a store-and-forward system with photographs or videos shared asynchronously, or remote patient monitoring.12 Although live video streaming uses a webcam, store-and-forward services involve sending photographs or videos electronically for later evaluation.12,13 Remote patient monitoring allows the collection of health-related data and transmission to a physician without the need for an office visit.13 Most states require physicians to have a license in the state in which the patient is located at the time of the encounter. Given the difficulty of applying for licensure in multiple states, several states started creating their own special licenses to allow out-of-state providers to offer services through telemedicine.14 The Federation of State Medical Boards then created the Interstate Medical Licensure Compact (IMLC) for an expedited process to apply for medical licensure in other states. The IMLC was formed to increase access to health care in underserved or rural areas including but not limited to the use of telemedicine.15 To qualify for IMLC, a physician must have a medical license in a state registered with the IMLC (ie, state of principal license) and have at least one of the following in their state of principal license: primary residence, 25% of their medical practice, a current employer, or US federal income taxes filed.15 The remaining states that do not have a licensing process for telemedicine allow practice in contiguous states or may provide temporary licenses dependent on the situation.14
Since 2017, billing codes for telemedicine have been the same as those used for in-person evaluation and management services with modifiers -95 or GQ added to the end of the code. Modifier -95 has been used for real-time telemedicine services, while modifier GQ has been used for store-and-forward services.16 For example, the code 99201, which is used to bill for new patients at outpatient visits, would become 99201-95 if performed using a live audio and video feed or 99201-GQ if information was sent electronically for later analysis. To receive reimbursement from Medicare, modifier -95 requires real-time communication using both audio and video; however, modifier GQ is only reimbursable in federal telemedicine demonstration programs in Alaska or Hawaii.12 Note that reimbursement is up to the discretion of private providers, and even Medicare reimbursement can vary from state to state.
In 2019, new Healthcare Common Procedure Coding System telemedicine codes were introduced to include virtual check-ins (G2012) and evaluation of patient-transmitted images and videos (G2010). G2010 is the first store-and-forward code that has the potential to be reimbursed outside of Alaska or Hawaii.3,12 G2012 allows providers to monitor the patients' well-being outside of the office setting, a cost-effective alternative if patients do not require a full visit. More detailed descriptions of the new codes can be found in Table 3.1
Final Thoughts
As insurance providers continue to better monitor health care costs, it is of utmost importance that physicians become more involved in accurately assessing their services and procedures, given that the changes in RVUs mirror the Centers for Medicare & Medicaid Services' utilization of the RUC's interpretation of our survey responses.1 The current billing codes attempt to better represent the work involved for each service, one example being the modification to more specific biopsy codes in 2019.
With the growth of technology, CPT and Healthcare Common Procedure Coding System codes also reflect a push toward more efficient health care delivery and broader coverage for provider services, as demonstrated by the introduction of new telemedicine codes as well as recent additions of noninvasive imaging codes. Although technology makes health care more cost-effective for patients, clinicians can still maintain their overall reimbursements by efficiently seeing an increasing number of patients; for example, a patient diagnosed noninvasively using RCM can then receive same-day care, which impacts patients' quality of life by minimizing travel time, number of office visits, and time taken off from work, while allowing providers to manage a higher patient volume more productively. The new CPT codes discussed here reflect the growth of medical technology potential, which increases our diagnostic capability, making it even more critical for physicians to engage with these developments.
As the US population continues to grow and patients become more aware of their health needs, payers are beginning to recognize the benefits of more efficient and cost-effective health care. With the implementation of the new Medicare Physician Fee Schedule on January 1, 2019, some old billing codes were revalued while others were replaced entirely with new codes.1 The restructuring of the standard biopsy codes now takes the complexity of different sampling techniques into consideration. Furthermore, Current Procedural Terminology (CPT) Category III tracking codes for some imaging devices (eg, optical coherence tomography) added in 2017 require more data before obtaining a Category I reimbursable code, while codes for other imaging devices such as reflectance confocal microscopy (RCM) remain relatively the same.2-4 Notably, the majority of the new 2019 telemedicine codes are applicable to dermatology.2,3 In this article, we discuss the new CPT codes for reporting diagnostic procedures, including biopsy, noninvasive imaging, and telemedicine services. We also provide a summary of the national average reimbursement rates for these procedures.
Background on Reimbursement
To better understand how reimbursement works, it is important to know that all billing codes are provided a relative value unit (RVU), a number representing the value of the work involved and cost of providing a service relative to other services.5 The total RVU consists of the work RVU (wRVU), practice expense RVU (peRVU), and malpractice expense RVU (mRVU). The wRVU represents the time, effort, and complexity involved in performing the service. The peRVU reflects the direct cost of supplies, personnel, and durable equipment involved in providing the service, excluding typical office overhead costs such as rent, utilities, and administrative staff. The mRVU is to cover the cost of malpractice insurance.5 The peRVU can be further specified as facility versus nonfacility services depending on where the service is performed.6 A facility peRVU is for services completed in a facility such as a hospital, outpatient hospital setting, or nursing home. The facility provides some of the involved supplies, personnel, and equipment for which they can recapture costs by separate reporting, resulting in a lower total RVU for the provider charges compared with nonfacility locations where the physician must provide these items.6 Many physicians may not be aware of how critical their role is in determining their own reimbursement rates by understanding RVUs and properly filling out Relative Value Scale Update Committee (RUC) surveys. If surveys sent to practitioners are accurately completed, RVUs have the potential to be fairly valued; however, if respondents are unaware of all of the components that are inherent to a procedure, they may end up minimizing the effort or time involved, which would skew the results and hurt those who perform the procedure. Rather than inputting appropriate preoperative and postoperative service times, many respondents often put 0s and 1s throughout the survey, which misrepresents the amount of time involved for a procedure. For example, inputting a preoperative time as 0 or 1 minute may severely underestimate the work involved for a procedure if the true preoperative time is 5 minutes. Such survey responses affect whether or not RVUs are valued appropriately.
The billing codes and their RVUs as well as Medicare payment values in your area can be found on the Centers for Medicare & Medicaid Services website.2,3 Table 1 provides a comparison of the old and new biopsy codes, and Table 2 shows the new RCM codes.


Biopsy Codes
Prior to 2019, biopsies were reimbursed using CPT code 11100 for the initial biopsy and 11101 for each additional biopsy.2 Called up for refinement in the RUC process, initial data from the Physician Practice Expense Information Survey pointed to the likelihood of different sampling techniques having different amounts of work being supplied by different techniques.1 Imaging modalities such as dermoscopy or RCM could help minimize the need for surgical biopsies. Dermoscopy, which has been proven to allow for more efficient and accurate diagnoses in dermatology, is reimbursed in Europe but not in the United States.7-9 In 2016, CPT codes 96931 through 96936 were created for RCM and are covered by most insurances.10 Optical coherence tomography, another noninvasive imaging technology, currently is not reimbursed but did receive Category III codes (0470T-0471T), also known as a tracking codes, in 2017.4 Category III codes are used for emerging technologies that have future potential but do not have enough US-based evidence to support receiving Category I CPT codes. The use of Category III codes allows for data collection on emerging technologies and services, with the potential to convert the Category III codes to Category I codes once certain criteria are met.11
Beginning in 2019, the standard biopsy codes 11100 and 11101 were replaced with 6 new codes to represent primary (11102, 11104, 11106) and add-on biopsies (11103, 11105, 11107) based on the sampling technique utilized and the thickness of the sample (Table 1). Previously, the biopsy codes did not reflect the complexity of the different biopsy techniques, whereas the new codes provide differentiation of the method of removal (ie, tangential, punch, incisional).2,3 The base code is dependent on whichever biopsy performed has the highest complexity, with incisional biopsy--a partial excision--being considered the most complex.3 Punch biopsy is considered the next level of complexity, followed by tangential biopsy. Each of the 6 new biopsy codes also received a new wRVU, which determines reimbursement under Medicare and most other insurers when combined with direct peRVU and mRVU. Additional biopsies, reported using the add-on codes, are reimbursed at a lower level than the base codes because of removal of duplicate inputs for preservice and postservice care.3
Telehealth Codes
Telemedicine services offer another form of imaging that providers can use to communicate remotely with patients through a live interactive video stream (with audio), a store-and-forward system with photographs or videos shared asynchronously, or remote patient monitoring.12 Although live video streaming uses a webcam, store-and-forward services involve sending photographs or videos electronically for later evaluation.12,13 Remote patient monitoring allows the collection of health-related data and transmission to a physician without the need for an office visit.13 Most states require physicians to have a license in the state in which the patient is located at the time of the encounter. Given the difficulty of applying for licensure in multiple states, several states started creating their own special licenses to allow out-of-state providers to offer services through telemedicine.14 The Federation of State Medical Boards then created the Interstate Medical Licensure Compact (IMLC) for an expedited process to apply for medical licensure in other states. The IMLC was formed to increase access to health care in underserved or rural areas including but not limited to the use of telemedicine.15 To qualify for IMLC, a physician must have a medical license in a state registered with the IMLC (ie, state of principal license) and have at least one of the following in their state of principal license: primary residence, 25% of their medical practice, a current employer, or US federal income taxes filed.15 The remaining states that do not have a licensing process for telemedicine allow practice in contiguous states or may provide temporary licenses dependent on the situation.14
Since 2017, billing codes for telemedicine have been the same as those used for in-person evaluation and management services with modifiers -95 or GQ added to the end of the code. Modifier -95 has been used for real-time telemedicine services, while modifier GQ has been used for store-and-forward services.16 For example, the code 99201, which is used to bill for new patients at outpatient visits, would become 99201-95 if performed using a live audio and video feed or 99201-GQ if information was sent electronically for later analysis. To receive reimbursement from Medicare, modifier -95 requires real-time communication using both audio and video; however, modifier GQ is only reimbursable in federal telemedicine demonstration programs in Alaska or Hawaii.12 Note that reimbursement is up to the discretion of private providers, and even Medicare reimbursement can vary from state to state.
In 2019, new Healthcare Common Procedure Coding System telemedicine codes were introduced to include virtual check-ins (G2012) and evaluation of patient-transmitted images and videos (G2010). G2010 is the first store-and-forward code that has the potential to be reimbursed outside of Alaska or Hawaii.3,12 G2012 allows providers to monitor the patients' well-being outside of the office setting, a cost-effective alternative if patients do not require a full visit. More detailed descriptions of the new codes can be found in Table 3.1
Final Thoughts
As insurance providers continue to better monitor health care costs, it is of utmost importance that physicians become more involved in accurately assessing their services and procedures, given that the changes in RVUs mirror the Centers for Medicare & Medicaid Services' utilization of the RUC's interpretation of our survey responses.1 The current billing codes attempt to better represent the work involved for each service, one example being the modification to more specific biopsy codes in 2019.
With the growth of technology, CPT and Healthcare Common Procedure Coding System codes also reflect a push toward more efficient health care delivery and broader coverage for provider services, as demonstrated by the introduction of new telemedicine codes as well as recent additions of noninvasive imaging codes. Although technology makes health care more cost-effective for patients, clinicians can still maintain their overall reimbursements by efficiently seeing an increasing number of patients; for example, a patient diagnosed noninvasively using RCM can then receive same-day care, which impacts patients' quality of life by minimizing travel time, number of office visits, and time taken off from work, while allowing providers to manage a higher patient volume more productively. The new CPT codes discussed here reflect the growth of medical technology potential, which increases our diagnostic capability, making it even more critical for physicians to engage with these developments.
- Centers for Medicare & Medicaid Services. Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; Quality Payment Program; Medicaid Promoting Interoperability Program; Quality Payment Program--Extreme and Uncontrollable Circumstance Policy for the 2019 MIPS Payment Year; Provisions From the Medicare Shared Savings Program-- Accountable Care Organizations--Pathways to Success; and Expanding the Use of Telehealth Services for the Treatment of Opioid Use Disorder Under the Substance Use-Disorder Prevention That Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act. Fed Registr. 2018;83(226):59452-60303. To be codified at 42 CFR §405, 410, 411, 414, 415, 425, and 495.
- Centers for Medicare & Medicaid Services. CY 2018 PFS Final Rule Addenda. https://www.cms.gov/Medicare/Medicare-Fee-for-Service Payment/PhysicianFeeSched/Downloads/CY2018-PFS-FR-Addenda.zip. Published 2018. Accessed March 28, 2019.
- Overview: Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed March 28, 2019.
- Medicare Learning Network. July 2017 update of the hospital outpatient prospective payment system (OPPS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM10122.pdf. Published 2017. Accessed March 21, 2019.
- Medicare Learning Network. Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/medcrephysfeeschedfctsht.pdf. Published February 2017. Accessed March 19, 2019.
- Medicare Learning Network. How to use the searchable Medicare Physician Fee Schedule (MPFS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/How_to_MPFS_Booklet_ICN901344.pdf. Published September 2017. Accessed March 19, 2019.
- Fox GN. Dermoscopy: an invaluable tool for evaluating skin lesions. Am Fam Physician. 2008;78:704, 706.
- Soyer HP, Argenziano G, Talamini R, et al. Is dermoscopy useful for the diagnosis of melanoma? Arch Dermatol. 2001;137:1361-1363.
- Kornek T, Schäfer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists' perspective. Dermatology. 2012;225:289-293.
- American Academy of Dermatology Association. New CPT coding updates for 2016. Derm Coding Consult. 2015;19:1-2. https://www.aad.org/File Library/Main navigation/Member resources and programs/Publications/DCC/DCC_Winter_2015.pdf. Published 2014. Accessed March 21, 2019.
- American Medical Association. CPT Category III codes. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/physicians/cpt/cpt-category3-codes-long-descriptors.pdf. Updated July 26, 2018. Accessed March 21, 2019.
- Medicare Learning Network. Telehealth services. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/TelehealthSrvcsfctsht.pdf. Accessed March 19, 2019.
