User login
Isotretinoin is used in the treatment of nodulocystic and severe papulopustular acne. During the treatment period, laboratory monitoring is recommended to identify the risk for complications such as hepatotoxicity, teratogenicity, rhabdomyolysis, hyperlipidemia, and pancreatitis.1 There is a lack of consensus of the frequency of follow-up of laboratory parameters during isotretinoin treatment. This study evaluated the changes in laboratory parameters used in daily practice for patients with acne who were treated with isotretinoin to determine the optimum test repetition frequency.
Materials and Methods
Statistical Analysis—The descriptive statistics of the measurements were presented as means, standard deviations, or medians (first and third quartiles). With respect to the normal distribution, the consistency of the measurements was evaluated with the Kolmogorov-Smirnov test, and small deviations from the normal distribution were observed. Changes in laboratory measurements were evaluated with simple repeated-measures analysis of variance, and changes that differed significantly were determined by a Holm-Sidak post hoc test. Relationships between total cumulative doses and laboratory measurements at second visits were evaluated by the Pearson correlation analysis. The statistical significance level was P<.05. SPSS Statistics 23 (IBM) was used in the calculations.
Results
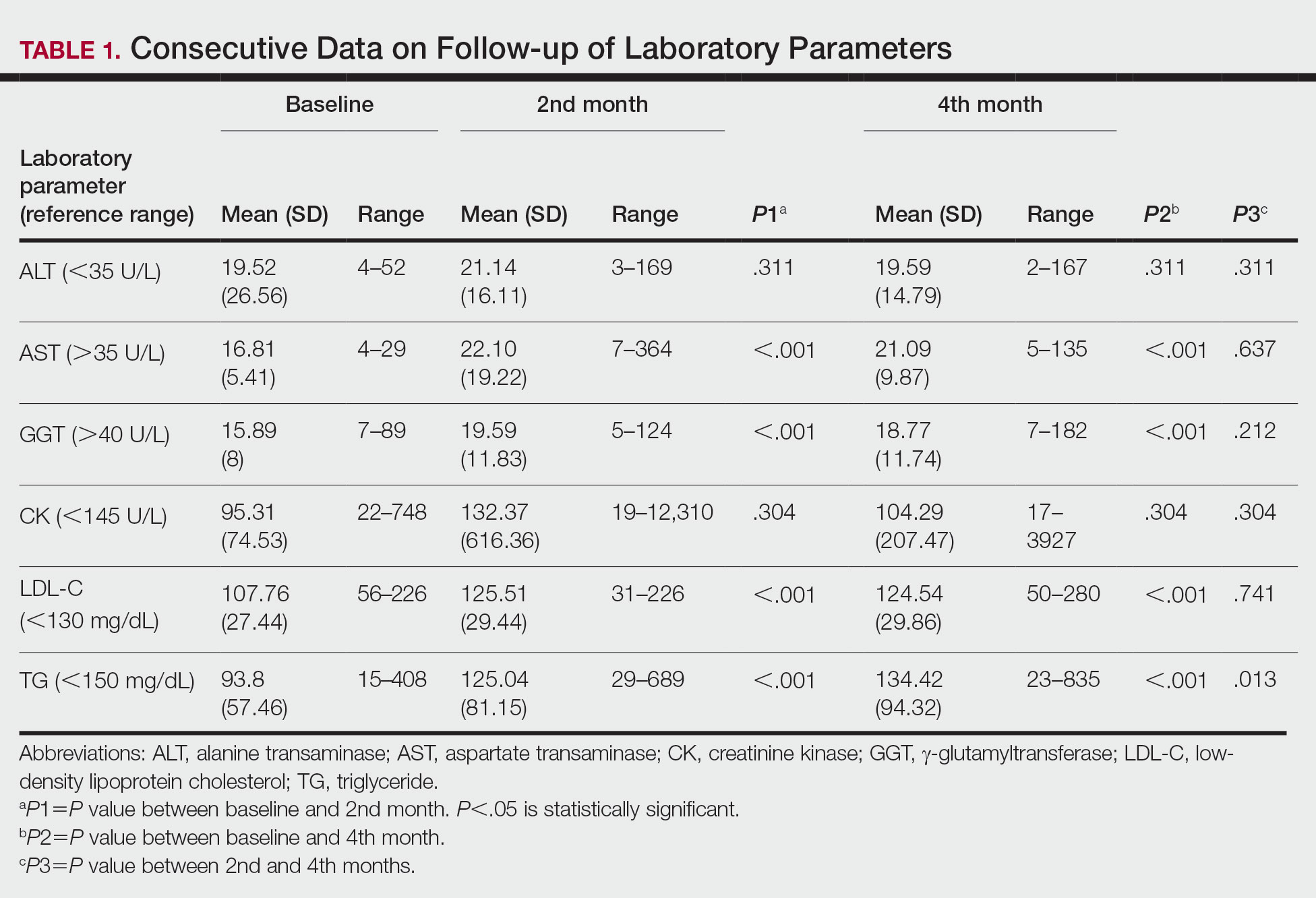
Consecutive Data at Baseline and Follow-up—A total of 415 patients with a mean age (SD) of 21.49 (7.25) years (range, 12–53 years) were included in our study. The mean total cumulative dose (SD) of the patients was 7267.27 (1878.4) mg. The consecutive data of the means of the laboratory parameters are shown in Table 1 and Figure 1. There was no significant change in the ALT levels between baseline and the fourth month as well as between the second- and fourth-month assessments (both P=.311).
