User login
Case
A 37-year-old woman presented to the ED with a 90-minute history of chest tightness and shortness of breath. She admitted to feeling anxious but denied nausea, vomiting, or diaphoresis. The patient was in good health overall and had no history of similar symptoms. The only medication she took on a regular basis was a combination oral contraceptive (OC). Regarding the patient’s social history, she admitted to smoking one-half of a pack of cigarettes per day and occasional alcohol use.
On physical examination, the patient’s vital signs were: heart rate (HR), 102 beats/min; blood pressure, 118/64 mm Hg; respiratory rate, 20 breaths/min; and temperature, 98.6˚F. Oxygen saturation was 95% on room air. The head, eyes, ears, nose, and throat examination was normal. The cardiopulmonary examination revealed slight tachycardia with a regular rhythm but no murmurs, rubs, or gallops; the lungs were clear to auscultation bilaterally. The abdominal examination revealed a soft, nontender abdomen, without mass, and no guarding or rebound was present. An examination of the lower extremities was not documented.
The emergency physician (EP) ordered laboratory studies, which included a complete blood count (CBC), basic metabolic profile (BMP), and troponin I level. A chest X-ray and electrocardiogram (ECG) were also ordered. The chest X-ray was interpreted as normal, and the ECG revealed mild sinus tachycardia with nonspecific ST-T segment changes in V1 through V3. The CBC and BMP were all within normal limits, but the troponin I level was slightly elevated.
Given the patient’s clinical presentation and slightly elevated troponin I level, the EP was concerned for an acute coronary syndrome (ACS) and admitted the patient to the care of the on-call cardiologist. Prior to transfer, the patient was given 325 mg of aspirin by mouth, but no anticoagulation therapy was ordered. The cardiologist, who evaluated the patient after she was admitted to the inpatient floor, was concerned the patient had a pulmonary embolism (PE), and ordered a stat computed tomography angiography (CTA) scan of the chest. While the patient was undergoing the chest CTA scan, she went into cardiac arrest. Despite aggressive resuscitative measures, the patient could not be revived and was pronounced dead. An autopsy revealed a PE as the cause of death.
Plaintiff’s Claim
The patient’s estate sued the EP for failure to properly diagnose the PE, stating the hospital was vicariously liable for the EP’s actions. The emergency medicine (EM) expert for the plaintiff opined that the decedent’s symptoms should have prompted the EP to suspect she was suffering from a PE, and he should have immediately ordered anticoagulation, a D-dimer test, or a chest CTA scan. The expert cardiologist for the plaintiff stated the EP should have immediately started the patient on anticoagulation prior to the chest CTA scan.
The Defense
The defense EM expert stated the defendant’s diagnosis of ACS was appropriate given the patient’s overall clinical presentation, and the defense expert cardiologist stated the standard of care did not require the EP to administer anticoagulation prior to her diagnosis of PE, since the bleeding risks outweighed the benefits.
Verdict
At trial, the jury returned a defense verdict.
Discussion
This is not the first (nor probably the last) malpractice case in this column to involve a missed PE. While there have been improvements to the tools we currently possess to evaluate patients for suspected PE, it remains a difficult condition to reliably and timely identify in the ED. Although the two predominating symptoms—shortness of breath and chest pain—are common presentations in the ED, each is associated with large differential diagnoses.
Acute Coronary Syndrome Versus Pulmonary Embolism
From what we know of the published details of this case, the patient had only one risk factor for ACS (cigarette smoking) and two risk factors for PE (cigarette smoking and estrogen-containing contraceptive use). The only abnormal physical finding (tachycardia) was slightly more suggestive of PE than ACS. This patient’s primary complaint was chest fullness and shortness of breath. According to the Prospective Investigation of Pulmonary Embolism Diagnosis II study, shortness of breath is the most common complaint in PE (73%), followed by pleuritic chest pain (44%).1
In ACS, which is more common in men versus women and in patients of both sexes over age 55 years, the clinical presentation most commonly involves chest pain that patients describe as a pressure or fullness (as demonstrated in this patient). Unfortunately, in certain patient populations (eg, women, elderly patients, patients with diabetes mellitus) the presenting complaint can be shortness of breath, weakness, or nausea and vomiting. In a study evaluating how frequently an acute PE can mimic ACS, Kukla et al2 found that one-third of patients with an acute PE can present with all of the manifestations suggestive of ACS (ie, chest pain, ECG changes, and elevated troponin).
