User login
Chronic pain is a worldwide cause of impairment. According to data from the 2016 National Health Interview Survey, an estimated 50 million American adults suffer from chronic pain, with 19.6 million adults suffering from high-impact chronic pain.1 This phenomenon is particularly prevalent in the older population. More than 25% of adults aged 65 to 74 years reported that they were often in pain in the past 3 months compared with just 10% of those adults between the ages of 18 and 44 years.2
The economic burdens of chronic pain disorders are well known. In 2010, Gaskin and Richard found that chronic pain has far-reaching consequences for the US economy, ranging from direct health care costs to lost productivity. This study estimated additional health care costs at about $300 billion yearly and lost productivity at $300 billion, bringing total annual costs to about $600 billion. This expense is more than heart disease alone ($309 billion), and cancer and diabetes mellitus ($243 billion and $188 billion respectively) combined.3
Opioid medications are powerful and effective pain-reducing agents that are indicated for short-term acute pain or long-term in the management of chronic, severe cancer-related pain.4 Although efficacious, use of these medications carries with it the inherent risks of abuse, misuse, addiction, and overdose.5 Since 1999, opioid-related overdose deaths have been on the rise. The CDC estimated that > 15,000 deaths were attributable specifically to prescription opioids in 2015.6 The estimates had risen to > 17,000 deaths in 2017, with the number increasing since that time.7 Cumulatively, the CDC estimates that > 200,000 deaths in the US between 1999 and 2017 are attributed to prescription opioid overdose, clearly marking this trend as a growing nationwide epidemic.8
In 2016, Florence and colleagues estimated costs associated with opioid overdose to be just shy of $80 billion in 2013 dollars.9 In October 2017, the US Department of Health and Human Services declared the opioid epidemic a public health emergency and committed $900 million to combating the crisis.10
An abundance of data exist analyzing outpatient prescribing and its impacts on opioid dependence, particularly postoperatively. A study by Brummett and colleagues indicated that the incidence of new persistent opioid use in patients who underwent surgery was 5.9% to 6.5% and did not differ between major and minor surgical procedures. This study concluded that new opioid use could be considered one of the most common complications after elective surgery.11 Similarly, in 2017 Makary and colleagues found that surgeons tend to overprescribe pain medications after procedures; some prescribing as many as 50 to 60 tablets to control pain after simple procedures.12 This is in stark contrast to pain guideline recommendations of no more than 10 tablets for most standard operative procedures.13
Sun and colleagues conducted a retrospective analysis of health care claims data in more than 18 million opioid-naïve patients who did and did not undergo surgery. Seven of the 11 surgical procedures were associated with an increased risk of chronic opioid use. The highest incidence of chronic opioid use in the first postoperative year was for total hip arthroplasty (1.4%, OR 5.10; 95% CI, 1.29-1.53). The study found that the risk factors most associated with chronic opioid use after surgery were male sex, aged > 50 years, and preoperative history of drug abuse, alcohol abuse, or depression, along with benzodiazepine use or antidepressant use.14 In a 2018 cohort study that evaluated predictors associated with transitioning to incident chronic opioid therapy, 4 factors were identified. These included opioid duration of action (adjusted odds ratio [AOR], 12.28; 95% CI, 8.1-06-18.72), the parent opioid compound (eg, tramadol vs codeine; AOR, 7.26; 95% CI, 5.20-10.13), the presence of conditions that are very likely to cause chronic pain (AOR, 5.47; 95% CI, 3.89-7.68), and drug use disorders (AOR, 4.02; 95% CI, 2.53-6.40).15
While there has been research into outpatient risk factors and medical practices that may contribute to chronic opioid use, a relative paucity of data exists on the contribution of hospitalization and inpatient opioid use on patient outcomes. A 2014 Canadian study assessed the impact of opioid use in the intensive care unit (ICU) on opioid use after discharge.16 This study included more than 2,500 patients who were admitted to a Canadian ICU between 2005 and 2008, and then followed after discharge for 48 months to quantify chronic opioid use. Nonopioid users increased from 87.8% in the early post-ICU period to 95.6% at 48 months after discharge. Preadmission chronic opioid use and prolonged hospital length of stay (LOS) were found to be associated with an increased risk of chronic opioid use after discharge.16 To date, there are no published studies that analyze the incidence of opioid-naïve veterans who convert to chronic opioid use after receiving opioids during an acute hospitalization.
In this retrospective analysis, we analyze the incidence of chronic opioid use after administration of opioids in the ICU as well as a variety of risk factors that may influence conversion to chronic opioid use.
Methods
This analysis was a single center, retrospective chart review conducted for patients admitted between July 1, 2017 and December 31, 2017 at the US Department of Veterans Affairs (VA) Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas. MEDVAMC is a 538-bed academic\teaching hospital serving about 130,000 veterans in Southeast Texas. The hospital has 3 ICUs (medical, cardiovascular, and surgical) and 38 total ICU beds. The study was approved by the Baylor College of Medicine Institutional Review Board and MEDVAMC Research and Development Review Board. Informed consent was not required.
Inclusion criteria consisted of patients aged ≥ 18 years admitted to the ICU in the above-specified time frame, who were administered a continuous infusion of an opioid for at least 12 hours. Patients were excluded if they were not opioid naïve prior to admission, defined as receiving > 30 days of opioids in the prior 12 months. Patients who died during hospital admission, never received an opioid despite having an active order, were hospital-to-hospital transfers, or were still admitted at the time of data collection were excluded from the analysis.
All pertinent data were collected using the VA Computerized Patient Record System (CPRS) and the Critical Care Manager (Picis Clinical Solutions) ICU monitoring application. Critical Care Manager was used to track the time frame, duration, and amounts of opioid infusions administered in the ICU. Patient demographic and preadmission data, including date of birth, age, race, history of substance use/alcohol use disorder (defined as a previous diagnosis) and previous opioid prescriptions within the past year were recorded. For the inpatient admission, the ICU LOS, hospital LOS, primary admission diagnosis, type of opioid medication administered, and total duration and dose of opioid administered were collected. After discharge, opioid medication fill data at 3, 6, and 12 months were collected. This information included names of any outpatient opioids filled, dosage unit, quantity, day supply, and number of refills.
