User login
Many fewer people will need to receive antibiotics as prophylaxis against infective endocarditis before undergoing dental procedures, according to new guidelines released by the American Heart Association.1 Now, the only patients to receive antibiotics will be those at highest risk, ie, those with a prosthetic heart valve, a history of endocarditis, certain forms of congenital heart disease, or valvulopathy after heart transplantation, and only before certain dental procedures.
Unfortunately, these guidelines are still based largely on expert opinion, with very little hard evidence to show that antibiotic therapy actually prevents infective endocarditis. Nevertheless, the new guidelines appear reasonable, and we believe they should be followed.
A RARE BUT LIFE-THREATENING INFECTION
Infective endocarditis is a rare but life-threatening infection, with an incidence in the United States of 10,000 to 20,000 new cases per year. Mortality rates for both native-valve endocarditis and prosthetic-valve endocarditis range from 20% to 30%.2,3 For the past half-century, antibiotic prophylaxis for dental procedures has been recommended for patients judged to be at risk of infective endocarditis, in hopes of preventing this dreaded infectious disease.
ENDOCARDIAL INJURY, THEN BACTERIAL SEEDING
A combination of events must occur to cause infective endocarditis. First, injury to the endocardial surface induces focal adherence of platelets and fibrin. Then, a bacteremic event seeds this aggregate with microorganisms, attracting more platelets and fibrin, allowing uninhibited microbial growth and the development of an inflammatory plaque or vegetation.
The magnitude and duration of bacteremia that produces this cascade of events is uncertain. Transient bacteremia occurs commonly, not only during procedures that cause trauma to mucosal surfaces or tissue but also with daily activities such as brushing teeth and chewing. The reported incidence of bacteremia during dental intervention ranges from 10% to 100%, and with daily brushing and flossing, from 20% to 68%.1
STAPHYLOCOCCI OVERTAKING VIRIDANS STREPTOCOCCI AS CAUSE
While historically the viridans group of streptococci has been responsible for the largest percentage of cases of both native-valve endocarditis and late-onset prosthetic-valve endocarditis, times have changed. In more recently reported series, Staphylococcus aureus appears more common, and unlikely to be susceptible to antibiotics recommended for dental prophylaxis. Other causative pathogens include coagulase-negative staphylococci, enterococci, gram-negative microorganisms, and fungi.
PREVIOUS GUIDELINES—1997
Previous American Heart Association guidelines4 separated patients into three risk categories for infective endocarditis. High-risk patients were those with prosthetic heart valves, a history of infective endocarditis, complex cyanotic congenital heart disease, or surgically constructed systemic pulmonary shunts. Moderate-risk patients had other congenital cardiac defects, hypertrophic cardiomyopathy, or acquired valvular heart disease including mitral valve prolapse with regurgitation. Negligible-risk patients—ie, most patients—included those with coronary artery bypass grafts, a permanent pacemaker, or mitral valve prolapse without regurgitation. Antibiotic prophylaxis was recommended only for patients in the high-risk and moderate-risk groups.
THOUGHTS AND CHALLENGES
Although prophylaxis has been a standard practice for years, its efficacy and cost-effectiveness have never been proven, owing to a lack of prospective randomized controlled trials. A sequential relationship between dental procedures and infective endocarditis can be demonstrated in only 4% to 7.5% of cases.5 Most cases of infective endocarditis are not preceded by dental procedures.
Furthermore, the data are limited and insufficient to substantiate the efficacy of antibiotics in preventing endocarditis in patients with high-risk cardiac conditions who undergo dental procedures.6 Failures have occurred even when the infecting microorganism was susceptible to the antibiotic given for prophylaxis. Since bacteremia occurs also during brushing and flossing of teeth, why give prophylaxis just for dental procedures? Moreover, the risks of causing adverse or anaphylactic reactions from antibiotics, as well as contributing to the nationwide antibiotic resistance problem, are issues not to be taken lightly.
Poor compliance with prophylaxis has been documented. Studies by Duval et al7 and others have shown that practitioners adhere to recommended dental prophylaxis programs only about 40% of the time, while only 22% of patients with predisposing cardiac conditions could recall taking their prescribed prophylactic antibiotics before an indicated procedure, as recommended.8
NEW GUIDELINES—2007
To address many of these concerns, the American Heart Association1 released extensively revised guidelines in 2007. They are more pragmatic, narrowly focused for a selected group of patients who have a greater lifetime risk of illness and death from infective endocarditis.
