User login
Which imaging modality is best for suspected stroke?
Patients exhibiting stroke symptoms should have brain imaging immediately within 3 hours of symptom onset (strength of recommendation [SOR]: A, based on systematic review). In the first 3 hours after a suspected cerebrovascular accident (CVA), noncontrast head computerized tomography (CT) is the gold standard for diagnosis of acute hemorrhagic stroke (SOR: C, based on expert panel consensus). However, the sensitivity for hemorrhage declines steeply 8 to 10 days after the event. Eligibility guidelines for acute thrombolytic therapy are currently based on use of CT to rule out acute hemorrhagic stroke.
Magnetic resonance imaging (MRI) may be equally accurate in diagnosing an acute hemorrhagic stroke if completed within 90 minutes of presentation for patients whose symptoms began fewer than 6 hours earlier (SOR: B, based on a single high-quality cohort study). MRI is more sensitive than CT for ischemic stroke in the first 24 hours of symptoms (SOR: B, based on systematic review of low-quality studies with consistent findings) and is more sensitive than CT in the diagnosis of hemorrhagic or ischemic stroke greater than 1 week after symptom onset (SOR: B, based on 1 high-quality prospective cohort study).
Evidence summary
The British National Health Service Health Technology Assessment (HTA) Programme published a systematic review on optimal brain imaging strategies for the diagnosis of stroke in July 2004.2 The HTA searched Medline and EMBASE from 1980 to 1999 and found 1903 studies relevant to diagnostic imaging for stroke. Only 25 studies reported the type of stroke diagnosed and the imaging reference standard. Thirteen of these 25 studies describe the time interval from symptom onset to imaging.
The HTA found a wide range of sensitivities for CT and MRI for both hemorrhagic and ischemic strokes at different time periods (TABLE) and noted that the quality of most of these studies was poor. Most of the studies identified were performed in academic stroke centers and had small sample sizes. Interpretation was masked in only 58% of studies. Few data were available on interobserver reliability, and neuroradiologists usually interpreted images. Two studies, totaling 165 patients, compared CT and MRI scans performed on the same day. However, the most “acute” time period reported was within 48 hours from symptom onset; neither study reported the order in which scans were performed, and only 1 study masked the neuroradiologist to the interpretation of the other modality.
After this systematic review, the HTA performed a prospective cohort study comparing CT with MRI obtained in random order on the day of presentation. They enrolled 228 patients presenting to a general hospital with stroke symptoms: (1) lasting longer than 1 day, but causing little or no decrease in function, or (2) lasting longer than 5 days. The mean time from onset of symptoms to scanning was 21.5 days. CT detected hemorrhagic stroke in 50%, and late hemorrhagic transformation in 20%, of those patients found to have hemorrhagic stroke on MRI, which was considered the criterion standard of chronic stroke diagnosis. The earliest hemorrhagic stroke missed by CT was 11 days old, and the latest hemorrhage correctly identified by CT was 14 days old.
An additional prospective cohort study comparing imaging modalities in the acute time frame has been published since the HTA review.2 The study enrolled 129 patients with stroke symptoms of less than 3 hours, as well as 71 patients with between 3 and 6 hours of symptoms. Patients underwent multimodal MRI (including gradient recalled echo and diffusion-weighted imaging) and noncontrast CT within 90 minutes of presentation. Two stroke specialists and 2 neuroradiologists, masked to clinical information, read the scans independently at a later date. Interrater reliability was good (= 0.75–0.94) for identifying acute hemorrhage. There was 96% concordance between the MRI and CT interpretations. The 4 hemorrhages “missed” by CT were hemorrhagic transformations of acute infarct, and the 4 hemorrhages “missed” by MRI were misclassified as chronic when they were acute.
The clinical implications of this study are uncertain. Without recognition of 1 imaging modality as the reference standard, it is difficult to say whether a hemorrhage “missed” on CT was a false negative on CT or a false positive on MRI.
TABLE
Range of sensitivities of CT and MRI for hemorrhagic and ischemic stroke
| NEUROIMAGING METHOD | TYPE OF STROKE | TIME SINCE SYMPTOM ONSET | |||
|---|---|---|---|---|---|
| >3 HOURS | >6 HOURS | <48 HOURS | >48 HOURS | ||
| Head CT without contrast | Sensitivity for hemorrhagic stroke | Evidence limited, assumed 100% in many studies | 86%–90%*3 | 93% | 17%–58% |
| Sensitivity for ischemic stroke | 64%–85% | 47%–80% | 23%–81% | 53%–74% | |
| Brain MRI | Sensitivity for hemorrhagic stroke | No MRI studies identified for this time frame | 86%–90%*3 | 46% | 38%–97% |
| Sensitivity for ischemic stroke | 65% | 84%–88% | 94%–98% | ||
| *Sensitivity=86% when other imaging modality used as gold standard, 90% when discharge diagnosis of acute hemorrhagic stroke used as gold standard. | |||||
| Source: Wardlaw et al, Health Technol Assess 20042; Kidwell et al, JAMA 2004.3 | |||||
Recommendations from others
In 2003, the Stroke Council, appointed by the American Heart Association, stated: “For most cases and at most institutions, CT remains the most important brain imaging test. A physician skilled in assessing CT studies should be available to interpret the scan (strength of recommendation grade B).” The Stroke Council further recommends that “[i]n patients seen within 6 hours of onset, CT currently may be preferred as the first imaging study because MRI detection of acute intracerebral hemorrhage has not been fully validated (strength of recommendation grade A).”4
The British National Health Service HTA Programme advises “scan all immediately” for diagnosing new neurological deficits with the understanding that CT scans were most available and cost-effective.2
CT without contrast still the best choice for assessing suspected acute stroke
Fred Grover, Jr, MD
Department of Family Medicine, University of Colorado
CT without contrast remains the best choice when assessing a patient for suspected stroke. For patients who are candidates for rtPA, this should be performed and read within 45 minutes of entering the emergency department. Remember that IV thrombolytics must be administered within 3 hours of stroke onset to be effective.
As Xenon-enhanced CT (XeCT) and Single Photon Emission CT (SPECT) become more available, these may be considered an adjunct to help risk-stratify patients prior to revascularization with a thrombolytic.1 After 48 hours, an MRI shows greater sensitivity in detecting both hemorrhagic and ischemic strokes.
1. Latchaw RE, Yonas H, Hunter GJ, et al. Guidelines and recommendations for perfusion imaging in cerebral ischemia: A scientific statement for healthcare professionals by the writing group on perfusion imaging, from the Council on Cardiovascular Radiology of the American Heart Association. Stroke 2003;34:1084-1104.
2. Wardlaw JM, Keir SL, Seymour J, et al. What is the best imaging strategy for acute stroke?. Health Technol Assess 2004;8:1-180.
3. Kidwell CS, Chalela JA, Saver JL, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA 2004;292:1823-1830.
4. Adams HP, Adams RJ, Brott T, et al. Guidelines for the early management of patients with ischemic stroke. Stroke 2003;34:1056-1083.
Patients exhibiting stroke symptoms should have brain imaging immediately within 3 hours of symptom onset (strength of recommendation [SOR]: A, based on systematic review). In the first 3 hours after a suspected cerebrovascular accident (CVA), noncontrast head computerized tomography (CT) is the gold standard for diagnosis of acute hemorrhagic stroke (SOR: C, based on expert panel consensus). However, the sensitivity for hemorrhage declines steeply 8 to 10 days after the event. Eligibility guidelines for acute thrombolytic therapy are currently based on use of CT to rule out acute hemorrhagic stroke.
Magnetic resonance imaging (MRI) may be equally accurate in diagnosing an acute hemorrhagic stroke if completed within 90 minutes of presentation for patients whose symptoms began fewer than 6 hours earlier (SOR: B, based on a single high-quality cohort study). MRI is more sensitive than CT for ischemic stroke in the first 24 hours of symptoms (SOR: B, based on systematic review of low-quality studies with consistent findings) and is more sensitive than CT in the diagnosis of hemorrhagic or ischemic stroke greater than 1 week after symptom onset (SOR: B, based on 1 high-quality prospective cohort study).
