User login
Septic arthritis predominantly involves the weight-bearing joints of the hip and knee, which account for nearly 60% of cases.1 In contrast, the shoulder joint is involved in 10% to 15% of cases, though this number may be higher among intravenous (IV) drug users.2 The most common causative organisms are the Staphylococcus species, followed closely by β-hemolytic streptococci, with these 2 groups accounting for more than 90% of all cases.3 The Streptococcus viridans group belongs to normal oral flora residing predominantly on the surface of teeth. Although well known for its ability to colonize heart valves and frequently cause bacterial endocarditis, this group has rarely been associated with septic arthritis. Furthermore, Streptococcus mitis, a subgroup of S viridans, has been implicated even less commonly.
In this article, we report a case of glenohumeral joint septic arthritis caused by S mitis. To our knowledge, such a case has not been previously reported in the English literature. Given the low virulence of this orally based bacterium, treating physicians must maintain clinical suspicion for the organism in the setting of persistent joint effusion and pain in association with periodontal disease or trauma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand-dominant 54-year-old man presented to Dr. Gruson with complaints of persistent right shoulder pain associated with worsening range of motion (ROM). Three weeks earlier, the patient reported being assaulted and noted progressive swelling about the right shoulder. He denied fevers, chills, or prior shoulder problems. Although his past medical history was remarkable for hepatitis C and diabetes, he was not taking any diabetic medications at that time. A review of systems was remarkable for poor dental hygiene, and the patient was missing several teeth, which he said had been knocked out during the assault. Physical examination revealed diffuse tenderness about the right shoulder and severe pain with all passive movement. The shoulder was pseudoparalyzed. There were no subcutaneous collections, wounds, or ecchymosis about the shoulder. Mild calor was noted on the right shoulder relative to the left. Radiographs of the right shoulder showed no acute osseous abnormalities.
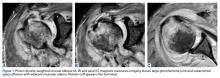
Magnetic resonance imaging (MRI), which was urgently obtained to assess the integrity of the rotator cuff and the location of the effusion, showed a large subacromial and glenohumeral joint effusion as well as diffuse muscular edema (Figures 1A-1C).
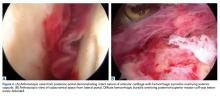
In light of the elevated infection findings of the laboratory tests and the positive culture, urgent arthroscopic irrigation and débridement of the right shoulder were indicated. Given the organism identified, transesophageal echocardiography was performed; there were no valvular vegetations. Creation of the posterior glenohumeral portal resulted in egress of turbid fluid, which was sent for culture. The subacromial space and the glenohumeral joint were thoroughly lavaged and the copious hemorrhagic synovitis débrided (Figures 2A, 2B).
The 8-week course of antibiotics normalized the patient’s ESR to 13 mm/h. Follow-up MRI showed improvement in the soft-tissue edema. Clinically, the patient reported minimal shoulder pain. He was undergoing physical therapy to regain strength and ROM.
Discussion
Staphylococcus aureus is the leading causative organism of septic arthritis, accounting for more than 60% of all cases.4 Conversely, the Streptococcus viridans group is rarely implicated in septic arthritis, accounting for <1% of cases.4S viridans is part of the commensal oral flora and has low virulence. This heterogeneous group is subdivided into S mitis, S salivarius, S anginosus, S mutans, and S bovis. The S mitis group is further subdivided into S sanguinis (formerly known as S sanguis) and S mitis. Infection by an organism of the S viridans group usually occurs on a previously injured focus, and the organism is a causative agent of bacterial endocarditis.5 Reported cases of septic arthritis caused by S viridans have predominantly involved the knee joint—with severe osteoarthritis, poor dental hygiene, and prior IV drug use identified as risk factors.5-7The shoulder joint is seldom involved in septic arthritis; estimated incidence is under 8%.8 Although overall incidence may rise in an increasingly elderly patient population, incidence of shoulder infection remains low.2,9
The main routes for developing septic arthritis include direct inoculation secondary to penetrating trauma or hematologic spread.10 Coatsworth and colleagues11 reported on iatrogenic S mitis septic arthritis of a shoulder arthroplasty during ultrasonography-guided aspiration by a technician who was not wearing a mask. Our institutional policy is to perform joint aspiration under strictly sterile conditions, which were adhered to in the present case. We surmise our patient developed transient bacteremia from the loss of several teeth, particularly given his poor dentition. Yombi and colleagues5 documented 2 cases of septic arthritis caused by Streptococcus gordonii, a relative of S sanguinis. One involved a previously replaced knee, and the other a native knee joint. Other cases of S viridans group septic arthritis have involved the knee,6,7,12,13 the sternoclavicular joint,14-16 and the acromioclavicular joint.17S sanguinis6,7,12,15,16 and S gordonii5 have been implicated in most cases, and an unspeciated S viridans in others.13,14,17 Concomitant periodontal disease has been reported in most cases as well,6,7,12,15 including our patient’s case. In the English-language literature, we found no other reports of S mitis as the causative agent of acute septic glenohumeral joint arthritis from hematogenous spread.
