User login
Fillers are products that are injected into soft tissue and are classified as either resorbable or nonresorbable (permanent). Several dermal and subcutaneous fillers for soft tissue augmentation currently are available. This article provides a brief review of the history of filler agents currently available for soft tissue augmentation. Cadaveric-derived fillers and implants will not be discussed, as these materials are expensive, are not all approved by the US Food and Drug Administration (FDA), and are more commonly used in burn victims than in cosmetic patients.
History of Fillers in Dermatology
The first known injectable agent was paraffin, but its use as a dermal filler was abandoned after complications including embolization, migration (ie, movement into surrounding tissue), and granuloma formation were reported.1 Silicone also was banned by the FDA for use as a soft tissue filler due to similar complications. Bovine collagen was the first agent to be approved by the FDA for cosmetic injection, and since then many filler agents have revolutionized a new era of cosmetic enhancement.1 Injectable soft tissue fillers now can be classified based on several characteristics, including site of placement (ie, dermal or subcutaneous), animal versus nonanimal derivation (eg, autologous, xenograft, semisynthetic, synthetic), and duration of effects (temporary [<6 months], long-lasting [6 months– 2 years], semipermanent [2–5 years], permanent [>5 years]).2
In the 19th century, early instances of soft tissue augmentation using dermal fillers included autologous fat harvested from the arms for correction of depressed facial defects and scars in a patient with tuberculous osteitis as well as injection of paraffin into the scrotum as a testicular prosthesis in a patient with advanced tuberculosis.1 Later, a technique using a syringe to transfer autologous fat from the extremities was used for facial soft tissue augmentation and contouring, and permanent facial soft tissue augmentation using liquid silicone also was performed. In 1981, purified bovine dermal collagen was first approved by the FDA as a xenogenic agent for dermal injection.3
In 2003, the FDA began to approve new fillers for temporary soft tissue augmentation.3 Approval of injectable purified human collagen derived from fibroblasts was followed by another class of new agents: hyaluronic acid fillers.1 A biodegradable non–animal based stabilized hyaluronic acid was followed by more agents derived from rooster combs. Further investigations and research have continued, and more long-lasting synthetic fillers have become available, including calcium hydroxylapatite and poly-L-lactic acid. A renewed interest in permanent agents and longer lasting products such as silicone oil and polymethyl methacrylate also has emerged.4
The Future of Fillers
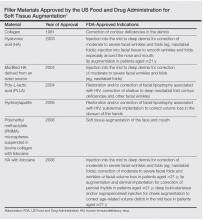
While earlier filler materials were limited, those used today are composed of a wide range of substances including collagen, hyaluronic acid, calcium hydroxylapatite, poly-L-lactic acid, and synthetic or manmade polymers (Table). The FDA has approved approximately 21 filler products for dermatologic indications, each with unique properties, advantages, and disadvantages.3 For example, there are several fillers that not only provide soft tissue augmentation but also stimulate collagen production. However, complications can occur, often many years after the initial treatment. Side effects such as swelling, erythema, and nodules may occur and in rare instances foreign body granulomas may develop and may be difficult to eradicate.5 Except for autologous fat, all fillers are foreign bodies and therefore can cause foreign body granulomatous reactions ranging from common (eg, with paraffin) to rare (eg, with hyaluronic acid) in occurrence.6 The clinical presentation of these reactions is variable, ranging from single to multiple nodules at the injection site to diffuse, hard swelling of the face accompanied by reddening of the skin.
With the increasing desire for a youthful appearance among the aging population, the pharmaceutical industry has responded by increasing the number of available treatment options to meet the demands of cosmetic patients, one of the fastest growing subpopulations in the field of dermatology. Fillers also have provided new options for patients who are unable to afford plastic surgery or those who are poor surgical candidates. The ideal filler material is nonallergenic, noncarcinogenic, and nonteratogenic. It should be stable, affordable, malleable, reversible, and durable with results that can be reproduced and should cause minimal inflammation, migration, and detectable changes. The ideal filler also should have predictable and consistent results, feel natural, take little time to administer, require minimal preparation, cause no patient downtime, and have a low risk for complications. Ideal administration should be painless, user-friendly, and conducted in an outpatient setting with minimal recovery and easy storage. Although no single filler is ideal for all patients, indications, and situations, residents should be aware of the properties and characteristics that make each product unique in order to optimize treatment in all patients.
As demands for cosmetic procedures increase, it is important to incorporate knowledge of cosmetic procedures (eg, fillers for soft tissue augmentation) in resident education and training. Although cosmetic dermatology has been featured prominently in dermatology residency, surveys have shown that residents desire more training in this area.7 Although lectures on soft tissue augmentation are popular topics in dermatology, hands-on experience performing these procedures varies widely depending on different training programs. My institution offers several lectures on cosmetic dermatology, and residents are able to perform procedures for soft tissue augmentation as the first assistant or first surgeon during our cosmetic clinic sessions twice weekly.