Evidence summary
The British National Health Service Health Technology Assessment (HTA) Programme published a systematic review on optimal brain imaging strategies for the diagnosis of stroke in July 2004.2 The HTA searched Medline and EMBASE from 1980 to 1999 and found 1903 studies relevant to diagnostic imaging for stroke. Only 25 studies reported the type of stroke diagnosed and the imaging reference standard. Thirteen of these 25 studies describe the time interval from symptom onset to imaging.
The HTA found a wide range of sensitivities for CT and MRI for both hemorrhagic and ischemic strokes at different time periods (TABLE) and noted that the quality of most of these studies was poor. Most of the studies identified were performed in academic stroke centers and had small sample sizes. Interpretation was masked in only 58% of studies. Few data were available on interobserver reliability, and neuroradiologists usually interpreted images. Two studies, totaling 165 patients, compared CT and MRI scans performed on the same day. However, the most “acute” time period reported was within 48 hours from symptom onset; neither study reported the order in which scans were performed, and only 1 study masked the neuroradiologist to the interpretation of the other modality.
After this systematic review, the HTA performed a prospective cohort study comparing CT with MRI obtained in random order on the day of presentation. They enrolled 228 patients presenting to a general hospital with stroke symptoms: (1) lasting longer than 1 day, but causing little or no decrease in function, or (2) lasting longer than 5 days. The mean time from onset of symptoms to scanning was 21.5 days. CT detected hemorrhagic stroke in 50%, and late hemorrhagic transformation in 20%, of those patients found to have hemorrhagic stroke on MRI, which was considered the criterion standard of chronic stroke diagnosis. The earliest hemorrhagic stroke missed by CT was 11 days old, and the latest hemorrhage correctly identified by CT was 14 days old.
An additional prospective cohort study comparing imaging modalities in the acute time frame has been published since the HTA review.2 The study enrolled 129 patients with stroke symptoms of less than 3 hours, as well as 71 patients with between 3 and 6 hours of symptoms. Patients underwent multimodal MRI (including gradient recalled echo and diffusion-weighted imaging) and noncontrast CT within 90 minutes of presentation. Two stroke specialists and 2 neuroradiologists, masked to clinical information, read the scans independently at a later date. Interrater reliability was good (= 0.75–0.94) for identifying acute hemorrhage. There was 96% concordance between the MRI and CT interpretations. The 4 hemorrhages “missed” by CT were hemorrhagic transformations of acute infarct, and the 4 hemorrhages “missed” by MRI were misclassified as chronic when they were acute.
The clinical implications of this study are uncertain. Without recognition of 1 imaging modality as the reference standard, it is difficult to say whether a hemorrhage “missed” on CT was a false negative on CT or a false positive on MRI.
TABLE
Range of sensitivities of CT and MRI for hemorrhagic and ischemic stroke
| NEUROIMAGING METHOD | TYPE OF STROKE | TIME SINCE SYMPTOM ONSET | |||
|---|---|---|---|---|---|
| >3 HOURS | >6 HOURS | <48 HOURS | >48 HOURS | ||
| Head CT without contrast | Sensitivity for hemorrhagic stroke | Evidence limited, assumed 100% in many studies | 86%–90%*3 | 93% | 17%–58% |
| Sensitivity for ischemic stroke | 64%–85% | 47%–80% | 23%–81% | 53%–74% | |
| Brain MRI | Sensitivity for hemorrhagic stroke | No MRI studies identified for this time frame | 86%–90%*3 | 46% | 38%–97% |
| Sensitivity for ischemic stroke | 65% | 84%–88% | 94%–98% | ||
| *Sensitivity=86% when other imaging modality used as gold standard, 90% when discharge diagnosis of acute hemorrhagic stroke used as gold standard. | |||||
| Source: Wardlaw et al, Health Technol Assess 20042; Kidwell et al, JAMA 2004.3 | |||||
Recommendations from others
In 2003, the Stroke Council, appointed by the American Heart Association, stated: “For most cases and at most institutions, CT remains the most important brain imaging test. A physician skilled in assessing CT studies should be available to interpret the scan (strength of recommendation grade B).” The Stroke Council further recommends that “[i]n patients seen within 6 hours of onset, CT currently may be preferred as the first imaging study because MRI detection of acute intracerebral hemorrhage has not been fully validated (strength of recommendation grade A).”4
The British National Health Service HTA Programme advises “scan all immediately” for diagnosing new neurological deficits with the understanding that CT scans were most available and cost-effective.2
CT without contrast still the best choice for assessing suspected acute stroke
Fred Grover, Jr, MD
Department of Family Medicine, University of Colorado
CT without contrast remains the best choice when assessing a patient for suspected stroke. For patients who are candidates for rtPA, this should be performed and read within 45 minutes of entering the emergency department. Remember that IV thrombolytics must be administered within 3 hours of stroke onset to be effective.
As Xenon-enhanced CT (XeCT) and Single Photon Emission CT (SPECT) become more available, these may be considered an adjunct to help risk-stratify patients prior to revascularization with a thrombolytic.1 After 48 hours, an MRI shows greater sensitivity in detecting both hemorrhagic and ischemic strokes.
Patients exhibiting stroke symptoms should have brain imaging immediately within 3 hours of symptom onset (strength of recommendation [SOR]: A, based on systematic review). In the first 3 hours after a suspected cerebrovascular accident (CVA), noncontrast head computerized tomography (CT) is the gold standard for diagnosis of acute hemorrhagic stroke (SOR: C, based on expert panel consensus). However, the sensitivity for hemorrhage declines steeply 8 to 10 days after the event. Eligibility guidelines for acute thrombolytic therapy are currently based on use of CT to rule out acute hemorrhagic stroke.
Magnetic resonance imaging (MRI) may be equally accurate in diagnosing an acute hemorrhagic stroke if completed within 90 minutes of presentation for patients whose symptoms began fewer than 6 hours earlier (SOR: B, based on a single high-quality cohort study). MRI is more sensitive than CT for ischemic stroke in the first 24 hours of symptoms (SOR: B, based on systematic review of low-quality studies with consistent findings) and is more sensitive than CT in the diagnosis of hemorrhagic or ischemic stroke greater than 1 week after symptom onset (SOR: B, based on 1 high-quality prospective cohort study).
Evidence summary
The British National Health Service Health Technology Assessment (HTA) Programme published a systematic review on optimal brain imaging strategies for the diagnosis of stroke in July 2004.2 The HTA searched Medline and EMBASE from 1980 to 1999 and found 1903 studies relevant to diagnostic imaging for stroke. Only 25 studies reported the type of stroke diagnosed and the imaging reference standard. Thirteen of these 25 studies describe the time interval from symptom onset to imaging.
The HTA found a wide range of sensitivities for CT and MRI for both hemorrhagic and ischemic strokes at different time periods (TABLE) and noted that the quality of most of these studies was poor. Most of the studies identified were performed in academic stroke centers and had small sample sizes. Interpretation was masked in only 58% of studies. Few data were available on interobserver reliability, and neuroradiologists usually interpreted images. Two studies, totaling 165 patients, compared CT and MRI scans performed on the same day. However, the most “acute” time period reported was within 48 hours from symptom onset; neither study reported the order in which scans were performed, and only 1 study masked the neuroradiologist to the interpretation of the other modality.