- Final policy, payment, and quality provisions in the Medicare Physician Fee Schedule for calendar year 2018. Centers for Medicare & Medicaid Services website. https://www.cms.gov/newsroom/fact-sheets/final-policy-payment-and-quality-provisions-medicare-physician-fee-schedule-calendar-year-2018. Published November 2, 2017. Accessed March 19, 2019.
- State Telehealth Laws & Reimbursement Policies. Sacramento, CA: Center for Connected Health Policy; 2018. https://www.cchpca.org/sites/default/files/2018-10/CCHP_50_State_Report_Fall_2018.pdf. Accessed March 19, 2019.
- The IMLC. Interstate Medical Licensure Compact website. https://imlcc.org/. Accessed March 19, 2019.
- Current Procedural Terminology 2018, Professional Edition. Chicago, IL: American Medical Association; 2018.
- Centers for Medicare & Medicaid Services. Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; Quality Payment Program; Medicaid Promoting Interoperability Program; Quality Payment Program--Extreme and Uncontrollable Circumstance Policy for the 2019 MIPS Payment Year; Provisions From the Medicare Shared Savings Program-- Accountable Care Organizations--Pathways to Success; and Expanding the Use of Telehealth Services for the Treatment of Opioid Use Disorder Under the Substance Use-Disorder Prevention That Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act. Fed Registr. 2018;83(226):59452-60303. To be codified at 42 CFR §405, 410, 411, 414, 415, 425, and 495.
- Centers for Medicare & Medicaid Services. CY 2018 PFS Final Rule Addenda. https://www.cms.gov/Medicare/Medicare-Fee-for-Service Payment/PhysicianFeeSched/Downloads/CY2018-PFS-FR-Addenda.zip. Published 2018. Accessed March 28, 2019.
- Overview: Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed March 28, 2019.
- Medicare Learning Network. July 2017 update of the hospital outpatient prospective payment system (OPPS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM10122.pdf. Published 2017. Accessed March 21, 2019.
- Medicare Learning Network. Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/medcrephysfeeschedfctsht.pdf. Published February 2017. Accessed March 19, 2019.
- Medicare Learning Network. How to use the searchable Medicare Physician Fee Schedule (MPFS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/How_to_MPFS_Booklet_ICN901344.pdf. Published September 2017. Accessed March 19, 2019.
- Fox GN. Dermoscopy: an invaluable tool for evaluating skin lesions. Am Fam Physician. 2008;78:704, 706.
- Soyer HP, Argenziano G, Talamini R, et al. Is dermoscopy useful for the diagnosis of melanoma? Arch Dermatol. 2001;137:1361-1363.
- Kornek T, Schäfer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists' perspective. Dermatology. 2012;225:289-293.
- American Academy of Dermatology Association. New CPT coding updates for 2016. Derm Coding Consult. 2015;19:1-2. https://www.aad.org/File Library/Main navigation/Member resources and programs/Publications/DCC/DCC_Winter_2015.pdf. Published 2014. Accessed March 21, 2019.
- American Medical Association. CPT Category III codes. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/physicians/cpt/cpt-category3-codes-long-descriptors.pdf. Updated July 26, 2018. Accessed March 21, 2019.
- Medicare Learning Network. Telehealth services. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/TelehealthSrvcsfctsht.pdf. Accessed March 19, 2019.
- Final policy, payment, and quality provisions in the Medicare Physician Fee Schedule for calendar year 2018. Centers for Medicare & Medicaid Services website. https://www.cms.gov/newsroom/fact-sheets/final-policy-payment-and-quality-provisions-medicare-physician-fee-schedule-calendar-year-2018. Published November 2, 2017. Accessed March 19, 2019.
- State Telehealth Laws & Reimbursement Policies. Sacramento, CA: Center for Connected Health Policy; 2018. https://www.cchpca.org/sites/default/files/2018-10/CCHP_50_State_Report_Fall_2018.pdf. Accessed March 19, 2019.
- The IMLC. Interstate Medical Licensure Compact website. https://imlcc.org/. Accessed March 19, 2019.
- Current Procedural Terminology 2018, Professional Edition. Chicago, IL: American Medical Association; 2018.
PRACTICE POINTS
- Reimbursement typically is proportional to the relative value unit (RVU), a number representing the value of the work involved and cost of providing a service relative to other services.
- The total RVU consists of the work RVU, practice expense RVU, and malpractice expense RVU.
- The new 2019 biopsy codes reflect the complexity of the sampling technique (ie, whether the biopsy is tangential, punch, or incisional).
- Accurate completion of Relative Value Scale Update Committee surveys sent to practitioners will allow RVUs to be valued appropriately.
Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is a rare acquired, cutaneous, reactive, vascular disorder that was originally thought to be a variant of cutaneous reactive angiomatosis (CREA) but is now considered to be on the spectrum of CREA. This article will focus on DDA and review the literature of prior case reports with brief descriptions of the differential diagnosis.
Case Report
A 43-year-old Haitian man presented to the clinic with a lesion on the left buttock that had developed over the last 6 years. The patient stated the lesion had been enlarging over the last several months. Upon examination, there was a large (15-cm diameter), indurated, hyperpigmented plaque covering the left buttock (Figure 1). The patient reported no medical or contributory family history. Upon review of systems, he described a burning sensation sometimes in the area of the lesion that would develop randomly throughout the year.
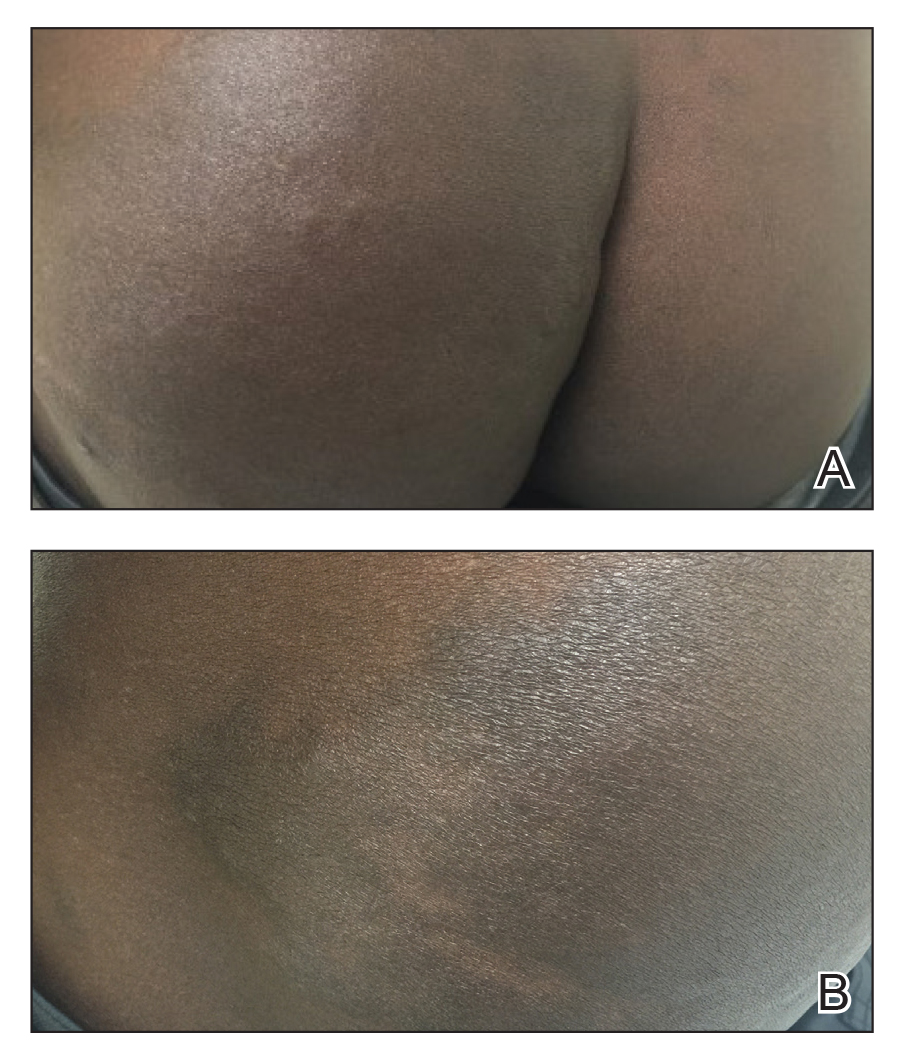
Three biopsies were performed, which revealed a collection of slightly dilated blood vessels with normal-appearing endothelial cells occupying the mid dermis and deep dermis (Figure 2). Immunohistochemical stains with antibodies were directed against human herpesvirus 8 (HHV-8), CD31, CD34, the cell surface glycoprotein podoplanin, Ki-67, and smooth muscle actin antigens, with appropriate controls. The vessel walls were positive for CD31, CD34, and smooth muscle actin, and negative for HHV-8 and podoplanin; Ki-67 was not increased. These histologic findings were consistent with a diagnosis of DDA. A detailed history was taken. The cause of DDA in our patient was uncertain.
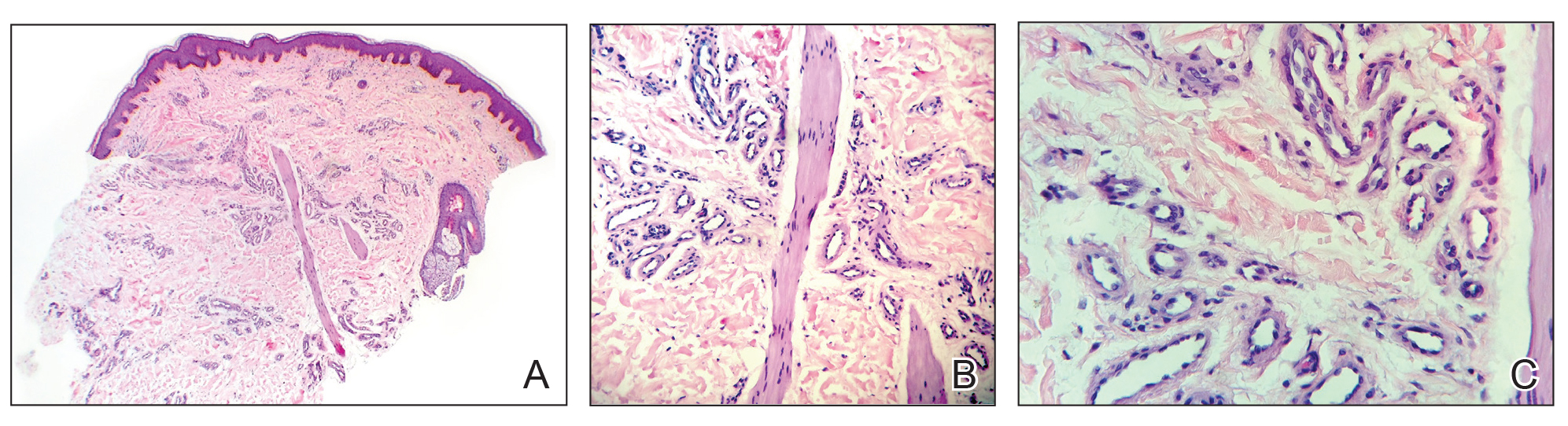
Comment
Classification and Epidemiology
Diffuse dermal angiomatosis is a rare acquired, cutaneous, reactive, vascular disorder first described by Krell et al1 in 1994. Diffuse dermal angiomatosis is benign and is classified in the group of cutaneous reactive angiomatoses,2 which are benign vascular disorders marked by intravascular and extravascular hyperplasia of endothelial cells that may or may not include pericytes.2 Diffuse dermal angiomatosis was originally described as a variant of CREA, which is characterized by hyperplasia of endothelial dermal cells and intravascular proliferation.3 However, DDA has more recently been identified as a distinct disorder on the spectrum of CREA rather than as a variant of CREA.2 Given the recent reclassification, not all physicians make this distinction. However, as more case reports of DDA are published, physicians continue to support this change.4 Nevertheless, DDA has been an established disorder since 1994.1
Vascular proliferation in DDA is hypothesized to stem from ischemia or inflammation.5 Peripheral vascular atherosclerosis has been associated with DDA.6 The epidemiology of DDA is not well known because of the rarity of the disease. We performed a more specific review of the literature by limiting the PubMed search of articles indexed for MEDLINE to the term diffuse dermal angiomatosis rather than a broader search including all reactive angioendotheliomatoses. Only 31 case reports have been published1,3-32; of them, only adults were affected. Most reported cases were in middle-aged females. A summary of the demographics of DDA is provided in the Table.1,3-32
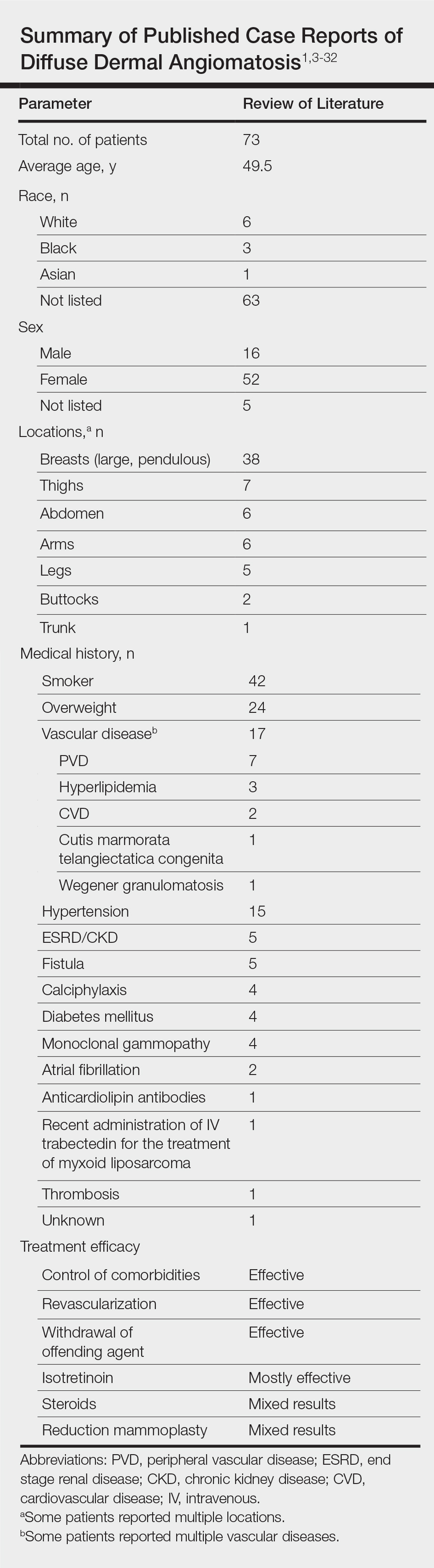
Pathophysiology
The pathophysiology of DDA remains unclear. It has been hypothesized that ischemia or inflammation creates local hypoxia, leading to an increase in vascular endothelial growth factor with subsequent endothelial proliferation and neovascularization.5 Rongioletti and Robora2 supported this hypothesis, proposing that occlusion or inflammation of the vasculature creates microthrombi and thus hypoxia. Afterward, histiocytes are recruited to reabsorb the microthrombi while hyperplasia of endothelial cells and pericytes ensues.7 Complete resolution of skin lesions following revascularization provides support for this theory.8
Etiology
Diffuse dermal angiomatosis is a rare complication of ischemia that may be secondary to atherosclerosis, arteriovenous fistula, or macromastia.9-11 In DDA of the breasts, ulcerations of fatty tissue occur due to trauma in these patients who have large pendulous breasts, causing angiogenesis resembling DDA histologically.2 One case of DDA was reported secondary to relative ischemia from cutis marmorata telangiectatica congenita,12 whereas another case highlighted Wegener granulomatosis as the cause of ischemia.7 There also have been reported cases associated with calciphylaxis and anticardiolipin antibiodies.13 In general, any medical condition that can lead to ischemia can cause DDA. Comorbid conditions for DDA include cardiovascular disease, hypertension, diabetes mellitus, and most often severe peripheral vascular disease. Many patients also have a history of smoking.14 Diffuse dermal angiomatosis rarely presents without underlying comorbidity, with only 1 case report of unknown cause (Table).