It is probably safe to assume the elevated troponin I level played a factor in influencing the EP to diagnose ACS, rather than pursuing an alternative diagnosis such as PE. Unfortunately, since both serum troponin T and I can be markers of right ventricle dysfunction, they are elevated in 30% to 50% of patients with moderate-to-large PE.3 However, neither serum troponin T nor troponin I is specific for myocardial infarction or unstable angina.
Pretest Probability: Wells Criteria
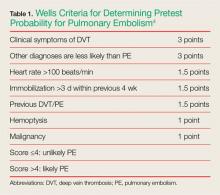
Determining pretest probability for any disease process is important when evaluating complaints in the ED; this is especially true for PE. One of the most frequently used tools for determining the likelihood of PE in ED patients is the Wells criteria (Table 1).4
Based on the published information available, the patient in this case would have scored a 1.5, placing her in the unlikely or low-risk category for PE. Patients whose Wells score places them in the low-risk group can benefit from serum D-dimer testing to help diagnose PE. However, serum D-dimer testing should not be ordered for patients in the likely or high-risk categories; these patients should instead be sent directly for imaging studies such as a chest CTA scan.
Pulmonary Embolism Rule-Out Criteria
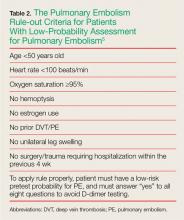
For patients whose Wells criteria score places them in the “unlikely group,” the PE rule-out criteria (PERC) can be used to determine the need for ordering a D-dimer. If all eight criteria are met, no further testing is necessary to exclude PE from the differential diagnosis (Table 2).5
Summary
Evaluating chest pain and shortness of breath in the ED is a humbling experience for even the most seasoned EP. Thoroughly reviewing the patient’s history and physical examination, and determining the pretest probability of disease entities high on the differential diagnoses list, go a long way in helping make the correct diagnosis—and in turn initiating possible life-saving interventions and treatment.
1. Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med. 2007;120(10):871-879.
2. Kukla P, Dlugopolski R, Krupa E, et al. How often pulmonary embolism mimics acute coronary syndrome? Kardiol Pol. 2011;69(3):235-240.
3. Meyer T, Binder L, Hruska N, Luthe H, Buchwald AB. Cardiac troponin I elevation in acute pulmonary embolism is associated with right ventricular dysfunction. J Am Coll Cardiol. 2000;36(5):1632-1636.
4. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416-420.
5. Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6(5):772-780. doi: 10.1111/j.1538-7836.2008.02944.x.
Case
A 37-year-old woman presented to the ED with a 90-minute history of chest tightness and shortness of breath. She admitted to feeling anxious but denied nausea, vomiting, or diaphoresis. The patient was in good health overall and had no history of similar symptoms. The only medication she took on a regular basis was a combination oral contraceptive (OC). Regarding the patient’s social history, she admitted to smoking one-half of a pack of cigarettes per day and occasional alcohol use.
On physical examination, the patient’s vital signs were: heart rate (HR), 102 beats/min; blood pressure, 118/64 mm Hg; respiratory rate, 20 breaths/min; and temperature, 98.6˚F. Oxygen saturation was 95% on room air. The head, eyes, ears, nose, and throat examination was normal. The cardiopulmonary examination revealed slight tachycardia with a regular rhythm but no murmurs, rubs, or gallops; the lungs were clear to auscultation bilaterally. The abdominal examination revealed a soft, nontender abdomen, without mass, and no guarding or rebound was present. An examination of the lower extremities was not documented.