The primary outcome for this study was to determine the incidence of chronic opioid use at 3, 6, and 12 months after discharge, defined as the percentage of patients receiving outpatient opioid prescriptions at each time point. Analyses were conducted to observe the effect of age, race, history of substance use or history of alcohol use (International Classification of Diseases documented diagnosis, 9th edition), ICU type (medical, surgical, or cardiovascular), surgical/nonsurgical admission, ICU LOS, hospital LOS, total time, and amount of opioids administered during admission on risk of conversion to chronic opioid use.
Descriptive statistics were calculated to analyze the incidence of chronic opioid use. Univariate logistic regression analysis, including ORs, 95% CIs, and P values, was conducted to determine the effects of the risk factors noted earlier on chronic opioid use at each time point. A multivariate logistic regression model was performed to assess the effect of multiple independent variables on opioid use at 3, 6, and 12 months. Statistical analysis was performed using StataCorp Stata SE.
Results
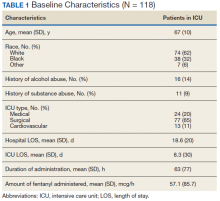
During the study period, 330 patients were admitted to the ICU. After applying inclusion/exclusion criteria, 118 patients were included in the final analysis. The most frequent reasons for exclusion from the study were patient death (n = 77), a past history of opioid use (n = 56), and not having received an opioid infusion for at least 12 hours (n = 68). The average age of the patients included was 67 years (Table 1). A total of 14% and 9% of patients, respectively, had a diagnosis of alcohol use disorder or substance use disorder recorded in CPRS. After admission, the most common location for these patients was the surgical ICU (65%). All patients were male. The average hospital LOS was 18.6 days , and the ICU LOS was 8.3 days. The average duration of administration for the opioid (fentanyl) infusion was 63 hours, and the average amount of fentanyl administered to each patient was 57.1 mcg/h.
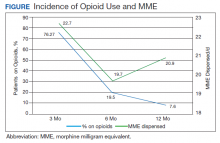
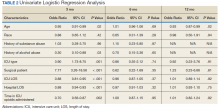
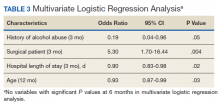
The incidence of opioid-naïve patients receiving opioids after discharge was 76.3% (n = 90) at 3 months, 19.5% (n = 23) at 6 months and 7.6% (n = 9) at 12 months (Figure). The daily morphine milligram equivalent (MME) of patients prescribed opioids at 3, 6, and 12 months was similar (3 months, 22.7; 6 months, 19.7; 12 months, 20.9). In the univariate regression analysis, several variables were found to be associated with converting to chronic opioid use. Prior history of alcohol use disorder (OR, 0.3; 95% CI, 0.10-0.88; P = .03), ICU type (OR, 3.9; 95% CI, 1.73-8.75; P = .001) and ICU LOS (OR, 0.88; 95% CI, 0.81-0.95; P = .01) had a statistically significant association on opioid use at 3 months. (Table 2). No variables evaluated had a statistically significant effect on chronic opioid use at 6 months, and only age (OR 0.93; 95% CI. 0.87-0.99; P = .02) was statistically significant at 12 months. In the multivariate logistic regression analysis, history of alcohol abuse, admission for surgery, and hospital LOS were significant at 3 months (Table 3).
Discussion
In this single-center analysis conducted at a VA academic hospital of opioid-naïve patients who were administered opioids in the ICU, the incidence of patients subsequently receiving outpatient opioid prescriptions at 12 months after discharge was 7.6%. There also was a decrease in the amount of opioids received by patients (daily MME) after discharge at 3, 6, and 12 months. This trend did not demonstrate the propensity for inpatient opioid use to convert opioid-naïve patients to chronic opioid users.
The most common outpatient opioids prescribed were hydrocodone/acetaminophen, morphine, and tramadol. Logistic regression showed few factors that correlated significantly with opioid use in the long-term (12 month) period. This finding is a deviation from the findings of Yaffe and colleagues who found hospital LOS to be one of the only predictors of long-term opioid use in their population (defined as use at 48 months).16 One important difference between our study and the Yaffe and colleagues study was that they evaluated all patients who were admitted to the ICU, regardless of the exposure to opioids during their inpatient stay. Consequently, this difference may have resulted in the differences in findings.
Strengths and Limitations
Location was a strength of our study, as this analysis was conducted at an integrated health care system that provides comprehensive inpatient and outpatient care. The VA uses a closed electronic health record, which allowed patients to be followed both in the inpatient and outpatient settings to determine which medications were prescribed at each time. In other health care systems this information would have been difficult to follow as patients often fill outpatient prescriptions at community pharmacies not affiliated with the treating hospital. However, any patient not using a VA prescriber for subsequent opioid prescriptions or patients who received opioids through other sources would not have had their continued opioid use captured. These data may be available in the states prescription monitoring program, but it was not available to query for research at this time.
This study also excluded chronic opioid users, which could have been another confounding factor to account for when analyzing the results. However, the primary objective of the study was to determine the impact of opioids prescribed in the ICU on converting previous opioid-naïve patients to chronic users. Finally, a multivariate logistic regression was incorporated to assess for factors that may predispose certain patients to convert to chronic opioid users. This analysis served to extend the applicability of our study by not only analyzing whether receiving opioids in the ICU contributed to chronic opioid use in the long-term, but also which populations may be at greatest risk. This information can be used in the future to target harm-reduction efforts toward high-risk hospitalized patients.
One limitation of this study was that it was conducted as a retrospective, single-center chart review in Houston, Texas. Because this was not a randomized controlled trial, it is difficult to imply any causation between exposure to opioids in the ICU and chronic use. In addition, because this study was conducted at a single site, the results may not be able to be generalized to other populations. VA populations tend to be elderly and predominantly male, as was reflected by the study population. These factors, along with regional variability in patient characteristics, may limit the generalizability of this study to older male patients located in Southeast Texas or other similar populations. Other limitations of this study also included the small sample size, limited period of follow-up obtained, and that other comorbidity information (pain scores during stay, use of nonopioid pain medications, past history of anxiety or depression, or other acute illnesses or surgeries that may have required opioid therapy during admission) was not collected.