The experts who wrote the guidelines agreed that evidence remains poor about which dental procedures increase the risk of infective endocarditis and the efficacy of antibiotic prophylaxis to prevent its development. They stress the importance of good oral hygiene and prevention of dental disease and argue persuasively that this will have a greater impact on decreasing the lifetime risk of infective endocarditis than will antibiotic prophylaxis.
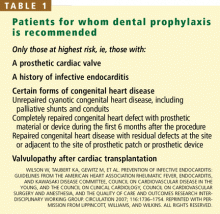
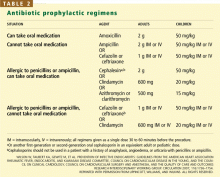
Prophylaxis is now recommended only for patients with a prosthetic heart valve, a history of infective endocarditis, certain forms of congenital heart disease, and valvulopathy after cardiac transplantation (Table 1), and only before procedures that involve manipulation of gingival tissue or the periapical region of teeth, or perforation of the oral mucosa. Excluded are routine dental cleaning and anesthetic injections through noninfected tissue, dental radiography, placement and adjustment of appliances, shedding of deciduous teeth, and bleeding from trauma to the lips.
CONTROVERSY WILL CONTINUE
The new guidelines for dental prophylaxis have been extensively revised and simplified. They are now focused only on patients who have a greater lifetime risk of illness and death from infective endocarditis. But what about patients who had previously been advised to take prophylaxis, such as those with mitral valve prolapse with regurgitation, who will not receive prophylaxis any more?
These guidelines will likely stir emotions, not only for practitioners who have strong desires to practice preventive medicine, but also for patients who have been taking prophylaxis in good faith per previous guidelines. They may feel abandoned. Unfortunately, funding for a prospective randomized clinical trial large enough to prove that antibiotic prophylaxis for dental procedures benefits patients is unlikely. That leaves us with the current recommendations, which are based on scientific evidence that currently exists and on expert opinion.
The intention of the guidelines is laudable. Of course, there will continue to be controversies with the new rules. Nevertheless, we believe they should be followed until there is more persuasive evidence to the contrary.
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007; 116:1736–1754.
- Hill EE, Herligers P, Claus P, Vanderschueren S, Herregods MC, Peetermans WE. Infective endocarditis: changing epidemiology and predictors of 6-month mortality: a prospective cohort study. Eur Heart J 2007; 28:196–203.
- Wang A, Athan E, Pappas PA, et al International Collaboration on Endocarditis-Prospective Cohort Study Investigators. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 2007; 297:1354–1361.
- Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1997; 277:1794–1801.
- Gendron R, Grenier D, Maheu-Robert LF. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes Infect 2000; 2:897–906.
- Strom BL, Abrutyn E, Berlin JA, et al. Dental and cardiac risk factors for infective endocarditis. A population-based, case-control study. Ann Intern Med 1998; 129:761–769.
- Duval X, Alla F, Hoen B, et al. Estimated risk of endocarditis in adults with predisposing cardiac conditions undergoing dental procedures with or without antibiotic prophylaxis. Clin Infect Dis 2006; 42:e102–e107.
- van der Meer JT, van Wijk W, Thompson J, Valkenburg HA, Michel MF. Awareness of need and actual use of prophylaxis: lack of patient compliance in the prevention of bacterial endocarditis. J Antimicrob Chemother 1992; 29:187–194.
Many fewer people will need to receive antibiotics as prophylaxis against infective endocarditis before undergoing dental procedures, according to new guidelines released by the American Heart Association.1 Now, the only patients to receive antibiotics will be those at highest risk, ie, those with a prosthetic heart valve, a history of endocarditis, certain forms of congenital heart disease, or valvulopathy after heart transplantation, and only before certain dental procedures.
Unfortunately, these guidelines are still based largely on expert opinion, with very little hard evidence to show that antibiotic therapy actually prevents infective endocarditis. Nevertheless, the new guidelines appear reasonable, and we believe they should be followed.
A RARE BUT LIFE-THREATENING INFECTION
Infective endocarditis is a rare but life-threatening infection, with an incidence in the United States of 10,000 to 20,000 new cases per year. Mortality rates for both native-valve endocarditis and prosthetic-valve endocarditis range from 20% to 30%.2,3 For the past half-century, antibiotic prophylaxis for dental procedures has been recommended for patients judged to be at risk of infective endocarditis, in hopes of preventing this dreaded infectious disease.
ENDOCARDIAL INJURY, THEN BACTERIAL SEEDING
A combination of events must occur to cause infective endocarditis. First, injury to the endocardial surface induces focal adherence of platelets and fibrin. Then, a bacteremic event seeds this aggregate with microorganisms, attracting more platelets and fibrin, allowing uninhibited microbial growth and the development of an inflammatory plaque or vegetation.