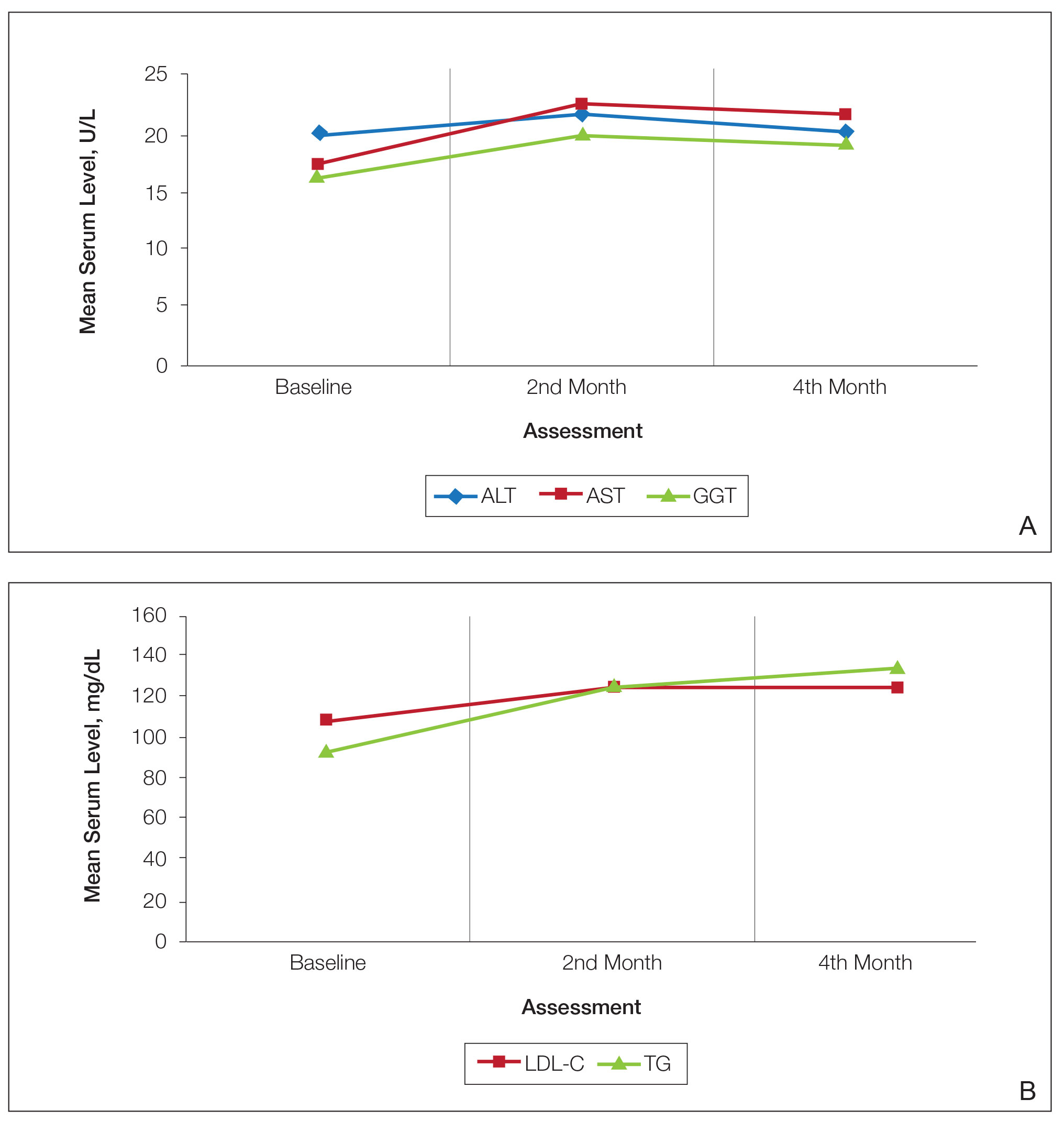
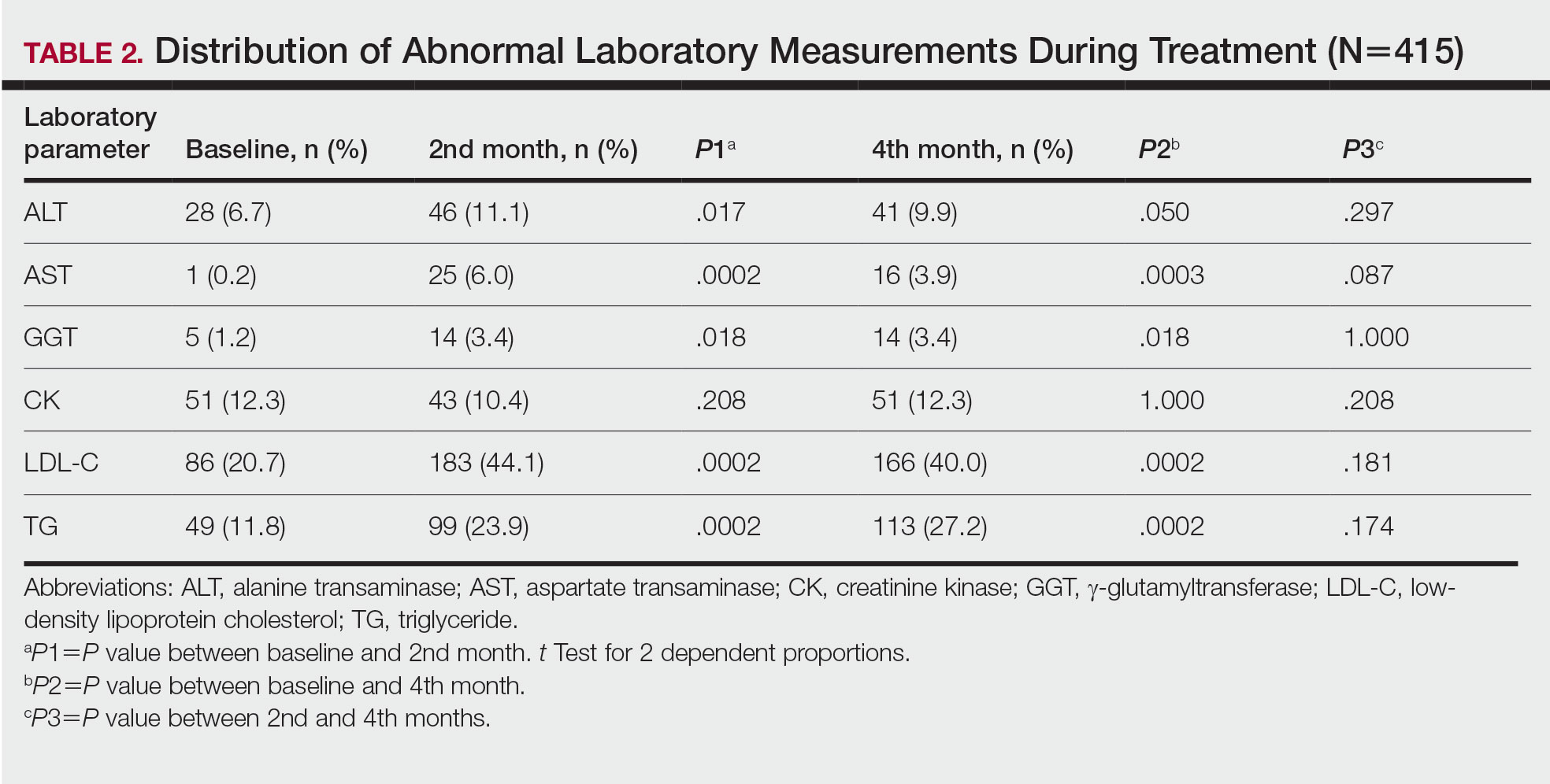
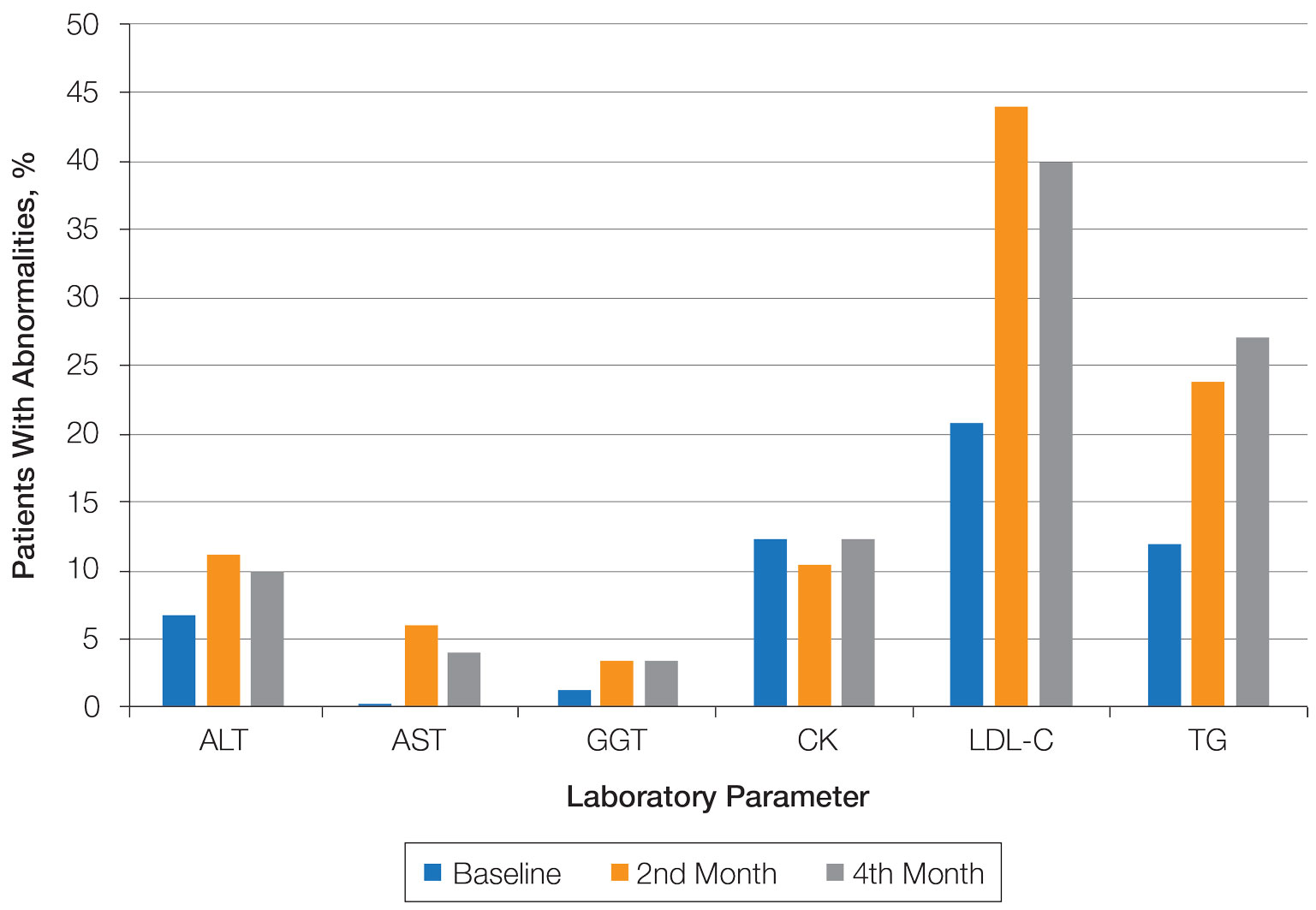
Abnormal Laboratory Measurements—The distribution of abnormal laboratory measurements during treatment is shown in Table 2 and Figure 2. Grade 3 or higher elevations of liver transaminases (ALT, AST) and GGT were observed in fewer than 2% of patients during treatment compared with baseline (grade 3 elevations of ALT and AST together in 2 patients; grade 4 AST elevation in 1 patient; grade 3 elevations of ALT, AST, and GGT combined in 1 patient; isolated grade 3 GGT elevation in 1 patient). All of the patients who developed grade 3 liver transaminases and isolated grade 3 GGT elevation had improved values when these were rechecked within 2 weeks.
In the patient who developed hepatotoxicity in the second month, the ALT level rose from a baseline of 19 U/L to 169 U/L, the AST level from a baseline of 19 U/L to 61 U/L, and the GGT level from a baseline of 24 U/L to 124 U/L. The patient was asymptomatic. Liver function test levels returned to reference range 4 weeks after discontinuation of therapy. Hepatotoxicity did not recur after treatment was re-administered.
The patient who developed grade 4 AST elevation (364 U/L) experienced fatigue and myalgia. He had done vigorous exercise up to 2 days before the test and also had a grade 4 CK elevation (12,310 U/L). He was thought to have isotretinoin-related rhabdomyolysis. His treatment was discontinued, and he was advised to hydrate and rest. Treatment was re-started after 2 weeks. With frequent laboratory monitoring and avoidance of vigorous physical activity, the patient completed the remaining course of isotretinoin without any laboratory abnormalities or symptoms.
Creatinine kinase abnormalities in the second and fourth months compared with baseline were not statistically significant. The patients with grade 3 or higher CK elevations, except for the case with rhabdomyolysis, had no clinical signs or other characteristic laboratory findings of rhabdomyolysis.
Hypercholesterolemia (LDL-C ≥130 mg/dL) occurred most frequently, with a maximum of 280 mg/dL in 1 patient (in the fourth month) and less than 250 mg/dL in all other patients. Hypercholesterolemia occurred in 183 (44.1%) patients in the second month and in 166 (40.0%) patients in the fourth month. However, baseline abnormalities also were frequent (86 [20.7%]), and hypercholesterolemia persisted in the second and fourth months in all of these patients.