Presentation, Histopathology, and Differential Diagnosis
Cutaneous reactive angiomatosis disorders present the same clinically, with multiple erythematous to violaceous purpuric patches and plaques that can progress to necrosis and ulceration. Lesions are widely distributed but are predisposed to the upper and lower extremities.2 The differential diagnosis of DDA includes CREA, acroangiodermatitis (pseudo–Kaposi sarcoma), or vascular malignancies such as Kaposi sarcoma and low-grade angiosarcoma.7
In DDA, lesions may be painful and sometimes have a central ulceration.15 They often are associated with notable peripheral vascular atherosclerotic disease and are mainly found on the lower extremities.12,16 Histologically, DDA presents as a diffuse proliferation of endothelial cells between collagen bundles. The endothelial cells are distributed throughout the papillary and reticular dermis and develop into vascular lumina.17 Furthermore, the proliferating endothelial cells are spindle shaped and contain vacuolated cytoplasm.14
Acroangiodermatitis, or pseudo–Kaposi sarcoma, presents as slow-growing, erythematous to violaceous, brown, or dusky macules, papules, or plaques of the legs.14 Histologically, acroangiodermatitis presents with relatively less proliferation of endothelial cells found intravascularly rather than extravascularly, as in DDA, forming new thick-walled vessels in a lobular pattern in the papillary dermis.14
Vascular malignancies, such as Kaposi sarcoma and angiosarcoma, may present similarly to DDA. Kaposi sarcoma, for example, presents as erythematous to violaceous patches, plaques, or nodules found mostly on the extremities.7 Histologically, spindle cells and vascular structures also are found but in a clefting pattern representative of Kaposi sarcoma (so-called vascular slits).7 Diffuse dermal angiomatosis and vascular malignancies can further be distinguished based on atypia of the proliferations and staining for HHV-8.7,14 Lastly, DDA differs from vascular tumors in that vascular tumors are reactive to locations of occluded vessels, with vascular proliferation ceasing once the underlying cause of hypoxia is removed.2
Treatment
There is no standard treatment of DDA.7 Treatment of the underlying cause of ischemia is the primary goal, which will cause the DDA to resolve in most cases. Stenting, removal of an arteriovenous fistula, or other forms of revascularization may be warranted.1,5,6,10,17,29,30
Reported medical therapies for DDA include systemic or topical corticosteroids used for their antiangiogenic properties with varying results.7 Isotretinoin also has been used, which has been found to be effective in several cases of DDA of the breast, though 1 study reported a subsequent elevated lipid profile, requiring a decrease in dosage.14,15,27,31
Most interestingly, a study by Sanz-Motilva et al16 demonstrated that control of comorbidities, especially smoking cessation, led to improvement, which highlights the importance of incorporating nonpharmacotherapy rather than initiating treatment solely with medication. The Table summarizes treatments used and their efficacy.
Conclusion
Diffuse dermal angiomatosis is associated with medical conditions that predispose an individual to ischemia. Although rare, DDA can present as painful and visibly disturbing lesions that can affect the daily life of afflicted patients. By reporting the few cases that do arise and reviewing prior cases and their treatments, physicians can consider DDA within the differential diagnosis and identify which treatment is most efficient for a given patient. For all DDA patients, strict control of comorbidities, especially smoking cessation, should be incorporated into the treatment plan. When DDA affects the breasts, isotretinoin appears to provide the best relief. Otherwise, treatment of the underlying cause, revascularization, withdrawal of the offending agent, or steroids seem to be the best treatment options.
- Krell JM, Sanchez RL, Solomon AR. Diffuse dermal angiomatosis: a variant of reactive cutaneous angioendotheliomatosis. J Cutan Pathol. 1994;21:363-370.
- Rongioletti F, Robora A. Cutaneous reactive angiomatoses: patterns and classification of reactive vascular proliferation. J Am Acad Dermatol. 2003;49:887-896.
- Crickx E, Saussine A, Vignon-Pennamen MD, et al. Diffuse dermal angiomatosis associated with severe atherosclerosis: two cases and review of the literature. Clin Exp Dermatol. 2015;40:521-524.
- Reusche R, Winocour S, Degnim A, et al. Diffuse dermal angiomatosis of the breast: a series of 22 cases from a single institution. Gland Surg. 2015;4:554-560.
- Sriphojanart T, Vachiramon V. Diffuse dermal angiomatosis: a clue to the diagnosis of atherosclerotic vascular disease. Case Rep Dermatol. 2015;7:100-106.
- Kimyai-Asadi A, Nousari HC, Ketabchi N, et al. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with atherosclerosis. J Am Acad Dermatol. 1999;40:257-259.
- Bassi A, Arunachalam M, Maio V, et al. Diffuse dermal angiomatosis in a patient with an iatrogenic arterio-venous fistula and Wegener’s granulomatosis. Acta Derm Venereol. 2013;93:93-94.
- Ormerod E, Miller K, Kennedy C. Diffuse dermal angiomatosis: a contributory factor to ulceration in a patient with renal transplant. Clin Exp Dermatol. 2015;40:48-51.
- Kim S, Elenitsas R, James WD. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with peripheral vascular atherosclerosis. Arch Dermatol. 2002;138:456-458.
- Requena L, Fariña MC, Renedo G, et al. Intravascular and diffuse dermal reactive angioendotheliomatosis secondary to iatrogenic arteriovenous fistulas. J Cutan Pathol. 1999;26:159-164.
- Villa MT, White LE, Petronic-Rosic V, et al. The treatment of diffuse dermal angiomatosis of the breast with reduction mammoplasty. Arch Dermatol. 2008;144:693-694.
- Halbesleben JJ, Cleveland MG, Stone MS. Diffuse dermal angiomatosis arising in cutis marmorata telangiectatica congenita. Arch Dermatol. 2010;146:1311-1313.
- Ferreli C, Atzori L, Pinna AL, et al. Diffuse dermal angiomatosis: a clinical mimicker of vasculitis associated with calciphylaxis and monoclonal gammopathy. G Ital Dermatol Venereol. 2015;150:115-121.
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347.
- Steele KT, Sullivan BJ, Wanat KA, et al. Diffuse dermal angiomatosis associated with calciphylaxis in a patient with end-stage renal disease.J Cutan Pathol. 2013;40:829-832.
- Sanz-Motilva V, Martorell-Calatayud A, Rongioletti F, et al. Diffuse dermal angiomatosis of the breast: clinical and histopathological features. Int J Dermatol. 2014;53:445-449.
- Kirkland CR, Hawayek LH, Mutasim DF. Atherosclerosis-induced diffuse dermal angiomatosis with fatal outcome. Arch Dermatol. 2010;146:684-685.
- Sommer S, Merchant WJ, Wilson CL. Diffuse dermal angiomatosis due to an iatrogenic arteriovenous fistula. Acta Derm Venereol. 2004;84:251-252.
- Corti MA, Rongioletti F, Borradori L, et al. Cutaneous reactive angiomatosis with combined histological pattern mimicking a cellulitis. Dermatology. 2013;227:226-230.
- Tollefson MM, McEvoy MT, Torgerson RR, et al. Diffuse dermal angiomatosis of the breast: clinicopathologic study of 5 patients. J Am Acad Dermatol. 2014;71:1212-1217.
- Walton K, Liggett J. Diffuse dermal angiomatosis: a case report. J Am Acad Dermatol. 2012;66(suppl 1):AB49.
- Mayor-Ibarguren A, Gómez-Fernández C, Beato-Merino MJ, et al. Diffuse reactive angioendotheliomatosis secondary to the administration of trabectedin and pegfilgrastim. Am J Dermatopathol. 2015;37:581-584.
- Lora V, Cota C, Cerroni L. Diffuse dermal angiomatosis of the abdomen. Eur J Dermatol. 2015;25:350-352.
- Pichardo RO, Lu D, Sangueza OP, et al. What is your diagnosis? diffuse dermal angiomatosis secondary to anticardiolipin antibodies. Am J Dermatopathol. 2002;24:502.
- Kutzner H, Requena L, Mentzel T, et al. Diffuse dermal angiomatosis. Hautarzt. 2002;53:808-812.
- McLaughlin ER, Morris R, Weiss SW, et al. Diffuse dermal angiomatosis of the breast: response to isotretinoin. J Am Acad Dermatol. 2001;45:462-465.
- Prinz Vavricka BM, Barry C, Victor T, et al. Diffuse dermal angiomatosis associated with calciphylaxis. Am J Dermatopathol. 2009;31:653-657.
- Müller CS, Wagner A, Pföhler C, et al. Cup-shaped painful ulcer of abdominal wall. Hautarzt. 2008;59:656-658.
- Draper BK, Boyd AS. Diffuse dermal angiomatosis. J Cutan Pathol. 2006;33:646-648.
- Adams BJ, Goldberg S, Massey HD, et al. A cause of unbearably painful breast, diffuse dermal angiomatosis. Gland Surg. 2012;1. doi:10.3978/j.issn.2227-684X.2012.07.02.
- Quatresooz P, Fumal I, Willemaers V, et al. Diffuse dermal angiomatosis: a previously undescribed pattern of immunoglobulin and complement deposits in two cases. Am J Dermatopathol. 2006;28:150-154.
- Morimoto K, Ioka H, Asada H, et al. Diffuse dermal angiomatosis. Eur J Vasc Endovasc Surg. 2011;42:381-383.
Diffuse dermal angiomatosis (DDA) is a rare acquired, cutaneous, reactive, vascular disorder that was originally thought to be a variant of cutaneous reactive angiomatosis (CREA) but is now considered to be on the spectrum of CREA. This article will focus on DDA and review the literature of prior case reports with brief descriptions of the differential diagnosis.
Case Report
A 43-year-old Haitian man presented to the clinic with a lesion on the left buttock that had developed over the last 6 years. The patient stated the lesion had been enlarging over the last several months. Upon examination, there was a large (15-cm diameter), indurated, hyperpigmented plaque covering the left buttock (Figure 1). The patient reported no medical or contributory family history. Upon review of systems, he described a burning sensation sometimes in the area of the lesion that would develop randomly throughout the year.

Three biopsies were performed, which revealed a collection of slightly dilated blood vessels with normal-appearing endothelial cells occupying the mid dermis and deep dermis (Figure 2). Immunohistochemical stains with antibodies were directed against human herpesvirus 8 (HHV-8), CD31, CD34, the cell surface glycoprotein podoplanin, Ki-67, and smooth muscle actin antigens, with appropriate controls. The vessel walls were positive for CD31, CD34, and smooth muscle actin, and negative for HHV-8 and podoplanin; Ki-67 was not increased. These histologic findings were consistent with a diagnosis of DDA. A detailed history was taken. The cause of DDA in our patient was uncertain.

