User login
A 30-year-old man experienced an episode of syncope while at work. He fully recovered consciousness within 2 minutes, but the emergency services team was called. As he was being loaded into the ambulance he again lost consciousness, and he was noted to be in ventricular fibrillation. Advanced cardiac life support was immediately started and continued for 50 minutes before a hemodynamically stable spontaneous rhythm was obtained.
On arrival at the emergency department of the local hospital, he was intubated to protect his airway, as he was comatose. A 12-lead electrocardiogram showed ST-segment elevations in leads V1, V2, and V3 and a wide QRS complex with an rSR′ pattern, consistent with right bundle branch block.
Mild therapeutic hypothermia was initiated by infusing intravenous saline solution chilled to 4°C and by applying cooling blankets, and he was transferred to our hospital on an emergency basis for further management. Here, hypothermia was maintained using an intravenous cooling catheter.
HYPOTHERMIA: BENEFICIAL, BUT SLOW TO BE ADOPTED
Mild therapeutic hypothermia is a recommended therapeutic intervention for out-of-hospital cardiac arrest due to ventricular fibrillation. Nonetheless, first-responders, emergency-room staff, and intensive-care teams have been slow to adopt and integrate it into a comprehensive postresuscitation strategy. This article summarizes the evidence supporting this therapy and how it is performed.
PROPOSED MECHANISMS OF BENEFIT
Mild therapeutic hypothermia is thought to protect against anoxic brain injury in survivors of cardiac arrest via several mechanisms:
- Decreasing neuronal metabolism in the early stage of ischemic injury
- Decreasing glucose and oxygen consumption by the brain,1 which reduces supply-demand mismatch
- Decreasing the release of excitatory amino acids (eg, glutamate) that normally trigger cytotoxic cascades in the intermediate phase of injury2
- Reducing the production of harmful reactive oxygen species3
- Maintaining cellular pH4
- Reducing cell death5
- Slowing the breakdown of the blood-brain barrier that worsens cerebral edema.6
CLINICAL DATA SUPPORTING HYPOTHERMIA
There has been an interest in therapeutic hypothermia for several decades. In the 1950s, it was used in small numbers of cases in a variety of cardiac arrest situations.7,8 Interest was rekindled in the mid-1990s after a number of animal studies suggested it might be beneficial in prolonged cerebral ischemia and anoxia,9,10 and reports of case-series described its use in adults with out-of-hospital cardiac arrest.11,12
In October 2002, the International Liaison Committee on Resuscitation (ILCOR), made up of executive members of several organizations including the American Heart Association, recommended that “unconscious adult patients with spontaneous circulation after out-of-hospital cardiac arrest” should be cooled to 32°C to 34°C [89.6°F–93.2°F] for 12 to 24 hours “when the initial rhythm was ventricular fibrillation.”13
Two large randomized trials
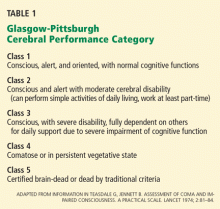
This position statement was based largely on the results of two randomized clinical trials published simultaneously earlier in 2002.14,15 These two trials were important not only because they were the largest randomized trials of this therapy to that point, but also because they used meaningful, prospectively defined clinical end points: all-cause mortality and degree of cognitive preservation as assessed using the Glasgow-Pittsburgh Cerebral Performance Category (CPC) scale.
Bernard et al14 performed a randomized trial in four centers in Australia, assigning 77 patients either to a goal temperature of 32°C to 34°C or to normothermia for 12 hours, with all other resuscitative measures being the same in both groups. The primary outcome measured was survival to hospital discharge with sufficient neurologic function to be discharged to home or to a rehabilitation facility, ie, a CPC score of 1 or 2.
In the hypothermia group, 21 (49%) of the 43 patients survived and had an outcome that was considered “good” (ie, they were discharged home or to a rehabilitation facility), compared with 9 (26%) of the 34 patients in the normothermia group (unadjusted odds ratio 2.65, 95% confidence interval [CI] 1.02–6.88, P = .046). Proportionally fewer patients in the hypothermia group died—22 (51%) of 43 vs 23 (68%) of 34; however, the difference was not statistically significant (P = .145).
The Hypothermia After Cardiac Arrest Study Group15 screened 3,551 European patients who suffered out-of-hospital cardiac arrest15; 275 patients were randomized to mild therapeutic hypothermia or normothermia for 24 hours. The primary outcome was the percentage of patients who had a CPC score of 1 or 2 (vs 3 to 5) at 6 months, and the secondary outcome was the rate of death at 6 months.
At 6 months, 75 (55%) of the 136 patients in the hypothermia group had a CPC score of 1 or 2, compared with 54 (39%) of the 137 patients in the normothermia group (P = .009). The rate of death was also lower with hypothermia: 55% vs 41% (P = .02).
In both trials, patients were included only if their cardiac arrest was witnessed, if their initial cardiac rhythm was ventricular fibrillation or pulseless ventricular tachycardia, if circulation spontaneously returned within 60 minutes with standard basic and advanced cardiac life support protocols, and if they were still comatose on arrival at the hospital. They were excluded if they were over age 75, if they had suffered a cerebrovascular accident at the time of cardiac arrest, or if the arrest was caused by trauma or drug overdose. In addition, the European trial excluded patients who suffered another cardiac arrest after the initial return of spontaneous circulation but before cooling was started.
The standard of care
In view of the available clinical data, the 2002 ILCOR guidelines and a 2005 statement from the American Heart Association advocated mild therapeutic hypothermia for survivors of out-of-hospital ventricular tachycardia or fibrillation.16 Subsequently, this therapy has become more widely practiced and accepted as the standard of care among critical-care providers.
Of note, some public health officials and local governments are strongly promoting this treatment for survivors of cardiac arrest in the community.17 More and more of these groups are mandating that these patients be transported only to hospitals that have therapeutic hypothermia protocols in place, bypassing those not equipped to provide this treatment.18
INDICATIONS, CONTRAINDICATIONS, AND GRAY AREAS
What are the indications and contraindications to the use of hypothermia after out-of-hospital cardiac arrest? What are some of the “gray areas”?
Indications. This treatment is indicated for comatose adults who have had a witnessed cardiac arrest, whose initial cardiac rhythm was ventricular fibrillation or pulseless ventricular tachycardia, and whose circulation spontaneously returned in less than 60 minutes with basic and advanced cardiac life support. This carries a class I recommendation, level of evidence B, and was recently reinforced in the 2010 update to the American Heart Association guidelines for cardiopulmonary resuscitation.19
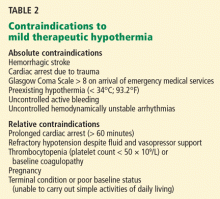
Absolute contraindications include hemorrhagic stroke (which must be proved by computed tomography) and cardiac arrest due to trauma (Table 2). Other major contraindications are a Glasgow Coma Scale score of 8 or higher before the initiation of mild therapeutic hypothermia, cardiac arrest due to drug overdose, and preexisting hypothermia (< 34°C) when first-responders arrive.
Relative contraindications include baseline coagulopathy and severe hypotension (mean atrial pressure < 60 mm Hg) that is not correctable by fluid infusion, vasopressors, or invasive hemodynamic support.
Gray areas. There are not enough data to make a firm recommendation about whether to apply mild therapeutic hypothermia if a witnessed cardiac arrest with ventricular fibrillation or ventricular tachycardia occurs in the hospital, but data from out-of-hospital cardiac arrest patients appear applicable for hospitalized patients.
The data are also quite limited and equivocal on its use for out-of-hospital cardiac arrest in patients whose initial cardiac rhythm is pulseless electrical activity or asystole,20,21 likely because of the competing risk of comorbidities and the resultant lower baseline survival rate in these patients.
Consequently, for in-hospital postarrest patients with any initial rhythm and for out-of-hospital cardiac arrest patients with rhythms other than ventricular tachycardia or ventricular fibrillation, the 2010 guideline recommendation on the use of mild therapeutic hypothermia is less enthusiastic (class IIb, level of evidence B).19
There are also few data on the use of mild therapeutic hypothermia in post-arrest patients in circulatory shock requiring vasopressors or intra-aortic balloon counterpulsation, largely limited to case series and comparisons with historical controls.22,23 Further investigation is clearly needed in these areas. Until then, it should be considered at the physician’s and the team’s discretion, on a case-by-case basis.
HYPOTHERMIA IN CASES OF VENTRICULAR FIBRILLATION AND ACUTE CORONARY SYNDROME
The value of coronary angiography after out-of-hospital cardiac arrest was first highlighted by Spaulding et al,24 who performed it urgently in 84 consecutive survivors of out-of-hospital cardiac arrest, 36 of whom had ST-segment elevation myocardial infarction (STEMI). Angiography uncovered an acute coronary occlusion in 40 (48%) of the 84 patients.
