User login
Lupus erythematosus tumidus (LET) is a relatively rare condition but may simply be underdiagnosed in the literature. It presents as urticarialike papules and plaques in sun-exposed areas, characterized by induration and erythema. Lesions occur on the face, neck, upper extremities, and trunk and heal without scarring.1,2 Rarely, lesions can show fine scaling and associated pruritus, but most often the lesions are asymptomatic.3
Case Report
A 45-year-old woman presented with 2 asymptomatic self-described bald spots on the top of the head of 2 months’ duration. The patient denied prior treatment of the lesions and noted one patch was resolving. She reported no involvement of the eyebrows, eyelashes, and axillary and pubic hair. A review of systems was negative. The patient denied personal or family history of lupus, thyroid disease, or vitiligo.
Clinical examination revealed a 1.1-cm round patch of nonscarring alopecia on the right vertex scalp and a 0.9-cm round patch of nonscarring alopecia with moderate hair regrowth on the left vertex scalp. There was no erythema, scaling, or induration. The rest of the scalp was normal in appearance and the eyebrows and eyelashes were uninvolved. The patient was diagnosed with alopecia areata and was treated with 10 mg/mL of intralesional triamcinolone once monthly for 4 months.
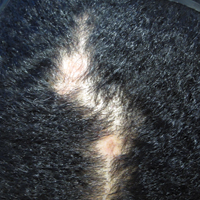
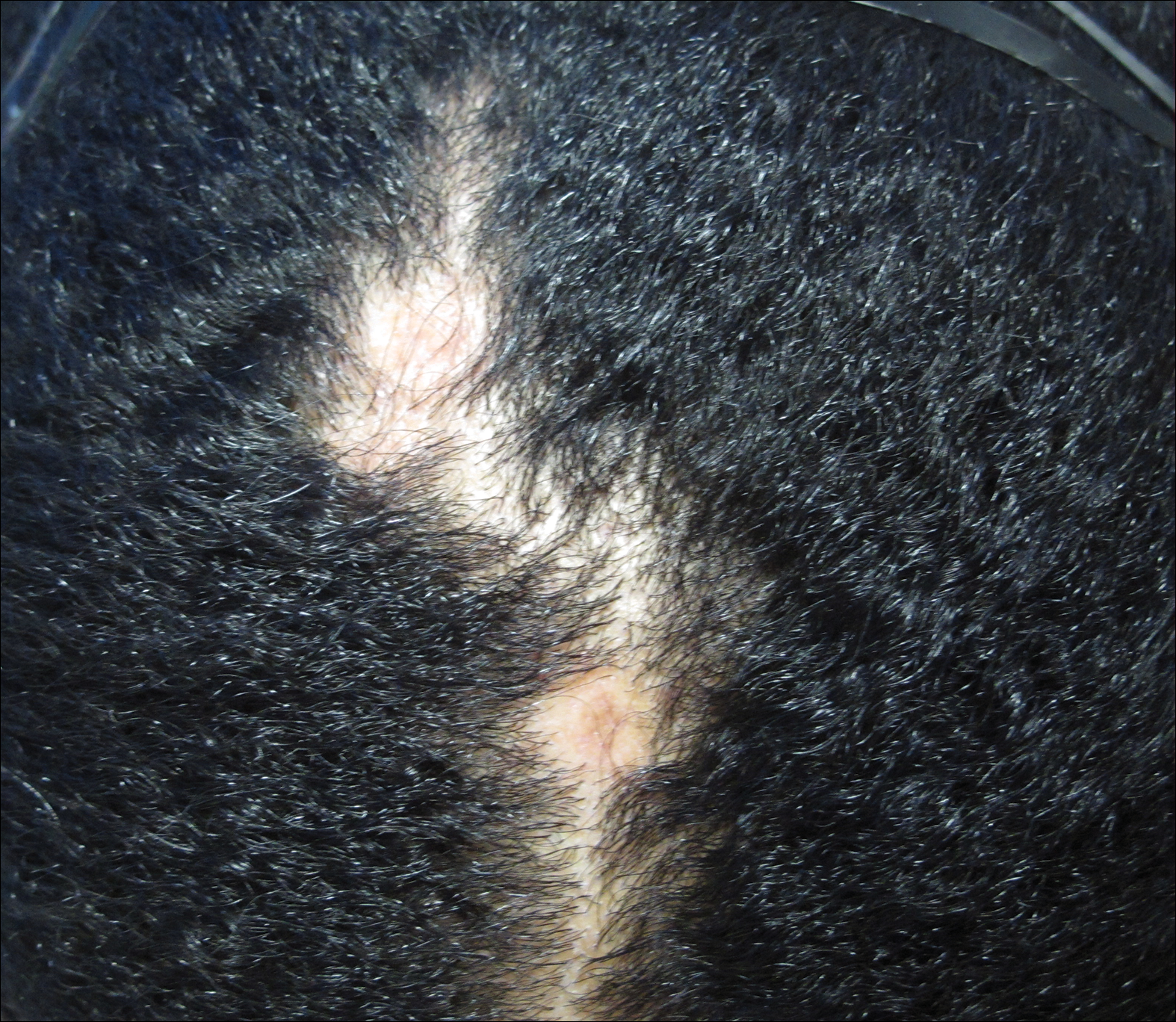
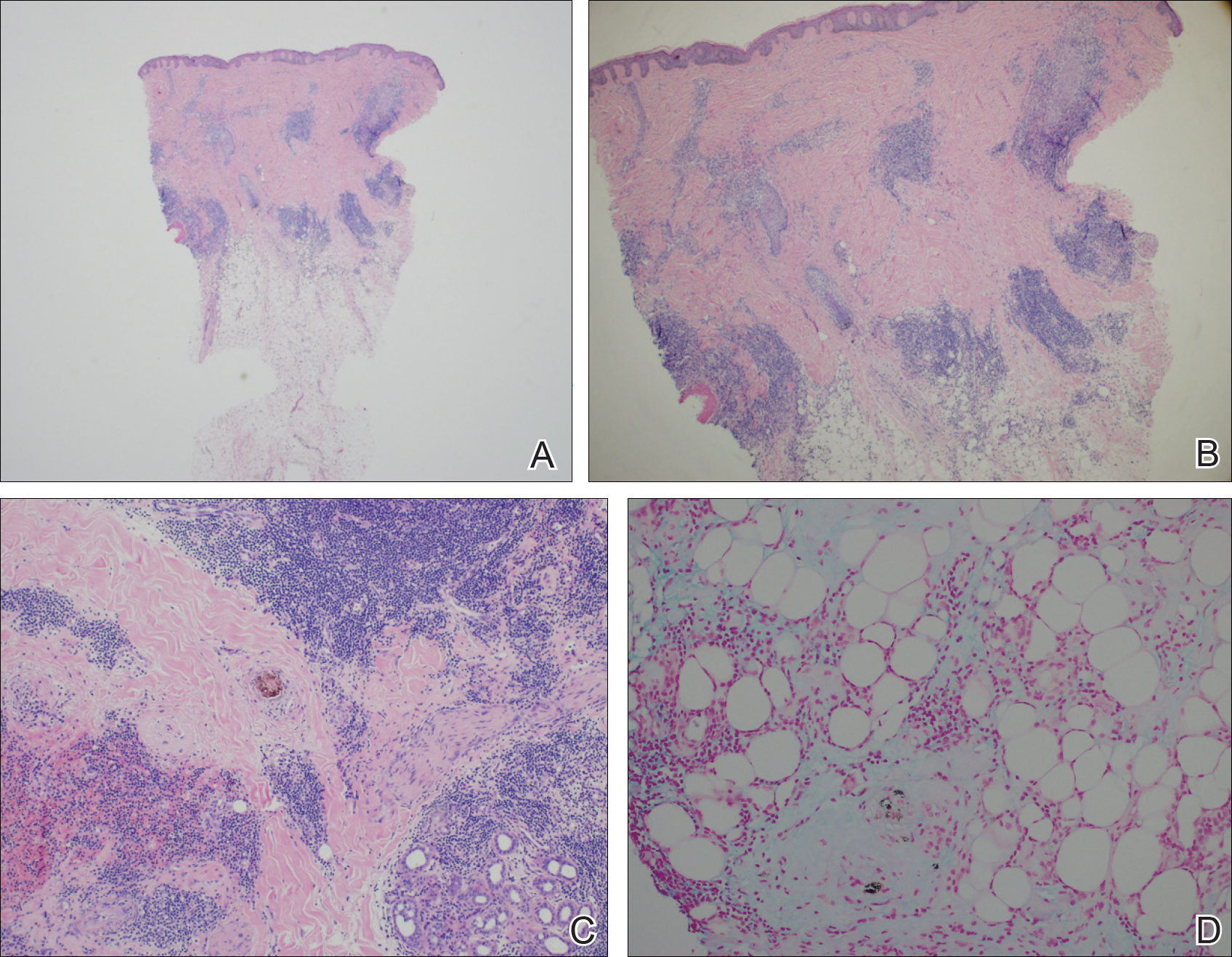
The patient initially showed improvement with moderate hair regrowth. After 4 months of treatment, she developed 3 new 1- to 1.5-cm erythematous alopecic patches on the vertex scalp and had worsening in the initial patches (Figure 1). Given the resistance to standard therapy and the onset of multiple new areas with evidence of inflammatory involvement, a punch biopsy was performed. Histopathologic examination revealed a fairly unremarkable epidermis and a dense dermal inflammatory infiltrate that was present both in the superficial and deep dermis (Figure 2). The inflammatory cells, which appeared to be predominantly comprised of lymphocytes, had a predilection for the vasculature but also were observed within the interstitial dermis. Additionally, mucin appeared to be slightly increased in the deep dermis. The lymphocytic phenotype was confirmed by immunohistochemical studies for CD20 and CD3. The most likely possibilities for this reaction pattern were LET, Jessner lymphocytic infiltrate of the skin (JLIS), gyrate erythema, and lymphoma; however, the immunohistochemical studies effectively ruled out lymphoma. Additionally, there was pronounced dermal mucin noted in the specimen. The patient was diagnosed with LET of the scalp based on the constellation of findings.
Comment
The classification of LET as a single unique entity or disease process sui generis has been in flux in the last decade. Its similarities to JLIS and other forms of chronic cutaneous lupus erythematosus (CCLE) have brought debate.4-6 In 1930, Gougerot and Burnier7 documented the first case of LET in the literature, describing smooth, infiltrated, erythematous lesions with no desquamation or other superficial changes seen in 5 patients.
In 2000, interest in LET and other forms of CCLE was increasing, and reports in the literature paralleled. That year, Kuhn et al4 reported 40 cases of LET, characterizing the clinical and histological features of each case to demonstrate that LET should be separate from other forms of CCLE. Until then, it is likely that many lesions that should have been classified as LET were instead classified as various forms of CCLE. The investigators maintained that LET also should be distinct from JLIS because it is associated with UV exposure.4 Kuhn et al8 reviewed phototesting in 60 patients with LET in 2001 and confirmed this subset was the most photosensitive type of lupus erythematosus.
