User login
Malpractice Counsel: Abdominal pain in an elderly patient
Case
An 89-year-old woman presented to the ED with the chief complaints of abdominal pain and nausea with vomiting. The patient stated that several hours prior, she had ingested an expired beverage, which she related to the sudden onset of her symptoms. The patient denied fever, chills, dysuria, or frequency. Her medical history was significant for chronic atrial fibrillation (AF) and congestive heart failure. The patient’s medications included metoprolol and furosemide; she was not on any anticoagulation medication.
On physical examination, the patient appeared her stated age, and was in moderate distress secondary to the abdominal pain. Vital signs were: temperature, 98.8oF; heart rate, 98 beats/min; respiratory rate, 20 breaths/min; and blood pressure, 116/72 mm Hg. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was unremarkable. On lung examination, breath sounds were equal bilaterally with bibasilar rales. The heart rhythm was irregularly irregular without murmurs, rubs, or gallops. The abdomen was soft to palpitation, but diffusely tender, without rebound, guarding, or mass. Rectal examination revealed normal tone and brown stool, and was trace positive for heme.
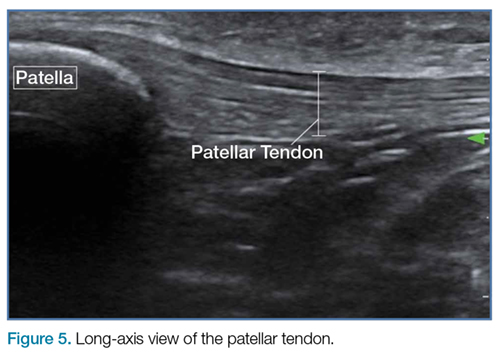
The emergency physician (EP) ordered an electrocardiogram (ECG), complete blood count, basic metabolic profile (BMP), urinalysis, and lipase test. The patient was administered intravenous (IV) normal saline at 75 cc/h, and morphine 4 mg and ondansetron 4 mg IV for the abdominal pain, nausea, and vomiting. She required several more doses of morphine due to the severity of the pain. The laboratory results included an elevated white blood count of 18.4 x 109/L with a left shift, but normal hemoglobin and hematocrit values. The ECG demonstrated AF with a controlled ventricular rate; there was no evidence of ischemia or injury. The BMP was remarkable for a slightly depressed potassium level (3.3 mEq/L), a decreased serum bicarbonate of 20 mEq/L, and evidence of renal insufficiency with a blood urea nitrogen of 28 mg/dL and a serum creatinine of 1.6 mg/dL. Given the ongoing severe pain, leukocytosis, metabolic acidosis, and lack of clear etiology, the EP ordered a computed tomography (CT) scan of the abdomen and pelvis; no IV contrast was ordered because of the abnormal renal function studies.
The radiologist interpreted the CT scan as essentially normal. The EP admitted the patient to the on-call hospitalist, who consulted both cardiology and gastroenterology services. During the night, the patient complained of increasing abdominal pain, and her abdomen became distended with peritoneal signs. She was taken emergently to the operating room in the early morning hours. A large segment of gangrenous small intestine was found upon exploration. The surgery was discontinued and comfort care measures were instituted. The patient died the following day.
The patient’s family sued the EP and the hospital for failure to make a timely diagnosis of mesenteric ischemia. They further stated that the EP should have ordered a CT angiogram (CTA) of the abdomen and pelvis. The defense argued that a contrast CT scan was contraindicated because of the patient’s poor renal function. A defense verdict was returned at trial.
Discussion
Elderly patients (defined as older than age 65 years) presenting to the ED with abdominal pain remain a diagnostic challenge for even the most seasoned clinician. While elderly patients with a chief complaint of abdominal pain represent only a small percentage of ED patients, approximately 50% to 66% of these patients will require hospitalization, while one-third will require a surgical intervention.1 The seriousness of this complaint in elderly patients is further emphasized by the fact that older patients with abdominal pain have a 6- to 8-fold increase in mortality compared to younger patients.2,3 This can be partially explained by the simple fact that the life-threatening causes of abdominal pain—abdominal aortic aneurysm, mesenteric ischemia, bowel perforation, volvulus, and acute bowel obstruction—occur more frequently (but not exclusively) in elderly patients. Historical risk factors for life-threatening causes of abdominal pain include: age older than 65 years, immunocompromised state, alcohol abuse, cardiovascular (CV) disease (eg, coronary artery disease, hypertension, AF), major comorbidities (eg, cancer, renal failure), and prior surgery or recent gastrointestinal instrumentation.1
The patient in this case had two risk factors for life-threatening causes of lower abdominal pain—age and AF. These are also two of the major risk factors for mesenteric ischemia, which was her ultimate diagnosis.
Acute mesenteric ischemia refers to the sudden onset of small intestinal hypoperfusion, frequently due to acute occlusion (embolism or thrombosis) of an intestinal artery, most commonly the superior mesenteric artery (SMA).4 The SMA supplies the entire small intestine except for the proximal duodenum. Other causes of acute mesenteric ischemia include venous occlusion (thrombosis) and nonocclusive mesenteric ischemia secondary to vasoconstriction from low-cardiac output or use of vasopressors.4
Thromboembolic occlusion of the SMA is the most common cause of acute mesenteric ischemia, accounting for 67% to 95% of cases.4 In addition to AF, the risk of arterial embolism is increased in patients with valvular disease, infective endocarditis, recent myocardial infarction, aortic atherosclerosis, or aortic aneurysm.4 Risk factors for thrombotic arterial occlusion include peripheral artery disease, advanced age, and low-cardiac output states.5
A frequent presentation of embolic mesenteric arterial ischemia, occurring in approximately one-third of cases, is an elderly patient with AF (or other source of embolism) and onset of severe, sudden abdominal pain out of proportion to physical examination. While nausea and vomiting are also common, bloody bowel movements are less frequent in the early course of the disease process.4 A history of a prior embolic event is present in approximately one-third of such patients.
On physical examination, the abdomen may be normal initially, or demonstrate only mild distention and tenderness without peritoneal signs. However, as the ischemia progresses, the abdomen becomes more distended, bowel sounds become absent, and peritoneal signs (ie, guarding and rebound) become apparent.6
The results of laboratory studies can suggest the diagnosis, but none are confirmatory. Laboratory findings may include a marked leukocytosis with left shift, an elevated hematocrit secondary to hemoconcentration, and metabolic acidosis. A helpful clinical pearl is to consider intestinal ischemia in the differential diagnosis of any patient with acute abdominal pain and metabolic acidosis.6 Serum lactate is frequently elevated (73%-94%) but a very nonspecific marker. Similarly, an arterial blood gas analysis may demonstrate metabolic acidosis. More recently, a normal D-dimer result has been used to help exclude the diagnosis of acute intestinal ischemia, since it is elevated in 96% of patients with the disease.6 Similar to lactate, an abnormal D-dimer result has a poor specificity (40%).6 Early in the disease course, nearly all laboratory studies may be normal.
Depending on the severity of the presentation, imaging can help make the definitive diagnosis. For patients with peritonitis or obvious bowel perforation, IV fluid resuscitation, IV antibiotics, and immediate surgical exploration are indicated. Plain radiographs of the abdomen offer little help, as many of the findings early in the disease course are nonspecific, and radiographs can be normal in 25% of cases.6 Ultrasound can identify arterial stenosis or occlusion of the SMA, but is frequently technically limited by the presence of air-filled loops of distended bowel.6 Magnetic resonance angiography has similar sensitivity and specificity as CTA for mesenteric arterial ischemia, and is actually more sensitive than CTA for mesenteric venous thrombosis; it also can be performed in patients with contrast allergy.6 However, CTA is performed more commonly because of its lower cost, greater speed, and wide availability.6 A CTA of the abdomen and pelvis (without oral contrast) is probably the best study for patients in whom mesenteric ischemia is high on the differential diagnosis.6 For patients with a less clear picture and a broader differential diagnosis, a CT scan of the abdomen/pelvis with both IV and oral contrast is preferred.7 Common findings on CT scan with IV/oral contrast in acute mesenteric ischemia include the following: bowel wall thickening, dilatation, stranding, bowel wall attenuation, abnormal enhancement, and pneumatosis. Unfortunately, many of these findings are nonspecific.7
Once the diagnosis of acute mesenteric ischemia is made, patients should be designated “nothing by mouth” and a nasogastric tube placed to decompress the bowel. These patients will require IV fluid resuscitation with normal saline. The amount and rate will depend on their clinical presentation and underlying CV status. Any electrolyte abnormalities should be corrected and broad spectrum IV antibiotics initiated. Vascular surgery or general surgery services should be consulted to determine the optimal management. Most patients with acute intestinal ischemia due to mesenteric arterial occlusion (or venous occlusive or nonocclusive mesenteric ischemia) will be started on anticoagulation, typically IV heparin, unless contraindications are present.6 Surgical treatment options include arterial embolectomy, arterial bypass, arterial stenting, arterial thrombolysis, or intra-arterial vasodilator infusion.
1. Kendall JL, Moreira ME. Evaluation of the adult with abdominal pain in the emergency department. UpToDate Web site. http://www.uptodate.com/contents/evaluation-of-the-adult-with-abdominal-pain-in-the-emergency-department. Updated September 29, 2016. Accessed November 30, 2016.
2. Lewis LM, Banet GA, Blanda M, Hustey FM, Meldon SW, Gerson LW. Etiology and clinical course of abdominal pain in senior patients: a prospective, multicenter study. J Gerontol A Biol Sci Med Sci. 2005;60(8):1071-1076.
3. Sanson TG, O’Keefe KP. Evaluation of abdominal pain in the elderly. Emerg Med Clin North Am. 1996;14(3):615.
4. Tendler DA, Lamont JT, Pearl G. Acute mesenteric arterial occlusion. UpToDate Web site. http://www.uptodate.com/contents/acute-mesenteric-arterial-occlusion. Updated May 27, 2015. Accessed November 30, 2016.
5. McKinsey JF, Gewertz BL. Acute mesenteric ischemia. Surg Clin North Am. 1997;77(2):307-318.
6. Tendler DA, Lamont JT. Overview of intestinal ischemia in adults. UpToDate Web site. http://www.uptodate.com/contents/overview-of-intestinal-ischemia-in-adults. Updated February 23, 2016. Accessed November 30, 2016.
7. Wiesner W. Khurana B, Ji H, Ros PR. CT of acute bowel ischemia. Radiology. 2003;226(3):635-650.
Case
An 89-year-old woman presented to the ED with the chief complaints of abdominal pain and nausea with vomiting. The patient stated that several hours prior, she had ingested an expired beverage, which she related to the sudden onset of her symptoms. The patient denied fever, chills, dysuria, or frequency. Her medical history was significant for chronic atrial fibrillation (AF) and congestive heart failure. The patient’s medications included metoprolol and furosemide; she was not on any anticoagulation medication.
On physical examination, the patient appeared her stated age, and was in moderate distress secondary to the abdominal pain. Vital signs were: temperature, 98.8oF; heart rate, 98 beats/min; respiratory rate, 20 breaths/min; and blood pressure, 116/72 mm Hg. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was unremarkable. On lung examination, breath sounds were equal bilaterally with bibasilar rales. The heart rhythm was irregularly irregular without murmurs, rubs, or gallops. The abdomen was soft to palpitation, but diffusely tender, without rebound, guarding, or mass. Rectal examination revealed normal tone and brown stool, and was trace positive for heme.
