User login
Background The combination of manual physical therapy and exercise provides important benefit for more than 80% of patients with knee osteoarthritis (OA). Our objective was to determine predictor variables for patients unlikely to respond to these interventions.
Methods We used a retrospective combined cohort study design to develop a preliminary clinical prediction rule (CPR). To determine useful predictors of nonsuccess, we used an extensive set of 167 baseline variables. These variables were extracted from standardized examination forms used with 101 patients (64 women and 37 men with a mean age of 60.5±11.8 and 63.6±9.3 years, respectively) in 2 previously published clinical trials. We classified patients based on whether they achieved a clinically meaningful benefit of at least 12% improvement in Western Ontario MacMaster (WOMAC) scores after 4 weeks of treatment using the smallest and most efficient subset of predictors.
Results The variables of patellofemoral pain, anterior cruciate ligament laxity, and height >1.71 m (5’7’’) comprise the CPR. Patients with at least 2 positive tests yielded a posttest probability of 88% for nonsuccess with this treatment (positive likelihood ratio=36.7). The overall prognostic accuracy of the CPR was 96%.
Conclusion Most patients with knee OA will benefit from a low-risk, cost-effective program of manual physical therapy and supporting exercise.1,2 The few patients who may not benefit from such a program are identifiable by a simple (preliminary) CPR. After validation, this rule could improve primary patient management, allowing more appropriate referrals and choices in intervention.
Although the exact cause of knee OA is unclear, its incidence increases with age and it is particularly prevalent among women and those who are obese and have occupations requiring heavy lifting and frequent kneeling or squatting.3-6 Lifelong sport-specific activity7,8 and joint injury9 also seem to increase the risk for knee OA. Knee malalignment also may predispose people to knee OA,10 and the presence of early degenerative changes predicts progression of the disease.11 The disability and pain associated with knee OA correlate with a loss of quadriceps femoris muscle strength and limited joint range of motion.12-14
Medications and surgery carry substantial risks. Pharmacologic interventions for knee OA include nonsteroidal anti-inflammatory drugs, acetaminophen, and cyclooxygenase-2-selective inhibitors.15-17 While each of these drugs reduces pain and improves function, potential side effects include gastrointestinal, cardiovascular, renal, and hepatic complications.16,18-21
Effective surgical options—most appropriate for advanced OA—include high-tibial osteotomy and total knee arthroplasty (TKA). There is good evidence that arthroscopic surgery is not an effective intervention for knee OA, yielding results for pain and function equivalent to those seen with knee capsule injections of saline, tidal irrigation, and placebo surgery.22-25 TKA reduces pain, improves function, and decreases arthritis-related costs in older individuals with advanced knee OA.26,27 However, this procedure is not without risk.28 Total knee replacement in patients younger than 55 years is associated with increased mortality.29 Reported adverse outcomes of TKA include death, deep vein thrombosis, pulmonary embolus, deep wound infections,30,31 arterial lacerations, amputations,32 postoperative ileus,33 fractures, joint stiffness, and ligamentous instability.34 Viscosupplementation reduces pain and improves function, most evident at 5 to 13 weeks posttreatment, with few reported serious complications and moderate rates of local complications.35
Physical therapy is beneficial for mild to moderate OA and confers very low risk. Both physical therapy and exercise programs for OA have demonstrated benefit in a variety of settings.36-42 As shown in 2 independently conducted randomized controlled trials (RCTs) (one placebo controlled and one with an alternate treatment comparison), manual physical therapy applied during a small number of clinical sessions and supplemented by home exercise yields large reductions in pain and stiffness and improvements in functional ability persisting to 1 year as measured on the WOMAC Osteoarthritis Index,1,2 a validated self-report outcome instrument for OA of the hip and knee.43 In these studies, 60% of subjects receiving manual physical therapy and exercise achieved more than 50% improvement in WOMAC scores (pain, stiffness, and function) postintervention. Additionally, 83% achieved more than the minimal clinically important difference (MCID) of 12% improvement.1,2 Physical therapy and exercise combined also decreased the need for TKA and long-term medication use.1,2
For an intervention that benefits most patients, there is clearly an interest in determining predictors of treatment failure44 to expedite referral for alternative care. When the time or resources required to attend physical therapy appointments would create financial or personal hardships, more appropriate interventions may be home-based physical therapy exercise programs or medications and injections. Equally important, patients for whom knee OA rehabilitation is predicted to fail can be reprioritized for physical therapy aimed at coexisting conditions or injuries such as a functionally limiting impingement syndrome of the shoulder or chronic degenerative back or hip conditions.
Methods
Using a retrospective combined-cohort study design, we reviewed baseline patient examinations from 2 RCTs1,2 to identify variables that indicate which individuals with knee OA are unlikely to benefit from manual physical therapy and exercise, and to thereby develop a preliminary CPR. We extracted data from the research folders of all study participants. The institutional review board of Brooke Army Medical Center determined that the study was exempt from review. From April to December 2008, we prepared an extensive database of examination findings and performed analyses to determine the variables that predict likely treatment nonsuccess with manual physical therapy and exercise. Improvement of <12% in the total WOMAC score after 4 weeks of treatment defined nonsuccess.45
Data sets from the previously published trials contained 22 variables measured at baseline that were potential predictors of nonsuccess. We combined these variables with an additional 145 variables manually retrieved from standardized examination forms used for each subject, for a total of 167 potential predictors. We combined only data from treatment groups receiving manual therapy and exercise.
We limited the extent of some examination procedures in the earlier studies, due to the high level of symptoms experienced by some subjects at rest and during the initial examination. For example, if there was severe pain with active knee flexion, we did not perform passive manual overpressure to flexion; nor did we record a finding. Thus, the total number of data points for each subject varied somewhat.
Data analysis
We compared success and nonsuccess groups with 2-tailed unpaired t-tests for continuous variables, and chi-square tests for categorical variables. We additionally performed logistic regression analysis on potential predictors that yielded P values <.10, using a forward conditional stepwise procedure with probability levels set to .05 for entry and .10 for removal from the model. Predictors retained by the final logistic regression model comprised the CPR.
We coded each patient in the data set as positive or negative for each predictor in the CPR. To determine a cut score, we dichotomized the single retained continuous predictor variable using receiver-operator characteristic (ROC) curve analysis and the Youden index.46 For each CPR level (ie, increasing number of predictors positive), we constructed a 2 × 2 contingency table with numbers of patients with true-positive test results, false-positive test results, true-negative test results, and false-negative test results. We characterized prognostic performance of the CPR by calculating sensitivity, specificity, and positive likelihood ratios for each level of positive predictors. To determine overall prognostic accuracy, we added true positives and true negatives and divided by the total number of patients in the cross tabulation.
For each CPR level, we derived posttest probabilities of nonsuccess from generalized pretest probability (incidence of treatment nonsuccess in the sample) and the positive likelihood ratios.47 Finally, to determine how consistently the CPR performed with subjects in the original studies,1,2 we generated separate cross-tabulations and prognostic accuracy statistics from each RCT.
Results
Baseline patient attributes are summarized in TABLE 1. Of the 101 subjects in the combined data set, 17 (16.8%) met the definition of nonsuccess. Among 47 continuous-scale variables available, 11 predictors significantly discriminated between those in the treatment success and nonsuccess groups. Among 120 categorical-scale variables, 15 predictors significantly discriminated between groups. We identified 6 potential predictors for entry into the final logistic regression analysis: height, assistive device type, prone knee bend degrees, baseline WOMAC visual analog scale (VAS) for difficulty descending stairs, anterior cruciate ligament (ACL) laxity, and pain with passive patellofemoral glide.
TABLE 1
Baseline descriptive summaries of patients (n=101)
| Sex, n (%) Men Women | 37 (36.6) 64 (63.4) |
| Age, y Mean±SD Range | 62.5±10.4 39-85 |
| Height, m Mean±SD Range | 1.66±0.1041 1.42-1.91 |
| Side(s) involved, n (%) Unilateral Bilateral | 63 (62.4) 38 (37.6) |
| Weight, kg Mean±SD Range | 84.5±17.8 48.6-132.7 |
| Duration of symptoms, mo Mean±SD Range | 76.1±87.9 1-480 |
| WOMAC (VAS) total baseline, mm Mean±SD Range | 1059.8±447.1 193-2289 |
| 6-minute walk test baseline, m Mean±SD Range | 425.6±114.8 118.2-683.3 |
| Physical activity relative to peers (self-report), n (%) Much more active Somewhat more active About the same Somewhat less active | 26 (26) 33 (33) 20 (20) 21 (21) |
| Radiographic severity score, n (%) 0 1 2 3 4 | 6 (6.1) 25 (25.5) 33 (33.7) 25 (25.5) 9 (9.2) |
| *Baseline data were available for all 101 subjects except for duration of symptoms (n=98); physical activity (n=100); and radiographic severity (n=98). VAS, visual analog scale; WOMAC, Western Ontario MacMaster. | |
The final regression model retained 3 predictors comprising the CPR: height, ACL laxity, and pain with passive patellofemoral glides. We dichotomized height with a cut point of 1.71 m (5’7”), which corresponded with a deflection point at the upper left extent of the ROC curve (area under the curve=0.72; 95% CI, 0.57-0.87; P=.001). We thus deemed a patient 1.71 m or taller as positive for nonsuccess. We considered a patient with laxity of the ACL as positive for nonsuccess if a test result on the Lachman test (or the anterior drawer test) was positive (any grade other than 0). We regarded passive patellofemoral glide as positive for nonsuccess if a patient reported pain with any direction of passive gliding motion imposed by the therapist. The final regression model was a good fit to the data: Hosmer & Lemeshow test χ2 = 2.90 (P=.940); Nagelkerke R2=0.680.
