User login
Genetic cancers are relatively uncommon but not rare. Although there has not been a comprehensive study of the incidence of cancers that are caused by an identifiable single gene mutation, it is estimated that they account for approximately 5% to 10% of all cancers, or 50,000 to 100,000 patients annually in the U.S.1 The hallmarks of a genetic cancer syndrome are early onset, multiple family members in multiple generations with cancer, bilateral cancer, and multiple cancers in the same person.
Until recently, the VA has not had a significant interest in genetic cancer risk assessment (GCRA). This is changing, however, because veterans with identified genetic risks for cancer can benefit from targeted screening and intervention strategies to lower their risk of dying of cancer. The value of GCRA was also recognized in the 2015 standards for accreditation of the American College of Surgeons, which include a requirement for programs to include a provision for GCRA.2
The 2 most common familial cancer syndromes are hereditary breast and ovarian cancer (HBOC) syndrome, which occurs in about 5% of all patients with breast cancer, and Lynch syndrome (LS), or hereditary nonpolyposis colorectal cancer (CRC) syndrome, which occurs in about 3% of all patients with CRC.3,4 Other familial cancer syndromes are rare: For example, familial adenomatous polyposis (FAP) accounts for 0.2% to 0.5% of all CRC cases.5
The Raymond G. Murphy VAMC in Albuquerque is the sole VA hospital in New Mexico. Its catchment area extends into southern Colorado, eastern Arizona, and western Texas. About 40 CRCs and 8 breast cancers are diagnosed at this facility yearly. Given the incidence of these familial cancer syndromes, one might expect to see 1 LS case/year, 1 HBOC case every 2 years, and 1 FAP or attenuated FAP case every 5 to 10 years.
Methods
In 2010, a GCRA clinic was set up to evaluate and manage treatment of veterans who might have inherited a genetic cancer syndrome. Prior to that, veterans with suspected genetic cancer family syndromes were referred to the University of New Mexico for evaluation and testing. Initially, the pathology department (PD) paid for genetic testing. However, due to the cost of testing, a formal budget for genetic testing was approved. Contracts were set up by the PD with outside laboratories for genetic testing services. For quality control, all veterans who were referred for genetic evaluation were seen by Dr. Lin.
The initial consultation consisted of construction of a family pedigree and evaluation, using available models or tables, such as the Myriad tables (BRCA), Penn II BRCA, or PREMM1,2,6 (LS), to estimate likelihood of finding a mutation. Veterans who had a 10% likelihood of finding a gene mutation were counseled, following the American Society of Clinical Oncology guidelines (Table 1). Those who consented to genetic testing signed a consent form and were given a copy of that form and a copy of their family pedigree. Because the VA covers the cost of counseling and testing, cost was not discussed.
Veterans had a follow-up visit to review the test results. Patients were counseled on treatment recommendations, including a copy of current consensus recommendations, and disclosure to the family. The recommendations were then included in the patient’s electronic medical record. For example, BRCA patients had a discussion of risks and benefits of various management options, including breast magnetic resonance imaging, prophylactic mastectomy, and prophylactic bilateral salpingo-oophorectomy, once childbearing was complete.
Results
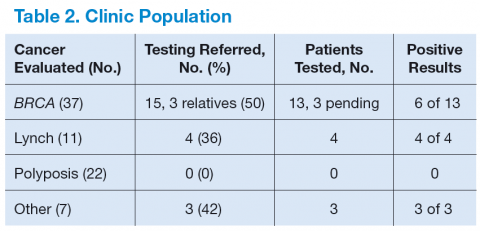
Table 2 shows the number of veterans referred to the GCRA clinic since it started in late 2010, categorized by the likely genetic syndrome, the number and percentage of veterans where genetic testing was recommended, and the results of testing. Four veterans, 2 with LS, 1 with CHEK2 mutation, and 1 with Peutz-Jeghers syndrome, were identified outside the VA system but were referred for counseling. One of the veterans with LS was referred by an outside provider who obtained a suspicious family history, and the other was identified via pathologic screening. The miscellaneous group included 1 veteran with MEN 1 and 1 veteran with Birt-Hogg-Dube.
There are a number of interesting results. Although the number of patients referred for LS was low, the number of annual referrals for possible BRCA was about equal to the number of patients with breast cancer who were diagnosed and treated yearly. Although this could have been due to pent up demand initially, the number of annual referrals has not decreased with time. Furthermore, the number of patients referred for polyposis has been considerably higher than would be expected by the rarity of attenuated FAP. Initially, patients with 10 to 20 polyps of any type were referred for evaluation. All but 1 had their first polyp diagnosed after the age of 50 years. Five veterans who were referred to GCRA had < 10 polyps lifetime, 3 veterans had between 10 and 20 polyps, and 12 veterans have had ≥ 20 adenomatous polyps over their lifetime. None seen to date have had a personal or family history of gastrointestinal (GI) cancer.