Final Thoughts
There are a variety of fillers on the horizon to improve aging and volume loss and the science behind cosmetic injections is evolving. Regardless of the filler material chosen, optimal results are yielded by the combination of patient expectations, physician judgment based on clinical experience, and injection technique.
1. Kontis TC, Rivkin A. The history of injectable facial fillers. Facial Plast Surg. 2009;25:67-72.
2. Ashinoff R. Overview: soft tissue augmentation. Clin Plast Surg. 2000;27:479-487.
3. Soft tissue fillers approved by the Center for Devices and Radiological Health. US Food and Drug Administration Web site. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm. Updated July 27, 2015. Accessed November 16, 2015.
4. Rohrich RJ, Ghavami A, Crosby MA. The role of hyaluronic acid fillers (restylane) in facial cosmetic surgery: review and technical considerations. Plast Reconstr Surg. 2007;120(suppl 6):S41-S54.
5. Braun M, Braun S. Nodule formation following lip augmentation using porcine collagen-derived filler. J Drugs Dermatol. 2008;7:579-581.
6. Lee JM, Kim YJ. Foreign body granulomas after the use of dermal fillers: pathophysiology, clinical appearance, histologic features, and treatment. Arch Plast Surg. 2015;42:232-239.
7. Kirby JS, Adgerson CN, Anderson BE. A survey of dermatology resident education in cosmetic procedures. J Am Acad Dermatol 2013;68:e23-e28.
Fillers are products that are injected into soft tissue and are classified as either resorbable or nonresorbable (permanent). Several dermal and subcutaneous fillers for soft tissue augmentation currently are available. This article provides a brief review of the history of filler agents currently available for soft tissue augmentation. Cadaveric-derived fillers and implants will not be discussed, as these materials are expensive, are not all approved by the US Food and Drug Administration (FDA), and are more commonly used in burn victims than in cosmetic patients.
History of Fillers in Dermatology
The first known injectable agent was paraffin, but its use as a dermal filler was abandoned after complications including embolization, migration (ie, movement into surrounding tissue), and granuloma formation were reported.1 Silicone also was banned by the FDA for use as a soft tissue filler due to similar complications. Bovine collagen was the first agent to be approved by the FDA for cosmetic injection, and since then many filler agents have revolutionized a new era of cosmetic enhancement.1 Injectable soft tissue fillers now can be classified based on several characteristics, including site of placement (ie, dermal or subcutaneous), animal versus nonanimal derivation (eg, autologous, xenograft, semisynthetic, synthetic), and duration of effects (temporary [<6 months], long-lasting [6 months– 2 years], semipermanent [2–5 years], permanent [>5 years]).2
In the 19th century, early instances of soft tissue augmentation using dermal fillers included autologous fat harvested from the arms for correction of depressed facial defects and scars in a patient with tuberculous osteitis as well as injection of paraffin into the scrotum as a testicular prosthesis in a patient with advanced tuberculosis.1 Later, a technique using a syringe to transfer autologous fat from the extremities was used for facial soft tissue augmentation and contouring, and permanent facial soft tissue augmentation using liquid silicone also was performed. In 1981, purified bovine dermal collagen was first approved by the FDA as a xenogenic agent for dermal injection.3
In 2003, the FDA began to approve new fillers for temporary soft tissue augmentation.3 Approval of injectable purified human collagen derived from fibroblasts was followed by another class of new agents: hyaluronic acid fillers.1 A biodegradable non–animal based stabilized hyaluronic acid was followed by more agents derived from rooster combs. Further investigations and research have continued, and more long-lasting synthetic fillers have become available, including calcium hydroxylapatite and poly-L-lactic acid. A renewed interest in permanent agents and longer lasting products such as silicone oil and polymethyl methacrylate also has emerged.4
The Future of Fillers
While earlier filler materials were limited, those used today are composed of a wide range of substances including collagen, hyaluronic acid, calcium hydroxylapatite, poly-L-lactic acid, and synthetic or manmade polymers (Table). The FDA has approved approximately 21 filler products for dermatologic indications, each with unique properties, advantages, and disadvantages.3 For example, there are several fillers that not only provide soft tissue augmentation but also stimulate collagen production. However, complications can occur, often many years after the initial treatment. Side effects such as swelling, erythema, and nodules may occur and in rare instances foreign body granulomas may develop and may be difficult to eradicate.5 Except for autologous fat, all fillers are foreign bodies and therefore can cause foreign body granulomatous reactions ranging from common (eg, with paraffin) to rare (eg, with hyaluronic acid) in occurrence.6 The clinical presentation of these reactions is variable, ranging from single to multiple nodules at the injection site to diffuse, hard swelling of the face accompanied by reddening of the skin.