The emergency physician (EP) ordered laboratory studies, which included a complete blood count (CBC), basic metabolic profile (BMP), and troponin I level. A chest X-ray and electrocardiogram (ECG) were also ordered. The chest X-ray was interpreted as normal, and the ECG revealed mild sinus tachycardia with nonspecific ST-T segment changes in V1 through V3. The CBC and BMP were all within normal limits, but the troponin I level was slightly elevated.
Given the patient’s clinical presentation and slightly elevated troponin I level, the EP was concerned for an acute coronary syndrome (ACS) and admitted the patient to the care of the on-call cardiologist. Prior to transfer, the patient was given 325 mg of aspirin by mouth, but no anticoagulation therapy was ordered. The cardiologist, who evaluated the patient after she was admitted to the inpatient floor, was concerned the patient had a pulmonary embolism (PE), and ordered a stat computed tomography angiography (CTA) scan of the chest. While the patient was undergoing the chest CTA scan, she went into cardiac arrest. Despite aggressive resuscitative measures, the patient could not be revived and was pronounced dead. An autopsy revealed a PE as the cause of death.
Plaintiff’s Claim
The patient’s estate sued the EP for failure to properly diagnose the PE, stating the hospital was vicariously liable for the EP’s actions. The emergency medicine (EM) expert for the plaintiff opined that the decedent’s symptoms should have prompted the EP to suspect she was suffering from a PE, and he should have immediately ordered anticoagulation, a D-dimer test, or a chest CTA scan. The expert cardiologist for the plaintiff stated the EP should have immediately started the patient on anticoagulation prior to the chest CTA scan.
The Defense
The defense EM expert stated the defendant’s diagnosis of ACS was appropriate given the patient’s overall clinical presentation, and the defense expert cardiologist stated the standard of care did not require the EP to administer anticoagulation prior to her diagnosis of PE, since the bleeding risks outweighed the benefits.
Verdict
At trial, the jury returned a defense verdict.
Discussion
This is not the first (nor probably the last) malpractice case in this column to involve a missed PE. While there have been improvements to the tools we currently possess to evaluate patients for suspected PE, it remains a difficult condition to reliably and timely identify in the ED. Although the two predominating symptoms—shortness of breath and chest pain—are common presentations in the ED, each is associated with large differential diagnoses.
Acute Coronary Syndrome Versus Pulmonary Embolism
From what we know of the published details of this case, the patient had only one risk factor for ACS (cigarette smoking) and two risk factors for PE (cigarette smoking and estrogen-containing contraceptive use). The only abnormal physical finding (tachycardia) was slightly more suggestive of PE than ACS. This patient’s primary complaint was chest fullness and shortness of breath. According to the Prospective Investigation of Pulmonary Embolism Diagnosis II study, shortness of breath is the most common complaint in PE (73%), followed by pleuritic chest pain (44%).1
In ACS, which is more common in men versus women and in patients of both sexes over age 55 years, the clinical presentation most commonly involves chest pain that patients describe as a pressure or fullness (as demonstrated in this patient). Unfortunately, in certain patient populations (eg, women, elderly patients, patients with diabetes mellitus) the presenting complaint can be shortness of breath, weakness, or nausea and vomiting. In a study evaluating how frequently an acute PE can mimic ACS, Kukla et al2 found that one-third of patients with an acute PE can present with all of the manifestations suggestive of ACS (ie, chest pain, ECG changes, and elevated troponin).
It is probably safe to assume the elevated troponin I level played a factor in influencing the EP to diagnose ACS, rather than pursuing an alternative diagnosis such as PE. Unfortunately, since both serum troponin T and I can be markers of right ventricle dysfunction, they are elevated in 30% to 50% of patients with moderate-to-large PE.3 However, neither serum troponin T nor troponin I is specific for myocardial infarction or unstable angina.
Pretest Probability: Wells Criteria
Determining pretest probability for any disease process is important when evaluating complaints in the ED; this is especially true for PE. One of the most frequently used tools for determining the likelihood of PE in ED patients is the Wells criteria (Table 1).4
Based on the published information available, the patient in this case would have scored a 1.5, placing her in the unlikely or low-risk category for PE. Patients whose Wells score places them in the low-risk group can benefit from serum D-dimer testing to help diagnose PE. However, serum D-dimer testing should not be ordered for patients in the likely or high-risk categories; these patients should instead be sent directly for imaging studies such as a chest CTA scan.