After this systematic review, the HTA performed a prospective cohort study comparing CT with MRI obtained in random order on the day of presentation. They enrolled 228 patients presenting to a general hospital with stroke symptoms: (1) lasting longer than 1 day, but causing little or no decrease in function, or (2) lasting longer than 5 days. The mean time from onset of symptoms to scanning was 21.5 days. CT detected hemorrhagic stroke in 50%, and late hemorrhagic transformation in 20%, of those patients found to have hemorrhagic stroke on MRI, which was considered the criterion standard of chronic stroke diagnosis. The earliest hemorrhagic stroke missed by CT was 11 days old, and the latest hemorrhage correctly identified by CT was 14 days old.
An additional prospective cohort study comparing imaging modalities in the acute time frame has been published since the HTA review.2 The study enrolled 129 patients with stroke symptoms of less than 3 hours, as well as 71 patients with between 3 and 6 hours of symptoms. Patients underwent multimodal MRI (including gradient recalled echo and diffusion-weighted imaging) and noncontrast CT within 90 minutes of presentation. Two stroke specialists and 2 neuroradiologists, masked to clinical information, read the scans independently at a later date. Interrater reliability was good (= 0.75–0.94) for identifying acute hemorrhage. There was 96% concordance between the MRI and CT interpretations. The 4 hemorrhages “missed” by CT were hemorrhagic transformations of acute infarct, and the 4 hemorrhages “missed” by MRI were misclassified as chronic when they were acute.
The clinical implications of this study are uncertain. Without recognition of 1 imaging modality as the reference standard, it is difficult to say whether a hemorrhage “missed” on CT was a false negative on CT or a false positive on MRI.
TABLE
Range of sensitivities of CT and MRI for hemorrhagic and ischemic stroke
| NEUROIMAGING METHOD | TYPE OF STROKE | TIME SINCE SYMPTOM ONSET | |||
|---|---|---|---|---|---|
| >3 HOURS | >6 HOURS | <48 HOURS | >48 HOURS | ||
| Head CT without contrast | Sensitivity for hemorrhagic stroke | Evidence limited, assumed 100% in many studies | 86%–90%*3 | 93% | 17%–58% |
| Sensitivity for ischemic stroke | 64%–85% | 47%–80% | 23%–81% | 53%–74% | |
| Brain MRI | Sensitivity for hemorrhagic stroke | No MRI studies identified for this time frame | 86%–90%*3 | 46% | 38%–97% |
| Sensitivity for ischemic stroke | 65% | 84%–88% | 94%–98% | ||
| *Sensitivity=86% when other imaging modality used as gold standard, 90% when discharge diagnosis of acute hemorrhagic stroke used as gold standard. | |||||
| Source: Wardlaw et al, Health Technol Assess 20042; Kidwell et al, JAMA 2004.3 | |||||
Recommendations from others
In 2003, the Stroke Council, appointed by the American Heart Association, stated: “For most cases and at most institutions, CT remains the most important brain imaging test. A physician skilled in assessing CT studies should be available to interpret the scan (strength of recommendation grade B).” The Stroke Council further recommends that “[i]n patients seen within 6 hours of onset, CT currently may be preferred as the first imaging study because MRI detection of acute intracerebral hemorrhage has not been fully validated (strength of recommendation grade A).”4
The British National Health Service HTA Programme advises “scan all immediately” for diagnosing new neurological deficits with the understanding that CT scans were most available and cost-effective.2
CT without contrast still the best choice for assessing suspected acute stroke
Fred Grover, Jr, MD
Department of Family Medicine, University of Colorado
CT without contrast remains the best choice when assessing a patient for suspected stroke. For patients who are candidates for rtPA, this should be performed and read within 45 minutes of entering the emergency department. Remember that IV thrombolytics must be administered within 3 hours of stroke onset to be effective.
As Xenon-enhanced CT (XeCT) and Single Photon Emission CT (SPECT) become more available, these may be considered an adjunct to help risk-stratify patients prior to revascularization with a thrombolytic.1 After 48 hours, an MRI shows greater sensitivity in detecting both hemorrhagic and ischemic strokes.
1. Latchaw RE, Yonas H, Hunter GJ, et al. Guidelines and recommendations for perfusion imaging in cerebral ischemia: A scientific statement for healthcare professionals by the writing group on perfusion imaging, from the Council on Cardiovascular Radiology of the American Heart Association. Stroke 2003;34:1084-1104.
2. Wardlaw JM, Keir SL, Seymour J, et al. What is the best imaging strategy for acute stroke?. Health Technol Assess 2004;8:1-180.
3. Kidwell CS, Chalela JA, Saver JL, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA 2004;292:1823-1830.
4. Adams HP, Adams RJ, Brott T, et al. Guidelines for the early management of patients with ischemic stroke. Stroke 2003;34:1056-1083.
1. Latchaw RE, Yonas H, Hunter GJ, et al. Guidelines and recommendations for perfusion imaging in cerebral ischemia: A scientific statement for healthcare professionals by the writing group on perfusion imaging, from the Council on Cardiovascular Radiology of the American Heart Association. Stroke 2003;34:1084-1104.
2. Wardlaw JM, Keir SL, Seymour J, et al. What is the best imaging strategy for acute stroke?. Health Technol Assess 2004;8:1-180.
3. Kidwell CS, Chalela JA, Saver JL, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA 2004;292:1823-1830.
4. Adams HP, Adams RJ, Brott T, et al. Guidelines for the early management of patients with ischemic stroke. Stroke 2003;34:1056-1083.
Evidence-based answers from the Family Physicians Inquiries Network
Do statins cause myopathy?
If statins (HMG-CoA reductase inhibitors) cause myopathy, the risk is very low (strength of recommendation [SOR]: A). There is no direct evidence to answer this question. A pooled analysis of randomized controlled trials found similar myopathy rates in patients taking statins and placebo. However, a large cohort study revealed a very small but statistically significant increased risk of myopathy in patients taking statins (number needed to harm=10,000/year).
Case reports suggest a myopathy risk for all statins, including fatal rhabdomyolysis. Risk of myopathy may increase with higher statin doses, certain comorbid states (eg, hypothyroidism, renal insufficiency [especially with diabetes], recent trauma, perioperative periods, advanced age, small body frame) and concurrent medications, including fibrates, cyclosporine, azole antifungals, and macrolide antibiotics (SOR: B). No studies have directly compared myopathy rates among statins, and there is no good evidence to suggest any differences. No controlled study has directly examined statin rechallenge in patients with previous myopathy; however, case reports and expert opinion support this practice (SOR: B).
Evidence summary
There is little evidence that statins cause myopathy. Synthesis is difficult because definitions of myopathy types differ among investigators. Proposed clinical syndrome definitions are myalgia (muscle weakness or ache with normal creatine kinase), myositis (symptoms with increased creatine kinase), and rhabdomyolysis (symptoms, markedly elevated creatine kinase, and renal insufficiency) as subsets of the more general term myopathy.1 The Table summarizes myopathy data from 30 statin trials analyzed in a recent systematic review, showing similar myopathy rates in statin and placebo patients.2 There may be a lower myopathy rate in these trials than in routine clinical practice because of stricter exclusion criteria and more intense monitoring.