This study was only able to review 118 patients for a follow-up duration of 1 year. In the Yaffe and colleagues study, more than 2,500 patients were followed over 4 years, which provided a more long-term overview of the clinical course of these patients and may be another reason for discrepant findings. However, this study did not actually assess the impact on administration of opioids on the development of chronic opioid use.16 Finally, the biggest limitation to this study may be the potential for confounding discharge prescriptions. After discharge, patients’ prescriptions were evaluated from discharge to 3 months, in between 3 and 6 months, and between 6 and 12 months for the presence of an opioid prescription. Due to this methodology, any opioid prescription a patient was discharged with was counted in the 3-month time point. Since many patients included in the study were admitted to the surgical ICU (65%), it was logical that they were discharged with opioids after their procedure. While including the immediate postdischarge prescription data was useful for evaluating the decrease in opioid use and incidence over time, it did cause the 3-month time point to appear overly inflated, potentially signaling that at 3 months after discharge many of these patients were still requiring opioid use.
The Society of Critical Care Medicine still recommends opioids as first-line therapy for non-neuropathic pain in the ICU setting.17 Additionally, postoperative pain can be difficult to manage in the surgical population and is often treated with opioids, though treatment with multimodal pain regimens is becoming more common.18 It is difficult to imagine that a finding that implicates opioid use in the hospital with conversion to chronic opioid use would prompt a cessation in the use of opioid in these settings, especially in the context of analgosedation related to mechanically ventilated patients. However, it would be plausible to use this knowledge to advocate for opioid-sparing therapies and consideration for weaning individuals at high risk for misuse after discharge from opioid-containing sedation or analgesia regimens in a timelier manner.
Though our findings did not show a clinically relevant increase in chronic opioid users, clinicians can still use this information to encourage targeted education and closer monitoring for those patients deemed as high risk at discharge to prevent unnecessary prolonged opioid use. By having more frequent follow-up in pain clinics, switching patients to nonopioid therapies after discharge, and ensuring high-risk patients are discharged with naloxone rescue kits, it would be possible to drastically reduce the number of potential overdoses for patients who previously required opioid therapy in the ICU.
Conclusion
After discharge, 7.6% of previously opioid-naïve patients who were treated with opioids in the ICU were still receiving prescriptions for opioids at 12 months. These findings did not suggest a clinically significant increase in the incidence of chronic opioid use after inpatient administration of opioids. However, these results prompt the need for larger, prospective, multicenter studies to evaluate the effect on hospitalization on converting to chronic opioid use and a deeper evaluation of other potential risk factors that may be present.
1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006.
2. Centers for Disease Control and Prevention. QuickStats: percentage of adults aged ≥18 years who often had pain in the past 3 months, by sex and age group. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6217a10.htm. Published May 3, 2103. Accessed February 7, 2020.
3. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715-724.
4. Jamison RN, Mao J. Opioid analgesics. Mayo Clin Proc. 2015;90(7):957-68.
5. DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: A Pathophysiologic Approach, 9e. McGraw Hill Professional; 2014.
6. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
7. Ahmad FB, Rossen LM, Spencer M, Warner M, Sutton P. Provisional drug overdose death counts. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Reviewed February 12, 2020. Accessed February 18, 2020.
8. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Revised January 2019. Accessed February 10, 2020.
9. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906.
10. HHS Acting Secretary declares public health emergency to address national opioid crisis [news release]. https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html. Published October 26, 2017. Accessed February 7, 2020.
11. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504.
12. Makary MA, Overton HN, Wang P. Overprescribing is major contributor to opioid crisis. BMJ. 2017;359:j4792.
13. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
14. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-93.
15. Thornton JD, Dwibedi N, Scott V, et al. Predictors of transitioning to incident chronic opioid therapy among working-age adults in the United States. Am Health Drug Benefits. 2018;11(1):12-21.
16. Yaffe PB, Green RS, Butler MB, Witter T. Is admission to the intensive care unit associated with chronic opioid use? A 4-year follow-up of intensive care unit survivors. J Intensive Care Med. 2017;327(7):429-435.
17. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873.
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157.
