User login
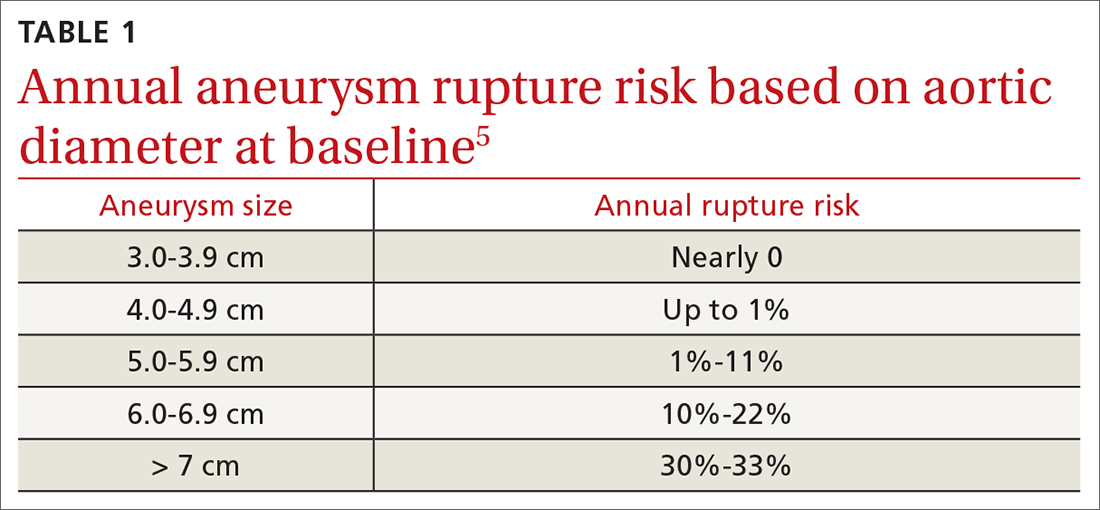
Ruptured abdominal aortic aneurysms (AAAs) caused about 6000 deaths annually in the United States between 2014 and 20201 and are associated with a pooled mortality rate of 81%.2 They result from a distinct degenerative process of the layers of the aortic wall.2 An AAA is defined as an abdominal aorta whose dilation is > 50% normal (more commonly, a diameter > 3 cm).3,4 The risk for rupture correlates closely with size; most ruptures occur in aneurysms > 5.5 cm3,4 (TABLE 15).
Most AAAs are asymptomatic and often go undetected until rupture, resulting in poor outcomes. Because of a low and declining prevalence of AAA and ruptured AAA in developed countries, screening recommendations target high-risk groups rather than the general population.4,6-8 This review summarizes risk factors, prevalence, and current evidence-based screening and management recommendations for AAA.
Who’s at risk?
Age is the most significant nonmodifiable risk factor, with AAA rupture uncommon in patients younger than 55 years.9 One retrospective study found the odds ratio (OR) for diagnosing AAA was 9.41 in adults ages 65 to 69 years (95% CI, 8.76-10.12; P < .0001) and 14.46 (95% CI, 13.45-15.55; P < .0001) in adults ages 70 to 74 years, compared to adults younger than 55 years.10
Smoking is the most potent modifiable risk factor for AAA. Among patients with AAA, > 90% have a history of smoking.4 The association between smoking and AAA is dose dependent, with an OR of 2.61 (95% CI, 2.47-2.74) in patients with a pack-per-year history < 5 years and 12.13 (95% CI, 11.66-12.61) in patients with a pack-per-year history > 35 years, compared to nonsmokers.10 The risk for AAA increases with smoking duration but decreases with cessation duration.4,10 Smoking cessation remains an important intervention, as active smokers have higher AAA rupture rates.11
Other risk factors for AAA include concomitant cardiovascular disease (CVD) such as coronary artery disease (CAD), cerebrovascular disease, atherosclerosis, dyslipidemia, and hypertension.10 Factors associated with reduced risk for AAA include African American race, Hispanic ethnicity, Asian ethnicity, diabetes, smoking cessation, consuming fruits and vegetables > 3 times per week, and exercising more than once per week.6,10
Prevalence declines but sex-based disparities in outcomes persist
The prevalence of AAA has declined in the United States and Europe in recent decades, correlating with declining rates of smoking.4,12 Reports published between 2011 and 2019 estimate that AAA prevalence in men older than 60 years has declined over time, with a prevalence of 1.2% to 3.3%.6 The prevalence of AAA has also decreased in women,6,13,14 estimated in 1 study to be as low as 0.74%.13 Similarly, deaths from ruptured AAA have declined markedly in the United States—by 70% between 1999 and 2016 according to 1 analysis.9
One striking difference in the male-female data is that although AAAs are more common in men, there is a 2- to 4-fold higher risk for rupture in women, who account for nearly half of all AAA-related deaths.9,10,15-17 The reasons for this heightened risk to women despite lower prevalence are not fully understood but are likely multifactorial and related to a general lack of screening for AAA in women, tendency for AAA to rupture at smaller diameters in women, rupture at an older age in women, and a history of worse surgical outcomes in women than men (though the gap in surgical outcomes appears to be closing).9,10,18
Continue to: While declines in AAA and AAA-related...
While declines in AAA and AAA-related death are largely attributed to lower smoking rates, other likely contributing factors include the implementation of screening programs, incidental detection during cross-sectional imaging, and improved surgical techniques and management of CV risk factors (eg, hypertension, hyperlipidemia).9,10
The benefits of screening older men
Randomized controlled trials (RCTs) have demonstrated the benefits of AAA screening programs. A meta-analysis of 4 populationbased RCTs of AAA screening in men ≥ 65 years demonstrated statistically significant reductions in AAA rupture (OR = 0.62; 95% CI, 0.55-0.70) and death from AAA (OR = 0.65; 95% CI, 0.57-0.74) over 12 to 15 years, with a number needed to screen (NNS) of 305 (95% CI, 248-411) to prevent 1 AAA-related death.18 The study also found screening decreases the rate of emergent surgeries for AAA (OR = 0.57; 95% CI, 0.48-0.68) while increasing the number of elective surgeries (OR = 1.44; 95% CI, 1.34-1.55) over 4 to 15 years.18
Only 1 study has demonstrated an improvement in all-cause mortality with screening programs, with a relatively small benefit (OR = 0.97; 95% CI, 0.94-0.99).19 Only 1 of the studies included women and, while underpowered, showed no difference in AAA-related death or rupture.20 Guidelines and recommendations of various countries and professional societies focus screening on subgroups at highest risk for AAA.4,6-8,18
Screening recommendations from USPSTF and others
The US Preventive Services Task Force (USPSTF) currently recommends one-time ultrasound screening for AAA in men ages 65 to 75 years who have ever smoked (commonly defined as having smoked > 100 cigarettes) in their lifetime.6 This grade “B” recommendation, initially made in 2005 and reaffirmed in the 2014 and 2019 USPSTF updates, recommends screening the highest-risk segment of the population (ie, older male smokers).
In men ages 65 to 75 years with no smoking history, rather than routine screening, the USPSTF recommends selectively offering screening based on the patient’s medical history, family history, risk factors, and personal values (with a “C” grade).6 The USPSTF continues to recommend against screening for AAA in women with no smoking history and no family history of AAA.6 According to the USPSTF, the evidence is insufficient to recommend for or against screening women ages 65 to 75 years who have ever smoked or have a family history of AAA (“I” statement).6
Continue to: One critique of the USPSTF recommendations
One critique of the USPSTF recommendations is that they fail to detect a significant portion of patients with AAA and AAA rupture. For example, in a retrospective analysis of 55,197 patients undergoing AAA repair, only 33% would have been detected by the USPSTF grade “B” recommendation to screen male smokers ages 65 to 75 years, and an analysis of AAA-related fatalities found 43% would be missed by USPSTF criteria.9,21
Screening guidelines from the Society for Vascular Surgery (SVS) are broader than those of the USPSTF, in an attempt to capture a larger percentage of the population at risk for AAA-related disease by extrapolating from epidemiologic data. The SVS guidelines include screening for women ages 65 to 75 years with a smoking history, screening men and women ages 65 to 75 years who have a first-degree relative with AAA, and consideration of screening patients older than 75 years if they are in good health and have a first-degree relative with AAA or a smoking history and have not been previously screened.4 However, these expanded recommendations are not supported by patient-oriented evidence.6
Attempts to broaden screening guidelines must be tempered by potential risks for harm, primarily overdiagnosis (ie, diagnosing AAAs that would not otherwise rise to clinical significance) and overtreatment (ie, resulting in unnecessary imaging, appointments, anxiety, or surgery). Negative psychological effects on quality of life after a diagnosis of AAA have not been shown to cause significant harm.6,18
A recent UK analysis found that screening programs for AAA in women modeled after those in men are not cost effective, with an NNS to prevent 1 death of 3900 in women vs 700 in men.15,18 Another recent trial of ultrasound screening in 5200 high-risk women ages 65 to 74 years found an AAA incidence of 0.29% (95% CI, 0.18%-0.48%) in which only 3 large aneurysms were identified.22
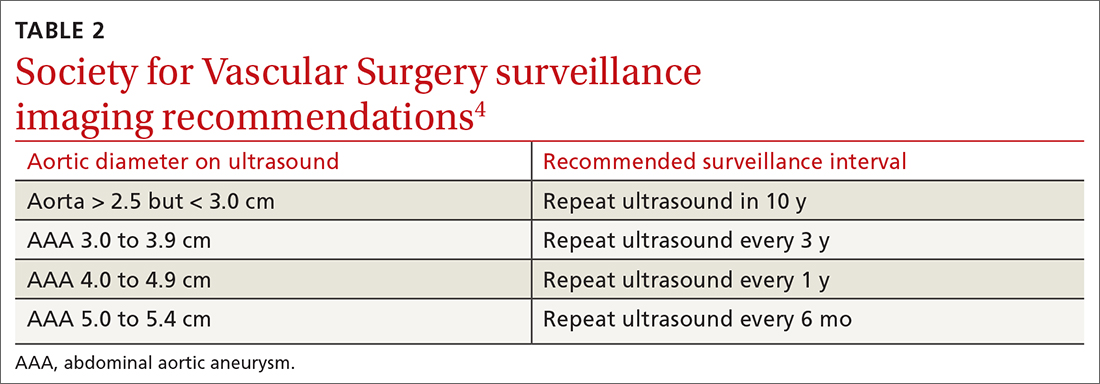
In the United States, rates of screening for AAA remain low.23 One study has shown electronic medical record–based reminders increased screening rates from 48% to 80%.24 Point-of-care bedside ultrasound performed by clinicians also could improve screening rates. Multiple studies have demonstrated that screening and diagnosis of AAA can be performed safely and effectively at the bedside by nonradiologists such as family physicians and emergency physicians.25-28 In 1 study, such exams added < 4 minutes to the patient encounter.26 Follow-up surveillance schedules for those identified as having a AAA are summarized in TABLE 2.4
Continue to: Management options
Management options: Immediate repair or surveillance?