A large well-done British epidemiologic cohort study (n=96,193) found an increased rate of myopathy (broadly defined, not requiring creatine kinase elevation) among patients taking statins, with an absolute rate difference of 1 per 10,000 person-years.3 A small study of 21 patients on statins with muscle symptoms but normal creatine kinases described 4 patients who were able to distinguish statins from placebo, with objective reversible weakness and abnormal muscle biopsies.4 Postmarket voluntary clinician reports point to statin myopathy in this and other countries;5-7 these include 3339 rhabdomyolysis FDA reports (1990 to March 2002).2
Though some assert differential myopathy rates among statins based on cell research, case reports, or differences in metabolic clearance, no studies directly compare clinical myopathy rates among statins. There is no good evidence of a differential myopathy risk among statins currently available in the US.1-2 Cerivastatin, however, was withdrawn from the US market because of a fatal rhabdomyolysis rate 16 to 80 times higher than other statins based on FDA reports using a denominator of prescription volume (3.16 fatal cases/million prescriptions vs 0.15 for the statin class as a whole).6
It is unknown whether previous myopathy, however defined, increases the risk of future myopathy with statin rechallenge. A tabular analysis of 74 published case reports of statin-associated rhabdomyolysis from a MEDLINE search covering 1985 to 2000 reported that in most cases the statin was safely restarted after stopping presumed interactive drugs (exact numbers not reported).8 In the AFCAPS/TexCAPS trial, 20 of 21 statin patients (out of a study population of 3304) who had elevated creatine kinase (>10 times the upper limits of normal) recovered with continued lovastatin treatment, while the other patient resumed treatment after a brief interruption without further elevations.9 The EXCEL trial (n=8245) of various lovastatin dosages included routine creatine kinase tests every 6 weeks for 48 weeks. Five lovastatin patients had muscle symptoms with creatine kinases >10 times the upper limits of normal, and in the 2 who continued treatment, symptoms and creatine kinase became normal.10 Of note, creatine kinase elevation of any kind at least once during 48 weeks occurred in 28.9% of placebo patients, arguing against routine creatine kinase screening in statin patients.
Case studies suggest an increased myopathy risk when statins are given with various medications, including fibrates, cyclosporine, azole antifungals, warfarin, nefazodone, and macrolide antibiotics.4,6,8 Pravastatin and fluvastatin, which are not metabolized by the P450 CYP3A4 pathway, may be safer to use because of fewer drug interactions.2,8 Likewise, certain comorbid states such as hypothyroidism, renal insufficiency (especially in patients with diabetes), recent trauma, and perioperative periods, as well as advanced age, small body frame, and multiple medications may increase statin myopathy risk.1,2,7,8
TABLE
Pooled myopathy data from 30 randomized controlled statin trials
| Trials | Total patients | Statin patients | Placebo patients | |
|---|---|---|---|---|
| Myalgia | 5 | 33,929 | 0.3–32.9% | 0–33.3% |
| Creatine kinase elevation | 9 | 33,921 | 0–0.64% | 0–0.58% |
| Myositis* | 18 | 58,237 | 0.17% | 0.15% |
| Rhabdomyolysis | 20 | 70,126 | 0.020% | 0.014% |
| *Creatine kinase >10 times upper limit of normal | ||||
| Adapted and calculated from Thompson PD, et al. JAMA 2003; 289:1681–1690.2 | ||||
Recommendations from others
A 2002 Clinical Advisory, jointly issued by the American College of Cardiology, the American Heart Association, and the National Heart, Lung and Blood Institute, asserted that statins carry a small but definite myopathy risk.1 It recommended against routine creatine kinase tests, reserving them for patients who develop muscle symptoms. It also recommended stopping statins when muscle symptoms with creatine kinase elevations >10 times the upper limits of normal occur, with consideration of restarting statins later at a lower dose if symptoms and elevated creatine kinase resolve. Careful monitoring of patients at higher risk of statin myopathy is also recommended.
Benefits of statins outweigh the risks
Joseph Saseen, PharmD, BCPS
University of Colorado Health Sciences Center, Denver
Contrary to popular belief, statin-associated myopathy is a rare adverse event. Drug-drug interactions and comorbid diseases (especially chronic kidney disease) increase myopathy risk. Given the overwhelming evidence demonstrating reduced morbidity and mortality with statins, benefits outweigh risks in patients with elevated low-density lipoprotein cholesterol. Data supporting myopathy management strategies are limited, but support stopping statin therapy in patients with myopathy (muscle aches/pain with elevated creatine kinase), and restarting, possibly with a different statin, after symptoms resolve. Myopathy should not be confused with myalgia (muscle aches/pain with normal creatine kinase). Myalgia requires interrupting treatment only for patients with persistent muscle aches/pain while on statin therapy.
1. Pasternak RC, Smith SC, Jr, Biarey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 2002;40:567-572.
2. Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003;289:1681-1690.
3. Gaist D, Rodriquez LA, Huerta C, Hallas J, Sindrup SH. Lipid-lowering drugs and risk of myopathy: a population-based follow-up study. Epidemiology 2001;12:565-569.
4. Phillips PS, Haas RH, Bannykh S, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med 2002;137:581-585.
5. Omar MA, Wilson JP. FDA adverse event reports on statin-associated rhabdomyolysis. Ann Pharmacother 2002;36:288-295.
6. Staffa JA, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med 2002;346:539-540.
7. Ucar M, Mjorndal T, Dahlqvist R. HMG-CoA reductase inhibitors and myotoxicity. Drug Saf 2000;6:441-447.
8. Omar MA, Wilson JP, Cox TS. Rhabdomyolysis and HMG-CoA reductase inhibitors. Ann Pharmacother 2001;35:1096-1107.
9. Downs JR, Clearfield M, Tyroler HA, et al. Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS): Additional perspectives on tolerability of long-term treatment with lovastatin. Am J Cardiol 2001;87:1074-1079.
10. Bradford RH, Shear CL, Chremos AN, et al. Expanded Clinical Evaluation of Lovastatin (EXCEL) study results. I. Efficacy in modifying plasma lipoproteins and adverse event profile in 8245 patients with moderate hypercholes-terolemia. Arch Intern Med 1991;151:43-49.
If statins (HMG-CoA reductase inhibitors) cause myopathy, the risk is very low (strength of recommendation [SOR]: A). There is no direct evidence to answer this question. A pooled analysis of randomized controlled trials found similar myopathy rates in patients taking statins and placebo. However, a large cohort study revealed a very small but statistically significant increased risk of myopathy in patients taking statins (number needed to harm=10,000/year).
Case reports suggest a myopathy risk for all statins, including fatal rhabdomyolysis. Risk of myopathy may increase with higher statin doses, certain comorbid states (eg, hypothyroidism, renal insufficiency [especially with diabetes], recent trauma, perioperative periods, advanced age, small body frame) and concurrent medications, including fibrates, cyclosporine, azole antifungals, and macrolide antibiotics (SOR: B). No studies have directly compared myopathy rates among statins, and there is no good evidence to suggest any differences. No controlled study has directly examined statin rechallenge in patients with previous myopathy; however, case reports and expert opinion support this practice (SOR: B).
Evidence summary
There is little evidence that statins cause myopathy. Synthesis is difficult because definitions of myopathy types differ among investigators. Proposed clinical syndrome definitions are myalgia (muscle weakness or ache with normal creatine kinase), myositis (symptoms with increased creatine kinase), and rhabdomyolysis (symptoms, markedly elevated creatine kinase, and renal insufficiency) as subsets of the more general term myopathy.1 The Table summarizes myopathy data from 30 statin trials analyzed in a recent systematic review, showing similar myopathy rates in statin and placebo patients.2 There may be a lower myopathy rate in these trials than in routine clinical practice because of stricter exclusion criteria and more intense monitoring.