Chronic pain is a worldwide cause of impairment. According to data from the 2016 National Health Interview Survey, an estimated 50 million American adults suffer from chronic pain, with 19.6 million adults suffering from high-impact chronic pain.1 This phenomenon is particularly prevalent in the older population. More than 25% of adults aged 65 to 74 years reported that they were often in pain in the past 3 months compared with just 10% of those adults between the ages of 18 and 44 years.2
The economic burdens of chronic pain disorders are well known. In 2010, Gaskin and Richard found that chronic pain has far-reaching consequences for the US economy, ranging from direct health care costs to lost productivity. This study estimated additional health care costs at about $300 billion yearly and lost productivity at $300 billion, bringing total annual costs to about $600 billion. This expense is more than heart disease alone ($309 billion), and cancer and diabetes mellitus ($243 billion and $188 billion respectively) combined.3
Opioid medications are powerful and effective pain-reducing agents that are indicated for short-term acute pain or long-term in the management of chronic, severe cancer-related pain.4 Although efficacious, use of these medications carries with it the inherent risks of abuse, misuse, addiction, and overdose.5 Since 1999, opioid-related overdose deaths have been on the rise. The CDC estimated that > 15,000 deaths were attributable specifically to prescription opioids in 2015.6 The estimates had risen to > 17,000 deaths in 2017, with the number increasing since that time.7 Cumulatively, the CDC estimates that > 200,000 deaths in the US between 1999 and 2017 are attributed to prescription opioid overdose, clearly marking this trend as a growing nationwide epidemic.8
In 2016, Florence and colleagues estimated costs associated with opioid overdose to be just shy of $80 billion in 2013 dollars.9 In October 2017, the US Department of Health and Human Services declared the opioid epidemic a public health emergency and committed $900 million to combating the crisis.10
An abundance of data exist analyzing outpatient prescribing and its impacts on opioid dependence, particularly postoperatively. A study by Brummett and colleagues indicated that the incidence of new persistent opioid use in patients who underwent surgery was 5.9% to 6.5% and did not differ between major and minor surgical procedures. This study concluded that new opioid use could be considered one of the most common complications after elective surgery.11 Similarly, in 2017 Makary and colleagues found that surgeons tend to overprescribe pain medications after procedures; some prescribing as many as 50 to 60 tablets to control pain after simple procedures.12 This is in stark contrast to pain guideline recommendations of no more than 10 tablets for most standard operative procedures.13
Sun and colleagues conducted a retrospective analysis of health care claims data in more than 18 million opioid-naïve patients who did and did not undergo surgery. Seven of the 11 surgical procedures were associated with an increased risk of chronic opioid use. The highest incidence of chronic opioid use in the first postoperative year was for total hip arthroplasty (1.4%, OR 5.10; 95% CI, 1.29-1.53). The study found that the risk factors most associated with chronic opioid use after surgery were male sex, aged > 50 years, and preoperative history of drug abuse, alcohol abuse, or depression, along with benzodiazepine use or antidepressant use.14 In a 2018 cohort study that evaluated predictors associated with transitioning to incident chronic opioid therapy, 4 factors were identified. These included opioid duration of action (adjusted odds ratio [AOR], 12.28; 95% CI, 8.1-06-18.72), the parent opioid compound (eg, tramadol vs codeine; AOR, 7.26; 95% CI, 5.20-10.13), the presence of conditions that are very likely to cause chronic pain (AOR, 5.47; 95% CI, 3.89-7.68), and drug use disorders (AOR, 4.02; 95% CI, 2.53-6.40).15
While there has been research into outpatient risk factors and medical practices that may contribute to chronic opioid use, a relative paucity of data exists on the contribution of hospitalization and inpatient opioid use on patient outcomes. A 2014 Canadian study assessed the impact of opioid use in the intensive care unit (ICU) on opioid use after discharge.16 This study included more than 2,500 patients who were admitted to a Canadian ICU between 2005 and 2008, and then followed after discharge for 48 months to quantify chronic opioid use. Nonopioid users increased from 87.8% in the early post-ICU period to 95.6% at 48 months after discharge. Preadmission chronic opioid use and prolonged hospital length of stay (LOS) were found to be associated with an increased risk of chronic opioid use after discharge.16 To date, there are no published studies that analyze the incidence of opioid-naïve veterans who convert to chronic opioid use after receiving opioids during an acute hospitalization.
In this retrospective analysis, we analyze the incidence of chronic opioid use after administration of opioids in the ICU as well as a variety of risk factors that may influence conversion to chronic opioid use.
Methods
This analysis was a single center, retrospective chart review conducted for patients admitted between July 1, 2017 and December 31, 2017 at the US Department of Veterans Affairs (VA) Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas. MEDVAMC is a 538-bed academic\teaching hospital serving about 130,000 veterans in Southeast Texas. The hospital has 3 ICUs (medical, cardiovascular, and surgical) and 38 total ICU beds. The study was approved by the Baylor College of Medicine Institutional Review Board and MEDVAMC Research and Development Review Board. Informed consent was not required.
Inclusion criteria consisted of patients aged ≥ 18 years admitted to the ICU in the above-specified time frame, who were administered a continuous infusion of an opioid for at least 12 hours. Patients were excluded if they were not opioid naïve prior to admission, defined as receiving > 30 days of opioids in the prior 12 months. Patients who died during hospital admission, never received an opioid despite having an active order, were hospital-to-hospital transfers, or were still admitted at the time of data collection were excluded from the analysis.
All pertinent data were collected using the VA Computerized Patient Record System (CPRS) and the Critical Care Manager (Picis Clinical Solutions) ICU monitoring application. Critical Care Manager was used to track the time frame, duration, and amounts of opioid infusions administered in the ICU. Patient demographic and preadmission data, including date of birth, age, race, history of substance use/alcohol use disorder (defined as a previous diagnosis) and previous opioid prescriptions within the past year were recorded. For the inpatient admission, the ICU LOS, hospital LOS, primary admission diagnosis, type of opioid medication administered, and total duration and dose of opioid administered were collected. After discharge, opioid medication fill data at 3, 6, and 12 months were collected. This information included names of any outpatient opioids filled, dosage unit, quantity, day supply, and number of refills.
The primary outcome for this study was to determine the incidence of chronic opioid use at 3, 6, and 12 months after discharge, defined as the percentage of patients receiving outpatient opioid prescriptions at each time point. Analyses were conducted to observe the effect of age, race, history of substance use or history of alcohol use (International Classification of Diseases documented diagnosis, 9th edition), ICU type (medical, surgical, or cardiovascular), surgical/nonsurgical admission, ICU LOS, hospital LOS, total time, and amount of opioids administered during admission on risk of conversion to chronic opioid use.
Descriptive statistics were calculated to analyze the incidence of chronic opioid use. Univariate logistic regression analysis, including ORs, 95% CIs, and P values, was conducted to determine the effects of the risk factors noted earlier on chronic opioid use at each time point. A multivariate logistic regression model was performed to assess the effect of multiple independent variables on opioid use at 3, 6, and 12 months. Statistical analysis was performed using StataCorp Stata SE.
Results
During the study period, 330 patients were admitted to the ICU. After applying inclusion/exclusion criteria, 118 patients were included in the final analysis. The most frequent reasons for exclusion from the study were patient death (n = 77), a past history of opioid use (n = 56), and not having received an opioid infusion for at least 12 hours (n = 68). The average age of the patients included was 67 years (Table 1). A total of 14% and 9% of patients, respectively, had a diagnosis of alcohol use disorder or substance use disorder recorded in CPRS. After admission, the most common location for these patients was the surgical ICU (65%). All patients were male. The average hospital LOS was 18.6 days , and the ICU LOS was 8.3 days. The average duration of administration for the opioid (fentanyl) infusion was 63 hours, and the average amount of fentanyl administered to each patient was 57.1 mcg/h.
