User login
Asymptomatic term infants whose mothers received adequate intrapartum antibiotic prophylaxis (defined as intravenous penicillin or ampicillin at least 4 hours before delivery) for group B streptococcal disease do not need work-up or treatment (strength of recommendation [SOR]: B, based on retrospective, population-based study). These infants should be observed for 48 hours, but may be discharged after 24 hours in circumstances where close follow-up is available (SOR: D, based on expert opinion).
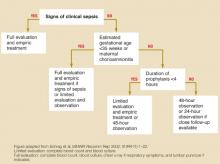
Symptomatic infants, premature infants (gestational age <35 weeks) of mothers who did not receive prophylaxis, and infants whose mothers had chorioamnionitis should receive a full evaluation (complete blood count, blood culture, and chest x-ray with or without a lumbar puncture) and an initial empiric antibiotic treatment with ampicillin or penicillin and gentamycin. If a term infant is not symptomatic and maternal antibiotic prophylaxis was not adequate, opinions differ as to whether to perform limited evaluation with empiric treatment or close observation (SOR: D, based on expert opinion). See Figure.
FIGURE
Management of infants born to mothers with group B streptococcal disease–positive cultures
Evidence summary
Intrapartum antibiotic prophylaxis has decreased the incidence of early-onset group B streptococcal disease by 65% in the last decade.1 A multicenter population-based study demonstrated that basing prophylaxis on screening cultures is twice as effective as risk stratification, a previously recommended strategy.2
Intrapartum prophylaxis of women who had positive group B streptococcal disease screening cultures at 35 weeks will prevent 70% of earlyonset disease and 89% of fatalities.3,4 As demonstrated by multicenter retrospective studies, infants aged <35 weeks are at significantly higher risk of group B streptococcal disease than term infants (relative risk=1.5–2.07), and mortality for premature infants with early-onset disease (25%–30%) is substantially higher than for term infants with early-onset disease (2%–8%).2,5
A large retrospective study demonstrated that infants who developed early-onset disease despite intrapartum prophylaxis developed the same clinical syndrome in the same time frame (78% of early-onset disease evident in first 24 hours and 96% by 48 hours) as infants whose mothers did not receive prophylaxis.6
The duration of adequate intrapartum antibiotic prophylaxis was initially set at 4 hours, based on a study measuring antibiotic penetration into amniotic fluid.7 A recent randomized trial, enrolling more than 4500 women, has confirmed this finding. The vertical transmission rate of group B streptococcal disease, as measured by neonatal colonization (as opposed to clinical illness), is 46% when antibiotic prophylaxis is started <1 hour before delivery, 2.9% when prophylaxis is given at 2 to 4 hours, and 1.2% when given at least 4 hours before delivery.8
Implementation of these guidelines is aided by the adoption of an institution-wide policy to support point of care decision-making.9 A retrospective study after the release of the 1996 Centers for Disease Control (CDC) guidelines concluded that hospitals with established group B streptococcal disease policies had significantly fewer cases of early-onset disease (P=.038).10
Recommendations from others
The 2002 Prevention of Perinatal Group B Streptococcal Disease Revised Guidelines from the CDC states: “a healthy-appearing infant whose mother received >4 hours of [intrapartum antibiotic prophylaxis] before delivery may be discharged home as early as 24 hours after delivery, assuming other discharge criteria have been met and that a person able to comply fully with instructions for home observation will be present … if these conditions are not met, the infant should remain in the hospital for at least 48 hours of observation and until criteria for discharge are achieved.”1
These guidelines strongly support universal prenatal screening and the use of intrapartum antibiotic prophylaxis. Both the American Academy of Pediatrics and the American College Obstetrics and Gynecology have endorsed the CDC’s revised guidelines.
Richard Nicholas, MD
Rose Family Medicine Residency, Denver, Colo
The question of appropriate care of the infant exposed to group B streptococcal disease arises frequently in any practice caring for newborns. These clear, evidence-based recommendations are helpful in guiding that care. The evidence supports watchful waiting for appropriately covered newborns, providing reassurance for both parents and physicians.
Unfortunately, little evidence exists to guide care in a setting that seems to be quite common: the term, asymptomatic infant born to a mother who, in labor, received less-than-adequate intrapartum antibiotic prophylaxis. Further research for this subgroup is needed; in the meantime, physicians who provide maternity or newborn care should work together to develop protocols that ensure adequate intrapartum antibiotic coverage for mothers with group B streptococcal disease.
1. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC MMWR Recomm Rep 2002;51(RR-11):1-22.
