User login
In treating hypertension, lower systolic pressure is better than higher—with caveats. This is the message of the Systolic Blood Pressure Intervention Trial (SPRINT),1 a large, federally funded study that was halted early when patients at high cardiovascular risk who were randomized to a goal systolic pressure of less than 120 mm Hg were found to have better outcomes, including lower rates of heart failure, death from cardiovascular causes, and death from any cause, than patients randomized to a goal of less than 140 mm Hg.
The caveats: the benefit came at a price of more adverse events. Also, the trial excluded patients who had diabetes mellitus or previous strokes, so it is uncertain if these subgroups would also benefit from intensive lowering of systolic pressure—and in earlier trials they did not.
This article reviews the trial design and protocol, summarizes the results, and briefly discusses the implications of these results.
BEFORE SPRINT
Hypertension is very common in adults in the United States, and is a risk factor for heart disease, stroke, heart failure, and kidney disease. The estimated prevalence of hypertension in the 2011–2014 National Health and Nutrition Examination Survey (NHANES) was 29%, and the prevalence increases with age (7.3% in those ages 18 to 39, 32.2% in those ages 40 to 59, and 64.9% in those ages 60 and older).2 Isolated systolic hypertension (ie, systolic blood pressure > 140 mm Hg with diastolic pressure < 90 mm Hg) is the most common form of hypertension after age 50.3
Clinical trials have provided substantial evidence that treating hypertension reduces the incidence of stroke, myocardial infarction, and heart failure.4,5 Although observational studies show a progressive and linear rise in cardiovascular risk as systolic blood pressure rises above 115 mm Hg,6 clinical trials in the general population have not documented benefits of lowering systolic pressure to this level.7–11 However, clinical trials that directly evaluated two different blood pressure goals in the general population showed benefit with achieving systolic blood pressure less than 150 mm Hg,7,9 with limited data on lower blood pressure targets.10–12
No benefit found in intensive systolic lowering in diabetes or after stroke
The Action to Control Cardiovascular Risk in Diabetes-Blood Pressure (ACCORD BP) trial13 in patients with type 2 diabetes found no benefit in lowering systolic pressure to less than 120 mm Hg compared with less than 140 mm Hg in terms of the trial’s primary composite cardiovascular outcome (ie, nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes). However, the intensively treated group in this trial did enjoy a benefit in terms of fewer stroke events.
The Secondary Prevention of Small Subcortical Strokes (SPS3) trial14 in patients with stroke found no significant benefit in lowering systolic pressure to less than 130 mm Hg compared with less than 150 mm Hg for overall risk of another stroke, but a significant benefit was noted in reduced risk of intracerebral hemorrhage.
Current guidelines, based on available evidence, advocate treatment to a systolic goal of less than 140 mm Hg in most patients, and recommend relaxing this goal to less than 150 mm Hg in the elderly.15,16
Given the uncertainty surrounding optimal systolic targets, SPRINT was designed to test the hypothesis that a goal of less than 120 mm Hg would reduce the risk of cardiovascular events more than the generally accepted systolic goal of less than 140 mm Hg.17 Patients with diabetes and stroke were excluded because a similar hypothesis was tested in the ACCORD BP and SPS3 trials, which included patients with these conditions.
SPRINT DESIGN
SPRINT was a randomized, controlled, open-label trial sponsored by the National Institutes of Health and conducted at 102 US sites.
Inclusion criteria. Participants had to be at least 50 years old, with systolic pressure of 130 to 180 mm Hg, and had to have at least one cardiovascular risk factor, eg:
- Clinical or subclinical cardiovascular disease (other than stroke)
- Chronic kidney disease, defined as estimated glomerular filtration rate (eGFR), calculated by the Modification of Diet in Renal Disease (MDRD) study equation, of 20 to less than 60 mL/min/1.73 m2
- Framingham risk score of 15% of more
- Age 75 or older.
Major exclusion criteria included:
- Diabetes
- Stroke
- Polycystic kidney disease
- Chronic kidney disease with an eGFR less than 20 mL/min/1.73 m2
- Proteinuria (excretion > 1 g/day).
Intensive vs standard treatment
Participants were randomized to receive intensive treatment (systolic goal < 120 mm Hg) or standard treatment (systolic goal < 140 mm Hg). Baseline antihypertensive medications were adjusted to achieve blood pressure goals based on randomization assignment.
Doses of medications were adjusted on the basis of an average of three seated office blood pressure measurements after a 5-minute period of rest, taken with an automated monitor (Omron Healthcare Model 907); the same monitor was used and the same protocol was followed at all participating sites. Blood pressure was also measured after standing for 1 minute to assess orthostatic change.
Lifestyle modifications were encouraged in both groups. There was no restriction on using any antihypertensive medication, and this was at the discretion of individual investigators. Thiazide-type diuretics were encouraged as first-line agents (with chlorthalidone encouraged as the primary thiazide-type diuretic).
Outcomes measured
The primary outcome was a composite of myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, stroke, acute decompensated heart failure, and cardiovascular mortality.
Secondary outcomes included individual components of the primary composite outcome, all-cause mortality, and the composite of primary outcome and all-cause mortality.
Renal outcomes were assessed as:
- Incident albuminuria (doubling of the urinary albumin-to-creatinine ratio from less than 10 mg/g to more than 10 mg/g)
- Composite of a 50% decrease in eGFR or development of end-stage renal disease requiring long-term dialysis or kidney transplantation (in those with baseline chronic kidney disease)
- A 30% decrease in eGFR (in those without chronic kidney disease).1,17
SPRINT also recruited participants to two nested substudies: SPRINT MIND and SPRINT MIND MRI, to study differences in cognitive outcomes and small-vessel ischemic disease between intensive treatment and standard treatment.
STUDY RESULTS
Older patients at risk, but without diabetes
Of 14,692 participants screened, 9,361 were enrolled in the study between 2010 and 2013. Baseline characteristics were comparable in both groups.
Demographics. The mean age of the participants was 67.9, and about 28% were 75 or older. About 36% were women, 58% white, 30% black, and 11% Hispanic.
Cardiovascular risk. The mean Framingham risk score was 20% (ie, they had a 20% risk of having a cardiovascular event within 10 years), and 61% of the participants had a risk score of at least 15%. Twenty percent already had cardiovascular disease.
Blood pressure. The average baseline blood pressure was 139.7/78.2 mm Hg. One-third of the participants had baseline systolic pressures of 132 mm Hg or less, another third had pressures in the range of 132 to 145, and the rest had 145 mm Hg or higher.
Renal function. The mean serum creatinine level was about 1.1 mg/dL. The mean eGFR was about 71 mL/min/1.73 m2 as calculated by the MDRD equation, and about 28% had eGFRs less than 60. The mean ratio of urinary albumin to creatinine was 44.1 mg/g in the intensive treatment group and 41.1 in the standard treatment group.
Other. The mean total cholesterol level was 190 mg/dL, fasting plasma glucose 99 mg/dL, and body mass index nearly 30 kg/m2.
Blood pressure during treatment
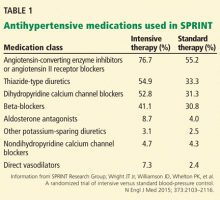
People in the intensive treatment group were taking a mean of 2.8 antihypertensive medications, and those in the standard treatment group were taking 1.8. Patients in the intensive group required greater use of all classes of medications to achieve goal systolic pressure (Table 1).