The incidence of opioid-naïve patients receiving opioids after discharge was 76.3% (n = 90) at 3 months, 19.5% (n = 23) at 6 months and 7.6% (n = 9) at 12 months (Figure). The daily morphine milligram equivalent (MME) of patients prescribed opioids at 3, 6, and 12 months was similar (3 months, 22.7; 6 months, 19.7; 12 months, 20.9). In the univariate regression analysis, several variables were found to be associated with converting to chronic opioid use. Prior history of alcohol use disorder (OR, 0.3; 95% CI, 0.10-0.88; P = .03), ICU type (OR, 3.9; 95% CI, 1.73-8.75; P = .001) and ICU LOS (OR, 0.88; 95% CI, 0.81-0.95; P = .01) had a statistically significant association on opioid use at 3 months. (Table 2). No variables evaluated had a statistically significant effect on chronic opioid use at 6 months, and only age (OR 0.93; 95% CI. 0.87-0.99; P = .02) was statistically significant at 12 months. In the multivariate logistic regression analysis, history of alcohol abuse, admission for surgery, and hospital LOS were significant at 3 months (Table 3).
Discussion
In this single-center analysis conducted at a VA academic hospital of opioid-naïve patients who were administered opioids in the ICU, the incidence of patients subsequently receiving outpatient opioid prescriptions at 12 months after discharge was 7.6%. There also was a decrease in the amount of opioids received by patients (daily MME) after discharge at 3, 6, and 12 months. This trend did not demonstrate the propensity for inpatient opioid use to convert opioid-naïve patients to chronic opioid users.
The most common outpatient opioids prescribed were hydrocodone/acetaminophen, morphine, and tramadol. Logistic regression showed few factors that correlated significantly with opioid use in the long-term (12 month) period. This finding is a deviation from the findings of Yaffe and colleagues who found hospital LOS to be one of the only predictors of long-term opioid use in their population (defined as use at 48 months).16 One important difference between our study and the Yaffe and colleagues study was that they evaluated all patients who were admitted to the ICU, regardless of the exposure to opioids during their inpatient stay. Consequently, this difference may have resulted in the differences in findings.
Strengths and Limitations
Location was a strength of our study, as this analysis was conducted at an integrated health care system that provides comprehensive inpatient and outpatient care. The VA uses a closed electronic health record, which allowed patients to be followed both in the inpatient and outpatient settings to determine which medications were prescribed at each time. In other health care systems this information would have been difficult to follow as patients often fill outpatient prescriptions at community pharmacies not affiliated with the treating hospital. However, any patient not using a VA prescriber for subsequent opioid prescriptions or patients who received opioids through other sources would not have had their continued opioid use captured. These data may be available in the states prescription monitoring program, but it was not available to query for research at this time.
This study also excluded chronic opioid users, which could have been another confounding factor to account for when analyzing the results. However, the primary objective of the study was to determine the impact of opioids prescribed in the ICU on converting previous opioid-naïve patients to chronic users. Finally, a multivariate logistic regression was incorporated to assess for factors that may predispose certain patients to convert to chronic opioid users. This analysis served to extend the applicability of our study by not only analyzing whether receiving opioids in the ICU contributed to chronic opioid use in the long-term, but also which populations may be at greatest risk. This information can be used in the future to target harm-reduction efforts toward high-risk hospitalized patients.
One limitation of this study was that it was conducted as a retrospective, single-center chart review in Houston, Texas. Because this was not a randomized controlled trial, it is difficult to imply any causation between exposure to opioids in the ICU and chronic use. In addition, because this study was conducted at a single site, the results may not be able to be generalized to other populations. VA populations tend to be elderly and predominantly male, as was reflected by the study population. These factors, along with regional variability in patient characteristics, may limit the generalizability of this study to older male patients located in Southeast Texas or other similar populations. Other limitations of this study also included the small sample size, limited period of follow-up obtained, and that other comorbidity information (pain scores during stay, use of nonopioid pain medications, past history of anxiety or depression, or other acute illnesses or surgeries that may have required opioid therapy during admission) was not collected.
This study was only able to review 118 patients for a follow-up duration of 1 year. In the Yaffe and colleagues study, more than 2,500 patients were followed over 4 years, which provided a more long-term overview of the clinical course of these patients and may be another reason for discrepant findings. However, this study did not actually assess the impact on administration of opioids on the development of chronic opioid use.16 Finally, the biggest limitation to this study may be the potential for confounding discharge prescriptions. After discharge, patients’ prescriptions were evaluated from discharge to 3 months, in between 3 and 6 months, and between 6 and 12 months for the presence of an opioid prescription. Due to this methodology, any opioid prescription a patient was discharged with was counted in the 3-month time point. Since many patients included in the study were admitted to the surgical ICU (65%), it was logical that they were discharged with opioids after their procedure. While including the immediate postdischarge prescription data was useful for evaluating the decrease in opioid use and incidence over time, it did cause the 3-month time point to appear overly inflated, potentially signaling that at 3 months after discharge many of these patients were still requiring opioid use.
The Society of Critical Care Medicine still recommends opioids as first-line therapy for non-neuropathic pain in the ICU setting.17 Additionally, postoperative pain can be difficult to manage in the surgical population and is often treated with opioids, though treatment with multimodal pain regimens is becoming more common.18 It is difficult to imagine that a finding that implicates opioid use in the hospital with conversion to chronic opioid use would prompt a cessation in the use of opioid in these settings, especially in the context of analgosedation related to mechanically ventilated patients. However, it would be plausible to use this knowledge to advocate for opioid-sparing therapies and consideration for weaning individuals at high risk for misuse after discharge from opioid-containing sedation or analgesia regimens in a timelier manner.
Though our findings did not show a clinically relevant increase in chronic opioid users, clinicians can still use this information to encourage targeted education and closer monitoring for those patients deemed as high risk at discharge to prevent unnecessary prolonged opioid use. By having more frequent follow-up in pain clinics, switching patients to nonopioid therapies after discharge, and ensuring high-risk patients are discharged with naloxone rescue kits, it would be possible to drastically reduce the number of potential overdoses for patients who previously required opioid therapy in the ICU.