Comment
Classification and Epidemiology
Diffuse dermal angiomatosis is a rare acquired, cutaneous, reactive, vascular disorder first described by Krell et al1 in 1994. Diffuse dermal angiomatosis is benign and is classified in the group of cutaneous reactive angiomatoses,2 which are benign vascular disorders marked by intravascular and extravascular hyperplasia of endothelial cells that may or may not include pericytes.2 Diffuse dermal angiomatosis was originally described as a variant of CREA, which is characterized by hyperplasia of endothelial dermal cells and intravascular proliferation.3 However, DDA has more recently been identified as a distinct disorder on the spectrum of CREA rather than as a variant of CREA.2 Given the recent reclassification, not all physicians make this distinction. However, as more case reports of DDA are published, physicians continue to support this change.4 Nevertheless, DDA has been an established disorder since 1994.1
Vascular proliferation in DDA is hypothesized to stem from ischemia or inflammation.5 Peripheral vascular atherosclerosis has been associated with DDA.6 The epidemiology of DDA is not well known because of the rarity of the disease. We performed a more specific review of the literature by limiting the PubMed search of articles indexed for MEDLINE to the term diffuse dermal angiomatosis rather than a broader search including all reactive angioendotheliomatoses. Only 31 case reports have been published1,3-32; of them, only adults were affected. Most reported cases were in middle-aged females. A summary of the demographics of DDA is provided in the Table.1,3-32

Pathophysiology
The pathophysiology of DDA remains unclear. It has been hypothesized that ischemia or inflammation creates local hypoxia, leading to an increase in vascular endothelial growth factor with subsequent endothelial proliferation and neovascularization.5 Rongioletti and Robora2 supported this hypothesis, proposing that occlusion or inflammation of the vasculature creates microthrombi and thus hypoxia. Afterward, histiocytes are recruited to reabsorb the microthrombi while hyperplasia of endothelial cells and pericytes ensues.7 Complete resolution of skin lesions following revascularization provides support for this theory.8
Etiology
Diffuse dermal angiomatosis is a rare complication of ischemia that may be secondary to atherosclerosis, arteriovenous fistula, or macromastia.9-11 In DDA of the breasts, ulcerations of fatty tissue occur due to trauma in these patients who have large pendulous breasts, causing angiogenesis resembling DDA histologically.2 One case of DDA was reported secondary to relative ischemia from cutis marmorata telangiectatica congenita,12 whereas another case highlighted Wegener granulomatosis as the cause of ischemia.7 There also have been reported cases associated with calciphylaxis and anticardiolipin antibiodies.13 In general, any medical condition that can lead to ischemia can cause DDA. Comorbid conditions for DDA include cardiovascular disease, hypertension, diabetes mellitus, and most often severe peripheral vascular disease. Many patients also have a history of smoking.14 Diffuse dermal angiomatosis rarely presents without underlying comorbidity, with only 1 case report of unknown cause (Table).
Presentation, Histopathology, and Differential Diagnosis
Cutaneous reactive angiomatosis disorders present the same clinically, with multiple erythematous to violaceous purpuric patches and plaques that can progress to necrosis and ulceration. Lesions are widely distributed but are predisposed to the upper and lower extremities.2 The differential diagnosis of DDA includes CREA, acroangiodermatitis (pseudo–Kaposi sarcoma), or vascular malignancies such as Kaposi sarcoma and low-grade angiosarcoma.7
In DDA, lesions may be painful and sometimes have a central ulceration.15 They often are associated with notable peripheral vascular atherosclerotic disease and are mainly found on the lower extremities.12,16 Histologically, DDA presents as a diffuse proliferation of endothelial cells between collagen bundles. The endothelial cells are distributed throughout the papillary and reticular dermis and develop into vascular lumina.17 Furthermore, the proliferating endothelial cells are spindle shaped and contain vacuolated cytoplasm.14
Acroangiodermatitis, or pseudo–Kaposi sarcoma, presents as slow-growing, erythematous to violaceous, brown, or dusky macules, papules, or plaques of the legs.14 Histologically, acroangiodermatitis presents with relatively less proliferation of endothelial cells found intravascularly rather than extravascularly, as in DDA, forming new thick-walled vessels in a lobular pattern in the papillary dermis.14
Vascular malignancies, such as Kaposi sarcoma and angiosarcoma, may present similarly to DDA. Kaposi sarcoma, for example, presents as erythematous to violaceous patches, plaques, or nodules found mostly on the extremities.7 Histologically, spindle cells and vascular structures also are found but in a clefting pattern representative of Kaposi sarcoma (so-called vascular slits).7 Diffuse dermal angiomatosis and vascular malignancies can further be distinguished based on atypia of the proliferations and staining for HHV-8.7,14 Lastly, DDA differs from vascular tumors in that vascular tumors are reactive to locations of occluded vessels, with vascular proliferation ceasing once the underlying cause of hypoxia is removed.2
Treatment
There is no standard treatment of DDA.7 Treatment of the underlying cause of ischemia is the primary goal, which will cause the DDA to resolve in most cases. Stenting, removal of an arteriovenous fistula, or other forms of revascularization may be warranted.1,5,6,10,17,29,30
Reported medical therapies for DDA include systemic or topical corticosteroids used for their antiangiogenic properties with varying results.7 Isotretinoin also has been used, which has been found to be effective in several cases of DDA of the breast, though 1 study reported a subsequent elevated lipid profile, requiring a decrease in dosage.14,15,27,31
Most interestingly, a study by Sanz-Motilva et al16 demonstrated that control of comorbidities, especially smoking cessation, led to improvement, which highlights the importance of incorporating nonpharmacotherapy rather than initiating treatment solely with medication. The Table summarizes treatments used and their efficacy.
Conclusion
Diffuse dermal angiomatosis is associated with medical conditions that predispose an individual to ischemia. Although rare, DDA can present as painful and visibly disturbing lesions that can affect the daily life of afflicted patients. By reporting the few cases that do arise and reviewing prior cases and their treatments, physicians can consider DDA within the differential diagnosis and identify which treatment is most efficient for a given patient. For all DDA patients, strict control of comorbidities, especially smoking cessation, should be incorporated into the treatment plan. When DDA affects the breasts, isotretinoin appears to provide the best relief. Otherwise, treatment of the underlying cause, revascularization, withdrawal of the offending agent, or steroids seem to be the best treatment options.
Diffuse dermal angiomatosis (DDA) is a rare acquired, cutaneous, reactive, vascular disorder that was originally thought to be a variant of cutaneous reactive angiomatosis (CREA) but is now considered to be on the spectrum of CREA. This article will focus on DDA and review the literature of prior case reports with brief descriptions of the differential diagnosis.
Case Report
A 43-year-old Haitian man presented to the clinic with a lesion on the left buttock that had developed over the last 6 years. The patient stated the lesion had been enlarging over the last several months. Upon examination, there was a large (15-cm diameter), indurated, hyperpigmented plaque covering the left buttock (Figure 1). The patient reported no medical or contributory family history. Upon review of systems, he described a burning sensation sometimes in the area of the lesion that would develop randomly throughout the year.

Three biopsies were performed, which revealed a collection of slightly dilated blood vessels with normal-appearing endothelial cells occupying the mid dermis and deep dermis (Figure 2). Immunohistochemical stains with antibodies were directed against human herpesvirus 8 (HHV-8), CD31, CD34, the cell surface glycoprotein podoplanin, Ki-67, and smooth muscle actin antigens, with appropriate controls. The vessel walls were positive for CD31, CD34, and smooth muscle actin, and negative for HHV-8 and podoplanin; Ki-67 was not increased. These histologic findings were consistent with a diagnosis of DDA. A detailed history was taken. The cause of DDA in our patient was uncertain.

Comment
Classification and Epidemiology
Diffuse dermal angiomatosis is a rare acquired, cutaneous, reactive, vascular disorder first described by Krell et al1 in 1994. Diffuse dermal angiomatosis is benign and is classified in the group of cutaneous reactive angiomatoses,2 which are benign vascular disorders marked by intravascular and extravascular hyperplasia of endothelial cells that may or may not include pericytes.2 Diffuse dermal angiomatosis was originally described as a variant of CREA, which is characterized by hyperplasia of endothelial dermal cells and intravascular proliferation.3 However, DDA has more recently been identified as a distinct disorder on the spectrum of CREA rather than as a variant of CREA.2 Given the recent reclassification, not all physicians make this distinction. However, as more case reports of DDA are published, physicians continue to support this change.4 Nevertheless, DDA has been an established disorder since 1994.1
Vascular proliferation in DDA is hypothesized to stem from ischemia or inflammation.5 Peripheral vascular atherosclerosis has been associated with DDA.6 The epidemiology of DDA is not well known because of the rarity of the disease. We performed a more specific review of the literature by limiting the PubMed search of articles indexed for MEDLINE to the term diffuse dermal angiomatosis rather than a broader search including all reactive angioendotheliomatoses. Only 31 case reports have been published1,3-32; of them, only adults were affected. Most reported cases were in middle-aged females. A summary of the demographics of DDA is provided in the Table.1,3-32

Pathophysiology
The pathophysiology of DDA remains unclear. It has been hypothesized that ischemia or inflammation creates local hypoxia, leading to an increase in vascular endothelial growth factor with subsequent endothelial proliferation and neovascularization.5 Rongioletti and Robora2 supported this hypothesis, proposing that occlusion or inflammation of the vasculature creates microthrombi and thus hypoxia. Afterward, histiocytes are recruited to reabsorb the microthrombi while hyperplasia of endothelial cells and pericytes ensues.7 Complete resolution of skin lesions following revascularization provides support for this theory.8
Etiology
Diffuse dermal angiomatosis is a rare complication of ischemia that may be secondary to atherosclerosis, arteriovenous fistula, or macromastia.9-11 In DDA of the breasts, ulcerations of fatty tissue occur due to trauma in these patients who have large pendulous breasts, causing angiogenesis resembling DDA histologically.2 One case of DDA was reported secondary to relative ischemia from cutis marmorata telangiectatica congenita,12 whereas another case highlighted Wegener granulomatosis as the cause of ischemia.7 There also have been reported cases associated with calciphylaxis and anticardiolipin antibiodies.13 In general, any medical condition that can lead to ischemia can cause DDA. Comorbid conditions for DDA include cardiovascular disease, hypertension, diabetes mellitus, and most often severe peripheral vascular disease. Many patients also have a history of smoking.14 Diffuse dermal angiomatosis rarely presents without underlying comorbidity, with only 1 case report of unknown cause (Table).
Presentation, Histopathology, and Differential Diagnosis
Cutaneous reactive angiomatosis disorders present the same clinically, with multiple erythematous to violaceous purpuric patches and plaques that can progress to necrosis and ulceration. Lesions are widely distributed but are predisposed to the upper and lower extremities.2 The differential diagnosis of DDA includes CREA, acroangiodermatitis (pseudo–Kaposi sarcoma), or vascular malignancies such as Kaposi sarcoma and low-grade angiosarcoma.7
In DDA, lesions may be painful and sometimes have a central ulceration.15 They often are associated with notable peripheral vascular atherosclerotic disease and are mainly found on the lower extremities.12,16 Histologically, DDA presents as a diffuse proliferation of endothelial cells between collagen bundles. The endothelial cells are distributed throughout the papillary and reticular dermis and develop into vascular lumina.17 Furthermore, the proliferating endothelial cells are spindle shaped and contain vacuolated cytoplasm.14
Acroangiodermatitis, or pseudo–Kaposi sarcoma, presents as slow-growing, erythematous to violaceous, brown, or dusky macules, papules, or plaques of the legs.14 Histologically, acroangiodermatitis presents with relatively less proliferation of endothelial cells found intravascularly rather than extravascularly, as in DDA, forming new thick-walled vessels in a lobular pattern in the papillary dermis.14
Vascular malignancies, such as Kaposi sarcoma and angiosarcoma, may present similarly to DDA. Kaposi sarcoma, for example, presents as erythematous to violaceous patches, plaques, or nodules found mostly on the extremities.7 Histologically, spindle cells and vascular structures also are found but in a clefting pattern representative of Kaposi sarcoma (so-called vascular slits).7 Diffuse dermal angiomatosis and vascular malignancies can further be distinguished based on atypia of the proliferations and staining for HHV-8.7,14 Lastly, DDA differs from vascular tumors in that vascular tumors are reactive to locations of occluded vessels, with vascular proliferation ceasing once the underlying cause of hypoxia is removed.2
Treatment
There is no standard treatment of DDA.7 Treatment of the underlying cause of ischemia is the primary goal, which will cause the DDA to resolve in most cases. Stenting, removal of an arteriovenous fistula, or other forms of revascularization may be warranted.1,5,6,10,17,29,30
Reported medical therapies for DDA include systemic or topical corticosteroids used for their antiangiogenic properties with varying results.7 Isotretinoin also has been used, which has been found to be effective in several cases of DDA of the breast, though 1 study reported a subsequent elevated lipid profile, requiring a decrease in dosage.14,15,27,31
Most interestingly, a study by Sanz-Motilva et al16 demonstrated that control of comorbidities, especially smoking cessation, led to improvement, which highlights the importance of incorporating nonpharmacotherapy rather than initiating treatment solely with medication. The Table summarizes treatments used and their efficacy.
Conclusion
Diffuse dermal angiomatosis is associated with medical conditions that predispose an individual to ischemia. Although rare, DDA can present as painful and visibly disturbing lesions that can affect the daily life of afflicted patients. By reporting the few cases that do arise and reviewing prior cases and their treatments, physicians can consider DDA within the differential diagnosis and identify which treatment is most efficient for a given patient. For all DDA patients, strict control of comorbidities, especially smoking cessation, should be incorporated into the treatment plan. When DDA affects the breasts, isotretinoin appears to provide the best relief. Otherwise, treatment of the underlying cause, revascularization, withdrawal of the offending agent, or steroids seem to be the best treatment options.
- Krell JM, Sanchez RL, Solomon AR. Diffuse dermal angiomatosis: a variant of reactive cutaneous angioendotheliomatosis. J Cutan Pathol. 1994;21:363-370.
- Rongioletti F, Robora A. Cutaneous reactive angiomatoses: patterns and classification of reactive vascular proliferation. J Am Acad Dermatol. 2003;49:887-896.
- Crickx E, Saussine A, Vignon-Pennamen MD, et al. Diffuse dermal angiomatosis associated with severe atherosclerosis: two cases and review of the literature. Clin Exp Dermatol. 2015;40:521-524.
- Reusche R, Winocour S, Degnim A, et al. Diffuse dermal angiomatosis of the breast: a series of 22 cases from a single institution. Gland Surg. 2015;4:554-560.
- Sriphojanart T, Vachiramon V. Diffuse dermal angiomatosis: a clue to the diagnosis of atherosclerotic vascular disease. Case Rep Dermatol. 2015;7:100-106.
- Kimyai-Asadi A, Nousari HC, Ketabchi N, et al. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with atherosclerosis. J Am Acad Dermatol. 1999;40:257-259.