It was observed that the patients with TG abnormalities increased continuously in the second (99 [23.9%]) and fourth (113 [27.2%]) months compared with baseline (49 [11.8%]). Grade 3 TG elevations were observed in 2.2% of patients (n=9; 5 patients in the second month, 4 patients in the fourth month) during treatment compared with baseline, and all patients had grade 1 or 2 hypertriglyceridemia at baseline. Of the patients with grade 3 TG elevation, 3 patients in the second month and 2 patients in the fourth month were obese at baseline. No grade 4 TG elevations were observed. Complications related to hyperlipidemia, such as pancreatitis, were observed in 1 patient. No patient terminated treatment because of lipid abnormalities. The treatment of our patients with major hypercholesterolemia and/or grade 3 hypertriglyceridemia was interrupted. The hyperlipidemia of these patients was controlled by a low-fat diet and a short-term dose reduction.
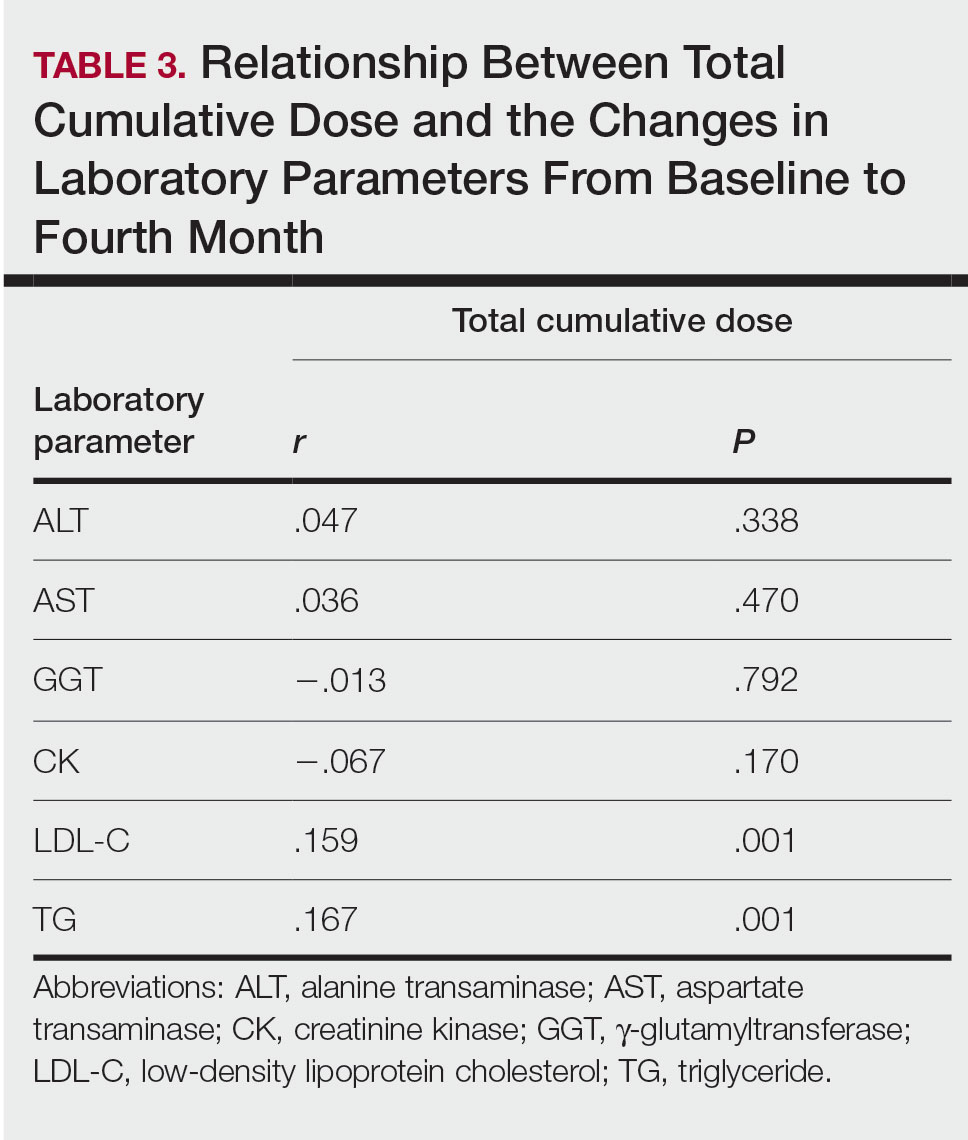
Relationship Between Total Cumulative Dose and Laboratory Parameters—The relationships between the total cumulative dose and changes up to the fourth month are presented in Table 3. As the total dose increased, the changes in TG and LDL-C levels significantly increased in the fourth month (both P=.001). However, the degree of these relationships was weak. No significant correlation was found between the periodic changes of other laboratory parameters and the total dose.
Comment
The parameters followed in our study show that TG levels tend to increase continuously from baseline during isotretinoin treatment, while ALT, AST, GGT, and LDL-C levels increase in the second month and decrease at 4 months. Although this same trend occurs with CK levels, the change was not statistically significant. The most common laboratory abnormality in our study was hyperlipidemia. Levels of LDL-C and TG were both found to be statistically elevated in the second and fourth months of treatment compared with baseline. Parthasarathy et al3 reported that obesity had an important role in the increase of lipid levels in patients using isotretinoin at baseline. In our study, 5 of 9 patients (55.6%) with grade 3 TG elevation were obese, which supports the theory that obesity plays an important role in the increase in lipid levels. Up-to-date laboratory follow-up of lipids suggests that there is no need to follow up serum lipids after the second month of treatment. Patients with risk factors for hyperlipidemia, such as abdominal obesity and familial hyperlipidemia, do not require further follow-up if there is no increase in serum lipids in the first month of treatment.1 The presence of grade 1 or 2 hypertriglyceridemia at baseline in all our patients with grade 3 TG elevation may suggest that periodic laboratory follow-up during isotretinoin treatment is necessary to detect patients with grade 3 and higher TG levels.