The emergency physician (EP) ordered an electrocardiogram (ECG), complete blood count, basic metabolic profile (BMP), urinalysis, and lipase test. The patient was administered intravenous (IV) normal saline at 75 cc/h, and morphine 4 mg and ondansetron 4 mg IV for the abdominal pain, nausea, and vomiting. She required several more doses of morphine due to the severity of the pain. The laboratory results included an elevated white blood count of 18.4 x 109/L with a left shift, but normal hemoglobin and hematocrit values. The ECG demonstrated AF with a controlled ventricular rate; there was no evidence of ischemia or injury. The BMP was remarkable for a slightly depressed potassium level (3.3 mEq/L), a decreased serum bicarbonate of 20 mEq/L, and evidence of renal insufficiency with a blood urea nitrogen of 28 mg/dL and a serum creatinine of 1.6 mg/dL. Given the ongoing severe pain, leukocytosis, metabolic acidosis, and lack of clear etiology, the EP ordered a computed tomography (CT) scan of the abdomen and pelvis; no IV contrast was ordered because of the abnormal renal function studies.
The radiologist interpreted the CT scan as essentially normal. The EP admitted the patient to the on-call hospitalist, who consulted both cardiology and gastroenterology services. During the night, the patient complained of increasing abdominal pain, and her abdomen became distended with peritoneal signs. She was taken emergently to the operating room in the early morning hours. A large segment of gangrenous small intestine was found upon exploration. The surgery was discontinued and comfort care measures were instituted. The patient died the following day.
The patient’s family sued the EP and the hospital for failure to make a timely diagnosis of mesenteric ischemia. They further stated that the EP should have ordered a CT angiogram (CTA) of the abdomen and pelvis. The defense argued that a contrast CT scan was contraindicated because of the patient’s poor renal function. A defense verdict was returned at trial.
Discussion
Elderly patients (defined as older than age 65 years) presenting to the ED with abdominal pain remain a diagnostic challenge for even the most seasoned clinician. While elderly patients with a chief complaint of abdominal pain represent only a small percentage of ED patients, approximately 50% to 66% of these patients will require hospitalization, while one-third will require a surgical intervention.1 The seriousness of this complaint in elderly patients is further emphasized by the fact that older patients with abdominal pain have a 6- to 8-fold increase in mortality compared to younger patients.2,3 This can be partially explained by the simple fact that the life-threatening causes of abdominal pain—abdominal aortic aneurysm, mesenteric ischemia, bowel perforation, volvulus, and acute bowel obstruction—occur more frequently (but not exclusively) in elderly patients. Historical risk factors for life-threatening causes of abdominal pain include: age older than 65 years, immunocompromised state, alcohol abuse, cardiovascular (CV) disease (eg, coronary artery disease, hypertension, AF), major comorbidities (eg, cancer, renal failure), and prior surgery or recent gastrointestinal instrumentation.1
The patient in this case had two risk factors for life-threatening causes of lower abdominal pain—age and AF. These are also two of the major risk factors for mesenteric ischemia, which was her ultimate diagnosis.
Acute mesenteric ischemia refers to the sudden onset of small intestinal hypoperfusion, frequently due to acute occlusion (embolism or thrombosis) of an intestinal artery, most commonly the superior mesenteric artery (SMA).4 The SMA supplies the entire small intestine except for the proximal duodenum. Other causes of acute mesenteric ischemia include venous occlusion (thrombosis) and nonocclusive mesenteric ischemia secondary to vasoconstriction from low-cardiac output or use of vasopressors.4
Thromboembolic occlusion of the SMA is the most common cause of acute mesenteric ischemia, accounting for 67% to 95% of cases.4 In addition to AF, the risk of arterial embolism is increased in patients with valvular disease, infective endocarditis, recent myocardial infarction, aortic atherosclerosis, or aortic aneurysm.4 Risk factors for thrombotic arterial occlusion include peripheral artery disease, advanced age, and low-cardiac output states.5
A frequent presentation of embolic mesenteric arterial ischemia, occurring in approximately one-third of cases, is an elderly patient with AF (or other source of embolism) and onset of severe, sudden abdominal pain out of proportion to physical examination. While nausea and vomiting are also common, bloody bowel movements are less frequent in the early course of the disease process.4 A history of a prior embolic event is present in approximately one-third of such patients.
On physical examination, the abdomen may be normal initially, or demonstrate only mild distention and tenderness without peritoneal signs. However, as the ischemia progresses, the abdomen becomes more distended, bowel sounds become absent, and peritoneal signs (ie, guarding and rebound) become apparent.6
The results of laboratory studies can suggest the diagnosis, but none are confirmatory. Laboratory findings may include a marked leukocytosis with left shift, an elevated hematocrit secondary to hemoconcentration, and metabolic acidosis. A helpful clinical pearl is to consider intestinal ischemia in the differential diagnosis of any patient with acute abdominal pain and metabolic acidosis.6 Serum lactate is frequently elevated (73%-94%) but a very nonspecific marker. Similarly, an arterial blood gas analysis may demonstrate metabolic acidosis. More recently, a normal D-dimer result has been used to help exclude the diagnosis of acute intestinal ischemia, since it is elevated in 96% of patients with the disease.6 Similar to lactate, an abnormal D-dimer result has a poor specificity (40%).6 Early in the disease course, nearly all laboratory studies may be normal.
Depending on the severity of the presentation, imaging can help make the definitive diagnosis. For patients with peritonitis or obvious bowel perforation, IV fluid resuscitation, IV antibiotics, and immediate surgical exploration are indicated. Plain radiographs of the abdomen offer little help, as many of the findings early in the disease course are nonspecific, and radiographs can be normal in 25% of cases.6 Ultrasound can identify arterial stenosis or occlusion of the SMA, but is frequently technically limited by the presence of air-filled loops of distended bowel.6 Magnetic resonance angiography has similar sensitivity and specificity as CTA for mesenteric arterial ischemia, and is actually more sensitive than CTA for mesenteric venous thrombosis; it also can be performed in patients with contrast allergy.6 However, CTA is performed more commonly because of its lower cost, greater speed, and wide availability.6 A CTA of the abdomen and pelvis (without oral contrast) is probably the best study for patients in whom mesenteric ischemia is high on the differential diagnosis.6 For patients with a less clear picture and a broader differential diagnosis, a CT scan of the abdomen/pelvis with both IV and oral contrast is preferred.7 Common findings on CT scan with IV/oral contrast in acute mesenteric ischemia include the following: bowel wall thickening, dilatation, stranding, bowel wall attenuation, abnormal enhancement, and pneumatosis. Unfortunately, many of these findings are nonspecific.7
Once the diagnosis of acute mesenteric ischemia is made, patients should be designated “nothing by mouth” and a nasogastric tube placed to decompress the bowel. These patients will require IV fluid resuscitation with normal saline. The amount and rate will depend on their clinical presentation and underlying CV status. Any electrolyte abnormalities should be corrected and broad spectrum IV antibiotics initiated. Vascular surgery or general surgery services should be consulted to determine the optimal management. Most patients with acute intestinal ischemia due to mesenteric arterial occlusion (or venous occlusive or nonocclusive mesenteric ischemia) will be started on anticoagulation, typically IV heparin, unless contraindications are present.6 Surgical treatment options include arterial embolectomy, arterial bypass, arterial stenting, arterial thrombolysis, or intra-arterial vasodilator infusion.
Case
An 89-year-old woman presented to the ED with the chief complaints of abdominal pain and nausea with vomiting. The patient stated that several hours prior, she had ingested an expired beverage, which she related to the sudden onset of her symptoms. The patient denied fever, chills, dysuria, or frequency. Her medical history was significant for chronic atrial fibrillation (AF) and congestive heart failure. The patient’s medications included metoprolol and furosemide; she was not on any anticoagulation medication.
On physical examination, the patient appeared her stated age, and was in moderate distress secondary to the abdominal pain. Vital signs were: temperature, 98.8oF; heart rate, 98 beats/min; respiratory rate, 20 breaths/min; and blood pressure, 116/72 mm Hg. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was unremarkable. On lung examination, breath sounds were equal bilaterally with bibasilar rales. The heart rhythm was irregularly irregular without murmurs, rubs, or gallops. The abdomen was soft to palpitation, but diffusely tender, without rebound, guarding, or mass. Rectal examination revealed normal tone and brown stool, and was trace positive for heme.
The emergency physician (EP) ordered an electrocardiogram (ECG), complete blood count, basic metabolic profile (BMP), urinalysis, and lipase test. The patient was administered intravenous (IV) normal saline at 75 cc/h, and morphine 4 mg and ondansetron 4 mg IV for the abdominal pain, nausea, and vomiting. She required several more doses of morphine due to the severity of the pain. The laboratory results included an elevated white blood count of 18.4 x 109/L with a left shift, but normal hemoglobin and hematocrit values. The ECG demonstrated AF with a controlled ventricular rate; there was no evidence of ischemia or injury. The BMP was remarkable for a slightly depressed potassium level (3.3 mEq/L), a decreased serum bicarbonate of 20 mEq/L, and evidence of renal insufficiency with a blood urea nitrogen of 28 mg/dL and a serum creatinine of 1.6 mg/dL. Given the ongoing severe pain, leukocytosis, metabolic acidosis, and lack of clear etiology, the EP ordered a computed tomography (CT) scan of the abdomen and pelvis; no IV contrast was ordered because of the abnormal renal function studies.
The radiologist interpreted the CT scan as essentially normal. The EP admitted the patient to the on-call hospitalist, who consulted both cardiology and gastroenterology services. During the night, the patient complained of increasing abdominal pain, and her abdomen became distended with peritoneal signs. She was taken emergently to the operating room in the early morning hours. A large segment of gangrenous small intestine was found upon exploration. The surgery was discontinued and comfort care measures were instituted. The patient died the following day.
The patient’s family sued the EP and the hospital for failure to make a timely diagnosis of mesenteric ischemia. They further stated that the EP should have ordered a CT angiogram (CTA) of the abdomen and pelvis. The defense argued that a contrast CT scan was contraindicated because of the patient’s poor renal function. A defense verdict was returned at trial.
Discussion
Elderly patients (defined as older than age 65 years) presenting to the ED with abdominal pain remain a diagnostic challenge for even the most seasoned clinician. While elderly patients with a chief complaint of abdominal pain represent only a small percentage of ED patients, approximately 50% to 66% of these patients will require hospitalization, while one-third will require a surgical intervention.1 The seriousness of this complaint in elderly patients is further emphasized by the fact that older patients with abdominal pain have a 6- to 8-fold increase in mortality compared to younger patients.2,3 This can be partially explained by the simple fact that the life-threatening causes of abdominal pain—abdominal aortic aneurysm, mesenteric ischemia, bowel perforation, volvulus, and acute bowel obstruction—occur more frequently (but not exclusively) in elderly patients. Historical risk factors for life-threatening causes of abdominal pain include: age older than 65 years, immunocompromised state, alcohol abuse, cardiovascular (CV) disease (eg, coronary artery disease, hypertension, AF), major comorbidities (eg, cancer, renal failure), and prior surgery or recent gastrointestinal instrumentation.1
The patient in this case had two risk factors for life-threatening causes of lower abdominal pain—age and AF. These are also two of the major risk factors for mesenteric ischemia, which was her ultimate diagnosis.