- Bassi A, Arunachalam M, Maio V, et al. Diffuse dermal angiomatosis in a patient with an iatrogenic arterio-venous fistula and Wegener’s granulomatosis. Acta Derm Venereol. 2013;93:93-94.
- Ormerod E, Miller K, Kennedy C. Diffuse dermal angiomatosis: a contributory factor to ulceration in a patient with renal transplant. Clin Exp Dermatol. 2015;40:48-51.
- Kim S, Elenitsas R, James WD. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with peripheral vascular atherosclerosis. Arch Dermatol. 2002;138:456-458.
- Requena L, Fariña MC, Renedo G, et al. Intravascular and diffuse dermal reactive angioendotheliomatosis secondary to iatrogenic arteriovenous fistulas. J Cutan Pathol. 1999;26:159-164.
- Villa MT, White LE, Petronic-Rosic V, et al. The treatment of diffuse dermal angiomatosis of the breast with reduction mammoplasty. Arch Dermatol. 2008;144:693-694.
- Halbesleben JJ, Cleveland MG, Stone MS. Diffuse dermal angiomatosis arising in cutis marmorata telangiectatica congenita. Arch Dermatol. 2010;146:1311-1313.
- Ferreli C, Atzori L, Pinna AL, et al. Diffuse dermal angiomatosis: a clinical mimicker of vasculitis associated with calciphylaxis and monoclonal gammopathy. G Ital Dermatol Venereol. 2015;150:115-121.
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347.
- Steele KT, Sullivan BJ, Wanat KA, et al. Diffuse dermal angiomatosis associated with calciphylaxis in a patient with end-stage renal disease.J Cutan Pathol. 2013;40:829-832.
- Sanz-Motilva V, Martorell-Calatayud A, Rongioletti F, et al. Diffuse dermal angiomatosis of the breast: clinical and histopathological features. Int J Dermatol. 2014;53:445-449.
- Kirkland CR, Hawayek LH, Mutasim DF. Atherosclerosis-induced diffuse dermal angiomatosis with fatal outcome. Arch Dermatol. 2010;146:684-685.
- Sommer S, Merchant WJ, Wilson CL. Diffuse dermal angiomatosis due to an iatrogenic arteriovenous fistula. Acta Derm Venereol. 2004;84:251-252.
- Corti MA, Rongioletti F, Borradori L, et al. Cutaneous reactive angiomatosis with combined histological pattern mimicking a cellulitis. Dermatology. 2013;227:226-230.
- Tollefson MM, McEvoy MT, Torgerson RR, et al. Diffuse dermal angiomatosis of the breast: clinicopathologic study of 5 patients. J Am Acad Dermatol. 2014;71:1212-1217.
- Walton K, Liggett J. Diffuse dermal angiomatosis: a case report. J Am Acad Dermatol. 2012;66(suppl 1):AB49.
- Mayor-Ibarguren A, Gómez-Fernández C, Beato-Merino MJ, et al. Diffuse reactive angioendotheliomatosis secondary to the administration of trabectedin and pegfilgrastim. Am J Dermatopathol. 2015;37:581-584.
- Lora V, Cota C, Cerroni L. Diffuse dermal angiomatosis of the abdomen. Eur J Dermatol. 2015;25:350-352.
- Pichardo RO, Lu D, Sangueza OP, et al. What is your diagnosis? diffuse dermal angiomatosis secondary to anticardiolipin antibodies. Am J Dermatopathol. 2002;24:502.
- Kutzner H, Requena L, Mentzel T, et al. Diffuse dermal angiomatosis. Hautarzt. 2002;53:808-812.
- McLaughlin ER, Morris R, Weiss SW, et al. Diffuse dermal angiomatosis of the breast: response to isotretinoin. J Am Acad Dermatol. 2001;45:462-465.
- Prinz Vavricka BM, Barry C, Victor T, et al. Diffuse dermal angiomatosis associated with calciphylaxis. Am J Dermatopathol. 2009;31:653-657.
- Müller CS, Wagner A, Pföhler C, et al. Cup-shaped painful ulcer of abdominal wall. Hautarzt. 2008;59:656-658.
- Draper BK, Boyd AS. Diffuse dermal angiomatosis. J Cutan Pathol. 2006;33:646-648.
- Adams BJ, Goldberg S, Massey HD, et al. A cause of unbearably painful breast, diffuse dermal angiomatosis. Gland Surg. 2012;1. doi:10.3978/j.issn.2227-684X.2012.07.02.
- Quatresooz P, Fumal I, Willemaers V, et al. Diffuse dermal angiomatosis: a previously undescribed pattern of immunoglobulin and complement deposits in two cases. Am J Dermatopathol. 2006;28:150-154.
- Morimoto K, Ioka H, Asada H, et al. Diffuse dermal angiomatosis. Eur J Vasc Endovasc Surg. 2011;42:381-383.
- Krell JM, Sanchez RL, Solomon AR. Diffuse dermal angiomatosis: a variant of reactive cutaneous angioendotheliomatosis. J Cutan Pathol. 1994;21:363-370.
- Rongioletti F, Robora A. Cutaneous reactive angiomatoses: patterns and classification of reactive vascular proliferation. J Am Acad Dermatol. 2003;49:887-896.
- Crickx E, Saussine A, Vignon-Pennamen MD, et al. Diffuse dermal angiomatosis associated with severe atherosclerosis: two cases and review of the literature. Clin Exp Dermatol. 2015;40:521-524.
- Reusche R, Winocour S, Degnim A, et al. Diffuse dermal angiomatosis of the breast: a series of 22 cases from a single institution. Gland Surg. 2015;4:554-560.
- Sriphojanart T, Vachiramon V. Diffuse dermal angiomatosis: a clue to the diagnosis of atherosclerotic vascular disease. Case Rep Dermatol. 2015;7:100-106.
- Kimyai-Asadi A, Nousari HC, Ketabchi N, et al. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with atherosclerosis. J Am Acad Dermatol. 1999;40:257-259.
- Bassi A, Arunachalam M, Maio V, et al. Diffuse dermal angiomatosis in a patient with an iatrogenic arterio-venous fistula and Wegener’s granulomatosis. Acta Derm Venereol. 2013;93:93-94.
- Ormerod E, Miller K, Kennedy C. Diffuse dermal angiomatosis: a contributory factor to ulceration in a patient with renal transplant. Clin Exp Dermatol. 2015;40:48-51.
- Kim S, Elenitsas R, James WD. Diffuse dermal angiomatosis: a variant of reactive angioendotheliomatosis associated with peripheral vascular atherosclerosis. Arch Dermatol. 2002;138:456-458.
- Requena L, Fariña MC, Renedo G, et al. Intravascular and diffuse dermal reactive angioendotheliomatosis secondary to iatrogenic arteriovenous fistulas. J Cutan Pathol. 1999;26:159-164.
- Villa MT, White LE, Petronic-Rosic V, et al. The treatment of diffuse dermal angiomatosis of the breast with reduction mammoplasty. Arch Dermatol. 2008;144:693-694.
- Halbesleben JJ, Cleveland MG, Stone MS. Diffuse dermal angiomatosis arising in cutis marmorata telangiectatica congenita. Arch Dermatol. 2010;146:1311-1313.
- Ferreli C, Atzori L, Pinna AL, et al. Diffuse dermal angiomatosis: a clinical mimicker of vasculitis associated with calciphylaxis and monoclonal gammopathy. G Ital Dermatol Venereol. 2015;150:115-121.
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347.
- Steele KT, Sullivan BJ, Wanat KA, et al. Diffuse dermal angiomatosis associated with calciphylaxis in a patient with end-stage renal disease.J Cutan Pathol. 2013;40:829-832.
- Sanz-Motilva V, Martorell-Calatayud A, Rongioletti F, et al. Diffuse dermal angiomatosis of the breast: clinical and histopathological features. Int J Dermatol. 2014;53:445-449.
- Kirkland CR, Hawayek LH, Mutasim DF. Atherosclerosis-induced diffuse dermal angiomatosis with fatal outcome. Arch Dermatol. 2010;146:684-685.
- Sommer S, Merchant WJ, Wilson CL. Diffuse dermal angiomatosis due to an iatrogenic arteriovenous fistula. Acta Derm Venereol. 2004;84:251-252.
- Corti MA, Rongioletti F, Borradori L, et al. Cutaneous reactive angiomatosis with combined histological pattern mimicking a cellulitis. Dermatology. 2013;227:226-230.
- Tollefson MM, McEvoy MT, Torgerson RR, et al. Diffuse dermal angiomatosis of the breast: clinicopathologic study of 5 patients. J Am Acad Dermatol. 2014;71:1212-1217.
- Walton K, Liggett J. Diffuse dermal angiomatosis: a case report. J Am Acad Dermatol. 2012;66(suppl 1):AB49.
- Mayor-Ibarguren A, Gómez-Fernández C, Beato-Merino MJ, et al. Diffuse reactive angioendotheliomatosis secondary to the administration of trabectedin and pegfilgrastim. Am J Dermatopathol. 2015;37:581-584.
- Lora V, Cota C, Cerroni L. Diffuse dermal angiomatosis of the abdomen. Eur J Dermatol. 2015;25:350-352.
- Pichardo RO, Lu D, Sangueza OP, et al. What is your diagnosis? diffuse dermal angiomatosis secondary to anticardiolipin antibodies. Am J Dermatopathol. 2002;24:502.
- Kutzner H, Requena L, Mentzel T, et al. Diffuse dermal angiomatosis. Hautarzt. 2002;53:808-812.
- McLaughlin ER, Morris R, Weiss SW, et al. Diffuse dermal angiomatosis of the breast: response to isotretinoin. J Am Acad Dermatol. 2001;45:462-465.
- Prinz Vavricka BM, Barry C, Victor T, et al. Diffuse dermal angiomatosis associated with calciphylaxis. Am J Dermatopathol. 2009;31:653-657.
- Müller CS, Wagner A, Pföhler C, et al. Cup-shaped painful ulcer of abdominal wall. Hautarzt. 2008;59:656-658.
- Draper BK, Boyd AS. Diffuse dermal angiomatosis. J Cutan Pathol. 2006;33:646-648.
- Adams BJ, Goldberg S, Massey HD, et al. A cause of unbearably painful breast, diffuse dermal angiomatosis. Gland Surg. 2012;1. doi:10.3978/j.issn.2227-684X.2012.07.02.
- Quatresooz P, Fumal I, Willemaers V, et al. Diffuse dermal angiomatosis: a previously undescribed pattern of immunoglobulin and complement deposits in two cases. Am J Dermatopathol. 2006;28:150-154.
- Morimoto K, Ioka H, Asada H, et al. Diffuse dermal angiomatosis. Eur J Vasc Endovasc Surg. 2011;42:381-383.
Practice Points
- Diffuse dermal angiomatosis is commonly reported in patients with hypoxic comorbidities such as smoking or vascular disease as well as in women with large pendulous breasts.
- Effective treatments include control of comorbidities, revascularization, withdrawal of the offending agent, steroids, and isotretinoin.
Mobile App Rankings in Dermatology
As technology continues to advance, so too does its accessibility to the general population. In 2013, 56% of Americans owned a smartphone versus 77% in 2017.1With the increase in mobile applications (apps) available, it is no surprise that the market has extended into the medical field, with dermatology being no exception.2 The majority of dermatology apps can be classified as teledermatology apps, followed by self-surveillance, disease guide, and reference apps. Additional types of dermatology apps include dermoscopy, conference, education, photograph storage and sharing, and journal apps, and others.2 In this study, we examined Apple App Store rankings to determine the types of dermatology apps that are most popular among patients and physicians.
METHODS
A popular app rankings analyzer (App Annie) was used to search for dermatology apps along with their App Store rankings.3 Although iOS is not the most popular mobile device operating system, we chose to evaluate app rankings via the App Store because iPhones are the top-selling individual phones of any kind in the United States.4
We performed our analysis on a single day (July 14, 2018) given that app rankings can change daily. We incorporated the following keywords, which were commonly used in other dermatology app studies: dermatology, psoriasis, rosacea, acne, skin cancer, melanoma, eczema, and teledermatology. The category ranking was defined as the rank of a free or paid app in the App Store’s top charts for the selected country (United States), market (Apple), and device (iPhone) within their app category (Medical). Inclusion criteria required a ranking in the top 1500 Medical apps and being categorized in the App Store as a Medical app. Exclusion criteria included apps that focused on cosmetics, private practice, direct advertisements, photograph editing, or claims to cure skin disease, as well as non–English-language apps. The App Store descriptions were assessed to determine the type of each app (eg, teledermatology, disease guide) and target audience (patient, physician, or both).
Another search was performed using the same keywords but within the Health and Fitness category to capture potentially more highly ranked apps among patients. We also conducted separate searches within the Medical category using the keywords billing, coding, and ICD (International Classification of Diseases) to evaluate rankings for billing/coding apps, as well as EMR and electronic medical records for electronic medical record (EMR) apps.
RESULTS
The initial search yielded 851 results, which was narrowed down to 29 apps after applying the exclusion criteria. Of note, prior to application of the exclusion criteria, one dermatology app that was considered to be a direct advertisement app claiming to cure acne was ranked fourth of 1500 apps in the Medical category. However, the majority of the search results were excluded because they were not popular enough to be ranked among the top 1500 apps. There were more ranked dermatology apps in the Medical category targeting patients than physicians; 18 of 29 (62%) qualifying apps targeted patients and 11 (38%) targeted physicians (Tables 1 and 2). No apps targeted both groups. The most common type of ranked app targeting patients was self-surveillance (11/18), and the most common type targeting physicians was reference (8/11). The highest ranked app targeting patients was a teledermatology app with a ranking of 184, and the highest ranked app targeting physicians was educational, ranked 353. The least common type of ranked apps targeting patients were “other” (2/18 [11%]; 1 prescription and 1 UV monitor app) and conference (1/18 [6%]). The least common type of ranked apps targeting physicians were education (2/11 [18%]) and dermoscopy (1/11 [9%]).