Pulmonary Embolism Rule-Out Criteria
For patients whose Wells criteria score places them in the “unlikely group,” the PE rule-out criteria (PERC) can be used to determine the need for ordering a D-dimer. If all eight criteria are met, no further testing is necessary to exclude PE from the differential diagnosis (Table 2).5
Summary
Evaluating chest pain and shortness of breath in the ED is a humbling experience for even the most seasoned EP. Thoroughly reviewing the patient’s history and physical examination, and determining the pretest probability of disease entities high on the differential diagnoses list, go a long way in helping make the correct diagnosis—and in turn initiating possible life-saving interventions and treatment.
Case
A 37-year-old woman presented to the ED with a 90-minute history of chest tightness and shortness of breath. She admitted to feeling anxious but denied nausea, vomiting, or diaphoresis. The patient was in good health overall and had no history of similar symptoms. The only medication she took on a regular basis was a combination oral contraceptive (OC). Regarding the patient’s social history, she admitted to smoking one-half of a pack of cigarettes per day and occasional alcohol use.
On physical examination, the patient’s vital signs were: heart rate (HR), 102 beats/min; blood pressure, 118/64 mm Hg; respiratory rate, 20 breaths/min; and temperature, 98.6˚F. Oxygen saturation was 95% on room air. The head, eyes, ears, nose, and throat examination was normal. The cardiopulmonary examination revealed slight tachycardia with a regular rhythm but no murmurs, rubs, or gallops; the lungs were clear to auscultation bilaterally. The abdominal examination revealed a soft, nontender abdomen, without mass, and no guarding or rebound was present. An examination of the lower extremities was not documented.
The emergency physician (EP) ordered laboratory studies, which included a complete blood count (CBC), basic metabolic profile (BMP), and troponin I level. A chest X-ray and electrocardiogram (ECG) were also ordered. The chest X-ray was interpreted as normal, and the ECG revealed mild sinus tachycardia with nonspecific ST-T segment changes in V1 through V3. The CBC and BMP were all within normal limits, but the troponin I level was slightly elevated.
Given the patient’s clinical presentation and slightly elevated troponin I level, the EP was concerned for an acute coronary syndrome (ACS) and admitted the patient to the care of the on-call cardiologist. Prior to transfer, the patient was given 325 mg of aspirin by mouth, but no anticoagulation therapy was ordered. The cardiologist, who evaluated the patient after she was admitted to the inpatient floor, was concerned the patient had a pulmonary embolism (PE), and ordered a stat computed tomography angiography (CTA) scan of the chest. While the patient was undergoing the chest CTA scan, she went into cardiac arrest. Despite aggressive resuscitative measures, the patient could not be revived and was pronounced dead. An autopsy revealed a PE as the cause of death.
Plaintiff’s Claim
The patient’s estate sued the EP for failure to properly diagnose the PE, stating the hospital was vicariously liable for the EP’s actions. The emergency medicine (EM) expert for the plaintiff opined that the decedent’s symptoms should have prompted the EP to suspect she was suffering from a PE, and he should have immediately ordered anticoagulation, a D-dimer test, or a chest CTA scan. The expert cardiologist for the plaintiff stated the EP should have immediately started the patient on anticoagulation prior to the chest CTA scan.
The Defense
The defense EM expert stated the defendant’s diagnosis of ACS was appropriate given the patient’s overall clinical presentation, and the defense expert cardiologist stated the standard of care did not require the EP to administer anticoagulation prior to her diagnosis of PE, since the bleeding risks outweighed the benefits.
Verdict
At trial, the jury returned a defense verdict.
Discussion
This is not the first (nor probably the last) malpractice case in this column to involve a missed PE. While there have been improvements to the tools we currently possess to evaluate patients for suspected PE, it remains a difficult condition to reliably and timely identify in the ED. Although the two predominating symptoms—shortness of breath and chest pain—are common presentations in the ED, each is associated with large differential diagnoses.