The lack of knowledge of other risk factors (eg, familial hyperlipidemia, insulin resistance) for hyperlipidemia in all patients at baseline may be a limitation of our study. Although hypercholesterolemia persisted in the follow-up of our patients with initial LDL-C abnormalities, hypercholesterolemia over 250 mg/dL was very rare (1 patient). Possible complications associated with serum lipid abnormalities are pancreatitis and metabolic syndrome.4 In our study, none of the patients with lipid abnormalities had any relevant clinical sequelae. The dose-dependent elevation of the changes in LDL-C and TG (Table 3) may be important to predict the significant elevation of lipids and the associated complications in patients with a high total cumulative dose target that may require a long treatment duration. However, considering the short follow-up periods in our patients, the absence of clinical sequelae may be misleading. There are differences in recommendations between the US and European guidelines for isotretinoin dosage. Although the US guidelines recommend a total cumulative dose target, the European guidelines recommend low-dose isotretinoin daily for at least 6 months instead of a cumulative dose.
Most liver transaminase abnormalities were detected in the second month. Abnormalities in GGT were seen in the second month and remained elevated at the next follow-up. However, clinically important grade 3 transaminase and GGT elevations were rare. It has been reported that GGT levels are more specific than transaminases in measuring hepatotoxicity.7 The fact that our patient with hepatotoxicity had a grade 3 GGT elevation in addition to grade 3 transaminase elevations supports that GGT elevation is more specific than transaminase levels in measuring hepatotoxicity. When these parameters were rechecked in our patients with grade 3 transaminase elevations, except in the case of hepatotoxicity, transaminase elevations did not recur, and GGT elevations did not accompany elevated transaminases, which suggested that transaminases may be elevated due to an extrahepatic origin (eg, hemolysis, exercise).
Rhabdomyolysis secondary to isotretinoin is rare in the literature of acne studies. In addition to clinical findings such as myalgia and fatigue, increased CK and abnormal liver enzymes, specifically AST, suggest the development of rhabdomyolysis.8 Our patient who developed rhabdomyolysis also had a recent history of vigorous exercise, grade 4 CK, and AST elevations. Other patients with isolated grade 3 CK elevations were informed about possible clinical signs of rhabdomyolysis, and they were able to complete their courses without any incident. According to a study by Landau et al,9 isotretinoin-associated hyperCKemia has been reported as benign. Similarly, our study found that isolated CK elevation during isotretinoin treatment may be misleading as a sign of rhabdomyolysis. Instead, CK monitoring may be more appropriate and cost-effective in patients with suspected clinical signs of rhabdomyolysis or in those with major elevations in transaminases, especially AST.
Conclusion
According to our study, hyperlipidemia was the most common complication in acne patients using isotretinoin. It may be appropriate to monitor the TG level at 2-month intervals in patients with grade 1 or 2 TG elevation at baseline to detect the possible risk for developing grade 3 hyperlipidemia. Periodic monitoring of LDL-C and TG levels may be appropriate, especially in patients who require a high total cumulative dose of isotretinoin. Clinically important liver enzyme abnormalities were rare in our study. Our findings support the idea that routine monthly monitoring of normal laboratory parameters is unnecessary and wasteful. Additionally, periodic monitoring of abnormal laboratory parameters should be considered on an individual basis.
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865.
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). August 9, 2006. Accessed June 12, 2023. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- Parthasarathy V, Shah N, Kirkorian AY. The utility of laboratory testing for pediatric patients undergoing isotretinoin treatment. Pediatr Dermatol. 2022;39:731-733.
- Sarkar T, Sarkar S, Patra A. Low-dose isotretinoin therapy and blood lipid abnormality: a case series with sixty patients. J Family Med Prim Care. 2018;7:171-174.
- Nast A, Dréno B, Bettoli V, et al. European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016;30:1261-1268.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Webster GF, Webster TG, Grimes LR. Laboratory tests in patients treated with isotretinoin: occurrence of liver and muscle abnormalities and failure of AST and ALT to predict liver abnormality. Dermatol Online J. 2017;23:13030/qt7rv7j80p.
- Raneses E, Schmidgal EC. Rhabdomyolysis caused by isotretinoin and exercise in an otherwise healthy female patient. Cureus. 2022;14:E25981.
- Landau M, Mesterman R, Ophir J, et al. Clinical significance of markedly elevated serum creatine kinase levels in patients with acne on isotretinoin. Acta Derm Venereol. 2001;81:350-352.
Isotretinoin is used in the treatment of nodulocystic and severe papulopustular acne. During the treatment period, laboratory monitoring is recommended to identify the risk for complications such as hepatotoxicity, teratogenicity, rhabdomyolysis, hyperlipidemia, and pancreatitis.1 There is a lack of consensus of the frequency of follow-up of laboratory parameters during isotretinoin treatment. This study evaluated the changes in laboratory parameters used in daily practice for patients with acne who were treated with isotretinoin to determine the optimum test repetition frequency.
Materials and Methods

Statistical Analysis—The descriptive statistics of the measurements were presented as means, standard deviations, or medians (first and third quartiles). With respect to the normal distribution, the consistency of the measurements was evaluated with the Kolmogorov-Smirnov test, and small deviations from the normal distribution were observed. Changes in laboratory measurements were evaluated with simple repeated-measures analysis of variance, and changes that differed significantly were determined by a Holm-Sidak post hoc test. Relationships between total cumulative doses and laboratory measurements at second visits were evaluated by the Pearson correlation analysis. The statistical significance level was P<.05. SPSS Statistics 23 (IBM) was used in the calculations.
Results
Consecutive Data at Baseline and Follow-up—A total of 415 patients with a mean age (SD) of 21.49 (7.25) years (range, 12–53 years) were included in our study. The mean total cumulative dose (SD) of the patients was 7267.27 (1878.4) mg. The consecutive data of the means of the laboratory parameters are shown in Table 1 and Figure 1. There was no significant change in the ALT levels between baseline and the fourth month as well as between the second- and fourth-month assessments (both P=.311).

Abnormal Laboratory Measurements—The distribution of abnormal laboratory measurements during treatment is shown in Table 2 and Figure 2. Grade 3 or higher elevations of liver transaminases (ALT, AST) and GGT were observed in fewer than 2% of patients during treatment compared with baseline (grade 3 elevations of ALT and AST together in 2 patients; grade 4 AST elevation in 1 patient; grade 3 elevations of ALT, AST, and GGT combined in 1 patient; isolated grade 3 GGT elevation in 1 patient). All of the patients who developed grade 3 liver transaminases and isolated grade 3 GGT elevation had improved values when these were rechecked within 2 weeks.

In the patient who developed hepatotoxicity in the second month, the ALT level rose from a baseline of 19 U/L to 169 U/L, the AST level from a baseline of 19 U/L to 61 U/L, and the GGT level from a baseline of 24 U/L to 124 U/L. The patient was asymptomatic. Liver function test levels returned to reference range 4 weeks after discontinuation of therapy. Hepatotoxicity did not recur after treatment was re-administered.