After diagnosing AAA, important decisions must be made regarding management, including indications for surgical repair, appropriate follow-up surveillance, and medications for secondary prevention and cardiovascular risk reduction.
EVAR vs open repair
The 2 main surgical strategies for aneurysm repair are open repair and endovascular repair (EVAR). In the United States, EVAR is becoming the more common approach and was used to repair asymptomatic aneurysms in > 80% of patients and ruptured aneurysms in 50% of patients.6 There have been multiple RCTs assessing EVAR and open repair for large and small aneurysms.29-34 Findings across these studies consistently show EVAR is associated with lower immediate (ie, 30-day) morbidity and mortality but no longer-term survival benefit compared to open repair.
EVAR procedures require ongoing long-term surveillance for endovascular leakage and other complications, resulting in an increased need for re-intervention.31,33,35 For these reasons, the National Institute for Health and Care Excellence (NICE) guidelines suggest open repair as the preferred modality.7 However, SVS and the American College of Cardiology Foundation/American Heart Association guidance support either EVAR or open repair, noting that open repair may be preferable in patients unable to engage in long-term follow-up surveillance.36

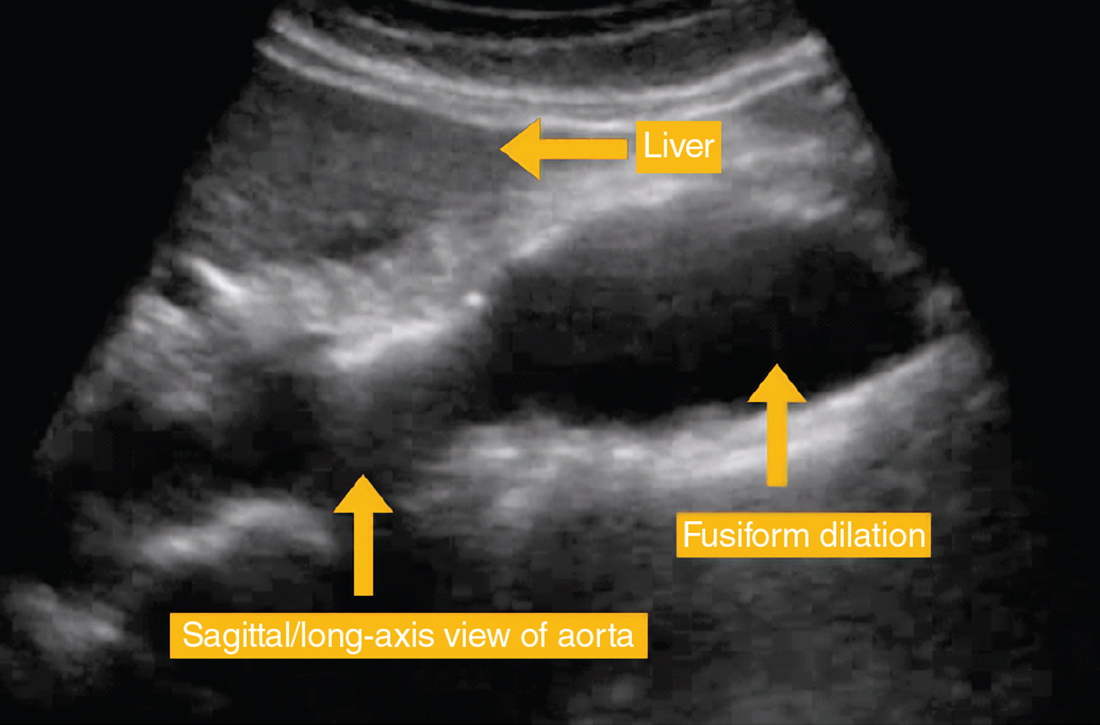
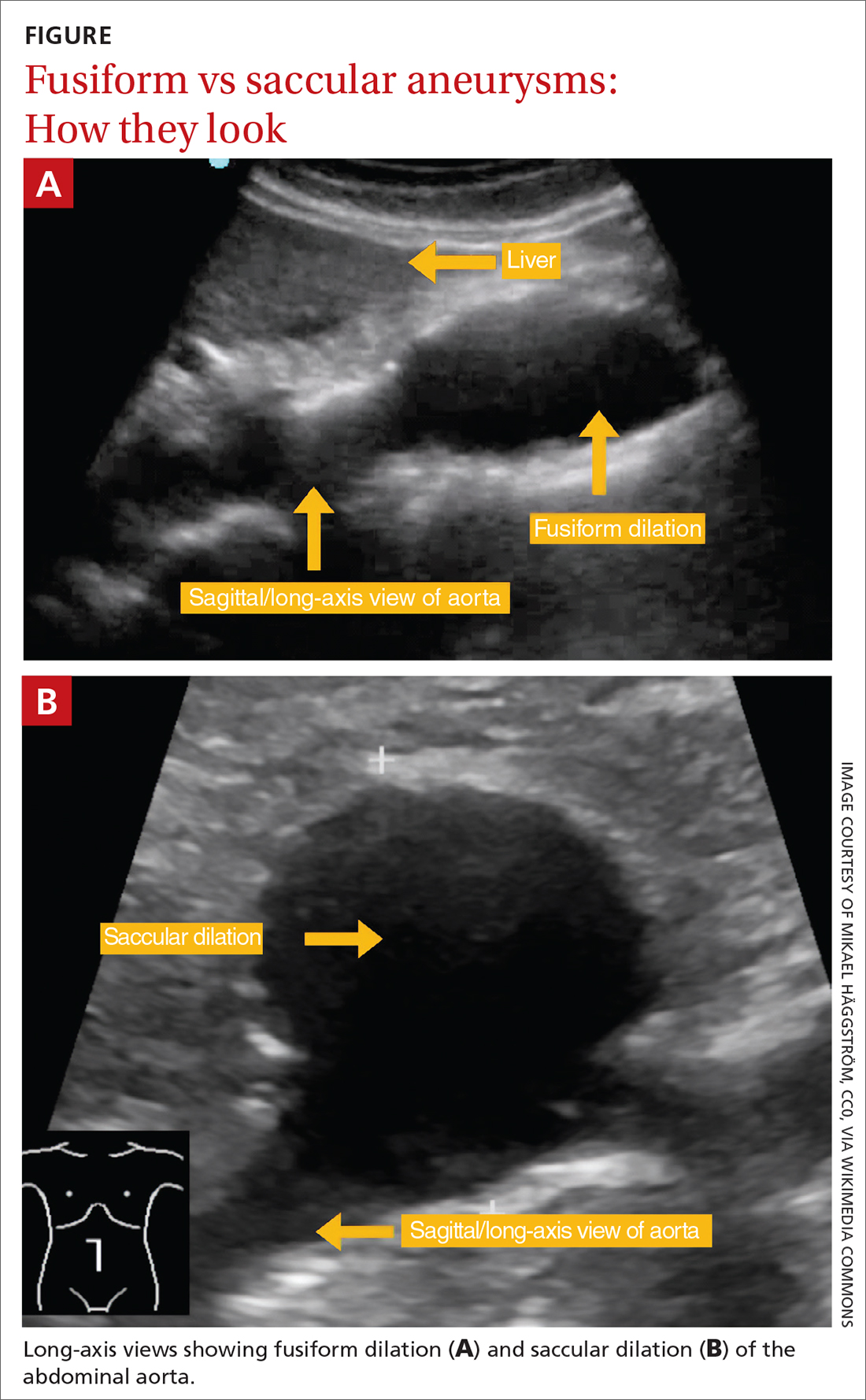
Indications for repair. In general, repair is indicated when an aneurysm reaches or exceeds 5.5 cm.4,7 Both SVS and NICE also recommend clinicians consider surgical repair of smaller, rapidly expanding aneurysms (> 1 cm over a 1-year period).4,7 Based on evidence suggesting a higher risk for rupture in women with smaller aneurysms,14,37 SVS recommends clinicians consider surgical repair in women with an AAA ≥ 5.0 cm. Several RCTs evaluating the benefits of immediate repair for smaller-sized aneurysms (4.0-5.5 cm) favored surveillance.38,39 Accepted indications for surgical repair are summarized in TABLE 3.4,7,34Surgical repair recommendations also are based on aneurysm morphology, which can be fusiform or saccular (FIGURE). More than 90% of AAAs are fusiform.40 Although saccular AAAs are less common, some studies suggest they are more prone to rupture than fusiform AAAs, and SVS guidelines suggest surgical repair of saccular aneurysms regardless of size.4,41,42
Perioperative and long-term risks. Both EVAR and open repair of AAA carry a high perioperative and long-term risk for death, as patients often have multiple comorbidities. A 2019 trial comparing EVAR to open repair with 14 years of follow-up reported death in 68% of patients in the EVAR group and 70% in the open repair group. 31 Among these deaths, 2.7% in the EVAR group and 3.7% in the open repair group were aneurysm related.31 The study also found a second surgical intervention was required in 19.8% of patients in the open repair group and 26.7% in the EVAR group.31
Continue to: When assessing perioperative risk...