Study halted early due to efficacy
Throughout the 3.26 years of follow-up, the average difference in systolic pressure between the two groups was 13.1 mm Hg, with a mean systolic pressure of 121.5 mm Hg in the intensive treatment group and 134.6 mm Hg in the standard treatment group. The mean diastolic blood pressure was 68.7 mm Hg in the intensive treatment group and 76.3 mm Hg in the standard treatment group.
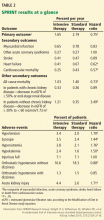
Although the study was planned to run for an average follow-up of 5 years, the National Heart, Lung, and Blood Institute terminated it early at a median of 3.26 years in view of lower rates of the primary outcome and of heart failure and death in the intensive treatment group (Table 2).
The effects on the primary outcome and mortality were consistent across the prespecified subgroups of age (< 75 vs ≥ 75), sex (female vs male), race (black vs nonblack), cardiovascular disease (presence or absence at baseline), prior chronic kidney disease (presence or absence at baseline), and across blood pressure tertiles (≤ 132 mm Hg, > 132 to < 145 mm Hg, ≥ 145 mm Hg).
Follow-up for assessment of cognitive outcomes (SPRINT MIND) and small-vessel ischemic disease (SPRINT MIND MRI) is ongoing.
WHAT DOES THIS MEAN?
SPRINT is the first large, adequately powered, randomized trial to demonstrate cardiovascular and mortality benefit from lowering the systolic blood pressure (goal < 120 mm Hg) in older patients at cardiovascular risk but without a history of diabetes mellitus or stroke.1
Most SPRINT patients had reasonably controlled blood pressure at baseline (the mean systolic pressure was 139.7 mm Hg, and two-thirds of participants had systolic pressure < 145 mm Hg). Of note, however, this trial excluded patients with systolic pressure higher than 180 mm Hg. There was excellent separation of systolic pressure between the two groups beginning at 1 year, which was consistent through the course of the trial.
The cardiovascular benefit in the intensive treatment group was predominantly driven by lower rates of heart failure (a 38% reduction in the intensive treatment group, P = .0002) and cardiovascular mortality (a 43% reduction in the intensive treatment group, P = .005), while there was no significant difference between the two groups in myocardial infarction or stroke. The beneficial effect on heart failure events is consistent with results from other trials including the Systolic Hypertension in the Elderly Program,7 Systolic Hypertension in Europe,8 and Hypertension in the Very Elderly Trial,9 all of which showed greatest risk reduction for heart failure events with systolic pressure-lowering (although to higher systolic levels than SPRINT).7–9 It is unclear why there was no beneficial effect on stroke events. The reduction in all-cause mortality in the intensive treatment group in SPRINT was greater than the reduction in cardiovascular deaths, which is also unexplained.
Although the study was terminated early due to efficacy (which introduces the possible bias that the estimated effect size will be too high), the number of primary end points reached was large (562 in the two groups combined), providing reassurance that the findings are valid. There was no blinding in the study (both participants and study investigators were aware of treatment assignment and study medications), but there was a structured assessment of outcomes and adverse events, with adjudication done by blinded reviewers.
SPRINT used an automated device for blood pressure measurement, which is known to reduce the “white coat” effect and correlates tightly with average daytime blood pressure done by ambulatory blood pressure monitoring.18 However, in clinical practice automated devices may not be available and a strict protocol for correct measurement may not be followed, with the possible result that blood pressure may be overestimated and overtreated.
What about diastolic pressure?
The trial, by design, focused on lowering systolic pressure (given the greater prevalence of isolated systolic hypertension with age), and the implications of lowering diastolic pressure are unclear. The issue of a J-shaped relationship between diastolic pressure and cardiovascular risk is debated in the literature: patients with a diastolic pressure of 60 to 65 mm Hg, especially those with existing coronary artery disease, may not tolerate aggressive blood pressure-lowering.19,20 Further analysis of this association (if any) from SPRINT will be helpful.
What about patients with diabetes?
Patients were excluded from SPRINT if they were under age 50, were at low cardiovascular risk, or had diabetes, raising the question of whether the results apply to these groups as well.
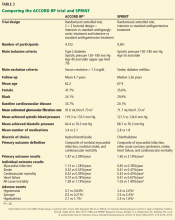
The question is particularly relevant in diabetes, as the ACCORD BP study, which used the same blood pressure targets as SPRINT, did not show a significant difference in the primary cardiovascular outcome between the intensive and standard treatments in patients with diabetes (Table 3).13 In ACCORD BP, the rate of the primary outcome was 12% lower in the intensive treatment group than in the standard treatment group, but the 95% confidence interval was –27% to +6%, so the finding was not statistically significant. However, the wide confidence interval does not exclude the possibility of a benefit that was comparable to that observed in SPRINT.
It has been speculated that ACCORD BP was underpowered to detect significant differences in the primary outcome.21 An analysis combining data from both trials indicated that effects on individual outcomes were generally consistent in both trials (with no significant heterogeneity noted).22 Also, the primary composite outcome in ACCORD did not include heart failure, which is particularly sensitive to blood pressure reduction.
Additionally, ACCORD BP had a 2 × 2 factorial design involving a simultaneous comparison of intensive vs standard glycemic control, which may have influenced the effects due to blood pressure. Indeed, a post hoc analysis showed that there was a significant 26% lower risk of the primary outcome in ACCORD BP patients who received intensive systolic pressure control plus standard glycemic control than in those receiving standard systolic control plus standard glycemic control.23
Are more adverse events an acceptable trade-off?
Adverse events, including acute kidney injury, were more frequent in the intensive therapy group in SPRINT.
Acute kidney injury was coded as an adverse event on the basis of this diagnosis being included in the hospital discharge summary (as a primary or main secondary diagnosis) and if considered by the safety officer to be one of the top three reasons for admission or continued hospitalization. Further analysis of renal events should be forthcoming.
People in the intensive treatment group, on average, needed one more medication than those in the standard treatment group. Some of the adverse events may be related to the antihypertensive medications taken (eg, electrolyte abnormalities such as hyponatremia and hypokalemia due to diuretic use), and others may be related to blood pressure-lowering (eg, acute kidney injury due to renal hypoperfusion).
At this point, the long-term effects of these adverse events, especially on kidney function, are not known. Patients enrolled in clinical trials tend to be healthier than patients seen in clinical practice; thus, the rate of adverse events reported in the trial may be lower than one would see in the real world.
Does lower systolic pressure protect or harm the kidneys?
SPRINT included patients with stage 3 and 4 chronic kidney disease (ie, with eGFR 20–50 mL/min/1.73 m2), but it was designed to assess cardiovascular outcomes, not the progression of chronic kidney disease. The trial excluded patients with diabetic nephropathy or high degrees of proteinuria.
Earlier randomized trials that focused on chronic kidney disease progression, including the MDRD24 and the African American Study of Kidney Disease and Hypertension,25 did not show benefit with more aggressive blood pressure-lowering (except in patients with higher degrees of proteinuria), and these trials were not powered to assess effects on cardiovascular outcomes.24,25
The Irbesartan Diabetic Nephropathy Trial,26,27 which was done in patients with overt diabetic nephropathy, showed that a progressively lower achieved systolic pressure down to 120 mm Hg predicted lower rates of heart failure, cardiovascular mortality, and renal events (although the trial target was ≤ 130/85 mm Hg and few participants achieved systolic pressure lower than 120 mm Hg).