Discussion
A genetic cancer risk assessment clinic was set up in a VA hospital and has been running successfully for 4 years. Although many parts of setting up such a clinic are common to a community GCRA clinic, there are also aspects that are specific to a VA setting.6
Because genetic testing is relatively expensive, a budget must be set up and approved by VA administration. This budget is based on the estimated number of veterans that will be referred yearly, the likely percentage that will need to be tested, and the cost of testing. Currently, the average cost of a single gene test is about $2,000 to $3,000. Some patients will need to have 2 to 4 genes tested. Furthermore, many centers are now moving to multigene testing, and the cost of these panels is about $10,000 or more, though this is less than the cumulative cost of the genes done individually.
Since there is currently no national VA contract for genetic cancer testing, each VA facility needs to negotiate contracts with outside laboratories. Several of these laboratories offer gene panel testing, but the panels vary from one laboratory to another.
Limiting the number of providers who can order genetic testing helps maintain quality control and ensure a comprehensive database of patient testing. At the Albuquerque VAMC, Dr. Lin is currently the only provider who can order genetic testing for cancer risk assessment. Nearly all GCRA consultations, from obtaining a detailed family history to providing education on the risks, benefits, and limitations of genetic testing, can be conducted via telemedicine. The VA GCRA program in Utah has established a number of telemedicine collaborations with VA facilities around the country, beginning with BRCA consultations and branching out into a national LS screening program.
The first few years of the program have shown some unexpected results, including a much higher referral rate for HBOC referrals than was anticipated. The reasons for this are not clear. The high rate of polyposis referrals can be attributed in large part to the robust CRC screening program in the VA system. Veterans are routinely screened for CRC with occult blood tests, and positive results are referred for colonoscopy. Nearly 400 veterans per year have a colonoscopy at the Albuquerque VAMC.
Because the VA screening program begins at age 50 years, nearly all the veterans referred to date have had their first polyp diagnosed at age ≥ 50 years. Unfortunately, the 1 patient who had polyps and CRC at a young age was not tested due to lack of budget when she was evaluated. By contrast, in a large study, the median age of first polyp diagnosis in patients with APC mutation was 30 years, and with biallelic MUTYH mutations was 47 years.7
The difficulty in distinguishing which veterans should be tested for attenuated FAP lies in the fact that age of onset and personal or family history alone or in
combination do not seem to be adequate discriminators to screen out low-risk veterans who do not need testing.7 Considering the number of veterans referred each year and the incidence of attenuated FAP, if every veteran who fit the current criteria of 20 adenomatous polyps lifetime were tested, about 35 to 70 veterans would have to be tested to detect 1 mutation carrier. The development of clinical criteria to identify low-risk patients would be very helpful.
On the other hand, referrals for LS were uncommon. This is consistent with results reported elsewhere.8 For this reason, diagnosis of LS has shifted from clinical identification to pathologic screening for the molecular hallmarks of LS in tumor specimens.8,9 Shortly after the GCRA clinic was established, a pathologist with an interest in GI malignancies developed and validated a pathologic screening program using immunohistochemistry (IHC) staining for mismatch repair (MMR) gene expression, with the assistance of a pathologist who had been involved in a community-based LS screening program.9 For the past 3 years, all CRC patients aged ≤ 60 years have been screened for loss of expression of MMR IHC. Patients identified have been seen in the GCRA clinic to discuss possible genetic testing. This screening program is now extending to all patients with CRC aged ≤ 70 years, in line with consensus recommendations.10
The Future
The lack of a national VA contract with outside laboratories for genetic testing means that each facility has to negotiate its own contract, which is a wasteful duplication of resources that needs to be addressed. Beyond this parochial concern, GCRA is undergoing a revolution in diagnosing and managing cancer risk. In the past, a careful family history was followed by selected single gene testing for mutations, using Sanger sequencing. However, many laboratories are now offering multigene testing using next-generation sequencing that can look at multiple genes, all the way up to whole genome sequencing. Current estimates for the actual cost to the laboratory for a whole genome using next-generation sequencing is about $1,000.
A number of laboratories also have been offering multigene panels for testing in patients with familial cancer syndromes. The genes in these panels include those with a well-documented association with known cancer syndromes as well as other genes where mutations may confer only a modestly increased risk. Furthermore, new genetic syndromes and new genes associated with known syndromes are being reported yearly.