Acute mesenteric ischemia refers to the sudden onset of small intestinal hypoperfusion, frequently due to acute occlusion (embolism or thrombosis) of an intestinal artery, most commonly the superior mesenteric artery (SMA).4 The SMA supplies the entire small intestine except for the proximal duodenum. Other causes of acute mesenteric ischemia include venous occlusion (thrombosis) and nonocclusive mesenteric ischemia secondary to vasoconstriction from low-cardiac output or use of vasopressors.4
Thromboembolic occlusion of the SMA is the most common cause of acute mesenteric ischemia, accounting for 67% to 95% of cases.4 In addition to AF, the risk of arterial embolism is increased in patients with valvular disease, infective endocarditis, recent myocardial infarction, aortic atherosclerosis, or aortic aneurysm.4 Risk factors for thrombotic arterial occlusion include peripheral artery disease, advanced age, and low-cardiac output states.5
A frequent presentation of embolic mesenteric arterial ischemia, occurring in approximately one-third of cases, is an elderly patient with AF (or other source of embolism) and onset of severe, sudden abdominal pain out of proportion to physical examination. While nausea and vomiting are also common, bloody bowel movements are less frequent in the early course of the disease process.4 A history of a prior embolic event is present in approximately one-third of such patients.
On physical examination, the abdomen may be normal initially, or demonstrate only mild distention and tenderness without peritoneal signs. However, as the ischemia progresses, the abdomen becomes more distended, bowel sounds become absent, and peritoneal signs (ie, guarding and rebound) become apparent.6
The results of laboratory studies can suggest the diagnosis, but none are confirmatory. Laboratory findings may include a marked leukocytosis with left shift, an elevated hematocrit secondary to hemoconcentration, and metabolic acidosis. A helpful clinical pearl is to consider intestinal ischemia in the differential diagnosis of any patient with acute abdominal pain and metabolic acidosis.6 Serum lactate is frequently elevated (73%-94%) but a very nonspecific marker. Similarly, an arterial blood gas analysis may demonstrate metabolic acidosis. More recently, a normal D-dimer result has been used to help exclude the diagnosis of acute intestinal ischemia, since it is elevated in 96% of patients with the disease.6 Similar to lactate, an abnormal D-dimer result has a poor specificity (40%).6 Early in the disease course, nearly all laboratory studies may be normal.
Depending on the severity of the presentation, imaging can help make the definitive diagnosis. For patients with peritonitis or obvious bowel perforation, IV fluid resuscitation, IV antibiotics, and immediate surgical exploration are indicated. Plain radiographs of the abdomen offer little help, as many of the findings early in the disease course are nonspecific, and radiographs can be normal in 25% of cases.6 Ultrasound can identify arterial stenosis or occlusion of the SMA, but is frequently technically limited by the presence of air-filled loops of distended bowel.6 Magnetic resonance angiography has similar sensitivity and specificity as CTA for mesenteric arterial ischemia, and is actually more sensitive than CTA for mesenteric venous thrombosis; it also can be performed in patients with contrast allergy.6 However, CTA is performed more commonly because of its lower cost, greater speed, and wide availability.6 A CTA of the abdomen and pelvis (without oral contrast) is probably the best study for patients in whom mesenteric ischemia is high on the differential diagnosis.6 For patients with a less clear picture and a broader differential diagnosis, a CT scan of the abdomen/pelvis with both IV and oral contrast is preferred.7 Common findings on CT scan with IV/oral contrast in acute mesenteric ischemia include the following: bowel wall thickening, dilatation, stranding, bowel wall attenuation, abnormal enhancement, and pneumatosis. Unfortunately, many of these findings are nonspecific.7
Once the diagnosis of acute mesenteric ischemia is made, patients should be designated “nothing by mouth” and a nasogastric tube placed to decompress the bowel. These patients will require IV fluid resuscitation with normal saline. The amount and rate will depend on their clinical presentation and underlying CV status. Any electrolyte abnormalities should be corrected and broad spectrum IV antibiotics initiated. Vascular surgery or general surgery services should be consulted to determine the optimal management. Most patients with acute intestinal ischemia due to mesenteric arterial occlusion (or venous occlusive or nonocclusive mesenteric ischemia) will be started on anticoagulation, typically IV heparin, unless contraindications are present.6 Surgical treatment options include arterial embolectomy, arterial bypass, arterial stenting, arterial thrombolysis, or intra-arterial vasodilator infusion.
1. Kendall JL, Moreira ME. Evaluation of the adult with abdominal pain in the emergency department. UpToDate Web site. http://www.uptodate.com/contents/evaluation-of-the-adult-with-abdominal-pain-in-the-emergency-department. Updated September 29, 2016. Accessed November 30, 2016.
2. Lewis LM, Banet GA, Blanda M, Hustey FM, Meldon SW, Gerson LW. Etiology and clinical course of abdominal pain in senior patients: a prospective, multicenter study. J Gerontol A Biol Sci Med Sci. 2005;60(8):1071-1076.
3. Sanson TG, O’Keefe KP. Evaluation of abdominal pain in the elderly. Emerg Med Clin North Am. 1996;14(3):615.
4. Tendler DA, Lamont JT, Pearl G. Acute mesenteric arterial occlusion. UpToDate Web site. http://www.uptodate.com/contents/acute-mesenteric-arterial-occlusion. Updated May 27, 2015. Accessed November 30, 2016.
5. McKinsey JF, Gewertz BL. Acute mesenteric ischemia. Surg Clin North Am. 1997;77(2):307-318.
6. Tendler DA, Lamont JT. Overview of intestinal ischemia in adults. UpToDate Web site. http://www.uptodate.com/contents/overview-of-intestinal-ischemia-in-adults. Updated February 23, 2016. Accessed November 30, 2016.
7. Wiesner W. Khurana B, Ji H, Ros PR. CT of acute bowel ischemia. Radiology. 2003;226(3):635-650.
1. Kendall JL, Moreira ME. Evaluation of the adult with abdominal pain in the emergency department. UpToDate Web site. http://www.uptodate.com/contents/evaluation-of-the-adult-with-abdominal-pain-in-the-emergency-department. Updated September 29, 2016. Accessed November 30, 2016.
2. Lewis LM, Banet GA, Blanda M, Hustey FM, Meldon SW, Gerson LW. Etiology and clinical course of abdominal pain in senior patients: a prospective, multicenter study. J Gerontol A Biol Sci Med Sci. 2005;60(8):1071-1076.
3. Sanson TG, O’Keefe KP. Evaluation of abdominal pain in the elderly. Emerg Med Clin North Am. 1996;14(3):615.
4. Tendler DA, Lamont JT, Pearl G. Acute mesenteric arterial occlusion. UpToDate Web site. http://www.uptodate.com/contents/acute-mesenteric-arterial-occlusion. Updated May 27, 2015. Accessed November 30, 2016.
5. McKinsey JF, Gewertz BL. Acute mesenteric ischemia. Surg Clin North Am. 1997;77(2):307-318.
6. Tendler DA, Lamont JT. Overview of intestinal ischemia in adults. UpToDate Web site. http://www.uptodate.com/contents/overview-of-intestinal-ischemia-in-adults. Updated February 23, 2016. Accessed November 30, 2016.
7. Wiesner W. Khurana B, Ji H, Ros PR. CT of acute bowel ischemia. Radiology. 2003;226(3):635-650.
Malpractice Counsel: Missed Nodule
Case
A 48-year-old man presented to the ED with a 2-day history of cough and congestion. He described the cough as gradual in onset and, though initially nonproductive, it was now productive of green sputum. He denied fevers or chills, chest pain, nausea, vomiting, or diarrhea, and complained of only mild shortness of breath. His medical history was significant for hypertension, which was well managed with daily lisinopril-hydrochlorothiazide. He admitted to smoking one pack of cigarettes per day for the past 25 years, but denied alcohol or illicit drug use.
On physical examination, the patient’s vital signs were: blood pressure, 112/64 mm Hg; heart rate, 84 beats/min; respiratory rate, 20 breaths/min; and temperature, 98oF. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was normal. Auscultation of the lungs revealed bilateral breath sounds with scattered, faint expiratory wheezing; the heart had a regular rate and rhythm, without murmurs, rubs, or gallops.
The emergency physician (EP) ordered posteroanterior and lateral chest X-rays (CXR), which he interpreted as normal. He also ordered an albuterol handheld nebulizer treatment for the patient. After the albuterol treatment, the patient felt he was breathing more easily. The frequency of his cough had also decreased following treatment and, on re-examination, he exhibited no wheezing and was given azithromycin 500 mg orally in the ED. The EP diagnosed the patient with acute bronchitis and discharged him home with an albuterol metered dose inhaler with a spacer, and a 4-day course of azithromycin. He also encouraged the patient to quit smoking.
The next day the radiologist’s official reading of the patient’s radiographs included the finding of a very small pulmonary nodule, which was seen only on the lateral X-ray. The radiologist recommended a repeat CXR or a computed tomography (CT) scan of the chest in 6 months.
Unfortunately, the EP never saw this information, and the patient was not contacted regarding the abnormal radiology finding and the need for follow-up. Approximately 20 months later, the patient was diagnosed with lung cancer with metastasis to the thoracic spine and liver. Despite chemotherapy and radiation treatment, he died from the cancer.
The patient’s family brought a malpractice suit against the EP, stating that the cancer could have been successfully treated prior to any metastasis if the patient had been informed of the abnormal radiology findings at his ED visit 20 months prior. The EP argued that he never saw the official radiology report, and therefore had no knowledge of the need for follow-up. At trial, a jury verdict was returned in favor of the defendant.
Discussion
Unfortunately, some version of this scenario occurs on a frequent basis. While imaging studies account for the majority of such cases, the same situation can occur with abnormal laboratory results, body-fluid cultures, or pathology reports in which an abnormality is identified (eg, positive blood culture, missed fracture) but, for a myriad of reasons, the critical information does not get related to the patient.
Because of the episodic nature of the practice of emergency medicine (EM), a process must be in place to ensure any “positive” test results or findings discovered after patient discharge are reviewed and compared to the ED diagnosis, and that any “misses” result in notifying the patient and/or his or her primary care physician and arranging follow-up. In cases such as the one presented here, a system issue existed—one that was not due to any fault or oversight of the EP. Ideally, EM leadership should work closely with leadership from radiology and laboratory services and hospital risk management to develop such a process—one that will be effective every day, including weekends and holidays.
Missed fractures on radiographs are a common cause of malpractice litigation against EPs. In one review by Kachalia et al1 examining malpractice claims involving EPs, missed fractures on radiographs accounted for 19% (the most common) of the 79 missed diagnoses identified in their study.In a similar study by Karcz et al,2 missed fractures ranked second in frequency and dollars lost in malpractice cases against EPs in Massachusetts.