A large well-done British epidemiologic cohort study (n=96,193) found an increased rate of myopathy (broadly defined, not requiring creatine kinase elevation) among patients taking statins, with an absolute rate difference of 1 per 10,000 person-years.3 A small study of 21 patients on statins with muscle symptoms but normal creatine kinases described 4 patients who were able to distinguish statins from placebo, with objective reversible weakness and abnormal muscle biopsies.4 Postmarket voluntary clinician reports point to statin myopathy in this and other countries;5-7 these include 3339 rhabdomyolysis FDA reports (1990 to March 2002).2
Though some assert differential myopathy rates among statins based on cell research, case reports, or differences in metabolic clearance, no studies directly compare clinical myopathy rates among statins. There is no good evidence of a differential myopathy risk among statins currently available in the US.1-2 Cerivastatin, however, was withdrawn from the US market because of a fatal rhabdomyolysis rate 16 to 80 times higher than other statins based on FDA reports using a denominator of prescription volume (3.16 fatal cases/million prescriptions vs 0.15 for the statin class as a whole).6
It is unknown whether previous myopathy, however defined, increases the risk of future myopathy with statin rechallenge. A tabular analysis of 74 published case reports of statin-associated rhabdomyolysis from a MEDLINE search covering 1985 to 2000 reported that in most cases the statin was safely restarted after stopping presumed interactive drugs (exact numbers not reported).8 In the AFCAPS/TexCAPS trial, 20 of 21 statin patients (out of a study population of 3304) who had elevated creatine kinase (>10 times the upper limits of normal) recovered with continued lovastatin treatment, while the other patient resumed treatment after a brief interruption without further elevations.9 The EXCEL trial (n=8245) of various lovastatin dosages included routine creatine kinase tests every 6 weeks for 48 weeks. Five lovastatin patients had muscle symptoms with creatine kinases >10 times the upper limits of normal, and in the 2 who continued treatment, symptoms and creatine kinase became normal.10 Of note, creatine kinase elevation of any kind at least once during 48 weeks occurred in 28.9% of placebo patients, arguing against routine creatine kinase screening in statin patients.
Case studies suggest an increased myopathy risk when statins are given with various medications, including fibrates, cyclosporine, azole antifungals, warfarin, nefazodone, and macrolide antibiotics.4,6,8 Pravastatin and fluvastatin, which are not metabolized by the P450 CYP3A4 pathway, may be safer to use because of fewer drug interactions.2,8 Likewise, certain comorbid states such as hypothyroidism, renal insufficiency (especially in patients with diabetes), recent trauma, and perioperative periods, as well as advanced age, small body frame, and multiple medications may increase statin myopathy risk.1,2,7,8
TABLE
Pooled myopathy data from 30 randomized controlled statin trials
| Trials | Total patients | Statin patients | Placebo patients | |
|---|---|---|---|---|
| Myalgia | 5 | 33,929 | 0.3–32.9% | 0–33.3% |
| Creatine kinase elevation | 9 | 33,921 | 0–0.64% | 0–0.58% |
| Myositis* | 18 | 58,237 | 0.17% | 0.15% |
| Rhabdomyolysis | 20 | 70,126 | 0.020% | 0.014% |
| *Creatine kinase >10 times upper limit of normal | ||||
| Adapted and calculated from Thompson PD, et al. JAMA 2003; 289:1681–1690.2 | ||||
Recommendations from others
A 2002 Clinical Advisory, jointly issued by the American College of Cardiology, the American Heart Association, and the National Heart, Lung and Blood Institute, asserted that statins carry a small but definite myopathy risk.1 It recommended against routine creatine kinase tests, reserving them for patients who develop muscle symptoms. It also recommended stopping statins when muscle symptoms with creatine kinase elevations >10 times the upper limits of normal occur, with consideration of restarting statins later at a lower dose if symptoms and elevated creatine kinase resolve. Careful monitoring of patients at higher risk of statin myopathy is also recommended.
Benefits of statins outweigh the risks
Joseph Saseen, PharmD, BCPS
University of Colorado Health Sciences Center, Denver
Contrary to popular belief, statin-associated myopathy is a rare adverse event. Drug-drug interactions and comorbid diseases (especially chronic kidney disease) increase myopathy risk. Given the overwhelming evidence demonstrating reduced morbidity and mortality with statins, benefits outweigh risks in patients with elevated low-density lipoprotein cholesterol. Data supporting myopathy management strategies are limited, but support stopping statin therapy in patients with myopathy (muscle aches/pain with elevated creatine kinase), and restarting, possibly with a different statin, after symptoms resolve. Myopathy should not be confused with myalgia (muscle aches/pain with normal creatine kinase). Myalgia requires interrupting treatment only for patients with persistent muscle aches/pain while on statin therapy.
If statins (HMG-CoA reductase inhibitors) cause myopathy, the risk is very low (strength of recommendation [SOR]: A). There is no direct evidence to answer this question. A pooled analysis of randomized controlled trials found similar myopathy rates in patients taking statins and placebo. However, a large cohort study revealed a very small but statistically significant increased risk of myopathy in patients taking statins (number needed to harm=10,000/year).
Case reports suggest a myopathy risk for all statins, including fatal rhabdomyolysis. Risk of myopathy may increase with higher statin doses, certain comorbid states (eg, hypothyroidism, renal insufficiency [especially with diabetes], recent trauma, perioperative periods, advanced age, small body frame) and concurrent medications, including fibrates, cyclosporine, azole antifungals, and macrolide antibiotics (SOR: B). No studies have directly compared myopathy rates among statins, and there is no good evidence to suggest any differences. No controlled study has directly examined statin rechallenge in patients with previous myopathy; however, case reports and expert opinion support this practice (SOR: B).
Evidence summary
There is little evidence that statins cause myopathy. Synthesis is difficult because definitions of myopathy types differ among investigators. Proposed clinical syndrome definitions are myalgia (muscle weakness or ache with normal creatine kinase), myositis (symptoms with increased creatine kinase), and rhabdomyolysis (symptoms, markedly elevated creatine kinase, and renal insufficiency) as subsets of the more general term myopathy.1 The Table summarizes myopathy data from 30 statin trials analyzed in a recent systematic review, showing similar myopathy rates in statin and placebo patients.2 There may be a lower myopathy rate in these trials than in routine clinical practice because of stricter exclusion criteria and more intense monitoring.
A large well-done British epidemiologic cohort study (n=96,193) found an increased rate of myopathy (broadly defined, not requiring creatine kinase elevation) among patients taking statins, with an absolute rate difference of 1 per 10,000 person-years.3 A small study of 21 patients on statins with muscle symptoms but normal creatine kinases described 4 patients who were able to distinguish statins from placebo, with objective reversible weakness and abnormal muscle biopsies.4 Postmarket voluntary clinician reports point to statin myopathy in this and other countries;5-7 these include 3339 rhabdomyolysis FDA reports (1990 to March 2002).2
Though some assert differential myopathy rates among statins based on cell research, case reports, or differences in metabolic clearance, no studies directly compare clinical myopathy rates among statins. There is no good evidence of a differential myopathy risk among statins currently available in the US.1-2 Cerivastatin, however, was withdrawn from the US market because of a fatal rhabdomyolysis rate 16 to 80 times higher than other statins based on FDA reports using a denominator of prescription volume (3.16 fatal cases/million prescriptions vs 0.15 for the statin class as a whole).6
It is unknown whether previous myopathy, however defined, increases the risk of future myopathy with statin rechallenge. A tabular analysis of 74 published case reports of statin-associated rhabdomyolysis from a MEDLINE search covering 1985 to 2000 reported that in most cases the statin was safely restarted after stopping presumed interactive drugs (exact numbers not reported).8 In the AFCAPS/TexCAPS trial, 20 of 21 statin patients (out of a study population of 3304) who had elevated creatine kinase (>10 times the upper limits of normal) recovered with continued lovastatin treatment, while the other patient resumed treatment after a brief interruption without further elevations.9 The EXCEL trial (n=8245) of various lovastatin dosages included routine creatine kinase tests every 6 weeks for 48 weeks. Five lovastatin patients had muscle symptoms with creatine kinases >10 times the upper limits of normal, and in the 2 who continued treatment, symptoms and creatine kinase became normal.10 Of note, creatine kinase elevation of any kind at least once during 48 weeks occurred in 28.9% of placebo patients, arguing against routine creatine kinase screening in statin patients.