Conclusion
After discharge, 7.6% of previously opioid-naïve patients who were treated with opioids in the ICU were still receiving prescriptions for opioids at 12 months. These findings did not suggest a clinically significant increase in the incidence of chronic opioid use after inpatient administration of opioids. However, these results prompt the need for larger, prospective, multicenter studies to evaluate the effect on hospitalization on converting to chronic opioid use and a deeper evaluation of other potential risk factors that may be present.
Chronic pain is a worldwide cause of impairment. According to data from the 2016 National Health Interview Survey, an estimated 50 million American adults suffer from chronic pain, with 19.6 million adults suffering from high-impact chronic pain.1 This phenomenon is particularly prevalent in the older population. More than 25% of adults aged 65 to 74 years reported that they were often in pain in the past 3 months compared with just 10% of those adults between the ages of 18 and 44 years.2
The economic burdens of chronic pain disorders are well known. In 2010, Gaskin and Richard found that chronic pain has far-reaching consequences for the US economy, ranging from direct health care costs to lost productivity. This study estimated additional health care costs at about $300 billion yearly and lost productivity at $300 billion, bringing total annual costs to about $600 billion. This expense is more than heart disease alone ($309 billion), and cancer and diabetes mellitus ($243 billion and $188 billion respectively) combined.3
Opioid medications are powerful and effective pain-reducing agents that are indicated for short-term acute pain or long-term in the management of chronic, severe cancer-related pain.4 Although efficacious, use of these medications carries with it the inherent risks of abuse, misuse, addiction, and overdose.5 Since 1999, opioid-related overdose deaths have been on the rise. The CDC estimated that > 15,000 deaths were attributable specifically to prescription opioids in 2015.6 The estimates had risen to > 17,000 deaths in 2017, with the number increasing since that time.7 Cumulatively, the CDC estimates that > 200,000 deaths in the US between 1999 and 2017 are attributed to prescription opioid overdose, clearly marking this trend as a growing nationwide epidemic.8
In 2016, Florence and colleagues estimated costs associated with opioid overdose to be just shy of $80 billion in 2013 dollars.9 In October 2017, the US Department of Health and Human Services declared the opioid epidemic a public health emergency and committed $900 million to combating the crisis.10
An abundance of data exist analyzing outpatient prescribing and its impacts on opioid dependence, particularly postoperatively. A study by Brummett and colleagues indicated that the incidence of new persistent opioid use in patients who underwent surgery was 5.9% to 6.5% and did not differ between major and minor surgical procedures. This study concluded that new opioid use could be considered one of the most common complications after elective surgery.11 Similarly, in 2017 Makary and colleagues found that surgeons tend to overprescribe pain medications after procedures; some prescribing as many as 50 to 60 tablets to control pain after simple procedures.12 This is in stark contrast to pain guideline recommendations of no more than 10 tablets for most standard operative procedures.13
Sun and colleagues conducted a retrospective analysis of health care claims data in more than 18 million opioid-naïve patients who did and did not undergo surgery. Seven of the 11 surgical procedures were associated with an increased risk of chronic opioid use. The highest incidence of chronic opioid use in the first postoperative year was for total hip arthroplasty (1.4%, OR 5.10; 95% CI, 1.29-1.53). The study found that the risk factors most associated with chronic opioid use after surgery were male sex, aged > 50 years, and preoperative history of drug abuse, alcohol abuse, or depression, along with benzodiazepine use or antidepressant use.14 In a 2018 cohort study that evaluated predictors associated with transitioning to incident chronic opioid therapy, 4 factors were identified. These included opioid duration of action (adjusted odds ratio [AOR], 12.28; 95% CI, 8.1-06-18.72), the parent opioid compound (eg, tramadol vs codeine; AOR, 7.26; 95% CI, 5.20-10.13), the presence of conditions that are very likely to cause chronic pain (AOR, 5.47; 95% CI, 3.89-7.68), and drug use disorders (AOR, 4.02; 95% CI, 2.53-6.40).15
While there has been research into outpatient risk factors and medical practices that may contribute to chronic opioid use, a relative paucity of data exists on the contribution of hospitalization and inpatient opioid use on patient outcomes. A 2014 Canadian study assessed the impact of opioid use in the intensive care unit (ICU) on opioid use after discharge.16 This study included more than 2,500 patients who were admitted to a Canadian ICU between 2005 and 2008, and then followed after discharge for 48 months to quantify chronic opioid use. Nonopioid users increased from 87.8% in the early post-ICU period to 95.6% at 48 months after discharge. Preadmission chronic opioid use and prolonged hospital length of stay (LOS) were found to be associated with an increased risk of chronic opioid use after discharge.16 To date, there are no published studies that analyze the incidence of opioid-naïve veterans who convert to chronic opioid use after receiving opioids during an acute hospitalization.
In this retrospective analysis, we analyze the incidence of chronic opioid use after administration of opioids in the ICU as well as a variety of risk factors that may influence conversion to chronic opioid use.
Methods
This analysis was a single center, retrospective chart review conducted for patients admitted between July 1, 2017 and December 31, 2017 at the US Department of Veterans Affairs (VA) Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas. MEDVAMC is a 538-bed academic\teaching hospital serving about 130,000 veterans in Southeast Texas. The hospital has 3 ICUs (medical, cardiovascular, and surgical) and 38 total ICU beds. The study was approved by the Baylor College of Medicine Institutional Review Board and MEDVAMC Research and Development Review Board. Informed consent was not required.
Inclusion criteria consisted of patients aged ≥ 18 years admitted to the ICU in the above-specified time frame, who were administered a continuous infusion of an opioid for at least 12 hours. Patients were excluded if they were not opioid naïve prior to admission, defined as receiving > 30 days of opioids in the prior 12 months. Patients who died during hospital admission, never received an opioid despite having an active order, were hospital-to-hospital transfers, or were still admitted at the time of data collection were excluded from the analysis.