IMPLICATIONS FOR MANAGEMENT
The recent estimates of hypertension prevalence and control from NHANES show that only about 53% of hypertensive adults have their blood pressure under control (defined as systolic pressure < 140 mm Hg and diastolic pressure < 90 mm Hg).2 Analysis of the NHANES 2007–2012 data showed that 16.7% or 8.2 million US adults with treated hypertension meet the eligibility criteria for SPRINT.28
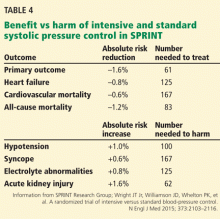
Although the SPRINT results support the notion that “lower is better,” the risks and benefits of intensive control will need to be balanced in individual patients. Table 4 shows the number needed to treat and number needed to harm in the trial.
More aggressive management of hypertension is challenging. The median systolic pressure achieved in the intensive group in SPRINT was just over 120 mm Hg, which implies that at least half of the participants in the intensive group did not achieve the goal of less than 120 mm Hg. While it may be reasonable to aim for systolic pressure of less than 120 or 125 mm Hg in patients who fit the SPRINT criteria and can tolerate intensive blood pressure lowering, it would be prudent to aim for a more conservative goal in elderly patients who are frail and at risk for falls, considering the higher incidence of specified adverse events in the intensive group.
Results of cognitive outcomes, as well as data related to quality of life, are still awaited. Long-term renal outcomes are also unclear.
As noted above, the question of generalizability of SPRINT results to patients with diabetes is open to debate. In our opinion, with currently available evidence, it is difficult to conclusively answer the question of whether a lower systolic target provides cardiovascular benefit in diabetes. It is also unclear whether similar beneficial results would be seen with intensive treatment in a population at low cardiovascular risk. The American Heart Association and the American College of Cardiology are in the process of formulating new hypertension guidelines, and evidence from SPRINT will inform any new recommendations.
As more medications will likely be needed for intensive systolic blood pressure control, side effects and tolerability of medications with polypharmacy and potential nonadherence with increasing complexity of medication regimens should be kept in mind. Lifestyle modifications will need to be emphasized and reinforced, with greater use of combination antihypertensive therapy.
The data from SPRINT indicate that lower systolic pressure is better, as long as untoward clinical events can be monitored and avoided or easily managed. Careful monitoring will likely entail more frequent clinic visits and more frequent assessment of renal function and electrolyte levels (participants in the intensive group in the trial were seen every month until goal was achieved). A team approach that includes pharmacists and nurse practitioners, along with optimal use of best practice algorithms and remote monitoring technology, will need to be implemented for efficient and effective care.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- Yoon SS, Fryar CD, Carroll MD. Hypertension prevalence and control among adults: United States, 2011–2014. NCHS data brief, no. 220. Hyattsville, MD: National Center for Health Statistics. 2015.
- Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 2001; 37:869–874.
- Neal B, MacMahon S, Chapman N; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration. Lancet 2000; 356:1955–1964.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Ogihara T, Saruta T, Rakugi H, et al; Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: Valsartan in Elderly Isolated Systolic Hypertension study. Hypertension 2010; 56:196–202.
- Liu L, Zhang Y, Liu G, Li W, Zhang X, Zanchetti A; FEVER Study Group. The Felodipine Event Reduction (FEVER) Study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens 2005; 23:2157–2172.
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382:507–515.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. Erratum in: JAMA. 2014; 311:1809.
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich) 2014; 16:14–26.
- Ambrosius WT, Sink KM, Foy CG, et al; SPRINT Study Research Group. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014; 11:532–546.
- Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Kaczorowski J. Conventional versus automated measurement of blood pressure in the office (CAMBO) trial. Fam Pract 2012; 29:376–382.
- Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006; 144:884–893.
- Boutitie F, Gueyffier F, Pocock S, Fagard R, Boissel JP; INDANA Project Steering Committee; INdividual Data ANalysis of Antihypertensive intervention. J-shaped relationship between blood pressure and mortality in hypertensive patients: new insights from a meta-analysis of individual-patient data. Ann Intern Med 2002; 136:438–448.
- Mancia G. Effects of intensive blood pressure control in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation 2010; 122:847–849.
- Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med 2015; 373:2175–2178.
- Margolis KL, O’Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care 2014; 37:1721–1728.
- Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease study. Ann Intern Med 1995; 123:754–762.
- Agodoa LY, Appel L, Bakris GL, et al; African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Berl T, Hunsicker LG, Lewis JB, et al; Collaborative Study Group. Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol 2005; 16:2170–2179.
- Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the Irbesartan Diabetic Nephropathy Trial: clinical implications and limitations. J Am Soc Nephrol 2005; 16:3027–3037.
- Bress AP, Tanner RM, Hess R, Colantonio LD, Shimbo D, Muntner P. Generalizability of results from the Systolic Blood Pressure Intervention Trial (SPRINT) to the US adult population. J Am Coll Cardiol 2015 Oct 31. doi: 10.1016/j.jacc.2015.10.037. Epub ahead of print.
In treating hypertension, lower systolic pressure is better than higher—with caveats. This is the message of the Systolic Blood Pressure Intervention Trial (SPRINT),1 a large, federally funded study that was halted early when patients at high cardiovascular risk who were randomized to a goal systolic pressure of less than 120 mm Hg were found to have better outcomes, including lower rates of heart failure, death from cardiovascular causes, and death from any cause, than patients randomized to a goal of less than 140 mm Hg.
The caveats: the benefit came at a price of more adverse events. Also, the trial excluded patients who had diabetes mellitus or previous strokes, so it is uncertain if these subgroups would also benefit from intensive lowering of systolic pressure—and in earlier trials they did not.
This article reviews the trial design and protocol, summarizes the results, and briefly discusses the implications of these results.
BEFORE SPRINT
Hypertension is very common in adults in the United States, and is a risk factor for heart disease, stroke, heart failure, and kidney disease. The estimated prevalence of hypertension in the 2011–2014 National Health and Nutrition Examination Survey (NHANES) was 29%, and the prevalence increases with age (7.3% in those ages 18 to 39, 32.2% in those ages 40 to 59, and 64.9% in those ages 60 and older).2 Isolated systolic hypertension (ie, systolic blood pressure > 140 mm Hg with diastolic pressure < 90 mm Hg) is the most common form of hypertension after age 50.3
Clinical trials have provided substantial evidence that treating hypertension reduces the incidence of stroke, myocardial infarction, and heart failure.4,5 Although observational studies show a progressive and linear rise in cardiovascular risk as systolic blood pressure rises above 115 mm Hg,6 clinical trials in the general population have not documented benefits of lowering systolic pressure to this level.7–11 However, clinical trials that directly evaluated two different blood pressure goals in the general population showed benefit with achieving systolic blood pressure less than 150 mm Hg,7,9 with limited data on lower blood pressure targets.10–12
No benefit found in intensive systolic lowering in diabetes or after stroke
The Action to Control Cardiovascular Risk in Diabetes-Blood Pressure (ACCORD BP) trial13 in patients with type 2 diabetes found no benefit in lowering systolic pressure to less than 120 mm Hg compared with less than 140 mm Hg in terms of the trial’s primary composite cardiovascular outcome (ie, nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes). However, the intensively treated group in this trial did enjoy a benefit in terms of fewer stroke events.
The Secondary Prevention of Small Subcortical Strokes (SPS3) trial14 in patients with stroke found no significant benefit in lowering systolic pressure to less than 130 mm Hg compared with less than 150 mm Hg for overall risk of another stroke, but a significant benefit was noted in reduced risk of intracerebral hemorrhage.