TABLE 2 presents prognostic accuracy profiles for each predictor in the CPR; TABLE 3 summarizes the accuracy for each level of the multivariate CPR. Values in TABLE 3 reflect complete sets of data for the 3 predictors found for 50 patients. Of those 50 patients, 6 (12%) were in the nonsuccess group.
TABLE 2
Prognostic accuracy statistics for individual predictors
| Predictor | Sensitivity (95% CI) | Specificity (95% CI) | Positive likelihood ratio (95% CI) | Posttest probability of nonsuccess* |
|---|---|---|---|---|
| Height ≥1.71 m | 0.65 (0.41-0.83) | 0.77 (0.67-0.85) | 2.86 (1.69-4.86) | 37% |
| ACL laxity | 0.27 (0.10-0.57) | 0.93 (0.83-0.97) | 3.68 (0.96-14.19) | 43% |
| Pain with passive patellofemoral glide in any direction | 0.71 (0.35-0.92) | 0.61 (0.47-0.74) | 1.84 (1.03-3.31) | 27% |
| ACL, anterior cruciate ligament; CI, confidence interval. *Assumes pretest probability of nonsuccess=17% (incidence in this sample). | ||||
With any 2 of the 3 tests positive, the CPR yielded a sensitivity of 83% (95% CI, 44%-97%), specificity of 98% (95% CI, 88%-100%), and positive likelihood ratio of 36.7 (95% CI, 5.1-263.0). Only 2 patients out of 50 were misclassified (one false positive and one false negative) at this level of the CPR, yielding an overall prognostic accuracy of 96% (95% CI, 87%-99%). Application of the positive likelihood ratio for a patient with any 2 positive tests yielded a posttest probability of 88% for nonsuccess with this treatment.
TABLE 3
Prognostic accuracy statistics for 3-level clinical prediction rule
| CPR level | Sensitivity (95% CI) | Specificity (95% CI) | Positive likelihood ratio (95% CI) | Posttest probability of nonsuccess* |
|---|---|---|---|---|
| All 3 tests positive | 0.21 (0.05-0.58) | 0.99 (0.90-1.00) | 19.29 (0.87-428.09) | 80% |
| At least 2 tests positive | 0.83 (0.44-0.97) | 0.98 (0.88-1.00) | 36.67 (5.11-263.01) | 88% |
| At least 1 test positive | 0.92 (0.56-0.99) | 0.48 (0.34-0.62) | 1.78 (1.26-2.52) | 27% |
| CI, confidence interval; CPR, clinical prediction rule. *Assumes pretest probability of nonsuccess=17% (incidence in this sample). | ||||
In the sensitivity analysis, the CPR performed similarly well for patients in each of the 2 original studies when applied separately to the groups of patients. Among the 30 patients from the first trial2 who had data for all 3 predictors in the CPR, only one was misclassified (a false positive), yielding a prognostic accuracy of 97% (95% CI, 83%-99%). Among the 20 patients from the second trial1 who had data for all 3 predictors, only one was misclassified (a false negative), yielding a prognostic accuracy of 95% (95% CI, 76%-99%).
DISCUSSION
Family physicians and physical therapists should be able to discuss with confidence how any given patient with knee OA will likely respond to treatment options. Our study is a preliminary step toward defining the population of patients with knee OA who are unlikely to benefit from manual physical therapy and exercise. We found such patients to be those with height >1.71 m, ACL laxity, and pain with passive glides of the patellofemoral joint.
A limitation of our study is the retrospective nature of gathering data. However, retrospective CPR derivation studies have made valuable contributions to many areas of medical practice.48-53 Additionally, if there had been uniformly available data across all patients, there may have been other, perhaps more powerful, predictors for treatment nonsuccess.
Actual cases of knee osteoarthritis (OA) evaluated by one of the authors (GD)
A 48-year-old female elementary teacher was referred for physical therapy due to right knee pain and a diagnosis of OA that was limiting her ability to climb stairs and squat to work with children in the classroom. Her goals were to be able to perform these physical activities with less pain and to reduce her anti-inflammatory medications. However, she also worried about taking time away from her job to attend physical therapy appointments. She was 1.63 m (5’4”) tall and had a body mass index of 27.5 kg/m2. Her knee was stable to ligamentous testing, with mild limitation and pain with active and passive movement of both the tibiofemoral and the patellofemoral joints. She had weakness of the quadriceps and hip abductors, and moderate tightness of the calf muscles in both lower extremities.
Given the presence of only a single predictor for nonsuccess (pain with passive movement of her patella), the likelihood that this patient would not respond to manual physical therapy and exercise was just 27%, according to the clinical prediction rule. The impairments to movement, strength, and flexibility found during the physical examination typically can be successfully addressed with manual physical therapy. Additionally, one of the patient’s goals was to reduce her medication use—a reported outcome of the clinical trials used for deriving the rule.1,2 This patient was a good candidate for the intervention, with an acceptably small chance of not achieving a clinically meaningful benefit.
A 50-year-old male soldier 1.95 m (6’5”) tall was referred for physical therapy to ameliorate chronic pain due to tricompartmental knee OA. He exhibited anterior ligamentous laxity and felt severe pain with manually performed passive patellar glides (FIGURES 1 AND 2). He also had a rotator cuff tear and a mild traumatic brain injury from a roadside bomb blast. With 3/3 predictors for failure, the likelihood of reducing this patient’s knee symptoms with manual therapy and exercise was just 20%. The physical therapist and referring physician jointly decided to focus a small number of physical therapy visits on the patient’s shoulder, while giving rehabilitation priority to ongoing cognitive therapy appointments.
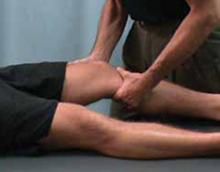
FIGURE 1
Lachman test
With the patient’s knee flexed at 30°, draw the proximal tibia anteriorly to observe movement of the tibia relative to the femur and thereby gauge anterior cruciate ligament integrity. Laxity is suggested by increased movement relative to the opposite knee.
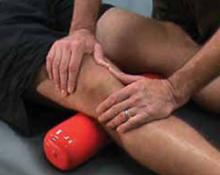
FIGURE 2
Passive patellofemoral glide
With the patient’s knee slightly flexed, apply light pressure to the medial border of the patella, moving it laterally and taking care not to compress the patella. Repeat the procedure superiorly, inferiorly, and medially. A positive test is pain experienced with any of the glides.
Patient height >1.71 m is the least intuitive of the predictors for nonsuccess, but that underscores the value of data-driven prediction rules. Variables regarded as unimportant in a typical clinical assessment may show clinical usefulness if validated in independent studies. It may be that in taller patients with knee OA, biomechanical forces are such that a positive response to conservative therapy is less likely—particularly in the presence of ligamentous laxity or patellofemoral dysfunction.
For most patients with knee OA, the combined intervention of manual physical therapy and exercise is clinically beneficial, relatively inexpensive, and has no known adverse effects.54 However, unique circumstances may increase the importance of determining the likelihood that a patient will benefit. A validated CPR will facilitate timely decisions for those relatively few patients requiring alternative interventions. Although the rule is preliminary and needs to be validated, these results provide current best evidence to define patients with knee OA who are unlikely to respond to manual physical therapy and exercise.
1. Deyle GD, Allison SC, Matekel RL, et al. Physical therapy treatment effectiveness for osteoarthritis of the knee: a randomized comparison of supervised clinical exercise and manual therapy procedures versus a home exercise program. Phys Ther. 2005;85:1301-1317.
2. Deyle GD, Henderson NE, Matekel RL, et al. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee. A randomized, controlled trial. Ann Intern Med. 2000;132:173-181
3. Sandmark H, Hogstedt C, Vingard E. Primary osteoarthrosis of the knee in men and women as a result of lifelong physical load from work. Scand J Work Environ Health. 2000;26:20-25.
4. Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40:728-733.
5. Jarvholm B, From C, Lewold S, et al. Incidence of surgically treated osteoarthritis in the hip and knee in male construction workers. Occup Environ Med. 2008;65:275-278.
6. Messier SP, Loeser RF, Mitchell MN, et al. Exercise and weight loss in obese older adults with knee osteoarthritis: a preliminary study. J Am Geriatr Soc. 2000;48:1062-1072.
7. Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;41:1343-1355.
8. Sandmark H, Vingard E. Sports and risk for severe osteoarthrosis of the knee. Scand J Med Sci Sports. 1999;9:279-284.
9. Gelber AC, Hochberg MC, Mead LA, et al. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133:321-328.
10. Cerejo R, Dunlop DD, Cahue S, et al. The influence of alignment on risk of knee osteoarthritis progression according to baseline stage of disease. Arthritis Rheum. 2002;46:2632-2636.
11. Wolfe F, Lane NE. The longterm outcome of osteoarthritis: rates and predictors of joint space narrowing in symptomatic patients with knee osteoarthritis. J Rheumatol. 2002;29:139-146.
12. Lewek MD, Rudolph KS, Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res. 2004;22:110-115.
13. Fitzgerald GK, Piva SR, Irrgang JJ. Reports of joint instability in knee osteoarthritis: its prevalence and relationship to physical function. Arthritis Rheum. 2004;51:941-946.