In general, the histopathologic and immunohistochemical studies in LET and JLIS can be quite similar. Relatively distinguishing histopathologic findings in JLIS include no evidence of epidermal atrophy, basal vacuolar change, or follicular plugging, as well as negative immunofluorescence studies. Both entities show a predominantly T-cell population with a smaller component of B cells and thus a distinction cannot be made based on relative proportions of T and B cells in lesions.2
In 2003, Alexiades-Armenakas et al6 determined immunohistochemical criteria for LET, finding a predominance of T cells and more CD4 lymphocytes than CD8 lymphocytes with a mean ratio of roughly 3 to 1. Their study results maintained LET should be classified as a form of CCLE due to the chronicity of the lesions, the serologic profile with negative anti–double-stranded DNA, anticentromere, anti-Smith, anti-Ro/Sjögren syndrome antigen A, anti-La/Sjögren syndrome antigen B, and anti-nuclear ribonucleoprotein antibodies and the rare association with systemic disease.6 This conclusion was further solidified by a review published that same year citing unique histopathological features when compared to subacute cutaneous LE and discoid lupus erythematosus.5
This case illustrates the importance of histologic evaluation in determining the correct diagnosis in a patient with alopecia areata recalcitrant to treatment. Including LET in the differential of alopecic patches on the scalp could prove beneficial for patients, as LET responds well to antimalarial drugs and photoprotection.9 This patient had a normal antinuclear antibody panel and no signs or symptoms of systemic lupus. It was recommended that she avoid sun exposure and begin treatment with hydroxychloroquine but she declined. At a follow-up visit 6 months later she reported the lesions had improved, but a permanent wig had been sewn over the area, so it could not be examined.
- Lee L, Werth V. Rheumatologic disease. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 3rd ed. Mosby Elsevier; 2008:615-629.
- Weedon D. The lichenoid reaction pattern. In: Weedon D. Skin Pathology. 2nd ed. Edinburgh, Scotland: Churchill Livingstone; 2002:35-70.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am Acad Dermatol. 1999;41:250-253.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Kuhn A, Sonntag M, Ruzicka T, et al. Histopathologic findings in lupus erythematosus tumidus: review of 80 patients. J Am Acad Dermatol. 2003;48:901-908.
- Alexiades-Armenakas MR, Baldassano M, Bince B, et al. Tumid lupus erythematosus: criteria for classification with immunohistochemical analysis. Arthritis Rheum. 2003;49:494-500.
- Gougerot H, Burnier R. Lupuse rythe mateux “tumidus.” Bull Soc Fr Dermatol Syph. 1930;37:1291-1292.
- Kuhn A, Sonntag M, Richter-Hintz D, et al. Phototesting in lupus erythematosus tumidus—review of 60 patients. Photochem Photobiol. 2001;73:532-536.
- Cozzani E, Christana K, Rongioletti F, et al. Lupus erythematosus tumidus: clinical, histopathological and serological aspects and therapy response of 21 patients. Eur J Dermatol. 2010;20:797-801.
Lupus erythematosus tumidus (LET) is a relatively rare condition but may simply be underdiagnosed in the literature. It presents as urticarialike papules and plaques in sun-exposed areas, characterized by induration and erythema. Lesions occur on the face, neck, upper extremities, and trunk and heal without scarring.1,2 Rarely, lesions can show fine scaling and associated pruritus, but most often the lesions are asymptomatic.3
Case Report
A 45-year-old woman presented with 2 asymptomatic self-described bald spots on the top of the head of 2 months’ duration. The patient denied prior treatment of the lesions and noted one patch was resolving. She reported no involvement of the eyebrows, eyelashes, and axillary and pubic hair. A review of systems was negative. The patient denied personal or family history of lupus, thyroid disease, or vitiligo.
Clinical examination revealed a 1.1-cm round patch of nonscarring alopecia on the right vertex scalp and a 0.9-cm round patch of nonscarring alopecia with moderate hair regrowth on the left vertex scalp. There was no erythema, scaling, or induration. The rest of the scalp was normal in appearance and the eyebrows and eyelashes were uninvolved. The patient was diagnosed with alopecia areata and was treated with 10 mg/mL of intralesional triamcinolone once monthly for 4 months.