The patient who developed grade 4 AST elevation (364 U/L) experienced fatigue and myalgia. He had done vigorous exercise up to 2 days before the test and also had a grade 4 CK elevation (12,310 U/L). He was thought to have isotretinoin-related rhabdomyolysis. His treatment was discontinued, and he was advised to hydrate and rest. Treatment was re-started after 2 weeks. With frequent laboratory monitoring and avoidance of vigorous physical activity, the patient completed the remaining course of isotretinoin without any laboratory abnormalities or symptoms.
Creatinine kinase abnormalities in the second and fourth months compared with baseline were not statistically significant. The patients with grade 3 or higher CK elevations, except for the case with rhabdomyolysis, had no clinical signs or other characteristic laboratory findings of rhabdomyolysis.
Hypercholesterolemia (LDL-C ≥130 mg/dL) occurred most frequently, with a maximum of 280 mg/dL in 1 patient (in the fourth month) and less than 250 mg/dL in all other patients. Hypercholesterolemia occurred in 183 (44.1%) patients in the second month and in 166 (40.0%) patients in the fourth month. However, baseline abnormalities also were frequent (86 [20.7%]), and hypercholesterolemia persisted in the second and fourth months in all of these patients.
It was observed that the patients with TG abnormalities increased continuously in the second (99 [23.9%]) and fourth (113 [27.2%]) months compared with baseline (49 [11.8%]). Grade 3 TG elevations were observed in 2.2% of patients (n=9; 5 patients in the second month, 4 patients in the fourth month) during treatment compared with baseline, and all patients had grade 1 or 2 hypertriglyceridemia at baseline. Of the patients with grade 3 TG elevation, 3 patients in the second month and 2 patients in the fourth month were obese at baseline. No grade 4 TG elevations were observed. Complications related to hyperlipidemia, such as pancreatitis, were observed in 1 patient. No patient terminated treatment because of lipid abnormalities. The treatment of our patients with major hypercholesterolemia and/or grade 3 hypertriglyceridemia was interrupted. The hyperlipidemia of these patients was controlled by a low-fat diet and a short-term dose reduction.
Relationship Between Total Cumulative Dose and Laboratory Parameters—The relationships between the total cumulative dose and changes up to the fourth month are presented in Table 3. As the total dose increased, the changes in TG and LDL-C levels significantly increased in the fourth month (both P=.001). However, the degree of these relationships was weak. No significant correlation was found between the periodic changes of other laboratory parameters and the total dose.

Comment
The parameters followed in our study show that TG levels tend to increase continuously from baseline during isotretinoin treatment, while ALT, AST, GGT, and LDL-C levels increase in the second month and decrease at 4 months. Although this same trend occurs with CK levels, the change was not statistically significant. The most common laboratory abnormality in our study was hyperlipidemia. Levels of LDL-C and TG were both found to be statistically elevated in the second and fourth months of treatment compared with baseline. Parthasarathy et al3 reported that obesity had an important role in the increase of lipid levels in patients using isotretinoin at baseline. In our study, 5 of 9 patients (55.6%) with grade 3 TG elevation were obese, which supports the theory that obesity plays an important role in the increase in lipid levels. Up-to-date laboratory follow-up of lipids suggests that there is no need to follow up serum lipids after the second month of treatment. Patients with risk factors for hyperlipidemia, such as abdominal obesity and familial hyperlipidemia, do not require further follow-up if there is no increase in serum lipids in the first month of treatment.1 The presence of grade 1 or 2 hypertriglyceridemia at baseline in all our patients with grade 3 TG elevation may suggest that periodic laboratory follow-up during isotretinoin treatment is necessary to detect patients with grade 3 and higher TG levels.
The lack of knowledge of other risk factors (eg, familial hyperlipidemia, insulin resistance) for hyperlipidemia in all patients at baseline may be a limitation of our study. Although hypercholesterolemia persisted in the follow-up of our patients with initial LDL-C abnormalities, hypercholesterolemia over 250 mg/dL was very rare (1 patient). Possible complications associated with serum lipid abnormalities are pancreatitis and metabolic syndrome.4 In our study, none of the patients with lipid abnormalities had any relevant clinical sequelae. The dose-dependent elevation of the changes in LDL-C and TG (Table 3) may be important to predict the significant elevation of lipids and the associated complications in patients with a high total cumulative dose target that may require a long treatment duration. However, considering the short follow-up periods in our patients, the absence of clinical sequelae may be misleading. There are differences in recommendations between the US and European guidelines for isotretinoin dosage. Although the US guidelines recommend a total cumulative dose target, the European guidelines recommend low-dose isotretinoin daily for at least 6 months instead of a cumulative dose.
Most liver transaminase abnormalities were detected in the second month. Abnormalities in GGT were seen in the second month and remained elevated at the next follow-up. However, clinically important grade 3 transaminase and GGT elevations were rare. It has been reported that GGT levels are more specific than transaminases in measuring hepatotoxicity.7 The fact that our patient with hepatotoxicity had a grade 3 GGT elevation in addition to grade 3 transaminase elevations supports that GGT elevation is more specific than transaminase levels in measuring hepatotoxicity. When these parameters were rechecked in our patients with grade 3 transaminase elevations, except in the case of hepatotoxicity, transaminase elevations did not recur, and GGT elevations did not accompany elevated transaminases, which suggested that transaminases may be elevated due to an extrahepatic origin (eg, hemolysis, exercise).
Rhabdomyolysis secondary to isotretinoin is rare in the literature of acne studies. In addition to clinical findings such as myalgia and fatigue, increased CK and abnormal liver enzymes, specifically AST, suggest the development of rhabdomyolysis.8 Our patient who developed rhabdomyolysis also had a recent history of vigorous exercise, grade 4 CK, and AST elevations. Other patients with isolated grade 3 CK elevations were informed about possible clinical signs of rhabdomyolysis, and they were able to complete their courses without any incident. According to a study by Landau et al,9 isotretinoin-associated hyperCKemia has been reported as benign. Similarly, our study found that isolated CK elevation during isotretinoin treatment may be misleading as a sign of rhabdomyolysis. Instead, CK monitoring may be more appropriate and cost-effective in patients with suspected clinical signs of rhabdomyolysis or in those with major elevations in transaminases, especially AST.