This revolution in technology and the virtual explosion in the amount of data generated have raised as many questions as answers.11 One joke in the genetic testing community goes: “$1,000 genome, $100,000 interpretation.” Among the remaining issues are how to counsel patients about the possible results from multigene testing, including the possibility of results that may be applicable to noncancer-related diagnoses; what to do about the unanticipated actionable finding (incidentaloma); how to interpret and treat a patient whose gene test results are at odds with the clinical family history; how to treat patients whose panel returns with a mutation in a gene that has only a minor increased risk for the cancers; how genes with modestly increased or decreased risk singly or in combination may modify highrisk gene expression; and how to address variants of unknown significance.
A general consensus has emerged that these questions will need much more research correlating genetic and clinical data to answer. As a result, many leading researchers have set up multi-institutional, international collaborative groups directed at specific syndromes, which pool data from many investigators to answer questions beyond the capability of any single investigator or group. These big data collaborative studies are already beginning to publish early results and seem to represent the future of genetic cancer risk assessment, a field that is at once dynamic, exciting, and confusing.4
A major question is whether and how the VA can cooperate with these international consortia. The VA has particular concerns about confidentiality based on past experience, but it also has a unique group of patients who could provide valuable contributions to our knowledge about genetic markers for disease, including cancer. A method for the VA system to provide data to collaborative groups who are advancing our knowledge of the genetic risk factors for cancer while protecting the confidentiality of veterans could provide a model for collaboration between the VA and non-VA health care systems.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer. 1996;77(11):2318-2324.
2. American College of Surgeons. Cancer Program Standards 2012: Ensuring Patient- Centered Care, v1.2.1. Chicago, IL: American College of Surgeons; 2012. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed July 6, 2015.
3. Campeau PM, Foulkes WD, Tischkowitz MD. Hereditary breast cancer: new genetic developments, new therapeutic avenues. Hum Genet. 2008;124(1):31-34.
4. Moreira L, Balaguer F, Lindor N, et al; EPICOLON Consortium. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308(15):1555-1565.
5. Bülow S, Faurschou Nielsen T, Bülow C, Bisgaard ML, Karlsen L, Moesgaard F. The incidence rate of familial adenomatous polyposis. Results from the Danish Polyposis Register. Int J Colorect Dis. 1996;11(2):88-91.
6. Duncan PR, Lin JT. Ingredients for success: a familial cancer clinic in an oncology
practice setting. J Oncol Pract. 2011;7(1):39-42.
7. Grover S, Kastrinos F, Steyerberg EW, et al. Prevalence and phenotypes of APC and MUTYH mutations in patients with multiple colorectal adenomas. JAMA. 2012;308(5):485-492.
8. Hampel H, de la Chapelle A. How do we approach the goal of identifying everybody with Lynch syndrome? Fam Cancer. 2013;12(2):313-317.
9. Duncan PR, Lin JT, Feddersen R. Prospective screening for Lynch syndrome (LS) in a cohort of colorectal cancer (CRC) surgical patients in a community hospital. J Clin Oncol. 2010;28(suppl; abstr 1535):15s.
10. Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Dis Colon Rectum. 2014;57(8):1025-1048.
11. Domchek SM, Bradbury A, Garber JE, Offit K, Robson ME. Multiplex genetic testing for cancer susceptibility: out on a high wire without a net? J Clin Oncol. 2013;31(10):1267-1270.
Genetic cancers are relatively uncommon but not rare. Although there has not been a comprehensive study of the incidence of cancers that are caused by an identifiable single gene mutation, it is estimated that they account for approximately 5% to 10% of all cancers, or 50,000 to 100,000 patients annually in the U.S.1 The hallmarks of a genetic cancer syndrome are early onset, multiple family members in multiple generations with cancer, bilateral cancer, and multiple cancers in the same person.
Until recently, the VA has not had a significant interest in genetic cancer risk assessment (GCRA). This is changing, however, because veterans with identified genetic risks for cancer can benefit from targeted screening and intervention strategies to lower their risk of dying of cancer. The value of GCRA was also recognized in the 2015 standards for accreditation of the American College of Surgeons, which include a requirement for programs to include a provision for GCRA.2
The 2 most common familial cancer syndromes are hereditary breast and ovarian cancer (HBOC) syndrome, which occurs in about 5% of all patients with breast cancer, and Lynch syndrome (LS), or hereditary nonpolyposis colorectal cancer (CRC) syndrome, which occurs in about 3% of all patients with CRC.3,4 Other familial cancer syndromes are rare: For example, familial adenomatous polyposis (FAP) accounts for 0.2% to 0.5% of all CRC cases.5
The Raymond G. Murphy VAMC in Albuquerque is the sole VA hospital in New Mexico. Its catchment area extends into southern Colorado, eastern Arizona, and western Texas. About 40 CRCs and 8 breast cancers are diagnosed at this facility yearly. Given the incidence of these familial cancer syndromes, one might expect to see 1 LS case/year, 1 HBOC case every 2 years, and 1 FAP or attenuated FAP case every 5 to 10 years.