In this series, ST-segment elevation was a strong predictor of acute coronary occlusion (odds ratio 4.3; 95% CI 1.6–2.0; P = .004). However, 9 patients without chest pain or ST elevation were also found to have an occluded infarct-related artery. Successful angioplasty was an independent predictor of survival, highlighting the importance of an angiographic definition in this population.
These findings were recently confirmed in the larger Parisian Region Out of Hospital Cardiac Arrest (PROCAT) registry in 435 patients who had no obvious extracardiac cause of arrest, for whom successful culprit coronary angioplasty was associated with survival.25
Angioplasty comes first, but neither treatment need be delayed
Efforts to induce hypothermia must not be allowed to delay the door-to-balloon time of post-arrest patients in the setting of STEMI. The top priority is establishing patency of the infarct-related artery with a goal of salvaging ischemic myocardium and obtaining mechanical and electrical stabilization.
Fortunately, mild therapeutic hypothermia does not necessarily delay emergency revascularization if hypothermia protocols are well established. In fact, induction of mild therapeutic hypothermia prior to or on arrival at the catheterization laboratory has been shown to be feasible and safe.26,27
We believe that all centers performing primary percutaneous coronary intervention for STEMI should have immediate access to and expertise in mild therapeutic hypothermia. Regional planning and integration of STEMI and out-of-hospital cardiac arrest networks will ensure that most patients with STEMI have access to this treatment when it is indicated.
Does hypothermia help the heart? Does it increase bleeding?
Researchers have been interested in therapeutic hypothermia as a means of reducing myocardial infarct size,28,29 but clinical trials have not shown a clear-cut benefit in this regard. However, these investigations have also added to the evidence that antiplatelet and anticoagulation therapy in patients undergoing mild therapeutic hypothermia does not result in a statistically significant excess of major bleeding events, which is a potential concern.
Of note, these studies were neither powered nor specifically designed to evaluate for major bleeding as an end point. Therefore, these complications should still be carefully monitored for.
IS THERE AN OPTIMAL TIME TO BEGIN MILD THERAPEUTIC HYPOTHERMIA?
Experimental data suggest that mild therapeutic hypothermia should be started as soon as possible after a comprehensive clinical evaluation indicates the patient is eligible.30–33 However, clinical data are not robustly in favor of starting it before the patient reaches the hospital rather than on hospital arrival.
In a recent randomized trial in 2,334 survivors of out-of-hospital cardiac arrest, outcomes were no better if hypothermia was started by paramedics than if it was started on arrival at the hospital (47.5% vs 52.6% discharged to home or rehabilitation; 95% CI 0.70–1.17; P = .43).34
Earlier data from smaller studies had suggested that prehospital initiation of hypothermia (for example, using chilled intravenous saline infusions) in carefully selected patients with out-of-hospital cardiac arrest was safe and showed a nonsignificant trend toward better outcomes.20,35
The randomized controlled trials that showed hypothermia to be beneficial used very slow cooling methods; consequently, it is reasonable to allow up to 6 hours from initial presentation to first-responders to start it. There are, however, no conclusive data in humans for or against starting it later than 6 hours after presentation. Most experts believe that its potential neurologic and mortality benefits are largely lost if it is delayed more than 6 hours.
The overall message from these data seems to be that, in patients who survive cardiac arrest outside the hospital with ventricular tachycardia or fibrillation, mild therapeutic hypothermia is effective and safe and should be started as soon as possible after arrival at the hospital.
METHODS FOR INDUCING AND MAINTAINING HYPOTHERMIA
Cooling the patient
To cool the patient and keep him or her cold, caregivers have used ice packs placed around the head, groin, and axillae; intravenous infusion of saline maintained at 4°C (39°F); and cooling-air blankets. More recently, thermal wraps and intravascular cooling catheters have been used.36–38 The newer methods are more effective in rapidly bringing patients to the target temperature of 32 to 34°C (usually within 3 or 4 hours) and keeping them within this range, and they auto-adjust their output on the basis of measured core temperature.
The Pre ROSC Intranasal Cooling Effectiveness (PRINCE) trial demonstrated the safety and efficacy of nasopharyngeal cooling using a perfluorocarbon aerosol given via a nasopharyngeal cannula in patients with out-of-hospital cardiac arrest.39
Monitoring the core temperature
The patient’s core temperature is most commonly monitored with a probe in the esophagus, bladder, rectum, or pulmonary artery.40
Of these, the bladder and rectum are considered “intermediate” monitoring sites, as their temperatures tend to lag behind the core temperature. Furthermore, the bladder temperature can be significantly altered by the flow of urine, which can vary considerably during the cooling and rewarming process.
Esophageal temperature monitoring is relatively noninvasive and tends to reliably and accurately reflect core temperature as long as the probe is placed far enough down (about 45 cm from the nose in an average adult) that it is not affected by proximity to the trachea.
Pulmonary artery catheters are considered the gold standard for core temperature monitoring, but they pose risks such as bloodstream infection and large-vessel damage. In practice, many patients admitted to the coronary intensive care unit after out-of-hospital cardiac arrest require pulmonary artery catheterization anyway for other indications, and in these situations it is the preferred method of monitoring the core temperature.
However, no approach is ideal in terms of measuring the temperature in the critical end organs. Rather, core temperature monitoring serves as a guide to help ensure consistent clinical practice in attaining and maintaining mild therapeutic hypothermia.
Preventing shivering
To achieve and maintain the goal temperature, the body’s natural response to a decrease in core temperature—shivering—must be watched for and eliminated. A number of drugs may be used for this purpose.41
Paralytic drugs are used to reduce shivering; nursing staff must be trained to monitor for signs of occult shivering (eg, jaw vibration) and adjust the dose of paralytic drug accordingly. Since the patients are paralyzed, they must also receive continuous intravenous sedation.
Other commonly used drugs that decrease the hypothalamic drive to shiver include buspirone (BuSpar), a serotonin 5HT-1A partial agonist, and meperidine (Demerol), an opiate agonist of kappa and mu receptors.
Rewarming after 24 hours
Rewarming is conventionally started after 24 hours of mild therapeutic hypothermia, at a rate no greater than 0.5°C (1°F) per hour.
Because sedation is used during the hypothermia period of 24 hours, a washout period for these medications is necessary, and the neurologic prognosis of cardiac arrest patients who undergo mild therapeutic hypothermia cannot be adequately assessed until 72 hours after rewarming.
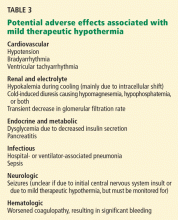
ADVERSE EFFECTS OF MILD THERAPEUTIC HYPOTHERMIA
Some of these effects are predictable. Decreasing the body temperature causes potassium to shift into the cells, and this same potassium will leave the intracellular space during the rewarming phase. For this reason, aggressive potassium repletion for mild hypokalemia (potassium levels of 3.0–3.5 mmol/L) during mild therapeutic hypothermia can result in dangerous hyperkalemia during rewarming and should generally be avoided.
As another example, the enzymes involved in coagulation are less effective at lower temperatures. Thus, if it occurs, active bleeding requiring transfusion warrants consideration of stopping the hypothermia.
Adverse effects should be watched for (eg, by checking electrolyte levels frequently, monitoring blood glucose, continuous electroencephalographic monitoring during the cooling phase, and avoiding placement of intracardiac catheters once the goal temperature is reached) and addressed as they happen. However, in a recent review of this subject42 the balance of evidence continued to indicate that the benefit of this treatment exceeds its risks.
OUR PATIENT RECOVERS
After 24 hours of therapeutic hypothermia, our patient was gradually rewarmed to a normal temperature, and sedation and paralysis were discontinued.
Analysis of his prearrest and postarrest 12-lead electrocardiograms revealed a type I Brugada pattern (coved ST elevation and negative T waves in V1, V2, and V3, caused by abnormal repolarization due to inherited mutations in SCN5A). Cardiac catheterization revealed normal coronary arteries, and MRI revealed no evidence of arrhythmogenic right ventricular cardiomyopathy or other structural abnormalities.
In the next 72 hours the patient was successfully extubated, and he gradually returned to full neurologic function. Before he went home a few days later, a single-lead cardioverter-defibrillator was implanted to prevent sudden cardiac death. All of his first-degree relatives were encouraged to undergo genetic screening for SCN5A mutations. The patient is currently back to his previous high level of functioning as a marketing manager, husband, and father of two young children.
- Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in mammalian central nervous system. J Cereb Blood Flow Metab 2003; 23:513–530.
- Nakashima K, Todd MM. Effects of hypothermia on the rate of excitatory amino acid release after ischemic depolarization. Stroke 1996; 27:913–918.
- Thoresen M, Satas S, Puka-Sundvall M, et al. Post-hypoxic hypothermia reduces cerebrocortical release of NO and excitotoxins. Neuroreport 1997; 8:3359–3362.
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009; 37(suppl 7):S186–S202.
- Yang D, Guo S, Zhang T, Li H. Hypothermia attenuates ischemia/reperfusion-induced endothelial cell apoptosis via alterations in apoptotic pathways and JNK signaling. FEBS Lett 2009; 583:2500–2506.