With the increasing desire for a youthful appearance among the aging population, the pharmaceutical industry has responded by increasing the number of available treatment options to meet the demands of cosmetic patients, one of the fastest growing subpopulations in the field of dermatology. Fillers also have provided new options for patients who are unable to afford plastic surgery or those who are poor surgical candidates. The ideal filler material is nonallergenic, noncarcinogenic, and nonteratogenic. It should be stable, affordable, malleable, reversible, and durable with results that can be reproduced and should cause minimal inflammation, migration, and detectable changes. The ideal filler also should have predictable and consistent results, feel natural, take little time to administer, require minimal preparation, cause no patient downtime, and have a low risk for complications. Ideal administration should be painless, user-friendly, and conducted in an outpatient setting with minimal recovery and easy storage. Although no single filler is ideal for all patients, indications, and situations, residents should be aware of the properties and characteristics that make each product unique in order to optimize treatment in all patients.
As demands for cosmetic procedures increase, it is important to incorporate knowledge of cosmetic procedures (eg, fillers for soft tissue augmentation) in resident education and training. Although cosmetic dermatology has been featured prominently in dermatology residency, surveys have shown that residents desire more training in this area.7 Although lectures on soft tissue augmentation are popular topics in dermatology, hands-on experience performing these procedures varies widely depending on different training programs. My institution offers several lectures on cosmetic dermatology, and residents are able to perform procedures for soft tissue augmentation as the first assistant or first surgeon during our cosmetic clinic sessions twice weekly.
Final Thoughts
There are a variety of fillers on the horizon to improve aging and volume loss and the science behind cosmetic injections is evolving. Regardless of the filler material chosen, optimal results are yielded by the combination of patient expectations, physician judgment based on clinical experience, and injection technique.
Fillers are products that are injected into soft tissue and are classified as either resorbable or nonresorbable (permanent). Several dermal and subcutaneous fillers for soft tissue augmentation currently are available. This article provides a brief review of the history of filler agents currently available for soft tissue augmentation. Cadaveric-derived fillers and implants will not be discussed, as these materials are expensive, are not all approved by the US Food and Drug Administration (FDA), and are more commonly used in burn victims than in cosmetic patients.
History of Fillers in Dermatology
The first known injectable agent was paraffin, but its use as a dermal filler was abandoned after complications including embolization, migration (ie, movement into surrounding tissue), and granuloma formation were reported.1 Silicone also was banned by the FDA for use as a soft tissue filler due to similar complications. Bovine collagen was the first agent to be approved by the FDA for cosmetic injection, and since then many filler agents have revolutionized a new era of cosmetic enhancement.1 Injectable soft tissue fillers now can be classified based on several characteristics, including site of placement (ie, dermal or subcutaneous), animal versus nonanimal derivation (eg, autologous, xenograft, semisynthetic, synthetic), and duration of effects (temporary [<6 months], long-lasting [6 months– 2 years], semipermanent [2–5 years], permanent [>5 years]).2
In the 19th century, early instances of soft tissue augmentation using dermal fillers included autologous fat harvested from the arms for correction of depressed facial defects and scars in a patient with tuberculous osteitis as well as injection of paraffin into the scrotum as a testicular prosthesis in a patient with advanced tuberculosis.1 Later, a technique using a syringe to transfer autologous fat from the extremities was used for facial soft tissue augmentation and contouring, and permanent facial soft tissue augmentation using liquid silicone also was performed. In 1981, purified bovine dermal collagen was first approved by the FDA as a xenogenic agent for dermal injection.3
In 2003, the FDA began to approve new fillers for temporary soft tissue augmentation.3 Approval of injectable purified human collagen derived from fibroblasts was followed by another class of new agents: hyaluronic acid fillers.1 A biodegradable non–animal based stabilized hyaluronic acid was followed by more agents derived from rooster combs. Further investigations and research have continued, and more long-lasting synthetic fillers have become available, including calcium hydroxylapatite and poly-L-lactic acid. A renewed interest in permanent agents and longer lasting products such as silicone oil and polymethyl methacrylate also has emerged.4
The Future of Fillers
While earlier filler materials were limited, those used today are composed of a wide range of substances including collagen, hyaluronic acid, calcium hydroxylapatite, poly-L-lactic acid, and synthetic or manmade polymers (Table). The FDA has approved approximately 21 filler products for dermatologic indications, each with unique properties, advantages, and disadvantages.3 For example, there are several fillers that not only provide soft tissue augmentation but also stimulate collagen production. However, complications can occur, often many years after the initial treatment. Side effects such as swelling, erythema, and nodules may occur and in rare instances foreign body granulomas may develop and may be difficult to eradicate.5 Except for autologous fat, all fillers are foreign bodies and therefore can cause foreign body granulomatous reactions ranging from common (eg, with paraffin) to rare (eg, with hyaluronic acid) in occurrence.6 The clinical presentation of these reactions is variable, ranging from single to multiple nodules at the injection site to diffuse, hard swelling of the face accompanied by reddening of the skin.