Current guidelines, based on available evidence, advocate treatment to a systolic goal of less than 140 mm Hg in most patients, and recommend relaxing this goal to less than 150 mm Hg in the elderly.15,16
Given the uncertainty surrounding optimal systolic targets, SPRINT was designed to test the hypothesis that a goal of less than 120 mm Hg would reduce the risk of cardiovascular events more than the generally accepted systolic goal of less than 140 mm Hg.17 Patients with diabetes and stroke were excluded because a similar hypothesis was tested in the ACCORD BP and SPS3 trials, which included patients with these conditions.
SPRINT DESIGN
SPRINT was a randomized, controlled, open-label trial sponsored by the National Institutes of Health and conducted at 102 US sites.
Inclusion criteria. Participants had to be at least 50 years old, with systolic pressure of 130 to 180 mm Hg, and had to have at least one cardiovascular risk factor, eg:
- Clinical or subclinical cardiovascular disease (other than stroke)
- Chronic kidney disease, defined as estimated glomerular filtration rate (eGFR), calculated by the Modification of Diet in Renal Disease (MDRD) study equation, of 20 to less than 60 mL/min/1.73 m2
- Framingham risk score of 15% of more
- Age 75 or older.
Major exclusion criteria included:
- Diabetes
- Stroke
- Polycystic kidney disease
- Chronic kidney disease with an eGFR less than 20 mL/min/1.73 m2
- Proteinuria (excretion > 1 g/day).
Intensive vs standard treatment
Participants were randomized to receive intensive treatment (systolic goal < 120 mm Hg) or standard treatment (systolic goal < 140 mm Hg). Baseline antihypertensive medications were adjusted to achieve blood pressure goals based on randomization assignment.
Doses of medications were adjusted on the basis of an average of three seated office blood pressure measurements after a 5-minute period of rest, taken with an automated monitor (Omron Healthcare Model 907); the same monitor was used and the same protocol was followed at all participating sites. Blood pressure was also measured after standing for 1 minute to assess orthostatic change.
Lifestyle modifications were encouraged in both groups. There was no restriction on using any antihypertensive medication, and this was at the discretion of individual investigators. Thiazide-type diuretics were encouraged as first-line agents (with chlorthalidone encouraged as the primary thiazide-type diuretic).
Outcomes measured
The primary outcome was a composite of myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, stroke, acute decompensated heart failure, and cardiovascular mortality.
Secondary outcomes included individual components of the primary composite outcome, all-cause mortality, and the composite of primary outcome and all-cause mortality.
Renal outcomes were assessed as:
- Incident albuminuria (doubling of the urinary albumin-to-creatinine ratio from less than 10 mg/g to more than 10 mg/g)
- Composite of a 50% decrease in eGFR or development of end-stage renal disease requiring long-term dialysis or kidney transplantation (in those with baseline chronic kidney disease)
- A 30% decrease in eGFR (in those without chronic kidney disease).1,17
SPRINT also recruited participants to two nested substudies: SPRINT MIND and SPRINT MIND MRI, to study differences in cognitive outcomes and small-vessel ischemic disease between intensive treatment and standard treatment.
STUDY RESULTS
Older patients at risk, but without diabetes
Of 14,692 participants screened, 9,361 were enrolled in the study between 2010 and 2013. Baseline characteristics were comparable in both groups.
Demographics. The mean age of the participants was 67.9, and about 28% were 75 or older. About 36% were women, 58% white, 30% black, and 11% Hispanic.
Cardiovascular risk. The mean Framingham risk score was 20% (ie, they had a 20% risk of having a cardiovascular event within 10 years), and 61% of the participants had a risk score of at least 15%. Twenty percent already had cardiovascular disease.
Blood pressure. The average baseline blood pressure was 139.7/78.2 mm Hg. One-third of the participants had baseline systolic pressures of 132 mm Hg or less, another third had pressures in the range of 132 to 145, and the rest had 145 mm Hg or higher.
Renal function. The mean serum creatinine level was about 1.1 mg/dL. The mean eGFR was about 71 mL/min/1.73 m2 as calculated by the MDRD equation, and about 28% had eGFRs less than 60. The mean ratio of urinary albumin to creatinine was 44.1 mg/g in the intensive treatment group and 41.1 in the standard treatment group.
Other. The mean total cholesterol level was 190 mg/dL, fasting plasma glucose 99 mg/dL, and body mass index nearly 30 kg/m2.
Blood pressure during treatment
People in the intensive treatment group were taking a mean of 2.8 antihypertensive medications, and those in the standard treatment group were taking 1.8. Patients in the intensive group required greater use of all classes of medications to achieve goal systolic pressure (Table 1).
Study halted early due to efficacy
Throughout the 3.26 years of follow-up, the average difference in systolic pressure between the two groups was 13.1 mm Hg, with a mean systolic pressure of 121.5 mm Hg in the intensive treatment group and 134.6 mm Hg in the standard treatment group. The mean diastolic blood pressure was 68.7 mm Hg in the intensive treatment group and 76.3 mm Hg in the standard treatment group.
Although the study was planned to run for an average follow-up of 5 years, the National Heart, Lung, and Blood Institute terminated it early at a median of 3.26 years in view of lower rates of the primary outcome and of heart failure and death in the intensive treatment group (Table 2).
The effects on the primary outcome and mortality were consistent across the prespecified subgroups of age (< 75 vs ≥ 75), sex (female vs male), race (black vs nonblack), cardiovascular disease (presence or absence at baseline), prior chronic kidney disease (presence or absence at baseline), and across blood pressure tertiles (≤ 132 mm Hg, > 132 to < 145 mm Hg, ≥ 145 mm Hg).
Follow-up for assessment of cognitive outcomes (SPRINT MIND) and small-vessel ischemic disease (SPRINT MIND MRI) is ongoing.
WHAT DOES THIS MEAN?
SPRINT is the first large, adequately powered, randomized trial to demonstrate cardiovascular and mortality benefit from lowering the systolic blood pressure (goal < 120 mm Hg) in older patients at cardiovascular risk but without a history of diabetes mellitus or stroke.1
Most SPRINT patients had reasonably controlled blood pressure at baseline (the mean systolic pressure was 139.7 mm Hg, and two-thirds of participants had systolic pressure < 145 mm Hg). Of note, however, this trial excluded patients with systolic pressure higher than 180 mm Hg. There was excellent separation of systolic pressure between the two groups beginning at 1 year, which was consistent through the course of the trial.
The cardiovascular benefit in the intensive treatment group was predominantly driven by lower rates of heart failure (a 38% reduction in the intensive treatment group, P = .0002) and cardiovascular mortality (a 43% reduction in the intensive treatment group, P = .005), while there was no significant difference between the two groups in myocardial infarction or stroke. The beneficial effect on heart failure events is consistent with results from other trials including the Systolic Hypertension in the Elderly Program,7 Systolic Hypertension in Europe,8 and Hypertension in the Very Elderly Trial,9 all of which showed greatest risk reduction for heart failure events with systolic pressure-lowering (although to higher systolic levels than SPRINT).7–9 It is unclear why there was no beneficial effect on stroke events. The reduction in all-cause mortality in the intensive treatment group in SPRINT was greater than the reduction in cardiovascular deaths, which is also unexplained.