2. Schrag SJ, Zell ER, Lynfield R, et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med 2002;347:233-239.
3. Boyer KM, Gotoff SP. Prevention of early-onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis. N Engl J Med 1986;314:1665-1669.
4. Schuchat A, Oxtoby M, Cochi S, et al. Population-based risk factors for neonatal group B streptococcal disease: results of a cohort study in metropolitan Atlanta. J Infect Dis 1990;162:672-677.
5. Cochi SL, Feldman RA. Estimating national incidence of group B streptococcal disease: the effect of adjusting for birth weight. Pediatr Infect Dis 1983;2:414-415.
6. Bromberger P, Lawrence JM, Braun D, Saunders B, Contreras R, Petitti DB. The influence of intrapartum antibiotics on the clinical spectrum of early-onset group B streptococcal infection in term infants. Pediatrics 2000;106:244-250.
7. Bray RE, Boe RW, Johnson WL. Transfer of ampicillin into fetus and amniotic fluid from maternal plasma in late pregnancy. Am J Obstet Gynecol 1966;96:938-942.
8. de Cueto M, Sanchez MJ, Sampedro A, Miranda JA, Herruzo AJ, Rosa-Fraile M. Timing of intrapartum ampicillin and prevention of vertical transmission of group B streptococcus. Obstet Gynecol 1998;91:112-114.
9. Clemens CJ, Gable EK. The development of a group B streptococcus prevention policy at a community hospital. J Perinatol 2002;22:523-525.
10. Factor SH, Whitney CG, Zywicki SS, Schuchat A. Effects of hospital policies based on 1996 group B streptococcal disease consensus guidelines. The Active Bacterial Core Surveillance Team. Obstet Gynecol 2000;95:377-382.
Asymptomatic term infants whose mothers received adequate intrapartum antibiotic prophylaxis (defined as intravenous penicillin or ampicillin at least 4 hours before delivery) for group B streptococcal disease do not need work-up or treatment (strength of recommendation [SOR]: B, based on retrospective, population-based study). These infants should be observed for 48 hours, but may be discharged after 24 hours in circumstances where close follow-up is available (SOR: D, based on expert opinion).
Symptomatic infants, premature infants (gestational age <35 weeks) of mothers who did not receive prophylaxis, and infants whose mothers had chorioamnionitis should receive a full evaluation (complete blood count, blood culture, and chest x-ray with or without a lumbar puncture) and an initial empiric antibiotic treatment with ampicillin or penicillin and gentamycin. If a term infant is not symptomatic and maternal antibiotic prophylaxis was not adequate, opinions differ as to whether to perform limited evaluation with empiric treatment or close observation (SOR: D, based on expert opinion). See Figure.
FIGURE
Management of infants born to mothers with group B streptococcal disease–positive cultures
Evidence summary
Intrapartum antibiotic prophylaxis has decreased the incidence of early-onset group B streptococcal disease by 65% in the last decade.1 A multicenter population-based study demonstrated that basing prophylaxis on screening cultures is twice as effective as risk stratification, a previously recommended strategy.2
Intrapartum prophylaxis of women who had positive group B streptococcal disease screening cultures at 35 weeks will prevent 70% of earlyonset disease and 89% of fatalities.3,4 As demonstrated by multicenter retrospective studies, infants aged <35 weeks are at significantly higher risk of group B streptococcal disease than term infants (relative risk=1.5–2.07), and mortality for premature infants with early-onset disease (25%–30%) is substantially higher than for term infants with early-onset disease (2%–8%).2,5
A large retrospective study demonstrated that infants who developed early-onset disease despite intrapartum prophylaxis developed the same clinical syndrome in the same time frame (78% of early-onset disease evident in first 24 hours and 96% by 48 hours) as infants whose mothers did not receive prophylaxis.6
The duration of adequate intrapartum antibiotic prophylaxis was initially set at 4 hours, based on a study measuring antibiotic penetration into amniotic fluid.7 A recent randomized trial, enrolling more than 4500 women, has confirmed this finding. The vertical transmission rate of group B streptococcal disease, as measured by neonatal colonization (as opposed to clinical illness), is 46% when antibiotic prophylaxis is started <1 hour before delivery, 2.9% when prophylaxis is given at 2 to 4 hours, and 1.2% when given at least 4 hours before delivery.8
Implementation of these guidelines is aided by the adoption of an institution-wide policy to support point of care decision-making.9 A retrospective study after the release of the 1996 Centers for Disease Control (CDC) guidelines concluded that hospitals with established group B streptococcal disease policies had significantly fewer cases of early-onset disease (P=.038).10
Recommendations from others
The 2002 Prevention of Perinatal Group B Streptococcal Disease Revised Guidelines from the CDC states: “a healthy-appearing infant whose mother received >4 hours of [intrapartum antibiotic prophylaxis] before delivery may be discharged home as early as 24 hours after delivery, assuming other discharge criteria have been met and that a person able to comply fully with instructions for home observation will be present … if these conditions are not met, the infant should remain in the hospital for at least 48 hours of observation and until criteria for discharge are achieved.”1
These guidelines strongly support universal prenatal screening and the use of intrapartum antibiotic prophylaxis. Both the American Academy of Pediatrics and the American College Obstetrics and Gynecology have endorsed the CDC’s revised guidelines.