When assessing perioperative risk, SVS guidelines recommend clinicians employ a shared decision-making approach with patients that incorporates Vascular Quality Initiative (VQI) mortality risk score.4 (VQI risk calculators are available at https://qxmd.com/vascular-study-group-new-england-decision-support-tools.43)
Medication management
Based on the close association of aortic aneurysm with atherosclerotic CVD (ASCVD), professional societies such as the European Society of Cardiology and European Atherosclerosis Society (ESC/EAS) have suggested aortic aneurysm is equivalent to ASCVD and should be managed medically in a similar manner to peripheral arterial disease.44 Indeed, many patients with AAA may have concomitant CAD or other arterial vascular diseases (eg, carotid, lower extremity).
Statins. In its guidelines, the ESC/EAS consider patients with AAA at “very high risk” for adverse CV events and suggest pharmacotherapy with high-intensity statins, adding ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors if needed, to reduce low-density lipoprotein cholesterol ≥ 50% from baseline, with a goal of < 55 mg/dL.44 Statin therapy additionally lowers all-cause postoperative mortality in patients undergoing AAA repair but does not affect the rate of aneurysm expansion.45
Aspirin and other anticoagulants. Although aspirin therapy may be indicated for the secondary prevention of other cardiovascular events that may coexist with AAA, it does not appear to affect the rate of growth or prevent rupture of aneurysms.46,47 In addition to aspirin, anticoagulants such as clopidogrel, enoxaparin, and warfarin are not recommended when the presence of AAA is the only indication.4
Other medications. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and antibiotics (eg, doxycycline) have been studied as a treatment for AAA. However, none has shown benefit in reducing aneurysm growth or rupture and they are not recommended for that sole purpose.4,48
Metformin. There is a negative association between diabetes and AAA expansion and rupture. Several cohort studies have indicated that this may be an independent effect driven primarily by exposure to metformin. While it is not unreasonable to consider this another important indication for metformin use in patients with diabetes, RCT evidence has yet to establish a role for metformin in patients without diabetes who have AAA.48,49
ACKNOWLEDGEMENT
The authors thank Gwen Wilson, MLS, AHIP, for her assistance with the literature searches performed in the preparation of this manuscript.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; [email protected]
1. CDC. Wide-ranging Online Data for Epidemiologic Research (WONDER) database. Accessed August 30, 2023. https://wonder.cdc.gov/ucd-icd10.html
2. Reimerink JJ, van der Laan MJ, Koelemay MJ, et al. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br J Surg. 2013;100:1405-1413. doi: 10.1002/bjs.9235
3. Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi: 10.1056/NEJMcp1401430
4. Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi: 10.1016/j.jvs.2017.10.044
5. Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41 suppl 1:S1-S58. doi: 10.1016/j.ejvs.2010.09.011
6. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:2211-2218. doi: 10.1001/jama.2019.18928
7. National Institute for Health and Care Excellence. Abdominal aortic aneurysm: diagnosis and management. NICE guideline [NG156]. March 19, 2020. Accessed June 30, 2023. www.nice.org.uk/guidance/ng156/chapter/recommendations
8. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
9. Abdulameer H, Al Taii H, Al-Kindi SG, et al. Epidemiology of fatal ruptured aortic aneurysms in the United States (1999-2016). J Vasc Surg. 2019;69:378-384.e2. doi: 10.1016/j.jvs.2018.03.435
10. Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539-548. doi: 10.1016/j.jvs.2010.05.090
11. [No authors listed] Smoking, lung function and the prognosis of abdominal aortic aneurysm. The UK Small Aneurysm Trial Participants. Eur J Vasc Endovasc Surg. 2000;19:636-642. doi: 10.1053/ejvs.2000.1066
12. Oliver-Williams C, Sweeting MJ, Turton G, et al. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br J Surg. 2018;105:68-74. doi: 10.1002/bjs.10715
13. Ulug P, Powell JT, Sweeting MJ, et al. Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. Br J Surg. 2016;103:1097-1104. doi: 10.1002/bjs.10225
14. Chabok M, Nicolaides A, Aslam M, et al. Risk factors associated with increased prevalence of abdominal aortic aneurysm in women. Br J Surg. 2016;103:1132-1138. doi: 10.1002/bjs.10179
15. Sweeting, MJ, Masconi KL, Jones E, et al. Analysis of clinical benefit, harms, and cost-effectiveness of screening women for abdominal aortic aneurysm. Lancet. 2018;392:487-495. doi: 10.1016/S0140-6736(18)31222-4
16. Sweeting MJ, Thompson SG, Brown LC, et al; RESCAN collaborators. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655-665. doi: 10.1002/bjs.8707
17. Skibba AA, Evans JR, Hopkins SP, et al. Reconsidering gender relative to risk of rupture in the contemporary management of abdominal aortic aneurysms. J Vasc Surg. 2015;62:1429-1436. doi: 10.1016/j.jvs.2015.07.079
18. Guirguis-Blake JM, Beil TL, Senger CA, et al. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:2219-2238. doi: 10.1001/jama.2019.17021
19. Thompson SG, Ashton HA, Gao L, et al; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656. doi: 10.1002/bjs.8897
20. Ashton HA, Gao L, Kim LG, et al. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696-701. doi: 10.1002/bjs.5780
21. Carnevale ML, Koleilat I, Lipsitz EC, et al. Extended screening guidelines for the diagnosis of abdominal aortic aneurysm. J Vasc Surg. 2020;72:1917-1926. doi: 10.1016/j.jvs.2020.03.047
22. Duncan A, Maslen C, Gibson C, et al. Ultrasound screening for abdominal aortic aneurysm in high-risk women. Br J Surg. 2021;108:1192-1198. doi: 10.1093/bjs/znab220
23. Shreibati JB, Baker LC, Hlatky MA, et al. Impact of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act on abdominal ultrasonography use among Medicare beneficiaries. Arch Intern Med. 2012;172:1456-1462. doi: 10.1001/archinternmed.2012.4268
24. Hye RJ, Smith AE, Wong GH, et al. Leveraging the electronic medical record to implement an abdominal aortic aneurysm screening program. J Vasc Surg. 2014;59:1535-1542. doi: 10.1016/j.jvs.2013.12.016
25. Rubano E, Mehta N, Caputo W, et al., Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013. 20:128-138. doi: 10.1111/acem.12080
26. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
27. Arnold MJ, Jonas CE, Carter RE. Point-of-care ultrasonography. Am Fam Physician. 2020;101:275-285.
28. Nixon G, Blattner K, Muirhead J, et al. Point-of-care ultrasound for FAST and AAA in rural New Zealand: quality and impact on patient care. Rural Remote Health. 2019;19:5027. doi: 10.22605/RRH5027
29. Lederle FA, Wilson SE, Johnson GR, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437-1444. doi: 10.1056/NEJMoa012573
30. Filardo G, Lederle FA, Ballard DJ, et al. Immediate open repair vs surveillance in patients with small abdominal aortic aneurysms: survival differences by aneurysm size. Mayo Clin Proc. 2013;88:910-919. doi: 10.1016/j.mayocp.2013.05.014
31. Lederle FA, Kyriakides TC, Stroupe KT, et al. Open versus endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2019;380:2126-2135. doi: 10.1056/NEJMoa1715955
32. Patel R, Sweeting MJ, Powell JT, et al., Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366-2374. doi: 10.1016/S0140-6736(16)31135-7
33. van Schaik TG, Yeung KK, Verhagen HJ, et al. Long-term survival and secondary procedures after open or endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2017;66:1379-1389. doi: 10.1016/j.jvs.2017.05.122
34. Powell JT, Brady AR, Brown, LC, et al; United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445-1452. doi: 10.1056/NEJMoa013527
35. Paravastu SC, Jayarajasingam R, Cottam R, et al. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev. 2014:CD004178. doi: 10.1002/14651858.CD004178.pub2
36. Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020-2045. doi: 10.1016/j.jacc.2011.08.023
37. Bhak RH, Wininger M, Johnson GR, et al. Factors associated with small abdominal aortic aneurysm expansion rate. JAMA Surg. 2015;150:44-50. doi: 10.1001/jamasurg.2014.2025
38. Ouriel K, Clair DG, Kent KC, et al; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg. 2010;51:1081-1087. doi: 10.1016/j.jvs.2009.10.113
39. Cao P, De Rango P, Verzini F, et al. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg. 2011;41:13-25. doi: 10.1016/j.ejvs.2010.08.026
40. Karthaus EG, Tong TML, Vahl A, et al; Dutch Society of Vascular Surgery, the Steering Committee of the Dutch Surgical Aneurysm Audit and the Dutch Institute for Clinical Auditing. Saccular abdominal aortic aneurysms: patient characteristics, clinical presentation, treatment, and outcomes in the Netherlands. Ann Surg. 2019;270:852-858. doi: 10.1097/SLA.0000000000003529
41. Nathan DP, Xu C, Pouch AM, et al. Increased wall stress of saccular versus fusiform aneurysms of the descending thoracic aorta. Ann Vasc Surg. 2011;25:1129-2237. doi: 10.1016/j.avsg.2011.07.008
42. Durojaye MS, Adeniyi TO, Alagbe OA. Multiple saccular aneurysms of the abdominal aorta: a case report and short review of risk factors for rupture on CT Scan. Ann Ib Postgrad Med. 2020;18:178-180.
43. Bertges DJ, Neal D, Schanzer A, et al. The Vascular Quality Initiative Cardiac Risk Index for prediction of myocardial infarction after vascular surgery. J Vasc Surg. 2016;64:1411-1421.e4. doi: 10.1016/j.jvs.2016.04.045
44. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. doi: 10.1093/eurheartj/ehz455
45. Twine CP, Williams IM. Systematic review and meta-analysis of the effects of statin therapy on abdominal aortic aneurysms. Br J Surg. 2011;98:346-353. doi: 10.1002/bjs.7343
46. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
47. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873-2926. doi: 10.1093/eurheartj/ehu281
48. Lederle FA, Noorbaloochi S, Nugent S, et al. Multicentre study of abdominal aortic aneurysm measurement and enlargement. Br J Surg. 2015;102:1480-1487. doi: 10.1002/bjs.9895
49. Itoga NK, Rothenberg KA, Suarez P, et al. Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J Vasc Surg. 2019;69:710-716.e3. doi: 10.1016/j.jvs.2018.06.19
Ruptured abdominal aortic aneurysms (AAAs) caused about 6000 deaths annually in the United States between 2014 and 20201 and are associated with a pooled mortality rate of 81%.2 They result from a distinct degenerative process of the layers of the aortic wall.2 An AAA is defined as an abdominal aorta whose dilation is > 50% normal (more commonly, a diameter > 3 cm).3,4 The risk for rupture correlates closely with size; most ruptures occur in aneurysms > 5.5 cm3,4 (TABLE 15).

Most AAAs are asymptomatic and often go undetected until rupture, resulting in poor outcomes. Because of a low and declining prevalence of AAA and ruptured AAA in developed countries, screening recommendations target high-risk groups rather than the general population.4,6-8 This review summarizes risk factors, prevalence, and current evidence-based screening and management recommendations for AAA.
Who’s at risk?
Age is the most significant nonmodifiable risk factor, with AAA rupture uncommon in patients younger than 55 years.9 One retrospective study found the odds ratio (OR) for diagnosing AAA was 9.41 in adults ages 65 to 69 years (95% CI, 8.76-10.12; P < .0001) and 14.46 (95% CI, 13.45-15.55; P < .0001) in adults ages 70 to 74 years, compared to adults younger than 55 years.10
Smoking is the most potent modifiable risk factor for AAA. Among patients with AAA, > 90% have a history of smoking.4 The association between smoking and AAA is dose dependent, with an OR of 2.61 (95% CI, 2.47-2.74) in patients with a pack-per-year history < 5 years and 12.13 (95% CI, 11.66-12.61) in patients with a pack-per-year history > 35 years, compared to nonsmokers.10 The risk for AAA increases with smoking duration but decreases with cessation duration.4,10 Smoking cessation remains an important intervention, as active smokers have higher AAA rupture rates.11
Other risk factors for AAA include concomitant cardiovascular disease (CVD) such as coronary artery disease (CAD), cerebrovascular disease, atherosclerosis, dyslipidemia, and hypertension.10 Factors associated with reduced risk for AAA include African American race, Hispanic ethnicity, Asian ethnicity, diabetes, smoking cessation, consuming fruits and vegetables > 3 times per week, and exercising more than once per week.6,10
Prevalence declines but sex-based disparities in outcomes persist
The prevalence of AAA has declined in the United States and Europe in recent decades, correlating with declining rates of smoking.4,12 Reports published between 2011 and 2019 estimate that AAA prevalence in men older than 60 years has declined over time, with a prevalence of 1.2% to 3.3%.6 The prevalence of AAA has also decreased in women,6,13,14 estimated in 1 study to be as low as 0.74%.13 Similarly, deaths from ruptured AAA have declined markedly in the United States—by 70% between 1999 and 2016 according to 1 analysis.9
One striking difference in the male-female data is that although AAAs are more common in men, there is a 2- to 4-fold higher risk for rupture in women, who account for nearly half of all AAA-related deaths.9,10,15-17 The reasons for this heightened risk to women despite lower prevalence are not fully understood but are likely multifactorial and related to a general lack of screening for AAA in women, tendency for AAA to rupture at smaller diameters in women, rupture at an older age in women, and a history of worse surgical outcomes in women than men (though the gap in surgical outcomes appears to be closing).9,10,18
Continue to: While declines in AAA and AAA-related...
While declines in AAA and AAA-related death are largely attributed to lower smoking rates, other likely contributing factors include the implementation of screening programs, incidental detection during cross-sectional imaging, and improved surgical techniques and management of CV risk factors (eg, hypertension, hyperlipidemia).9,10
The benefits of screening older men
Randomized controlled trials (RCTs) have demonstrated the benefits of AAA screening programs. A meta-analysis of 4 populationbased RCTs of AAA screening in men ≥ 65 years demonstrated statistically significant reductions in AAA rupture (OR = 0.62; 95% CI, 0.55-0.70) and death from AAA (OR = 0.65; 95% CI, 0.57-0.74) over 12 to 15 years, with a number needed to screen (NNS) of 305 (95% CI, 248-411) to prevent 1 AAA-related death.18 The study also found screening decreases the rate of emergent surgeries for AAA (OR = 0.57; 95% CI, 0.48-0.68) while increasing the number of elective surgeries (OR = 1.44; 95% CI, 1.34-1.55) over 4 to 15 years.18
Only 1 study has demonstrated an improvement in all-cause mortality with screening programs, with a relatively small benefit (OR = 0.97; 95% CI, 0.94-0.99).19 Only 1 of the studies included women and, while underpowered, showed no difference in AAA-related death or rupture.20 Guidelines and recommendations of various countries and professional societies focus screening on subgroups at highest risk for AAA.4,6-8,18
Screening recommendations from USPSTF and others
The US Preventive Services Task Force (USPSTF) currently recommends one-time ultrasound screening for AAA in men ages 65 to 75 years who have ever smoked (commonly defined as having smoked > 100 cigarettes) in their lifetime.6 This grade “B” recommendation, initially made in 2005 and reaffirmed in the 2014 and 2019 USPSTF updates, recommends screening the highest-risk segment of the population (ie, older male smokers).