While missed lesions on CXR do not occur with the same frequency as missed fractures, the results are much more devastating when the lesion turns out to be malignant. Three common areas where such lesions are missed on CXR include: the apex of the lung, obscured by overlying clavicle and ribs; the retrocardiac region (as in the patient in this case); and the lung bases obscured by the diaphragm.
Emergency physicians are neither trained nor expected to identify every single abnormality—especially subtle radiographic abnormalities. This is why there are radiology overreads, and a system or process must be in place to ensure patients are informed of any positive findings and to arrange proper follow-up.
1. Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49(2):196-205.
2. Karcz A, Korn R, Burke MC, et al. Malpractice claims against emergency physicians in Massachusetts: 1975-1993. Am J Emerg Med. 1996;14(4):341-345.
Case
A 48-year-old man presented to the ED with a 2-day history of cough and congestion. He described the cough as gradual in onset and, though initially nonproductive, it was now productive of green sputum. He denied fevers or chills, chest pain, nausea, vomiting, or diarrhea, and complained of only mild shortness of breath. His medical history was significant for hypertension, which was well managed with daily lisinopril-hydrochlorothiazide. He admitted to smoking one pack of cigarettes per day for the past 25 years, but denied alcohol or illicit drug use.
On physical examination, the patient’s vital signs were: blood pressure, 112/64 mm Hg; heart rate, 84 beats/min; respiratory rate, 20 breaths/min; and temperature, 98oF. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was normal. Auscultation of the lungs revealed bilateral breath sounds with scattered, faint expiratory wheezing; the heart had a regular rate and rhythm, without murmurs, rubs, or gallops.
The emergency physician (EP) ordered posteroanterior and lateral chest X-rays (CXR), which he interpreted as normal. He also ordered an albuterol handheld nebulizer treatment for the patient. After the albuterol treatment, the patient felt he was breathing more easily. The frequency of his cough had also decreased following treatment and, on re-examination, he exhibited no wheezing and was given azithromycin 500 mg orally in the ED. The EP diagnosed the patient with acute bronchitis and discharged him home with an albuterol metered dose inhaler with a spacer, and a 4-day course of azithromycin. He also encouraged the patient to quit smoking.
The next day the radiologist’s official reading of the patient’s radiographs included the finding of a very small pulmonary nodule, which was seen only on the lateral X-ray. The radiologist recommended a repeat CXR or a computed tomography (CT) scan of the chest in 6 months.
Unfortunately, the EP never saw this information, and the patient was not contacted regarding the abnormal radiology finding and the need for follow-up. Approximately 20 months later, the patient was diagnosed with lung cancer with metastasis to the thoracic spine and liver. Despite chemotherapy and radiation treatment, he died from the cancer.
The patient’s family brought a malpractice suit against the EP, stating that the cancer could have been successfully treated prior to any metastasis if the patient had been informed of the abnormal radiology findings at his ED visit 20 months prior. The EP argued that he never saw the official radiology report, and therefore had no knowledge of the need for follow-up. At trial, a jury verdict was returned in favor of the defendant.
Discussion
Unfortunately, some version of this scenario occurs on a frequent basis. While imaging studies account for the majority of such cases, the same situation can occur with abnormal laboratory results, body-fluid cultures, or pathology reports in which an abnormality is identified (eg, positive blood culture, missed fracture) but, for a myriad of reasons, the critical information does not get related to the patient.
Because of the episodic nature of the practice of emergency medicine (EM), a process must be in place to ensure any “positive” test results or findings discovered after patient discharge are reviewed and compared to the ED diagnosis, and that any “misses” result in notifying the patient and/or his or her primary care physician and arranging follow-up. In cases such as the one presented here, a system issue existed—one that was not due to any fault or oversight of the EP. Ideally, EM leadership should work closely with leadership from radiology and laboratory services and hospital risk management to develop such a process—one that will be effective every day, including weekends and holidays.
Missed fractures on radiographs are a common cause of malpractice litigation against EPs. In one review by Kachalia et al1 examining malpractice claims involving EPs, missed fractures on radiographs accounted for 19% (the most common) of the 79 missed diagnoses identified in their study.In a similar study by Karcz et al,2 missed fractures ranked second in frequency and dollars lost in malpractice cases against EPs in Massachusetts.
While missed lesions on CXR do not occur with the same frequency as missed fractures, the results are much more devastating when the lesion turns out to be malignant. Three common areas where such lesions are missed on CXR include: the apex of the lung, obscured by overlying clavicle and ribs; the retrocardiac region (as in the patient in this case); and the lung bases obscured by the diaphragm.
Emergency physicians are neither trained nor expected to identify every single abnormality—especially subtle radiographic abnormalities. This is why there are radiology overreads, and a system or process must be in place to ensure patients are informed of any positive findings and to arrange proper follow-up.
Case
A 48-year-old man presented to the ED with a 2-day history of cough and congestion. He described the cough as gradual in onset and, though initially nonproductive, it was now productive of green sputum. He denied fevers or chills, chest pain, nausea, vomiting, or diarrhea, and complained of only mild shortness of breath. His medical history was significant for hypertension, which was well managed with daily lisinopril-hydrochlorothiazide. He admitted to smoking one pack of cigarettes per day for the past 25 years, but denied alcohol or illicit drug use.
On physical examination, the patient’s vital signs were: blood pressure, 112/64 mm Hg; heart rate, 84 beats/min; respiratory rate, 20 breaths/min; and temperature, 98oF. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was normal. Auscultation of the lungs revealed bilateral breath sounds with scattered, faint expiratory wheezing; the heart had a regular rate and rhythm, without murmurs, rubs, or gallops.
The emergency physician (EP) ordered posteroanterior and lateral chest X-rays (CXR), which he interpreted as normal. He also ordered an albuterol handheld nebulizer treatment for the patient. After the albuterol treatment, the patient felt he was breathing more easily. The frequency of his cough had also decreased following treatment and, on re-examination, he exhibited no wheezing and was given azithromycin 500 mg orally in the ED. The EP diagnosed the patient with acute bronchitis and discharged him home with an albuterol metered dose inhaler with a spacer, and a 4-day course of azithromycin. He also encouraged the patient to quit smoking.
The next day the radiologist’s official reading of the patient’s radiographs included the finding of a very small pulmonary nodule, which was seen only on the lateral X-ray. The radiologist recommended a repeat CXR or a computed tomography (CT) scan of the chest in 6 months.
Unfortunately, the EP never saw this information, and the patient was not contacted regarding the abnormal radiology finding and the need for follow-up. Approximately 20 months later, the patient was diagnosed with lung cancer with metastasis to the thoracic spine and liver. Despite chemotherapy and radiation treatment, he died from the cancer.
The patient’s family brought a malpractice suit against the EP, stating that the cancer could have been successfully treated prior to any metastasis if the patient had been informed of the abnormal radiology findings at his ED visit 20 months prior. The EP argued that he never saw the official radiology report, and therefore had no knowledge of the need for follow-up. At trial, a jury verdict was returned in favor of the defendant.
Discussion
Unfortunately, some version of this scenario occurs on a frequent basis. While imaging studies account for the majority of such cases, the same situation can occur with abnormal laboratory results, body-fluid cultures, or pathology reports in which an abnormality is identified (eg, positive blood culture, missed fracture) but, for a myriad of reasons, the critical information does not get related to the patient.
Because of the episodic nature of the practice of emergency medicine (EM), a process must be in place to ensure any “positive” test results or findings discovered after patient discharge are reviewed and compared to the ED diagnosis, and that any “misses” result in notifying the patient and/or his or her primary care physician and arranging follow-up. In cases such as the one presented here, a system issue existed—one that was not due to any fault or oversight of the EP. Ideally, EM leadership should work closely with leadership from radiology and laboratory services and hospital risk management to develop such a process—one that will be effective every day, including weekends and holidays.
Missed fractures on radiographs are a common cause of malpractice litigation against EPs. In one review by Kachalia et al1 examining malpractice claims involving EPs, missed fractures on radiographs accounted for 19% (the most common) of the 79 missed diagnoses identified in their study.In a similar study by Karcz et al,2 missed fractures ranked second in frequency and dollars lost in malpractice cases against EPs in Massachusetts.
While missed lesions on CXR do not occur with the same frequency as missed fractures, the results are much more devastating when the lesion turns out to be malignant. Three common areas where such lesions are missed on CXR include: the apex of the lung, obscured by overlying clavicle and ribs; the retrocardiac region (as in the patient in this case); and the lung bases obscured by the diaphragm.
Emergency physicians are neither trained nor expected to identify every single abnormality—especially subtle radiographic abnormalities. This is why there are radiology overreads, and a system or process must be in place to ensure patients are informed of any positive findings and to arrange proper follow-up.
1. Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49(2):196-205.
2. Karcz A, Korn R, Burke MC, et al. Malpractice claims against emergency physicians in Massachusetts: 1975-1993. Am J Emerg Med. 1996;14(4):341-345.
1. Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49(2):196-205.
2. Karcz A, Korn R, Burke MC, et al. Malpractice claims against emergency physicians in Massachusetts: 1975-1993. Am J Emerg Med. 1996;14(4):341-345.
Malpractice Counsel: Acute Pulmonary Embolism Masquerading as Acute Coronary Syndrome
Case
A 37-year-old woman presented to the ED with a 90-minute history of chest tightness and shortness of breath. She admitted to feeling anxious but denied nausea, vomiting, or diaphoresis. The patient was in good health overall and had no history of similar symptoms. The only medication she took on a regular basis was a combination oral contraceptive (OC). Regarding the patient’s social history, she admitted to smoking one-half of a pack of cigarettes per day and occasional alcohol use.
On physical examination, the patient’s vital signs were: heart rate (HR), 102 beats/min; blood pressure, 118/64 mm Hg; respiratory rate, 20 breaths/min; and temperature, 98.6˚F. Oxygen saturation was 95% on room air. The head, eyes, ears, nose, and throat examination was normal. The cardiopulmonary examination revealed slight tachycardia with a regular rhythm but no murmurs, rubs, or gallops; the lungs were clear to auscultation bilaterally. The abdominal examination revealed a soft, nontender abdomen, without mass, and no guarding or rebound was present. An examination of the lower extremities was not documented.
The emergency physician (EP) ordered laboratory studies, which included a complete blood count (CBC), basic metabolic profile (BMP), and troponin I level. A chest X-ray and electrocardiogram (ECG) were also ordered. The chest X-ray was interpreted as normal, and the ECG revealed mild sinus tachycardia with nonspecific ST-T segment changes in V1 through V3. The CBC and BMP were all within normal limits, but the troponin I level was slightly elevated.
Given the patient’s clinical presentation and slightly elevated troponin I level, the EP was concerned for an acute coronary syndrome (ACS) and admitted the patient to the care of the on-call cardiologist. Prior to transfer, the patient was given 325 mg of aspirin by mouth, but no anticoagulation therapy was ordered. The cardiologist, who evaluated the patient after she was admitted to the inpatient floor, was concerned the patient had a pulmonary embolism (PE), and ordered a stat computed tomography angiography (CTA) scan of the chest. While the patient was undergoing the chest CTA scan, she went into cardiac arrest. Despite aggressive resuscitative measures, the patient could not be revived and was pronounced dead. An autopsy revealed a PE as the cause of death.