All pertinent data were collected using the VA Computerized Patient Record System (CPRS) and the Critical Care Manager (Picis Clinical Solutions) ICU monitoring application. Critical Care Manager was used to track the time frame, duration, and amounts of opioid infusions administered in the ICU. Patient demographic and preadmission data, including date of birth, age, race, history of substance use/alcohol use disorder (defined as a previous diagnosis) and previous opioid prescriptions within the past year were recorded. For the inpatient admission, the ICU LOS, hospital LOS, primary admission diagnosis, type of opioid medication administered, and total duration and dose of opioid administered were collected. After discharge, opioid medication fill data at 3, 6, and 12 months were collected. This information included names of any outpatient opioids filled, dosage unit, quantity, day supply, and number of refills.
The primary outcome for this study was to determine the incidence of chronic opioid use at 3, 6, and 12 months after discharge, defined as the percentage of patients receiving outpatient opioid prescriptions at each time point. Analyses were conducted to observe the effect of age, race, history of substance use or history of alcohol use (International Classification of Diseases documented diagnosis, 9th edition), ICU type (medical, surgical, or cardiovascular), surgical/nonsurgical admission, ICU LOS, hospital LOS, total time, and amount of opioids administered during admission on risk of conversion to chronic opioid use.
Descriptive statistics were calculated to analyze the incidence of chronic opioid use. Univariate logistic regression analysis, including ORs, 95% CIs, and P values, was conducted to determine the effects of the risk factors noted earlier on chronic opioid use at each time point. A multivariate logistic regression model was performed to assess the effect of multiple independent variables on opioid use at 3, 6, and 12 months. Statistical analysis was performed using StataCorp Stata SE.
Results
During the study period, 330 patients were admitted to the ICU. After applying inclusion/exclusion criteria, 118 patients were included in the final analysis. The most frequent reasons for exclusion from the study were patient death (n = 77), a past history of opioid use (n = 56), and not having received an opioid infusion for at least 12 hours (n = 68). The average age of the patients included was 67 years (Table 1). A total of 14% and 9% of patients, respectively, had a diagnosis of alcohol use disorder or substance use disorder recorded in CPRS. After admission, the most common location for these patients was the surgical ICU (65%). All patients were male. The average hospital LOS was 18.6 days , and the ICU LOS was 8.3 days. The average duration of administration for the opioid (fentanyl) infusion was 63 hours, and the average amount of fentanyl administered to each patient was 57.1 mcg/h.
The incidence of opioid-naïve patients receiving opioids after discharge was 76.3% (n = 90) at 3 months, 19.5% (n = 23) at 6 months and 7.6% (n = 9) at 12 months (Figure). The daily morphine milligram equivalent (MME) of patients prescribed opioids at 3, 6, and 12 months was similar (3 months, 22.7; 6 months, 19.7; 12 months, 20.9). In the univariate regression analysis, several variables were found to be associated with converting to chronic opioid use. Prior history of alcohol use disorder (OR, 0.3; 95% CI, 0.10-0.88; P = .03), ICU type (OR, 3.9; 95% CI, 1.73-8.75; P = .001) and ICU LOS (OR, 0.88; 95% CI, 0.81-0.95; P = .01) had a statistically significant association on opioid use at 3 months. (Table 2). No variables evaluated had a statistically significant effect on chronic opioid use at 6 months, and only age (OR 0.93; 95% CI. 0.87-0.99; P = .02) was statistically significant at 12 months. In the multivariate logistic regression analysis, history of alcohol abuse, admission for surgery, and hospital LOS were significant at 3 months (Table 3).
Discussion
In this single-center analysis conducted at a VA academic hospital of opioid-naïve patients who were administered opioids in the ICU, the incidence of patients subsequently receiving outpatient opioid prescriptions at 12 months after discharge was 7.6%. There also was a decrease in the amount of opioids received by patients (daily MME) after discharge at 3, 6, and 12 months. This trend did not demonstrate the propensity for inpatient opioid use to convert opioid-naïve patients to chronic opioid users.
The most common outpatient opioids prescribed were hydrocodone/acetaminophen, morphine, and tramadol. Logistic regression showed few factors that correlated significantly with opioid use in the long-term (12 month) period. This finding is a deviation from the findings of Yaffe and colleagues who found hospital LOS to be one of the only predictors of long-term opioid use in their population (defined as use at 48 months).16 One important difference between our study and the Yaffe and colleagues study was that they evaluated all patients who were admitted to the ICU, regardless of the exposure to opioids during their inpatient stay. Consequently, this difference may have resulted in the differences in findings.
Strengths and Limitations
Location was a strength of our study, as this analysis was conducted at an integrated health care system that provides comprehensive inpatient and outpatient care. The VA uses a closed electronic health record, which allowed patients to be followed both in the inpatient and outpatient settings to determine which medications were prescribed at each time. In other health care systems this information would have been difficult to follow as patients often fill outpatient prescriptions at community pharmacies not affiliated with the treating hospital. However, any patient not using a VA prescriber for subsequent opioid prescriptions or patients who received opioids through other sources would not have had their continued opioid use captured. These data may be available in the states prescription monitoring program, but it was not available to query for research at this time.
This study also excluded chronic opioid users, which could have been another confounding factor to account for when analyzing the results. However, the primary objective of the study was to determine the impact of opioids prescribed in the ICU on converting previous opioid-naïve patients to chronic users. Finally, a multivariate logistic regression was incorporated to assess for factors that may predispose certain patients to convert to chronic opioid users. This analysis served to extend the applicability of our study by not only analyzing whether receiving opioids in the ICU contributed to chronic opioid use in the long-term, but also which populations may be at greatest risk. This information can be used in the future to target harm-reduction efforts toward high-risk hospitalized patients.