14. Fitzgerald GK, Piva SR, Irrgang JJ, et al. Quadriceps activation failure as a moderator of the relationship between quadriceps strength and physical function in individuals with knee osteoarthritis. Arthritis Rheum. 2004;51:40-48.
15. Scott DL, Berry H, Capell H, et al. The long-term effects of non-steroidal anti-inflammatory drugs in osteoarthritis of the knee: a randomized placebo-controlled trial. Rheumatology (Oxford). 2000;39:1095-1101.
16. Towheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257.-
17. Kivitz A, Fairfax M, Sheldon EA, et al. Comparison of the effectiveness and tolerability of lidocaine patch 5% versus celecoxib for osteoarthritis-related knee pain: post hoc analysis of a 12 week, prospective, randomized, active-controlled, open-label, parallel-group trial in adults. Clin Ther. 2008;30:2366-2377.
18. Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081-1091.
19. Psaty BM, Furberg CD. COX-2 inhibitors—lessons in drug safety. N Engl J Med. 2005;352:1133-1135.
20. Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071-1080.
21. Wittenberg RH, Schell E, Krehan G, et al. First-dose analgesic effect of the cyclo-oxygenase-2 selective inhibitor lumiracoxib in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled comparison with celecoxib [NCT00267215]. Arthritis Res Ther. 2006;8:R35.-
22. Bradley JD, Heilman DK, Katz BP, et al. Tidal irrigation as treatment for knee osteoarthritis: a sham-controlled, randomized, double-blinded evaluation. Arthritis Rheum. 2002;46:100-108.
23. Chang RW, Falconer J, Stulberg SD, et al. A randomized, controlled trial of arthroscopic surgery versus closed-needle joint lavage for patients with osteoarthritis of the knee. Arthritis Rheum. 1993;36:289-296.
24. Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81-88.
25. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, et al. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118.-
26. Hawker GA, Badley EM, Croxford R, et al. A population-based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47:732-741.
27. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169:1113-1121.
28. Hamel MB, Toth M, Legedza A, et al. Joint replacement surgery in elderly patients with severe osteoarthritis of the hip or knee: decision making, postoperative recovery, and clinical outcomes. Arch Intern Med. 2008;168:1430-1440.
29. Robertsson O, Stefansdottir A, Lidgren L, et al. Increased long-term mortality in patients less than 55 years old who have undergone knee replacement for osteoarthritis: results from the Swedish Knee Arthroplasty Register. J Bone Joint Surg Br. 2007;89:599-603.
30. SooHoo NF, Lieberman JR, Ko CY, et al. Factors predicting complication rates following total knee replacement. J Bone Joint Surg Am. 2006;88:480-485.
31. Solomon DH, Chibnik LB, Losina E, et al. Development of a preliminary index that predicts adverse events after total knee replacement. Arthritis Rheum. 2006;54:1536-1542.
32. Abularrage CJ, Weiswasser JM, Dezee KJ, et al. Predictors of lower extremity arterial injury after total knee or total hip arthroplasty. J Vasc Surg. 2008;47:803-807.
33. Parvizi J, Han SB, Tarity TD, et al. Postoperative ileus after total joint arthroplasty. J Arthroplasty. 2008;23:360-365.
34. Pinaroli A, Piedade SR, Servien E, et al. Intraoperative fractures and ligament tears during total knee arthroplasty. A 1795 posterostabilized TKA continuous series. Orthop Traumatol Surg Res. 2009;95:183-189
35. Bellamy N, Campbell J, Robinson V, et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321.-
36. Baker K, McAlindon T. Exercise for knee osteoarthritis. Curr Opin Rheumatol. 2000;12:456-463.
37. Baker KR, Nelson ME, Felson DT, et al. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol. 2001;28:1655-1665.
38. O’Reilly SC, Muir KR, Doherty M. Effectiveness of home exercise on pain and disability from osteoarthritis of the knee: a randomised controlled trial. Ann Rheum Dis. 1999;58:15-19.
39. Petrella RJ, Bartha C. Home based exercise therapy for older patients with knee osteoarthritis: a randomized clinical trial. J Rheumatol. 2000;27:2215-2221.
40. van Baar ME, Dekker J, Oostendorp RA, et al. Effectiveness of exercise in patients with osteoarthritis of hip or knee: nine months’ follow up. Ann Rheum Dis. 2001;60:1123-1130.
41. Silva LE, Valim V, Pessanha AP, et al. Hydrotherapy versus conventional land-based exercise for the management of patients with osteoarthritis of the knee: a randomized clinical trial. Phys Ther. 2008;88:12-21.
42. Hinman RS, Heywood SE, Day AR. Aquatic physical therapy for hip and knee osteoarthritis: results of a single-blind randomized controlled trial. Phys Ther. 2007;87:32-43.
43. Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29:2473-2476.
44. Fritz JM. Clinical prediction rules in physical therapy: coming of age? J Orthop Sports Phys Ther. 2009;39:159-161
45. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45:384-391.
46. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32-35.
47. Fritz JM, Wainner RS. Examining diagnostic tests: an evidence-based perspective. Phys Ther. 2001;81:1546-1564.
48. van Walraven C, Hart RG, Wells GA, et al. A clinical prediction rule to identify patients with atrial fibrillation and a low risk for stroke while taking aspirin. Arch Intern Med. 2003;163:936-943.
49. Predictors of thromboembolism in atrial fibrillation: I. Clinical features of patients at risk. The Stroke Prevention in Atrial Fibrillation Investigators. Ann Intern Med. 1992;116:1-5
50. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041-1046.
51. Aujesky D, Obrosky DS, Stone RA, et al. A prediction rule to identify low-risk patients with pulmonary embolism. Arch Intern Med. 2006;166:169-175.
52. Espana PP, Capelastegui A, Gorordo I, et al. Development and validation of a clinical prediction rule for severe community-acquired pneumonia. Am J Respir Crit Care Med. 2006;174:1249-1256.
53. Kuijpers T, van der Heijden GJ, Vergouwe Y, et al. Good generalizability of a prediction rule for prediction of persistent shoulder pain in the short term. J Clin Epidemiol. 2007;60:947-953.
54. Ludica CA. Can a program of manual physical therapy and supervised exercise improve the symptoms of osteoarthritis of the knee? J Fam Pract. 2000;49:466-467
CORRESPONDENCE Gail D. Deyle, PT, DSc, Orthopaedic Manual Physical Therapy Fellowship, 3551 Roger Brooke Drive, Brooke Army Medical Center, Ft. Sam Houston, TX 78234; [email protected]
Background The combination of manual physical therapy and exercise provides important benefit for more than 80% of patients with knee osteoarthritis (OA). Our objective was to determine predictor variables for patients unlikely to respond to these interventions.
Methods We used a retrospective combined cohort study design to develop a preliminary clinical prediction rule (CPR). To determine useful predictors of nonsuccess, we used an extensive set of 167 baseline variables. These variables were extracted from standardized examination forms used with 101 patients (64 women and 37 men with a mean age of 60.5±11.8 and 63.6±9.3 years, respectively) in 2 previously published clinical trials. We classified patients based on whether they achieved a clinically meaningful benefit of at least 12% improvement in Western Ontario MacMaster (WOMAC) scores after 4 weeks of treatment using the smallest and most efficient subset of predictors.
Results The variables of patellofemoral pain, anterior cruciate ligament laxity, and height >1.71 m (5’7’’) comprise the CPR. Patients with at least 2 positive tests yielded a posttest probability of 88% for nonsuccess with this treatment (positive likelihood ratio=36.7). The overall prognostic accuracy of the CPR was 96%.
Conclusion Most patients with knee OA will benefit from a low-risk, cost-effective program of manual physical therapy and supporting exercise.1,2 The few patients who may not benefit from such a program are identifiable by a simple (preliminary) CPR. After validation, this rule could improve primary patient management, allowing more appropriate referrals and choices in intervention.
Although the exact cause of knee OA is unclear, its incidence increases with age and it is particularly prevalent among women and those who are obese and have occupations requiring heavy lifting and frequent kneeling or squatting.3-6 Lifelong sport-specific activity7,8 and joint injury9 also seem to increase the risk for knee OA. Knee malalignment also may predispose people to knee OA,10 and the presence of early degenerative changes predicts progression of the disease.11 The disability and pain associated with knee OA correlate with a loss of quadriceps femoris muscle strength and limited joint range of motion.12-14
Medications and surgery carry substantial risks. Pharmacologic interventions for knee OA include nonsteroidal anti-inflammatory drugs, acetaminophen, and cyclooxygenase-2-selective inhibitors.15-17 While each of these drugs reduces pain and improves function, potential side effects include gastrointestinal, cardiovascular, renal, and hepatic complications.16,18-21
Effective surgical options—most appropriate for advanced OA—include high-tibial osteotomy and total knee arthroplasty (TKA). There is good evidence that arthroscopic surgery is not an effective intervention for knee OA, yielding results for pain and function equivalent to those seen with knee capsule injections of saline, tidal irrigation, and placebo surgery.22-25 TKA reduces pain, improves function, and decreases arthritis-related costs in older individuals with advanced knee OA.26,27 However, this procedure is not without risk.28 Total knee replacement in patients younger than 55 years is associated with increased mortality.29 Reported adverse outcomes of TKA include death, deep vein thrombosis, pulmonary embolus, deep wound infections,30,31 arterial lacerations, amputations,32 postoperative ileus,33 fractures, joint stiffness, and ligamentous instability.34 Viscosupplementation reduces pain and improves function, most evident at 5 to 13 weeks posttreatment, with few reported serious complications and moderate rates of local complications.35
Physical therapy is beneficial for mild to moderate OA and confers very low risk. Both physical therapy and exercise programs for OA have demonstrated benefit in a variety of settings.36-42 As shown in 2 independently conducted randomized controlled trials (RCTs) (one placebo controlled and one with an alternate treatment comparison), manual physical therapy applied during a small number of clinical sessions and supplemented by home exercise yields large reductions in pain and stiffness and improvements in functional ability persisting to 1 year as measured on the WOMAC Osteoarthritis Index,1,2 a validated self-report outcome instrument for OA of the hip and knee.43 In these studies, 60% of subjects receiving manual physical therapy and exercise achieved more than 50% improvement in WOMAC scores (pain, stiffness, and function) postintervention. Additionally, 83% achieved more than the minimal clinically important difference (MCID) of 12% improvement.1,2 Physical therapy and exercise combined also decreased the need for TKA and long-term medication use.1,2
For an intervention that benefits most patients, there is clearly an interest in determining predictors of treatment failure44 to expedite referral for alternative care. When the time or resources required to attend physical therapy appointments would create financial or personal hardships, more appropriate interventions may be home-based physical therapy exercise programs or medications and injections. Equally important, patients for whom knee OA rehabilitation is predicted to fail can be reprioritized for physical therapy aimed at coexisting conditions or injuries such as a functionally limiting impingement syndrome of the shoulder or chronic degenerative back or hip conditions.