Although the study was terminated early due to efficacy (which introduces the possible bias that the estimated effect size will be too high), the number of primary end points reached was large (562 in the two groups combined), providing reassurance that the findings are valid. There was no blinding in the study (both participants and study investigators were aware of treatment assignment and study medications), but there was a structured assessment of outcomes and adverse events, with adjudication done by blinded reviewers.
SPRINT used an automated device for blood pressure measurement, which is known to reduce the “white coat” effect and correlates tightly with average daytime blood pressure done by ambulatory blood pressure monitoring.18 However, in clinical practice automated devices may not be available and a strict protocol for correct measurement may not be followed, with the possible result that blood pressure may be overestimated and overtreated.
What about diastolic pressure?
The trial, by design, focused on lowering systolic pressure (given the greater prevalence of isolated systolic hypertension with age), and the implications of lowering diastolic pressure are unclear. The issue of a J-shaped relationship between diastolic pressure and cardiovascular risk is debated in the literature: patients with a diastolic pressure of 60 to 65 mm Hg, especially those with existing coronary artery disease, may not tolerate aggressive blood pressure-lowering.19,20 Further analysis of this association (if any) from SPRINT will be helpful.
What about patients with diabetes?
Patients were excluded from SPRINT if they were under age 50, were at low cardiovascular risk, or had diabetes, raising the question of whether the results apply to these groups as well.
The question is particularly relevant in diabetes, as the ACCORD BP study, which used the same blood pressure targets as SPRINT, did not show a significant difference in the primary cardiovascular outcome between the intensive and standard treatments in patients with diabetes (Table 3).13 In ACCORD BP, the rate of the primary outcome was 12% lower in the intensive treatment group than in the standard treatment group, but the 95% confidence interval was –27% to +6%, so the finding was not statistically significant. However, the wide confidence interval does not exclude the possibility of a benefit that was comparable to that observed in SPRINT.
It has been speculated that ACCORD BP was underpowered to detect significant differences in the primary outcome.21 An analysis combining data from both trials indicated that effects on individual outcomes were generally consistent in both trials (with no significant heterogeneity noted).22 Also, the primary composite outcome in ACCORD did not include heart failure, which is particularly sensitive to blood pressure reduction.
Additionally, ACCORD BP had a 2 × 2 factorial design involving a simultaneous comparison of intensive vs standard glycemic control, which may have influenced the effects due to blood pressure. Indeed, a post hoc analysis showed that there was a significant 26% lower risk of the primary outcome in ACCORD BP patients who received intensive systolic pressure control plus standard glycemic control than in those receiving standard systolic control plus standard glycemic control.23
Are more adverse events an acceptable trade-off?
Adverse events, including acute kidney injury, were more frequent in the intensive therapy group in SPRINT.
Acute kidney injury was coded as an adverse event on the basis of this diagnosis being included in the hospital discharge summary (as a primary or main secondary diagnosis) and if considered by the safety officer to be one of the top three reasons for admission or continued hospitalization. Further analysis of renal events should be forthcoming.
People in the intensive treatment group, on average, needed one more medication than those in the standard treatment group. Some of the adverse events may be related to the antihypertensive medications taken (eg, electrolyte abnormalities such as hyponatremia and hypokalemia due to diuretic use), and others may be related to blood pressure-lowering (eg, acute kidney injury due to renal hypoperfusion).
At this point, the long-term effects of these adverse events, especially on kidney function, are not known. Patients enrolled in clinical trials tend to be healthier than patients seen in clinical practice; thus, the rate of adverse events reported in the trial may be lower than one would see in the real world.
Does lower systolic pressure protect or harm the kidneys?
SPRINT included patients with stage 3 and 4 chronic kidney disease (ie, with eGFR 20–50 mL/min/1.73 m2), but it was designed to assess cardiovascular outcomes, not the progression of chronic kidney disease. The trial excluded patients with diabetic nephropathy or high degrees of proteinuria.
Earlier randomized trials that focused on chronic kidney disease progression, including the MDRD24 and the African American Study of Kidney Disease and Hypertension,25 did not show benefit with more aggressive blood pressure-lowering (except in patients with higher degrees of proteinuria), and these trials were not powered to assess effects on cardiovascular outcomes.24,25
The Irbesartan Diabetic Nephropathy Trial,26,27 which was done in patients with overt diabetic nephropathy, showed that a progressively lower achieved systolic pressure down to 120 mm Hg predicted lower rates of heart failure, cardiovascular mortality, and renal events (although the trial target was ≤ 130/85 mm Hg and few participants achieved systolic pressure lower than 120 mm Hg).
IMPLICATIONS FOR MANAGEMENT
The recent estimates of hypertension prevalence and control from NHANES show that only about 53% of hypertensive adults have their blood pressure under control (defined as systolic pressure < 140 mm Hg and diastolic pressure < 90 mm Hg).2 Analysis of the NHANES 2007–2012 data showed that 16.7% or 8.2 million US adults with treated hypertension meet the eligibility criteria for SPRINT.28
Although the SPRINT results support the notion that “lower is better,” the risks and benefits of intensive control will need to be balanced in individual patients. Table 4 shows the number needed to treat and number needed to harm in the trial.
More aggressive management of hypertension is challenging. The median systolic pressure achieved in the intensive group in SPRINT was just over 120 mm Hg, which implies that at least half of the participants in the intensive group did not achieve the goal of less than 120 mm Hg. While it may be reasonable to aim for systolic pressure of less than 120 or 125 mm Hg in patients who fit the SPRINT criteria and can tolerate intensive blood pressure lowering, it would be prudent to aim for a more conservative goal in elderly patients who are frail and at risk for falls, considering the higher incidence of specified adverse events in the intensive group.
Results of cognitive outcomes, as well as data related to quality of life, are still awaited. Long-term renal outcomes are also unclear.
As noted above, the question of generalizability of SPRINT results to patients with diabetes is open to debate. In our opinion, with currently available evidence, it is difficult to conclusively answer the question of whether a lower systolic target provides cardiovascular benefit in diabetes. It is also unclear whether similar beneficial results would be seen with intensive treatment in a population at low cardiovascular risk. The American Heart Association and the American College of Cardiology are in the process of formulating new hypertension guidelines, and evidence from SPRINT will inform any new recommendations.
As more medications will likely be needed for intensive systolic blood pressure control, side effects and tolerability of medications with polypharmacy and potential nonadherence with increasing complexity of medication regimens should be kept in mind. Lifestyle modifications will need to be emphasized and reinforced, with greater use of combination antihypertensive therapy.
The data from SPRINT indicate that lower systolic pressure is better, as long as untoward clinical events can be monitored and avoided or easily managed. Careful monitoring will likely entail more frequent clinic visits and more frequent assessment of renal function and electrolyte levels (participants in the intensive group in the trial were seen every month until goal was achieved). A team approach that includes pharmacists and nurse practitioners, along with optimal use of best practice algorithms and remote monitoring technology, will need to be implemented for efficient and effective care.
In treating hypertension, lower systolic pressure is better than higher—with caveats. This is the message of the Systolic Blood Pressure Intervention Trial (SPRINT),1 a large, federally funded study that was halted early when patients at high cardiovascular risk who were randomized to a goal systolic pressure of less than 120 mm Hg were found to have better outcomes, including lower rates of heart failure, death from cardiovascular causes, and death from any cause, than patients randomized to a goal of less than 140 mm Hg.
The caveats: the benefit came at a price of more adverse events. Also, the trial excluded patients who had diabetes mellitus or previous strokes, so it is uncertain if these subgroups would also benefit from intensive lowering of systolic pressure—and in earlier trials they did not.