- Karibe H, Zarow GJ, Graham SH, Weinstein PR. Mild intraischemic hypothermia reduces postischemic hyperperfusion, delayed postischemic hypoperfusion, blood-brain barrier disruption, brain edema, and neuronal damage volume after temporary focal cerebral ischemia in rats. J Cereb Blood Flow Metab 1994; 14:620–627.
- Benson DW, Williams GR, Spencer FC, Yates AJ. The use of hypothermia after cardiac arrest. Anesth Analg 1959; 38:423–428.
- Williams GR, Spencer FC. The clinical use of hypothermia following cardiac arrest. Ann Surg 1958; 148:462–468.
- Baker CJ, Onesti ST, Barth KN, Prestigiacomo CJ, Solomon RA. Hypothermic protection following middle cerebral artery occlusion in the rat. Surg Neurol 1991; 36:175–180.
- Ridenour TR, Warner DS, Todd MM, McAllister AC. Mild hypothermia reduces infarct size resulting from temporary but not permanent focal ischemia in rats. Stroke 1992; 23:733–738.
- Bernard SA, Jones BM, Horne MK. Clinical trial of induced hypothermia in comatose survivors of out-of-hospital cardiac arrest. Ann Emerg Med 1997; 30:146–153.
- Yanagawa Y, Ishihara S, Norio H, et al. Preliminary clinical outcome study of mild resuscitative hypothermia after out-of-hospital cardiopulmonary arrest. Resuscitation 1998; 39:61–66.
- Nolan JP, Morley PT, Vanden Hoek TL, et al; International Liaison Committee on Resuscitation. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation 2003; 108:118–121.
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002; 346:557–563.
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002; 346:549–556.
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2005; 112(suppl 24):IV1–IV203.
- Hartocollis A. “City Pushes Cooling Therapy for Cardiac Arrest”. New York Times, December4th, 2008,A1. http://www.nytimes.com/2008/12/04/nyregion/04cool.html. Accessed May 31, , 2011.
- Nichol G, Aufderheide TP, Eigel B, et al; American Heart Association Emergency Cardiovascular Care Committee. Regional systems of care for out-of-hospital cardiac arrest: A policy statement from the American Heart Association. Circulation 2010; 121:709–729.
- Field JM, Hazinski MF, Sayre MR, et al. Part 1: executive summary: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010; 122(suppl 3):S640–S56.
- Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation 2001; 51:275–281.
- Kim F, Olsufka M, Longstreth WT, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation 2007; 115:3064–3070.
- Hovdenes J, Laake JH, Aaberge L, Haugaa H, Bugge JF. Therapeutic hypothermia after out-of-hospital cardiac arrest: experiences with patients treated with percutaneous coronary intervention and cardiogenic shock. Acta Anaesthesiol Scand 2007; 51:137–142.
- Skulec R, Kovarnik T, Dostalova G, Kolar J, Linhart A. Induction of mild hypothermia in cardiac arrest survivors presenting with cardiogenic shock syndrome. Acta Anaesthesiol Scand 2008; 52:188–194.
- Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med 1997; 336:1629–1633.
- Dumas F, Cariou A, Manzo-Silberman S, et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ Cardiovasc Interv 2010; 3:200–207.
- Knafelj R, Radsel P, Ploj T, Noc M. Primary percutaneous coronary intervention and mild induced hypothermia in comatose survivors of ventricular fibrillation with ST-elevation acute myocardial infarction. Resuscitation 2007; 74:227–234.
- Wolfrum S, Pierau C, Radke PW, Schunkert H, Kurowski V. Mild therapeutic hypothermia in patients after out-of-hospital cardiac arrest due to acute ST-segment elevation myocardial infarction undergoing immediate percutaneous coronary intervention. Crit Care Med 2008; 36:1780–1786.
- O’Neill WW, on behalf of the COOL-MI Investigators. Cooling as an adjunct to primary PCI for myocardial infarction. Presented at Transcatheter Cardiovascular Therapeutics. Washington, DC; 2004.
- Grines CL, on behalf of the ICE-IT Investigators. Intravascular cooling adjunctive to percutaneous coronary intervention for acute myocardial infarction. Presented at Transcatheter Cardiovascular Therapeutics. Washington, DC; 2004.
- Weil MH, Gazmuri RJ. Hypothermia after cardiac arrest. Crit Care Med 1991; 19:315.
- Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation 2004; 109:2786–2791.
- Zhao D, Abella BS, Beiser DG, et al. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation 2008; 77:242–249.
- Jia X, Koenig MA, Shin HC, et al. Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation 2008; 76:431–442.
- Bernard SA, Smith K, Cameron P, et al; Rapid Infusion of Cold Hartmanns (RICH) Investigators. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation 2010; 122:737–742.
- Bruel C, Parienti JJ, Marie W, et al. Mild hypothermia during advanced life support: a preliminary study in out-of-hospital cardiac arrest. Crit Care 2008; 12:R31.
- Pichon N, Amiel JB, François B, Dugard A, Etchecopar C, Vignon P. Efficacy of and tolerance to mild induced hypothermia after out-of-hospital cardiac arrest using an endovascular cooling system. Crit Care 2007; 11:R71.
- Wolff B, Machill K, Schumacher D, Schulzki I, Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int J Cardiol 2009; 133:223–228.
- Heard KJ, Peberdy MA, Sayre MR, et al. A randomized controlled trial comparing the Arctic Sun to standard cooling for induction of hypothermia after cardiac arrest. Resuscitation 2010; 81:9–14.
- Castrén M, Nordberg P, Svensson L, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation 2010; 122:729–736.
- Insler SR, Sessler DI. Perioperative thermoregulation and temperature monitoring. Anesthesiol Clin 2006; 24:823–837.
- Weant KA, Martin JE, Humphries RL, Cook AM. Pharmacologic options for reducing the shivering response to therapeutic hypothermia. Pharmacotherapy 2010; 30:830–841.
- Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med 2010; 363:1256–1264.
A 30-year-old man experienced an episode of syncope while at work. He fully recovered consciousness within 2 minutes, but the emergency services team was called. As he was being loaded into the ambulance he again lost consciousness, and he was noted to be in ventricular fibrillation. Advanced cardiac life support was immediately started and continued for 50 minutes before a hemodynamically stable spontaneous rhythm was obtained.
On arrival at the emergency department of the local hospital, he was intubated to protect his airway, as he was comatose. A 12-lead electrocardiogram showed ST-segment elevations in leads V1, V2, and V3 and a wide QRS complex with an rSR′ pattern, consistent with right bundle branch block.
Mild therapeutic hypothermia was initiated by infusing intravenous saline solution chilled to 4°C and by applying cooling blankets, and he was transferred to our hospital on an emergency basis for further management. Here, hypothermia was maintained using an intravenous cooling catheter.
HYPOTHERMIA: BENEFICIAL, BUT SLOW TO BE ADOPTED
Mild therapeutic hypothermia is a recommended therapeutic intervention for out-of-hospital cardiac arrest due to ventricular fibrillation. Nonetheless, first-responders, emergency-room staff, and intensive-care teams have been slow to adopt and integrate it into a comprehensive postresuscitation strategy. This article summarizes the evidence supporting this therapy and how it is performed.
PROPOSED MECHANISMS OF BENEFIT
Mild therapeutic hypothermia is thought to protect against anoxic brain injury in survivors of cardiac arrest via several mechanisms:
- Decreasing neuronal metabolism in the early stage of ischemic injury
- Decreasing glucose and oxygen consumption by the brain,1 which reduces supply-demand mismatch
- Decreasing the release of excitatory amino acids (eg, glutamate) that normally trigger cytotoxic cascades in the intermediate phase of injury2
- Reducing the production of harmful reactive oxygen species3
- Maintaining cellular pH4
- Reducing cell death5
- Slowing the breakdown of the blood-brain barrier that worsens cerebral edema.6
CLINICAL DATA SUPPORTING HYPOTHERMIA
There has been an interest in therapeutic hypothermia for several decades. In the 1950s, it was used in small numbers of cases in a variety of cardiac arrest situations.7,8 Interest was rekindled in the mid-1990s after a number of animal studies suggested it might be beneficial in prolonged cerebral ischemia and anoxia,9,10 and reports of case-series described its use in adults with out-of-hospital cardiac arrest.11,12
In October 2002, the International Liaison Committee on Resuscitation (ILCOR), made up of executive members of several organizations including the American Heart Association, recommended that “unconscious adult patients with spontaneous circulation after out-of-hospital cardiac arrest” should be cooled to 32°C to 34°C [89.6°F–93.2°F] for 12 to 24 hours “when the initial rhythm was ventricular fibrillation.”13
Two large randomized trials
This position statement was based largely on the results of two randomized clinical trials published simultaneously earlier in 2002.14,15 These two trials were important not only because they were the largest randomized trials of this therapy to that point, but also because they used meaningful, prospectively defined clinical end points: all-cause mortality and degree of cognitive preservation as assessed using the Glasgow-Pittsburgh Cerebral Performance Category (CPC) scale.