There should be no delay in diagnosing septic arthritis, and infected material should be removed from the joint. In animal models, complete joint destruction occurred only 5 weeks after inoculation with Staphylococcus aureus.10 Garofalo and colleagues18 reported a trend toward improved functional outcomes after earlier operative treatment. The choice of open surgical drainage vs repeat needle aspiration seems to be of little consequence, as both have good long-term outcomes, but open surgical drainage seems to result in better long-term functional ROM.2,9 However, results of a recent study suggested surgical treatment is not always superior to medical treatment for septic arthritis in native joints.19 In some cases involving S viridans species, treatment consisted of a combination of IV antibiotics and onetime or repeat aspiration;6,12-15 treatment in the remaining cases was surgical débridement.5,7,16,17 Given that S viridans is associated with bacterial endocarditis, echocardiography is essential if this organism is to be identified. Medical management and antibiotic treatment should be initiated after consultation with medical and infectious disease specialists.19We have reported a case of septic shoulder caused by S mitis, a low-virulence organism seldom associated with joint infection. The patient’s infection likely resulted from hematogenous spread from the oral cavity (dentition was poor). Urgent aspiration of the joint and baseline infection laboratory tests are recommended. MRI of the shoulder may show an effusion. Urgent arthroscopic irrigation and débridement can yield good clinical outcomes.
Am J Orthop. 2016;45(6):E343-E346. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Mathews CJ, Kingsley G, Field M, et al. Management of septic arthritis: a systematic review. Ann Rheum Dis. 2007;66(4):440-445.
2. Leslie BM, Harris JM 3rd, Driscoll D. Septic arthritis of the shoulder in adults. J Bone Joint Surg Am. 1989;71(10):1516-1522.
3. Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology. 2001;40(1):24-30.
4. Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiere JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61(3):267-269.
5. Yombi J, Belkhir L, Jonckheere S, et al. Streptococcus gordonii septic arthritis: two cases and review of literature. BMC Infect Dis. 2012;12:215.
6. Papaioannides D, Boniatsi L, Korantzopoulos P, Sinapidis D, Giotis C. Acute septic arthritis due to Streptococcus sanguis. Med Princ Pract. 2006;15(1):77-79.
7. Edson RS, Osmon DR, Berry DJ. Septic arthritis due to Streptococcus sanguis. Mayo Clin Proc. 2002;77(7):709-710.
8. Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK health district 1982-1991. Ann Rheum Dis. 1999;58(4):214-219.
9. Lossos IS, Yossepowitch O, Kandel L, Yardeni D, Arber N. Septic arthritis of the glenohumeral joint. A report of 11 cases and review of the literature. Medicine. 1998;77(3):177-187.
10. Esterhai JL Jr, Gelb I. Adult septic arthritis. Orthop Clin North Am. 1991;22(3):503-514.
11. Coatsworth NR, Huntington PG, Giuffre B, Kotsiou G. The doctor and the mask: iatrogenic septic arthritis caused by Streptoccocus mitis. Med J Aust. 2013;198(5):285-286.
12. Patrick MR, Lewis D. Short of a length: Streptococcus sanguis knee infection from dental source. Br J Rheumatol. 1992;31(8):569.