Methods
Using a retrospective combined-cohort study design, we reviewed baseline patient examinations from 2 RCTs1,2 to identify variables that indicate which individuals with knee OA are unlikely to benefit from manual physical therapy and exercise, and to thereby develop a preliminary CPR. We extracted data from the research folders of all study participants. The institutional review board of Brooke Army Medical Center determined that the study was exempt from review. From April to December 2008, we prepared an extensive database of examination findings and performed analyses to determine the variables that predict likely treatment nonsuccess with manual physical therapy and exercise. Improvement of <12% in the total WOMAC score after 4 weeks of treatment defined nonsuccess.45
Data sets from the previously published trials contained 22 variables measured at baseline that were potential predictors of nonsuccess. We combined these variables with an additional 145 variables manually retrieved from standardized examination forms used for each subject, for a total of 167 potential predictors. We combined only data from treatment groups receiving manual therapy and exercise.
We limited the extent of some examination procedures in the earlier studies, due to the high level of symptoms experienced by some subjects at rest and during the initial examination. For example, if there was severe pain with active knee flexion, we did not perform passive manual overpressure to flexion; nor did we record a finding. Thus, the total number of data points for each subject varied somewhat.
Data analysis
We compared success and nonsuccess groups with 2-tailed unpaired t-tests for continuous variables, and chi-square tests for categorical variables. We additionally performed logistic regression analysis on potential predictors that yielded P values <.10, using a forward conditional stepwise procedure with probability levels set to .05 for entry and .10 for removal from the model. Predictors retained by the final logistic regression model comprised the CPR.
We coded each patient in the data set as positive or negative for each predictor in the CPR. To determine a cut score, we dichotomized the single retained continuous predictor variable using receiver-operator characteristic (ROC) curve analysis and the Youden index.46 For each CPR level (ie, increasing number of predictors positive), we constructed a 2 × 2 contingency table with numbers of patients with true-positive test results, false-positive test results, true-negative test results, and false-negative test results. We characterized prognostic performance of the CPR by calculating sensitivity, specificity, and positive likelihood ratios for each level of positive predictors. To determine overall prognostic accuracy, we added true positives and true negatives and divided by the total number of patients in the cross tabulation.
For each CPR level, we derived posttest probabilities of nonsuccess from generalized pretest probability (incidence of treatment nonsuccess in the sample) and the positive likelihood ratios.47 Finally, to determine how consistently the CPR performed with subjects in the original studies,1,2 we generated separate cross-tabulations and prognostic accuracy statistics from each RCT.
Results
Baseline patient attributes are summarized in TABLE 1. Of the 101 subjects in the combined data set, 17 (16.8%) met the definition of nonsuccess. Among 47 continuous-scale variables available, 11 predictors significantly discriminated between those in the treatment success and nonsuccess groups. Among 120 categorical-scale variables, 15 predictors significantly discriminated between groups. We identified 6 potential predictors for entry into the final logistic regression analysis: height, assistive device type, prone knee bend degrees, baseline WOMAC visual analog scale (VAS) for difficulty descending stairs, anterior cruciate ligament (ACL) laxity, and pain with passive patellofemoral glide.
TABLE 1
Baseline descriptive summaries of patients (n=101)
| Sex, n (%) Men Women | 37 (36.6) 64 (63.4) |
| Age, y Mean±SD Range | 62.5±10.4 39-85 |
| Height, m Mean±SD Range | 1.66±0.1041 1.42-1.91 |
| Side(s) involved, n (%) Unilateral Bilateral | 63 (62.4) 38 (37.6) |
| Weight, kg Mean±SD Range | 84.5±17.8 48.6-132.7 |
| Duration of symptoms, mo Mean±SD Range | 76.1±87.9 1-480 |
| WOMAC (VAS) total baseline, mm Mean±SD Range | 1059.8±447.1 193-2289 |
| 6-minute walk test baseline, m Mean±SD Range | 425.6±114.8 118.2-683.3 |
| Physical activity relative to peers (self-report), n (%) Much more active Somewhat more active About the same Somewhat less active | 26 (26) 33 (33) 20 (20) 21 (21) |
| Radiographic severity score, n (%) 0 1 2 3 4 | 6 (6.1) 25 (25.5) 33 (33.7) 25 (25.5) 9 (9.2) |
| *Baseline data were available for all 101 subjects except for duration of symptoms (n=98); physical activity (n=100); and radiographic severity (n=98). VAS, visual analog scale; WOMAC, Western Ontario MacMaster. | |
The final regression model retained 3 predictors comprising the CPR: height, ACL laxity, and pain with passive patellofemoral glides. We dichotomized height with a cut point of 1.71 m (5’7”), which corresponded with a deflection point at the upper left extent of the ROC curve (area under the curve=0.72; 95% CI, 0.57-0.87; P=.001). We thus deemed a patient 1.71 m or taller as positive for nonsuccess. We considered a patient with laxity of the ACL as positive for nonsuccess if a test result on the Lachman test (or the anterior drawer test) was positive (any grade other than 0). We regarded passive patellofemoral glide as positive for nonsuccess if a patient reported pain with any direction of passive gliding motion imposed by the therapist. The final regression model was a good fit to the data: Hosmer & Lemeshow test χ2 = 2.90 (P=.940); Nagelkerke R2=0.680.
TABLE 2 presents prognostic accuracy profiles for each predictor in the CPR; TABLE 3 summarizes the accuracy for each level of the multivariate CPR. Values in TABLE 3 reflect complete sets of data for the 3 predictors found for 50 patients. Of those 50 patients, 6 (12%) were in the nonsuccess group.
TABLE 2
Prognostic accuracy statistics for individual predictors
| Predictor | Sensitivity (95% CI) | Specificity (95% CI) | Positive likelihood ratio (95% CI) | Posttest probability of nonsuccess* |
|---|---|---|---|---|
| Height ≥1.71 m | 0.65 (0.41-0.83) | 0.77 (0.67-0.85) | 2.86 (1.69-4.86) | 37% |
| ACL laxity | 0.27 (0.10-0.57) | 0.93 (0.83-0.97) | 3.68 (0.96-14.19) | 43% |
| Pain with passive patellofemoral glide in any direction | 0.71 (0.35-0.92) | 0.61 (0.47-0.74) | 1.84 (1.03-3.31) | 27% |
| ACL, anterior cruciate ligament; CI, confidence interval. *Assumes pretest probability of nonsuccess=17% (incidence in this sample). | ||||
With any 2 of the 3 tests positive, the CPR yielded a sensitivity of 83% (95% CI, 44%-97%), specificity of 98% (95% CI, 88%-100%), and positive likelihood ratio of 36.7 (95% CI, 5.1-263.0). Only 2 patients out of 50 were misclassified (one false positive and one false negative) at this level of the CPR, yielding an overall prognostic accuracy of 96% (95% CI, 87%-99%). Application of the positive likelihood ratio for a patient with any 2 positive tests yielded a posttest probability of 88% for nonsuccess with this treatment.
TABLE 3
Prognostic accuracy statistics for 3-level clinical prediction rule
| CPR level | Sensitivity (95% CI) | Specificity (95% CI) | Positive likelihood ratio (95% CI) | Posttest probability of nonsuccess* |
|---|---|---|---|---|
| All 3 tests positive | 0.21 (0.05-0.58) | 0.99 (0.90-1.00) | 19.29 (0.87-428.09) | 80% |
| At least 2 tests positive | 0.83 (0.44-0.97) | 0.98 (0.88-1.00) | 36.67 (5.11-263.01) | 88% |
| At least 1 test positive | 0.92 (0.56-0.99) | 0.48 (0.34-0.62) | 1.78 (1.26-2.52) | 27% |
| CI, confidence interval; CPR, clinical prediction rule. *Assumes pretest probability of nonsuccess=17% (incidence in this sample). | ||||
In the sensitivity analysis, the CPR performed similarly well for patients in each of the 2 original studies when applied separately to the groups of patients. Among the 30 patients from the first trial2 who had data for all 3 predictors in the CPR, only one was misclassified (a false positive), yielding a prognostic accuracy of 97% (95% CI, 83%-99%). Among the 20 patients from the second trial1 who had data for all 3 predictors, only one was misclassified (a false negative), yielding a prognostic accuracy of 95% (95% CI, 76%-99%).