Acute Coronary Syndrome Versus Pulmonary Embolism
From what we know of the published details of this case, the patient had only one risk factor for ACS (cigarette smoking) and two risk factors for PE (cigarette smoking and estrogen-containing contraceptive use). The only abnormal physical finding (tachycardia) was slightly more suggestive of PE than ACS. This patient’s primary complaint was chest fullness and shortness of breath. According to the Prospective Investigation of Pulmonary Embolism Diagnosis II study, shortness of breath is the most common complaint in PE (73%), followed by pleuritic chest pain (44%).1
In ACS, which is more common in men versus women and in patients of both sexes over age 55 years, the clinical presentation most commonly involves chest pain that patients describe as a pressure or fullness (as demonstrated in this patient). Unfortunately, in certain patient populations (eg, women, elderly patients, patients with diabetes mellitus) the presenting complaint can be shortness of breath, weakness, or nausea and vomiting. In a study evaluating how frequently an acute PE can mimic ACS, Kukla et al2 found that one-third of patients with an acute PE can present with all of the manifestations suggestive of ACS (ie, chest pain, ECG changes, and elevated troponin).
It is probably safe to assume the elevated troponin I level played a factor in influencing the EP to diagnose ACS, rather than pursuing an alternative diagnosis such as PE. Unfortunately, since both serum troponin T and I can be markers of right ventricle dysfunction, they are elevated in 30% to 50% of patients with moderate-to-large PE.3 However, neither serum troponin T nor troponin I is specific for myocardial infarction or unstable angina.
Pretest Probability: Wells Criteria
Determining pretest probability for any disease process is important when evaluating complaints in the ED; this is especially true for PE. One of the most frequently used tools for determining the likelihood of PE in ED patients is the Wells criteria (Table 1).4
Based on the published information available, the patient in this case would have scored a 1.5, placing her in the unlikely or low-risk category for PE. Patients whose Wells score places them in the low-risk group can benefit from serum D-dimer testing to help diagnose PE. However, serum D-dimer testing should not be ordered for patients in the likely or high-risk categories; these patients should instead be sent directly for imaging studies such as a chest CTA scan.
Pulmonary Embolism Rule-Out Criteria
For patients whose Wells criteria score places them in the “unlikely group,” the PE rule-out criteria (PERC) can be used to determine the need for ordering a D-dimer. If all eight criteria are met, no further testing is necessary to exclude PE from the differential diagnosis (Table 2).5
Summary
Evaluating chest pain and shortness of breath in the ED is a humbling experience for even the most seasoned EP. Thoroughly reviewing the patient’s history and physical examination, and determining the pretest probability of disease entities high on the differential diagnoses list, go a long way in helping make the correct diagnosis—and in turn initiating possible life-saving interventions and treatment.
1. Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med. 2007;120(10):871-879.
2. Kukla P, Dlugopolski R, Krupa E, et al. How often pulmonary embolism mimics acute coronary syndrome? Kardiol Pol. 2011;69(3):235-240.
3. Meyer T, Binder L, Hruska N, Luthe H, Buchwald AB. Cardiac troponin I elevation in acute pulmonary embolism is associated with right ventricular dysfunction. J Am Coll Cardiol. 2000;36(5):1632-1636.
4. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416-420.
5. Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6(5):772-780. doi: 10.1111/j.1538-7836.2008.02944.x.
1. Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med. 2007;120(10):871-879.
2. Kukla P, Dlugopolski R, Krupa E, et al. How often pulmonary embolism mimics acute coronary syndrome? Kardiol Pol. 2011;69(3):235-240.
3. Meyer T, Binder L, Hruska N, Luthe H, Buchwald AB. Cardiac troponin I elevation in acute pulmonary embolism is associated with right ventricular dysfunction. J Am Coll Cardiol. 2000;36(5):1632-1636.
4. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416-420.
5. Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6(5):772-780. doi: 10.1111/j.1538-7836.2008.02944.x.