In men ages 65 to 75 years with no smoking history, rather than routine screening, the USPSTF recommends selectively offering screening based on the patient’s medical history, family history, risk factors, and personal values (with a “C” grade).6 The USPSTF continues to recommend against screening for AAA in women with no smoking history and no family history of AAA.6 According to the USPSTF, the evidence is insufficient to recommend for or against screening women ages 65 to 75 years who have ever smoked or have a family history of AAA (“I” statement).6
Continue to: One critique of the USPSTF recommendations
One critique of the USPSTF recommendations is that they fail to detect a significant portion of patients with AAA and AAA rupture. For example, in a retrospective analysis of 55,197 patients undergoing AAA repair, only 33% would have been detected by the USPSTF grade “B” recommendation to screen male smokers ages 65 to 75 years, and an analysis of AAA-related fatalities found 43% would be missed by USPSTF criteria.9,21
Screening guidelines from the Society for Vascular Surgery (SVS) are broader than those of the USPSTF, in an attempt to capture a larger percentage of the population at risk for AAA-related disease by extrapolating from epidemiologic data. The SVS guidelines include screening for women ages 65 to 75 years with a smoking history, screening men and women ages 65 to 75 years who have a first-degree relative with AAA, and consideration of screening patients older than 75 years if they are in good health and have a first-degree relative with AAA or a smoking history and have not been previously screened.4 However, these expanded recommendations are not supported by patient-oriented evidence.6
Attempts to broaden screening guidelines must be tempered by potential risks for harm, primarily overdiagnosis (ie, diagnosing AAAs that would not otherwise rise to clinical significance) and overtreatment (ie, resulting in unnecessary imaging, appointments, anxiety, or surgery). Negative psychological effects on quality of life after a diagnosis of AAA have not been shown to cause significant harm.6,18
A recent UK analysis found that screening programs for AAA in women modeled after those in men are not cost effective, with an NNS to prevent 1 death of 3900 in women vs 700 in men.15,18 Another recent trial of ultrasound screening in 5200 high-risk women ages 65 to 74 years found an AAA incidence of 0.29% (95% CI, 0.18%-0.48%) in which only 3 large aneurysms were identified.22
In the United States, rates of screening for AAA remain low.23 One study has shown electronic medical record–based reminders increased screening rates from 48% to 80%.24 Point-of-care bedside ultrasound performed by clinicians also could improve screening rates. Multiple studies have demonstrated that screening and diagnosis of AAA can be performed safely and effectively at the bedside by nonradiologists such as family physicians and emergency physicians.25-28 In 1 study, such exams added < 4 minutes to the patient encounter.26 Follow-up surveillance schedules for those identified as having a AAA are summarized in TABLE 2.4

Continue to: Management options
Management options: Immediate repair or surveillance?
After diagnosing AAA, important decisions must be made regarding management, including indications for surgical repair, appropriate follow-up surveillance, and medications for secondary prevention and cardiovascular risk reduction.
EVAR vs open repair
The 2 main surgical strategies for aneurysm repair are open repair and endovascular repair (EVAR). In the United States, EVAR is becoming the more common approach and was used to repair asymptomatic aneurysms in > 80% of patients and ruptured aneurysms in 50% of patients.6 There have been multiple RCTs assessing EVAR and open repair for large and small aneurysms.29-34 Findings across these studies consistently show EVAR is associated with lower immediate (ie, 30-day) morbidity and mortality but no longer-term survival benefit compared to open repair.
EVAR procedures require ongoing long-term surveillance for endovascular leakage and other complications, resulting in an increased need for re-intervention.31,33,35 For these reasons, the National Institute for Health and Care Excellence (NICE) guidelines suggest open repair as the preferred modality.7 However, SVS and the American College of Cardiology Foundation/American Heart Association guidance support either EVAR or open repair, noting that open repair may be preferable in patients unable to engage in long-term follow-up surveillance.36

Indications for repair. In general, repair is indicated when an aneurysm reaches or exceeds 5.5 cm.4,7 Both SVS and NICE also recommend clinicians consider surgical repair of smaller, rapidly expanding aneurysms (> 1 cm over a 1-year period).4,7 Based on evidence suggesting a higher risk for rupture in women with smaller aneurysms,14,37 SVS recommends clinicians consider surgical repair in women with an AAA ≥ 5.0 cm. Several RCTs evaluating the benefits of immediate repair for smaller-sized aneurysms (4.0-5.5 cm) favored surveillance.38,39 Accepted indications for surgical repair are summarized in TABLE 3.4,7,34Surgical repair recommendations also are based on aneurysm morphology, which can be fusiform or saccular (FIGURE). More than 90% of AAAs are fusiform.40 Although saccular AAAs are less common, some studies suggest they are more prone to rupture than fusiform AAAs, and SVS guidelines suggest surgical repair of saccular aneurysms regardless of size.4,41,42

Perioperative and long-term risks. Both EVAR and open repair of AAA carry a high perioperative and long-term risk for death, as patients often have multiple comorbidities. A 2019 trial comparing EVAR to open repair with 14 years of follow-up reported death in 68% of patients in the EVAR group and 70% in the open repair group. 31 Among these deaths, 2.7% in the EVAR group and 3.7% in the open repair group were aneurysm related.31 The study also found a second surgical intervention was required in 19.8% of patients in the open repair group and 26.7% in the EVAR group.31
Continue to: When assessing perioperative risk...
When assessing perioperative risk, SVS guidelines recommend clinicians employ a shared decision-making approach with patients that incorporates Vascular Quality Initiative (VQI) mortality risk score.4 (VQI risk calculators are available at https://qxmd.com/vascular-study-group-new-england-decision-support-tools.43)
Medication management
Based on the close association of aortic aneurysm with atherosclerotic CVD (ASCVD), professional societies such as the European Society of Cardiology and European Atherosclerosis Society (ESC/EAS) have suggested aortic aneurysm is equivalent to ASCVD and should be managed medically in a similar manner to peripheral arterial disease.44 Indeed, many patients with AAA may have concomitant CAD or other arterial vascular diseases (eg, carotid, lower extremity).
Statins. In its guidelines, the ESC/EAS consider patients with AAA at “very high risk” for adverse CV events and suggest pharmacotherapy with high-intensity statins, adding ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors if needed, to reduce low-density lipoprotein cholesterol ≥ 50% from baseline, with a goal of < 55 mg/dL.44 Statin therapy additionally lowers all-cause postoperative mortality in patients undergoing AAA repair but does not affect the rate of aneurysm expansion.45
Aspirin and other anticoagulants. Although aspirin therapy may be indicated for the secondary prevention of other cardiovascular events that may coexist with AAA, it does not appear to affect the rate of growth or prevent rupture of aneurysms.46,47 In addition to aspirin, anticoagulants such as clopidogrel, enoxaparin, and warfarin are not recommended when the presence of AAA is the only indication.4
Other medications. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and antibiotics (eg, doxycycline) have been studied as a treatment for AAA. However, none has shown benefit in reducing aneurysm growth or rupture and they are not recommended for that sole purpose.4,48
Metformin. There is a negative association between diabetes and AAA expansion and rupture. Several cohort studies have indicated that this may be an independent effect driven primarily by exposure to metformin. While it is not unreasonable to consider this another important indication for metformin use in patients with diabetes, RCT evidence has yet to establish a role for metformin in patients without diabetes who have AAA.48,49
ACKNOWLEDGEMENT
The authors thank Gwen Wilson, MLS, AHIP, for her assistance with the literature searches performed in the preparation of this manuscript.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; [email protected]
Ruptured abdominal aortic aneurysms (AAAs) caused about 6000 deaths annually in the United States between 2014 and 20201 and are associated with a pooled mortality rate of 81%.2 They result from a distinct degenerative process of the layers of the aortic wall.2 An AAA is defined as an abdominal aorta whose dilation is > 50% normal (more commonly, a diameter > 3 cm).3,4 The risk for rupture correlates closely with size; most ruptures occur in aneurysms > 5.5 cm3,4 (TABLE 15).

Most AAAs are asymptomatic and often go undetected until rupture, resulting in poor outcomes. Because of a low and declining prevalence of AAA and ruptured AAA in developed countries, screening recommendations target high-risk groups rather than the general population.4,6-8 This review summarizes risk factors, prevalence, and current evidence-based screening and management recommendations for AAA.
Who’s at risk?
Age is the most significant nonmodifiable risk factor, with AAA rupture uncommon in patients younger than 55 years.9 One retrospective study found the odds ratio (OR) for diagnosing AAA was 9.41 in adults ages 65 to 69 years (95% CI, 8.76-10.12; P < .0001) and 14.46 (95% CI, 13.45-15.55; P < .0001) in adults ages 70 to 74 years, compared to adults younger than 55 years.10
Smoking is the most potent modifiable risk factor for AAA. Among patients with AAA, > 90% have a history of smoking.4 The association between smoking and AAA is dose dependent, with an OR of 2.61 (95% CI, 2.47-2.74) in patients with a pack-per-year history < 5 years and 12.13 (95% CI, 11.66-12.61) in patients with a pack-per-year history > 35 years, compared to nonsmokers.10 The risk for AAA increases with smoking duration but decreases with cessation duration.4,10 Smoking cessation remains an important intervention, as active smokers have higher AAA rupture rates.11
Other risk factors for AAA include concomitant cardiovascular disease (CVD) such as coronary artery disease (CAD), cerebrovascular disease, atherosclerosis, dyslipidemia, and hypertension.10 Factors associated with reduced risk for AAA include African American race, Hispanic ethnicity, Asian ethnicity, diabetes, smoking cessation, consuming fruits and vegetables > 3 times per week, and exercising more than once per week.6,10
Prevalence declines but sex-based disparities in outcomes persist
The prevalence of AAA has declined in the United States and Europe in recent decades, correlating with declining rates of smoking.4,12 Reports published between 2011 and 2019 estimate that AAA prevalence in men older than 60 years has declined over time, with a prevalence of 1.2% to 3.3%.6 The prevalence of AAA has also decreased in women,6,13,14 estimated in 1 study to be as low as 0.74%.13 Similarly, deaths from ruptured AAA have declined markedly in the United States—by 70% between 1999 and 2016 according to 1 analysis.9
One striking difference in the male-female data is that although AAAs are more common in men, there is a 2- to 4-fold higher risk for rupture in women, who account for nearly half of all AAA-related deaths.9,10,15-17 The reasons for this heightened risk to women despite lower prevalence are not fully understood but are likely multifactorial and related to a general lack of screening for AAA in women, tendency for AAA to rupture at smaller diameters in women, rupture at an older age in women, and a history of worse surgical outcomes in women than men (though the gap in surgical outcomes appears to be closing).9,10,18
Continue to: While declines in AAA and AAA-related...