Richard Nicholas, MD
Rose Family Medicine Residency, Denver, Colo
The question of appropriate care of the infant exposed to group B streptococcal disease arises frequently in any practice caring for newborns. These clear, evidence-based recommendations are helpful in guiding that care. The evidence supports watchful waiting for appropriately covered newborns, providing reassurance for both parents and physicians.
Unfortunately, little evidence exists to guide care in a setting that seems to be quite common: the term, asymptomatic infant born to a mother who, in labor, received less-than-adequate intrapartum antibiotic prophylaxis. Further research for this subgroup is needed; in the meantime, physicians who provide maternity or newborn care should work together to develop protocols that ensure adequate intrapartum antibiotic coverage for mothers with group B streptococcal disease.
Asymptomatic term infants whose mothers received adequate intrapartum antibiotic prophylaxis (defined as intravenous penicillin or ampicillin at least 4 hours before delivery) for group B streptococcal disease do not need work-up or treatment (strength of recommendation [SOR]: B, based on retrospective, population-based study). These infants should be observed for 48 hours, but may be discharged after 24 hours in circumstances where close follow-up is available (SOR: D, based on expert opinion).
Symptomatic infants, premature infants (gestational age <35 weeks) of mothers who did not receive prophylaxis, and infants whose mothers had chorioamnionitis should receive a full evaluation (complete blood count, blood culture, and chest x-ray with or without a lumbar puncture) and an initial empiric antibiotic treatment with ampicillin or penicillin and gentamycin. If a term infant is not symptomatic and maternal antibiotic prophylaxis was not adequate, opinions differ as to whether to perform limited evaluation with empiric treatment or close observation (SOR: D, based on expert opinion). See Figure.
FIGURE
Management of infants born to mothers with group B streptococcal disease–positive cultures
Evidence summary
Intrapartum antibiotic prophylaxis has decreased the incidence of early-onset group B streptococcal disease by 65% in the last decade.1 A multicenter population-based study demonstrated that basing prophylaxis on screening cultures is twice as effective as risk stratification, a previously recommended strategy.2
Intrapartum prophylaxis of women who had positive group B streptococcal disease screening cultures at 35 weeks will prevent 70% of earlyonset disease and 89% of fatalities.3,4 As demonstrated by multicenter retrospective studies, infants aged <35 weeks are at significantly higher risk of group B streptococcal disease than term infants (relative risk=1.5–2.07), and mortality for premature infants with early-onset disease (25%–30%) is substantially higher than for term infants with early-onset disease (2%–8%).2,5
A large retrospective study demonstrated that infants who developed early-onset disease despite intrapartum prophylaxis developed the same clinical syndrome in the same time frame (78% of early-onset disease evident in first 24 hours and 96% by 48 hours) as infants whose mothers did not receive prophylaxis.6
The duration of adequate intrapartum antibiotic prophylaxis was initially set at 4 hours, based on a study measuring antibiotic penetration into amniotic fluid.7 A recent randomized trial, enrolling more than 4500 women, has confirmed this finding. The vertical transmission rate of group B streptococcal disease, as measured by neonatal colonization (as opposed to clinical illness), is 46% when antibiotic prophylaxis is started <1 hour before delivery, 2.9% when prophylaxis is given at 2 to 4 hours, and 1.2% when given at least 4 hours before delivery.8
Implementation of these guidelines is aided by the adoption of an institution-wide policy to support point of care decision-making.9 A retrospective study after the release of the 1996 Centers for Disease Control (CDC) guidelines concluded that hospitals with established group B streptococcal disease policies had significantly fewer cases of early-onset disease (P=.038).10
Recommendations from others
The 2002 Prevention of Perinatal Group B Streptococcal Disease Revised Guidelines from the CDC states: “a healthy-appearing infant whose mother received >4 hours of [intrapartum antibiotic prophylaxis] before delivery may be discharged home as early as 24 hours after delivery, assuming other discharge criteria have been met and that a person able to comply fully with instructions for home observation will be present … if these conditions are not met, the infant should remain in the hospital for at least 48 hours of observation and until criteria for discharge are achieved.”1
These guidelines strongly support universal prenatal screening and the use of intrapartum antibiotic prophylaxis. Both the American Academy of Pediatrics and the American College Obstetrics and Gynecology have endorsed the CDC’s revised guidelines.