Plaintiff’s Claim
The patient’s estate sued the EP for failure to properly diagnose the PE, stating the hospital was vicariously liable for the EP’s actions. The emergency medicine (EM) expert for the plaintiff opined that the decedent’s symptoms should have prompted the EP to suspect she was suffering from a PE, and he should have immediately ordered anticoagulation, a D-dimer test, or a chest CTA scan. The expert cardiologist for the plaintiff stated the EP should have immediately started the patient on anticoagulation prior to the chest CTA scan.
The Defense
The defense EM expert stated the defendant’s diagnosis of ACS was appropriate given the patient’s overall clinical presentation, and the defense expert cardiologist stated the standard of care did not require the EP to administer anticoagulation prior to her diagnosis of PE, since the bleeding risks outweighed the benefits.
Verdict
At trial, the jury returned a defense verdict.
Discussion
This is not the first (nor probably the last) malpractice case in this column to involve a missed PE. While there have been improvements to the tools we currently possess to evaluate patients for suspected PE, it remains a difficult condition to reliably and timely identify in the ED. Although the two predominating symptoms—shortness of breath and chest pain—are common presentations in the ED, each is associated with large differential diagnoses.
Acute Coronary Syndrome Versus Pulmonary Embolism
From what we know of the published details of this case, the patient had only one risk factor for ACS (cigarette smoking) and two risk factors for PE (cigarette smoking and estrogen-containing contraceptive use). The only abnormal physical finding (tachycardia) was slightly more suggestive of PE than ACS. This patient’s primary complaint was chest fullness and shortness of breath. According to the Prospective Investigation of Pulmonary Embolism Diagnosis II study, shortness of breath is the most common complaint in PE (73%), followed by pleuritic chest pain (44%).1
In ACS, which is more common in men versus women and in patients of both sexes over age 55 years, the clinical presentation most commonly involves chest pain that patients describe as a pressure or fullness (as demonstrated in this patient). Unfortunately, in certain patient populations (eg, women, elderly patients, patients with diabetes mellitus) the presenting complaint can be shortness of breath, weakness, or nausea and vomiting. In a study evaluating how frequently an acute PE can mimic ACS, Kukla et al2 found that one-third of patients with an acute PE can present with all of the manifestations suggestive of ACS (ie, chest pain, ECG changes, and elevated troponin).
It is probably safe to assume the elevated troponin I level played a factor in influencing the EP to diagnose ACS, rather than pursuing an alternative diagnosis such as PE. Unfortunately, since both serum troponin T and I can be markers of right ventricle dysfunction, they are elevated in 30% to 50% of patients with moderate-to-large PE.3 However, neither serum troponin T nor troponin I is specific for myocardial infarction or unstable angina.
Pretest Probability: Wells Criteria
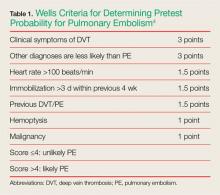
Determining pretest probability for any disease process is important when evaluating complaints in the ED; this is especially true for PE. One of the most frequently used tools for determining the likelihood of PE in ED patients is the Wells criteria (Table 1).4
Based on the published information available, the patient in this case would have scored a 1.5, placing her in the unlikely or low-risk category for PE. Patients whose Wells score places them in the low-risk group can benefit from serum D-dimer testing to help diagnose PE. However, serum D-dimer testing should not be ordered for patients in the likely or high-risk categories; these patients should instead be sent directly for imaging studies such as a chest CTA scan.
Pulmonary Embolism Rule-Out Criteria
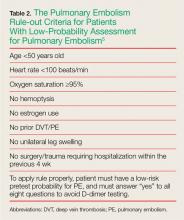
For patients whose Wells criteria score places them in the “unlikely group,” the PE rule-out criteria (PERC) can be used to determine the need for ordering a D-dimer. If all eight criteria are met, no further testing is necessary to exclude PE from the differential diagnosis (Table 2).5
Summary
Evaluating chest pain and shortness of breath in the ED is a humbling experience for even the most seasoned EP. Thoroughly reviewing the patient’s history and physical examination, and determining the pretest probability of disease entities high on the differential diagnoses list, go a long way in helping make the correct diagnosis—and in turn initiating possible life-saving interventions and treatment.
1. Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med. 2007;120(10):871-879.
2. Kukla P, Dlugopolski R, Krupa E, et al. How often pulmonary embolism mimics acute coronary syndrome? Kardiol Pol. 2011;69(3):235-240.
3. Meyer T, Binder L, Hruska N, Luthe H, Buchwald AB. Cardiac troponin I elevation in acute pulmonary embolism is associated with right ventricular dysfunction. J Am Coll Cardiol. 2000;36(5):1632-1636.
4. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416-420.
5. Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6(5):772-780. doi: 10.1111/j.1538-7836.2008.02944.x.
Case
A 37-year-old woman presented to the ED with a 90-minute history of chest tightness and shortness of breath. She admitted to feeling anxious but denied nausea, vomiting, or diaphoresis. The patient was in good health overall and had no history of similar symptoms. The only medication she took on a regular basis was a combination oral contraceptive (OC). Regarding the patient’s social history, she admitted to smoking one-half of a pack of cigarettes per day and occasional alcohol use.
On physical examination, the patient’s vital signs were: heart rate (HR), 102 beats/min; blood pressure, 118/64 mm Hg; respiratory rate, 20 breaths/min; and temperature, 98.6˚F. Oxygen saturation was 95% on room air. The head, eyes, ears, nose, and throat examination was normal. The cardiopulmonary examination revealed slight tachycardia with a regular rhythm but no murmurs, rubs, or gallops; the lungs were clear to auscultation bilaterally. The abdominal examination revealed a soft, nontender abdomen, without mass, and no guarding or rebound was present. An examination of the lower extremities was not documented.
The emergency physician (EP) ordered laboratory studies, which included a complete blood count (CBC), basic metabolic profile (BMP), and troponin I level. A chest X-ray and electrocardiogram (ECG) were also ordered. The chest X-ray was interpreted as normal, and the ECG revealed mild sinus tachycardia with nonspecific ST-T segment changes in V1 through V3. The CBC and BMP were all within normal limits, but the troponin I level was slightly elevated.
Given the patient’s clinical presentation and slightly elevated troponin I level, the EP was concerned for an acute coronary syndrome (ACS) and admitted the patient to the care of the on-call cardiologist. Prior to transfer, the patient was given 325 mg of aspirin by mouth, but no anticoagulation therapy was ordered. The cardiologist, who evaluated the patient after she was admitted to the inpatient floor, was concerned the patient had a pulmonary embolism (PE), and ordered a stat computed tomography angiography (CTA) scan of the chest. While the patient was undergoing the chest CTA scan, she went into cardiac arrest. Despite aggressive resuscitative measures, the patient could not be revived and was pronounced dead. An autopsy revealed a PE as the cause of death.
Plaintiff’s Claim
The patient’s estate sued the EP for failure to properly diagnose the PE, stating the hospital was vicariously liable for the EP’s actions. The emergency medicine (EM) expert for the plaintiff opined that the decedent’s symptoms should have prompted the EP to suspect she was suffering from a PE, and he should have immediately ordered anticoagulation, a D-dimer test, or a chest CTA scan. The expert cardiologist for the plaintiff stated the EP should have immediately started the patient on anticoagulation prior to the chest CTA scan.
The Defense
The defense EM expert stated the defendant’s diagnosis of ACS was appropriate given the patient’s overall clinical presentation, and the defense expert cardiologist stated the standard of care did not require the EP to administer anticoagulation prior to her diagnosis of PE, since the bleeding risks outweighed the benefits.
Verdict
At trial, the jury returned a defense verdict.
Discussion
This is not the first (nor probably the last) malpractice case in this column to involve a missed PE. While there have been improvements to the tools we currently possess to evaluate patients for suspected PE, it remains a difficult condition to reliably and timely identify in the ED. Although the two predominating symptoms—shortness of breath and chest pain—are common presentations in the ED, each is associated with large differential diagnoses.
Acute Coronary Syndrome Versus Pulmonary Embolism
From what we know of the published details of this case, the patient had only one risk factor for ACS (cigarette smoking) and two risk factors for PE (cigarette smoking and estrogen-containing contraceptive use). The only abnormal physical finding (tachycardia) was slightly more suggestive of PE than ACS. This patient’s primary complaint was chest fullness and shortness of breath. According to the Prospective Investigation of Pulmonary Embolism Diagnosis II study, shortness of breath is the most common complaint in PE (73%), followed by pleuritic chest pain (44%).1
In ACS, which is more common in men versus women and in patients of both sexes over age 55 years, the clinical presentation most commonly involves chest pain that patients describe as a pressure or fullness (as demonstrated in this patient). Unfortunately, in certain patient populations (eg, women, elderly patients, patients with diabetes mellitus) the presenting complaint can be shortness of breath, weakness, or nausea and vomiting. In a study evaluating how frequently an acute PE can mimic ACS, Kukla et al2 found that one-third of patients with an acute PE can present with all of the manifestations suggestive of ACS (ie, chest pain, ECG changes, and elevated troponin).
It is probably safe to assume the elevated troponin I level played a factor in influencing the EP to diagnose ACS, rather than pursuing an alternative diagnosis such as PE. Unfortunately, since both serum troponin T and I can be markers of right ventricle dysfunction, they are elevated in 30% to 50% of patients with moderate-to-large PE.3 However, neither serum troponin T nor troponin I is specific for myocardial infarction or unstable angina.
Pretest Probability: Wells Criteria
Determining pretest probability for any disease process is important when evaluating complaints in the ED; this is especially true for PE. One of the most frequently used tools for determining the likelihood of PE in ED patients is the Wells criteria (Table 1).4
Based on the published information available, the patient in this case would have scored a 1.5, placing her in the unlikely or low-risk category for PE. Patients whose Wells score places them in the low-risk group can benefit from serum D-dimer testing to help diagnose PE. However, serum D-dimer testing should not be ordered for patients in the likely or high-risk categories; these patients should instead be sent directly for imaging studies such as a chest CTA scan.
Pulmonary Embolism Rule-Out Criteria
For patients whose Wells criteria score places them in the “unlikely group,” the PE rule-out criteria (PERC) can be used to determine the need for ordering a D-dimer. If all eight criteria are met, no further testing is necessary to exclude PE from the differential diagnosis (Table 2).5
Summary
Evaluating chest pain and shortness of breath in the ED is a humbling experience for even the most seasoned EP. Thoroughly reviewing the patient’s history and physical examination, and determining the pretest probability of disease entities high on the differential diagnoses list, go a long way in helping make the correct diagnosis—and in turn initiating possible life-saving interventions and treatment.
Case
A 37-year-old woman presented to the ED with a 90-minute history of chest tightness and shortness of breath. She admitted to feeling anxious but denied nausea, vomiting, or diaphoresis. The patient was in good health overall and had no history of similar symptoms. The only medication she took on a regular basis was a combination oral contraceptive (OC). Regarding the patient’s social history, she admitted to smoking one-half of a pack of cigarettes per day and occasional alcohol use.