The patient initially showed improvement with moderate hair regrowth. After 4 months of treatment, she developed 3 new 1- to 1.5-cm erythematous alopecic patches on the vertex scalp and had worsening in the initial patches (Figure 1). Given the resistance to standard therapy and the onset of multiple new areas with evidence of inflammatory involvement, a punch biopsy was performed. Histopathologic examination revealed a fairly unremarkable epidermis and a dense dermal inflammatory infiltrate that was present both in the superficial and deep dermis (Figure 2). The inflammatory cells, which appeared to be predominantly comprised of lymphocytes, had a predilection for the vasculature but also were observed within the interstitial dermis. Additionally, mucin appeared to be slightly increased in the deep dermis. The lymphocytic phenotype was confirmed by immunohistochemical studies for CD20 and CD3. The most likely possibilities for this reaction pattern were LET, Jessner lymphocytic infiltrate of the skin (JLIS), gyrate erythema, and lymphoma; however, the immunohistochemical studies effectively ruled out lymphoma. Additionally, there was pronounced dermal mucin noted in the specimen. The patient was diagnosed with LET of the scalp based on the constellation of findings.


Comment
The classification of LET as a single unique entity or disease process sui generis has been in flux in the last decade. Its similarities to JLIS and other forms of chronic cutaneous lupus erythematosus (CCLE) have brought debate.4-6 In 1930, Gougerot and Burnier7 documented the first case of LET in the literature, describing smooth, infiltrated, erythematous lesions with no desquamation or other superficial changes seen in 5 patients.
In 2000, interest in LET and other forms of CCLE was increasing, and reports in the literature paralleled. That year, Kuhn et al4 reported 40 cases of LET, characterizing the clinical and histological features of each case to demonstrate that LET should be separate from other forms of CCLE. Until then, it is likely that many lesions that should have been classified as LET were instead classified as various forms of CCLE. The investigators maintained that LET also should be distinct from JLIS because it is associated with UV exposure.4 Kuhn et al8 reviewed phototesting in 60 patients with LET in 2001 and confirmed this subset was the most photosensitive type of lupus erythematosus.
In general, the histopathologic and immunohistochemical studies in LET and JLIS can be quite similar. Relatively distinguishing histopathologic findings in JLIS include no evidence of epidermal atrophy, basal vacuolar change, or follicular plugging, as well as negative immunofluorescence studies. Both entities show a predominantly T-cell population with a smaller component of B cells and thus a distinction cannot be made based on relative proportions of T and B cells in lesions.2
In 2003, Alexiades-Armenakas et al6 determined immunohistochemical criteria for LET, finding a predominance of T cells and more CD4 lymphocytes than CD8 lymphocytes with a mean ratio of roughly 3 to 1. Their study results maintained LET should be classified as a form of CCLE due to the chronicity of the lesions, the serologic profile with negative anti–double-stranded DNA, anticentromere, anti-Smith, anti-Ro/Sjögren syndrome antigen A, anti-La/Sjögren syndrome antigen B, and anti-nuclear ribonucleoprotein antibodies and the rare association with systemic disease.6 This conclusion was further solidified by a review published that same year citing unique histopathological features when compared to subacute cutaneous LE and discoid lupus erythematosus.5
This case illustrates the importance of histologic evaluation in determining the correct diagnosis in a patient with alopecia areata recalcitrant to treatment. Including LET in the differential of alopecic patches on the scalp could prove beneficial for patients, as LET responds well to antimalarial drugs and photoprotection.9 This patient had a normal antinuclear antibody panel and no signs or symptoms of systemic lupus. It was recommended that she avoid sun exposure and begin treatment with hydroxychloroquine but she declined. At a follow-up visit 6 months later she reported the lesions had improved, but a permanent wig had been sewn over the area, so it could not be examined.