13. Barbadillo C, Trujillo A, Cuende E, Mazzucchelli R, Mulero J, Andreu JL. Septic arthritis due to Streptococcus viridans. Clin Exp Rheumatol. 1990;8(5):520-521.
14. Mata P, Molins A, de Oya M. Sternal arthritis caused by Streptococcus viridans in a heroin addict [in Spanish]. Med Clin. 1984;83(16):689.
15. Mandac I, Prkacin I, Sabljar Matovinovic M, Sustercic D. Septic arthritis due to Streptococcus sanguis. Coll Antropol. 2010;34(2):661-664.
16. Nitsche JF, Vaughan JH, Williams G, Curd JG. Septic sternoclavicular arthritis with Pasteurella multocida and Streptococcus sanguis. Arthritis Rheum. 1982;25(4):467-469.
17. Blankstein A, Amsallem JL, Rubenstein E, Horoszowski H, Farin I. Septic arthritis of the acromioclavicular joint. Arch Orthop Trauma Surg. 1985;103(6):417-418.
18. Garofalo R, Flanagin B, Cesari E, Vinci E, Conti M, Castagna A. Destructive septic arthritis of shoulder in adults. Musculoskelet Surg. 2014;98(supp 1):S35-S39.
19. Ravindran V, Logan I, Bourke BE. Medical vs surgical treatment for the native joint in septic arthritis: a 6-year, single UK academic centre experience. Rheumatology. 2009;48(10):1320-1322.
Septic arthritis predominantly involves the weight-bearing joints of the hip and knee, which account for nearly 60% of cases.1 In contrast, the shoulder joint is involved in 10% to 15% of cases, though this number may be higher among intravenous (IV) drug users.2 The most common causative organisms are the Staphylococcus species, followed closely by β-hemolytic streptococci, with these 2 groups accounting for more than 90% of all cases.3 The Streptococcus viridans group belongs to normal oral flora residing predominantly on the surface of teeth. Although well known for its ability to colonize heart valves and frequently cause bacterial endocarditis, this group has rarely been associated with septic arthritis. Furthermore, Streptococcus mitis, a subgroup of S viridans, has been implicated even less commonly.
In this article, we report a case of glenohumeral joint septic arthritis caused by S mitis. To our knowledge, such a case has not been previously reported in the English literature. Given the low virulence of this orally based bacterium, treating physicians must maintain clinical suspicion for the organism in the setting of persistent joint effusion and pain in association with periodontal disease or trauma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand-dominant 54-year-old man presented to Dr. Gruson with complaints of persistent right shoulder pain associated with worsening range of motion (ROM). Three weeks earlier, the patient reported being assaulted and noted progressive swelling about the right shoulder. He denied fevers, chills, or prior shoulder problems. Although his past medical history was remarkable for hepatitis C and diabetes, he was not taking any diabetic medications at that time. A review of systems was remarkable for poor dental hygiene, and the patient was missing several teeth, which he said had been knocked out during the assault. Physical examination revealed diffuse tenderness about the right shoulder and severe pain with all passive movement. The shoulder was pseudoparalyzed. There were no subcutaneous collections, wounds, or ecchymosis about the shoulder. Mild calor was noted on the right shoulder relative to the left. Radiographs of the right shoulder showed no acute osseous abnormalities.
Magnetic resonance imaging (MRI), which was urgently obtained to assess the integrity of the rotator cuff and the location of the effusion, showed a large subacromial and glenohumeral joint effusion as well as diffuse muscular edema (Figures 1A-1C).
In light of the elevated infection findings of the laboratory tests and the positive culture, urgent arthroscopic irrigation and débridement of the right shoulder were indicated. Given the organism identified, transesophageal echocardiography was performed; there were no valvular vegetations. Creation of the posterior glenohumeral portal resulted in egress of turbid fluid, which was sent for culture. The subacromial space and the glenohumeral joint were thoroughly lavaged and the copious hemorrhagic synovitis débrided (Figures 2A, 2B).
The 8-week course of antibiotics normalized the patient’s ESR to 13 mm/h. Follow-up MRI showed improvement in the soft-tissue edema. Clinically, the patient reported minimal shoulder pain. He was undergoing physical therapy to regain strength and ROM.