Case studies suggest an increased myopathy risk when statins are given with various medications, including fibrates, cyclosporine, azole antifungals, warfarin, nefazodone, and macrolide antibiotics.4,6,8 Pravastatin and fluvastatin, which are not metabolized by the P450 CYP3A4 pathway, may be safer to use because of fewer drug interactions.2,8 Likewise, certain comorbid states such as hypothyroidism, renal insufficiency (especially in patients with diabetes), recent trauma, and perioperative periods, as well as advanced age, small body frame, and multiple medications may increase statin myopathy risk.1,2,7,8
TABLE
Pooled myopathy data from 30 randomized controlled statin trials
| Trials | Total patients | Statin patients | Placebo patients | |
|---|---|---|---|---|
| Myalgia | 5 | 33,929 | 0.3–32.9% | 0–33.3% |
| Creatine kinase elevation | 9 | 33,921 | 0–0.64% | 0–0.58% |
| Myositis* | 18 | 58,237 | 0.17% | 0.15% |
| Rhabdomyolysis | 20 | 70,126 | 0.020% | 0.014% |
| *Creatine kinase >10 times upper limit of normal | ||||
| Adapted and calculated from Thompson PD, et al. JAMA 2003; 289:1681–1690.2 | ||||
Recommendations from others
A 2002 Clinical Advisory, jointly issued by the American College of Cardiology, the American Heart Association, and the National Heart, Lung and Blood Institute, asserted that statins carry a small but definite myopathy risk.1 It recommended against routine creatine kinase tests, reserving them for patients who develop muscle symptoms. It also recommended stopping statins when muscle symptoms with creatine kinase elevations >10 times the upper limits of normal occur, with consideration of restarting statins later at a lower dose if symptoms and elevated creatine kinase resolve. Careful monitoring of patients at higher risk of statin myopathy is also recommended.
Benefits of statins outweigh the risks
Joseph Saseen, PharmD, BCPS
University of Colorado Health Sciences Center, Denver
Contrary to popular belief, statin-associated myopathy is a rare adverse event. Drug-drug interactions and comorbid diseases (especially chronic kidney disease) increase myopathy risk. Given the overwhelming evidence demonstrating reduced morbidity and mortality with statins, benefits outweigh risks in patients with elevated low-density lipoprotein cholesterol. Data supporting myopathy management strategies are limited, but support stopping statin therapy in patients with myopathy (muscle aches/pain with elevated creatine kinase), and restarting, possibly with a different statin, after symptoms resolve. Myopathy should not be confused with myalgia (muscle aches/pain with normal creatine kinase). Myalgia requires interrupting treatment only for patients with persistent muscle aches/pain while on statin therapy.
1. Pasternak RC, Smith SC, Jr, Biarey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 2002;40:567-572.
2. Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003;289:1681-1690.
3. Gaist D, Rodriquez LA, Huerta C, Hallas J, Sindrup SH. Lipid-lowering drugs and risk of myopathy: a population-based follow-up study. Epidemiology 2001;12:565-569.
4. Phillips PS, Haas RH, Bannykh S, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med 2002;137:581-585.
5. Omar MA, Wilson JP. FDA adverse event reports on statin-associated rhabdomyolysis. Ann Pharmacother 2002;36:288-295.
6. Staffa JA, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med 2002;346:539-540.
7. Ucar M, Mjorndal T, Dahlqvist R. HMG-CoA reductase inhibitors and myotoxicity. Drug Saf 2000;6:441-447.
8. Omar MA, Wilson JP, Cox TS. Rhabdomyolysis and HMG-CoA reductase inhibitors. Ann Pharmacother 2001;35:1096-1107.
9. Downs JR, Clearfield M, Tyroler HA, et al. Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS): Additional perspectives on tolerability of long-term treatment with lovastatin. Am J Cardiol 2001;87:1074-1079.
10. Bradford RH, Shear CL, Chremos AN, et al. Expanded Clinical Evaluation of Lovastatin (EXCEL) study results. I. Efficacy in modifying plasma lipoproteins and adverse event profile in 8245 patients with moderate hypercholes-terolemia. Arch Intern Med 1991;151:43-49.
1. Pasternak RC, Smith SC, Jr, Biarey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 2002;40:567-572.
2. Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003;289:1681-1690.
3. Gaist D, Rodriquez LA, Huerta C, Hallas J, Sindrup SH. Lipid-lowering drugs and risk of myopathy: a population-based follow-up study. Epidemiology 2001;12:565-569.
4. Phillips PS, Haas RH, Bannykh S, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med 2002;137:581-585.
5. Omar MA, Wilson JP. FDA adverse event reports on statin-associated rhabdomyolysis. Ann Pharmacother 2002;36:288-295.
6. Staffa JA, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med 2002;346:539-540.
7. Ucar M, Mjorndal T, Dahlqvist R. HMG-CoA reductase inhibitors and myotoxicity. Drug Saf 2000;6:441-447.
8. Omar MA, Wilson JP, Cox TS. Rhabdomyolysis and HMG-CoA reductase inhibitors. Ann Pharmacother 2001;35:1096-1107.
9. Downs JR, Clearfield M, Tyroler HA, et al. Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS): Additional perspectives on tolerability of long-term treatment with lovastatin. Am J Cardiol 2001;87:1074-1079.
10. Bradford RH, Shear CL, Chremos AN, et al. Expanded Clinical Evaluation of Lovastatin (EXCEL) study results. I. Efficacy in modifying plasma lipoproteins and adverse event profile in 8245 patients with moderate hypercholes-terolemia. Arch Intern Med 1991;151:43-49.
Evidence-based answers from the Family Physicians Inquiries Network
How should we manage infants at risk for group B streptococcal disease?
Asymptomatic term infants whose mothers received adequate intrapartum antibiotic prophylaxis (defined as intravenous penicillin or ampicillin at least 4 hours before delivery) for group B streptococcal disease do not need work-up or treatment (strength of recommendation [SOR]: B, based on retrospective, population-based study). These infants should be observed for 48 hours, but may be discharged after 24 hours in circumstances where close follow-up is available (SOR: D, based on expert opinion).
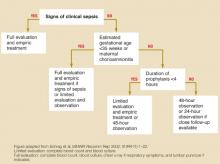
Symptomatic infants, premature infants (gestational age <35 weeks) of mothers who did not receive prophylaxis, and infants whose mothers had chorioamnionitis should receive a full evaluation (complete blood count, blood culture, and chest x-ray with or without a lumbar puncture) and an initial empiric antibiotic treatment with ampicillin or penicillin and gentamycin. If a term infant is not symptomatic and maternal antibiotic prophylaxis was not adequate, opinions differ as to whether to perform limited evaluation with empiric treatment or close observation (SOR: D, based on expert opinion). See Figure.
FIGURE
Management of infants born to mothers with group B streptococcal disease–positive cultures
Evidence summary
Intrapartum antibiotic prophylaxis has decreased the incidence of early-onset group B streptococcal disease by 65% in the last decade.1 A multicenter population-based study demonstrated that basing prophylaxis on screening cultures is twice as effective as risk stratification, a previously recommended strategy.2
Intrapartum prophylaxis of women who had positive group B streptococcal disease screening cultures at 35 weeks will prevent 70% of earlyonset disease and 89% of fatalities.3,4 As demonstrated by multicenter retrospective studies, infants aged <35 weeks are at significantly higher risk of group B streptococcal disease than term infants (relative risk=1.5–2.07), and mortality for premature infants with early-onset disease (25%–30%) is substantially higher than for term infants with early-onset disease (2%–8%).2,5
A large retrospective study demonstrated that infants who developed early-onset disease despite intrapartum prophylaxis developed the same clinical syndrome in the same time frame (78% of early-onset disease evident in first 24 hours and 96% by 48 hours) as infants whose mothers did not receive prophylaxis.6
The duration of adequate intrapartum antibiotic prophylaxis was initially set at 4 hours, based on a study measuring antibiotic penetration into amniotic fluid.7 A recent randomized trial, enrolling more than 4500 women, has confirmed this finding. The vertical transmission rate of group B streptococcal disease, as measured by neonatal colonization (as opposed to clinical illness), is 46% when antibiotic prophylaxis is started <1 hour before delivery, 2.9% when prophylaxis is given at 2 to 4 hours, and 1.2% when given at least 4 hours before delivery.8
Implementation of these guidelines is aided by the adoption of an institution-wide policy to support point of care decision-making.9 A retrospective study after the release of the 1996 Centers for Disease Control (CDC) guidelines concluded that hospitals with established group B streptococcal disease policies had significantly fewer cases of early-onset disease (P=.038).10
Recommendations from others
The 2002 Prevention of Perinatal Group B Streptococcal Disease Revised Guidelines from the CDC states: “a healthy-appearing infant whose mother received >4 hours of [intrapartum antibiotic prophylaxis] before delivery may be discharged home as early as 24 hours after delivery, assuming other discharge criteria have been met and that a person able to comply fully with instructions for home observation will be present … if these conditions are not met, the infant should remain in the hospital for at least 48 hours of observation and until criteria for discharge are achieved.”1
These guidelines strongly support universal prenatal screening and the use of intrapartum antibiotic prophylaxis. Both the American Academy of Pediatrics and the American College Obstetrics and Gynecology have endorsed the CDC’s revised guidelines.