Lupus erythematosus tumidus (LET) is a relatively rare condition but may simply be underdiagnosed in the literature. It presents as urticarialike papules and plaques in sun-exposed areas, characterized by induration and erythema. Lesions occur on the face, neck, upper extremities, and trunk and heal without scarring.1,2 Rarely, lesions can show fine scaling and associated pruritus, but most often the lesions are asymptomatic.3
Case Report
A 45-year-old woman presented with 2 asymptomatic self-described bald spots on the top of the head of 2 months’ duration. The patient denied prior treatment of the lesions and noted one patch was resolving. She reported no involvement of the eyebrows, eyelashes, and axillary and pubic hair. A review of systems was negative. The patient denied personal or family history of lupus, thyroid disease, or vitiligo.
Clinical examination revealed a 1.1-cm round patch of nonscarring alopecia on the right vertex scalp and a 0.9-cm round patch of nonscarring alopecia with moderate hair regrowth on the left vertex scalp. There was no erythema, scaling, or induration. The rest of the scalp was normal in appearance and the eyebrows and eyelashes were uninvolved. The patient was diagnosed with alopecia areata and was treated with 10 mg/mL of intralesional triamcinolone once monthly for 4 months.
The patient initially showed improvement with moderate hair regrowth. After 4 months of treatment, she developed 3 new 1- to 1.5-cm erythematous alopecic patches on the vertex scalp and had worsening in the initial patches (Figure 1). Given the resistance to standard therapy and the onset of multiple new areas with evidence of inflammatory involvement, a punch biopsy was performed. Histopathologic examination revealed a fairly unremarkable epidermis and a dense dermal inflammatory infiltrate that was present both in the superficial and deep dermis (Figure 2). The inflammatory cells, which appeared to be predominantly comprised of lymphocytes, had a predilection for the vasculature but also were observed within the interstitial dermis. Additionally, mucin appeared to be slightly increased in the deep dermis. The lymphocytic phenotype was confirmed by immunohistochemical studies for CD20 and CD3. The most likely possibilities for this reaction pattern were LET, Jessner lymphocytic infiltrate of the skin (JLIS), gyrate erythema, and lymphoma; however, the immunohistochemical studies effectively ruled out lymphoma. Additionally, there was pronounced dermal mucin noted in the specimen. The patient was diagnosed with LET of the scalp based on the constellation of findings.


Comment
The classification of LET as a single unique entity or disease process sui generis has been in flux in the last decade. Its similarities to JLIS and other forms of chronic cutaneous lupus erythematosus (CCLE) have brought debate.4-6 In 1930, Gougerot and Burnier7 documented the first case of LET in the literature, describing smooth, infiltrated, erythematous lesions with no desquamation or other superficial changes seen in 5 patients.
In 2000, interest in LET and other forms of CCLE was increasing, and reports in the literature paralleled. That year, Kuhn et al4 reported 40 cases of LET, characterizing the clinical and histological features of each case to demonstrate that LET should be separate from other forms of CCLE. Until then, it is likely that many lesions that should have been classified as LET were instead classified as various forms of CCLE. The investigators maintained that LET also should be distinct from JLIS because it is associated with UV exposure.4 Kuhn et al8 reviewed phototesting in 60 patients with LET in 2001 and confirmed this subset was the most photosensitive type of lupus erythematosus.