This article reviews the trial design and protocol, summarizes the results, and briefly discusses the implications of these results.
BEFORE SPRINT
Hypertension is very common in adults in the United States, and is a risk factor for heart disease, stroke, heart failure, and kidney disease. The estimated prevalence of hypertension in the 2011–2014 National Health and Nutrition Examination Survey (NHANES) was 29%, and the prevalence increases with age (7.3% in those ages 18 to 39, 32.2% in those ages 40 to 59, and 64.9% in those ages 60 and older).2 Isolated systolic hypertension (ie, systolic blood pressure > 140 mm Hg with diastolic pressure < 90 mm Hg) is the most common form of hypertension after age 50.3
Clinical trials have provided substantial evidence that treating hypertension reduces the incidence of stroke, myocardial infarction, and heart failure.4,5 Although observational studies show a progressive and linear rise in cardiovascular risk as systolic blood pressure rises above 115 mm Hg,6 clinical trials in the general population have not documented benefits of lowering systolic pressure to this level.7–11 However, clinical trials that directly evaluated two different blood pressure goals in the general population showed benefit with achieving systolic blood pressure less than 150 mm Hg,7,9 with limited data on lower blood pressure targets.10–12
No benefit found in intensive systolic lowering in diabetes or after stroke
The Action to Control Cardiovascular Risk in Diabetes-Blood Pressure (ACCORD BP) trial13 in patients with type 2 diabetes found no benefit in lowering systolic pressure to less than 120 mm Hg compared with less than 140 mm Hg in terms of the trial’s primary composite cardiovascular outcome (ie, nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes). However, the intensively treated group in this trial did enjoy a benefit in terms of fewer stroke events.
The Secondary Prevention of Small Subcortical Strokes (SPS3) trial14 in patients with stroke found no significant benefit in lowering systolic pressure to less than 130 mm Hg compared with less than 150 mm Hg for overall risk of another stroke, but a significant benefit was noted in reduced risk of intracerebral hemorrhage.
Current guidelines, based on available evidence, advocate treatment to a systolic goal of less than 140 mm Hg in most patients, and recommend relaxing this goal to less than 150 mm Hg in the elderly.15,16
Given the uncertainty surrounding optimal systolic targets, SPRINT was designed to test the hypothesis that a goal of less than 120 mm Hg would reduce the risk of cardiovascular events more than the generally accepted systolic goal of less than 140 mm Hg.17 Patients with diabetes and stroke were excluded because a similar hypothesis was tested in the ACCORD BP and SPS3 trials, which included patients with these conditions.
SPRINT DESIGN
SPRINT was a randomized, controlled, open-label trial sponsored by the National Institutes of Health and conducted at 102 US sites.
Inclusion criteria. Participants had to be at least 50 years old, with systolic pressure of 130 to 180 mm Hg, and had to have at least one cardiovascular risk factor, eg:
- Clinical or subclinical cardiovascular disease (other than stroke)
- Chronic kidney disease, defined as estimated glomerular filtration rate (eGFR), calculated by the Modification of Diet in Renal Disease (MDRD) study equation, of 20 to less than 60 mL/min/1.73 m2
- Framingham risk score of 15% of more
- Age 75 or older.
Major exclusion criteria included:
- Diabetes
- Stroke
- Polycystic kidney disease
- Chronic kidney disease with an eGFR less than 20 mL/min/1.73 m2
- Proteinuria (excretion > 1 g/day).
Intensive vs standard treatment
Participants were randomized to receive intensive treatment (systolic goal < 120 mm Hg) or standard treatment (systolic goal < 140 mm Hg). Baseline antihypertensive medications were adjusted to achieve blood pressure goals based on randomization assignment.
Doses of medications were adjusted on the basis of an average of three seated office blood pressure measurements after a 5-minute period of rest, taken with an automated monitor (Omron Healthcare Model 907); the same monitor was used and the same protocol was followed at all participating sites. Blood pressure was also measured after standing for 1 minute to assess orthostatic change.
Lifestyle modifications were encouraged in both groups. There was no restriction on using any antihypertensive medication, and this was at the discretion of individual investigators. Thiazide-type diuretics were encouraged as first-line agents (with chlorthalidone encouraged as the primary thiazide-type diuretic).
Outcomes measured
The primary outcome was a composite of myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, stroke, acute decompensated heart failure, and cardiovascular mortality.
Secondary outcomes included individual components of the primary composite outcome, all-cause mortality, and the composite of primary outcome and all-cause mortality.
Renal outcomes were assessed as:
- Incident albuminuria (doubling of the urinary albumin-to-creatinine ratio from less than 10 mg/g to more than 10 mg/g)
- Composite of a 50% decrease in eGFR or development of end-stage renal disease requiring long-term dialysis or kidney transplantation (in those with baseline chronic kidney disease)
- A 30% decrease in eGFR (in those without chronic kidney disease).1,17
SPRINT also recruited participants to two nested substudies: SPRINT MIND and SPRINT MIND MRI, to study differences in cognitive outcomes and small-vessel ischemic disease between intensive treatment and standard treatment.
STUDY RESULTS
Older patients at risk, but without diabetes
Of 14,692 participants screened, 9,361 were enrolled in the study between 2010 and 2013. Baseline characteristics were comparable in both groups.
Demographics. The mean age of the participants was 67.9, and about 28% were 75 or older. About 36% were women, 58% white, 30% black, and 11% Hispanic.
Cardiovascular risk. The mean Framingham risk score was 20% (ie, they had a 20% risk of having a cardiovascular event within 10 years), and 61% of the participants had a risk score of at least 15%. Twenty percent already had cardiovascular disease.
Blood pressure. The average baseline blood pressure was 139.7/78.2 mm Hg. One-third of the participants had baseline systolic pressures of 132 mm Hg or less, another third had pressures in the range of 132 to 145, and the rest had 145 mm Hg or higher.
Renal function. The mean serum creatinine level was about 1.1 mg/dL. The mean eGFR was about 71 mL/min/1.73 m2 as calculated by the MDRD equation, and about 28% had eGFRs less than 60. The mean ratio of urinary albumin to creatinine was 44.1 mg/g in the intensive treatment group and 41.1 in the standard treatment group.
Other. The mean total cholesterol level was 190 mg/dL, fasting plasma glucose 99 mg/dL, and body mass index nearly 30 kg/m2.
Blood pressure during treatment
People in the intensive treatment group were taking a mean of 2.8 antihypertensive medications, and those in the standard treatment group were taking 1.8. Patients in the intensive group required greater use of all classes of medications to achieve goal systolic pressure (Table 1).
Study halted early due to efficacy
Throughout the 3.26 years of follow-up, the average difference in systolic pressure between the two groups was 13.1 mm Hg, with a mean systolic pressure of 121.5 mm Hg in the intensive treatment group and 134.6 mm Hg in the standard treatment group. The mean diastolic blood pressure was 68.7 mm Hg in the intensive treatment group and 76.3 mm Hg in the standard treatment group.
Although the study was planned to run for an average follow-up of 5 years, the National Heart, Lung, and Blood Institute terminated it early at a median of 3.26 years in view of lower rates of the primary outcome and of heart failure and death in the intensive treatment group (Table 2).