Richard Nicholas, MD
Rose Family Medicine Residency, Denver, Colo
The question of appropriate care of the infant exposed to group B streptococcal disease arises frequently in any practice caring for newborns. These clear, evidence-based recommendations are helpful in guiding that care. The evidence supports watchful waiting for appropriately covered newborns, providing reassurance for both parents and physicians.
Unfortunately, little evidence exists to guide care in a setting that seems to be quite common: the term, asymptomatic infant born to a mother who, in labor, received less-than-adequate intrapartum antibiotic prophylaxis. Further research for this subgroup is needed; in the meantime, physicians who provide maternity or newborn care should work together to develop protocols that ensure adequate intrapartum antibiotic coverage for mothers with group B streptococcal disease.
1. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC MMWR Recomm Rep 2002;51(RR-11):1-22.
2. Schrag SJ, Zell ER, Lynfield R, et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med 2002;347:233-239.
3. Boyer KM, Gotoff SP. Prevention of early-onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis. N Engl J Med 1986;314:1665-1669.
4. Schuchat A, Oxtoby M, Cochi S, et al. Population-based risk factors for neonatal group B streptococcal disease: results of a cohort study in metropolitan Atlanta. J Infect Dis 1990;162:672-677.
5. Cochi SL, Feldman RA. Estimating national incidence of group B streptococcal disease: the effect of adjusting for birth weight. Pediatr Infect Dis 1983;2:414-415.
6. Bromberger P, Lawrence JM, Braun D, Saunders B, Contreras R, Petitti DB. The influence of intrapartum antibiotics on the clinical spectrum of early-onset group B streptococcal infection in term infants. Pediatrics 2000;106:244-250.
7. Bray RE, Boe RW, Johnson WL. Transfer of ampicillin into fetus and amniotic fluid from maternal plasma in late pregnancy. Am J Obstet Gynecol 1966;96:938-942.
8. de Cueto M, Sanchez MJ, Sampedro A, Miranda JA, Herruzo AJ, Rosa-Fraile M. Timing of intrapartum ampicillin and prevention of vertical transmission of group B streptococcus. Obstet Gynecol 1998;91:112-114.
9. Clemens CJ, Gable EK. The development of a group B streptococcus prevention policy at a community hospital. J Perinatol 2002;22:523-525.
10. Factor SH, Whitney CG, Zywicki SS, Schuchat A. Effects of hospital policies based on 1996 group B streptococcal disease consensus guidelines. The Active Bacterial Core Surveillance Team. Obstet Gynecol 2000;95:377-382.
1. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC MMWR Recomm Rep 2002;51(RR-11):1-22.
2. Schrag SJ, Zell ER, Lynfield R, et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med 2002;347:233-239.
3. Boyer KM, Gotoff SP. Prevention of early-onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis. N Engl J Med 1986;314:1665-1669.
4. Schuchat A, Oxtoby M, Cochi S, et al. Population-based risk factors for neonatal group B streptococcal disease: results of a cohort study in metropolitan Atlanta. J Infect Dis 1990;162:672-677.
5. Cochi SL, Feldman RA. Estimating national incidence of group B streptococcal disease: the effect of adjusting for birth weight. Pediatr Infect Dis 1983;2:414-415.
6. Bromberger P, Lawrence JM, Braun D, Saunders B, Contreras R, Petitti DB. The influence of intrapartum antibiotics on the clinical spectrum of early-onset group B streptococcal infection in term infants. Pediatrics 2000;106:244-250.
7. Bray RE, Boe RW, Johnson WL. Transfer of ampicillin into fetus and amniotic fluid from maternal plasma in late pregnancy. Am J Obstet Gynecol 1966;96:938-942.
8. de Cueto M, Sanchez MJ, Sampedro A, Miranda JA, Herruzo AJ, Rosa-Fraile M. Timing of intrapartum ampicillin and prevention of vertical transmission of group B streptococcus. Obstet Gynecol 1998;91:112-114.
9. Clemens CJ, Gable EK. The development of a group B streptococcus prevention policy at a community hospital. J Perinatol 2002;22:523-525.
10. Factor SH, Whitney CG, Zywicki SS, Schuchat A. Effects of hospital policies based on 1996 group B streptococcal disease consensus guidelines. The Active Bacterial Core Surveillance Team. Obstet Gynecol 2000;95:377-382.
Evidence-based answers from the Family Physicians Inquiries Network