Bernard et al14 performed a randomized trial in four centers in Australia, assigning 77 patients either to a goal temperature of 32°C to 34°C or to normothermia for 12 hours, with all other resuscitative measures being the same in both groups. The primary outcome measured was survival to hospital discharge with sufficient neurologic function to be discharged to home or to a rehabilitation facility, ie, a CPC score of 1 or 2.
In the hypothermia group, 21 (49%) of the 43 patients survived and had an outcome that was considered “good” (ie, they were discharged home or to a rehabilitation facility), compared with 9 (26%) of the 34 patients in the normothermia group (unadjusted odds ratio 2.65, 95% confidence interval [CI] 1.02–6.88, P = .046). Proportionally fewer patients in the hypothermia group died—22 (51%) of 43 vs 23 (68%) of 34; however, the difference was not statistically significant (P = .145).
The Hypothermia After Cardiac Arrest Study Group15 screened 3,551 European patients who suffered out-of-hospital cardiac arrest15; 275 patients were randomized to mild therapeutic hypothermia or normothermia for 24 hours. The primary outcome was the percentage of patients who had a CPC score of 1 or 2 (vs 3 to 5) at 6 months, and the secondary outcome was the rate of death at 6 months.
At 6 months, 75 (55%) of the 136 patients in the hypothermia group had a CPC score of 1 or 2, compared with 54 (39%) of the 137 patients in the normothermia group (P = .009). The rate of death was also lower with hypothermia: 55% vs 41% (P = .02).
In both trials, patients were included only if their cardiac arrest was witnessed, if their initial cardiac rhythm was ventricular fibrillation or pulseless ventricular tachycardia, if circulation spontaneously returned within 60 minutes with standard basic and advanced cardiac life support protocols, and if they were still comatose on arrival at the hospital. They were excluded if they were over age 75, if they had suffered a cerebrovascular accident at the time of cardiac arrest, or if the arrest was caused by trauma or drug overdose. In addition, the European trial excluded patients who suffered another cardiac arrest after the initial return of spontaneous circulation but before cooling was started.
The standard of care
In view of the available clinical data, the 2002 ILCOR guidelines and a 2005 statement from the American Heart Association advocated mild therapeutic hypothermia for survivors of out-of-hospital ventricular tachycardia or fibrillation.16 Subsequently, this therapy has become more widely practiced and accepted as the standard of care among critical-care providers.
Of note, some public health officials and local governments are strongly promoting this treatment for survivors of cardiac arrest in the community.17 More and more of these groups are mandating that these patients be transported only to hospitals that have therapeutic hypothermia protocols in place, bypassing those not equipped to provide this treatment.18
INDICATIONS, CONTRAINDICATIONS, AND GRAY AREAS
What are the indications and contraindications to the use of hypothermia after out-of-hospital cardiac arrest? What are some of the “gray areas”?
Indications. This treatment is indicated for comatose adults who have had a witnessed cardiac arrest, whose initial cardiac rhythm was ventricular fibrillation or pulseless ventricular tachycardia, and whose circulation spontaneously returned in less than 60 minutes with basic and advanced cardiac life support. This carries a class I recommendation, level of evidence B, and was recently reinforced in the 2010 update to the American Heart Association guidelines for cardiopulmonary resuscitation.19
Absolute contraindications include hemorrhagic stroke (which must be proved by computed tomography) and cardiac arrest due to trauma (Table 2). Other major contraindications are a Glasgow Coma Scale score of 8 or higher before the initiation of mild therapeutic hypothermia, cardiac arrest due to drug overdose, and preexisting hypothermia (< 34°C) when first-responders arrive.
Relative contraindications include baseline coagulopathy and severe hypotension (mean atrial pressure < 60 mm Hg) that is not correctable by fluid infusion, vasopressors, or invasive hemodynamic support.
Gray areas. There are not enough data to make a firm recommendation about whether to apply mild therapeutic hypothermia if a witnessed cardiac arrest with ventricular fibrillation or ventricular tachycardia occurs in the hospital, but data from out-of-hospital cardiac arrest patients appear applicable for hospitalized patients.
The data are also quite limited and equivocal on its use for out-of-hospital cardiac arrest in patients whose initial cardiac rhythm is pulseless electrical activity or asystole,20,21 likely because of the competing risk of comorbidities and the resultant lower baseline survival rate in these patients.
Consequently, for in-hospital postarrest patients with any initial rhythm and for out-of-hospital cardiac arrest patients with rhythms other than ventricular tachycardia or ventricular fibrillation, the 2010 guideline recommendation on the use of mild therapeutic hypothermia is less enthusiastic (class IIb, level of evidence B).19
There are also few data on the use of mild therapeutic hypothermia in post-arrest patients in circulatory shock requiring vasopressors or intra-aortic balloon counterpulsation, largely limited to case series and comparisons with historical controls.22,23 Further investigation is clearly needed in these areas. Until then, it should be considered at the physician’s and the team’s discretion, on a case-by-case basis.
HYPOTHERMIA IN CASES OF VENTRICULAR FIBRILLATION AND ACUTE CORONARY SYNDROME
The value of coronary angiography after out-of-hospital cardiac arrest was first highlighted by Spaulding et al,24 who performed it urgently in 84 consecutive survivors of out-of-hospital cardiac arrest, 36 of whom had ST-segment elevation myocardial infarction (STEMI). Angiography uncovered an acute coronary occlusion in 40 (48%) of the 84 patients.
In this series, ST-segment elevation was a strong predictor of acute coronary occlusion (odds ratio 4.3; 95% CI 1.6–2.0; P = .004). However, 9 patients without chest pain or ST elevation were also found to have an occluded infarct-related artery. Successful angioplasty was an independent predictor of survival, highlighting the importance of an angiographic definition in this population.
These findings were recently confirmed in the larger Parisian Region Out of Hospital Cardiac Arrest (PROCAT) registry in 435 patients who had no obvious extracardiac cause of arrest, for whom successful culprit coronary angioplasty was associated with survival.25
Angioplasty comes first, but neither treatment need be delayed
Efforts to induce hypothermia must not be allowed to delay the door-to-balloon time of post-arrest patients in the setting of STEMI. The top priority is establishing patency of the infarct-related artery with a goal of salvaging ischemic myocardium and obtaining mechanical and electrical stabilization.
Fortunately, mild therapeutic hypothermia does not necessarily delay emergency revascularization if hypothermia protocols are well established. In fact, induction of mild therapeutic hypothermia prior to or on arrival at the catheterization laboratory has been shown to be feasible and safe.26,27
We believe that all centers performing primary percutaneous coronary intervention for STEMI should have immediate access to and expertise in mild therapeutic hypothermia. Regional planning and integration of STEMI and out-of-hospital cardiac arrest networks will ensure that most patients with STEMI have access to this treatment when it is indicated.
Does hypothermia help the heart? Does it increase bleeding?
Researchers have been interested in therapeutic hypothermia as a means of reducing myocardial infarct size,28,29 but clinical trials have not shown a clear-cut benefit in this regard. However, these investigations have also added to the evidence that antiplatelet and anticoagulation therapy in patients undergoing mild therapeutic hypothermia does not result in a statistically significant excess of major bleeding events, which is a potential concern.
Of note, these studies were neither powered nor specifically designed to evaluate for major bleeding as an end point. Therefore, these complications should still be carefully monitored for.
IS THERE AN OPTIMAL TIME TO BEGIN MILD THERAPEUTIC HYPOTHERMIA?
Experimental data suggest that mild therapeutic hypothermia should be started as soon as possible after a comprehensive clinical evaluation indicates the patient is eligible.30–33 However, clinical data are not robustly in favor of starting it before the patient reaches the hospital rather than on hospital arrival.
In a recent randomized trial in 2,334 survivors of out-of-hospital cardiac arrest, outcomes were no better if hypothermia was started by paramedics than if it was started on arrival at the hospital (47.5% vs 52.6% discharged to home or rehabilitation; 95% CI 0.70–1.17; P = .43).34
Earlier data from smaller studies had suggested that prehospital initiation of hypothermia (for example, using chilled intravenous saline infusions) in carefully selected patients with out-of-hospital cardiac arrest was safe and showed a nonsignificant trend toward better outcomes.20,35
The randomized controlled trials that showed hypothermia to be beneficial used very slow cooling methods; consequently, it is reasonable to allow up to 6 hours from initial presentation to first-responders to start it. There are, however, no conclusive data in humans for or against starting it later than 6 hours after presentation. Most experts believe that its potential neurologic and mortality benefits are largely lost if it is delayed more than 6 hours.
The overall message from these data seems to be that, in patients who survive cardiac arrest outside the hospital with ventricular tachycardia or fibrillation, mild therapeutic hypothermia is effective and safe and should be started as soon as possible after arrival at the hospital.