On physical examination, the patient’s vital signs were: heart rate (HR), 102 beats/min; blood pressure, 118/64 mm Hg; respiratory rate, 20 breaths/min; and temperature, 98.6˚F. Oxygen saturation was 95% on room air. The head, eyes, ears, nose, and throat examination was normal. The cardiopulmonary examination revealed slight tachycardia with a regular rhythm but no murmurs, rubs, or gallops; the lungs were clear to auscultation bilaterally. The abdominal examination revealed a soft, nontender abdomen, without mass, and no guarding or rebound was present. An examination of the lower extremities was not documented.
The emergency physician (EP) ordered laboratory studies, which included a complete blood count (CBC), basic metabolic profile (BMP), and troponin I level. A chest X-ray and electrocardiogram (ECG) were also ordered. The chest X-ray was interpreted as normal, and the ECG revealed mild sinus tachycardia with nonspecific ST-T segment changes in V1 through V3. The CBC and BMP were all within normal limits, but the troponin I level was slightly elevated.
Given the patient’s clinical presentation and slightly elevated troponin I level, the EP was concerned for an acute coronary syndrome (ACS) and admitted the patient to the care of the on-call cardiologist. Prior to transfer, the patient was given 325 mg of aspirin by mouth, but no anticoagulation therapy was ordered. The cardiologist, who evaluated the patient after she was admitted to the inpatient floor, was concerned the patient had a pulmonary embolism (PE), and ordered a stat computed tomography angiography (CTA) scan of the chest. While the patient was undergoing the chest CTA scan, she went into cardiac arrest. Despite aggressive resuscitative measures, the patient could not be revived and was pronounced dead. An autopsy revealed a PE as the cause of death.
Plaintiff’s Claim
The patient’s estate sued the EP for failure to properly diagnose the PE, stating the hospital was vicariously liable for the EP’s actions. The emergency medicine (EM) expert for the plaintiff opined that the decedent’s symptoms should have prompted the EP to suspect she was suffering from a PE, and he should have immediately ordered anticoagulation, a D-dimer test, or a chest CTA scan. The expert cardiologist for the plaintiff stated the EP should have immediately started the patient on anticoagulation prior to the chest CTA scan.
The Defense
The defense EM expert stated the defendant’s diagnosis of ACS was appropriate given the patient’s overall clinical presentation, and the defense expert cardiologist stated the standard of care did not require the EP to administer anticoagulation prior to her diagnosis of PE, since the bleeding risks outweighed the benefits.
Verdict
At trial, the jury returned a defense verdict.
Discussion
This is not the first (nor probably the last) malpractice case in this column to involve a missed PE. While there have been improvements to the tools we currently possess to evaluate patients for suspected PE, it remains a difficult condition to reliably and timely identify in the ED. Although the two predominating symptoms—shortness of breath and chest pain—are common presentations in the ED, each is associated with large differential diagnoses.
Acute Coronary Syndrome Versus Pulmonary Embolism
From what we know of the published details of this case, the patient had only one risk factor for ACS (cigarette smoking) and two risk factors for PE (cigarette smoking and estrogen-containing contraceptive use). The only abnormal physical finding (tachycardia) was slightly more suggestive of PE than ACS. This patient’s primary complaint was chest fullness and shortness of breath. According to the Prospective Investigation of Pulmonary Embolism Diagnosis II study, shortness of breath is the most common complaint in PE (73%), followed by pleuritic chest pain (44%).1
In ACS, which is more common in men versus women and in patients of both sexes over age 55 years, the clinical presentation most commonly involves chest pain that patients describe as a pressure or fullness (as demonstrated in this patient). Unfortunately, in certain patient populations (eg, women, elderly patients, patients with diabetes mellitus) the presenting complaint can be shortness of breath, weakness, or nausea and vomiting. In a study evaluating how frequently an acute PE can mimic ACS, Kukla et al2 found that one-third of patients with an acute PE can present with all of the manifestations suggestive of ACS (ie, chest pain, ECG changes, and elevated troponin).
It is probably safe to assume the elevated troponin I level played a factor in influencing the EP to diagnose ACS, rather than pursuing an alternative diagnosis such as PE. Unfortunately, since both serum troponin T and I can be markers of right ventricle dysfunction, they are elevated in 30% to 50% of patients with moderate-to-large PE.3 However, neither serum troponin T nor troponin I is specific for myocardial infarction or unstable angina.
Pretest Probability: Wells Criteria
Determining pretest probability for any disease process is important when evaluating complaints in the ED; this is especially true for PE. One of the most frequently used tools for determining the likelihood of PE in ED patients is the Wells criteria (Table 1).4
Based on the published information available, the patient in this case would have scored a 1.5, placing her in the unlikely or low-risk category for PE. Patients whose Wells score places them in the low-risk group can benefit from serum D-dimer testing to help diagnose PE. However, serum D-dimer testing should not be ordered for patients in the likely or high-risk categories; these patients should instead be sent directly for imaging studies such as a chest CTA scan.
Pulmonary Embolism Rule-Out Criteria
For patients whose Wells criteria score places them in the “unlikely group,” the PE rule-out criteria (PERC) can be used to determine the need for ordering a D-dimer. If all eight criteria are met, no further testing is necessary to exclude PE from the differential diagnosis (Table 2).5
Summary
Evaluating chest pain and shortness of breath in the ED is a humbling experience for even the most seasoned EP. Thoroughly reviewing the patient’s history and physical examination, and determining the pretest probability of disease entities high on the differential diagnoses list, go a long way in helping make the correct diagnosis—and in turn initiating possible life-saving interventions and treatment.
1. Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med. 2007;120(10):871-879.
2. Kukla P, Dlugopolski R, Krupa E, et al. How often pulmonary embolism mimics acute coronary syndrome? Kardiol Pol. 2011;69(3):235-240.
3. Meyer T, Binder L, Hruska N, Luthe H, Buchwald AB. Cardiac troponin I elevation in acute pulmonary embolism is associated with right ventricular dysfunction. J Am Coll Cardiol. 2000;36(5):1632-1636.
4. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416-420.
5. Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6(5):772-780. doi: 10.1111/j.1538-7836.2008.02944.x.
1. Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med. 2007;120(10):871-879.
2. Kukla P, Dlugopolski R, Krupa E, et al. How often pulmonary embolism mimics acute coronary syndrome? Kardiol Pol. 2011;69(3):235-240.
3. Meyer T, Binder L, Hruska N, Luthe H, Buchwald AB. Cardiac troponin I elevation in acute pulmonary embolism is associated with right ventricular dysfunction. J Am Coll Cardiol. 2000;36(5):1632-1636.
4. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416-420.
5. Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6(5):772-780. doi: 10.1111/j.1538-7836.2008.02944.x.
Malpractice Counsel: Retained foreign body, ruptured esophagus
Retained Foreign Body
A 15-year-old male adolescent was brought to the ED by his father for evaluation of lacerations on the teenager’s left forearm, which were caused by a shattered glass door. The accident happened approximately 45 minutes prior to the patient’s arrival at the ED. The patient was up to date on all of his immunizations, including tetanus, and had no significant medical history.
On physical examination, the patient’s vital signs were all normal. He was noted to have two lacerations on the volar aspect of the distal one-third of his left forearm. One laceration measured 2.5 cm, running diagonally on the forearm; the other laceration was approximately 2 cm, running horizontally on the forearm. The bleeding from both wound sites was easily controlled with pressure.
The emergency physician (EP) did not document a neurological examination of the left wrist and hand. He did, however, note that the patient had a 2+ radial pulse and good capillary refill. The EP irrigated the wounds thoroughly and sutured the two lacerations. There was no documentation on file of wound exploration or imaging studies. The patient returned 1 week after discharge from the ED for a wound check, and again 6 weeks later. On both occasions, he continued to complain of pain and decreased function of his left thumb and index finger.
Since the patient’s condition did not improve, his father took him to an orthopedic surgeon. The orthopedist ordered a magnetic resonance imaging (MRI) study of the left forearm, which demonstrated a complete tear of one of the patient’s flexor tendons. The orthopedist thought it was too late to repair the tendon and referred the patient to physical therapy. As the patient continued to complain of pain and decreased function of his left thumb, he consulted a second orthopedist, who decided to surgically explore the wound to determine the cause of the patient’s continued pain and loss of thumb function. Surgical exploration revealed a piece of glass measuring 3.5 x 2 cm retained in the patient’s forearm. The orthopedist removed the glass, irrigated the wound thoroughly, and closed the incision, after which the patient’s thumb function improved considerably and his pain resolved.
The patient’s family sued the EP and the hospital, arguing that the wound should have been explored and the glass removed on the initial ED visit. They further stated that if these steps were performed initially, the patient would not have required multiple imaging studies and surgery. At trial, the jury returned a defense verdict.
Discussion
Approximately 11 million wounds are treated in US EDs each year.1 Proper management of lacerations and wounds requires more than sutures or staples. The EP must also evaluate for associated injuries (eg, tendon laceration, vascular injury), and the possibility of a retained foreign body. It is also important to ensure the patient is up to date on his or her tetanus immunization.
As with most areas of medicine, a good history and physical examination are essential. The mechanism of injury will often be the first clue to the risk of a retained foreign body. For example, shattered glass or porcelain carries a much higher risk of retention compared to a laceration from a box cutter.
The age of the injury is also important in determining the best management approach and the risk of infection. In a study by Brancto,1 wounds closed within 19 hours of injury had a 92% rate of healing without infection, compared to only 77% of those closed after 19 hours. In addition, determination of a patient’s allergy status to anesthetics and antibiotics ensures safe and appropriate treatment.
On physical examination, the wound should be described in sufficient detail (eg, length, shape), and a distal neurovascular examination should be completed and documented. This involves testing the patient’s motor strength, sensation, adequacy of pulses, and capillary refill. When examining the extremities, flexion and extension strength should also be assessed and documented.
After a wound is prepped and anesthetized, it should be explored. Often a patient may have excellent flexor or extensor strength on testing, but have a near-complete tendon laceration on visual inspection. Similarly, the wound should be explored for foreign bodies. It is important to identify and remove foreign bodies because of the associated increased risk of infection, pain, and delayed healing.1 Occasionally, a wound may need to be extended to remove a foreign body.
Unfortunately, visual inspection of a wound, especially a deep one, is not highly sensitive. If a physician has a high index of suspicion for a retained foreign body but is unable to identify one on examination, imaging studies should be ordered. Conventional plain radiography, ultrasonography, computed tomography (CT), and MRI studies can all be used to identify foreign bodies. Each of these modalities has its unique advantages and disadvantages. A recent study by Pattamapaspong et al2 compared the accuracy of radiography, CT, and MRI in detecting foreign bodies in the foot. In this study, researchers placed various types of foreign bodies, including fresh wood, dry wood, glass, porcelain, and plastic—all measuring 5 x 2 mm— in cadaver feet.2 The overall sensitivity and specificity for foreign body detection was 29% and 100%, respectively, for radiographs; 63% and 98%, respectively, for CT; and 58% and 100%, respectively, for MRI.2 Interestingly, CT was superior to MRI in identifying water-rich fresh wood.2 A similar study by Aras et al3 compared the sensitivity of plain radiographs, CT, and ultrasound in detecting foreign bodies in the face. The foreign bodies used in this study measured 1 x 1 x 1 cm and included metal, glass, wood, stone, acrylic, graphite, and polyoxybenzylmethylenglycolanhydride (ie, Bakelite).3 In this study, ultrasound identified superficial foreign bodies with low radiopacity in body tissues more effectively than CT or plain radiographs.3 In a review by Karabay4 of traumatic wrist and hand injuries, ultrasound was considered the best modality to identify and locate both opaque and radiolucent foreign bodies in the soft tissue.