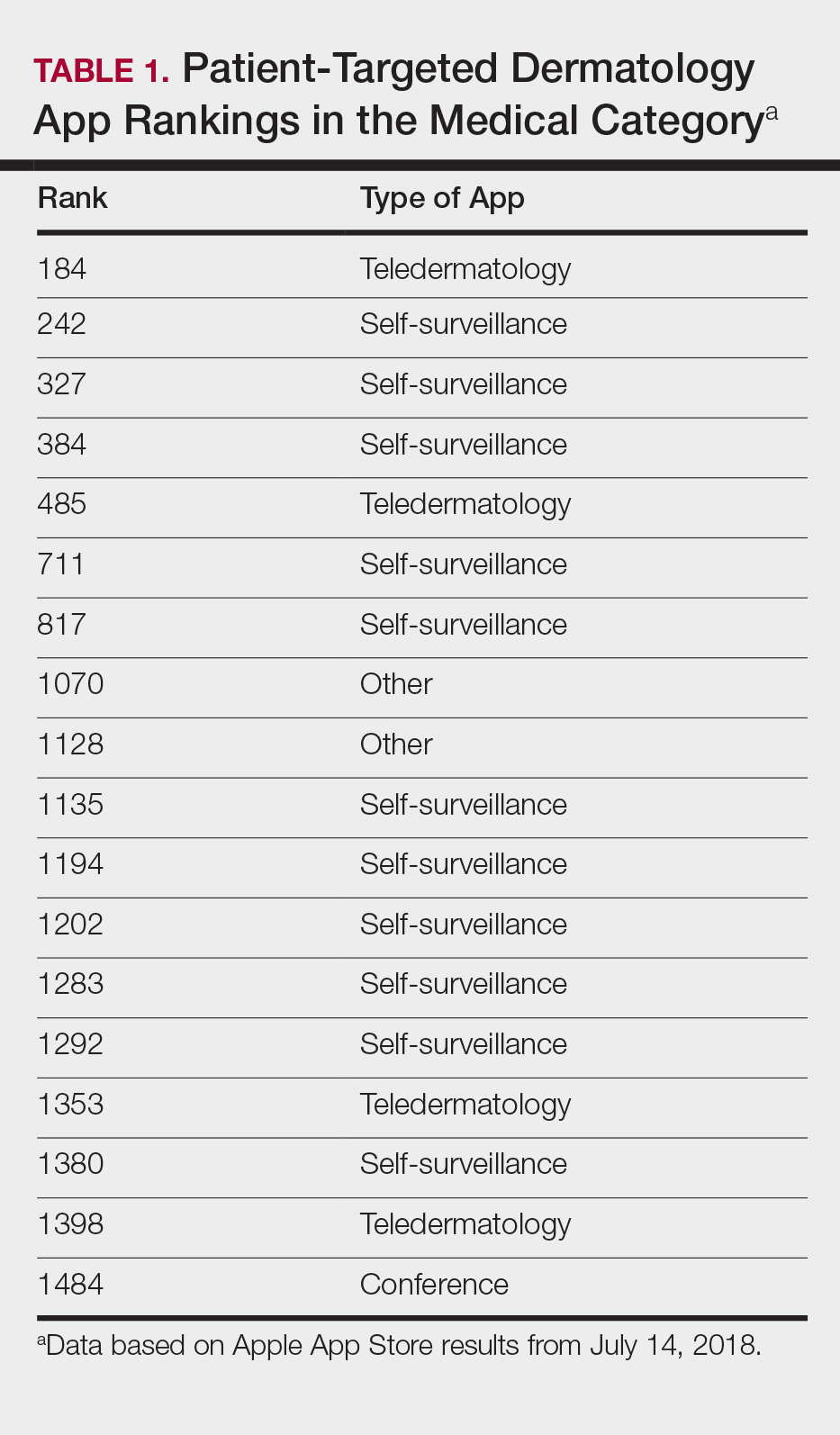
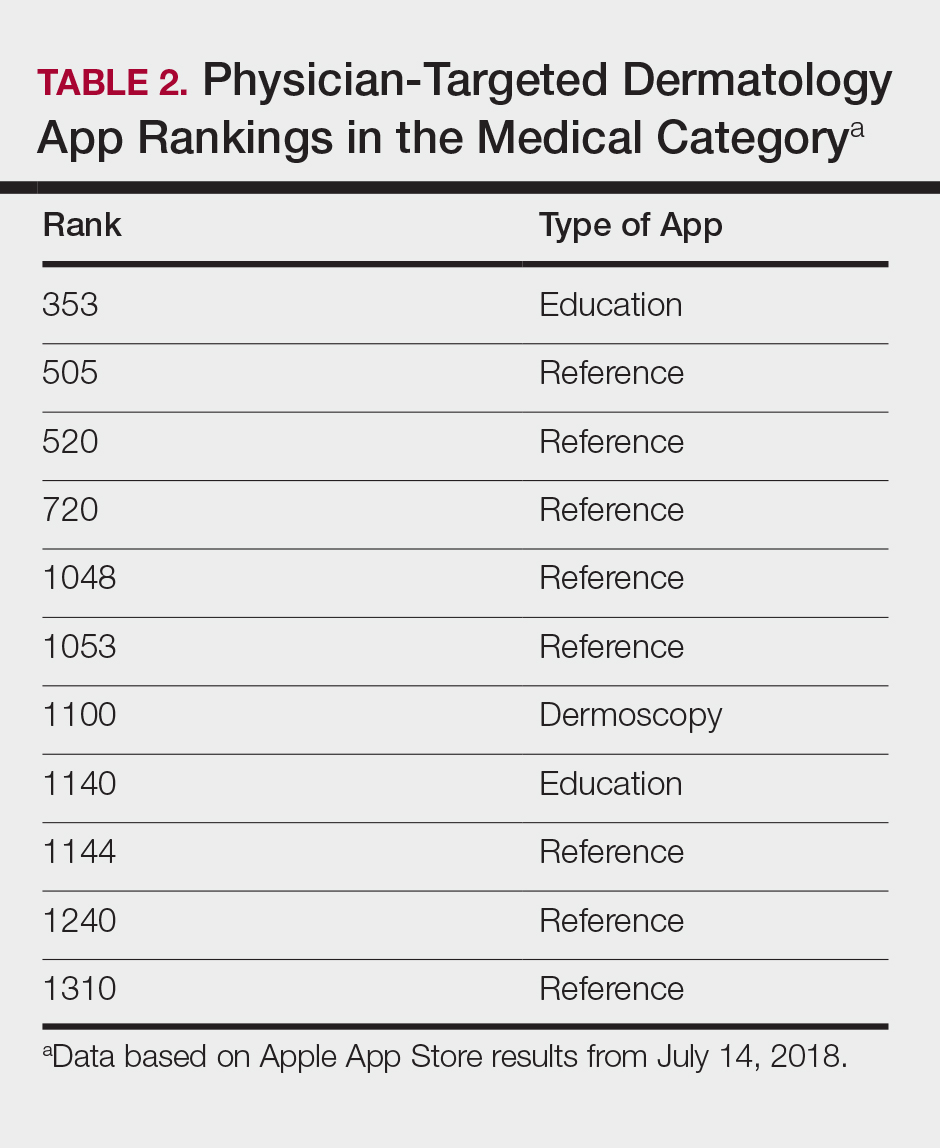
Our search of the Health and Fitness category yielded 6 apps, all targeting patients; 3 (50%) were self-surveillance apps, and 3 (50%) were classified as other (2 UV monitors and a conferencing app for cancer emotional support)(Table 3).

Our search of the Medical category for billing/coding and EMR apps yielded 232 and 164 apps, respectively; of them, 49 (21%) and 54 (33%) apps were ranked. These apps did not overlap with the dermatology-related search criteria; thus, we were not able to ascertain how many of these apps were used specifically by health care providers in dermatology.
COMMENT
Patient Apps
The most common apps used by patients are fitness and nutrition tracker apps categorized as Health and Fitness5,6; however, the majority of ranked dermatology apps are categorized as Medical per our findings. In a study of 557 dermatology patients, it was found that among the health-related apps they used, the most common apps after fitness/nutrition were references, followed by patient portals, self-surveillance, and emotional assistance apps.6 Our search was consistent with these findings, suggesting that the most desired dermatology apps by patients are those that allow them to be proactive with their health. It is no surprise that the top-ranked app targeting patients was a teledermatology app, followed by multiple self-surveillance apps. The highest ranked self-surveillance app in the Health and Fitness category focused on monitoring the effects of nutrition on symptoms of diseases including skin disorders, while the highest ranked (as well as the majority of) self-surveillance apps in the Medical category encompassed mole monitoring and cancer risk calculators.
Benefits of the ranked dermatology apps in the Medical and Health and Fitness categories targeting patients include more immediate access to health care and education. Despite this popularity among patients, Masud et al7 demonstrated that only 20.5% (9/44) of dermatology apps targeting patients may be reliable resources based on a rubric created by the investigators. Overall, there remains a research gap for a standardized scientific approach to evaluating app validity and reliability.
Teledermatology
Teledermatology apps are the most common dermatology apps,2 allowing for remote evaluation of patients through either live consultations or transmittance of medical information for later review by board-certified physicians.8 Features common to many teledermatology apps include accessibility on Android (Google Inc) and iOS as well as a web version. Security and Health Insurance Portability and Accountability Act compliance is especially important and is enforced through user authentications, data encryption, and automatic logout features. Data is not stored locally and is secured on a private server with backup. Referring providers and consultants often can communicate within the app. Insurance providers also may cover teledermatology services, and if not, the out-of-pocket costs often are affordable.
The highest-ranked patient app (ranked 184 in the Medical category) was a teledermatology app that did not meet the American Telemedicine Association standards for teledermatology apps.9 The popularity of this app among patients may have been attributable to multiple ease-of-use and turnaround time features. The user interface was simplistic, and the design was appealing to the eye. The entry field options were minimal to avoid confusion. The turnaround time to receive a diagnosis depended on 1 of 3 options, including a more rapid response for an increased cost. Ease of use was the highlight of this app at the cost of accuracy, as the limited amount of information that users were required to provide physicians compromised diagnostic accuracy in this app.
For comparison, we chose a nonranked (and thus less frequently used) teledermatology app that had previously undergone scientific evaluation using 13 evaluation criteria specific to teledermatology.10 The app also met the American Telemedicine Association standard for teledermatology apps.9 The app was originally a broader telemedicine app but featured a section specific to teledermatology. The user interface was simple but professional, almost resembling an EMR. The input fields included a comprehensive history that permitted a better evaluation of a lesion but might be tedious for users. This app boasted professionalism and accuracy, but from a user standpoint, it may have been too time-consuming.
Striking a balance between ensuring proper care versus appealing to patients is a difficult but important task. Based on this study, it appears that popular patient apps may in fact have less scientific rationale and therefore potentially less accuracy.
Self-surveillance
Although self-surveillance apps did not account for the highest-ranked app, they were the most frequently ranked app type in our study. Most of the ranked self-surveillance apps in the Medical category were for monitoring lesions over time to assess for changes. These apps help users take photographs that are well organized in a single, easy-to-find location. Some apps were risk calculators that assessed the risk for malignancies using a questionnaire. The majority of these self-surveillance apps were specific to skin cancer detection. Of note, one of the ranked self-surveillance apps assessed drug effectiveness by monitoring clinical appearance and symptoms. The lowest ranked self-surveillance app in the top 1500 ranked Medical apps in our search monitored cancer symptoms not specific to dermatology. Although this app had a low ranking (1380/1500), it received a high number of reviews and was well rated at 4.8 out of 5 stars; therefore, it seemed more helpful than the other higher-ranked apps targeting patients, which had higher rankings but minimal to no reviews or ratings. A comparison of the ease-of-use features of all the ranked patient-targeted self-surveillance apps in the Medical category is provided in Table 4.