METHODS FOR INDUCING AND MAINTAINING HYPOTHERMIA
Cooling the patient
To cool the patient and keep him or her cold, caregivers have used ice packs placed around the head, groin, and axillae; intravenous infusion of saline maintained at 4°C (39°F); and cooling-air blankets. More recently, thermal wraps and intravascular cooling catheters have been used.36–38 The newer methods are more effective in rapidly bringing patients to the target temperature of 32 to 34°C (usually within 3 or 4 hours) and keeping them within this range, and they auto-adjust their output on the basis of measured core temperature.
The Pre ROSC Intranasal Cooling Effectiveness (PRINCE) trial demonstrated the safety and efficacy of nasopharyngeal cooling using a perfluorocarbon aerosol given via a nasopharyngeal cannula in patients with out-of-hospital cardiac arrest.39
Monitoring the core temperature
The patient’s core temperature is most commonly monitored with a probe in the esophagus, bladder, rectum, or pulmonary artery.40
Of these, the bladder and rectum are considered “intermediate” monitoring sites, as their temperatures tend to lag behind the core temperature. Furthermore, the bladder temperature can be significantly altered by the flow of urine, which can vary considerably during the cooling and rewarming process.
Esophageal temperature monitoring is relatively noninvasive and tends to reliably and accurately reflect core temperature as long as the probe is placed far enough down (about 45 cm from the nose in an average adult) that it is not affected by proximity to the trachea.
Pulmonary artery catheters are considered the gold standard for core temperature monitoring, but they pose risks such as bloodstream infection and large-vessel damage. In practice, many patients admitted to the coronary intensive care unit after out-of-hospital cardiac arrest require pulmonary artery catheterization anyway for other indications, and in these situations it is the preferred method of monitoring the core temperature.
However, no approach is ideal in terms of measuring the temperature in the critical end organs. Rather, core temperature monitoring serves as a guide to help ensure consistent clinical practice in attaining and maintaining mild therapeutic hypothermia.
Preventing shivering
To achieve and maintain the goal temperature, the body’s natural response to a decrease in core temperature—shivering—must be watched for and eliminated. A number of drugs may be used for this purpose.41
Paralytic drugs are used to reduce shivering; nursing staff must be trained to monitor for signs of occult shivering (eg, jaw vibration) and adjust the dose of paralytic drug accordingly. Since the patients are paralyzed, they must also receive continuous intravenous sedation.
Other commonly used drugs that decrease the hypothalamic drive to shiver include buspirone (BuSpar), a serotonin 5HT-1A partial agonist, and meperidine (Demerol), an opiate agonist of kappa and mu receptors.
Rewarming after 24 hours
Rewarming is conventionally started after 24 hours of mild therapeutic hypothermia, at a rate no greater than 0.5°C (1°F) per hour.
Because sedation is used during the hypothermia period of 24 hours, a washout period for these medications is necessary, and the neurologic prognosis of cardiac arrest patients who undergo mild therapeutic hypothermia cannot be adequately assessed until 72 hours after rewarming.
ADVERSE EFFECTS OF MILD THERAPEUTIC HYPOTHERMIA
Some of these effects are predictable. Decreasing the body temperature causes potassium to shift into the cells, and this same potassium will leave the intracellular space during the rewarming phase. For this reason, aggressive potassium repletion for mild hypokalemia (potassium levels of 3.0–3.5 mmol/L) during mild therapeutic hypothermia can result in dangerous hyperkalemia during rewarming and should generally be avoided.
As another example, the enzymes involved in coagulation are less effective at lower temperatures. Thus, if it occurs, active bleeding requiring transfusion warrants consideration of stopping the hypothermia.
Adverse effects should be watched for (eg, by checking electrolyte levels frequently, monitoring blood glucose, continuous electroencephalographic monitoring during the cooling phase, and avoiding placement of intracardiac catheters once the goal temperature is reached) and addressed as they happen. However, in a recent review of this subject42 the balance of evidence continued to indicate that the benefit of this treatment exceeds its risks.
OUR PATIENT RECOVERS
After 24 hours of therapeutic hypothermia, our patient was gradually rewarmed to a normal temperature, and sedation and paralysis were discontinued.
Analysis of his prearrest and postarrest 12-lead electrocardiograms revealed a type I Brugada pattern (coved ST elevation and negative T waves in V1, V2, and V3, caused by abnormal repolarization due to inherited mutations in SCN5A). Cardiac catheterization revealed normal coronary arteries, and MRI revealed no evidence of arrhythmogenic right ventricular cardiomyopathy or other structural abnormalities.
In the next 72 hours the patient was successfully extubated, and he gradually returned to full neurologic function. Before he went home a few days later, a single-lead cardioverter-defibrillator was implanted to prevent sudden cardiac death. All of his first-degree relatives were encouraged to undergo genetic screening for SCN5A mutations. The patient is currently back to his previous high level of functioning as a marketing manager, husband, and father of two young children.
A 30-year-old man experienced an episode of syncope while at work. He fully recovered consciousness within 2 minutes, but the emergency services team was called. As he was being loaded into the ambulance he again lost consciousness, and he was noted to be in ventricular fibrillation. Advanced cardiac life support was immediately started and continued for 50 minutes before a hemodynamically stable spontaneous rhythm was obtained.
On arrival at the emergency department of the local hospital, he was intubated to protect his airway, as he was comatose. A 12-lead electrocardiogram showed ST-segment elevations in leads V1, V2, and V3 and a wide QRS complex with an rSR′ pattern, consistent with right bundle branch block.
Mild therapeutic hypothermia was initiated by infusing intravenous saline solution chilled to 4°C and by applying cooling blankets, and he was transferred to our hospital on an emergency basis for further management. Here, hypothermia was maintained using an intravenous cooling catheter.
HYPOTHERMIA: BENEFICIAL, BUT SLOW TO BE ADOPTED
Mild therapeutic hypothermia is a recommended therapeutic intervention for out-of-hospital cardiac arrest due to ventricular fibrillation. Nonetheless, first-responders, emergency-room staff, and intensive-care teams have been slow to adopt and integrate it into a comprehensive postresuscitation strategy. This article summarizes the evidence supporting this therapy and how it is performed.
PROPOSED MECHANISMS OF BENEFIT
Mild therapeutic hypothermia is thought to protect against anoxic brain injury in survivors of cardiac arrest via several mechanisms:
- Decreasing neuronal metabolism in the early stage of ischemic injury
- Decreasing glucose and oxygen consumption by the brain,1 which reduces supply-demand mismatch
- Decreasing the release of excitatory amino acids (eg, glutamate) that normally trigger cytotoxic cascades in the intermediate phase of injury2
- Reducing the production of harmful reactive oxygen species3
- Maintaining cellular pH4
- Reducing cell death5
- Slowing the breakdown of the blood-brain barrier that worsens cerebral edema.6
CLINICAL DATA SUPPORTING HYPOTHERMIA
There has been an interest in therapeutic hypothermia for several decades. In the 1950s, it was used in small numbers of cases in a variety of cardiac arrest situations.7,8 Interest was rekindled in the mid-1990s after a number of animal studies suggested it might be beneficial in prolonged cerebral ischemia and anoxia,9,10 and reports of case-series described its use in adults with out-of-hospital cardiac arrest.11,12
In October 2002, the International Liaison Committee on Resuscitation (ILCOR), made up of executive members of several organizations including the American Heart Association, recommended that “unconscious adult patients with spontaneous circulation after out-of-hospital cardiac arrest” should be cooled to 32°C to 34°C [89.6°F–93.2°F] for 12 to 24 hours “when the initial rhythm was ventricular fibrillation.”13
Two large randomized trials
This position statement was based largely on the results of two randomized clinical trials published simultaneously earlier in 2002.14,15 These two trials were important not only because they were the largest randomized trials of this therapy to that point, but also because they used meaningful, prospectively defined clinical end points: all-cause mortality and degree of cognitive preservation as assessed using the Glasgow-Pittsburgh Cerebral Performance Category (CPC) scale.
Bernard et al14 performed a randomized trial in four centers in Australia, assigning 77 patients either to a goal temperature of 32°C to 34°C or to normothermia for 12 hours, with all other resuscitative measures being the same in both groups. The primary outcome measured was survival to hospital discharge with sufficient neurologic function to be discharged to home or to a rehabilitation facility, ie, a CPC score of 1 or 2.
In the hypothermia group, 21 (49%) of the 43 patients survived and had an outcome that was considered “good” (ie, they were discharged home or to a rehabilitation facility), compared with 9 (26%) of the 34 patients in the normothermia group (unadjusted odds ratio 2.65, 95% confidence interval [CI] 1.02–6.88, P = .046). Proportionally fewer patients in the hypothermia group died—22 (51%) of 43 vs 23 (68%) of 34; however, the difference was not statistically significant (P = .145).
The Hypothermia After Cardiac Arrest Study Group15 screened 3,551 European patients who suffered out-of-hospital cardiac arrest15; 275 patients were randomized to mild therapeutic hypothermia or normothermia for 24 hours. The primary outcome was the percentage of patients who had a CPC score of 1 or 2 (vs 3 to 5) at 6 months, and the secondary outcome was the rate of death at 6 months.