The magnitude and duration of bacteremia that produces this cascade of events is uncertain. Transient bacteremia occurs commonly, not only during procedures that cause trauma to mucosal surfaces or tissue but also with daily activities such as brushing teeth and chewing. The reported incidence of bacteremia during dental intervention ranges from 10% to 100%, and with daily brushing and flossing, from 20% to 68%.1
STAPHYLOCOCCI OVERTAKING VIRIDANS STREPTOCOCCI AS CAUSE
While historically the viridans group of streptococci has been responsible for the largest percentage of cases of both native-valve endocarditis and late-onset prosthetic-valve endocarditis, times have changed. In more recently reported series, Staphylococcus aureus appears more common, and unlikely to be susceptible to antibiotics recommended for dental prophylaxis. Other causative pathogens include coagulase-negative staphylococci, enterococci, gram-negative microorganisms, and fungi.
PREVIOUS GUIDELINES—1997
Previous American Heart Association guidelines4 separated patients into three risk categories for infective endocarditis. High-risk patients were those with prosthetic heart valves, a history of infective endocarditis, complex cyanotic congenital heart disease, or surgically constructed systemic pulmonary shunts. Moderate-risk patients had other congenital cardiac defects, hypertrophic cardiomyopathy, or acquired valvular heart disease including mitral valve prolapse with regurgitation. Negligible-risk patients—ie, most patients—included those with coronary artery bypass grafts, a permanent pacemaker, or mitral valve prolapse without regurgitation. Antibiotic prophylaxis was recommended only for patients in the high-risk and moderate-risk groups.
THOUGHTS AND CHALLENGES
Although prophylaxis has been a standard practice for years, its efficacy and cost-effectiveness have never been proven, owing to a lack of prospective randomized controlled trials. A sequential relationship between dental procedures and infective endocarditis can be demonstrated in only 4% to 7.5% of cases.5 Most cases of infective endocarditis are not preceded by dental procedures.
Furthermore, the data are limited and insufficient to substantiate the efficacy of antibiotics in preventing endocarditis in patients with high-risk cardiac conditions who undergo dental procedures.6 Failures have occurred even when the infecting microorganism was susceptible to the antibiotic given for prophylaxis. Since bacteremia occurs also during brushing and flossing of teeth, why give prophylaxis just for dental procedures? Moreover, the risks of causing adverse or anaphylactic reactions from antibiotics, as well as contributing to the nationwide antibiotic resistance problem, are issues not to be taken lightly.
Poor compliance with prophylaxis has been documented. Studies by Duval et al7 and others have shown that practitioners adhere to recommended dental prophylaxis programs only about 40% of the time, while only 22% of patients with predisposing cardiac conditions could recall taking their prescribed prophylactic antibiotics before an indicated procedure, as recommended.8
NEW GUIDELINES—2007
To address many of these concerns, the American Heart Association1 released extensively revised guidelines in 2007. They are more pragmatic, narrowly focused for a selected group of patients who have a greater lifetime risk of illness and death from infective endocarditis.
The experts who wrote the guidelines agreed that evidence remains poor about which dental procedures increase the risk of infective endocarditis and the efficacy of antibiotic prophylaxis to prevent its development. They stress the importance of good oral hygiene and prevention of dental disease and argue persuasively that this will have a greater impact on decreasing the lifetime risk of infective endocarditis than will antibiotic prophylaxis.
Prophylaxis is now recommended only for patients with a prosthetic heart valve, a history of infective endocarditis, certain forms of congenital heart disease, and valvulopathy after cardiac transplantation (Table 1), and only before procedures that involve manipulation of gingival tissue or the periapical region of teeth, or perforation of the oral mucosa. Excluded are routine dental cleaning and anesthetic injections through noninfected tissue, dental radiography, placement and adjustment of appliances, shedding of deciduous teeth, and bleeding from trauma to the lips.
CONTROVERSY WILL CONTINUE
The new guidelines for dental prophylaxis have been extensively revised and simplified. They are now focused only on patients who have a greater lifetime risk of illness and death from infective endocarditis. But what about patients who had previously been advised to take prophylaxis, such as those with mitral valve prolapse with regurgitation, who will not receive prophylaxis any more?
These guidelines will likely stir emotions, not only for practitioners who have strong desires to practice preventive medicine, but also for patients who have been taking prophylaxis in good faith per previous guidelines. They may feel abandoned. Unfortunately, funding for a prospective randomized clinical trial large enough to prove that antibiotic prophylaxis for dental procedures benefits patients is unlikely. That leaves us with the current recommendations, which are based on scientific evidence that currently exists and on expert opinion.