DISCUSSION
Family physicians and physical therapists should be able to discuss with confidence how any given patient with knee OA will likely respond to treatment options. Our study is a preliminary step toward defining the population of patients with knee OA who are unlikely to benefit from manual physical therapy and exercise. We found such patients to be those with height >1.71 m, ACL laxity, and pain with passive glides of the patellofemoral joint.
A limitation of our study is the retrospective nature of gathering data. However, retrospective CPR derivation studies have made valuable contributions to many areas of medical practice.48-53 Additionally, if there had been uniformly available data across all patients, there may have been other, perhaps more powerful, predictors for treatment nonsuccess.
Actual cases of knee osteoarthritis (OA) evaluated by one of the authors (GD)
A 48-year-old female elementary teacher was referred for physical therapy due to right knee pain and a diagnosis of OA that was limiting her ability to climb stairs and squat to work with children in the classroom. Her goals were to be able to perform these physical activities with less pain and to reduce her anti-inflammatory medications. However, she also worried about taking time away from her job to attend physical therapy appointments. She was 1.63 m (5’4”) tall and had a body mass index of 27.5 kg/m2. Her knee was stable to ligamentous testing, with mild limitation and pain with active and passive movement of both the tibiofemoral and the patellofemoral joints. She had weakness of the quadriceps and hip abductors, and moderate tightness of the calf muscles in both lower extremities.
Given the presence of only a single predictor for nonsuccess (pain with passive movement of her patella), the likelihood that this patient would not respond to manual physical therapy and exercise was just 27%, according to the clinical prediction rule. The impairments to movement, strength, and flexibility found during the physical examination typically can be successfully addressed with manual physical therapy. Additionally, one of the patient’s goals was to reduce her medication use—a reported outcome of the clinical trials used for deriving the rule.1,2 This patient was a good candidate for the intervention, with an acceptably small chance of not achieving a clinically meaningful benefit.
A 50-year-old male soldier 1.95 m (6’5”) tall was referred for physical therapy to ameliorate chronic pain due to tricompartmental knee OA. He exhibited anterior ligamentous laxity and felt severe pain with manually performed passive patellar glides (FIGURES 1 AND 2). He also had a rotator cuff tear and a mild traumatic brain injury from a roadside bomb blast. With 3/3 predictors for failure, the likelihood of reducing this patient’s knee symptoms with manual therapy and exercise was just 20%. The physical therapist and referring physician jointly decided to focus a small number of physical therapy visits on the patient’s shoulder, while giving rehabilitation priority to ongoing cognitive therapy appointments.
FIGURE 1
Lachman test
With the patient’s knee flexed at 30°, draw the proximal tibia anteriorly to observe movement of the tibia relative to the femur and thereby gauge anterior cruciate ligament integrity. Laxity is suggested by increased movement relative to the opposite knee.
FIGURE 2
Passive patellofemoral glide
With the patient’s knee slightly flexed, apply light pressure to the medial border of the patella, moving it laterally and taking care not to compress the patella. Repeat the procedure superiorly, inferiorly, and medially. A positive test is pain experienced with any of the glides.
Patient height >1.71 m is the least intuitive of the predictors for nonsuccess, but that underscores the value of data-driven prediction rules. Variables regarded as unimportant in a typical clinical assessment may show clinical usefulness if validated in independent studies. It may be that in taller patients with knee OA, biomechanical forces are such that a positive response to conservative therapy is less likely—particularly in the presence of ligamentous laxity or patellofemoral dysfunction.
For most patients with knee OA, the combined intervention of manual physical therapy and exercise is clinically beneficial, relatively inexpensive, and has no known adverse effects.54 However, unique circumstances may increase the importance of determining the likelihood that a patient will benefit. A validated CPR will facilitate timely decisions for those relatively few patients requiring alternative interventions. Although the rule is preliminary and needs to be validated, these results provide current best evidence to define patients with knee OA who are unlikely to respond to manual physical therapy and exercise.
Background The combination of manual physical therapy and exercise provides important benefit for more than 80% of patients with knee osteoarthritis (OA). Our objective was to determine predictor variables for patients unlikely to respond to these interventions.
Methods We used a retrospective combined cohort study design to develop a preliminary clinical prediction rule (CPR). To determine useful predictors of nonsuccess, we used an extensive set of 167 baseline variables. These variables were extracted from standardized examination forms used with 101 patients (64 women and 37 men with a mean age of 60.5±11.8 and 63.6±9.3 years, respectively) in 2 previously published clinical trials. We classified patients based on whether they achieved a clinically meaningful benefit of at least 12% improvement in Western Ontario MacMaster (WOMAC) scores after 4 weeks of treatment using the smallest and most efficient subset of predictors.
Results The variables of patellofemoral pain, anterior cruciate ligament laxity, and height >1.71 m (5’7’’) comprise the CPR. Patients with at least 2 positive tests yielded a posttest probability of 88% for nonsuccess with this treatment (positive likelihood ratio=36.7). The overall prognostic accuracy of the CPR was 96%.
Conclusion Most patients with knee OA will benefit from a low-risk, cost-effective program of manual physical therapy and supporting exercise.1,2 The few patients who may not benefit from such a program are identifiable by a simple (preliminary) CPR. After validation, this rule could improve primary patient management, allowing more appropriate referrals and choices in intervention.
Although the exact cause of knee OA is unclear, its incidence increases with age and it is particularly prevalent among women and those who are obese and have occupations requiring heavy lifting and frequent kneeling or squatting.3-6 Lifelong sport-specific activity7,8 and joint injury9 also seem to increase the risk for knee OA. Knee malalignment also may predispose people to knee OA,10 and the presence of early degenerative changes predicts progression of the disease.11 The disability and pain associated with knee OA correlate with a loss of quadriceps femoris muscle strength and limited joint range of motion.12-14
Medications and surgery carry substantial risks. Pharmacologic interventions for knee OA include nonsteroidal anti-inflammatory drugs, acetaminophen, and cyclooxygenase-2-selective inhibitors.15-17 While each of these drugs reduces pain and improves function, potential side effects include gastrointestinal, cardiovascular, renal, and hepatic complications.16,18-21
Effective surgical options—most appropriate for advanced OA—include high-tibial osteotomy and total knee arthroplasty (TKA). There is good evidence that arthroscopic surgery is not an effective intervention for knee OA, yielding results for pain and function equivalent to those seen with knee capsule injections of saline, tidal irrigation, and placebo surgery.22-25 TKA reduces pain, improves function, and decreases arthritis-related costs in older individuals with advanced knee OA.26,27 However, this procedure is not without risk.28 Total knee replacement in patients younger than 55 years is associated with increased mortality.29 Reported adverse outcomes of TKA include death, deep vein thrombosis, pulmonary embolus, deep wound infections,30,31 arterial lacerations, amputations,32 postoperative ileus,33 fractures, joint stiffness, and ligamentous instability.34 Viscosupplementation reduces pain and improves function, most evident at 5 to 13 weeks posttreatment, with few reported serious complications and moderate rates of local complications.35
Physical therapy is beneficial for mild to moderate OA and confers very low risk. Both physical therapy and exercise programs for OA have demonstrated benefit in a variety of settings.36-42 As shown in 2 independently conducted randomized controlled trials (RCTs) (one placebo controlled and one with an alternate treatment comparison), manual physical therapy applied during a small number of clinical sessions and supplemented by home exercise yields large reductions in pain and stiffness and improvements in functional ability persisting to 1 year as measured on the WOMAC Osteoarthritis Index,1,2 a validated self-report outcome instrument for OA of the hip and knee.43 In these studies, 60% of subjects receiving manual physical therapy and exercise achieved more than 50% improvement in WOMAC scores (pain, stiffness, and function) postintervention. Additionally, 83% achieved more than the minimal clinically important difference (MCID) of 12% improvement.1,2 Physical therapy and exercise combined also decreased the need for TKA and long-term medication use.1,2
For an intervention that benefits most patients, there is clearly an interest in determining predictors of treatment failure44 to expedite referral for alternative care. When the time or resources required to attend physical therapy appointments would create financial or personal hardships, more appropriate interventions may be home-based physical therapy exercise programs or medications and injections. Equally important, patients for whom knee OA rehabilitation is predicted to fail can be reprioritized for physical therapy aimed at coexisting conditions or injuries such as a functionally limiting impingement syndrome of the shoulder or chronic degenerative back or hip conditions.
Methods
Using a retrospective combined-cohort study design, we reviewed baseline patient examinations from 2 RCTs1,2 to identify variables that indicate which individuals with knee OA are unlikely to benefit from manual physical therapy and exercise, and to thereby develop a preliminary CPR. We extracted data from the research folders of all study participants. The institutional review board of Brooke Army Medical Center determined that the study was exempt from review. From April to December 2008, we prepared an extensive database of examination findings and performed analyses to determine the variables that predict likely treatment nonsuccess with manual physical therapy and exercise. Improvement of <12% in the total WOMAC score after 4 weeks of treatment defined nonsuccess.45
Data sets from the previously published trials contained 22 variables measured at baseline that were potential predictors of nonsuccess. We combined these variables with an additional 145 variables manually retrieved from standardized examination forms used for each subject, for a total of 167 potential predictors. We combined only data from treatment groups receiving manual therapy and exercise.
We limited the extent of some examination procedures in the earlier studies, due to the high level of symptoms experienced by some subjects at rest and during the initial examination. For example, if there was severe pain with active knee flexion, we did not perform passive manual overpressure to flexion; nor did we record a finding. Thus, the total number of data points for each subject varied somewhat.
Data analysis
We compared success and nonsuccess groups with 2-tailed unpaired t-tests for continuous variables, and chi-square tests for categorical variables. We additionally performed logistic regression analysis on potential predictors that yielded P values <.10, using a forward conditional stepwise procedure with probability levels set to .05 for entry and .10 for removal from the model. Predictors retained by the final logistic regression model comprised the CPR.
We coded each patient in the data set as positive or negative for each predictor in the CPR. To determine a cut score, we dichotomized the single retained continuous predictor variable using receiver-operator characteristic (ROC) curve analysis and the Youden index.46 For each CPR level (ie, increasing number of predictors positive), we constructed a 2 × 2 contingency table with numbers of patients with true-positive test results, false-positive test results, true-negative test results, and false-negative test results. We characterized prognostic performance of the CPR by calculating sensitivity, specificity, and positive likelihood ratios for each level of positive predictors. To determine overall prognostic accuracy, we added true positives and true negatives and divided by the total number of patients in the cross tabulation.
For each CPR level, we derived posttest probabilities of nonsuccess from generalized pretest probability (incidence of treatment nonsuccess in the sample) and the positive likelihood ratios.47 Finally, to determine how consistently the CPR performed with subjects in the original studies,1,2 we generated separate cross-tabulations and prognostic accuracy statistics from each RCT.
Results
Baseline patient attributes are summarized in TABLE 1. Of the 101 subjects in the combined data set, 17 (16.8%) met the definition of nonsuccess. Among 47 continuous-scale variables available, 11 predictors significantly discriminated between those in the treatment success and nonsuccess groups. Among 120 categorical-scale variables, 15 predictors significantly discriminated between groups. We identified 6 potential predictors for entry into the final logistic regression analysis: height, assistive device type, prone knee bend degrees, baseline WOMAC visual analog scale (VAS) for difficulty descending stairs, anterior cruciate ligament (ACL) laxity, and pain with passive patellofemoral glide.
TABLE 1
Baseline descriptive summaries of patients (n=101)
| Sex, n (%) Men Women | 37 (36.6) 64 (63.4) |
| Age, y Mean±SD Range | 62.5±10.4 39-85 |
| Height, m Mean±SD Range | 1.66±0.1041 1.42-1.91 |
| Side(s) involved, n (%) Unilateral Bilateral | 63 (62.4) 38 (37.6) |
| Weight, kg Mean±SD Range | 84.5±17.8 48.6-132.7 |
| Duration of symptoms, mo Mean±SD Range | 76.1±87.9 1-480 |
| WOMAC (VAS) total baseline, mm Mean±SD Range | 1059.8±447.1 193-2289 |
| 6-minute walk test baseline, m Mean±SD Range | 425.6±114.8 118.2-683.3 |
| Physical activity relative to peers (self-report), n (%) Much more active Somewhat more active About the same Somewhat less active | 26 (26) 33 (33) 20 (20) 21 (21) |
| Radiographic severity score, n (%) 0 1 2 3 4 | 6 (6.1) 25 (25.5) 33 (33.7) 25 (25.5) 9 (9.2) |
| *Baseline data were available for all 101 subjects except for duration of symptoms (n=98); physical activity (n=100); and radiographic severity (n=98). VAS, visual analog scale; WOMAC, Western Ontario MacMaster. | |
The final regression model retained 3 predictors comprising the CPR: height, ACL laxity, and pain with passive patellofemoral glides. We dichotomized height with a cut point of 1.71 m (5’7”), which corresponded with a deflection point at the upper left extent of the ROC curve (area under the curve=0.72; 95% CI, 0.57-0.87; P=.001). We thus deemed a patient 1.71 m or taller as positive for nonsuccess. We considered a patient with laxity of the ACL as positive for nonsuccess if a test result on the Lachman test (or the anterior drawer test) was positive (any grade other than 0). We regarded passive patellofemoral glide as positive for nonsuccess if a patient reported pain with any direction of passive gliding motion imposed by the therapist. The final regression model was a good fit to the data: Hosmer & Lemeshow test χ2 = 2.90 (P=.940); Nagelkerke R2=0.680.
TABLE 2 presents prognostic accuracy profiles for each predictor in the CPR; TABLE 3 summarizes the accuracy for each level of the multivariate CPR. Values in TABLE 3 reflect complete sets of data for the 3 predictors found for 50 patients. Of those 50 patients, 6 (12%) were in the nonsuccess group.
TABLE 2
Prognostic accuracy statistics for individual predictors
| Predictor | Sensitivity (95% CI) | Specificity (95% CI) | Positive likelihood ratio (95% CI) | Posttest probability of nonsuccess* |
|---|---|---|---|---|
| Height ≥1.71 m | 0.65 (0.41-0.83) | 0.77 (0.67-0.85) | 2.86 (1.69-4.86) | 37% |
| ACL laxity | 0.27 (0.10-0.57) | 0.93 (0.83-0.97) | 3.68 (0.96-14.19) | 43% |
| Pain with passive patellofemoral glide in any direction | 0.71 (0.35-0.92) | 0.61 (0.47-0.74) | 1.84 (1.03-3.31) | 27% |
| ACL, anterior cruciate ligament; CI, confidence interval. *Assumes pretest probability of nonsuccess=17% (incidence in this sample). | ||||
With any 2 of the 3 tests positive, the CPR yielded a sensitivity of 83% (95% CI, 44%-97%), specificity of 98% (95% CI, 88%-100%), and positive likelihood ratio of 36.7 (95% CI, 5.1-263.0). Only 2 patients out of 50 were misclassified (one false positive and one false negative) at this level of the CPR, yielding an overall prognostic accuracy of 96% (95% CI, 87%-99%). Application of the positive likelihood ratio for a patient with any 2 positive tests yielded a posttest probability of 88% for nonsuccess with this treatment.
TABLE 3
Prognostic accuracy statistics for 3-level clinical prediction rule
| CPR level | Sensitivity (95% CI) | Specificity (95% CI) | Positive likelihood ratio (95% CI) | Posttest probability of nonsuccess* |
|---|---|---|---|---|
| All 3 tests positive | 0.21 (0.05-0.58) | 0.99 (0.90-1.00) | 19.29 (0.87-428.09) | 80% |
| At least 2 tests positive | 0.83 (0.44-0.97) | 0.98 (0.88-1.00) | 36.67 (5.11-263.01) | 88% |
| At least 1 test positive | 0.92 (0.56-0.99) | 0.48 (0.34-0.62) | 1.78 (1.26-2.52) | 27% |
| CI, confidence interval; CPR, clinical prediction rule. *Assumes pretest probability of nonsuccess=17% (incidence in this sample). | ||||
In the sensitivity analysis, the CPR performed similarly well for patients in each of the 2 original studies when applied separately to the groups of patients. Among the 30 patients from the first trial2 who had data for all 3 predictors in the CPR, only one was misclassified (a false positive), yielding a prognostic accuracy of 97% (95% CI, 83%-99%). Among the 20 patients from the second trial1 who had data for all 3 predictors, only one was misclassified (a false negative), yielding a prognostic accuracy of 95% (95% CI, 76%-99%).
DISCUSSION
Family physicians and physical therapists should be able to discuss with confidence how any given patient with knee OA will likely respond to treatment options. Our study is a preliminary step toward defining the population of patients with knee OA who are unlikely to benefit from manual physical therapy and exercise. We found such patients to be those with height >1.71 m, ACL laxity, and pain with passive glides of the patellofemoral joint.
A limitation of our study is the retrospective nature of gathering data. However, retrospective CPR derivation studies have made valuable contributions to many areas of medical practice.48-53 Additionally, if there had been uniformly available data across all patients, there may have been other, perhaps more powerful, predictors for treatment nonsuccess.
Actual cases of knee osteoarthritis (OA) evaluated by one of the authors (GD)
A 48-year-old female elementary teacher was referred for physical therapy due to right knee pain and a diagnosis of OA that was limiting her ability to climb stairs and squat to work with children in the classroom. Her goals were to be able to perform these physical activities with less pain and to reduce her anti-inflammatory medications. However, she also worried about taking time away from her job to attend physical therapy appointments. She was 1.63 m (5’4”) tall and had a body mass index of 27.5 kg/m2. Her knee was stable to ligamentous testing, with mild limitation and pain with active and passive movement of both the tibiofemoral and the patellofemoral joints. She had weakness of the quadriceps and hip abductors, and moderate tightness of the calf muscles in both lower extremities.
Given the presence of only a single predictor for nonsuccess (pain with passive movement of her patella), the likelihood that this patient would not respond to manual physical therapy and exercise was just 27%, according to the clinical prediction rule. The impairments to movement, strength, and flexibility found during the physical examination typically can be successfully addressed with manual physical therapy. Additionally, one of the patient’s goals was to reduce her medication use—a reported outcome of the clinical trials used for deriving the rule.1,2 This patient was a good candidate for the intervention, with an acceptably small chance of not achieving a clinically meaningful benefit.
A 50-year-old male soldier 1.95 m (6’5”) tall was referred for physical therapy to ameliorate chronic pain due to tricompartmental knee OA. He exhibited anterior ligamentous laxity and felt severe pain with manually performed passive patellar glides (FIGURES 1 AND 2). He also had a rotator cuff tear and a mild traumatic brain injury from a roadside bomb blast. With 3/3 predictors for failure, the likelihood of reducing this patient’s knee symptoms with manual therapy and exercise was just 20%. The physical therapist and referring physician jointly decided to focus a small number of physical therapy visits on the patient’s shoulder, while giving rehabilitation priority to ongoing cognitive therapy appointments.
FIGURE 1
Lachman test
With the patient’s knee flexed at 30°, draw the proximal tibia anteriorly to observe movement of the tibia relative to the femur and thereby gauge anterior cruciate ligament integrity. Laxity is suggested by increased movement relative to the opposite knee.
FIGURE 2
Passive patellofemoral glide
With the patient’s knee slightly flexed, apply light pressure to the medial border of the patella, moving it laterally and taking care not to compress the patella. Repeat the procedure superiorly, inferiorly, and medially. A positive test is pain experienced with any of the glides.
Patient height >1.71 m is the least intuitive of the predictors for nonsuccess, but that underscores the value of data-driven prediction rules. Variables regarded as unimportant in a typical clinical assessment may show clinical usefulness if validated in independent studies. It may be that in taller patients with knee OA, biomechanical forces are such that a positive response to conservative therapy is less likely—particularly in the presence of ligamentous laxity or patellofemoral dysfunction.
For most patients with knee OA, the combined intervention of manual physical therapy and exercise is clinically beneficial, relatively inexpensive, and has no known adverse effects.54 However, unique circumstances may increase the importance of determining the likelihood that a patient will benefit. A validated CPR will facilitate timely decisions for those relatively few patients requiring alternative interventions. Although the rule is preliminary and needs to be validated, these results provide current best evidence to define patients with knee OA who are unlikely to respond to manual physical therapy and exercise.
1. Deyle GD, Allison SC, Matekel RL, et al. Physical therapy treatment effectiveness for osteoarthritis of the knee: a randomized comparison of supervised clinical exercise and manual therapy procedures versus a home exercise program. Phys Ther. 2005;85:1301-1317.
2. Deyle GD, Henderson NE, Matekel RL, et al. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee. A randomized, controlled trial. Ann Intern Med. 2000;132:173-181
3. Sandmark H, Hogstedt C, Vingard E. Primary osteoarthrosis of the knee in men and women as a result of lifelong physical load from work. Scand J Work Environ Health. 2000;26:20-25.
4. Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40:728-733.
5. Jarvholm B, From C, Lewold S, et al. Incidence of surgically treated osteoarthritis in the hip and knee in male construction workers. Occup Environ Med. 2008;65:275-278.
6. Messier SP, Loeser RF, Mitchell MN, et al. Exercise and weight loss in obese older adults with knee osteoarthritis: a preliminary study. J Am Geriatr Soc. 2000;48:1062-1072.
7. Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;41:1343-1355.
8. Sandmark H, Vingard E. Sports and risk for severe osteoarthrosis of the knee. Scand J Med Sci Sports. 1999;9:279-284.
9. Gelber AC, Hochberg MC, Mead LA, et al. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133:321-328.
10. Cerejo R, Dunlop DD, Cahue S, et al. The influence of alignment on risk of knee osteoarthritis progression according to baseline stage of disease. Arthritis Rheum. 2002;46:2632-2636.
11. Wolfe F, Lane NE. The longterm outcome of osteoarthritis: rates and predictors of joint space narrowing in symptomatic patients with knee osteoarthritis. J Rheumatol. 2002;29:139-146.
12. Lewek MD, Rudolph KS, Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res. 2004;22:110-115.
13. Fitzgerald GK, Piva SR, Irrgang JJ. Reports of joint instability in knee osteoarthritis: its prevalence and relationship to physical function. Arthritis Rheum. 2004;51:941-946.
14. Fitzgerald GK, Piva SR, Irrgang JJ, et al. Quadriceps activation failure as a moderator of the relationship between quadriceps strength and physical function in individuals with knee osteoarthritis. Arthritis Rheum. 2004;51:40-48.
15. Scott DL, Berry H, Capell H, et al. The long-term effects of non-steroidal anti-inflammatory drugs in osteoarthritis of the knee: a randomized placebo-controlled trial. Rheumatology (Oxford). 2000;39:1095-1101.
16. Towheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257.-
17. Kivitz A, Fairfax M, Sheldon EA, et al. Comparison of the effectiveness and tolerability of lidocaine patch 5% versus celecoxib for osteoarthritis-related knee pain: post hoc analysis of a 12 week, prospective, randomized, active-controlled, open-label, parallel-group trial in adults. Clin Ther. 2008;30:2366-2377.
18. Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081-1091.
19. Psaty BM, Furberg CD. COX-2 inhibitors—lessons in drug safety. N Engl J Med. 2005;352:1133-1135.
20. Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071-1080.
21. Wittenberg RH, Schell E, Krehan G, et al. First-dose analgesic effect of the cyclo-oxygenase-2 selective inhibitor lumiracoxib in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled comparison with celecoxib [NCT00267215]. Arthritis Res Ther. 2006;8:R35.-
22. Bradley JD, Heilman DK, Katz BP, et al. Tidal irrigation as treatment for knee osteoarthritis: a sham-controlled, randomized, double-blinded evaluation. Arthritis Rheum. 2002;46:100-108.
23. Chang RW, Falconer J, Stulberg SD, et al. A randomized, controlled trial of arthroscopic surgery versus closed-needle joint lavage for patients with osteoarthritis of the knee. Arthritis Rheum. 1993;36:289-296.
24. Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81-88.
25. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, et al. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118.-
26. Hawker GA, Badley EM, Croxford R, et al. A population-based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47:732-741.
27. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169:1113-1121.
28. Hamel MB, Toth M, Legedza A, et al. Joint replacement surgery in elderly patients with severe osteoarthritis of the hip or knee: decision making, postoperative recovery, and clinical outcomes. Arch Intern Med. 2008;168:1430-1440.
29. Robertsson O, Stefansdottir A, Lidgren L, et al. Increased long-term mortality in patients less than 55 years old who have undergone knee replacement for osteoarthritis: results from the Swedish Knee Arthroplasty Register. J Bone Joint Surg Br. 2007;89:599-603.
30. SooHoo NF, Lieberman JR, Ko CY, et al. Factors predicting complication rates following total knee replacement. J Bone Joint Surg Am. 2006;88:480-485.
31. Solomon DH, Chibnik LB, Losina E, et al. Development of a preliminary index that predicts adverse events after total knee replacement. Arthritis Rheum. 2006;54:1536-1542.
32. Abularrage CJ, Weiswasser JM, Dezee KJ, et al. Predictors of lower extremity arterial injury after total knee or total hip arthroplasty. J Vasc Surg. 2008;47:803-807.
33. Parvizi J, Han SB, Tarity TD, et al. Postoperative ileus after total joint arthroplasty. J Arthroplasty. 2008;23:360-365.
34. Pinaroli A, Piedade SR, Servien E, et al. Intraoperative fractures and ligament tears during total knee arthroplasty. A 1795 posterostabilized TKA continuous series. Orthop Traumatol Surg Res. 2009;95:183-189
35. Bellamy N, Campbell J, Robinson V, et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321.-
36. Baker K, McAlindon T. Exercise for knee osteoarthritis. Curr Opin Rheumatol. 2000;12:456-463.
37. Baker KR, Nelson ME, Felson DT, et al. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol. 2001;28:1655-1665.
38. O’Reilly SC, Muir KR, Doherty M. Effectiveness of home exercise on pain and disability from osteoarthritis of the knee: a randomised controlled trial. Ann Rheum Dis. 1999;58:15-19.
39. Petrella RJ, Bartha C. Home based exercise therapy for older patients with knee osteoarthritis: a randomized clinical trial. J Rheumatol. 2000;27:2215-2221.
40. van Baar ME, Dekker J, Oostendorp RA, et al. Effectiveness of exercise in patients with osteoarthritis of hip or knee: nine months’ follow up. Ann Rheum Dis. 2001;60:1123-1130.
41. Silva LE, Valim V, Pessanha AP, et al. Hydrotherapy versus conventional land-based exercise for the management of patients with osteoarthritis of the knee: a randomized clinical trial. Phys Ther. 2008;88:12-21.
42. Hinman RS, Heywood SE, Day AR. Aquatic physical therapy for hip and knee osteoarthritis: results of a single-blind randomized controlled trial. Phys Ther. 2007;87:32-43.
43. Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29:2473-2476.
44. Fritz JM. Clinical prediction rules in physical therapy: coming of age? J Orthop Sports Phys Ther. 2009;39:159-161
45. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45:384-391.
46. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32-35.
47. Fritz JM, Wainner RS. Examining diagnostic tests: an evidence-based perspective. Phys Ther. 2001;81:1546-1564.
48. van Walraven C, Hart RG, Wells GA, et al. A clinical prediction rule to identify patients with atrial fibrillation and a low risk for stroke while taking aspirin. Arch Intern Med. 2003;163:936-943.
49. Predictors of thromboembolism in atrial fibrillation: I. Clinical features of patients at risk. The Stroke Prevention in Atrial Fibrillation Investigators. Ann Intern Med. 1992;116:1-5
50. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041-1046.
51. Aujesky D, Obrosky DS, Stone RA, et al. A prediction rule to identify low-risk patients with pulmonary embolism. Arch Intern Med. 2006;166:169-175.
52. Espana PP, Capelastegui A, Gorordo I, et al. Development and validation of a clinical prediction rule for severe community-acquired pneumonia. Am J Respir Crit Care Med. 2006;174:1249-1256.
53. Kuijpers T, van der Heijden GJ, Vergouwe Y, et al. Good generalizability of a prediction rule for prediction of persistent shoulder pain in the short term. J Clin Epidemiol. 2007;60:947-953.
54. Ludica CA. Can a program of manual physical therapy and supervised exercise improve the symptoms of osteoarthritis of the knee? J Fam Pract. 2000;49:466-467
CORRESPONDENCE Gail D. Deyle, PT, DSc, Orthopaedic Manual Physical Therapy Fellowship, 3551 Roger Brooke Drive, Brooke Army Medical Center, Ft. Sam Houston, TX 78234; [email protected]
1. Deyle GD, Allison SC, Matekel RL, et al. Physical therapy treatment effectiveness for osteoarthritis of the knee: a randomized comparison of supervised clinical exercise and manual therapy procedures versus a home exercise program. Phys Ther. 2005;85:1301-1317.
2. Deyle GD, Henderson NE, Matekel RL, et al. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee. A randomized, controlled trial. Ann Intern Med. 2000;132:173-181
3. Sandmark H, Hogstedt C, Vingard E. Primary osteoarthrosis of the knee in men and women as a result of lifelong physical load from work. Scand J Work Environ Health. 2000;26:20-25.
4. Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40:728-733.
5. Jarvholm B, From C, Lewold S, et al. Incidence of surgically treated osteoarthritis in the hip and knee in male construction workers. Occup Environ Med. 2008;65:275-278.
6. Messier SP, Loeser RF, Mitchell MN, et al. Exercise and weight loss in obese older adults with knee osteoarthritis: a preliminary study. J Am Geriatr Soc. 2000;48:1062-1072.
7. Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998;41:1343-1355.
8. Sandmark H, Vingard E. Sports and risk for severe osteoarthrosis of the knee. Scand J Med Sci Sports. 1999;9:279-284.
9. Gelber AC, Hochberg MC, Mead LA, et al. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133:321-328.
10. Cerejo R, Dunlop DD, Cahue S, et al. The influence of alignment on risk of knee osteoarthritis progression according to baseline stage of disease. Arthritis Rheum. 2002;46:2632-2636.
11. Wolfe F, Lane NE. The longterm outcome of osteoarthritis: rates and predictors of joint space narrowing in symptomatic patients with knee osteoarthritis. J Rheumatol. 2002;29:139-146.
12. Lewek MD, Rudolph KS, Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res. 2004;22:110-115.
13. Fitzgerald GK, Piva SR, Irrgang JJ. Reports of joint instability in knee osteoarthritis: its prevalence and relationship to physical function. Arthritis Rheum. 2004;51:941-946.
14. Fitzgerald GK, Piva SR, Irrgang JJ, et al. Quadriceps activation failure as a moderator of the relationship between quadriceps strength and physical function in individuals with knee osteoarthritis. Arthritis Rheum. 2004;51:40-48.
15. Scott DL, Berry H, Capell H, et al. The long-term effects of non-steroidal anti-inflammatory drugs in osteoarthritis of the knee: a randomized placebo-controlled trial. Rheumatology (Oxford). 2000;39:1095-1101.
16. Towheed TE, Maxwell L, Judd MG, et al. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257.-
17. Kivitz A, Fairfax M, Sheldon EA, et al. Comparison of the effectiveness and tolerability of lidocaine patch 5% versus celecoxib for osteoarthritis-related knee pain: post hoc analysis of a 12 week, prospective, randomized, active-controlled, open-label, parallel-group trial in adults. Clin Ther. 2008;30:2366-2377.
18. Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081-1091.
19. Psaty BM, Furberg CD. COX-2 inhibitors—lessons in drug safety. N Engl J Med. 2005;352:1133-1135.
20. Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071-1080.
21. Wittenberg RH, Schell E, Krehan G, et al. First-dose analgesic effect of the cyclo-oxygenase-2 selective inhibitor lumiracoxib in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled comparison with celecoxib [NCT00267215]. Arthritis Res Ther. 2006;8:R35.-
22. Bradley JD, Heilman DK, Katz BP, et al. Tidal irrigation as treatment for knee osteoarthritis: a sham-controlled, randomized, double-blinded evaluation. Arthritis Rheum. 2002;46:100-108.
23. Chang RW, Falconer J, Stulberg SD, et al. A randomized, controlled trial of arthroscopic surgery versus closed-needle joint lavage for patients with osteoarthritis of the knee. Arthritis Rheum. 1993;36:289-296.
24. Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81-88.
25. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, et al. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118.-
26. Hawker GA, Badley EM, Croxford R, et al. A population-based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47:732-741.
27. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169:1113-1121.
28. Hamel MB, Toth M, Legedza A, et al. Joint replacement surgery in elderly patients with severe osteoarthritis of the hip or knee: decision making, postoperative recovery, and clinical outcomes. Arch Intern Med. 2008;168:1430-1440.
29. Robertsson O, Stefansdottir A, Lidgren L, et al. Increased long-term mortality in patients less than 55 years old who have undergone knee replacement for osteoarthritis: results from the Swedish Knee Arthroplasty Register. J Bone Joint Surg Br. 2007;89:599-603.
30. SooHoo NF, Lieberman JR, Ko CY, et al. Factors predicting complication rates following total knee replacement. J Bone Joint Surg Am. 2006;88:480-485.
31. Solomon DH, Chibnik LB, Losina E, et al. Development of a preliminary index that predicts adverse events after total knee replacement. Arthritis Rheum. 2006;54:1536-1542.
32. Abularrage CJ, Weiswasser JM, Dezee KJ, et al. Predictors of lower extremity arterial injury after total knee or total hip arthroplasty. J Vasc Surg. 2008;47:803-807.
33. Parvizi J, Han SB, Tarity TD, et al. Postoperative ileus after total joint arthroplasty. J Arthroplasty. 2008;23:360-365.
34. Pinaroli A, Piedade SR, Servien E, et al. Intraoperative fractures and ligament tears during total knee arthroplasty. A 1795 posterostabilized TKA continuous series. Orthop Traumatol Surg Res. 2009;95:183-189
35. Bellamy N, Campbell J, Robinson V, et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321.-
36. Baker K, McAlindon T. Exercise for knee osteoarthritis. Curr Opin Rheumatol. 2000;12:456-463.
37. Baker KR, Nelson ME, Felson DT, et al. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol. 2001;28:1655-1665.
38. O’Reilly SC, Muir KR, Doherty M. Effectiveness of home exercise on pain and disability from osteoarthritis of the knee: a randomised controlled trial. Ann Rheum Dis. 1999;58:15-19.
39. Petrella RJ, Bartha C. Home based exercise therapy for older patients with knee osteoarthritis: a randomized clinical trial. J Rheumatol. 2000;27:2215-2221.
40. van Baar ME, Dekker J, Oostendorp RA, et al. Effectiveness of exercise in patients with osteoarthritis of hip or knee: nine months’ follow up. Ann Rheum Dis. 2001;60:1123-1130.
41. Silva LE, Valim V, Pessanha AP, et al. Hydrotherapy versus conventional land-based exercise for the management of patients with osteoarthritis of the knee: a randomized clinical trial. Phys Ther. 2008;88:12-21.
42. Hinman RS, Heywood SE, Day AR. Aquatic physical therapy for hip and knee osteoarthritis: results of a single-blind randomized controlled trial. Phys Ther. 2007;87:32-43.
43. Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29:2473-2476.
44. Fritz JM. Clinical prediction rules in physical therapy: coming of age? J Orthop Sports Phys Ther. 2009;39:159-161
45. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45:384-391.
46. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32-35.
47. Fritz JM, Wainner RS. Examining diagnostic tests: an evidence-based perspective. Phys Ther. 2001;81:1546-1564.
48. van Walraven C, Hart RG, Wells GA, et al. A clinical prediction rule to identify patients with atrial fibrillation and a low risk for stroke while taking aspirin. Arch Intern Med. 2003;163:936-943.
49. Predictors of thromboembolism in atrial fibrillation: I. Clinical features of patients at risk. The Stroke Prevention in Atrial Fibrillation Investigators. Ann Intern Med. 1992;116:1-5
50. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041-1046.
51. Aujesky D, Obrosky DS, Stone RA, et al. A prediction rule to identify low-risk patients with pulmonary embolism. Arch Intern Med. 2006;166:169-175.
52. Espana PP, Capelastegui A, Gorordo I, et al. Development and validation of a clinical prediction rule for severe community-acquired pneumonia. Am J Respir Crit Care Med. 2006;174:1249-1256.
53. Kuijpers T, van der Heijden GJ, Vergouwe Y, et al. Good generalizability of a prediction rule for prediction of persistent shoulder pain in the short term. J Clin Epidemiol. 2007;60:947-953.
54. Ludica CA. Can a program of manual physical therapy and supervised exercise improve the symptoms of osteoarthritis of the knee? J Fam Pract. 2000;49:466-467
CORRESPONDENCE Gail D. Deyle, PT, DSc, Orthopaedic Manual Physical Therapy Fellowship, 3551 Roger Brooke Drive, Brooke Army Medical Center, Ft. Sam Houston, TX 78234; [email protected]