While declines in AAA and AAA-related death are largely attributed to lower smoking rates, other likely contributing factors include the implementation of screening programs, incidental detection during cross-sectional imaging, and improved surgical techniques and management of CV risk factors (eg, hypertension, hyperlipidemia).9,10
The benefits of screening older men
Randomized controlled trials (RCTs) have demonstrated the benefits of AAA screening programs. A meta-analysis of 4 populationbased RCTs of AAA screening in men ≥ 65 years demonstrated statistically significant reductions in AAA rupture (OR = 0.62; 95% CI, 0.55-0.70) and death from AAA (OR = 0.65; 95% CI, 0.57-0.74) over 12 to 15 years, with a number needed to screen (NNS) of 305 (95% CI, 248-411) to prevent 1 AAA-related death.18 The study also found screening decreases the rate of emergent surgeries for AAA (OR = 0.57; 95% CI, 0.48-0.68) while increasing the number of elective surgeries (OR = 1.44; 95% CI, 1.34-1.55) over 4 to 15 years.18
Only 1 study has demonstrated an improvement in all-cause mortality with screening programs, with a relatively small benefit (OR = 0.97; 95% CI, 0.94-0.99).19 Only 1 of the studies included women and, while underpowered, showed no difference in AAA-related death or rupture.20 Guidelines and recommendations of various countries and professional societies focus screening on subgroups at highest risk for AAA.4,6-8,18
Screening recommendations from USPSTF and others
The US Preventive Services Task Force (USPSTF) currently recommends one-time ultrasound screening for AAA in men ages 65 to 75 years who have ever smoked (commonly defined as having smoked > 100 cigarettes) in their lifetime.6 This grade “B” recommendation, initially made in 2005 and reaffirmed in the 2014 and 2019 USPSTF updates, recommends screening the highest-risk segment of the population (ie, older male smokers).
In men ages 65 to 75 years with no smoking history, rather than routine screening, the USPSTF recommends selectively offering screening based on the patient’s medical history, family history, risk factors, and personal values (with a “C” grade).6 The USPSTF continues to recommend against screening for AAA in women with no smoking history and no family history of AAA.6 According to the USPSTF, the evidence is insufficient to recommend for or against screening women ages 65 to 75 years who have ever smoked or have a family history of AAA (“I” statement).6
Continue to: One critique of the USPSTF recommendations
One critique of the USPSTF recommendations is that they fail to detect a significant portion of patients with AAA and AAA rupture. For example, in a retrospective analysis of 55,197 patients undergoing AAA repair, only 33% would have been detected by the USPSTF grade “B” recommendation to screen male smokers ages 65 to 75 years, and an analysis of AAA-related fatalities found 43% would be missed by USPSTF criteria.9,21
Screening guidelines from the Society for Vascular Surgery (SVS) are broader than those of the USPSTF, in an attempt to capture a larger percentage of the population at risk for AAA-related disease by extrapolating from epidemiologic data. The SVS guidelines include screening for women ages 65 to 75 years with a smoking history, screening men and women ages 65 to 75 years who have a first-degree relative with AAA, and consideration of screening patients older than 75 years if they are in good health and have a first-degree relative with AAA or a smoking history and have not been previously screened.4 However, these expanded recommendations are not supported by patient-oriented evidence.6
Attempts to broaden screening guidelines must be tempered by potential risks for harm, primarily overdiagnosis (ie, diagnosing AAAs that would not otherwise rise to clinical significance) and overtreatment (ie, resulting in unnecessary imaging, appointments, anxiety, or surgery). Negative psychological effects on quality of life after a diagnosis of AAA have not been shown to cause significant harm.6,18
A recent UK analysis found that screening programs for AAA in women modeled after those in men are not cost effective, with an NNS to prevent 1 death of 3900 in women vs 700 in men.15,18 Another recent trial of ultrasound screening in 5200 high-risk women ages 65 to 74 years found an AAA incidence of 0.29% (95% CI, 0.18%-0.48%) in which only 3 large aneurysms were identified.22
In the United States, rates of screening for AAA remain low.23 One study has shown electronic medical record–based reminders increased screening rates from 48% to 80%.24 Point-of-care bedside ultrasound performed by clinicians also could improve screening rates. Multiple studies have demonstrated that screening and diagnosis of AAA can be performed safely and effectively at the bedside by nonradiologists such as family physicians and emergency physicians.25-28 In 1 study, such exams added < 4 minutes to the patient encounter.26 Follow-up surveillance schedules for those identified as having a AAA are summarized in TABLE 2.4

Continue to: Management options
Management options: Immediate repair or surveillance?
After diagnosing AAA, important decisions must be made regarding management, including indications for surgical repair, appropriate follow-up surveillance, and medications for secondary prevention and cardiovascular risk reduction.
EVAR vs open repair
The 2 main surgical strategies for aneurysm repair are open repair and endovascular repair (EVAR). In the United States, EVAR is becoming the more common approach and was used to repair asymptomatic aneurysms in > 80% of patients and ruptured aneurysms in 50% of patients.6 There have been multiple RCTs assessing EVAR and open repair for large and small aneurysms.29-34 Findings across these studies consistently show EVAR is associated with lower immediate (ie, 30-day) morbidity and mortality but no longer-term survival benefit compared to open repair.
EVAR procedures require ongoing long-term surveillance for endovascular leakage and other complications, resulting in an increased need for re-intervention.31,33,35 For these reasons, the National Institute for Health and Care Excellence (NICE) guidelines suggest open repair as the preferred modality.7 However, SVS and the American College of Cardiology Foundation/American Heart Association guidance support either EVAR or open repair, noting that open repair may be preferable in patients unable to engage in long-term follow-up surveillance.36

Indications for repair. In general, repair is indicated when an aneurysm reaches or exceeds 5.5 cm.4,7 Both SVS and NICE also recommend clinicians consider surgical repair of smaller, rapidly expanding aneurysms (> 1 cm over a 1-year period).4,7 Based on evidence suggesting a higher risk for rupture in women with smaller aneurysms,14,37 SVS recommends clinicians consider surgical repair in women with an AAA ≥ 5.0 cm. Several RCTs evaluating the benefits of immediate repair for smaller-sized aneurysms (4.0-5.5 cm) favored surveillance.38,39 Accepted indications for surgical repair are summarized in TABLE 3.4,7,34Surgical repair recommendations also are based on aneurysm morphology, which can be fusiform or saccular (FIGURE). More than 90% of AAAs are fusiform.40 Although saccular AAAs are less common, some studies suggest they are more prone to rupture than fusiform AAAs, and SVS guidelines suggest surgical repair of saccular aneurysms regardless of size.4,41,42

Perioperative and long-term risks. Both EVAR and open repair of AAA carry a high perioperative and long-term risk for death, as patients often have multiple comorbidities. A 2019 trial comparing EVAR to open repair with 14 years of follow-up reported death in 68% of patients in the EVAR group and 70% in the open repair group. 31 Among these deaths, 2.7% in the EVAR group and 3.7% in the open repair group were aneurysm related.31 The study also found a second surgical intervention was required in 19.8% of patients in the open repair group and 26.7% in the EVAR group.31
Continue to: When assessing perioperative risk...
When assessing perioperative risk, SVS guidelines recommend clinicians employ a shared decision-making approach with patients that incorporates Vascular Quality Initiative (VQI) mortality risk score.4 (VQI risk calculators are available at https://qxmd.com/vascular-study-group-new-england-decision-support-tools.43)
Medication management
Based on the close association of aortic aneurysm with atherosclerotic CVD (ASCVD), professional societies such as the European Society of Cardiology and European Atherosclerosis Society (ESC/EAS) have suggested aortic aneurysm is equivalent to ASCVD and should be managed medically in a similar manner to peripheral arterial disease.44 Indeed, many patients with AAA may have concomitant CAD or other arterial vascular diseases (eg, carotid, lower extremity).