Methods
In 2010, a GCRA clinic was set up to evaluate and manage treatment of veterans who might have inherited a genetic cancer syndrome. Prior to that, veterans with suspected genetic cancer family syndromes were referred to the University of New Mexico for evaluation and testing. Initially, the pathology department (PD) paid for genetic testing. However, due to the cost of testing, a formal budget for genetic testing was approved. Contracts were set up by the PD with outside laboratories for genetic testing services. For quality control, all veterans who were referred for genetic evaluation were seen by Dr. Lin.
The initial consultation consisted of construction of a family pedigree and evaluation, using available models or tables, such as the Myriad tables (BRCA), Penn II BRCA, or PREMM1,2,6 (LS), to estimate likelihood of finding a mutation. Veterans who had a 10% likelihood of finding a gene mutation were counseled, following the American Society of Clinical Oncology guidelines (Table 1). Those who consented to genetic testing signed a consent form and were given a copy of that form and a copy of their family pedigree. Because the VA covers the cost of counseling and testing, cost was not discussed.
Veterans had a follow-up visit to review the test results. Patients were counseled on treatment recommendations, including a copy of current consensus recommendations, and disclosure to the family. The recommendations were then included in the patient’s electronic medical record. For example, BRCA patients had a discussion of risks and benefits of various management options, including breast magnetic resonance imaging, prophylactic mastectomy, and prophylactic bilateral salpingo-oophorectomy, once childbearing was complete.
Results
Table 2 shows the number of veterans referred to the GCRA clinic since it started in late 2010, categorized by the likely genetic syndrome, the number and percentage of veterans where genetic testing was recommended, and the results of testing. Four veterans, 2 with LS, 1 with CHEK2 mutation, and 1 with Peutz-Jeghers syndrome, were identified outside the VA system but were referred for counseling. One of the veterans with LS was referred by an outside provider who obtained a suspicious family history, and the other was identified via pathologic screening. The miscellaneous group included 1 veteran with MEN 1 and 1 veteran with Birt-Hogg-Dube.
There are a number of interesting results. Although the number of patients referred for LS was low, the number of annual referrals for possible BRCA was about equal to the number of patients with breast cancer who were diagnosed and treated yearly. Although this could have been due to pent up demand initially, the number of annual referrals has not decreased with time. Furthermore, the number of patients referred for polyposis has been considerably higher than would be expected by the rarity of attenuated FAP. Initially, patients with 10 to 20 polyps of any type were referred for evaluation. All but 1 had their first polyp diagnosed after the age of 50 years. Five veterans who were referred to GCRA had < 10 polyps lifetime, 3 veterans had between 10 and 20 polyps, and 12 veterans have had ≥ 20 adenomatous polyps over their lifetime. None seen to date have had a personal or family history of gastrointestinal (GI) cancer.
Discussion
A genetic cancer risk assessment clinic was set up in a VA hospital and has been running successfully for 4 years. Although many parts of setting up such a clinic are common to a community GCRA clinic, there are also aspects that are specific to a VA setting.6
Because genetic testing is relatively expensive, a budget must be set up and approved by VA administration. This budget is based on the estimated number of veterans that will be referred yearly, the likely percentage that will need to be tested, and the cost of testing. Currently, the average cost of a single gene test is about $2,000 to $3,000. Some patients will need to have 2 to 4 genes tested. Furthermore, many centers are now moving to multigene testing, and the cost of these panels is about $10,000 or more, though this is less than the cumulative cost of the genes done individually.
Since there is currently no national VA contract for genetic cancer testing, each VA facility needs to negotiate contracts with outside laboratories. Several of these laboratories offer gene panel testing, but the panels vary from one laboratory to another.
Limiting the number of providers who can order genetic testing helps maintain quality control and ensure a comprehensive database of patient testing. At the Albuquerque VAMC, Dr. Lin is currently the only provider who can order genetic testing for cancer risk assessment. Nearly all GCRA consultations, from obtaining a detailed family history to providing education on the risks, benefits, and limitations of genetic testing, can be conducted via telemedicine. The VA GCRA program in Utah has established a number of telemedicine collaborations with VA facilities around the country, beginning with BRCA consultations and branching out into a national LS screening program.