If a foreign body is identified but cannot be removed, consultation with a surgical service is required. Depending on the local referral pattern, this might be general surgery, plastic surgery, or hand surgery. Unless there is an acute nerve or vascular injury, patients rarely require immediate surgery. In most cases, the wound can be closed loosely until the surgeon can remove the foreign body in the operating room and/or with aid of fluoroscopy at a later time. Depending on the size, material, and location of the foreign body, the surgeon might even elect to simply observe.
The bottom-line lesson from this case: depending on the mechanism of injury, EPs must maintain a high index of suspicion for retained foreign bodies in traumatic wounds. In addition to wound exploration, imaging studies should be used in patients at high risk for a retained foreign body, such as those injured with broken glass or porcelain, but in whom no foreign body is found on wound exploration.
Ruptured Esophagus
A 78-year-old man presented to the ED with symptoms of choking and chest discomfort. The patient stated that he had experienced a sudden onset of difficulty swallowing, along with chest pain, while he was eating dinner at a restaurant earlier that evening. The patient initially thought he had a piece of carrot stuck in his throat. He denied any previous history of similar symptoms. He complained of mild shortness of breath, but denied any drooling or vomiting. His medical history was significant for hypertension, which was controlled with medication. He denied tobacco or alcohol use and had no known drug allergies.
On physical examination, the patient’s vital signs were: heart rate (HR), 106 beats/minute; blood pressure (BP), 144/82 mm Hg; respiratory rate, 22 breaths/minute, and temperature, 98.6°F. Oxygen saturation was 95% on room air. The patient’s oropharynx appeared normal and without foreign body obstruction; his lungs were clear to auscultation bilaterally; and his HR was tachycardic but with a regular rhythm. Other than mild diaphoresis, the remainder of the physical examination was normal.
The EP ordered a complete blood count (CBC), a basic metabolic profile (BMP), and a portable chest X-ray, which the EP interpreted as normal. In addition, an intravenous (IV) saline lock was placed, and the patient was given morphine 4 mg IV and ondansetron 4 mg IV. He was also placed on 2 L of oxygen via nasal cannula. Since the patient continued to complain of chest pain and dysphagia, the EP consulted with a gastroenterologist; unfortunately, there was no documentation of this.
The EP admitted the patient to the floor with a diagnosis of esophageal obstruction, probably secondary to a piece of carrot. During the night, the patient’s shortness of breath worsened, requiring an increase in supplemental oxygen. The next morning, the patient’s HR increased to 120 beats/minute; his BP dropped to 96/50 mm Hg, and he developed a low-grade fever. He was transferred to the intensive care unit, where he was started on IV fluid resuscitation with normal saline and broad spectrum antibiotics. A CT scan of the chest was also ordered, which revealed an esophageal perforation. The patient was taken immediately to the operating room; surgery revealed a large esophageal perforation with evidence of mediastinitis and gross contamination of the left hemithorax. The patient died 2 days later.
The patient’s family sued the EP for failure to diagnose and treat the esophageal perforation in a timely manner. The EP argued that the patient’s symptoms were consistent with an obstruction, not esophageal perforation. The defendant also argued that the initial chest X-ray was normal. The case was resolved for $800,000 prior to going to trial.
Discussion
Esophageal perforation is a true medical emergency that requires timely diagnosis and management because morbidity and mortality are directly related to the time to treatment. Unfortunately, esophageal perforation can be a difficult diagnosis due to its relative rarity and variability in clinical presentation.
More than 50% of all esophageal perforations are iatrogenic, primarily as a complication of endoscopy.1 Other causes of perforation include spontaneous perforation or Boerhaave syndrome (15%), foreign body (12%), trauma (9%), and malignancy (1%).1 Anatomically, perforation tends to occur in the areas of the esophagus that are most narrow—eg, cricopharyngeus muscle, area of broncho-aortic constriction, and esophagogastric junction.1
Food impactions, not surprisingly, tend to occur in these same areas of the esophagus. In addition, there are structural esophageal abnormalities that increase the risk of food impaction, including diverticula, webs, rings, strictures, achalasia, and tumors.2 Since food impaction can result in an esophageal perforation, there is a significant overlap in the initial presentation of these two conditions. However, in cases of perforation, signs and symptoms of shock predominate as time progresses due to esophageal contents leaking into the mediastinal and pleural spaces.
Patients with a food impaction will often complain of an acute onset of dysphagia, difficulty in handling secretions, choking, drooling, retrosternal fullness, regurgitation of undigested food, and wheezing.2 Perforation can cause severe chest pain, tachypnea, dyspnea, fever, and shock.2
A chest X-ray is typically the initial imaging study for suspected esophageal perforation. Since most spontaneous perforations occur through the left posterolateral wall of the distal esophagus, a new left pleural effusion can frequently be seen on X-ray. Mediastinal emphysema is highly suspicious for perforation, but the condition takes time to develop; therefore, its absence on X-ray does not exclude perforation. In the setting of a normal chest X-ray and ongoing esophageal symptoms, further investigation is required, usually via CT scan or endoscopy. Computed tomography, because of its availability and speed, is usually the preferred study to confirm the diagnosis.
Once an esophageal perforation is confirmed or is highly suspected, the patient will require IV fluid resuscitation, IV broad-spectrum antibiotic treatment, and emergency surgical consultation. As previously stated, esophageal perforation is associated with a high mortality rate, and time is critical to successful management.
- Retained Foreign Body
1. Brancto JC. Minor wound preparation and irrigation. http://www.uptodate.com/contents/minor-wound-preparation-and-irrigation. Accessed June 1, 2016.
2. Pattamapaspong N, Srisuwan T, Sivasomboon C, et al. Accuracy of radiography, computed tomography and magnetic resonance imaging in diagnosing foreign bodies in the foot. Radiol Med. 2013;118(2):303-310.
3. Aras MH, Miloglu O, Barutcugil C, Kantarci M, Ozcan E, Harorli A. Comparison of the sensitivity for detecting foreign bodies among conventional plain radiography, computed tomography and ultrasonography. Dentomaxillofac Radiol. 2010;39(2):72-78.
4. Karabay N. US findings in traumatic wrist and hand injuries. Diagn Interv Radiol. 2013;19(4):320-325.
- Ruptured Esophagus
1. Raymond DP, Jones C. Surgical management of esophageal perforation. http://www.uptodate.com/contents/surgical-management-of-esophageal-perforation. Accessed June 27, 2016.
2. Triadafilopoulos G. Ingested foreign bodies and food impaction in adults. http://www.uptodate.com/contents/ingested-foreign-bodies-and-food-impactions-in-adults. Accessed June 27, 2016.
Retained Foreign Body
A 15-year-old male adolescent was brought to the ED by his father for evaluation of lacerations on the teenager’s left forearm, which were caused by a shattered glass door. The accident happened approximately 45 minutes prior to the patient’s arrival at the ED. The patient was up to date on all of his immunizations, including tetanus, and had no significant medical history.
On physical examination, the patient’s vital signs were all normal. He was noted to have two lacerations on the volar aspect of the distal one-third of his left forearm. One laceration measured 2.5 cm, running diagonally on the forearm; the other laceration was approximately 2 cm, running horizontally on the forearm. The bleeding from both wound sites was easily controlled with pressure.
The emergency physician (EP) did not document a neurological examination of the left wrist and hand. He did, however, note that the patient had a 2+ radial pulse and good capillary refill. The EP irrigated the wounds thoroughly and sutured the two lacerations. There was no documentation on file of wound exploration or imaging studies. The patient returned 1 week after discharge from the ED for a wound check, and again 6 weeks later. On both occasions, he continued to complain of pain and decreased function of his left thumb and index finger.
Since the patient’s condition did not improve, his father took him to an orthopedic surgeon. The orthopedist ordered a magnetic resonance imaging (MRI) study of the left forearm, which demonstrated a complete tear of one of the patient’s flexor tendons. The orthopedist thought it was too late to repair the tendon and referred the patient to physical therapy. As the patient continued to complain of pain and decreased function of his left thumb, he consulted a second orthopedist, who decided to surgically explore the wound to determine the cause of the patient’s continued pain and loss of thumb function. Surgical exploration revealed a piece of glass measuring 3.5 x 2 cm retained in the patient’s forearm. The orthopedist removed the glass, irrigated the wound thoroughly, and closed the incision, after which the patient’s thumb function improved considerably and his pain resolved.
The patient’s family sued the EP and the hospital, arguing that the wound should have been explored and the glass removed on the initial ED visit. They further stated that if these steps were performed initially, the patient would not have required multiple imaging studies and surgery. At trial, the jury returned a defense verdict.
Discussion
Approximately 11 million wounds are treated in US EDs each year.1 Proper management of lacerations and wounds requires more than sutures or staples. The EP must also evaluate for associated injuries (eg, tendon laceration, vascular injury), and the possibility of a retained foreign body. It is also important to ensure the patient is up to date on his or her tetanus immunization.
As with most areas of medicine, a good history and physical examination are essential. The mechanism of injury will often be the first clue to the risk of a retained foreign body. For example, shattered glass or porcelain carries a much higher risk of retention compared to a laceration from a box cutter.
The age of the injury is also important in determining the best management approach and the risk of infection. In a study by Brancto,1 wounds closed within 19 hours of injury had a 92% rate of healing without infection, compared to only 77% of those closed after 19 hours. In addition, determination of a patient’s allergy status to anesthetics and antibiotics ensures safe and appropriate treatment.
On physical examination, the wound should be described in sufficient detail (eg, length, shape), and a distal neurovascular examination should be completed and documented. This involves testing the patient’s motor strength, sensation, adequacy of pulses, and capillary refill. When examining the extremities, flexion and extension strength should also be assessed and documented.
After a wound is prepped and anesthetized, it should be explored. Often a patient may have excellent flexor or extensor strength on testing, but have a near-complete tendon laceration on visual inspection. Similarly, the wound should be explored for foreign bodies. It is important to identify and remove foreign bodies because of the associated increased risk of infection, pain, and delayed healing.1 Occasionally, a wound may need to be extended to remove a foreign body.
Unfortunately, visual inspection of a wound, especially a deep one, is not highly sensitive. If a physician has a high index of suspicion for a retained foreign body but is unable to identify one on examination, imaging studies should be ordered. Conventional plain radiography, ultrasonography, computed tomography (CT), and MRI studies can all be used to identify foreign bodies. Each of these modalities has its unique advantages and disadvantages. A recent study by Pattamapaspong et al2 compared the accuracy of radiography, CT, and MRI in detecting foreign bodies in the foot. In this study, researchers placed various types of foreign bodies, including fresh wood, dry wood, glass, porcelain, and plastic—all measuring 5 x 2 mm— in cadaver feet.2 The overall sensitivity and specificity for foreign body detection was 29% and 100%, respectively, for radiographs; 63% and 98%, respectively, for CT; and 58% and 100%, respectively, for MRI.2 Interestingly, CT was superior to MRI in identifying water-rich fresh wood.2 A similar study by Aras et al3 compared the sensitivity of plain radiographs, CT, and ultrasound in detecting foreign bodies in the face. The foreign bodies used in this study measured 1 x 1 x 1 cm and included metal, glass, wood, stone, acrylic, graphite, and polyoxybenzylmethylenglycolanhydride (ie, Bakelite).3 In this study, ultrasound identified superficial foreign bodies with low radiopacity in body tissues more effectively than CT or plain radiographs.3 In a review by Karabay4 of traumatic wrist and hand injuries, ultrasound was considered the best modality to identify and locate both opaque and radiolucent foreign bodies in the soft tissue.