At 6 months, 75 (55%) of the 136 patients in the hypothermia group had a CPC score of 1 or 2, compared with 54 (39%) of the 137 patients in the normothermia group (P = .009). The rate of death was also lower with hypothermia: 55% vs 41% (P = .02).
In both trials, patients were included only if their cardiac arrest was witnessed, if their initial cardiac rhythm was ventricular fibrillation or pulseless ventricular tachycardia, if circulation spontaneously returned within 60 minutes with standard basic and advanced cardiac life support protocols, and if they were still comatose on arrival at the hospital. They were excluded if they were over age 75, if they had suffered a cerebrovascular accident at the time of cardiac arrest, or if the arrest was caused by trauma or drug overdose. In addition, the European trial excluded patients who suffered another cardiac arrest after the initial return of spontaneous circulation but before cooling was started.
The standard of care
In view of the available clinical data, the 2002 ILCOR guidelines and a 2005 statement from the American Heart Association advocated mild therapeutic hypothermia for survivors of out-of-hospital ventricular tachycardia or fibrillation.16 Subsequently, this therapy has become more widely practiced and accepted as the standard of care among critical-care providers.
Of note, some public health officials and local governments are strongly promoting this treatment for survivors of cardiac arrest in the community.17 More and more of these groups are mandating that these patients be transported only to hospitals that have therapeutic hypothermia protocols in place, bypassing those not equipped to provide this treatment.18
INDICATIONS, CONTRAINDICATIONS, AND GRAY AREAS
What are the indications and contraindications to the use of hypothermia after out-of-hospital cardiac arrest? What are some of the “gray areas”?
Indications. This treatment is indicated for comatose adults who have had a witnessed cardiac arrest, whose initial cardiac rhythm was ventricular fibrillation or pulseless ventricular tachycardia, and whose circulation spontaneously returned in less than 60 minutes with basic and advanced cardiac life support. This carries a class I recommendation, level of evidence B, and was recently reinforced in the 2010 update to the American Heart Association guidelines for cardiopulmonary resuscitation.19
Absolute contraindications include hemorrhagic stroke (which must be proved by computed tomography) and cardiac arrest due to trauma (Table 2). Other major contraindications are a Glasgow Coma Scale score of 8 or higher before the initiation of mild therapeutic hypothermia, cardiac arrest due to drug overdose, and preexisting hypothermia (< 34°C) when first-responders arrive.
Relative contraindications include baseline coagulopathy and severe hypotension (mean atrial pressure < 60 mm Hg) that is not correctable by fluid infusion, vasopressors, or invasive hemodynamic support.
Gray areas. There are not enough data to make a firm recommendation about whether to apply mild therapeutic hypothermia if a witnessed cardiac arrest with ventricular fibrillation or ventricular tachycardia occurs in the hospital, but data from out-of-hospital cardiac arrest patients appear applicable for hospitalized patients.
The data are also quite limited and equivocal on its use for out-of-hospital cardiac arrest in patients whose initial cardiac rhythm is pulseless electrical activity or asystole,20,21 likely because of the competing risk of comorbidities and the resultant lower baseline survival rate in these patients.
Consequently, for in-hospital postarrest patients with any initial rhythm and for out-of-hospital cardiac arrest patients with rhythms other than ventricular tachycardia or ventricular fibrillation, the 2010 guideline recommendation on the use of mild therapeutic hypothermia is less enthusiastic (class IIb, level of evidence B).19
There are also few data on the use of mild therapeutic hypothermia in post-arrest patients in circulatory shock requiring vasopressors or intra-aortic balloon counterpulsation, largely limited to case series and comparisons with historical controls.22,23 Further investigation is clearly needed in these areas. Until then, it should be considered at the physician’s and the team’s discretion, on a case-by-case basis.
HYPOTHERMIA IN CASES OF VENTRICULAR FIBRILLATION AND ACUTE CORONARY SYNDROME
The value of coronary angiography after out-of-hospital cardiac arrest was first highlighted by Spaulding et al,24 who performed it urgently in 84 consecutive survivors of out-of-hospital cardiac arrest, 36 of whom had ST-segment elevation myocardial infarction (STEMI). Angiography uncovered an acute coronary occlusion in 40 (48%) of the 84 patients.
In this series, ST-segment elevation was a strong predictor of acute coronary occlusion (odds ratio 4.3; 95% CI 1.6–2.0; P = .004). However, 9 patients without chest pain or ST elevation were also found to have an occluded infarct-related artery. Successful angioplasty was an independent predictor of survival, highlighting the importance of an angiographic definition in this population.
These findings were recently confirmed in the larger Parisian Region Out of Hospital Cardiac Arrest (PROCAT) registry in 435 patients who had no obvious extracardiac cause of arrest, for whom successful culprit coronary angioplasty was associated with survival.25
Angioplasty comes first, but neither treatment need be delayed
Efforts to induce hypothermia must not be allowed to delay the door-to-balloon time of post-arrest patients in the setting of STEMI. The top priority is establishing patency of the infarct-related artery with a goal of salvaging ischemic myocardium and obtaining mechanical and electrical stabilization.
Fortunately, mild therapeutic hypothermia does not necessarily delay emergency revascularization if hypothermia protocols are well established. In fact, induction of mild therapeutic hypothermia prior to or on arrival at the catheterization laboratory has been shown to be feasible and safe.26,27
We believe that all centers performing primary percutaneous coronary intervention for STEMI should have immediate access to and expertise in mild therapeutic hypothermia. Regional planning and integration of STEMI and out-of-hospital cardiac arrest networks will ensure that most patients with STEMI have access to this treatment when it is indicated.
Does hypothermia help the heart? Does it increase bleeding?
Researchers have been interested in therapeutic hypothermia as a means of reducing myocardial infarct size,28,29 but clinical trials have not shown a clear-cut benefit in this regard. However, these investigations have also added to the evidence that antiplatelet and anticoagulation therapy in patients undergoing mild therapeutic hypothermia does not result in a statistically significant excess of major bleeding events, which is a potential concern.
Of note, these studies were neither powered nor specifically designed to evaluate for major bleeding as an end point. Therefore, these complications should still be carefully monitored for.
IS THERE AN OPTIMAL TIME TO BEGIN MILD THERAPEUTIC HYPOTHERMIA?
Experimental data suggest that mild therapeutic hypothermia should be started as soon as possible after a comprehensive clinical evaluation indicates the patient is eligible.30–33 However, clinical data are not robustly in favor of starting it before the patient reaches the hospital rather than on hospital arrival.
In a recent randomized trial in 2,334 survivors of out-of-hospital cardiac arrest, outcomes were no better if hypothermia was started by paramedics than if it was started on arrival at the hospital (47.5% vs 52.6% discharged to home or rehabilitation; 95% CI 0.70–1.17; P = .43).34
Earlier data from smaller studies had suggested that prehospital initiation of hypothermia (for example, using chilled intravenous saline infusions) in carefully selected patients with out-of-hospital cardiac arrest was safe and showed a nonsignificant trend toward better outcomes.20,35
The randomized controlled trials that showed hypothermia to be beneficial used very slow cooling methods; consequently, it is reasonable to allow up to 6 hours from initial presentation to first-responders to start it. There are, however, no conclusive data in humans for or against starting it later than 6 hours after presentation. Most experts believe that its potential neurologic and mortality benefits are largely lost if it is delayed more than 6 hours.
The overall message from these data seems to be that, in patients who survive cardiac arrest outside the hospital with ventricular tachycardia or fibrillation, mild therapeutic hypothermia is effective and safe and should be started as soon as possible after arrival at the hospital.
METHODS FOR INDUCING AND MAINTAINING HYPOTHERMIA
Cooling the patient
To cool the patient and keep him or her cold, caregivers have used ice packs placed around the head, groin, and axillae; intravenous infusion of saline maintained at 4°C (39°F); and cooling-air blankets. More recently, thermal wraps and intravascular cooling catheters have been used.36–38 The newer methods are more effective in rapidly bringing patients to the target temperature of 32 to 34°C (usually within 3 or 4 hours) and keeping them within this range, and they auto-adjust their output on the basis of measured core temperature.
The Pre ROSC Intranasal Cooling Effectiveness (PRINCE) trial demonstrated the safety and efficacy of nasopharyngeal cooling using a perfluorocarbon aerosol given via a nasopharyngeal cannula in patients with out-of-hospital cardiac arrest.39
Monitoring the core temperature
The patient’s core temperature is most commonly monitored with a probe in the esophagus, bladder, rectum, or pulmonary artery.40
Of these, the bladder and rectum are considered “intermediate” monitoring sites, as their temperatures tend to lag behind the core temperature. Furthermore, the bladder temperature can be significantly altered by the flow of urine, which can vary considerably during the cooling and rewarming process.