With the increasing desire for a youthful appearance among the aging population, the pharmaceutical industry has responded by increasing the number of available treatment options to meet the demands of cosmetic patients, one of the fastest growing subpopulations in the field of dermatology. Fillers also have provided new options for patients who are unable to afford plastic surgery or those who are poor surgical candidates. The ideal filler material is nonallergenic, noncarcinogenic, and nonteratogenic. It should be stable, affordable, malleable, reversible, and durable with results that can be reproduced and should cause minimal inflammation, migration, and detectable changes. The ideal filler also should have predictable and consistent results, feel natural, take little time to administer, require minimal preparation, cause no patient downtime, and have a low risk for complications. Ideal administration should be painless, user-friendly, and conducted in an outpatient setting with minimal recovery and easy storage. Although no single filler is ideal for all patients, indications, and situations, residents should be aware of the properties and characteristics that make each product unique in order to optimize treatment in all patients.
As demands for cosmetic procedures increase, it is important to incorporate knowledge of cosmetic procedures (eg, fillers for soft tissue augmentation) in resident education and training. Although cosmetic dermatology has been featured prominently in dermatology residency, surveys have shown that residents desire more training in this area.7 Although lectures on soft tissue augmentation are popular topics in dermatology, hands-on experience performing these procedures varies widely depending on different training programs. My institution offers several lectures on cosmetic dermatology, and residents are able to perform procedures for soft tissue augmentation as the first assistant or first surgeon during our cosmetic clinic sessions twice weekly.
Final Thoughts
There are a variety of fillers on the horizon to improve aging and volume loss and the science behind cosmetic injections is evolving. Regardless of the filler material chosen, optimal results are yielded by the combination of patient expectations, physician judgment based on clinical experience, and injection technique.
1. Kontis TC, Rivkin A. The history of injectable facial fillers. Facial Plast Surg. 2009;25:67-72.
2. Ashinoff R. Overview: soft tissue augmentation. Clin Plast Surg. 2000;27:479-487.
3. Soft tissue fillers approved by the Center for Devices and Radiological Health. US Food and Drug Administration Web site. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm. Updated July 27, 2015. Accessed November 16, 2015.
4. Rohrich RJ, Ghavami A, Crosby MA. The role of hyaluronic acid fillers (restylane) in facial cosmetic surgery: review and technical considerations. Plast Reconstr Surg. 2007;120(suppl 6):S41-S54.
5. Braun M, Braun S. Nodule formation following lip augmentation using porcine collagen-derived filler. J Drugs Dermatol. 2008;7:579-581.
6. Lee JM, Kim YJ. Foreign body granulomas after the use of dermal fillers: pathophysiology, clinical appearance, histologic features, and treatment. Arch Plast Surg. 2015;42:232-239.
7. Kirby JS, Adgerson CN, Anderson BE. A survey of dermatology resident education in cosmetic procedures. J Am Acad Dermatol 2013;68:e23-e28.
1. Kontis TC, Rivkin A. The history of injectable facial fillers. Facial Plast Surg. 2009;25:67-72.
2. Ashinoff R. Overview: soft tissue augmentation. Clin Plast Surg. 2000;27:479-487.
3. Soft tissue fillers approved by the Center for Devices and Radiological Health. US Food and Drug Administration Web site. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm. Updated July 27, 2015. Accessed November 16, 2015.
4. Rohrich RJ, Ghavami A, Crosby MA. The role of hyaluronic acid fillers (restylane) in facial cosmetic surgery: review and technical considerations. Plast Reconstr Surg. 2007;120(suppl 6):S41-S54.
5. Braun M, Braun S. Nodule formation following lip augmentation using porcine collagen-derived filler. J Drugs Dermatol. 2008;7:579-581.
6. Lee JM, Kim YJ. Foreign body granulomas after the use of dermal fillers: pathophysiology, clinical appearance, histologic features, and treatment. Arch Plast Surg. 2015;42:232-239.
7. Kirby JS, Adgerson CN, Anderson BE. A survey of dermatology resident education in cosmetic procedures. J Am Acad Dermatol 2013;68:e23-e28.