Richard Nicholas, MD
Rose Family Medicine Residency, Denver, Colo
The question of appropriate care of the infant exposed to group B streptococcal disease arises frequently in any practice caring for newborns. These clear, evidence-based recommendations are helpful in guiding that care. The evidence supports watchful waiting for appropriately covered newborns, providing reassurance for both parents and physicians.
Unfortunately, little evidence exists to guide care in a setting that seems to be quite common: the term, asymptomatic infant born to a mother who, in labor, received less-than-adequate intrapartum antibiotic prophylaxis. Further research for this subgroup is needed; in the meantime, physicians who provide maternity or newborn care should work together to develop protocols that ensure adequate intrapartum antibiotic coverage for mothers with group B streptococcal disease.
1. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC MMWR Recomm Rep 2002;51(RR-11):1-22.
2. Schrag SJ, Zell ER, Lynfield R, et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med 2002;347:233-239.
3. Boyer KM, Gotoff SP. Prevention of early-onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis. N Engl J Med 1986;314:1665-1669.
4. Schuchat A, Oxtoby M, Cochi S, et al. Population-based risk factors for neonatal group B streptococcal disease: results of a cohort study in metropolitan Atlanta. J Infect Dis 1990;162:672-677.
5. Cochi SL, Feldman RA. Estimating national incidence of group B streptococcal disease: the effect of adjusting for birth weight. Pediatr Infect Dis 1983;2:414-415.
6. Bromberger P, Lawrence JM, Braun D, Saunders B, Contreras R, Petitti DB. The influence of intrapartum antibiotics on the clinical spectrum of early-onset group B streptococcal infection in term infants. Pediatrics 2000;106:244-250.
7. Bray RE, Boe RW, Johnson WL. Transfer of ampicillin into fetus and amniotic fluid from maternal plasma in late pregnancy. Am J Obstet Gynecol 1966;96:938-942.
8. de Cueto M, Sanchez MJ, Sampedro A, Miranda JA, Herruzo AJ, Rosa-Fraile M. Timing of intrapartum ampicillin and prevention of vertical transmission of group B streptococcus. Obstet Gynecol 1998;91:112-114.
9. Clemens CJ, Gable EK. The development of a group B streptococcus prevention policy at a community hospital. J Perinatol 2002;22:523-525.
10. Factor SH, Whitney CG, Zywicki SS, Schuchat A. Effects of hospital policies based on 1996 group B streptococcal disease consensus guidelines. The Active Bacterial Core Surveillance Team. Obstet Gynecol 2000;95:377-382.
Asymptomatic term infants whose mothers received adequate intrapartum antibiotic prophylaxis (defined as intravenous penicillin or ampicillin at least 4 hours before delivery) for group B streptococcal disease do not need work-up or treatment (strength of recommendation [SOR]: B, based on retrospective, population-based study). These infants should be observed for 48 hours, but may be discharged after 24 hours in circumstances where close follow-up is available (SOR: D, based on expert opinion).
Symptomatic infants, premature infants (gestational age <35 weeks) of mothers who did not receive prophylaxis, and infants whose mothers had chorioamnionitis should receive a full evaluation (complete blood count, blood culture, and chest x-ray with or without a lumbar puncture) and an initial empiric antibiotic treatment with ampicillin or penicillin and gentamycin. If a term infant is not symptomatic and maternal antibiotic prophylaxis was not adequate, opinions differ as to whether to perform limited evaluation with empiric treatment or close observation (SOR: D, based on expert opinion). See Figure.
FIGURE
Management of infants born to mothers with group B streptococcal disease–positive cultures
Evidence summary
Intrapartum antibiotic prophylaxis has decreased the incidence of early-onset group B streptococcal disease by 65% in the last decade.1 A multicenter population-based study demonstrated that basing prophylaxis on screening cultures is twice as effective as risk stratification, a previously recommended strategy.2
Intrapartum prophylaxis of women who had positive group B streptococcal disease screening cultures at 35 weeks will prevent 70% of earlyonset disease and 89% of fatalities.3,4 As demonstrated by multicenter retrospective studies, infants aged <35 weeks are at significantly higher risk of group B streptococcal disease than term infants (relative risk=1.5–2.07), and mortality for premature infants with early-onset disease (25%–30%) is substantially higher than for term infants with early-onset disease (2%–8%).2,5
A large retrospective study demonstrated that infants who developed early-onset disease despite intrapartum prophylaxis developed the same clinical syndrome in the same time frame (78% of early-onset disease evident in first 24 hours and 96% by 48 hours) as infants whose mothers did not receive prophylaxis.6
The duration of adequate intrapartum antibiotic prophylaxis was initially set at 4 hours, based on a study measuring antibiotic penetration into amniotic fluid.7 A recent randomized trial, enrolling more than 4500 women, has confirmed this finding. The vertical transmission rate of group B streptococcal disease, as measured by neonatal colonization (as opposed to clinical illness), is 46% when antibiotic prophylaxis is started <1 hour before delivery, 2.9% when prophylaxis is given at 2 to 4 hours, and 1.2% when given at least 4 hours before delivery.8
Implementation of these guidelines is aided by the adoption of an institution-wide policy to support point of care decision-making.9 A retrospective study after the release of the 1996 Centers for Disease Control (CDC) guidelines concluded that hospitals with established group B streptococcal disease policies had significantly fewer cases of early-onset disease (P=.038).10
Recommendations from others
The 2002 Prevention of Perinatal Group B Streptococcal Disease Revised Guidelines from the CDC states: “a healthy-appearing infant whose mother received >4 hours of [intrapartum antibiotic prophylaxis] before delivery may be discharged home as early as 24 hours after delivery, assuming other discharge criteria have been met and that a person able to comply fully with instructions for home observation will be present … if these conditions are not met, the infant should remain in the hospital for at least 48 hours of observation and until criteria for discharge are achieved.”1
These guidelines strongly support universal prenatal screening and the use of intrapartum antibiotic prophylaxis. Both the American Academy of Pediatrics and the American College Obstetrics and Gynecology have endorsed the CDC’s revised guidelines.
Richard Nicholas, MD
Rose Family Medicine Residency, Denver, Colo
The question of appropriate care of the infant exposed to group B streptococcal disease arises frequently in any practice caring for newborns. These clear, evidence-based recommendations are helpful in guiding that care. The evidence supports watchful waiting for appropriately covered newborns, providing reassurance for both parents and physicians.
Unfortunately, little evidence exists to guide care in a setting that seems to be quite common: the term, asymptomatic infant born to a mother who, in labor, received less-than-adequate intrapartum antibiotic prophylaxis. Further research for this subgroup is needed; in the meantime, physicians who provide maternity or newborn care should work together to develop protocols that ensure adequate intrapartum antibiotic coverage for mothers with group B streptococcal disease.