The intention of the guidelines is laudable. Of course, there will continue to be controversies with the new rules. Nevertheless, we believe they should be followed until there is more persuasive evidence to the contrary.
Many fewer people will need to receive antibiotics as prophylaxis against infective endocarditis before undergoing dental procedures, according to new guidelines released by the American Heart Association.1 Now, the only patients to receive antibiotics will be those at highest risk, ie, those with a prosthetic heart valve, a history of endocarditis, certain forms of congenital heart disease, or valvulopathy after heart transplantation, and only before certain dental procedures.
Unfortunately, these guidelines are still based largely on expert opinion, with very little hard evidence to show that antibiotic therapy actually prevents infective endocarditis. Nevertheless, the new guidelines appear reasonable, and we believe they should be followed.
A RARE BUT LIFE-THREATENING INFECTION
Infective endocarditis is a rare but life-threatening infection, with an incidence in the United States of 10,000 to 20,000 new cases per year. Mortality rates for both native-valve endocarditis and prosthetic-valve endocarditis range from 20% to 30%.2,3 For the past half-century, antibiotic prophylaxis for dental procedures has been recommended for patients judged to be at risk of infective endocarditis, in hopes of preventing this dreaded infectious disease.
ENDOCARDIAL INJURY, THEN BACTERIAL SEEDING
A combination of events must occur to cause infective endocarditis. First, injury to the endocardial surface induces focal adherence of platelets and fibrin. Then, a bacteremic event seeds this aggregate with microorganisms, attracting more platelets and fibrin, allowing uninhibited microbial growth and the development of an inflammatory plaque or vegetation.
The magnitude and duration of bacteremia that produces this cascade of events is uncertain. Transient bacteremia occurs commonly, not only during procedures that cause trauma to mucosal surfaces or tissue but also with daily activities such as brushing teeth and chewing. The reported incidence of bacteremia during dental intervention ranges from 10% to 100%, and with daily brushing and flossing, from 20% to 68%.1
STAPHYLOCOCCI OVERTAKING VIRIDANS STREPTOCOCCI AS CAUSE
While historically the viridans group of streptococci has been responsible for the largest percentage of cases of both native-valve endocarditis and late-onset prosthetic-valve endocarditis, times have changed. In more recently reported series, Staphylococcus aureus appears more common, and unlikely to be susceptible to antibiotics recommended for dental prophylaxis. Other causative pathogens include coagulase-negative staphylococci, enterococci, gram-negative microorganisms, and fungi.
PREVIOUS GUIDELINES—1997
Previous American Heart Association guidelines4 separated patients into three risk categories for infective endocarditis. High-risk patients were those with prosthetic heart valves, a history of infective endocarditis, complex cyanotic congenital heart disease, or surgically constructed systemic pulmonary shunts. Moderate-risk patients had other congenital cardiac defects, hypertrophic cardiomyopathy, or acquired valvular heart disease including mitral valve prolapse with regurgitation. Negligible-risk patients—ie, most patients—included those with coronary artery bypass grafts, a permanent pacemaker, or mitral valve prolapse without regurgitation. Antibiotic prophylaxis was recommended only for patients in the high-risk and moderate-risk groups.
THOUGHTS AND CHALLENGES
Although prophylaxis has been a standard practice for years, its efficacy and cost-effectiveness have never been proven, owing to a lack of prospective randomized controlled trials. A sequential relationship between dental procedures and infective endocarditis can be demonstrated in only 4% to 7.5% of cases.5 Most cases of infective endocarditis are not preceded by dental procedures.
Furthermore, the data are limited and insufficient to substantiate the efficacy of antibiotics in preventing endocarditis in patients with high-risk cardiac conditions who undergo dental procedures.6 Failures have occurred even when the infecting microorganism was susceptible to the antibiotic given for prophylaxis. Since bacteremia occurs also during brushing and flossing of teeth, why give prophylaxis just for dental procedures? Moreover, the risks of causing adverse or anaphylactic reactions from antibiotics, as well as contributing to the nationwide antibiotic resistance problem, are issues not to be taken lightly.
Poor compliance with prophylaxis has been documented. Studies by Duval et al7 and others have shown that practitioners adhere to recommended dental prophylaxis programs only about 40% of the time, while only 22% of patients with predisposing cardiac conditions could recall taking their prescribed prophylactic antibiotics before an indicated procedure, as recommended.8
NEW GUIDELINES—2007
To address many of these concerns, the American Heart Association1 released extensively revised guidelines in 2007. They are more pragmatic, narrowly focused for a selected group of patients who have a greater lifetime risk of illness and death from infective endocarditis.