Conclusion
According to our study, hyperlipidemia was the most common complication in acne patients using isotretinoin. It may be appropriate to monitor the TG level at 2-month intervals in patients with grade 1 or 2 TG elevation at baseline to detect the possible risk for developing grade 3 hyperlipidemia. Periodic monitoring of LDL-C and TG levels may be appropriate, especially in patients who require a high total cumulative dose of isotretinoin. Clinically important liver enzyme abnormalities were rare in our study. Our findings support the idea that routine monthly monitoring of normal laboratory parameters is unnecessary and wasteful. Additionally, periodic monitoring of abnormal laboratory parameters should be considered on an individual basis.
Isotretinoin is used in the treatment of nodulocystic and severe papulopustular acne. During the treatment period, laboratory monitoring is recommended to identify the risk for complications such as hepatotoxicity, teratogenicity, rhabdomyolysis, hyperlipidemia, and pancreatitis.1 There is a lack of consensus of the frequency of follow-up of laboratory parameters during isotretinoin treatment. This study evaluated the changes in laboratory parameters used in daily practice for patients with acne who were treated with isotretinoin to determine the optimum test repetition frequency.
Materials and Methods

Statistical Analysis—The descriptive statistics of the measurements were presented as means, standard deviations, or medians (first and third quartiles). With respect to the normal distribution, the consistency of the measurements was evaluated with the Kolmogorov-Smirnov test, and small deviations from the normal distribution were observed. Changes in laboratory measurements were evaluated with simple repeated-measures analysis of variance, and changes that differed significantly were determined by a Holm-Sidak post hoc test. Relationships between total cumulative doses and laboratory measurements at second visits were evaluated by the Pearson correlation analysis. The statistical significance level was P<.05. SPSS Statistics 23 (IBM) was used in the calculations.
Results
Consecutive Data at Baseline and Follow-up—A total of 415 patients with a mean age (SD) of 21.49 (7.25) years (range, 12–53 years) were included in our study. The mean total cumulative dose (SD) of the patients was 7267.27 (1878.4) mg. The consecutive data of the means of the laboratory parameters are shown in Table 1 and Figure 1. There was no significant change in the ALT levels between baseline and the fourth month as well as between the second- and fourth-month assessments (both P=.311).

Abnormal Laboratory Measurements—The distribution of abnormal laboratory measurements during treatment is shown in Table 2 and Figure 2. Grade 3 or higher elevations of liver transaminases (ALT, AST) and GGT were observed in fewer than 2% of patients during treatment compared with baseline (grade 3 elevations of ALT and AST together in 2 patients; grade 4 AST elevation in 1 patient; grade 3 elevations of ALT, AST, and GGT combined in 1 patient; isolated grade 3 GGT elevation in 1 patient). All of the patients who developed grade 3 liver transaminases and isolated grade 3 GGT elevation had improved values when these were rechecked within 2 weeks.

In the patient who developed hepatotoxicity in the second month, the ALT level rose from a baseline of 19 U/L to 169 U/L, the AST level from a baseline of 19 U/L to 61 U/L, and the GGT level from a baseline of 24 U/L to 124 U/L. The patient was asymptomatic. Liver function test levels returned to reference range 4 weeks after discontinuation of therapy. Hepatotoxicity did not recur after treatment was re-administered.

The patient who developed grade 4 AST elevation (364 U/L) experienced fatigue and myalgia. He had done vigorous exercise up to 2 days before the test and also had a grade 4 CK elevation (12,310 U/L). He was thought to have isotretinoin-related rhabdomyolysis. His treatment was discontinued, and he was advised to hydrate and rest. Treatment was re-started after 2 weeks. With frequent laboratory monitoring and avoidance of vigorous physical activity, the patient completed the remaining course of isotretinoin without any laboratory abnormalities or symptoms.
Creatinine kinase abnormalities in the second and fourth months compared with baseline were not statistically significant. The patients with grade 3 or higher CK elevations, except for the case with rhabdomyolysis, had no clinical signs or other characteristic laboratory findings of rhabdomyolysis.
Hypercholesterolemia (LDL-C ≥130 mg/dL) occurred most frequently, with a maximum of 280 mg/dL in 1 patient (in the fourth month) and less than 250 mg/dL in all other patients. Hypercholesterolemia occurred in 183 (44.1%) patients in the second month and in 166 (40.0%) patients in the fourth month. However, baseline abnormalities also were frequent (86 [20.7%]), and hypercholesterolemia persisted in the second and fourth months in all of these patients.
It was observed that the patients with TG abnormalities increased continuously in the second (99 [23.9%]) and fourth (113 [27.2%]) months compared with baseline (49 [11.8%]). Grade 3 TG elevations were observed in 2.2% of patients (n=9; 5 patients in the second month, 4 patients in the fourth month) during treatment compared with baseline, and all patients had grade 1 or 2 hypertriglyceridemia at baseline. Of the patients with grade 3 TG elevation, 3 patients in the second month and 2 patients in the fourth month were obese at baseline. No grade 4 TG elevations were observed. Complications related to hyperlipidemia, such as pancreatitis, were observed in 1 patient. No patient terminated treatment because of lipid abnormalities. The treatment of our patients with major hypercholesterolemia and/or grade 3 hypertriglyceridemia was interrupted. The hyperlipidemia of these patients was controlled by a low-fat diet and a short-term dose reduction.
Relationship Between Total Cumulative Dose and Laboratory Parameters—The relationships between the total cumulative dose and changes up to the fourth month are presented in Table 3. As the total dose increased, the changes in TG and LDL-C levels significantly increased in the fourth month (both P=.001). However, the degree of these relationships was weak. No significant correlation was found between the periodic changes of other laboratory parameters and the total dose.

Comment
The parameters followed in our study show that TG levels tend to increase continuously from baseline during isotretinoin treatment, while ALT, AST, GGT, and LDL-C levels increase in the second month and decrease at 4 months. Although this same trend occurs with CK levels, the change was not statistically significant. The most common laboratory abnormality in our study was hyperlipidemia. Levels of LDL-C and TG were both found to be statistically elevated in the second and fourth months of treatment compared with baseline. Parthasarathy et al3 reported that obesity had an important role in the increase of lipid levels in patients using isotretinoin at baseline. In our study, 5 of 9 patients (55.6%) with grade 3 TG elevation were obese, which supports the theory that obesity plays an important role in the increase in lipid levels. Up-to-date laboratory follow-up of lipids suggests that there is no need to follow up serum lipids after the second month of treatment. Patients with risk factors for hyperlipidemia, such as abdominal obesity and familial hyperlipidemia, do not require further follow-up if there is no increase in serum lipids in the first month of treatment.1 The presence of grade 1 or 2 hypertriglyceridemia at baseline in all our patients with grade 3 TG elevation may suggest that periodic laboratory follow-up during isotretinoin treatment is necessary to detect patients with grade 3 and higher TG levels.