Discussion
Staphylococcus aureus is the leading causative organism of septic arthritis, accounting for more than 60% of all cases.4 Conversely, the Streptococcus viridans group is rarely implicated in septic arthritis, accounting for <1% of cases.4S viridans is part of the commensal oral flora and has low virulence. This heterogeneous group is subdivided into S mitis, S salivarius, S anginosus, S mutans, and S bovis. The S mitis group is further subdivided into S sanguinis (formerly known as S sanguis) and S mitis. Infection by an organism of the S viridans group usually occurs on a previously injured focus, and the organism is a causative agent of bacterial endocarditis.5 Reported cases of septic arthritis caused by S viridans have predominantly involved the knee joint—with severe osteoarthritis, poor dental hygiene, and prior IV drug use identified as risk factors.5-7The shoulder joint is seldom involved in septic arthritis; estimated incidence is under 8%.8 Although overall incidence may rise in an increasingly elderly patient population, incidence of shoulder infection remains low.2,9
The main routes for developing septic arthritis include direct inoculation secondary to penetrating trauma or hematologic spread.10 Coatsworth and colleagues11 reported on iatrogenic S mitis septic arthritis of a shoulder arthroplasty during ultrasonography-guided aspiration by a technician who was not wearing a mask. Our institutional policy is to perform joint aspiration under strictly sterile conditions, which were adhered to in the present case. We surmise our patient developed transient bacteremia from the loss of several teeth, particularly given his poor dentition. Yombi and colleagues5 documented 2 cases of septic arthritis caused by Streptococcus gordonii, a relative of S sanguinis. One involved a previously replaced knee, and the other a native knee joint. Other cases of S viridans group septic arthritis have involved the knee,6,7,12,13 the sternoclavicular joint,14-16 and the acromioclavicular joint.17S sanguinis6,7,12,15,16 and S gordonii5 have been implicated in most cases, and an unspeciated S viridans in others.13,14,17 Concomitant periodontal disease has been reported in most cases as well,6,7,12,15 including our patient’s case. In the English-language literature, we found no other reports of S mitis as the causative agent of acute septic glenohumeral joint arthritis from hematogenous spread.
There should be no delay in diagnosing septic arthritis, and infected material should be removed from the joint. In animal models, complete joint destruction occurred only 5 weeks after inoculation with Staphylococcus aureus.10 Garofalo and colleagues18 reported a trend toward improved functional outcomes after earlier operative treatment. The choice of open surgical drainage vs repeat needle aspiration seems to be of little consequence, as both have good long-term outcomes, but open surgical drainage seems to result in better long-term functional ROM.2,9 However, results of a recent study suggested surgical treatment is not always superior to medical treatment for septic arthritis in native joints.19 In some cases involving S viridans species, treatment consisted of a combination of IV antibiotics and onetime or repeat aspiration;6,12-15 treatment in the remaining cases was surgical débridement.5,7,16,17 Given that S viridans is associated with bacterial endocarditis, echocardiography is essential if this organism is to be identified. Medical management and antibiotic treatment should be initiated after consultation with medical and infectious disease specialists.19We have reported a case of septic shoulder caused by S mitis, a low-virulence organism seldom associated with joint infection. The patient’s infection likely resulted from hematogenous spread from the oral cavity (dentition was poor). Urgent aspiration of the joint and baseline infection laboratory tests are recommended. MRI of the shoulder may show an effusion. Urgent arthroscopic irrigation and débridement can yield good clinical outcomes.
Am J Orthop. 2016;45(6):E343-E346. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Septic arthritis predominantly involves the weight-bearing joints of the hip and knee, which account for nearly 60% of cases.1 In contrast, the shoulder joint is involved in 10% to 15% of cases, though this number may be higher among intravenous (IV) drug users.2 The most common causative organisms are the Staphylococcus species, followed closely by β-hemolytic streptococci, with these 2 groups accounting for more than 90% of all cases.3 The Streptococcus viridans group belongs to normal oral flora residing predominantly on the surface of teeth. Although well known for its ability to colonize heart valves and frequently cause bacterial endocarditis, this group has rarely been associated with septic arthritis. Furthermore, Streptococcus mitis, a subgroup of S viridans, has been implicated even less commonly.