Statins. In its guidelines, the ESC/EAS consider patients with AAA at “very high risk” for adverse CV events and suggest pharmacotherapy with high-intensity statins, adding ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors if needed, to reduce low-density lipoprotein cholesterol ≥ 50% from baseline, with a goal of < 55 mg/dL.44 Statin therapy additionally lowers all-cause postoperative mortality in patients undergoing AAA repair but does not affect the rate of aneurysm expansion.45
Aspirin and other anticoagulants. Although aspirin therapy may be indicated for the secondary prevention of other cardiovascular events that may coexist with AAA, it does not appear to affect the rate of growth or prevent rupture of aneurysms.46,47 In addition to aspirin, anticoagulants such as clopidogrel, enoxaparin, and warfarin are not recommended when the presence of AAA is the only indication.4
Other medications. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and antibiotics (eg, doxycycline) have been studied as a treatment for AAA. However, none has shown benefit in reducing aneurysm growth or rupture and they are not recommended for that sole purpose.4,48
Metformin. There is a negative association between diabetes and AAA expansion and rupture. Several cohort studies have indicated that this may be an independent effect driven primarily by exposure to metformin. While it is not unreasonable to consider this another important indication for metformin use in patients with diabetes, RCT evidence has yet to establish a role for metformin in patients without diabetes who have AAA.48,49
ACKNOWLEDGEMENT
The authors thank Gwen Wilson, MLS, AHIP, for her assistance with the literature searches performed in the preparation of this manuscript.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; [email protected]
1. CDC. Wide-ranging Online Data for Epidemiologic Research (WONDER) database. Accessed August 30, 2023. https://wonder.cdc.gov/ucd-icd10.html
2. Reimerink JJ, van der Laan MJ, Koelemay MJ, et al. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br J Surg. 2013;100:1405-1413. doi: 10.1002/bjs.9235
3. Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi: 10.1056/NEJMcp1401430
4. Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi: 10.1016/j.jvs.2017.10.044
5. Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41 suppl 1:S1-S58. doi: 10.1016/j.ejvs.2010.09.011
6. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:2211-2218. doi: 10.1001/jama.2019.18928
7. National Institute for Health and Care Excellence. Abdominal aortic aneurysm: diagnosis and management. NICE guideline [NG156]. March 19, 2020. Accessed June 30, 2023. www.nice.org.uk/guidance/ng156/chapter/recommendations
8. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
9. Abdulameer H, Al Taii H, Al-Kindi SG, et al. Epidemiology of fatal ruptured aortic aneurysms in the United States (1999-2016). J Vasc Surg. 2019;69:378-384.e2. doi: 10.1016/j.jvs.2018.03.435
10. Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539-548. doi: 10.1016/j.jvs.2010.05.090
11. [No authors listed] Smoking, lung function and the prognosis of abdominal aortic aneurysm. The UK Small Aneurysm Trial Participants. Eur J Vasc Endovasc Surg. 2000;19:636-642. doi: 10.1053/ejvs.2000.1066
12. Oliver-Williams C, Sweeting MJ, Turton G, et al. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br J Surg. 2018;105:68-74. doi: 10.1002/bjs.10715
13. Ulug P, Powell JT, Sweeting MJ, et al. Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. Br J Surg. 2016;103:1097-1104. doi: 10.1002/bjs.10225
14. Chabok M, Nicolaides A, Aslam M, et al. Risk factors associated with increased prevalence of abdominal aortic aneurysm in women. Br J Surg. 2016;103:1132-1138. doi: 10.1002/bjs.10179
15. Sweeting, MJ, Masconi KL, Jones E, et al. Analysis of clinical benefit, harms, and cost-effectiveness of screening women for abdominal aortic aneurysm. Lancet. 2018;392:487-495. doi: 10.1016/S0140-6736(18)31222-4
16. Sweeting MJ, Thompson SG, Brown LC, et al; RESCAN collaborators. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655-665. doi: 10.1002/bjs.8707
17. Skibba AA, Evans JR, Hopkins SP, et al. Reconsidering gender relative to risk of rupture in the contemporary management of abdominal aortic aneurysms. J Vasc Surg. 2015;62:1429-1436. doi: 10.1016/j.jvs.2015.07.079
18. Guirguis-Blake JM, Beil TL, Senger CA, et al. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:2219-2238. doi: 10.1001/jama.2019.17021
19. Thompson SG, Ashton HA, Gao L, et al; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656. doi: 10.1002/bjs.8897
20. Ashton HA, Gao L, Kim LG, et al. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696-701. doi: 10.1002/bjs.5780
21. Carnevale ML, Koleilat I, Lipsitz EC, et al. Extended screening guidelines for the diagnosis of abdominal aortic aneurysm. J Vasc Surg. 2020;72:1917-1926. doi: 10.1016/j.jvs.2020.03.047
22. Duncan A, Maslen C, Gibson C, et al. Ultrasound screening for abdominal aortic aneurysm in high-risk women. Br J Surg. 2021;108:1192-1198. doi: 10.1093/bjs/znab220
23. Shreibati JB, Baker LC, Hlatky MA, et al. Impact of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act on abdominal ultrasonography use among Medicare beneficiaries. Arch Intern Med. 2012;172:1456-1462. doi: 10.1001/archinternmed.2012.4268
24. Hye RJ, Smith AE, Wong GH, et al. Leveraging the electronic medical record to implement an abdominal aortic aneurysm screening program. J Vasc Surg. 2014;59:1535-1542. doi: 10.1016/j.jvs.2013.12.016
25. Rubano E, Mehta N, Caputo W, et al., Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013. 20:128-138. doi: 10.1111/acem.12080
26. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
27. Arnold MJ, Jonas CE, Carter RE. Point-of-care ultrasonography. Am Fam Physician. 2020;101:275-285.
28. Nixon G, Blattner K, Muirhead J, et al. Point-of-care ultrasound for FAST and AAA in rural New Zealand: quality and impact on patient care. Rural Remote Health. 2019;19:5027. doi: 10.22605/RRH5027
29. Lederle FA, Wilson SE, Johnson GR, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437-1444. doi: 10.1056/NEJMoa012573
30. Filardo G, Lederle FA, Ballard DJ, et al. Immediate open repair vs surveillance in patients with small abdominal aortic aneurysms: survival differences by aneurysm size. Mayo Clin Proc. 2013;88:910-919. doi: 10.1016/j.mayocp.2013.05.014
31. Lederle FA, Kyriakides TC, Stroupe KT, et al. Open versus endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2019;380:2126-2135. doi: 10.1056/NEJMoa1715955
32. Patel R, Sweeting MJ, Powell JT, et al., Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366-2374. doi: 10.1016/S0140-6736(16)31135-7
33. van Schaik TG, Yeung KK, Verhagen HJ, et al. Long-term survival and secondary procedures after open or endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2017;66:1379-1389. doi: 10.1016/j.jvs.2017.05.122
34. Powell JT, Brady AR, Brown, LC, et al; United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445-1452. doi: 10.1056/NEJMoa013527
35. Paravastu SC, Jayarajasingam R, Cottam R, et al. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev. 2014:CD004178. doi: 10.1002/14651858.CD004178.pub2
36. Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020-2045. doi: 10.1016/j.jacc.2011.08.023
37. Bhak RH, Wininger M, Johnson GR, et al. Factors associated with small abdominal aortic aneurysm expansion rate. JAMA Surg. 2015;150:44-50. doi: 10.1001/jamasurg.2014.2025
38. Ouriel K, Clair DG, Kent KC, et al; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg. 2010;51:1081-1087. doi: 10.1016/j.jvs.2009.10.113
39. Cao P, De Rango P, Verzini F, et al. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg. 2011;41:13-25. doi: 10.1016/j.ejvs.2010.08.026
40. Karthaus EG, Tong TML, Vahl A, et al; Dutch Society of Vascular Surgery, the Steering Committee of the Dutch Surgical Aneurysm Audit and the Dutch Institute for Clinical Auditing. Saccular abdominal aortic aneurysms: patient characteristics, clinical presentation, treatment, and outcomes in the Netherlands. Ann Surg. 2019;270:852-858. doi: 10.1097/SLA.0000000000003529
41. Nathan DP, Xu C, Pouch AM, et al. Increased wall stress of saccular versus fusiform aneurysms of the descending thoracic aorta. Ann Vasc Surg. 2011;25:1129-2237. doi: 10.1016/j.avsg.2011.07.008
42. Durojaye MS, Adeniyi TO, Alagbe OA. Multiple saccular aneurysms of the abdominal aorta: a case report and short review of risk factors for rupture on CT Scan. Ann Ib Postgrad Med. 2020;18:178-180.