Esophageal temperature monitoring is relatively noninvasive and tends to reliably and accurately reflect core temperature as long as the probe is placed far enough down (about 45 cm from the nose in an average adult) that it is not affected by proximity to the trachea.
Pulmonary artery catheters are considered the gold standard for core temperature monitoring, but they pose risks such as bloodstream infection and large-vessel damage. In practice, many patients admitted to the coronary intensive care unit after out-of-hospital cardiac arrest require pulmonary artery catheterization anyway for other indications, and in these situations it is the preferred method of monitoring the core temperature.
However, no approach is ideal in terms of measuring the temperature in the critical end organs. Rather, core temperature monitoring serves as a guide to help ensure consistent clinical practice in attaining and maintaining mild therapeutic hypothermia.
Preventing shivering
To achieve and maintain the goal temperature, the body’s natural response to a decrease in core temperature—shivering—must be watched for and eliminated. A number of drugs may be used for this purpose.41
Paralytic drugs are used to reduce shivering; nursing staff must be trained to monitor for signs of occult shivering (eg, jaw vibration) and adjust the dose of paralytic drug accordingly. Since the patients are paralyzed, they must also receive continuous intravenous sedation.
Other commonly used drugs that decrease the hypothalamic drive to shiver include buspirone (BuSpar), a serotonin 5HT-1A partial agonist, and meperidine (Demerol), an opiate agonist of kappa and mu receptors.
Rewarming after 24 hours
Rewarming is conventionally started after 24 hours of mild therapeutic hypothermia, at a rate no greater than 0.5°C (1°F) per hour.
Because sedation is used during the hypothermia period of 24 hours, a washout period for these medications is necessary, and the neurologic prognosis of cardiac arrest patients who undergo mild therapeutic hypothermia cannot be adequately assessed until 72 hours after rewarming.
ADVERSE EFFECTS OF MILD THERAPEUTIC HYPOTHERMIA
Some of these effects are predictable. Decreasing the body temperature causes potassium to shift into the cells, and this same potassium will leave the intracellular space during the rewarming phase. For this reason, aggressive potassium repletion for mild hypokalemia (potassium levels of 3.0–3.5 mmol/L) during mild therapeutic hypothermia can result in dangerous hyperkalemia during rewarming and should generally be avoided.
As another example, the enzymes involved in coagulation are less effective at lower temperatures. Thus, if it occurs, active bleeding requiring transfusion warrants consideration of stopping the hypothermia.
Adverse effects should be watched for (eg, by checking electrolyte levels frequently, monitoring blood glucose, continuous electroencephalographic monitoring during the cooling phase, and avoiding placement of intracardiac catheters once the goal temperature is reached) and addressed as they happen. However, in a recent review of this subject42 the balance of evidence continued to indicate that the benefit of this treatment exceeds its risks.
OUR PATIENT RECOVERS
After 24 hours of therapeutic hypothermia, our patient was gradually rewarmed to a normal temperature, and sedation and paralysis were discontinued.
Analysis of his prearrest and postarrest 12-lead electrocardiograms revealed a type I Brugada pattern (coved ST elevation and negative T waves in V1, V2, and V3, caused by abnormal repolarization due to inherited mutations in SCN5A). Cardiac catheterization revealed normal coronary arteries, and MRI revealed no evidence of arrhythmogenic right ventricular cardiomyopathy or other structural abnormalities.
In the next 72 hours the patient was successfully extubated, and he gradually returned to full neurologic function. Before he went home a few days later, a single-lead cardioverter-defibrillator was implanted to prevent sudden cardiac death. All of his first-degree relatives were encouraged to undergo genetic screening for SCN5A mutations. The patient is currently back to his previous high level of functioning as a marketing manager, husband, and father of two young children.
- Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in mammalian central nervous system. J Cereb Blood Flow Metab 2003; 23:513–530.
- Nakashima K, Todd MM. Effects of hypothermia on the rate of excitatory amino acid release after ischemic depolarization. Stroke 1996; 27:913–918.
- Thoresen M, Satas S, Puka-Sundvall M, et al. Post-hypoxic hypothermia reduces cerebrocortical release of NO and excitotoxins. Neuroreport 1997; 8:3359–3362.
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009; 37(suppl 7):S186–S202.
- Yang D, Guo S, Zhang T, Li H. Hypothermia attenuates ischemia/reperfusion-induced endothelial cell apoptosis via alterations in apoptotic pathways and JNK signaling. FEBS Lett 2009; 583:2500–2506.
- Karibe H, Zarow GJ, Graham SH, Weinstein PR. Mild intraischemic hypothermia reduces postischemic hyperperfusion, delayed postischemic hypoperfusion, blood-brain barrier disruption, brain edema, and neuronal damage volume after temporary focal cerebral ischemia in rats. J Cereb Blood Flow Metab 1994; 14:620–627.
- Benson DW, Williams GR, Spencer FC, Yates AJ. The use of hypothermia after cardiac arrest. Anesth Analg 1959; 38:423–428.
- Williams GR, Spencer FC. The clinical use of hypothermia following cardiac arrest. Ann Surg 1958; 148:462–468.
- Baker CJ, Onesti ST, Barth KN, Prestigiacomo CJ, Solomon RA. Hypothermic protection following middle cerebral artery occlusion in the rat. Surg Neurol 1991; 36:175–180.
- Ridenour TR, Warner DS, Todd MM, McAllister AC. Mild hypothermia reduces infarct size resulting from temporary but not permanent focal ischemia in rats. Stroke 1992; 23:733–738.
- Bernard SA, Jones BM, Horne MK. Clinical trial of induced hypothermia in comatose survivors of out-of-hospital cardiac arrest. Ann Emerg Med 1997; 30:146–153.
- Yanagawa Y, Ishihara S, Norio H, et al. Preliminary clinical outcome study of mild resuscitative hypothermia after out-of-hospital cardiopulmonary arrest. Resuscitation 1998; 39:61–66.
- Nolan JP, Morley PT, Vanden Hoek TL, et al; International Liaison Committee on Resuscitation. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation 2003; 108:118–121.
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002; 346:557–563.
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002; 346:549–556.
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2005; 112(suppl 24):IV1–IV203.
- Hartocollis A. “City Pushes Cooling Therapy for Cardiac Arrest”. New York Times, December4th, 2008,A1. http://www.nytimes.com/2008/12/04/nyregion/04cool.html. Accessed May 31, , 2011.
- Nichol G, Aufderheide TP, Eigel B, et al; American Heart Association Emergency Cardiovascular Care Committee. Regional systems of care for out-of-hospital cardiac arrest: A policy statement from the American Heart Association. Circulation 2010; 121:709–729.
- Field JM, Hazinski MF, Sayre MR, et al. Part 1: executive summary: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010; 122(suppl 3):S640–S56.
- Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation 2001; 51:275–281.
- Kim F, Olsufka M, Longstreth WT, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation 2007; 115:3064–3070.
- Hovdenes J, Laake JH, Aaberge L, Haugaa H, Bugge JF. Therapeutic hypothermia after out-of-hospital cardiac arrest: experiences with patients treated with percutaneous coronary intervention and cardiogenic shock. Acta Anaesthesiol Scand 2007; 51:137–142.
- Skulec R, Kovarnik T, Dostalova G, Kolar J, Linhart A. Induction of mild hypothermia in cardiac arrest survivors presenting with cardiogenic shock syndrome. Acta Anaesthesiol Scand 2008; 52:188–194.
- Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med 1997; 336:1629–1633.
- Dumas F, Cariou A, Manzo-Silberman S, et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ Cardiovasc Interv 2010; 3:200–207.
- Knafelj R, Radsel P, Ploj T, Noc M. Primary percutaneous coronary intervention and mild induced hypothermia in comatose survivors of ventricular fibrillation with ST-elevation acute myocardial infarction. Resuscitation 2007; 74:227–234.
- Wolfrum S, Pierau C, Radke PW, Schunkert H, Kurowski V. Mild therapeutic hypothermia in patients after out-of-hospital cardiac arrest due to acute ST-segment elevation myocardial infarction undergoing immediate percutaneous coronary intervention. Crit Care Med 2008; 36:1780–1786.
- O’Neill WW, on behalf of the COOL-MI Investigators. Cooling as an adjunct to primary PCI for myocardial infarction. Presented at Transcatheter Cardiovascular Therapeutics. Washington, DC; 2004.
- Grines CL, on behalf of the ICE-IT Investigators. Intravascular cooling adjunctive to percutaneous coronary intervention for acute myocardial infarction. Presented at Transcatheter Cardiovascular Therapeutics. Washington, DC; 2004.
- Weil MH, Gazmuri RJ. Hypothermia after cardiac arrest. Crit Care Med 1991; 19:315.
- Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation 2004; 109:2786–2791.
- Zhao D, Abella BS, Beiser DG, et al. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation 2008; 77:242–249.
- Jia X, Koenig MA, Shin HC, et al. Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation 2008; 76:431–442.