In this article, we report a case of glenohumeral joint septic arthritis caused by S mitis. To our knowledge, such a case has not been previously reported in the English literature. Given the low virulence of this orally based bacterium, treating physicians must maintain clinical suspicion for the organism in the setting of persistent joint effusion and pain in association with periodontal disease or trauma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A right-hand-dominant 54-year-old man presented to Dr. Gruson with complaints of persistent right shoulder pain associated with worsening range of motion (ROM). Three weeks earlier, the patient reported being assaulted and noted progressive swelling about the right shoulder. He denied fevers, chills, or prior shoulder problems. Although his past medical history was remarkable for hepatitis C and diabetes, he was not taking any diabetic medications at that time. A review of systems was remarkable for poor dental hygiene, and the patient was missing several teeth, which he said had been knocked out during the assault. Physical examination revealed diffuse tenderness about the right shoulder and severe pain with all passive movement. The shoulder was pseudoparalyzed. There were no subcutaneous collections, wounds, or ecchymosis about the shoulder. Mild calor was noted on the right shoulder relative to the left. Radiographs of the right shoulder showed no acute osseous abnormalities.
Magnetic resonance imaging (MRI), which was urgently obtained to assess the integrity of the rotator cuff and the location of the effusion, showed a large subacromial and glenohumeral joint effusion as well as diffuse muscular edema (Figures 1A-1C).
In light of the elevated infection findings of the laboratory tests and the positive culture, urgent arthroscopic irrigation and débridement of the right shoulder were indicated. Given the organism identified, transesophageal echocardiography was performed; there were no valvular vegetations. Creation of the posterior glenohumeral portal resulted in egress of turbid fluid, which was sent for culture. The subacromial space and the glenohumeral joint were thoroughly lavaged and the copious hemorrhagic synovitis débrided (Figures 2A, 2B).
The 8-week course of antibiotics normalized the patient’s ESR to 13 mm/h. Follow-up MRI showed improvement in the soft-tissue edema. Clinically, the patient reported minimal shoulder pain. He was undergoing physical therapy to regain strength and ROM.
Discussion
Staphylococcus aureus is the leading causative organism of septic arthritis, accounting for more than 60% of all cases.4 Conversely, the Streptococcus viridans group is rarely implicated in septic arthritis, accounting for <1% of cases.4S viridans is part of the commensal oral flora and has low virulence. This heterogeneous group is subdivided into S mitis, S salivarius, S anginosus, S mutans, and S bovis. The S mitis group is further subdivided into S sanguinis (formerly known as S sanguis) and S mitis. Infection by an organism of the S viridans group usually occurs on a previously injured focus, and the organism is a causative agent of bacterial endocarditis.5 Reported cases of septic arthritis caused by S viridans have predominantly involved the knee joint—with severe osteoarthritis, poor dental hygiene, and prior IV drug use identified as risk factors.5-7The shoulder joint is seldom involved in septic arthritis; estimated incidence is under 8%.8 Although overall incidence may rise in an increasingly elderly patient population, incidence of shoulder infection remains low.2,9
The main routes for developing septic arthritis include direct inoculation secondary to penetrating trauma or hematologic spread.10 Coatsworth and colleagues11 reported on iatrogenic S mitis septic arthritis of a shoulder arthroplasty during ultrasonography-guided aspiration by a technician who was not wearing a mask. Our institutional policy is to perform joint aspiration under strictly sterile conditions, which were adhered to in the present case. We surmise our patient developed transient bacteremia from the loss of several teeth, particularly given his poor dentition. Yombi and colleagues5 documented 2 cases of septic arthritis caused by Streptococcus gordonii, a relative of S sanguinis. One involved a previously replaced knee, and the other a native knee joint. Other cases of S viridans group septic arthritis have involved the knee,6,7,12,13 the sternoclavicular joint,14-16 and the acromioclavicular joint.17S sanguinis6,7,12,15,16 and S gordonii5 have been implicated in most cases, and an unspeciated S viridans in others.13,14,17 Concomitant periodontal disease has been reported in most cases as well,6,7,12,15 including our patient’s case. In the English-language literature, we found no other reports of S mitis as the causative agent of acute septic glenohumeral joint arthritis from hematogenous spread.