43. Bertges DJ, Neal D, Schanzer A, et al. The Vascular Quality Initiative Cardiac Risk Index for prediction of myocardial infarction after vascular surgery. J Vasc Surg. 2016;64:1411-1421.e4. doi: 10.1016/j.jvs.2016.04.045
44. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. doi: 10.1093/eurheartj/ehz455
45. Twine CP, Williams IM. Systematic review and meta-analysis of the effects of statin therapy on abdominal aortic aneurysms. Br J Surg. 2011;98:346-353. doi: 10.1002/bjs.7343
46. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
47. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873-2926. doi: 10.1093/eurheartj/ehu281
48. Lederle FA, Noorbaloochi S, Nugent S, et al. Multicentre study of abdominal aortic aneurysm measurement and enlargement. Br J Surg. 2015;102:1480-1487. doi: 10.1002/bjs.9895
49. Itoga NK, Rothenberg KA, Suarez P, et al. Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J Vasc Surg. 2019;69:710-716.e3. doi: 10.1016/j.jvs.2018.06.19
1. CDC. Wide-ranging Online Data for Epidemiologic Research (WONDER) database. Accessed August 30, 2023. https://wonder.cdc.gov/ucd-icd10.html
2. Reimerink JJ, van der Laan MJ, Koelemay MJ, et al. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br J Surg. 2013;100:1405-1413. doi: 10.1002/bjs.9235
3. Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101-2108. doi: 10.1056/NEJMcp1401430
4. Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2-77.e2. doi: 10.1016/j.jvs.2017.10.044
5. Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41 suppl 1:S1-S58. doi: 10.1016/j.ejvs.2010.09.011
6. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:2211-2218. doi: 10.1001/jama.2019.18928
7. National Institute for Health and Care Excellence. Abdominal aortic aneurysm: diagnosis and management. NICE guideline [NG156]. March 19, 2020. Accessed June 30, 2023. www.nice.org.uk/guidance/ng156/chapter/recommendations
8. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
9. Abdulameer H, Al Taii H, Al-Kindi SG, et al. Epidemiology of fatal ruptured aortic aneurysms in the United States (1999-2016). J Vasc Surg. 2019;69:378-384.e2. doi: 10.1016/j.jvs.2018.03.435
10. Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539-548. doi: 10.1016/j.jvs.2010.05.090
11. [No authors listed] Smoking, lung function and the prognosis of abdominal aortic aneurysm. The UK Small Aneurysm Trial Participants. Eur J Vasc Endovasc Surg. 2000;19:636-642. doi: 10.1053/ejvs.2000.1066
12. Oliver-Williams C, Sweeting MJ, Turton G, et al. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br J Surg. 2018;105:68-74. doi: 10.1002/bjs.10715
13. Ulug P, Powell JT, Sweeting MJ, et al. Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. Br J Surg. 2016;103:1097-1104. doi: 10.1002/bjs.10225
14. Chabok M, Nicolaides A, Aslam M, et al. Risk factors associated with increased prevalence of abdominal aortic aneurysm in women. Br J Surg. 2016;103:1132-1138. doi: 10.1002/bjs.10179
15. Sweeting, MJ, Masconi KL, Jones E, et al. Analysis of clinical benefit, harms, and cost-effectiveness of screening women for abdominal aortic aneurysm. Lancet. 2018;392:487-495. doi: 10.1016/S0140-6736(18)31222-4
16. Sweeting MJ, Thompson SG, Brown LC, et al; RESCAN collaborators. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655-665. doi: 10.1002/bjs.8707
17. Skibba AA, Evans JR, Hopkins SP, et al. Reconsidering gender relative to risk of rupture in the contemporary management of abdominal aortic aneurysms. J Vasc Surg. 2015;62:1429-1436. doi: 10.1016/j.jvs.2015.07.079
18. Guirguis-Blake JM, Beil TL, Senger CA, et al. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;322:2219-2238. doi: 10.1001/jama.2019.17021
19. Thompson SG, Ashton HA, Gao L, et al; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99:1649-1656. doi: 10.1002/bjs.8897
20. Ashton HA, Gao L, Kim LG, et al. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg. 2007;94:696-701. doi: 10.1002/bjs.5780
21. Carnevale ML, Koleilat I, Lipsitz EC, et al. Extended screening guidelines for the diagnosis of abdominal aortic aneurysm. J Vasc Surg. 2020;72:1917-1926. doi: 10.1016/j.jvs.2020.03.047
22. Duncan A, Maslen C, Gibson C, et al. Ultrasound screening for abdominal aortic aneurysm in high-risk women. Br J Surg. 2021;108:1192-1198. doi: 10.1093/bjs/znab220
23. Shreibati JB, Baker LC, Hlatky MA, et al. Impact of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act on abdominal ultrasonography use among Medicare beneficiaries. Arch Intern Med. 2012;172:1456-1462. doi: 10.1001/archinternmed.2012.4268
24. Hye RJ, Smith AE, Wong GH, et al. Leveraging the electronic medical record to implement an abdominal aortic aneurysm screening program. J Vasc Surg. 2014;59:1535-1542. doi: 10.1016/j.jvs.2013.12.016
25. Rubano E, Mehta N, Caputo W, et al., Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013. 20:128-138. doi: 10.1111/acem.12080
26. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
27. Arnold MJ, Jonas CE, Carter RE. Point-of-care ultrasonography. Am Fam Physician. 2020;101:275-285.
28. Nixon G, Blattner K, Muirhead J, et al. Point-of-care ultrasound for FAST and AAA in rural New Zealand: quality and impact on patient care. Rural Remote Health. 2019;19:5027. doi: 10.22605/RRH5027
29. Lederle FA, Wilson SE, Johnson GR, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437-1444. doi: 10.1056/NEJMoa012573
30. Filardo G, Lederle FA, Ballard DJ, et al. Immediate open repair vs surveillance in patients with small abdominal aortic aneurysms: survival differences by aneurysm size. Mayo Clin Proc. 2013;88:910-919. doi: 10.1016/j.mayocp.2013.05.014
31. Lederle FA, Kyriakides TC, Stroupe KT, et al. Open versus endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2019;380:2126-2135. doi: 10.1056/NEJMoa1715955
32. Patel R, Sweeting MJ, Powell JT, et al., Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366-2374. doi: 10.1016/S0140-6736(16)31135-7
33. van Schaik TG, Yeung KK, Verhagen HJ, et al. Long-term survival and secondary procedures after open or endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2017;66:1379-1389. doi: 10.1016/j.jvs.2017.05.122
34. Powell JT, Brady AR, Brown, LC, et al; United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445-1452. doi: 10.1056/NEJMoa013527
35. Paravastu SC, Jayarajasingam R, Cottam R, et al. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev. 2014:CD004178. doi: 10.1002/14651858.CD004178.pub2
36. Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020-2045. doi: 10.1016/j.jacc.2011.08.023
37. Bhak RH, Wininger M, Johnson GR, et al. Factors associated with small abdominal aortic aneurysm expansion rate. JAMA Surg. 2015;150:44-50. doi: 10.1001/jamasurg.2014.2025
38. Ouriel K, Clair DG, Kent KC, et al; Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg. 2010;51:1081-1087. doi: 10.1016/j.jvs.2009.10.113
39. Cao P, De Rango P, Verzini F, et al. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg. 2011;41:13-25. doi: 10.1016/j.ejvs.2010.08.026
40. Karthaus EG, Tong TML, Vahl A, et al; Dutch Society of Vascular Surgery, the Steering Committee of the Dutch Surgical Aneurysm Audit and the Dutch Institute for Clinical Auditing. Saccular abdominal aortic aneurysms: patient characteristics, clinical presentation, treatment, and outcomes in the Netherlands. Ann Surg. 2019;270:852-858. doi: 10.1097/SLA.0000000000003529
41. Nathan DP, Xu C, Pouch AM, et al. Increased wall stress of saccular versus fusiform aneurysms of the descending thoracic aorta. Ann Vasc Surg. 2011;25:1129-2237. doi: 10.1016/j.avsg.2011.07.008
42. Durojaye MS, Adeniyi TO, Alagbe OA. Multiple saccular aneurysms of the abdominal aorta: a case report and short review of risk factors for rupture on CT Scan. Ann Ib Postgrad Med. 2020;18:178-180.
43. Bertges DJ, Neal D, Schanzer A, et al. The Vascular Quality Initiative Cardiac Risk Index for prediction of myocardial infarction after vascular surgery. J Vasc Surg. 2016;64:1411-1421.e4. doi: 10.1016/j.jvs.2016.04.045
44. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111-188. doi: 10.1093/eurheartj/ehz455
45. Twine CP, Williams IM. Systematic review and meta-analysis of the effects of statin therapy on abdominal aortic aneurysms. Br J Surg. 2011;98:346-353. doi: 10.1002/bjs.7343
46. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
47. Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873-2926. doi: 10.1093/eurheartj/ehu281
48. Lederle FA, Noorbaloochi S, Nugent S, et al. Multicentre study of abdominal aortic aneurysm measurement and enlargement. Br J Surg. 2015;102:1480-1487. doi: 10.1002/bjs.9895
49. Itoga NK, Rothenberg KA, Suarez P, et al. Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J Vasc Surg. 2019;69:710-716.e3. doi: 10.1016/j.jvs.2018.06.19
PRACTICE RECOMMENDATIONS
› Perform a one-time abdominal aortic aneurysm (AAA) screening ultrasound in men ages 65 to 75 years who have ever smoked. B
› Consider performing a one-time AAA screening ultrasound in women ages 65 to 75 years who have ever smoked. C
› Prescribe high-intensity statin therapy for men and women with atherosclerotic AAA. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series