The first few years of the program have shown some unexpected results, including a much higher referral rate for HBOC referrals than was anticipated. The reasons for this are not clear. The high rate of polyposis referrals can be attributed in large part to the robust CRC screening program in the VA system. Veterans are routinely screened for CRC with occult blood tests, and positive results are referred for colonoscopy. Nearly 400 veterans per year have a colonoscopy at the Albuquerque VAMC.
Because the VA screening program begins at age 50 years, nearly all the veterans referred to date have had their first polyp diagnosed at age ≥ 50 years. Unfortunately, the 1 patient who had polyps and CRC at a young age was not tested due to lack of budget when she was evaluated. By contrast, in a large study, the median age of first polyp diagnosis in patients with APC mutation was 30 years, and with biallelic MUTYH mutations was 47 years.7
The difficulty in distinguishing which veterans should be tested for attenuated FAP lies in the fact that age of onset and personal or family history alone or in
combination do not seem to be adequate discriminators to screen out low-risk veterans who do not need testing.7 Considering the number of veterans referred each year and the incidence of attenuated FAP, if every veteran who fit the current criteria of 20 adenomatous polyps lifetime were tested, about 35 to 70 veterans would have to be tested to detect 1 mutation carrier. The development of clinical criteria to identify low-risk patients would be very helpful.
On the other hand, referrals for LS were uncommon. This is consistent with results reported elsewhere.8 For this reason, diagnosis of LS has shifted from clinical identification to pathologic screening for the molecular hallmarks of LS in tumor specimens.8,9 Shortly after the GCRA clinic was established, a pathologist with an interest in GI malignancies developed and validated a pathologic screening program using immunohistochemistry (IHC) staining for mismatch repair (MMR) gene expression, with the assistance of a pathologist who had been involved in a community-based LS screening program.9 For the past 3 years, all CRC patients aged ≤ 60 years have been screened for loss of expression of MMR IHC. Patients identified have been seen in the GCRA clinic to discuss possible genetic testing. This screening program is now extending to all patients with CRC aged ≤ 70 years, in line with consensus recommendations.10
The Future
The lack of a national VA contract with outside laboratories for genetic testing means that each facility has to negotiate its own contract, which is a wasteful duplication of resources that needs to be addressed. Beyond this parochial concern, GCRA is undergoing a revolution in diagnosing and managing cancer risk. In the past, a careful family history was followed by selected single gene testing for mutations, using Sanger sequencing. However, many laboratories are now offering multigene testing using next-generation sequencing that can look at multiple genes, all the way up to whole genome sequencing. Current estimates for the actual cost to the laboratory for a whole genome using next-generation sequencing is about $1,000.
A number of laboratories also have been offering multigene panels for testing in patients with familial cancer syndromes. The genes in these panels include those with a well-documented association with known cancer syndromes as well as other genes where mutations may confer only a modestly increased risk. Furthermore, new genetic syndromes and new genes associated with known syndromes are being reported yearly.
This revolution in technology and the virtual explosion in the amount of data generated have raised as many questions as answers.11 One joke in the genetic testing community goes: “$1,000 genome, $100,000 interpretation.” Among the remaining issues are how to counsel patients about the possible results from multigene testing, including the possibility of results that may be applicable to noncancer-related diagnoses; what to do about the unanticipated actionable finding (incidentaloma); how to interpret and treat a patient whose gene test results are at odds with the clinical family history; how to treat patients whose panel returns with a mutation in a gene that has only a minor increased risk for the cancers; how genes with modestly increased or decreased risk singly or in combination may modify highrisk gene expression; and how to address variants of unknown significance.
A general consensus has emerged that these questions will need much more research correlating genetic and clinical data to answer. As a result, many leading researchers have set up multi-institutional, international collaborative groups directed at specific syndromes, which pool data from many investigators to answer questions beyond the capability of any single investigator or group. These big data collaborative studies are already beginning to publish early results and seem to represent the future of genetic cancer risk assessment, a field that is at once dynamic, exciting, and confusing.4
A major question is whether and how the VA can cooperate with these international consortia. The VA has particular concerns about confidentiality based on past experience, but it also has a unique group of patients who could provide valuable contributions to our knowledge about genetic markers for disease, including cancer. A method for the VA system to provide data to collaborative groups who are advancing our knowledge of the genetic risk factors for cancer while protecting the confidentiality of veterans could provide a model for collaboration between the VA and non-VA health care systems.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Genetic cancers are relatively uncommon but not rare. Although there has not been a comprehensive study of the incidence of cancers that are caused by an identifiable single gene mutation, it is estimated that they account for approximately 5% to 10% of all cancers, or 50,000 to 100,000 patients annually in the U.S.1 The hallmarks of a genetic cancer syndrome are early onset, multiple family members in multiple generations with cancer, bilateral cancer, and multiple cancers in the same person.