Asymptomatic term infants whose mothers received adequate intrapartum antibiotic prophylaxis (defined as intravenous penicillin or ampicillin at least 4 hours before delivery) for group B streptococcal disease do not need work-up or treatment (strength of recommendation [SOR]: B, based on retrospective, population-based study). These infants should be observed for 48 hours, but may be discharged after 24 hours in circumstances where close follow-up is available (SOR: D, based on expert opinion).
Symptomatic infants, premature infants (gestational age <35 weeks) of mothers who did not receive prophylaxis, and infants whose mothers had chorioamnionitis should receive a full evaluation (complete blood count, blood culture, and chest x-ray with or without a lumbar puncture) and an initial empiric antibiotic treatment with ampicillin or penicillin and gentamycin. If a term infant is not symptomatic and maternal antibiotic prophylaxis was not adequate, opinions differ as to whether to perform limited evaluation with empiric treatment or close observation (SOR: D, based on expert opinion). See Figure.
FIGURE
Management of infants born to mothers with group B streptococcal disease–positive cultures
Evidence summary
Intrapartum antibiotic prophylaxis has decreased the incidence of early-onset group B streptococcal disease by 65% in the last decade.1 A multicenter population-based study demonstrated that basing prophylaxis on screening cultures is twice as effective as risk stratification, a previously recommended strategy.2
Intrapartum prophylaxis of women who had positive group B streptococcal disease screening cultures at 35 weeks will prevent 70% of earlyonset disease and 89% of fatalities.3,4 As demonstrated by multicenter retrospective studies, infants aged <35 weeks are at significantly higher risk of group B streptococcal disease than term infants (relative risk=1.5–2.07), and mortality for premature infants with early-onset disease (25%–30%) is substantially higher than for term infants with early-onset disease (2%–8%).2,5
A large retrospective study demonstrated that infants who developed early-onset disease despite intrapartum prophylaxis developed the same clinical syndrome in the same time frame (78% of early-onset disease evident in first 24 hours and 96% by 48 hours) as infants whose mothers did not receive prophylaxis.6
The duration of adequate intrapartum antibiotic prophylaxis was initially set at 4 hours, based on a study measuring antibiotic penetration into amniotic fluid.7 A recent randomized trial, enrolling more than 4500 women, has confirmed this finding. The vertical transmission rate of group B streptococcal disease, as measured by neonatal colonization (as opposed to clinical illness), is 46% when antibiotic prophylaxis is started <1 hour before delivery, 2.9% when prophylaxis is given at 2 to 4 hours, and 1.2% when given at least 4 hours before delivery.8
Implementation of these guidelines is aided by the adoption of an institution-wide policy to support point of care decision-making.9 A retrospective study after the release of the 1996 Centers for Disease Control (CDC) guidelines concluded that hospitals with established group B streptococcal disease policies had significantly fewer cases of early-onset disease (P=.038).10
Recommendations from others
The 2002 Prevention of Perinatal Group B Streptococcal Disease Revised Guidelines from the CDC states: “a healthy-appearing infant whose mother received >4 hours of [intrapartum antibiotic prophylaxis] before delivery may be discharged home as early as 24 hours after delivery, assuming other discharge criteria have been met and that a person able to comply fully with instructions for home observation will be present … if these conditions are not met, the infant should remain in the hospital for at least 48 hours of observation and until criteria for discharge are achieved.”1
These guidelines strongly support universal prenatal screening and the use of intrapartum antibiotic prophylaxis. Both the American Academy of Pediatrics and the American College Obstetrics and Gynecology have endorsed the CDC’s revised guidelines.
Richard Nicholas, MD
Rose Family Medicine Residency, Denver, Colo
The question of appropriate care of the infant exposed to group B streptococcal disease arises frequently in any practice caring for newborns. These clear, evidence-based recommendations are helpful in guiding that care. The evidence supports watchful waiting for appropriately covered newborns, providing reassurance for both parents and physicians.
Unfortunately, little evidence exists to guide care in a setting that seems to be quite common: the term, asymptomatic infant born to a mother who, in labor, received less-than-adequate intrapartum antibiotic prophylaxis. Further research for this subgroup is needed; in the meantime, physicians who provide maternity or newborn care should work together to develop protocols that ensure adequate intrapartum antibiotic coverage for mothers with group B streptococcal disease.
1. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC MMWR Recomm Rep 2002;51(RR-11):1-22.
2. Schrag SJ, Zell ER, Lynfield R, et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med 2002;347:233-239.
3. Boyer KM, Gotoff SP. Prevention of early-onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis. N Engl J Med 1986;314:1665-1669.
4. Schuchat A, Oxtoby M, Cochi S, et al. Population-based risk factors for neonatal group B streptococcal disease: results of a cohort study in metropolitan Atlanta. J Infect Dis 1990;162:672-677.
5. Cochi SL, Feldman RA. Estimating national incidence of group B streptococcal disease: the effect of adjusting for birth weight. Pediatr Infect Dis 1983;2:414-415.
6. Bromberger P, Lawrence JM, Braun D, Saunders B, Contreras R, Petitti DB. The influence of intrapartum antibiotics on the clinical spectrum of early-onset group B streptococcal infection in term infants. Pediatrics 2000;106:244-250.
7. Bray RE, Boe RW, Johnson WL. Transfer of ampicillin into fetus and amniotic fluid from maternal plasma in late pregnancy. Am J Obstet Gynecol 1966;96:938-942.
8. de Cueto M, Sanchez MJ, Sampedro A, Miranda JA, Herruzo AJ, Rosa-Fraile M. Timing of intrapartum ampicillin and prevention of vertical transmission of group B streptococcus. Obstet Gynecol 1998;91:112-114.
9. Clemens CJ, Gable EK. The development of a group B streptococcus prevention policy at a community hospital. J Perinatol 2002;22:523-525.
10. Factor SH, Whitney CG, Zywicki SS, Schuchat A. Effects of hospital policies based on 1996 group B streptococcal disease consensus guidelines. The Active Bacterial Core Surveillance Team. Obstet Gynecol 2000;95:377-382.
1. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC MMWR Recomm Rep 2002;51(RR-11):1-22.
2. Schrag SJ, Zell ER, Lynfield R, et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med 2002;347:233-239.
3. Boyer KM, Gotoff SP. Prevention of early-onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis. N Engl J Med 1986;314:1665-1669.
4. Schuchat A, Oxtoby M, Cochi S, et al. Population-based risk factors for neonatal group B streptococcal disease: results of a cohort study in metropolitan Atlanta. J Infect Dis 1990;162:672-677.
5. Cochi SL, Feldman RA. Estimating national incidence of group B streptococcal disease: the effect of adjusting for birth weight. Pediatr Infect Dis 1983;2:414-415.
6. Bromberger P, Lawrence JM, Braun D, Saunders B, Contreras R, Petitti DB. The influence of intrapartum antibiotics on the clinical spectrum of early-onset group B streptococcal infection in term infants. Pediatrics 2000;106:244-250.
7. Bray RE, Boe RW, Johnson WL. Transfer of ampicillin into fetus and amniotic fluid from maternal plasma in late pregnancy. Am J Obstet Gynecol 1966;96:938-942.
8. de Cueto M, Sanchez MJ, Sampedro A, Miranda JA, Herruzo AJ, Rosa-Fraile M. Timing of intrapartum ampicillin and prevention of vertical transmission of group B streptococcus. Obstet Gynecol 1998;91:112-114.
9. Clemens CJ, Gable EK. The development of a group B streptococcus prevention policy at a community hospital. J Perinatol 2002;22:523-525.
10. Factor SH, Whitney CG, Zywicki SS, Schuchat A. Effects of hospital policies based on 1996 group B streptococcal disease consensus guidelines. The Active Bacterial Core Surveillance Team. Obstet Gynecol 2000;95:377-382.
Evidence-based answers from the Family Physicians Inquiries Network