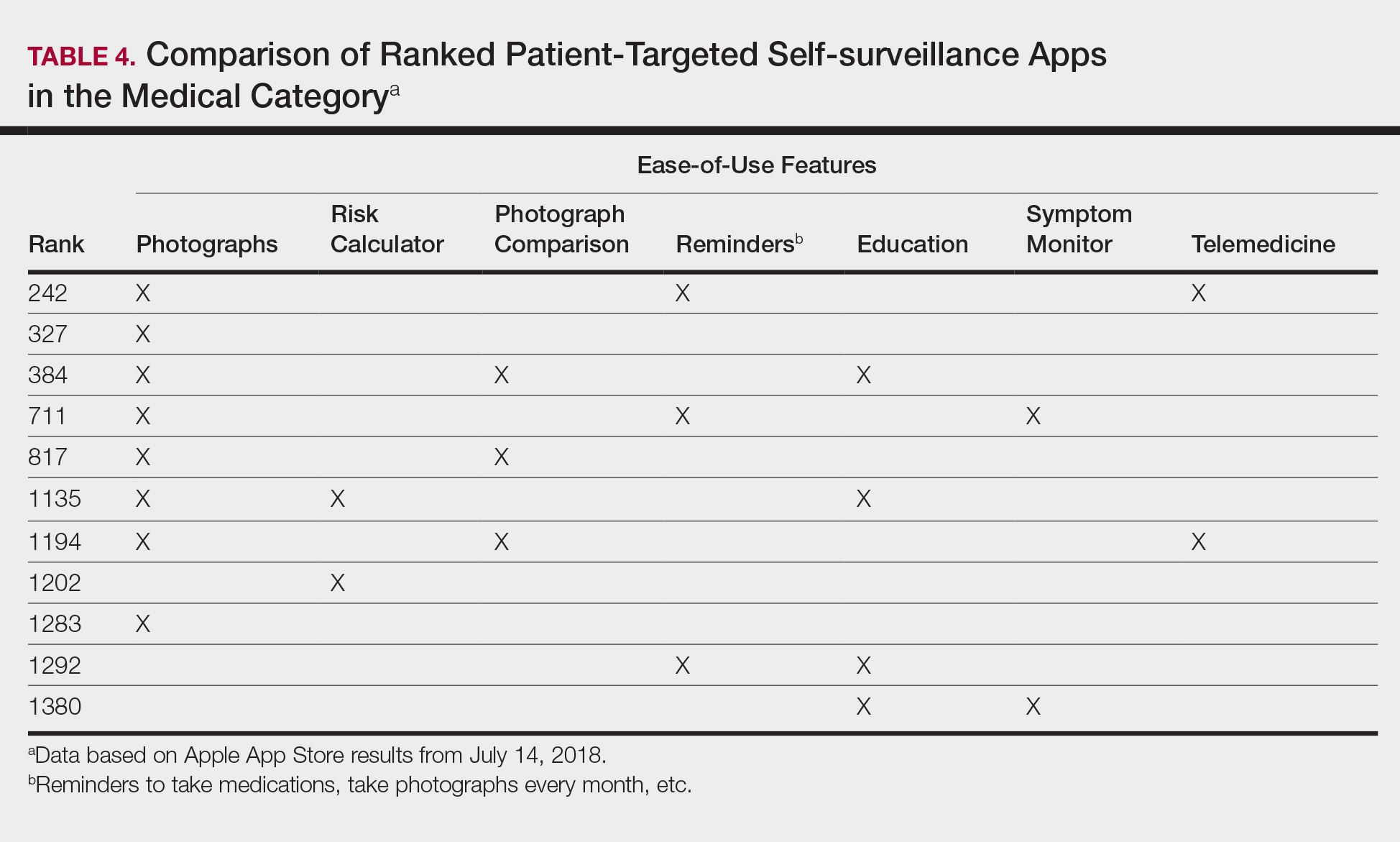
Physician Apps
After examining the results of apps targeting physicians, we realized that the data may be accurate but may not be as representative of all currently practicing dermatology providers. Given the increased usage of apps among younger age groups,11 our data may be skewed toward medical students and residents, supported by the fact that the top-ranked physician app in our study was an education app and the majority were reference apps. Future studies are needed to reexamine app ranking as this age group transitions from entry-level health care providers in the next 5 to 10 years. These findings also suggest less frequent app use among more veteran health care providers within our specific search parameters. Therefore, we decided to do subsequent searches for available billing/coding and EMR apps, which were many, but as mentioned above, none were specific to dermatology.
General Dermatology References
Most of the dermatology reference apps were formatted as e-books; however, other apps such as the Amazon Kindle app (categorized under Books) providing access to multiple e-books within one app were not included. Some apps included study aid features (eg, flash cards, quizzes), and topics spanned both dermatology and dermatopathology. Apps provide a unique way for on-the-go studying for dermatologists in training, and if the usage continues to grow, there may be a need for increased formal integration in dermatology education in the future.
Journals
Journal apps were not among those listed in the top-ranked apps we evaluated, which we suspect may be because journals were categorized differently from one journal to the next; for example, the Journal of the American Academy of Dermatology was ranked 1168 in the Magazines and Newspapers category. On the other hand, Dermatology World was ranked 1363 in the Reference category. An article’s citation affects the publishing journal’s impact factor, which is one of the most important variables in measuring a journal’s influence. In the future, there may be other variables that could aid in understanding journal impact as it relates to the journal’s accessibility.
Limitations
Our study did not look at Android apps. The top chart apps in the Android and Apple App Stores use undisclosed algorithms likely involving different characteristics such as number of downloads, frequency of updates, number of reviews, ratings, and more. Thus, the rankings across these different markets would not be comparable. Although our choice of keywords stemmed from the majority of prior studies looking at dermatology apps, our search was limited due to the use of these specific keywords. To avoid skewing data by cross-comparison of noncomparable categories, we could not compare apps in the Medical category versus those in other categories.
CONCLUSION
There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps. As app usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in our education, and as such, it will become more critical to develop formal scientific standards. Given these future trends, we may need to increase our current literature and understanding of apps in dermatology with regard to their impact on both patients and health care providers.
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center website. http://www.pewglobal.org/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/#table. Published June 19, 2018. Accessed August 28, 2018.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018;24. pii:13030/qt3hs7n9z6.
- App Annie website. https://www.appannie.com/top/. Accessed August 28, 2018.
- Number of iPhone users in the United States from 2012 to 2016 (in millions). Statista website. https://www.statista.com/statistics/232790/forecast-of-apple-users-in-the-us/. Accessed August 28, 2018.
- Burkhart C. Medical mobile apps and dermatology. Cutis. 2012;90:278-281.
- Wolf JA, Moreau JF, Patton TJ, et al. Prevalence and impact of health-related internet and smartphone use among dermatology patients. Cutis. 2015;95:323-328.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Walocko FM, Tejasvi T. Teledermatology applications in skin cancer diagnosis. Dermatol Clin. 2017;35:559-563.
- Krupinski E, Burdick A, Pak H, et al. American Telemedicine Association’s practice guidelines for teledermatology. Telemed J E Health. 2008;14:289-302.
- Ho B, Lee M, Armstrong AW. Evaluation criteria for mobile teledermatology applications and comparison of major mobile teledermatology applications. Telemed J E Health. 2013;19:678-682.
- Number of mobile app hours per smartphone and tablet app user in the United States in June 2016, by age group. Statista website. https://www.statista.com/statistics/323522/us-user-mobile-app-engagement-age/. Accessed September 18, 2018.
As technology continues to advance, so too does its accessibility to the general population. In 2013, 56% of Americans owned a smartphone versus 77% in 2017.1With the increase in mobile applications (apps) available, it is no surprise that the market has extended into the medical field, with dermatology being no exception.2 The majority of dermatology apps can be classified as teledermatology apps, followed by self-surveillance, disease guide, and reference apps. Additional types of dermatology apps include dermoscopy, conference, education, photograph storage and sharing, and journal apps, and others.2 In this study, we examined Apple App Store rankings to determine the types of dermatology apps that are most popular among patients and physicians.
METHODS
A popular app rankings analyzer (App Annie) was used to search for dermatology apps along with their App Store rankings.3 Although iOS is not the most popular mobile device operating system, we chose to evaluate app rankings via the App Store because iPhones are the top-selling individual phones of any kind in the United States.4
We performed our analysis on a single day (July 14, 2018) given that app rankings can change daily. We incorporated the following keywords, which were commonly used in other dermatology app studies: dermatology, psoriasis, rosacea, acne, skin cancer, melanoma, eczema, and teledermatology. The category ranking was defined as the rank of a free or paid app in the App Store’s top charts for the selected country (United States), market (Apple), and device (iPhone) within their app category (Medical). Inclusion criteria required a ranking in the top 1500 Medical apps and being categorized in the App Store as a Medical app. Exclusion criteria included apps that focused on cosmetics, private practice, direct advertisements, photograph editing, or claims to cure skin disease, as well as non–English-language apps. The App Store descriptions were assessed to determine the type of each app (eg, teledermatology, disease guide) and target audience (patient, physician, or both).
Another search was performed using the same keywords but within the Health and Fitness category to capture potentially more highly ranked apps among patients. We also conducted separate searches within the Medical category using the keywords billing, coding, and ICD (International Classification of Diseases) to evaluate rankings for billing/coding apps, as well as EMR and electronic medical records for electronic medical record (EMR) apps.
RESULTS
The initial search yielded 851 results, which was narrowed down to 29 apps after applying the exclusion criteria. Of note, prior to application of the exclusion criteria, one dermatology app that was considered to be a direct advertisement app claiming to cure acne was ranked fourth of 1500 apps in the Medical category. However, the majority of the search results were excluded because they were not popular enough to be ranked among the top 1500 apps. There were more ranked dermatology apps in the Medical category targeting patients than physicians; 18 of 29 (62%) qualifying apps targeted patients and 11 (38%) targeted physicians (Tables 1 and 2). No apps targeted both groups. The most common type of ranked app targeting patients was self-surveillance (11/18), and the most common type targeting physicians was reference (8/11). The highest ranked app targeting patients was a teledermatology app with a ranking of 184, and the highest ranked app targeting physicians was educational, ranked 353. The least common type of ranked apps targeting patients were “other” (2/18 [11%]; 1 prescription and 1 UV monitor app) and conference (1/18 [6%]). The least common type of ranked apps targeting physicians were education (2/11 [18%]) and dermoscopy (1/11 [9%]).


Our search of the Health and Fitness category yielded 6 apps, all targeting patients; 3 (50%) were self-surveillance apps, and 3 (50%) were classified as other (2 UV monitors and a conferencing app for cancer emotional support)(Table 3).

Our search of the Medical category for billing/coding and EMR apps yielded 232 and 164 apps, respectively; of them, 49 (21%) and 54 (33%) apps were ranked. These apps did not overlap with the dermatology-related search criteria; thus, we were not able to ascertain how many of these apps were used specifically by health care providers in dermatology.
COMMENT
Patient Apps
The most common apps used by patients are fitness and nutrition tracker apps categorized as Health and Fitness5,6; however, the majority of ranked dermatology apps are categorized as Medical per our findings. In a study of 557 dermatology patients, it was found that among the health-related apps they used, the most common apps after fitness/nutrition were references, followed by patient portals, self-surveillance, and emotional assistance apps.6 Our search was consistent with these findings, suggesting that the most desired dermatology apps by patients are those that allow them to be proactive with their health. It is no surprise that the top-ranked app targeting patients was a teledermatology app, followed by multiple self-surveillance apps. The highest ranked self-surveillance app in the Health and Fitness category focused on monitoring the effects of nutrition on symptoms of diseases including skin disorders, while the highest ranked (as well as the majority of) self-surveillance apps in the Medical category encompassed mole monitoring and cancer risk calculators.
Benefits of the ranked dermatology apps in the Medical and Health and Fitness categories targeting patients include more immediate access to health care and education. Despite this popularity among patients, Masud et al7 demonstrated that only 20.5% (9/44) of dermatology apps targeting patients may be reliable resources based on a rubric created by the investigators. Overall, there remains a research gap for a standardized scientific approach to evaluating app validity and reliability.
Teledermatology
Teledermatology apps are the most common dermatology apps,2 allowing for remote evaluation of patients through either live consultations or transmittance of medical information for later review by board-certified physicians.8 Features common to many teledermatology apps include accessibility on Android (Google Inc) and iOS as well as a web version. Security and Health Insurance Portability and Accountability Act compliance is especially important and is enforced through user authentications, data encryption, and automatic logout features. Data is not stored locally and is secured on a private server with backup. Referring providers and consultants often can communicate within the app. Insurance providers also may cover teledermatology services, and if not, the out-of-pocket costs often are affordable.
The highest-ranked patient app (ranked 184 in the Medical category) was a teledermatology app that did not meet the American Telemedicine Association standards for teledermatology apps.9 The popularity of this app among patients may have been attributable to multiple ease-of-use and turnaround time features. The user interface was simplistic, and the design was appealing to the eye. The entry field options were minimal to avoid confusion. The turnaround time to receive a diagnosis depended on 1 of 3 options, including a more rapid response for an increased cost. Ease of use was the highlight of this app at the cost of accuracy, as the limited amount of information that users were required to provide physicians compromised diagnostic accuracy in this app.
For comparison, we chose a nonranked (and thus less frequently used) teledermatology app that had previously undergone scientific evaluation using 13 evaluation criteria specific to teledermatology.10 The app also met the American Telemedicine Association standard for teledermatology apps.9 The app was originally a broader telemedicine app but featured a section specific to teledermatology. The user interface was simple but professional, almost resembling an EMR. The input fields included a comprehensive history that permitted a better evaluation of a lesion but might be tedious for users. This app boasted professionalism and accuracy, but from a user standpoint, it may have been too time-consuming.
Striking a balance between ensuring proper care versus appealing to patients is a difficult but important task. Based on this study, it appears that popular patient apps may in fact have less scientific rationale and therefore potentially less accuracy.
Self-surveillance
Although self-surveillance apps did not account for the highest-ranked app, they were the most frequently ranked app type in our study. Most of the ranked self-surveillance apps in the Medical category were for monitoring lesions over time to assess for changes. These apps help users take photographs that are well organized in a single, easy-to-find location. Some apps were risk calculators that assessed the risk for malignancies using a questionnaire. The majority of these self-surveillance apps were specific to skin cancer detection. Of note, one of the ranked self-surveillance apps assessed drug effectiveness by monitoring clinical appearance and symptoms. The lowest ranked self-surveillance app in the top 1500 ranked Medical apps in our search monitored cancer symptoms not specific to dermatology. Although this app had a low ranking (1380/1500), it received a high number of reviews and was well rated at 4.8 out of 5 stars; therefore, it seemed more helpful than the other higher-ranked apps targeting patients, which had higher rankings but minimal to no reviews or ratings. A comparison of the ease-of-use features of all the ranked patient-targeted self-surveillance apps in the Medical category is provided in Table 4.

Physician Apps
After examining the results of apps targeting physicians, we realized that the data may be accurate but may not be as representative of all currently practicing dermatology providers. Given the increased usage of apps among younger age groups,11 our data may be skewed toward medical students and residents, supported by the fact that the top-ranked physician app in our study was an education app and the majority were reference apps. Future studies are needed to reexamine app ranking as this age group transitions from entry-level health care providers in the next 5 to 10 years. These findings also suggest less frequent app use among more veteran health care providers within our specific search parameters. Therefore, we decided to do subsequent searches for available billing/coding and EMR apps, which were many, but as mentioned above, none were specific to dermatology.
General Dermatology References
Most of the dermatology reference apps were formatted as e-books; however, other apps such as the Amazon Kindle app (categorized under Books) providing access to multiple e-books within one app were not included. Some apps included study aid features (eg, flash cards, quizzes), and topics spanned both dermatology and dermatopathology. Apps provide a unique way for on-the-go studying for dermatologists in training, and if the usage continues to grow, there may be a need for increased formal integration in dermatology education in the future.