The effects on the primary outcome and mortality were consistent across the prespecified subgroups of age (< 75 vs ≥ 75), sex (female vs male), race (black vs nonblack), cardiovascular disease (presence or absence at baseline), prior chronic kidney disease (presence or absence at baseline), and across blood pressure tertiles (≤ 132 mm Hg, > 132 to < 145 mm Hg, ≥ 145 mm Hg).
Follow-up for assessment of cognitive outcomes (SPRINT MIND) and small-vessel ischemic disease (SPRINT MIND MRI) is ongoing.
WHAT DOES THIS MEAN?
SPRINT is the first large, adequately powered, randomized trial to demonstrate cardiovascular and mortality benefit from lowering the systolic blood pressure (goal < 120 mm Hg) in older patients at cardiovascular risk but without a history of diabetes mellitus or stroke.1
Most SPRINT patients had reasonably controlled blood pressure at baseline (the mean systolic pressure was 139.7 mm Hg, and two-thirds of participants had systolic pressure < 145 mm Hg). Of note, however, this trial excluded patients with systolic pressure higher than 180 mm Hg. There was excellent separation of systolic pressure between the two groups beginning at 1 year, which was consistent through the course of the trial.
The cardiovascular benefit in the intensive treatment group was predominantly driven by lower rates of heart failure (a 38% reduction in the intensive treatment group, P = .0002) and cardiovascular mortality (a 43% reduction in the intensive treatment group, P = .005), while there was no significant difference between the two groups in myocardial infarction or stroke. The beneficial effect on heart failure events is consistent with results from other trials including the Systolic Hypertension in the Elderly Program,7 Systolic Hypertension in Europe,8 and Hypertension in the Very Elderly Trial,9 all of which showed greatest risk reduction for heart failure events with systolic pressure-lowering (although to higher systolic levels than SPRINT).7–9 It is unclear why there was no beneficial effect on stroke events. The reduction in all-cause mortality in the intensive treatment group in SPRINT was greater than the reduction in cardiovascular deaths, which is also unexplained.
Although the study was terminated early due to efficacy (which introduces the possible bias that the estimated effect size will be too high), the number of primary end points reached was large (562 in the two groups combined), providing reassurance that the findings are valid. There was no blinding in the study (both participants and study investigators were aware of treatment assignment and study medications), but there was a structured assessment of outcomes and adverse events, with adjudication done by blinded reviewers.
SPRINT used an automated device for blood pressure measurement, which is known to reduce the “white coat” effect and correlates tightly with average daytime blood pressure done by ambulatory blood pressure monitoring.18 However, in clinical practice automated devices may not be available and a strict protocol for correct measurement may not be followed, with the possible result that blood pressure may be overestimated and overtreated.
What about diastolic pressure?
The trial, by design, focused on lowering systolic pressure (given the greater prevalence of isolated systolic hypertension with age), and the implications of lowering diastolic pressure are unclear. The issue of a J-shaped relationship between diastolic pressure and cardiovascular risk is debated in the literature: patients with a diastolic pressure of 60 to 65 mm Hg, especially those with existing coronary artery disease, may not tolerate aggressive blood pressure-lowering.19,20 Further analysis of this association (if any) from SPRINT will be helpful.
What about patients with diabetes?
Patients were excluded from SPRINT if they were under age 50, were at low cardiovascular risk, or had diabetes, raising the question of whether the results apply to these groups as well.
The question is particularly relevant in diabetes, as the ACCORD BP study, which used the same blood pressure targets as SPRINT, did not show a significant difference in the primary cardiovascular outcome between the intensive and standard treatments in patients with diabetes (Table 3).13 In ACCORD BP, the rate of the primary outcome was 12% lower in the intensive treatment group than in the standard treatment group, but the 95% confidence interval was –27% to +6%, so the finding was not statistically significant. However, the wide confidence interval does not exclude the possibility of a benefit that was comparable to that observed in SPRINT.
It has been speculated that ACCORD BP was underpowered to detect significant differences in the primary outcome.21 An analysis combining data from both trials indicated that effects on individual outcomes were generally consistent in both trials (with no significant heterogeneity noted).22 Also, the primary composite outcome in ACCORD did not include heart failure, which is particularly sensitive to blood pressure reduction.
Additionally, ACCORD BP had a 2 × 2 factorial design involving a simultaneous comparison of intensive vs standard glycemic control, which may have influenced the effects due to blood pressure. Indeed, a post hoc analysis showed that there was a significant 26% lower risk of the primary outcome in ACCORD BP patients who received intensive systolic pressure control plus standard glycemic control than in those receiving standard systolic control plus standard glycemic control.23
Are more adverse events an acceptable trade-off?
Adverse events, including acute kidney injury, were more frequent in the intensive therapy group in SPRINT.
Acute kidney injury was coded as an adverse event on the basis of this diagnosis being included in the hospital discharge summary (as a primary or main secondary diagnosis) and if considered by the safety officer to be one of the top three reasons for admission or continued hospitalization. Further analysis of renal events should be forthcoming.
People in the intensive treatment group, on average, needed one more medication than those in the standard treatment group. Some of the adverse events may be related to the antihypertensive medications taken (eg, electrolyte abnormalities such as hyponatremia and hypokalemia due to diuretic use), and others may be related to blood pressure-lowering (eg, acute kidney injury due to renal hypoperfusion).
At this point, the long-term effects of these adverse events, especially on kidney function, are not known. Patients enrolled in clinical trials tend to be healthier than patients seen in clinical practice; thus, the rate of adverse events reported in the trial may be lower than one would see in the real world.
Does lower systolic pressure protect or harm the kidneys?
SPRINT included patients with stage 3 and 4 chronic kidney disease (ie, with eGFR 20–50 mL/min/1.73 m2), but it was designed to assess cardiovascular outcomes, not the progression of chronic kidney disease. The trial excluded patients with diabetic nephropathy or high degrees of proteinuria.
Earlier randomized trials that focused on chronic kidney disease progression, including the MDRD24 and the African American Study of Kidney Disease and Hypertension,25 did not show benefit with more aggressive blood pressure-lowering (except in patients with higher degrees of proteinuria), and these trials were not powered to assess effects on cardiovascular outcomes.24,25
The Irbesartan Diabetic Nephropathy Trial,26,27 which was done in patients with overt diabetic nephropathy, showed that a progressively lower achieved systolic pressure down to 120 mm Hg predicted lower rates of heart failure, cardiovascular mortality, and renal events (although the trial target was ≤ 130/85 mm Hg and few participants achieved systolic pressure lower than 120 mm Hg).
IMPLICATIONS FOR MANAGEMENT
The recent estimates of hypertension prevalence and control from NHANES show that only about 53% of hypertensive adults have their blood pressure under control (defined as systolic pressure < 140 mm Hg and diastolic pressure < 90 mm Hg).2 Analysis of the NHANES 2007–2012 data showed that 16.7% or 8.2 million US adults with treated hypertension meet the eligibility criteria for SPRINT.28
Although the SPRINT results support the notion that “lower is better,” the risks and benefits of intensive control will need to be balanced in individual patients. Table 4 shows the number needed to treat and number needed to harm in the trial.
More aggressive management of hypertension is challenging. The median systolic pressure achieved in the intensive group in SPRINT was just over 120 mm Hg, which implies that at least half of the participants in the intensive group did not achieve the goal of less than 120 mm Hg. While it may be reasonable to aim for systolic pressure of less than 120 or 125 mm Hg in patients who fit the SPRINT criteria and can tolerate intensive blood pressure lowering, it would be prudent to aim for a more conservative goal in elderly patients who are frail and at risk for falls, considering the higher incidence of specified adverse events in the intensive group.