There should be no delay in diagnosing septic arthritis, and infected material should be removed from the joint. In animal models, complete joint destruction occurred only 5 weeks after inoculation with Staphylococcus aureus.10 Garofalo and colleagues18 reported a trend toward improved functional outcomes after earlier operative treatment. The choice of open surgical drainage vs repeat needle aspiration seems to be of little consequence, as both have good long-term outcomes, but open surgical drainage seems to result in better long-term functional ROM.2,9 However, results of a recent study suggested surgical treatment is not always superior to medical treatment for septic arthritis in native joints.19 In some cases involving S viridans species, treatment consisted of a combination of IV antibiotics and onetime or repeat aspiration;6,12-15 treatment in the remaining cases was surgical débridement.5,7,16,17 Given that S viridans is associated with bacterial endocarditis, echocardiography is essential if this organism is to be identified. Medical management and antibiotic treatment should be initiated after consultation with medical and infectious disease specialists.19We have reported a case of septic shoulder caused by S mitis, a low-virulence organism seldom associated with joint infection. The patient’s infection likely resulted from hematogenous spread from the oral cavity (dentition was poor). Urgent aspiration of the joint and baseline infection laboratory tests are recommended. MRI of the shoulder may show an effusion. Urgent arthroscopic irrigation and débridement can yield good clinical outcomes.
Am J Orthop. 2016;45(6):E343-E346. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Mathews CJ, Kingsley G, Field M, et al. Management of septic arthritis: a systematic review. Ann Rheum Dis. 2007;66(4):440-445.
2. Leslie BM, Harris JM 3rd, Driscoll D. Septic arthritis of the shoulder in adults. J Bone Joint Surg Am. 1989;71(10):1516-1522.
3. Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology. 2001;40(1):24-30.
4. Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiere JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61(3):267-269.
5. Yombi J, Belkhir L, Jonckheere S, et al. Streptococcus gordonii septic arthritis: two cases and review of literature. BMC Infect Dis. 2012;12:215.
6. Papaioannides D, Boniatsi L, Korantzopoulos P, Sinapidis D, Giotis C. Acute septic arthritis due to Streptococcus sanguis. Med Princ Pract. 2006;15(1):77-79.
7. Edson RS, Osmon DR, Berry DJ. Septic arthritis due to Streptococcus sanguis. Mayo Clin Proc. 2002;77(7):709-710.
8. Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK health district 1982-1991. Ann Rheum Dis. 1999;58(4):214-219.
9. Lossos IS, Yossepowitch O, Kandel L, Yardeni D, Arber N. Septic arthritis of the glenohumeral joint. A report of 11 cases and review of the literature. Medicine. 1998;77(3):177-187.
10. Esterhai JL Jr, Gelb I. Adult septic arthritis. Orthop Clin North Am. 1991;22(3):503-514.
11. Coatsworth NR, Huntington PG, Giuffre B, Kotsiou G. The doctor and the mask: iatrogenic septic arthritis caused by Streptoccocus mitis. Med J Aust. 2013;198(5):285-286.
12. Patrick MR, Lewis D. Short of a length: Streptococcus sanguis knee infection from dental source. Br J Rheumatol. 1992;31(8):569.
13. Barbadillo C, Trujillo A, Cuende E, Mazzucchelli R, Mulero J, Andreu JL. Septic arthritis due to Streptococcus viridans. Clin Exp Rheumatol. 1990;8(5):520-521.
14. Mata P, Molins A, de Oya M. Sternal arthritis caused by Streptococcus viridans in a heroin addict [in Spanish]. Med Clin. 1984;83(16):689.
15. Mandac I, Prkacin I, Sabljar Matovinovic M, Sustercic D. Septic arthritis due to Streptococcus sanguis. Coll Antropol. 2010;34(2):661-664.
16. Nitsche JF, Vaughan JH, Williams G, Curd JG. Septic sternoclavicular arthritis with Pasteurella multocida and Streptococcus sanguis. Arthritis Rheum. 1982;25(4):467-469.