In general, the histopathologic and immunohistochemical studies in LET and JLIS can be quite similar. Relatively distinguishing histopathologic findings in JLIS include no evidence of epidermal atrophy, basal vacuolar change, or follicular plugging, as well as negative immunofluorescence studies. Both entities show a predominantly T-cell population with a smaller component of B cells and thus a distinction cannot be made based on relative proportions of T and B cells in lesions.2
In 2003, Alexiades-Armenakas et al6 determined immunohistochemical criteria for LET, finding a predominance of T cells and more CD4 lymphocytes than CD8 lymphocytes with a mean ratio of roughly 3 to 1. Their study results maintained LET should be classified as a form of CCLE due to the chronicity of the lesions, the serologic profile with negative anti–double-stranded DNA, anticentromere, anti-Smith, anti-Ro/Sjögren syndrome antigen A, anti-La/Sjögren syndrome antigen B, and anti-nuclear ribonucleoprotein antibodies and the rare association with systemic disease.6 This conclusion was further solidified by a review published that same year citing unique histopathological features when compared to subacute cutaneous LE and discoid lupus erythematosus.5
This case illustrates the importance of histologic evaluation in determining the correct diagnosis in a patient with alopecia areata recalcitrant to treatment. Including LET in the differential of alopecic patches on the scalp could prove beneficial for patients, as LET responds well to antimalarial drugs and photoprotection.9 This patient had a normal antinuclear antibody panel and no signs or symptoms of systemic lupus. It was recommended that she avoid sun exposure and begin treatment with hydroxychloroquine but she declined. At a follow-up visit 6 months later she reported the lesions had improved, but a permanent wig had been sewn over the area, so it could not be examined.
- Lee L, Werth V. Rheumatologic disease. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 3rd ed. Mosby Elsevier; 2008:615-629.
- Weedon D. The lichenoid reaction pattern. In: Weedon D. Skin Pathology. 2nd ed. Edinburgh, Scotland: Churchill Livingstone; 2002:35-70.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am Acad Dermatol. 1999;41:250-253.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Kuhn A, Sonntag M, Ruzicka T, et al. Histopathologic findings in lupus erythematosus tumidus: review of 80 patients. J Am Acad Dermatol. 2003;48:901-908.
- Alexiades-Armenakas MR, Baldassano M, Bince B, et al. Tumid lupus erythematosus: criteria for classification with immunohistochemical analysis. Arthritis Rheum. 2003;49:494-500.
- Gougerot H, Burnier R. Lupuse rythe mateux “tumidus.” Bull Soc Fr Dermatol Syph. 1930;37:1291-1292.
- Kuhn A, Sonntag M, Richter-Hintz D, et al. Phototesting in lupus erythematosus tumidus—review of 60 patients. Photochem Photobiol. 2001;73:532-536.
- Cozzani E, Christana K, Rongioletti F, et al. Lupus erythematosus tumidus: clinical, histopathological and serological aspects and therapy response of 21 patients. Eur J Dermatol. 2010;20:797-801.
- Lee L, Werth V. Rheumatologic disease. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 3rd ed. Mosby Elsevier; 2008:615-629.
- Weedon D. The lichenoid reaction pattern. In: Weedon D. Skin Pathology. 2nd ed. Edinburgh, Scotland: Churchill Livingstone; 2002:35-70.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am Acad Dermatol. 1999;41:250-253.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Kuhn A, Sonntag M, Ruzicka T, et al. Histopathologic findings in lupus erythematosus tumidus: review of 80 patients. J Am Acad Dermatol. 2003;48:901-908.
- Alexiades-Armenakas MR, Baldassano M, Bince B, et al. Tumid lupus erythematosus: criteria for classification with immunohistochemical analysis. Arthritis Rheum. 2003;49:494-500.
- Gougerot H, Burnier R. Lupuse rythe mateux “tumidus.” Bull Soc Fr Dermatol Syph. 1930;37:1291-1292.
- Kuhn A, Sonntag M, Richter-Hintz D, et al. Phototesting in lupus erythematosus tumidus—review of 60 patients. Photochem Photobiol. 2001;73:532-536.
- Cozzani E, Christana K, Rongioletti F, et al. Lupus erythematosus tumidus: clinical, histopathological and serological aspects and therapy response of 21 patients. Eur J Dermatol. 2010;20:797-801.
Practice Points
- Lupus erythematosus tumidus (LET) of the scalp can mimic alopecia areata on clinical presentation.
- A unique variant of chronic cutaneous lupus erythematosus, LET presents in sun-exposed areas without any corresponding systemic signs.
- Lupus erythematosus tumidus may respond well to antimalarial drugs.