The experts who wrote the guidelines agreed that evidence remains poor about which dental procedures increase the risk of infective endocarditis and the efficacy of antibiotic prophylaxis to prevent its development. They stress the importance of good oral hygiene and prevention of dental disease and argue persuasively that this will have a greater impact on decreasing the lifetime risk of infective endocarditis than will antibiotic prophylaxis.
Prophylaxis is now recommended only for patients with a prosthetic heart valve, a history of infective endocarditis, certain forms of congenital heart disease, and valvulopathy after cardiac transplantation (Table 1), and only before procedures that involve manipulation of gingival tissue or the periapical region of teeth, or perforation of the oral mucosa. Excluded are routine dental cleaning and anesthetic injections through noninfected tissue, dental radiography, placement and adjustment of appliances, shedding of deciduous teeth, and bleeding from trauma to the lips.
CONTROVERSY WILL CONTINUE
The new guidelines for dental prophylaxis have been extensively revised and simplified. They are now focused only on patients who have a greater lifetime risk of illness and death from infective endocarditis. But what about patients who had previously been advised to take prophylaxis, such as those with mitral valve prolapse with regurgitation, who will not receive prophylaxis any more?
These guidelines will likely stir emotions, not only for practitioners who have strong desires to practice preventive medicine, but also for patients who have been taking prophylaxis in good faith per previous guidelines. They may feel abandoned. Unfortunately, funding for a prospective randomized clinical trial large enough to prove that antibiotic prophylaxis for dental procedures benefits patients is unlikely. That leaves us with the current recommendations, which are based on scientific evidence that currently exists and on expert opinion.
The intention of the guidelines is laudable. Of course, there will continue to be controversies with the new rules. Nevertheless, we believe they should be followed until there is more persuasive evidence to the contrary.
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007; 116:1736–1754.
- Hill EE, Herligers P, Claus P, Vanderschueren S, Herregods MC, Peetermans WE. Infective endocarditis: changing epidemiology and predictors of 6-month mortality: a prospective cohort study. Eur Heart J 2007; 28:196–203.
- Wang A, Athan E, Pappas PA, et al International Collaboration on Endocarditis-Prospective Cohort Study Investigators. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 2007; 297:1354–1361.
- Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1997; 277:1794–1801.
- Gendron R, Grenier D, Maheu-Robert LF. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes Infect 2000; 2:897–906.
- Strom BL, Abrutyn E, Berlin JA, et al. Dental and cardiac risk factors for infective endocarditis. A population-based, case-control study. Ann Intern Med 1998; 129:761–769.
- Duval X, Alla F, Hoen B, et al. Estimated risk of endocarditis in adults with predisposing cardiac conditions undergoing dental procedures with or without antibiotic prophylaxis. Clin Infect Dis 2006; 42:e102–e107.
- van der Meer JT, van Wijk W, Thompson J, Valkenburg HA, Michel MF. Awareness of need and actual use of prophylaxis: lack of patient compliance in the prevention of bacterial endocarditis. J Antimicrob Chemother 1992; 29:187–194.
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007; 116:1736–1754.
- Hill EE, Herligers P, Claus P, Vanderschueren S, Herregods MC, Peetermans WE. Infective endocarditis: changing epidemiology and predictors of 6-month mortality: a prospective cohort study. Eur Heart J 2007; 28:196–203.
- Wang A, Athan E, Pappas PA, et al International Collaboration on Endocarditis-Prospective Cohort Study Investigators. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 2007; 297:1354–1361.
- Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA 1997; 277:1794–1801.
- Gendron R, Grenier D, Maheu-Robert LF. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes Infect 2000; 2:897–906.
- Strom BL, Abrutyn E, Berlin JA, et al. Dental and cardiac risk factors for infective endocarditis. A population-based, case-control study. Ann Intern Med 1998; 129:761–769.
- Duval X, Alla F, Hoen B, et al. Estimated risk of endocarditis in adults with predisposing cardiac conditions undergoing dental procedures with or without antibiotic prophylaxis. Clin Infect Dis 2006; 42:e102–e107.
- van der Meer JT, van Wijk W, Thompson J, Valkenburg HA, Michel MF. Awareness of need and actual use of prophylaxis: lack of patient compliance in the prevention of bacterial endocarditis. J Antimicrob Chemother 1992; 29:187–194.