Until recently, the VA has not had a significant interest in genetic cancer risk assessment (GCRA). This is changing, however, because veterans with identified genetic risks for cancer can benefit from targeted screening and intervention strategies to lower their risk of dying of cancer. The value of GCRA was also recognized in the 2015 standards for accreditation of the American College of Surgeons, which include a requirement for programs to include a provision for GCRA.2
The 2 most common familial cancer syndromes are hereditary breast and ovarian cancer (HBOC) syndrome, which occurs in about 5% of all patients with breast cancer, and Lynch syndrome (LS), or hereditary nonpolyposis colorectal cancer (CRC) syndrome, which occurs in about 3% of all patients with CRC.3,4 Other familial cancer syndromes are rare: For example, familial adenomatous polyposis (FAP) accounts for 0.2% to 0.5% of all CRC cases.5
The Raymond G. Murphy VAMC in Albuquerque is the sole VA hospital in New Mexico. Its catchment area extends into southern Colorado, eastern Arizona, and western Texas. About 40 CRCs and 8 breast cancers are diagnosed at this facility yearly. Given the incidence of these familial cancer syndromes, one might expect to see 1 LS case/year, 1 HBOC case every 2 years, and 1 FAP or attenuated FAP case every 5 to 10 years.
Methods
In 2010, a GCRA clinic was set up to evaluate and manage treatment of veterans who might have inherited a genetic cancer syndrome. Prior to that, veterans with suspected genetic cancer family syndromes were referred to the University of New Mexico for evaluation and testing. Initially, the pathology department (PD) paid for genetic testing. However, due to the cost of testing, a formal budget for genetic testing was approved. Contracts were set up by the PD with outside laboratories for genetic testing services. For quality control, all veterans who were referred for genetic evaluation were seen by Dr. Lin.
The initial consultation consisted of construction of a family pedigree and evaluation, using available models or tables, such as the Myriad tables (BRCA), Penn II BRCA, or PREMM1,2,6 (LS), to estimate likelihood of finding a mutation. Veterans who had a 10% likelihood of finding a gene mutation were counseled, following the American Society of Clinical Oncology guidelines (Table 1). Those who consented to genetic testing signed a consent form and were given a copy of that form and a copy of their family pedigree. Because the VA covers the cost of counseling and testing, cost was not discussed.
Veterans had a follow-up visit to review the test results. Patients were counseled on treatment recommendations, including a copy of current consensus recommendations, and disclosure to the family. The recommendations were then included in the patient’s electronic medical record. For example, BRCA patients had a discussion of risks and benefits of various management options, including breast magnetic resonance imaging, prophylactic mastectomy, and prophylactic bilateral salpingo-oophorectomy, once childbearing was complete.
Results
Table 2 shows the number of veterans referred to the GCRA clinic since it started in late 2010, categorized by the likely genetic syndrome, the number and percentage of veterans where genetic testing was recommended, and the results of testing. Four veterans, 2 with LS, 1 with CHEK2 mutation, and 1 with Peutz-Jeghers syndrome, were identified outside the VA system but were referred for counseling. One of the veterans with LS was referred by an outside provider who obtained a suspicious family history, and the other was identified via pathologic screening. The miscellaneous group included 1 veteran with MEN 1 and 1 veteran with Birt-Hogg-Dube.
There are a number of interesting results. Although the number of patients referred for LS was low, the number of annual referrals for possible BRCA was about equal to the number of patients with breast cancer who were diagnosed and treated yearly. Although this could have been due to pent up demand initially, the number of annual referrals has not decreased with time. Furthermore, the number of patients referred for polyposis has been considerably higher than would be expected by the rarity of attenuated FAP. Initially, patients with 10 to 20 polyps of any type were referred for evaluation. All but 1 had their first polyp diagnosed after the age of 50 years. Five veterans who were referred to GCRA had < 10 polyps lifetime, 3 veterans had between 10 and 20 polyps, and 12 veterans have had ≥ 20 adenomatous polyps over their lifetime. None seen to date have had a personal or family history of gastrointestinal (GI) cancer.
Discussion
A genetic cancer risk assessment clinic was set up in a VA hospital and has been running successfully for 4 years. Although many parts of setting up such a clinic are common to a community GCRA clinic, there are also aspects that are specific to a VA setting.6
Because genetic testing is relatively expensive, a budget must be set up and approved by VA administration. This budget is based on the estimated number of veterans that will be referred yearly, the likely percentage that will need to be tested, and the cost of testing. Currently, the average cost of a single gene test is about $2,000 to $3,000. Some patients will need to have 2 to 4 genes tested. Furthermore, many centers are now moving to multigene testing, and the cost of these panels is about $10,000 or more, though this is less than the cumulative cost of the genes done individually.