Journals
Journal apps were not among those listed in the top-ranked apps we evaluated, which we suspect may be because journals were categorized differently from one journal to the next; for example, the Journal of the American Academy of Dermatology was ranked 1168 in the Magazines and Newspapers category. On the other hand, Dermatology World was ranked 1363 in the Reference category. An article’s citation affects the publishing journal’s impact factor, which is one of the most important variables in measuring a journal’s influence. In the future, there may be other variables that could aid in understanding journal impact as it relates to the journal’s accessibility.
Limitations
Our study did not look at Android apps. The top chart apps in the Android and Apple App Stores use undisclosed algorithms likely involving different characteristics such as number of downloads, frequency of updates, number of reviews, ratings, and more. Thus, the rankings across these different markets would not be comparable. Although our choice of keywords stemmed from the majority of prior studies looking at dermatology apps, our search was limited due to the use of these specific keywords. To avoid skewing data by cross-comparison of noncomparable categories, we could not compare apps in the Medical category versus those in other categories.
CONCLUSION
There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps. As app usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in our education, and as such, it will become more critical to develop formal scientific standards. Given these future trends, we may need to increase our current literature and understanding of apps in dermatology with regard to their impact on both patients and health care providers.
As technology continues to advance, so too does its accessibility to the general population. In 2013, 56% of Americans owned a smartphone versus 77% in 2017.1With the increase in mobile applications (apps) available, it is no surprise that the market has extended into the medical field, with dermatology being no exception.2 The majority of dermatology apps can be classified as teledermatology apps, followed by self-surveillance, disease guide, and reference apps. Additional types of dermatology apps include dermoscopy, conference, education, photograph storage and sharing, and journal apps, and others.2 In this study, we examined Apple App Store rankings to determine the types of dermatology apps that are most popular among patients and physicians.
METHODS
A popular app rankings analyzer (App Annie) was used to search for dermatology apps along with their App Store rankings.3 Although iOS is not the most popular mobile device operating system, we chose to evaluate app rankings via the App Store because iPhones are the top-selling individual phones of any kind in the United States.4
We performed our analysis on a single day (July 14, 2018) given that app rankings can change daily. We incorporated the following keywords, which were commonly used in other dermatology app studies: dermatology, psoriasis, rosacea, acne, skin cancer, melanoma, eczema, and teledermatology. The category ranking was defined as the rank of a free or paid app in the App Store’s top charts for the selected country (United States), market (Apple), and device (iPhone) within their app category (Medical). Inclusion criteria required a ranking in the top 1500 Medical apps and being categorized in the App Store as a Medical app. Exclusion criteria included apps that focused on cosmetics, private practice, direct advertisements, photograph editing, or claims to cure skin disease, as well as non–English-language apps. The App Store descriptions were assessed to determine the type of each app (eg, teledermatology, disease guide) and target audience (patient, physician, or both).
Another search was performed using the same keywords but within the Health and Fitness category to capture potentially more highly ranked apps among patients. We also conducted separate searches within the Medical category using the keywords billing, coding, and ICD (International Classification of Diseases) to evaluate rankings for billing/coding apps, as well as EMR and electronic medical records for electronic medical record (EMR) apps.
RESULTS
The initial search yielded 851 results, which was narrowed down to 29 apps after applying the exclusion criteria. Of note, prior to application of the exclusion criteria, one dermatology app that was considered to be a direct advertisement app claiming to cure acne was ranked fourth of 1500 apps in the Medical category. However, the majority of the search results were excluded because they were not popular enough to be ranked among the top 1500 apps. There were more ranked dermatology apps in the Medical category targeting patients than physicians; 18 of 29 (62%) qualifying apps targeted patients and 11 (38%) targeted physicians (Tables 1 and 2). No apps targeted both groups. The most common type of ranked app targeting patients was self-surveillance (11/18), and the most common type targeting physicians was reference (8/11). The highest ranked app targeting patients was a teledermatology app with a ranking of 184, and the highest ranked app targeting physicians was educational, ranked 353. The least common type of ranked apps targeting patients were “other” (2/18 [11%]; 1 prescription and 1 UV monitor app) and conference (1/18 [6%]). The least common type of ranked apps targeting physicians were education (2/11 [18%]) and dermoscopy (1/11 [9%]).


Our search of the Health and Fitness category yielded 6 apps, all targeting patients; 3 (50%) were self-surveillance apps, and 3 (50%) were classified as other (2 UV monitors and a conferencing app for cancer emotional support)(Table 3).

Our search of the Medical category for billing/coding and EMR apps yielded 232 and 164 apps, respectively; of them, 49 (21%) and 54 (33%) apps were ranked. These apps did not overlap with the dermatology-related search criteria; thus, we were not able to ascertain how many of these apps were used specifically by health care providers in dermatology.
COMMENT
Patient Apps
The most common apps used by patients are fitness and nutrition tracker apps categorized as Health and Fitness5,6; however, the majority of ranked dermatology apps are categorized as Medical per our findings. In a study of 557 dermatology patients, it was found that among the health-related apps they used, the most common apps after fitness/nutrition were references, followed by patient portals, self-surveillance, and emotional assistance apps.6 Our search was consistent with these findings, suggesting that the most desired dermatology apps by patients are those that allow them to be proactive with their health. It is no surprise that the top-ranked app targeting patients was a teledermatology app, followed by multiple self-surveillance apps. The highest ranked self-surveillance app in the Health and Fitness category focused on monitoring the effects of nutrition on symptoms of diseases including skin disorders, while the highest ranked (as well as the majority of) self-surveillance apps in the Medical category encompassed mole monitoring and cancer risk calculators.
Benefits of the ranked dermatology apps in the Medical and Health and Fitness categories targeting patients include more immediate access to health care and education. Despite this popularity among patients, Masud et al7 demonstrated that only 20.5% (9/44) of dermatology apps targeting patients may be reliable resources based on a rubric created by the investigators. Overall, there remains a research gap for a standardized scientific approach to evaluating app validity and reliability.
Teledermatology
Teledermatology apps are the most common dermatology apps,2 allowing for remote evaluation of patients through either live consultations or transmittance of medical information for later review by board-certified physicians.8 Features common to many teledermatology apps include accessibility on Android (Google Inc) and iOS as well as a web version. Security and Health Insurance Portability and Accountability Act compliance is especially important and is enforced through user authentications, data encryption, and automatic logout features. Data is not stored locally and is secured on a private server with backup. Referring providers and consultants often can communicate within the app. Insurance providers also may cover teledermatology services, and if not, the out-of-pocket costs often are affordable.
The highest-ranked patient app (ranked 184 in the Medical category) was a teledermatology app that did not meet the American Telemedicine Association standards for teledermatology apps.9 The popularity of this app among patients may have been attributable to multiple ease-of-use and turnaround time features. The user interface was simplistic, and the design was appealing to the eye. The entry field options were minimal to avoid confusion. The turnaround time to receive a diagnosis depended on 1 of 3 options, including a more rapid response for an increased cost. Ease of use was the highlight of this app at the cost of accuracy, as the limited amount of information that users were required to provide physicians compromised diagnostic accuracy in this app.
For comparison, we chose a nonranked (and thus less frequently used) teledermatology app that had previously undergone scientific evaluation using 13 evaluation criteria specific to teledermatology.10 The app also met the American Telemedicine Association standard for teledermatology apps.9 The app was originally a broader telemedicine app but featured a section specific to teledermatology. The user interface was simple but professional, almost resembling an EMR. The input fields included a comprehensive history that permitted a better evaluation of a lesion but might be tedious for users. This app boasted professionalism and accuracy, but from a user standpoint, it may have been too time-consuming.
Striking a balance between ensuring proper care versus appealing to patients is a difficult but important task. Based on this study, it appears that popular patient apps may in fact have less scientific rationale and therefore potentially less accuracy.
Self-surveillance
Although self-surveillance apps did not account for the highest-ranked app, they were the most frequently ranked app type in our study. Most of the ranked self-surveillance apps in the Medical category were for monitoring lesions over time to assess for changes. These apps help users take photographs that are well organized in a single, easy-to-find location. Some apps were risk calculators that assessed the risk for malignancies using a questionnaire. The majority of these self-surveillance apps were specific to skin cancer detection. Of note, one of the ranked self-surveillance apps assessed drug effectiveness by monitoring clinical appearance and symptoms. The lowest ranked self-surveillance app in the top 1500 ranked Medical apps in our search monitored cancer symptoms not specific to dermatology. Although this app had a low ranking (1380/1500), it received a high number of reviews and was well rated at 4.8 out of 5 stars; therefore, it seemed more helpful than the other higher-ranked apps targeting patients, which had higher rankings but minimal to no reviews or ratings. A comparison of the ease-of-use features of all the ranked patient-targeted self-surveillance apps in the Medical category is provided in Table 4.

Physician Apps
After examining the results of apps targeting physicians, we realized that the data may be accurate but may not be as representative of all currently practicing dermatology providers. Given the increased usage of apps among younger age groups,11 our data may be skewed toward medical students and residents, supported by the fact that the top-ranked physician app in our study was an education app and the majority were reference apps. Future studies are needed to reexamine app ranking as this age group transitions from entry-level health care providers in the next 5 to 10 years. These findings also suggest less frequent app use among more veteran health care providers within our specific search parameters. Therefore, we decided to do subsequent searches for available billing/coding and EMR apps, which were many, but as mentioned above, none were specific to dermatology.
General Dermatology References
Most of the dermatology reference apps were formatted as e-books; however, other apps such as the Amazon Kindle app (categorized under Books) providing access to multiple e-books within one app were not included. Some apps included study aid features (eg, flash cards, quizzes), and topics spanned both dermatology and dermatopathology. Apps provide a unique way for on-the-go studying for dermatologists in training, and if the usage continues to grow, there may be a need for increased formal integration in dermatology education in the future.
Journals
Journal apps were not among those listed in the top-ranked apps we evaluated, which we suspect may be because journals were categorized differently from one journal to the next; for example, the Journal of the American Academy of Dermatology was ranked 1168 in the Magazines and Newspapers category. On the other hand, Dermatology World was ranked 1363 in the Reference category. An article’s citation affects the publishing journal’s impact factor, which is one of the most important variables in measuring a journal’s influence. In the future, there may be other variables that could aid in understanding journal impact as it relates to the journal’s accessibility.
Limitations
Our study did not look at Android apps. The top chart apps in the Android and Apple App Stores use undisclosed algorithms likely involving different characteristics such as number of downloads, frequency of updates, number of reviews, ratings, and more. Thus, the rankings across these different markets would not be comparable. Although our choice of keywords stemmed from the majority of prior studies looking at dermatology apps, our search was limited due to the use of these specific keywords. To avoid skewing data by cross-comparison of noncomparable categories, we could not compare apps in the Medical category versus those in other categories.
CONCLUSION
There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps. As app usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in our education, and as such, it will become more critical to develop formal scientific standards. Given these future trends, we may need to increase our current literature and understanding of apps in dermatology with regard to their impact on both patients and health care providers.
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center website. http://www.pewglobal.org/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/#table. Published June 19, 2018. Accessed August 28, 2018.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018;24. pii:13030/qt3hs7n9z6.
- App Annie website. https://www.appannie.com/top/. Accessed August 28, 2018.
- Number of iPhone users in the United States from 2012 to 2016 (in millions). Statista website. https://www.statista.com/statistics/232790/forecast-of-apple-users-in-the-us/. Accessed August 28, 2018.
- Burkhart C. Medical mobile apps and dermatology. Cutis. 2012;90:278-281.
- Wolf JA, Moreau JF, Patton TJ, et al. Prevalence and impact of health-related internet and smartphone use among dermatology patients. Cutis. 2015;95:323-328.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Walocko FM, Tejasvi T. Teledermatology applications in skin cancer diagnosis. Dermatol Clin. 2017;35:559-563.
- Krupinski E, Burdick A, Pak H, et al. American Telemedicine Association’s practice guidelines for teledermatology. Telemed J E Health. 2008;14:289-302.
- Ho B, Lee M, Armstrong AW. Evaluation criteria for mobile teledermatology applications and comparison of major mobile teledermatology applications. Telemed J E Health. 2013;19:678-682.
- Number of mobile app hours per smartphone and tablet app user in the United States in June 2016, by age group. Statista website. https://www.statista.com/statistics/323522/us-user-mobile-app-engagement-age/. Accessed September 18, 2018.
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center website. http://www.pewglobal.org/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/#table. Published June 19, 2018. Accessed August 28, 2018.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018;24. pii:13030/qt3hs7n9z6.
- App Annie website. https://www.appannie.com/top/. Accessed August 28, 2018.
- Number of iPhone users in the United States from 2012 to 2016 (in millions). Statista website. https://www.statista.com/statistics/232790/forecast-of-apple-users-in-the-us/. Accessed August 28, 2018.
- Burkhart C. Medical mobile apps and dermatology. Cutis. 2012;90:278-281.
- Wolf JA, Moreau JF, Patton TJ, et al. Prevalence and impact of health-related internet and smartphone use among dermatology patients. Cutis. 2015;95:323-328.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Walocko FM, Tejasvi T. Teledermatology applications in skin cancer diagnosis. Dermatol Clin. 2017;35:559-563.
- Krupinski E, Burdick A, Pak H, et al. American Telemedicine Association’s practice guidelines for teledermatology. Telemed J E Health. 2008;14:289-302.
- Ho B, Lee M, Armstrong AW. Evaluation criteria for mobile teledermatology applications and comparison of major mobile teledermatology applications. Telemed J E Health. 2013;19:678-682.
- Number of mobile app hours per smartphone and tablet app user in the United States in June 2016, by age group. Statista website. https://www.statista.com/statistics/323522/us-user-mobile-app-engagement-age/. Accessed September 18, 2018.
Practice Points
- As mobile application (app) usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in dermatology education. As such, it will become more critical to develop formal scientific standards.
- The most desired dermatology apps for patients were apps that allowed them to be proactive with their health.
- There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps.