User login
Case Report
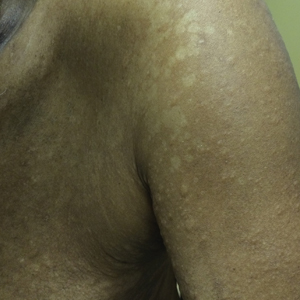
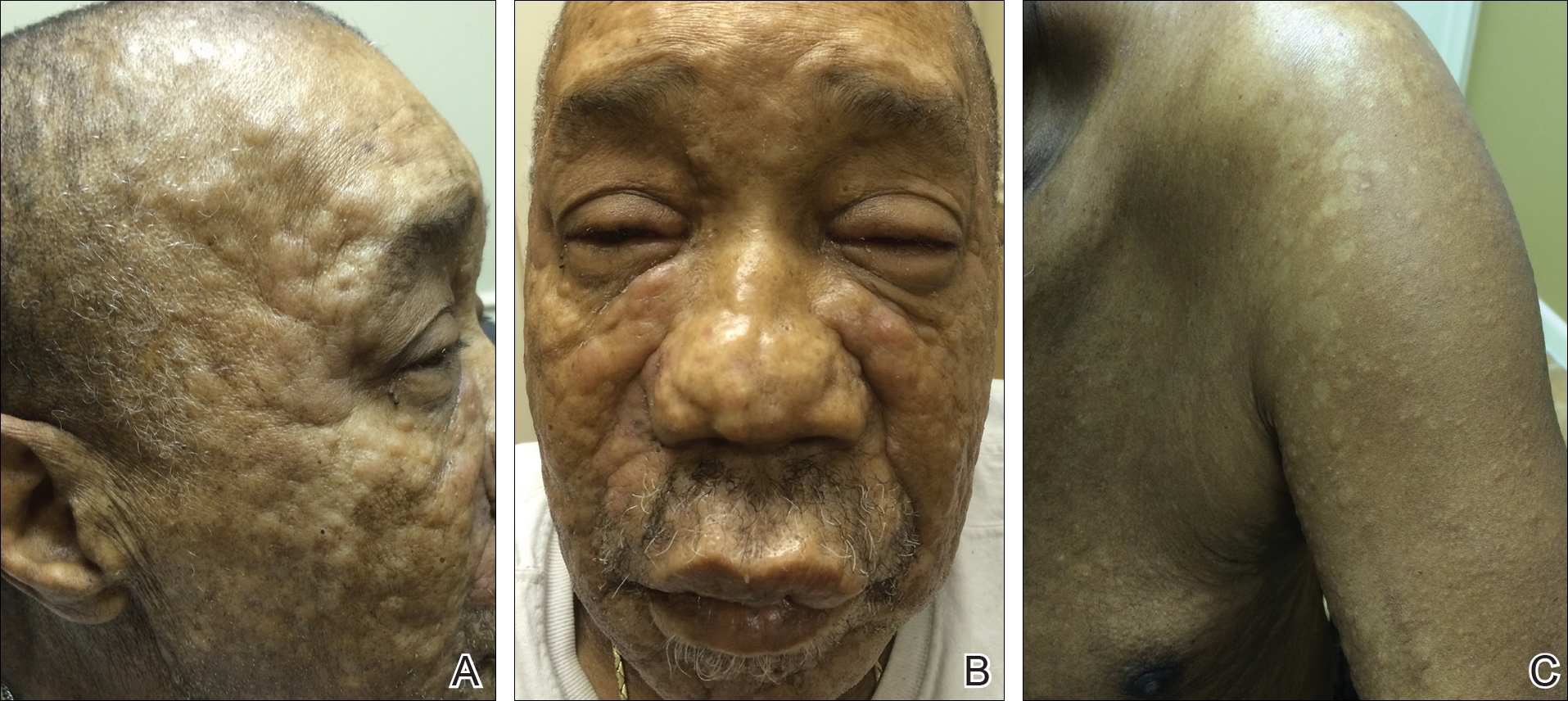
A 66-year-old man with a history of type 2 diabetes mellitus presented with considerable muscle weakness and infiltrative, flesh-colored plaques on the face, trunk, and arms of 3 months’ duration. The patient required the use of a wheelchair due to muscle weakness. On physical examination he had diffuse, infiltrative, flesh-colored plaques on the entire face (Figure 1A), trunk, and arms. The eyelids and lips were swollen, and the nose was distorted due to the infiltrative plaques (Figure 1B). Additionally, there were hypopigmented macules and patches scattered among the infiltrative plaques on the face, trunk, and arms (Figure 1C).
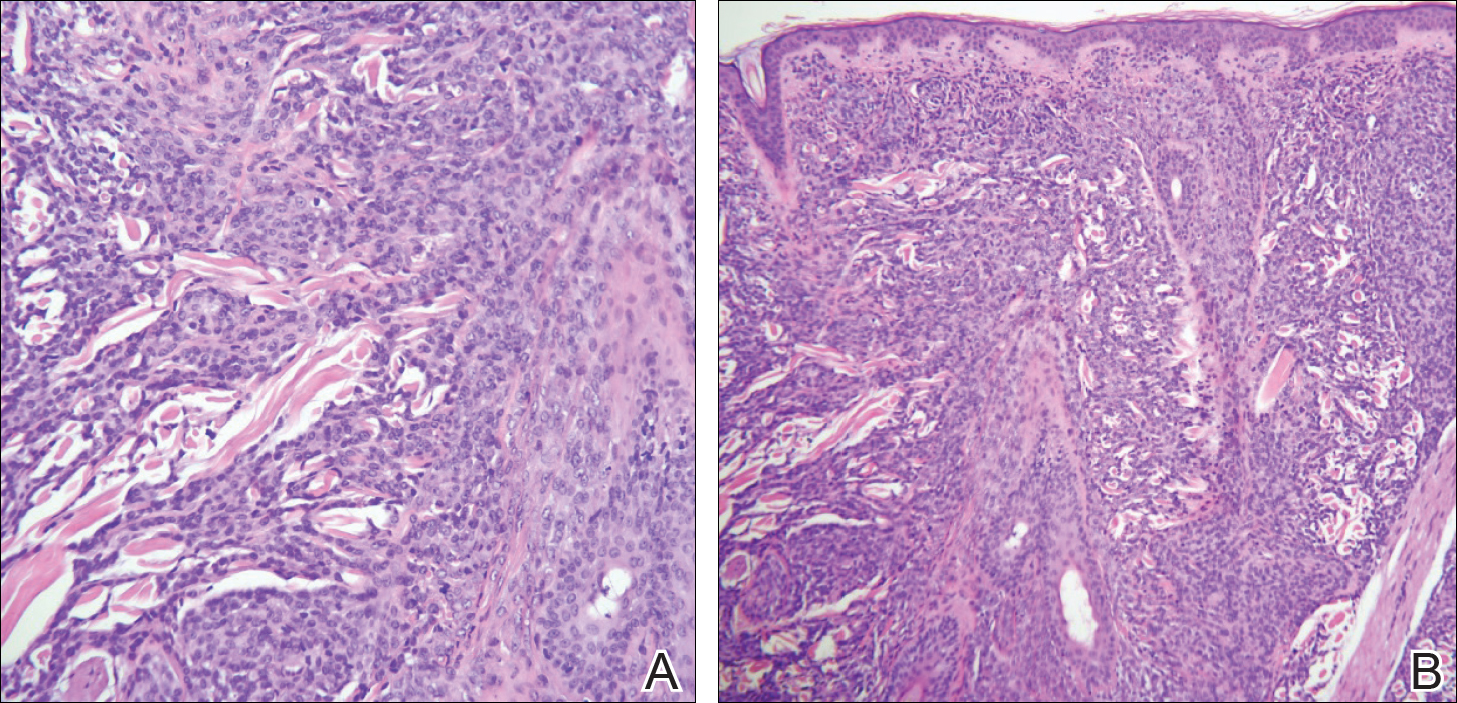
Punch biopsy specimens were obtained from the left cheek and left upper arm and were submitted for histologic examination with routine hematoxylin and eosin staining (Figure 2). Histopathology showed infiltrating and diffuse monomorphic cells in the dermis with large and hyperchromatic nuclei. Some nuclei were cleaved or folded in configuration. The cells displayed ample surrounding cytoplasm, which was finely granular or vacuolated. The infiltrate was accentuated in the perifollicular adventitial dermis. Immunohistochemistry was positive for CD33 and negative for CD3, CD20, and myeloperoxidase. Additionally, periodic acid–Schiff and Fite stains were negative for microorganisms. These morphologic and immunohistochemical findings were consistent with acute myeloid leukemia (AML). Further testing with complete blood cell count, peripheral blood smear, and bone marrow biopsy confirmed the diagnosis of AML. The patient subsequently died 5 weeks later.
Comment
Presentation of LC
Thirty percent to 40% of leukemia patients present with a variety of nonspecific cutaneous signs, including those related to hemorrhage, infection, and drug eruptions, as well as paraneoplastic lesions.1 Cutaneous signs of leukemia are less commonly due to leukemia cutis (LC), defined as the neoplastic infiltration of the skin or subcutaneous tissue by leukemic cells. The clinical presentation of LC varies, making it difficult to diagnose without immunohistochemistry. It can pre-sent as single or multiple erythematous papules and/or nodules, infiltrated plaques, macules, palpable purpura, ulcers, ecchymoses, and/or vesicles.2 Leukemia cutis most often presents on the head, neck, trunk, and sites of current or prior trauma. Gingival hyperplasia is another associated finding in the acute monocytic and myelomonocytic types of AML.3 Additionally, chloromas or granulocytic sarcomas are dermal nodules that can pre-sent in myelogenous leukemia.4
LC and AML
Leukemia cutis most commonly is observed in AML compared to the other types of leukemia. The myelomonocytic and monocytic subtypes of AML are most often implicated.5,6 The majority of patients with LC present with a pre-established (55%–77%) or simultaneous diagnosis of systemic leukemia (23%–38%).
Histopathology
In LC, histology typically reveals a normal epidermis and nodular or diffuse infiltrating cells in the dermis. The cells can appear monomorphic, atypical, or immature, and there is occasional single-filing between collagen bundles. Causative types of neoplasms can be distinguished based on their morphologic, immunophenotypic, and cytogenetic properties.8-10
Incidence
Of the acute leukemias, AML accounts for the highest prevalence in adults,11 with an annual incidence of 14,590 cases in the United States.12 The incidence of AML increases with age; the mean age of patients diagnosed with AML is 67 years.12 Risk is increased with a history of exposure to radiotherapy, chemotherapy, or cigarette smoke; preexisting myeloproliferative or myelodysplastic syndromes and mutations in DNA repair (eg, Fanconi anemia); neutropenia (eg, from elastase mutations); and Down syndrome.13
Diagnosis
More than 20% blasts in the bone marrow is required for a diagnosis of AML.14 Specific to AML is the presence of large immature precursor cells with a granular cytoplasm and, when present, highly diagnostic Auer rods.12Acute myeloid leukemia can be distinguished by staining for myeloperoxidase; Sudan Black B; or the antigens CD13, CD33, or c-kit.15
In our case, CD33 was positive, which is a characteristic finding in AML. Myeloperoxidase also can be positive in AML; however, in our case it was negative, and it can be an insensitive marker in the context of LC. Although most cases of LC present concurrently with bone marrow infiltration, some cases present before systemic involvement; for example, granulocytic sarcomas can occur months earlier than the development of systemic leukemia. Thus, early detection by a dermatologist is essential. Depending on the lesion’s appearance, the differential diagnoses can include lymphoma, drug eruptions, infectious etiologies, sarcoidosis, metastases from other malignancies, and blistering dermatoses.
Management
Systemic therapy should be the cornerstone of therapy. Induction therapy includes the combined use of cytarabine (except in acute promyelocytic leukemia [M3], for which all-trans retinoic acid is indicated) and anthracycline derivatives in a “7+3” regimen to achieve complete remission. Specifically, cytarabine (100–200 mg/m2) typically is continuously administered intravenously for 7 days combined with intravenous administration of either daunorubicin (60–90 mg/m2) or idarubicin (12 mg/m2) on days 1, 2, and 3. Postremission therapy is highly individualized depending on patients’ prognostic factors and is indicated to reduce the likelihood of relapse and to improve patient mortality. High doses of cytarabine and hematopoietic stem cell transplantation commonly are utilized.12 Resolution of hematologic atypia may result in complete or partial resolution of LC.10
Conclusion
We diagnosed AML with systemic involvement in our patient based on the cutaneous manifestation of LC. Diagnosis of LC relies on immunohistochemistry and strong clinical suspicion, as cutaneous findings are diverse and nonspecific. Early recognition is essential, as LC in the context of systemic involvement portends a poor prognosis. Our patient died 5 weeks following initial presentation.
- Rao AG, Danturty I. Leukemia cutis. Indian J Dermatol. 2012;57:504.
- Su WPD, Buechner SA, Chin-Yang L. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol. 1984;11:121-128.
- Kumar M, Nair V, Mishra L, et al. Gingival hyperplasia—a clue to the diagnosis of acute leukemia? Arch Oral Sci Res. 2012;2:165-168.
- Winfield H, Smoller B. Other lymphoproliferative and myeloproliferative diseases. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Mosby/Elsevier; 2012:2037-2048.
- Babina T, Miller L, Thomas B. Leukemia cutis. J Drugs Dermatol. 2012;11:416-417.
- Tziotzios C, Makrygeorgou A. Leukemia cutis. Cleve Clin J Med. 2011;78:226-227.
- Ratnam KV, Khor CJL, Su WPD. Leukemia cutis. Dermatol Clin 1994;12:419-431.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Buechner SA, Li CY, Su WP. Leukemia cutis. a histopathologic study of 42 cases. Am J Dermatopathol. 1985;7:109-119.
- Wagner G, Fenchel K, Back W, et al. Leukemia cutis—epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27-36.
- O’Donnell MR, Abboud CN, Altman J, et al. NCCN Clinical Practice Guidelines acute myeloid leukemia. J Natl Compr Canc Netw. 2012;10:984-1021.
- Marcucci G, Bloomfield CD. Acute myeloid leukemia. In: Kasper DL, Fauci AS, Hauser SL, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015:678-686.
- Aster JC, DeAngelo DJ. Acute leukemias. In: Bunn HF, Aster JC, eds. Pathophysiology of Blood Disorders. New York, NY: McGraw-Hill; 2010:244-259.
- Damon LE, Andreadis C. Blood disorders. In: Papadakis MA, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis & Treatment 2016. New York, NY: McGraw-Hill; 2016:495-541.
- Parikh SA, Jabbour E, Koller CA. Adult acute myeloid leukemia. In: Kantarjian HM, Wolff RA, eds. The MD Anderson Manual of Medical Oncology. 2nd ed. New York, NY: McGraw-Hill; 2011:15-32.
Case Report
A 66-year-old man with a history of type 2 diabetes mellitus presented with considerable muscle weakness and infiltrative, flesh-colored plaques on the face, trunk, and arms of 3 months’ duration. The patient required the use of a wheelchair due to muscle weakness. On physical examination he had diffuse, infiltrative, flesh-colored plaques on the entire face (Figure 1A), trunk, and arms. The eyelids and lips were swollen, and the nose was distorted due to the infiltrative plaques (Figure 1B). Additionally, there were hypopigmented macules and patches scattered among the infiltrative plaques on the face, trunk, and arms (Figure 1C).

Punch biopsy specimens were obtained from the left cheek and left upper arm and were submitted for histologic examination with routine hematoxylin and eosin staining (Figure 2). Histopathology showed infiltrating and diffuse monomorphic cells in the dermis with large and hyperchromatic nuclei. Some nuclei were cleaved or folded in configuration. The cells displayed ample surrounding cytoplasm, which was finely granular or vacuolated. The infiltrate was accentuated in the perifollicular adventitial dermis. Immunohistochemistry was positive for CD33 and negative for CD3, CD20, and myeloperoxidase. Additionally, periodic acid–Schiff and Fite stains were negative for microorganisms. These morphologic and immunohistochemical findings were consistent with acute myeloid leukemia (AML). Further testing with complete blood cell count, peripheral blood smear, and bone marrow biopsy confirmed the diagnosis of AML. The patient subsequently died 5 weeks later.

Comment
Presentation of LC
Thirty percent to 40% of leukemia patients present with a variety of nonspecific cutaneous signs, including those related to hemorrhage, infection, and drug eruptions, as well as paraneoplastic lesions.1 Cutaneous signs of leukemia are less commonly due to leukemia cutis (LC), defined as the neoplastic infiltration of the skin or subcutaneous tissue by leukemic cells. The clinical presentation of LC varies, making it difficult to diagnose without immunohistochemistry. It can pre-sent as single or multiple erythematous papules and/or nodules, infiltrated plaques, macules, palpable purpura, ulcers, ecchymoses, and/or vesicles.2 Leukemia cutis most often presents on the head, neck, trunk, and sites of current or prior trauma. Gingival hyperplasia is another associated finding in the acute monocytic and myelomonocytic types of AML.3 Additionally, chloromas or granulocytic sarcomas are dermal nodules that can pre-sent in myelogenous leukemia.4
LC and AML
Leukemia cutis most commonly is observed in AML compared to the other types of leukemia. The myelomonocytic and monocytic subtypes of AML are most often implicated.5,6 The majority of patients with LC present with a pre-established (55%–77%) or simultaneous diagnosis of systemic leukemia (23%–38%).
Histopathology
In LC, histology typically reveals a normal epidermis and nodular or diffuse infiltrating cells in the dermis. The cells can appear monomorphic, atypical, or immature, and there is occasional single-filing between collagen bundles. Causative types of neoplasms can be distinguished based on their morphologic, immunophenotypic, and cytogenetic properties.8-10
Incidence
Of the acute leukemias, AML accounts for the highest prevalence in adults,11 with an annual incidence of 14,590 cases in the United States.12 The incidence of AML increases with age; the mean age of patients diagnosed with AML is 67 years.12 Risk is increased with a history of exposure to radiotherapy, chemotherapy, or cigarette smoke; preexisting myeloproliferative or myelodysplastic syndromes and mutations in DNA repair (eg, Fanconi anemia); neutropenia (eg, from elastase mutations); and Down syndrome.13
Diagnosis
More than 20% blasts in the bone marrow is required for a diagnosis of AML.14 Specific to AML is the presence of large immature precursor cells with a granular cytoplasm and, when present, highly diagnostic Auer rods.12Acute myeloid leukemia can be distinguished by staining for myeloperoxidase; Sudan Black B; or the antigens CD13, CD33, or c-kit.15
In our case, CD33 was positive, which is a characteristic finding in AML. Myeloperoxidase also can be positive in AML; however, in our case it was negative, and it can be an insensitive marker in the context of LC. Although most cases of LC present concurrently with bone marrow infiltration, some cases present before systemic involvement; for example, granulocytic sarcomas can occur months earlier than the development of systemic leukemia. Thus, early detection by a dermatologist is essential. Depending on the lesion’s appearance, the differential diagnoses can include lymphoma, drug eruptions, infectious etiologies, sarcoidosis, metastases from other malignancies, and blistering dermatoses.
Management
Systemic therapy should be the cornerstone of therapy. Induction therapy includes the combined use of cytarabine (except in acute promyelocytic leukemia [M3], for which all-trans retinoic acid is indicated) and anthracycline derivatives in a “7+3” regimen to achieve complete remission. Specifically, cytarabine (100–200 mg/m2) typically is continuously administered intravenously for 7 days combined with intravenous administration of either daunorubicin (60–90 mg/m2) or idarubicin (12 mg/m2) on days 1, 2, and 3. Postremission therapy is highly individualized depending on patients’ prognostic factors and is indicated to reduce the likelihood of relapse and to improve patient mortality. High doses of cytarabine and hematopoietic stem cell transplantation commonly are utilized.12 Resolution of hematologic atypia may result in complete or partial resolution of LC.10
Conclusion
We diagnosed AML with systemic involvement in our patient based on the cutaneous manifestation of LC. Diagnosis of LC relies on immunohistochemistry and strong clinical suspicion, as cutaneous findings are diverse and nonspecific. Early recognition is essential, as LC in the context of systemic involvement portends a poor prognosis. Our patient died 5 weeks following initial presentation.
Case Report
A 66-year-old man with a history of type 2 diabetes mellitus presented with considerable muscle weakness and infiltrative, flesh-colored plaques on the face, trunk, and arms of 3 months’ duration. The patient required the use of a wheelchair due to muscle weakness. On physical examination he had diffuse, infiltrative, flesh-colored plaques on the entire face (Figure 1A), trunk, and arms. The eyelids and lips were swollen, and the nose was distorted due to the infiltrative plaques (Figure 1B). Additionally, there were hypopigmented macules and patches scattered among the infiltrative plaques on the face, trunk, and arms (Figure 1C).

Punch biopsy specimens were obtained from the left cheek and left upper arm and were submitted for histologic examination with routine hematoxylin and eosin staining (Figure 2). Histopathology showed infiltrating and diffuse monomorphic cells in the dermis with large and hyperchromatic nuclei. Some nuclei were cleaved or folded in configuration. The cells displayed ample surrounding cytoplasm, which was finely granular or vacuolated. The infiltrate was accentuated in the perifollicular adventitial dermis. Immunohistochemistry was positive for CD33 and negative for CD3, CD20, and myeloperoxidase. Additionally, periodic acid–Schiff and Fite stains were negative for microorganisms. These morphologic and immunohistochemical findings were consistent with acute myeloid leukemia (AML). Further testing with complete blood cell count, peripheral blood smear, and bone marrow biopsy confirmed the diagnosis of AML. The patient subsequently died 5 weeks later.

Comment
Presentation of LC
Thirty percent to 40% of leukemia patients present with a variety of nonspecific cutaneous signs, including those related to hemorrhage, infection, and drug eruptions, as well as paraneoplastic lesions.1 Cutaneous signs of leukemia are less commonly due to leukemia cutis (LC), defined as the neoplastic infiltration of the skin or subcutaneous tissue by leukemic cells. The clinical presentation of LC varies, making it difficult to diagnose without immunohistochemistry. It can pre-sent as single or multiple erythematous papules and/or nodules, infiltrated plaques, macules, palpable purpura, ulcers, ecchymoses, and/or vesicles.2 Leukemia cutis most often presents on the head, neck, trunk, and sites of current or prior trauma. Gingival hyperplasia is another associated finding in the acute monocytic and myelomonocytic types of AML.3 Additionally, chloromas or granulocytic sarcomas are dermal nodules that can pre-sent in myelogenous leukemia.4
LC and AML
Leukemia cutis most commonly is observed in AML compared to the other types of leukemia. The myelomonocytic and monocytic subtypes of AML are most often implicated.5,6 The majority of patients with LC present with a pre-established (55%–77%) or simultaneous diagnosis of systemic leukemia (23%–38%).
Histopathology
In LC, histology typically reveals a normal epidermis and nodular or diffuse infiltrating cells in the dermis. The cells can appear monomorphic, atypical, or immature, and there is occasional single-filing between collagen bundles. Causative types of neoplasms can be distinguished based on their morphologic, immunophenotypic, and cytogenetic properties.8-10
Incidence
Of the acute leukemias, AML accounts for the highest prevalence in adults,11 with an annual incidence of 14,590 cases in the United States.12 The incidence of AML increases with age; the mean age of patients diagnosed with AML is 67 years.12 Risk is increased with a history of exposure to radiotherapy, chemotherapy, or cigarette smoke; preexisting myeloproliferative or myelodysplastic syndromes and mutations in DNA repair (eg, Fanconi anemia); neutropenia (eg, from elastase mutations); and Down syndrome.13
Diagnosis
More than 20% blasts in the bone marrow is required for a diagnosis of AML.14 Specific to AML is the presence of large immature precursor cells with a granular cytoplasm and, when present, highly diagnostic Auer rods.12Acute myeloid leukemia can be distinguished by staining for myeloperoxidase; Sudan Black B; or the antigens CD13, CD33, or c-kit.15
In our case, CD33 was positive, which is a characteristic finding in AML. Myeloperoxidase also can be positive in AML; however, in our case it was negative, and it can be an insensitive marker in the context of LC. Although most cases of LC present concurrently with bone marrow infiltration, some cases present before systemic involvement; for example, granulocytic sarcomas can occur months earlier than the development of systemic leukemia. Thus, early detection by a dermatologist is essential. Depending on the lesion’s appearance, the differential diagnoses can include lymphoma, drug eruptions, infectious etiologies, sarcoidosis, metastases from other malignancies, and blistering dermatoses.
Management
Systemic therapy should be the cornerstone of therapy. Induction therapy includes the combined use of cytarabine (except in acute promyelocytic leukemia [M3], for which all-trans retinoic acid is indicated) and anthracycline derivatives in a “7+3” regimen to achieve complete remission. Specifically, cytarabine (100–200 mg/m2) typically is continuously administered intravenously for 7 days combined with intravenous administration of either daunorubicin (60–90 mg/m2) or idarubicin (12 mg/m2) on days 1, 2, and 3. Postremission therapy is highly individualized depending on patients’ prognostic factors and is indicated to reduce the likelihood of relapse and to improve patient mortality. High doses of cytarabine and hematopoietic stem cell transplantation commonly are utilized.12 Resolution of hematologic atypia may result in complete or partial resolution of LC.10
Conclusion
We diagnosed AML with systemic involvement in our patient based on the cutaneous manifestation of LC. Diagnosis of LC relies on immunohistochemistry and strong clinical suspicion, as cutaneous findings are diverse and nonspecific. Early recognition is essential, as LC in the context of systemic involvement portends a poor prognosis. Our patient died 5 weeks following initial presentation.
- Rao AG, Danturty I. Leukemia cutis. Indian J Dermatol. 2012;57:504.
- Su WPD, Buechner SA, Chin-Yang L. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol. 1984;11:121-128.
- Kumar M, Nair V, Mishra L, et al. Gingival hyperplasia—a clue to the diagnosis of acute leukemia? Arch Oral Sci Res. 2012;2:165-168.
- Winfield H, Smoller B. Other lymphoproliferative and myeloproliferative diseases. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Mosby/Elsevier; 2012:2037-2048.
- Babina T, Miller L, Thomas B. Leukemia cutis. J Drugs Dermatol. 2012;11:416-417.
- Tziotzios C, Makrygeorgou A. Leukemia cutis. Cleve Clin J Med. 2011;78:226-227.
- Ratnam KV, Khor CJL, Su WPD. Leukemia cutis. Dermatol Clin 1994;12:419-431.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Buechner SA, Li CY, Su WP. Leukemia cutis. a histopathologic study of 42 cases. Am J Dermatopathol. 1985;7:109-119.
- Wagner G, Fenchel K, Back W, et al. Leukemia cutis—epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27-36.
- O’Donnell MR, Abboud CN, Altman J, et al. NCCN Clinical Practice Guidelines acute myeloid leukemia. J Natl Compr Canc Netw. 2012;10:984-1021.
- Marcucci G, Bloomfield CD. Acute myeloid leukemia. In: Kasper DL, Fauci AS, Hauser SL, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015:678-686.
- Aster JC, DeAngelo DJ. Acute leukemias. In: Bunn HF, Aster JC, eds. Pathophysiology of Blood Disorders. New York, NY: McGraw-Hill; 2010:244-259.
- Damon LE, Andreadis C. Blood disorders. In: Papadakis MA, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis & Treatment 2016. New York, NY: McGraw-Hill; 2016:495-541.
- Parikh SA, Jabbour E, Koller CA. Adult acute myeloid leukemia. In: Kantarjian HM, Wolff RA, eds. The MD Anderson Manual of Medical Oncology. 2nd ed. New York, NY: McGraw-Hill; 2011:15-32.
- Rao AG, Danturty I. Leukemia cutis. Indian J Dermatol. 2012;57:504.
- Su WPD, Buechner SA, Chin-Yang L. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol. 1984;11:121-128.
- Kumar M, Nair V, Mishra L, et al. Gingival hyperplasia—a clue to the diagnosis of acute leukemia? Arch Oral Sci Res. 2012;2:165-168.
- Winfield H, Smoller B. Other lymphoproliferative and myeloproliferative diseases. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Mosby/Elsevier; 2012:2037-2048.
- Babina T, Miller L, Thomas B. Leukemia cutis. J Drugs Dermatol. 2012;11:416-417.
- Tziotzios C, Makrygeorgou A. Leukemia cutis. Cleve Clin J Med. 2011;78:226-227.
- Ratnam KV, Khor CJL, Su WPD. Leukemia cutis. Dermatol Clin 1994;12:419-431.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Buechner SA, Li CY, Su WP. Leukemia cutis. a histopathologic study of 42 cases. Am J Dermatopathol. 1985;7:109-119.
- Wagner G, Fenchel K, Back W, et al. Leukemia cutis—epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27-36.
- O’Donnell MR, Abboud CN, Altman J, et al. NCCN Clinical Practice Guidelines acute myeloid leukemia. J Natl Compr Canc Netw. 2012;10:984-1021.
- Marcucci G, Bloomfield CD. Acute myeloid leukemia. In: Kasper DL, Fauci AS, Hauser SL, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015:678-686.
- Aster JC, DeAngelo DJ. Acute leukemias. In: Bunn HF, Aster JC, eds. Pathophysiology of Blood Disorders. New York, NY: McGraw-Hill; 2010:244-259.
- Damon LE, Andreadis C. Blood disorders. In: Papadakis MA, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis & Treatment 2016. New York, NY: McGraw-Hill; 2016:495-541.
- Parikh SA, Jabbour E, Koller CA. Adult acute myeloid leukemia. In: Kantarjian HM, Wolff RA, eds. The MD Anderson Manual of Medical Oncology. 2nd ed. New York, NY: McGraw-Hill; 2011:15-32.
Practice Points
- Leukemia cutis (LC) describes cutaneous and/or subcutaneous infiltration by leukemic cells and most commonly occurs in patients with acute myeloid leukemia.
- The vast majority of patients presenting with LC already have systemic involvement.
- Cutaneous presentation of LC is diverse, thus diagnosis often is dependent on immunohisto-chemical findings.