One limitation of this study was that it was conducted as a retrospective, single-center chart review in Houston, Texas. Because this was not a randomized controlled trial, it is difficult to imply any causation between exposure to opioids in the ICU and chronic use. In addition, because this study was conducted at a single site, the results may not be able to be generalized to other populations. VA populations tend to be elderly and predominantly male, as was reflected by the study population. These factors, along with regional variability in patient characteristics, may limit the generalizability of this study to older male patients located in Southeast Texas or other similar populations. Other limitations of this study also included the small sample size, limited period of follow-up obtained, and that other comorbidity information (pain scores during stay, use of nonopioid pain medications, past history of anxiety or depression, or other acute illnesses or surgeries that may have required opioid therapy during admission) was not collected.
This study was only able to review 118 patients for a follow-up duration of 1 year. In the Yaffe and colleagues study, more than 2,500 patients were followed over 4 years, which provided a more long-term overview of the clinical course of these patients and may be another reason for discrepant findings. However, this study did not actually assess the impact on administration of opioids on the development of chronic opioid use.16 Finally, the biggest limitation to this study may be the potential for confounding discharge prescriptions. After discharge, patients’ prescriptions were evaluated from discharge to 3 months, in between 3 and 6 months, and between 6 and 12 months for the presence of an opioid prescription. Due to this methodology, any opioid prescription a patient was discharged with was counted in the 3-month time point. Since many patients included in the study were admitted to the surgical ICU (65%), it was logical that they were discharged with opioids after their procedure. While including the immediate postdischarge prescription data was useful for evaluating the decrease in opioid use and incidence over time, it did cause the 3-month time point to appear overly inflated, potentially signaling that at 3 months after discharge many of these patients were still requiring opioid use.
The Society of Critical Care Medicine still recommends opioids as first-line therapy for non-neuropathic pain in the ICU setting.17 Additionally, postoperative pain can be difficult to manage in the surgical population and is often treated with opioids, though treatment with multimodal pain regimens is becoming more common.18 It is difficult to imagine that a finding that implicates opioid use in the hospital with conversion to chronic opioid use would prompt a cessation in the use of opioid in these settings, especially in the context of analgosedation related to mechanically ventilated patients. However, it would be plausible to use this knowledge to advocate for opioid-sparing therapies and consideration for weaning individuals at high risk for misuse after discharge from opioid-containing sedation or analgesia regimens in a timelier manner.
Though our findings did not show a clinically relevant increase in chronic opioid users, clinicians can still use this information to encourage targeted education and closer monitoring for those patients deemed as high risk at discharge to prevent unnecessary prolonged opioid use. By having more frequent follow-up in pain clinics, switching patients to nonopioid therapies after discharge, and ensuring high-risk patients are discharged with naloxone rescue kits, it would be possible to drastically reduce the number of potential overdoses for patients who previously required opioid therapy in the ICU.
Conclusion
After discharge, 7.6% of previously opioid-naïve patients who were treated with opioids in the ICU were still receiving prescriptions for opioids at 12 months. These findings did not suggest a clinically significant increase in the incidence of chronic opioid use after inpatient administration of opioids. However, these results prompt the need for larger, prospective, multicenter studies to evaluate the effect on hospitalization on converting to chronic opioid use and a deeper evaluation of other potential risk factors that may be present.
1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006.
2. Centers for Disease Control and Prevention. QuickStats: percentage of adults aged ≥18 years who often had pain in the past 3 months, by sex and age group. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6217a10.htm. Published May 3, 2103. Accessed February 7, 2020.
3. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715-724.
4. Jamison RN, Mao J. Opioid analgesics. Mayo Clin Proc. 2015;90(7):957-68.
5. DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: A Pathophysiologic Approach, 9e. McGraw Hill Professional; 2014.
6. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
7. Ahmad FB, Rossen LM, Spencer M, Warner M, Sutton P. Provisional drug overdose death counts. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Reviewed February 12, 2020. Accessed February 18, 2020.
8. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Revised January 2019. Accessed February 10, 2020.
9. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906.
10. HHS Acting Secretary declares public health emergency to address national opioid crisis [news release]. https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html. Published October 26, 2017. Accessed February 7, 2020.
11. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504.
12. Makary MA, Overton HN, Wang P. Overprescribing is major contributor to opioid crisis. BMJ. 2017;359:j4792.
13. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
14. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-93.
15. Thornton JD, Dwibedi N, Scott V, et al. Predictors of transitioning to incident chronic opioid therapy among working-age adults in the United States. Am Health Drug Benefits. 2018;11(1):12-21.
16. Yaffe PB, Green RS, Butler MB, Witter T. Is admission to the intensive care unit associated with chronic opioid use? A 4-year follow-up of intensive care unit survivors. J Intensive Care Med. 2017;327(7):429-435.
17. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873.
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157.
1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006.
2. Centers for Disease Control and Prevention. QuickStats: percentage of adults aged ≥18 years who often had pain in the past 3 months, by sex and age group. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6217a10.htm. Published May 3, 2103. Accessed February 7, 2020.
3. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715-724.
4. Jamison RN, Mao J. Opioid analgesics. Mayo Clin Proc. 2015;90(7):957-68.
5. DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: A Pathophysiologic Approach, 9e. McGraw Hill Professional; 2014.
6. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
7. Ahmad FB, Rossen LM, Spencer M, Warner M, Sutton P. Provisional drug overdose death counts. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Reviewed February 12, 2020. Accessed February 18, 2020.
8. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Revised January 2019. Accessed February 10, 2020.
9. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906.
10. HHS Acting Secretary declares public health emergency to address national opioid crisis [news release]. https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html. Published October 26, 2017. Accessed February 7, 2020.
11. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504.
12. Makary MA, Overton HN, Wang P. Overprescribing is major contributor to opioid crisis. BMJ. 2017;359:j4792.
13. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
14. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-93.
15. Thornton JD, Dwibedi N, Scott V, et al. Predictors of transitioning to incident chronic opioid therapy among working-age adults in the United States. Am Health Drug Benefits. 2018;11(1):12-21.
16. Yaffe PB, Green RS, Butler MB, Witter T. Is admission to the intensive care unit associated with chronic opioid use? A 4-year follow-up of intensive care unit survivors. J Intensive Care Med. 2017;327(7):429-435.
17. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873.
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157.