Since there is currently no national VA contract for genetic cancer testing, each VA facility needs to negotiate contracts with outside laboratories. Several of these laboratories offer gene panel testing, but the panels vary from one laboratory to another.
Limiting the number of providers who can order genetic testing helps maintain quality control and ensure a comprehensive database of patient testing. At the Albuquerque VAMC, Dr. Lin is currently the only provider who can order genetic testing for cancer risk assessment. Nearly all GCRA consultations, from obtaining a detailed family history to providing education on the risks, benefits, and limitations of genetic testing, can be conducted via telemedicine. The VA GCRA program in Utah has established a number of telemedicine collaborations with VA facilities around the country, beginning with BRCA consultations and branching out into a national LS screening program.
The first few years of the program have shown some unexpected results, including a much higher referral rate for HBOC referrals than was anticipated. The reasons for this are not clear. The high rate of polyposis referrals can be attributed in large part to the robust CRC screening program in the VA system. Veterans are routinely screened for CRC with occult blood tests, and positive results are referred for colonoscopy. Nearly 400 veterans per year have a colonoscopy at the Albuquerque VAMC.
Because the VA screening program begins at age 50 years, nearly all the veterans referred to date have had their first polyp diagnosed at age ≥ 50 years. Unfortunately, the 1 patient who had polyps and CRC at a young age was not tested due to lack of budget when she was evaluated. By contrast, in a large study, the median age of first polyp diagnosis in patients with APC mutation was 30 years, and with biallelic MUTYH mutations was 47 years.7
The difficulty in distinguishing which veterans should be tested for attenuated FAP lies in the fact that age of onset and personal or family history alone or in
combination do not seem to be adequate discriminators to screen out low-risk veterans who do not need testing.7 Considering the number of veterans referred each year and the incidence of attenuated FAP, if every veteran who fit the current criteria of 20 adenomatous polyps lifetime were tested, about 35 to 70 veterans would have to be tested to detect 1 mutation carrier. The development of clinical criteria to identify low-risk patients would be very helpful.
On the other hand, referrals for LS were uncommon. This is consistent with results reported elsewhere.8 For this reason, diagnosis of LS has shifted from clinical identification to pathologic screening for the molecular hallmarks of LS in tumor specimens.8,9 Shortly after the GCRA clinic was established, a pathologist with an interest in GI malignancies developed and validated a pathologic screening program using immunohistochemistry (IHC) staining for mismatch repair (MMR) gene expression, with the assistance of a pathologist who had been involved in a community-based LS screening program.9 For the past 3 years, all CRC patients aged ≤ 60 years have been screened for loss of expression of MMR IHC. Patients identified have been seen in the GCRA clinic to discuss possible genetic testing. This screening program is now extending to all patients with CRC aged ≤ 70 years, in line with consensus recommendations.10
The Future
The lack of a national VA contract with outside laboratories for genetic testing means that each facility has to negotiate its own contract, which is a wasteful duplication of resources that needs to be addressed. Beyond this parochial concern, GCRA is undergoing a revolution in diagnosing and managing cancer risk. In the past, a careful family history was followed by selected single gene testing for mutations, using Sanger sequencing. However, many laboratories are now offering multigene testing using next-generation sequencing that can look at multiple genes, all the way up to whole genome sequencing. Current estimates for the actual cost to the laboratory for a whole genome using next-generation sequencing is about $1,000.
A number of laboratories also have been offering multigene panels for testing in patients with familial cancer syndromes. The genes in these panels include those with a well-documented association with known cancer syndromes as well as other genes where mutations may confer only a modestly increased risk. Furthermore, new genetic syndromes and new genes associated with known syndromes are being reported yearly.
This revolution in technology and the virtual explosion in the amount of data generated have raised as many questions as answers.11 One joke in the genetic testing community goes: “$1,000 genome, $100,000 interpretation.” Among the remaining issues are how to counsel patients about the possible results from multigene testing, including the possibility of results that may be applicable to noncancer-related diagnoses; what to do about the unanticipated actionable finding (incidentaloma); how to interpret and treat a patient whose gene test results are at odds with the clinical family history; how to treat patients whose panel returns with a mutation in a gene that has only a minor increased risk for the cancers; how genes with modestly increased or decreased risk singly or in combination may modify highrisk gene expression; and how to address variants of unknown significance.