- Bernard SA, Smith K, Cameron P, et al; Rapid Infusion of Cold Hartmanns (RICH) Investigators. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation 2010; 122:737–742.
- Bruel C, Parienti JJ, Marie W, et al. Mild hypothermia during advanced life support: a preliminary study in out-of-hospital cardiac arrest. Crit Care 2008; 12:R31.
- Pichon N, Amiel JB, François B, Dugard A, Etchecopar C, Vignon P. Efficacy of and tolerance to mild induced hypothermia after out-of-hospital cardiac arrest using an endovascular cooling system. Crit Care 2007; 11:R71.
- Wolff B, Machill K, Schumacher D, Schulzki I, Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int J Cardiol 2009; 133:223–228.
- Heard KJ, Peberdy MA, Sayre MR, et al. A randomized controlled trial comparing the Arctic Sun to standard cooling for induction of hypothermia after cardiac arrest. Resuscitation 2010; 81:9–14.
- Castrén M, Nordberg P, Svensson L, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation 2010; 122:729–736.
- Insler SR, Sessler DI. Perioperative thermoregulation and temperature monitoring. Anesthesiol Clin 2006; 24:823–837.
- Weant KA, Martin JE, Humphries RL, Cook AM. Pharmacologic options for reducing the shivering response to therapeutic hypothermia. Pharmacotherapy 2010; 30:830–841.
- Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med 2010; 363:1256–1264.
- Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in mammalian central nervous system. J Cereb Blood Flow Metab 2003; 23:513–530.
- Nakashima K, Todd MM. Effects of hypothermia on the rate of excitatory amino acid release after ischemic depolarization. Stroke 1996; 27:913–918.
- Thoresen M, Satas S, Puka-Sundvall M, et al. Post-hypoxic hypothermia reduces cerebrocortical release of NO and excitotoxins. Neuroreport 1997; 8:3359–3362.
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009; 37(suppl 7):S186–S202.
- Yang D, Guo S, Zhang T, Li H. Hypothermia attenuates ischemia/reperfusion-induced endothelial cell apoptosis via alterations in apoptotic pathways and JNK signaling. FEBS Lett 2009; 583:2500–2506.
- Karibe H, Zarow GJ, Graham SH, Weinstein PR. Mild intraischemic hypothermia reduces postischemic hyperperfusion, delayed postischemic hypoperfusion, blood-brain barrier disruption, brain edema, and neuronal damage volume after temporary focal cerebral ischemia in rats. J Cereb Blood Flow Metab 1994; 14:620–627.
- Benson DW, Williams GR, Spencer FC, Yates AJ. The use of hypothermia after cardiac arrest. Anesth Analg 1959; 38:423–428.
- Williams GR, Spencer FC. The clinical use of hypothermia following cardiac arrest. Ann Surg 1958; 148:462–468.
- Baker CJ, Onesti ST, Barth KN, Prestigiacomo CJ, Solomon RA. Hypothermic protection following middle cerebral artery occlusion in the rat. Surg Neurol 1991; 36:175–180.
- Ridenour TR, Warner DS, Todd MM, McAllister AC. Mild hypothermia reduces infarct size resulting from temporary but not permanent focal ischemia in rats. Stroke 1992; 23:733–738.
- Bernard SA, Jones BM, Horne MK. Clinical trial of induced hypothermia in comatose survivors of out-of-hospital cardiac arrest. Ann Emerg Med 1997; 30:146–153.
- Yanagawa Y, Ishihara S, Norio H, et al. Preliminary clinical outcome study of mild resuscitative hypothermia after out-of-hospital cardiopulmonary arrest. Resuscitation 1998; 39:61–66.
- Nolan JP, Morley PT, Vanden Hoek TL, et al; International Liaison Committee on Resuscitation. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation 2003; 108:118–121.
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002; 346:557–563.
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002; 346:549–556.
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2005; 112(suppl 24):IV1–IV203.
- Hartocollis A. “City Pushes Cooling Therapy for Cardiac Arrest”. New York Times, December4th, 2008,A1. http://www.nytimes.com/2008/12/04/nyregion/04cool.html. Accessed May 31, , 2011.
- Nichol G, Aufderheide TP, Eigel B, et al; American Heart Association Emergency Cardiovascular Care Committee. Regional systems of care for out-of-hospital cardiac arrest: A policy statement from the American Heart Association. Circulation 2010; 121:709–729.
- Field JM, Hazinski MF, Sayre MR, et al. Part 1: executive summary: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010; 122(suppl 3):S640–S56.
- Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation 2001; 51:275–281.
- Kim F, Olsufka M, Longstreth WT, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation 2007; 115:3064–3070.
- Hovdenes J, Laake JH, Aaberge L, Haugaa H, Bugge JF. Therapeutic hypothermia after out-of-hospital cardiac arrest: experiences with patients treated with percutaneous coronary intervention and cardiogenic shock. Acta Anaesthesiol Scand 2007; 51:137–142.
- Skulec R, Kovarnik T, Dostalova G, Kolar J, Linhart A. Induction of mild hypothermia in cardiac arrest survivors presenting with cardiogenic shock syndrome. Acta Anaesthesiol Scand 2008; 52:188–194.
- Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med 1997; 336:1629–1633.
- Dumas F, Cariou A, Manzo-Silberman S, et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circ Cardiovasc Interv 2010; 3:200–207.
- Knafelj R, Radsel P, Ploj T, Noc M. Primary percutaneous coronary intervention and mild induced hypothermia in comatose survivors of ventricular fibrillation with ST-elevation acute myocardial infarction. Resuscitation 2007; 74:227–234.
- Wolfrum S, Pierau C, Radke PW, Schunkert H, Kurowski V. Mild therapeutic hypothermia in patients after out-of-hospital cardiac arrest due to acute ST-segment elevation myocardial infarction undergoing immediate percutaneous coronary intervention. Crit Care Med 2008; 36:1780–1786.
- O’Neill WW, on behalf of the COOL-MI Investigators. Cooling as an adjunct to primary PCI for myocardial infarction. Presented at Transcatheter Cardiovascular Therapeutics. Washington, DC; 2004.
- Grines CL, on behalf of the ICE-IT Investigators. Intravascular cooling adjunctive to percutaneous coronary intervention for acute myocardial infarction. Presented at Transcatheter Cardiovascular Therapeutics. Washington, DC; 2004.
- Weil MH, Gazmuri RJ. Hypothermia after cardiac arrest. Crit Care Med 1991; 19:315.
- Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation 2004; 109:2786–2791.
- Zhao D, Abella BS, Beiser DG, et al. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation 2008; 77:242–249.
- Jia X, Koenig MA, Shin HC, et al. Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation 2008; 76:431–442.
- Bernard SA, Smith K, Cameron P, et al; Rapid Infusion of Cold Hartmanns (RICH) Investigators. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation 2010; 122:737–742.
- Bruel C, Parienti JJ, Marie W, et al. Mild hypothermia during advanced life support: a preliminary study in out-of-hospital cardiac arrest. Crit Care 2008; 12:R31.
- Pichon N, Amiel JB, François B, Dugard A, Etchecopar C, Vignon P. Efficacy of and tolerance to mild induced hypothermia after out-of-hospital cardiac arrest using an endovascular cooling system. Crit Care 2007; 11:R71.
- Wolff B, Machill K, Schumacher D, Schulzki I, Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int J Cardiol 2009; 133:223–228.
- Heard KJ, Peberdy MA, Sayre MR, et al. A randomized controlled trial comparing the Arctic Sun to standard cooling for induction of hypothermia after cardiac arrest. Resuscitation 2010; 81:9–14.
- Castrén M, Nordberg P, Svensson L, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation 2010; 122:729–736.
- Insler SR, Sessler DI. Perioperative thermoregulation and temperature monitoring. Anesthesiol Clin 2006; 24:823–837.
- Weant KA, Martin JE, Humphries RL, Cook AM. Pharmacologic options for reducing the shivering response to therapeutic hypothermia. Pharmacotherapy 2010; 30:830–841.
- Holzer M. Targeted temperature management for comatose survivors of cardiac arrest. N Engl J Med 2010; 363:1256–1264.
KEY POINTS
- This treatment is indicated for comatose adult patients who have had a witnessed cardiac arrest, whose initial cardiac rhythm is ventricular fibrillation or pulseless ventricular tachycardia, and who have return of spontaneous circulation with basic and advanced cardiac life support.
- Contraindications include hemorrhagic stroke, a Glasgow Coma Scale score of 8 or higher, cardiac arrest due to drug overdose, and preexisting hypothermia. Relative contraindications include baseline coagulopathy and severe hypotension (mean arterial pressure < 60 mm Hg) that is not correctable by fluid infusion, vasopressors, or invasive hemodynamic support.
- Adverse effects have included hypokalemia, bradyarrhythmia, ventricular tachycardia, hypotension, seizures, hyperglycemia, a transient decrease in the glomerular filtration rate, abnormal coagulation studies, and an increased incidence of pneumonia and sepsis.