The lack of knowledge of other risk factors (eg, familial hyperlipidemia, insulin resistance) for hyperlipidemia in all patients at baseline may be a limitation of our study. Although hypercholesterolemia persisted in the follow-up of our patients with initial LDL-C abnormalities, hypercholesterolemia over 250 mg/dL was very rare (1 patient). Possible complications associated with serum lipid abnormalities are pancreatitis and metabolic syndrome.4 In our study, none of the patients with lipid abnormalities had any relevant clinical sequelae. The dose-dependent elevation of the changes in LDL-C and TG (Table 3) may be important to predict the significant elevation of lipids and the associated complications in patients with a high total cumulative dose target that may require a long treatment duration. However, considering the short follow-up periods in our patients, the absence of clinical sequelae may be misleading. There are differences in recommendations between the US and European guidelines for isotretinoin dosage. Although the US guidelines recommend a total cumulative dose target, the European guidelines recommend low-dose isotretinoin daily for at least 6 months instead of a cumulative dose.
Most liver transaminase abnormalities were detected in the second month. Abnormalities in GGT were seen in the second month and remained elevated at the next follow-up. However, clinically important grade 3 transaminase and GGT elevations were rare. It has been reported that GGT levels are more specific than transaminases in measuring hepatotoxicity.7 The fact that our patient with hepatotoxicity had a grade 3 GGT elevation in addition to grade 3 transaminase elevations supports that GGT elevation is more specific than transaminase levels in measuring hepatotoxicity. When these parameters were rechecked in our patients with grade 3 transaminase elevations, except in the case of hepatotoxicity, transaminase elevations did not recur, and GGT elevations did not accompany elevated transaminases, which suggested that transaminases may be elevated due to an extrahepatic origin (eg, hemolysis, exercise).
Rhabdomyolysis secondary to isotretinoin is rare in the literature of acne studies. In addition to clinical findings such as myalgia and fatigue, increased CK and abnormal liver enzymes, specifically AST, suggest the development of rhabdomyolysis.8 Our patient who developed rhabdomyolysis also had a recent history of vigorous exercise, grade 4 CK, and AST elevations. Other patients with isolated grade 3 CK elevations were informed about possible clinical signs of rhabdomyolysis, and they were able to complete their courses without any incident. According to a study by Landau et al,9 isotretinoin-associated hyperCKemia has been reported as benign. Similarly, our study found that isolated CK elevation during isotretinoin treatment may be misleading as a sign of rhabdomyolysis. Instead, CK monitoring may be more appropriate and cost-effective in patients with suspected clinical signs of rhabdomyolysis or in those with major elevations in transaminases, especially AST.
Conclusion
According to our study, hyperlipidemia was the most common complication in acne patients using isotretinoin. It may be appropriate to monitor the TG level at 2-month intervals in patients with grade 1 or 2 TG elevation at baseline to detect the possible risk for developing grade 3 hyperlipidemia. Periodic monitoring of LDL-C and TG levels may be appropriate, especially in patients who require a high total cumulative dose of isotretinoin. Clinically important liver enzyme abnormalities were rare in our study. Our findings support the idea that routine monthly monitoring of normal laboratory parameters is unnecessary and wasteful. Additionally, periodic monitoring of abnormal laboratory parameters should be considered on an individual basis.
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865.
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). August 9, 2006. Accessed June 12, 2023. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- Parthasarathy V, Shah N, Kirkorian AY. The utility of laboratory testing for pediatric patients undergoing isotretinoin treatment. Pediatr Dermatol. 2022;39:731-733.
- Sarkar T, Sarkar S, Patra A. Low-dose isotretinoin therapy and blood lipid abnormality: a case series with sixty patients. J Family Med Prim Care. 2018;7:171-174.
- Nast A, Dréno B, Bettoli V, et al. European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016;30:1261-1268.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Webster GF, Webster TG, Grimes LR. Laboratory tests in patients treated with isotretinoin: occurrence of liver and muscle abnormalities and failure of AST and ALT to predict liver abnormality. Dermatol Online J. 2017;23:13030/qt7rv7j80p.
- Raneses E, Schmidgal EC. Rhabdomyolysis caused by isotretinoin and exercise in an otherwise healthy female patient. Cureus. 2022;14:E25981.
- Landau M, Mesterman R, Ophir J, et al. Clinical significance of markedly elevated serum creatine kinase levels in patients with acne on isotretinoin. Acta Derm Venereol. 2001;81:350-352.
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865.
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). August 9, 2006. Accessed June 12, 2023. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- Parthasarathy V, Shah N, Kirkorian AY. The utility of laboratory testing for pediatric patients undergoing isotretinoin treatment. Pediatr Dermatol. 2022;39:731-733.
- Sarkar T, Sarkar S, Patra A. Low-dose isotretinoin therapy and blood lipid abnormality: a case series with sixty patients. J Family Med Prim Care. 2018;7:171-174.
- Nast A, Dréno B, Bettoli V, et al. European evidence-based (S3) guideline for the treatment of acne - update 2016 - short version. J Eur Acad Dermatol Venereol. 2016;30:1261-1268.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.
- Webster GF, Webster TG, Grimes LR. Laboratory tests in patients treated with isotretinoin: occurrence of liver and muscle abnormalities and failure of AST and ALT to predict liver abnormality. Dermatol Online J. 2017;23:13030/qt7rv7j80p.
- Raneses E, Schmidgal EC. Rhabdomyolysis caused by isotretinoin and exercise in an otherwise healthy female patient. Cureus. 2022;14:E25981.
- Landau M, Mesterman R, Ophir J, et al. Clinical significance of markedly elevated serum creatine kinase levels in patients with acne on isotretinoin. Acta Derm Venereol. 2001;81:350-352.
Practice Points
- Hyperlipidemia was the most common complication in patients with acne using isotretinoin.
- It may be appropriate to monitor triglyceride levels at 2-month intervals in patients with grade 1 or 2 triglyceride elevation at baseline to detect the possible risk for developing grade 3 hyperlipidemia.
- Routine monthly monitoring of normal laboratory parameters is unnecessary and wasteful. Periodic monitoring of abnormal laboratory parameters should be considered on an individual basis.