A general consensus has emerged that these questions will need much more research correlating genetic and clinical data to answer. As a result, many leading researchers have set up multi-institutional, international collaborative groups directed at specific syndromes, which pool data from many investigators to answer questions beyond the capability of any single investigator or group. These big data collaborative studies are already beginning to publish early results and seem to represent the future of genetic cancer risk assessment, a field that is at once dynamic, exciting, and confusing.4
A major question is whether and how the VA can cooperate with these international consortia. The VA has particular concerns about confidentiality based on past experience, but it also has a unique group of patients who could provide valuable contributions to our knowledge about genetic markers for disease, including cancer. A method for the VA system to provide data to collaborative groups who are advancing our knowledge of the genetic risk factors for cancer while protecting the confidentiality of veterans could provide a model for collaboration between the VA and non-VA health care systems.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer. 1996;77(11):2318-2324.
2. American College of Surgeons. Cancer Program Standards 2012: Ensuring Patient- Centered Care, v1.2.1. Chicago, IL: American College of Surgeons; 2012. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed July 6, 2015.
3. Campeau PM, Foulkes WD, Tischkowitz MD. Hereditary breast cancer: new genetic developments, new therapeutic avenues. Hum Genet. 2008;124(1):31-34.
4. Moreira L, Balaguer F, Lindor N, et al; EPICOLON Consortium. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308(15):1555-1565.
5. Bülow S, Faurschou Nielsen T, Bülow C, Bisgaard ML, Karlsen L, Moesgaard F. The incidence rate of familial adenomatous polyposis. Results from the Danish Polyposis Register. Int J Colorect Dis. 1996;11(2):88-91.
6. Duncan PR, Lin JT. Ingredients for success: a familial cancer clinic in an oncology
practice setting. J Oncol Pract. 2011;7(1):39-42.
7. Grover S, Kastrinos F, Steyerberg EW, et al. Prevalence and phenotypes of APC and MUTYH mutations in patients with multiple colorectal adenomas. JAMA. 2012;308(5):485-492.
8. Hampel H, de la Chapelle A. How do we approach the goal of identifying everybody with Lynch syndrome? Fam Cancer. 2013;12(2):313-317.
9. Duncan PR, Lin JT, Feddersen R. Prospective screening for Lynch syndrome (LS) in a cohort of colorectal cancer (CRC) surgical patients in a community hospital. J Clin Oncol. 2010;28(suppl; abstr 1535):15s.
10. Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Dis Colon Rectum. 2014;57(8):1025-1048.
11. Domchek SM, Bradbury A, Garber JE, Offit K, Robson ME. Multiplex genetic testing for cancer susceptibility: out on a high wire without a net? J Clin Oncol. 2013;31(10):1267-1270.
1. Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer. 1996;77(11):2318-2324.
2. American College of Surgeons. Cancer Program Standards 2012: Ensuring Patient- Centered Care, v1.2.1. Chicago, IL: American College of Surgeons; 2012. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed July 6, 2015.
3. Campeau PM, Foulkes WD, Tischkowitz MD. Hereditary breast cancer: new genetic developments, new therapeutic avenues. Hum Genet. 2008;124(1):31-34.
4. Moreira L, Balaguer F, Lindor N, et al; EPICOLON Consortium. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308(15):1555-1565.
5. Bülow S, Faurschou Nielsen T, Bülow C, Bisgaard ML, Karlsen L, Moesgaard F. The incidence rate of familial adenomatous polyposis. Results from the Danish Polyposis Register. Int J Colorect Dis. 1996;11(2):88-91.
6. Duncan PR, Lin JT. Ingredients for success: a familial cancer clinic in an oncology
practice setting. J Oncol Pract. 2011;7(1):39-42.
7. Grover S, Kastrinos F, Steyerberg EW, et al. Prevalence and phenotypes of APC and MUTYH mutations in patients with multiple colorectal adenomas. JAMA. 2012;308(5):485-492.
8. Hampel H, de la Chapelle A. How do we approach the goal of identifying everybody with Lynch syndrome? Fam Cancer. 2013;12(2):313-317.
9. Duncan PR, Lin JT, Feddersen R. Prospective screening for Lynch syndrome (LS) in a cohort of colorectal cancer (CRC) surgical patients in a community hospital. J Clin Oncol. 2010;28(suppl; abstr 1535):15s.
10. Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Dis Colon Rectum. 2014;57(8):1025-1048.
11. Domchek SM, Bradbury A, Garber JE, Offit K, Robson ME. Multiplex genetic testing for cancer susceptibility: out on a high wire without a net? J Clin Oncol. 2013;31(10):1267-1270.