Results of cognitive outcomes, as well as data related to quality of life, are still awaited. Long-term renal outcomes are also unclear.
As noted above, the question of generalizability of SPRINT results to patients with diabetes is open to debate. In our opinion, with currently available evidence, it is difficult to conclusively answer the question of whether a lower systolic target provides cardiovascular benefit in diabetes. It is also unclear whether similar beneficial results would be seen with intensive treatment in a population at low cardiovascular risk. The American Heart Association and the American College of Cardiology are in the process of formulating new hypertension guidelines, and evidence from SPRINT will inform any new recommendations.
As more medications will likely be needed for intensive systolic blood pressure control, side effects and tolerability of medications with polypharmacy and potential nonadherence with increasing complexity of medication regimens should be kept in mind. Lifestyle modifications will need to be emphasized and reinforced, with greater use of combination antihypertensive therapy.
The data from SPRINT indicate that lower systolic pressure is better, as long as untoward clinical events can be monitored and avoided or easily managed. Careful monitoring will likely entail more frequent clinic visits and more frequent assessment of renal function and electrolyte levels (participants in the intensive group in the trial were seen every month until goal was achieved). A team approach that includes pharmacists and nurse practitioners, along with optimal use of best practice algorithms and remote monitoring technology, will need to be implemented for efficient and effective care.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- Yoon SS, Fryar CD, Carroll MD. Hypertension prevalence and control among adults: United States, 2011–2014. NCHS data brief, no. 220. Hyattsville, MD: National Center for Health Statistics. 2015.
- Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 2001; 37:869–874.
- Neal B, MacMahon S, Chapman N; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration. Lancet 2000; 356:1955–1964.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Ogihara T, Saruta T, Rakugi H, et al; Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: Valsartan in Elderly Isolated Systolic Hypertension study. Hypertension 2010; 56:196–202.
- Liu L, Zhang Y, Liu G, Li W, Zhang X, Zanchetti A; FEVER Study Group. The Felodipine Event Reduction (FEVER) Study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens 2005; 23:2157–2172.
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382:507–515.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. Erratum in: JAMA. 2014; 311:1809.
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich) 2014; 16:14–26.
- Ambrosius WT, Sink KM, Foy CG, et al; SPRINT Study Research Group. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014; 11:532–546.
- Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Kaczorowski J. Conventional versus automated measurement of blood pressure in the office (CAMBO) trial. Fam Pract 2012; 29:376–382.
- Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006; 144:884–893.
- Boutitie F, Gueyffier F, Pocock S, Fagard R, Boissel JP; INDANA Project Steering Committee; INdividual Data ANalysis of Antihypertensive intervention. J-shaped relationship between blood pressure and mortality in hypertensive patients: new insights from a meta-analysis of individual-patient data. Ann Intern Med 2002; 136:438–448.
- Mancia G. Effects of intensive blood pressure control in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation 2010; 122:847–849.
- Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med 2015; 373:2175–2178.
- Margolis KL, O’Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care 2014; 37:1721–1728.
- Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease study. Ann Intern Med 1995; 123:754–762.
- Agodoa LY, Appel L, Bakris GL, et al; African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Berl T, Hunsicker LG, Lewis JB, et al; Collaborative Study Group. Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol 2005; 16:2170–2179.
- Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the Irbesartan Diabetic Nephropathy Trial: clinical implications and limitations. J Am Soc Nephrol 2005; 16:3027–3037.
- Bress AP, Tanner RM, Hess R, Colantonio LD, Shimbo D, Muntner P. Generalizability of results from the Systolic Blood Pressure Intervention Trial (SPRINT) to the US adult population. J Am Coll Cardiol 2015 Oct 31. doi: 10.1016/j.jacc.2015.10.037. Epub ahead of print.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- Yoon SS, Fryar CD, Carroll MD. Hypertension prevalence and control among adults: United States, 2011–2014. NCHS data brief, no. 220. Hyattsville, MD: National Center for Health Statistics. 2015.
- Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 2001; 37:869–874.
- Neal B, MacMahon S, Chapman N; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration. Lancet 2000; 356:1955–1964.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913.
- SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991; 265:3255–3264.
- Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997; 350:757–764.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31:2115–2127.
- Ogihara T, Saruta T, Rakugi H, et al; Valsartan in Elderly Isolated Systolic Hypertension Study Group. Target blood pressure for treatment of isolated systolic hypertension in the elderly: Valsartan in Elderly Isolated Systolic Hypertension study. Hypertension 2010; 56:196–202.
- Liu L, Zhang Y, Liu G, Li W, Zhang X, Zanchetti A; FEVER Study Group. The Felodipine Event Reduction (FEVER) Study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens 2005; 23:2157–2172.
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382:507–515.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. Erratum in: JAMA. 2014; 311:1809.
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich) 2014; 16:14–26.
- Ambrosius WT, Sink KM, Foy CG, et al; SPRINT Study Research Group. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014; 11:532–546.
- Myers MG, Godwin M, Dawes M, Kiss A, Tobe SW, Kaczorowski J. Conventional versus automated measurement of blood pressure in the office (CAMBO) trial. Fam Pract 2012; 29:376–382.
- Messerli FH, Mancia G, Conti CR, et al. Dogma disputed: can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 2006; 144:884–893.
- Boutitie F, Gueyffier F, Pocock S, Fagard R, Boissel JP; INDANA Project Steering Committee; INdividual Data ANalysis of Antihypertensive intervention. J-shaped relationship between blood pressure and mortality in hypertensive patients: new insights from a meta-analysis of individual-patient data. Ann Intern Med 2002; 136:438–448.
- Mancia G. Effects of intensive blood pressure control in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation 2010; 122:847–849.
- Perkovic V, Rodgers A. Redefining blood-pressure targets—SPRINT starts the marathon. N Engl J Med 2015; 373:2175–2178.
- Margolis KL, O’Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care 2014; 37:1721–1728.
- Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease study. Ann Intern Med 1995; 123:754–762.
- Agodoa LY, Appel L, Bakris GL, et al; African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Berl T, Hunsicker LG, Lewis JB, et al; Collaborative Study Group. Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol 2005; 16:2170–2179.
- Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the Irbesartan Diabetic Nephropathy Trial: clinical implications and limitations. J Am Soc Nephrol 2005; 16:3027–3037.
- Bress AP, Tanner RM, Hess R, Colantonio LD, Shimbo D, Muntner P. Generalizability of results from the Systolic Blood Pressure Intervention Trial (SPRINT) to the US adult population. J Am Coll Cardiol 2015 Oct 31. doi: 10.1016/j.jacc.2015.10.037. Epub ahead of print.
KEY POINTS
- SPRINT is the first large prospective randomized trial to show evidence of cardiovascular and mortality benefit for intensive lowering of systolic blood pressure (goal < 120 mm Hg) in older patients at cardiovascular risk, but without a history of diabetes mellitus or stroke.
- A similar trial in patients with type 2 diabetes mellitus did not show significant benefit of intensive treatment.
- Intensive treatment was associated with more adverse events, including hypotension, syncope, electrolyte abnormalities, and acute kidney injury.
- It is unclear if these results can be extrapolated to patients with a history of diabetes or stroke, younger patients, or those with low cardiovascular risk.
- Healthcare providers should engage patients in a shared decision-making process, with discussion of the benefits and risks associated with intensive lowering of blood pressure.