17. Blankstein A, Amsallem JL, Rubenstein E, Horoszowski H, Farin I. Septic arthritis of the acromioclavicular joint. Arch Orthop Trauma Surg. 1985;103(6):417-418.
18. Garofalo R, Flanagin B, Cesari E, Vinci E, Conti M, Castagna A. Destructive septic arthritis of shoulder in adults. Musculoskelet Surg. 2014;98(supp 1):S35-S39.
19. Ravindran V, Logan I, Bourke BE. Medical vs surgical treatment for the native joint in septic arthritis: a 6-year, single UK academic centre experience. Rheumatology. 2009;48(10):1320-1322.
1. Mathews CJ, Kingsley G, Field M, et al. Management of septic arthritis: a systematic review. Ann Rheum Dis. 2007;66(4):440-445.
2. Leslie BM, Harris JM 3rd, Driscoll D. Septic arthritis of the shoulder in adults. J Bone Joint Surg Am. 1989;71(10):1516-1522.
3. Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology. 2001;40(1):24-30.
4. Dubost JJ, Soubrier M, De Champs C, Ristori JM, Bussiere JL, Sauvezie B. No changes in the distribution of organisms responsible for septic arthritis over a 20 year period. Ann Rheum Dis. 2002;61(3):267-269.
5. Yombi J, Belkhir L, Jonckheere S, et al. Streptococcus gordonii septic arthritis: two cases and review of literature. BMC Infect Dis. 2012;12:215.
6. Papaioannides D, Boniatsi L, Korantzopoulos P, Sinapidis D, Giotis C. Acute septic arthritis due to Streptococcus sanguis. Med Princ Pract. 2006;15(1):77-79.
7. Edson RS, Osmon DR, Berry DJ. Septic arthritis due to Streptococcus sanguis. Mayo Clin Proc. 2002;77(7):709-710.
8. Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK health district 1982-1991. Ann Rheum Dis. 1999;58(4):214-219.
9. Lossos IS, Yossepowitch O, Kandel L, Yardeni D, Arber N. Septic arthritis of the glenohumeral joint. A report of 11 cases and review of the literature. Medicine. 1998;77(3):177-187.
10. Esterhai JL Jr, Gelb I. Adult septic arthritis. Orthop Clin North Am. 1991;22(3):503-514.
11. Coatsworth NR, Huntington PG, Giuffre B, Kotsiou G. The doctor and the mask: iatrogenic septic arthritis caused by Streptoccocus mitis. Med J Aust. 2013;198(5):285-286.
12. Patrick MR, Lewis D. Short of a length: Streptococcus sanguis knee infection from dental source. Br J Rheumatol. 1992;31(8):569.
13. Barbadillo C, Trujillo A, Cuende E, Mazzucchelli R, Mulero J, Andreu JL. Septic arthritis due to Streptococcus viridans. Clin Exp Rheumatol. 1990;8(5):520-521.
14. Mata P, Molins A, de Oya M. Sternal arthritis caused by Streptococcus viridans in a heroin addict [in Spanish]. Med Clin. 1984;83(16):689.
15. Mandac I, Prkacin I, Sabljar Matovinovic M, Sustercic D. Septic arthritis due to Streptococcus sanguis. Coll Antropol. 2010;34(2):661-664.
16. Nitsche JF, Vaughan JH, Williams G, Curd JG. Septic sternoclavicular arthritis with Pasteurella multocida and Streptococcus sanguis. Arthritis Rheum. 1982;25(4):467-469.
17. Blankstein A, Amsallem JL, Rubenstein E, Horoszowski H, Farin I. Septic arthritis of the acromioclavicular joint. Arch Orthop Trauma Surg. 1985;103(6):417-418.
18. Garofalo R, Flanagin B, Cesari E, Vinci E, Conti M, Castagna A. Destructive septic arthritis of shoulder in adults. Musculoskelet Surg. 2014;98(supp 1):S35-S39.
19. Ravindran V, Logan I, Bourke BE. Medical vs surgical treatment for the native joint in septic arthritis: a 6-year, single UK academic centre experience. Rheumatology. 2009;48(10):1320-1322.