If a foreign body is identified but cannot be removed, consultation with a surgical service is required. Depending on the local referral pattern, this might be general surgery, plastic surgery, or hand surgery. Unless there is an acute nerve or vascular injury, patients rarely require immediate surgery. In most cases, the wound can be closed loosely until the surgeon can remove the foreign body in the operating room and/or with aid of fluoroscopy at a later time. Depending on the size, material, and location of the foreign body, the surgeon might even elect to simply observe.
The bottom-line lesson from this case: depending on the mechanism of injury, EPs must maintain a high index of suspicion for retained foreign bodies in traumatic wounds. In addition to wound exploration, imaging studies should be used in patients at high risk for a retained foreign body, such as those injured with broken glass or porcelain, but in whom no foreign body is found on wound exploration.
Ruptured Esophagus
A 78-year-old man presented to the ED with symptoms of choking and chest discomfort. The patient stated that he had experienced a sudden onset of difficulty swallowing, along with chest pain, while he was eating dinner at a restaurant earlier that evening. The patient initially thought he had a piece of carrot stuck in his throat. He denied any previous history of similar symptoms. He complained of mild shortness of breath, but denied any drooling or vomiting. His medical history was significant for hypertension, which was controlled with medication. He denied tobacco or alcohol use and had no known drug allergies.
On physical examination, the patient’s vital signs were: heart rate (HR), 106 beats/minute; blood pressure (BP), 144/82 mm Hg; respiratory rate, 22 breaths/minute, and temperature, 98.6°F. Oxygen saturation was 95% on room air. The patient’s oropharynx appeared normal and without foreign body obstruction; his lungs were clear to auscultation bilaterally; and his HR was tachycardic but with a regular rhythm. Other than mild diaphoresis, the remainder of the physical examination was normal.
The EP ordered a complete blood count (CBC), a basic metabolic profile (BMP), and a portable chest X-ray, which the EP interpreted as normal. In addition, an intravenous (IV) saline lock was placed, and the patient was given morphine 4 mg IV and ondansetron 4 mg IV. He was also placed on 2 L of oxygen via nasal cannula. Since the patient continued to complain of chest pain and dysphagia, the EP consulted with a gastroenterologist; unfortunately, there was no documentation of this.
The EP admitted the patient to the floor with a diagnosis of esophageal obstruction, probably secondary to a piece of carrot. During the night, the patient’s shortness of breath worsened, requiring an increase in supplemental oxygen. The next morning, the patient’s HR increased to 120 beats/minute; his BP dropped to 96/50 mm Hg, and he developed a low-grade fever. He was transferred to the intensive care unit, where he was started on IV fluid resuscitation with normal saline and broad spectrum antibiotics. A CT scan of the chest was also ordered, which revealed an esophageal perforation. The patient was taken immediately to the operating room; surgery revealed a large esophageal perforation with evidence of mediastinitis and gross contamination of the left hemithorax. The patient died 2 days later.
The patient’s family sued the EP for failure to diagnose and treat the esophageal perforation in a timely manner. The EP argued that the patient’s symptoms were consistent with an obstruction, not esophageal perforation. The defendant also argued that the initial chest X-ray was normal. The case was resolved for $800,000 prior to going to trial.
Discussion
Esophageal perforation is a true medical emergency that requires timely diagnosis and management because morbidity and mortality are directly related to the time to treatment. Unfortunately, esophageal perforation can be a difficult diagnosis due to its relative rarity and variability in clinical presentation.
More than 50% of all esophageal perforations are iatrogenic, primarily as a complication of endoscopy.1 Other causes of perforation include spontaneous perforation or Boerhaave syndrome (15%), foreign body (12%), trauma (9%), and malignancy (1%).1 Anatomically, perforation tends to occur in the areas of the esophagus that are most narrow—eg, cricopharyngeus muscle, area of broncho-aortic constriction, and esophagogastric junction.1
Food impactions, not surprisingly, tend to occur in these same areas of the esophagus. In addition, there are structural esophageal abnormalities that increase the risk of food impaction, including diverticula, webs, rings, strictures, achalasia, and tumors.2 Since food impaction can result in an esophageal perforation, there is a significant overlap in the initial presentation of these two conditions. However, in cases of perforation, signs and symptoms of shock predominate as time progresses due to esophageal contents leaking into the mediastinal and pleural spaces.
Patients with a food impaction will often complain of an acute onset of dysphagia, difficulty in handling secretions, choking, drooling, retrosternal fullness, regurgitation of undigested food, and wheezing.2 Perforation can cause severe chest pain, tachypnea, dyspnea, fever, and shock.2
A chest X-ray is typically the initial imaging study for suspected esophageal perforation. Since most spontaneous perforations occur through the left posterolateral wall of the distal esophagus, a new left pleural effusion can frequently be seen on X-ray. Mediastinal emphysema is highly suspicious for perforation, but the condition takes time to develop; therefore, its absence on X-ray does not exclude perforation. In the setting of a normal chest X-ray and ongoing esophageal symptoms, further investigation is required, usually via CT scan or endoscopy. Computed tomography, because of its availability and speed, is usually the preferred study to confirm the diagnosis.
Once an esophageal perforation is confirmed or is highly suspected, the patient will require IV fluid resuscitation, IV broad-spectrum antibiotic treatment, and emergency surgical consultation. As previously stated, esophageal perforation is associated with a high mortality rate, and time is critical to successful management.
Retained Foreign Body
A 15-year-old male adolescent was brought to the ED by his father for evaluation of lacerations on the teenager’s left forearm, which were caused by a shattered glass door. The accident happened approximately 45 minutes prior to the patient’s arrival at the ED. The patient was up to date on all of his immunizations, including tetanus, and had no significant medical history.
On physical examination, the patient’s vital signs were all normal. He was noted to have two lacerations on the volar aspect of the distal one-third of his left forearm. One laceration measured 2.5 cm, running diagonally on the forearm; the other laceration was approximately 2 cm, running horizontally on the forearm. The bleeding from both wound sites was easily controlled with pressure.
The emergency physician (EP) did not document a neurological examination of the left wrist and hand. He did, however, note that the patient had a 2+ radial pulse and good capillary refill. The EP irrigated the wounds thoroughly and sutured the two lacerations. There was no documentation on file of wound exploration or imaging studies. The patient returned 1 week after discharge from the ED for a wound check, and again 6 weeks later. On both occasions, he continued to complain of pain and decreased function of his left thumb and index finger.
Since the patient’s condition did not improve, his father took him to an orthopedic surgeon. The orthopedist ordered a magnetic resonance imaging (MRI) study of the left forearm, which demonstrated a complete tear of one of the patient’s flexor tendons. The orthopedist thought it was too late to repair the tendon and referred the patient to physical therapy. As the patient continued to complain of pain and decreased function of his left thumb, he consulted a second orthopedist, who decided to surgically explore the wound to determine the cause of the patient’s continued pain and loss of thumb function. Surgical exploration revealed a piece of glass measuring 3.5 x 2 cm retained in the patient’s forearm. The orthopedist removed the glass, irrigated the wound thoroughly, and closed the incision, after which the patient’s thumb function improved considerably and his pain resolved.
The patient’s family sued the EP and the hospital, arguing that the wound should have been explored and the glass removed on the initial ED visit. They further stated that if these steps were performed initially, the patient would not have required multiple imaging studies and surgery. At trial, the jury returned a defense verdict.
Discussion
Approximately 11 million wounds are treated in US EDs each year.1 Proper management of lacerations and wounds requires more than sutures or staples. The EP must also evaluate for associated injuries (eg, tendon laceration, vascular injury), and the possibility of a retained foreign body. It is also important to ensure the patient is up to date on his or her tetanus immunization.
As with most areas of medicine, a good history and physical examination are essential. The mechanism of injury will often be the first clue to the risk of a retained foreign body. For example, shattered glass or porcelain carries a much higher risk of retention compared to a laceration from a box cutter.
The age of the injury is also important in determining the best management approach and the risk of infection. In a study by Brancto,1 wounds closed within 19 hours of injury had a 92% rate of healing without infection, compared to only 77% of those closed after 19 hours. In addition, determination of a patient’s allergy status to anesthetics and antibiotics ensures safe and appropriate treatment.
On physical examination, the wound should be described in sufficient detail (eg, length, shape), and a distal neurovascular examination should be completed and documented. This involves testing the patient’s motor strength, sensation, adequacy of pulses, and capillary refill. When examining the extremities, flexion and extension strength should also be assessed and documented.
After a wound is prepped and anesthetized, it should be explored. Often a patient may have excellent flexor or extensor strength on testing, but have a near-complete tendon laceration on visual inspection. Similarly, the wound should be explored for foreign bodies. It is important to identify and remove foreign bodies because of the associated increased risk of infection, pain, and delayed healing.1 Occasionally, a wound may need to be extended to remove a foreign body.
Unfortunately, visual inspection of a wound, especially a deep one, is not highly sensitive. If a physician has a high index of suspicion for a retained foreign body but is unable to identify one on examination, imaging studies should be ordered. Conventional plain radiography, ultrasonography, computed tomography (CT), and MRI studies can all be used to identify foreign bodies. Each of these modalities has its unique advantages and disadvantages. A recent study by Pattamapaspong et al2 compared the accuracy of radiography, CT, and MRI in detecting foreign bodies in the foot. In this study, researchers placed various types of foreign bodies, including fresh wood, dry wood, glass, porcelain, and plastic—all measuring 5 x 2 mm— in cadaver feet.2 The overall sensitivity and specificity for foreign body detection was 29% and 100%, respectively, for radiographs; 63% and 98%, respectively, for CT; and 58% and 100%, respectively, for MRI.2 Interestingly, CT was superior to MRI in identifying water-rich fresh wood.2 A similar study by Aras et al3 compared the sensitivity of plain radiographs, CT, and ultrasound in detecting foreign bodies in the face. The foreign bodies used in this study measured 1 x 1 x 1 cm and included metal, glass, wood, stone, acrylic, graphite, and polyoxybenzylmethylenglycolanhydride (ie, Bakelite).3 In this study, ultrasound identified superficial foreign bodies with low radiopacity in body tissues more effectively than CT or plain radiographs.3 In a review by Karabay4 of traumatic wrist and hand injuries, ultrasound was considered the best modality to identify and locate both opaque and radiolucent foreign bodies in the soft tissue.
If a foreign body is identified but cannot be removed, consultation with a surgical service is required. Depending on the local referral pattern, this might be general surgery, plastic surgery, or hand surgery. Unless there is an acute nerve or vascular injury, patients rarely require immediate surgery. In most cases, the wound can be closed loosely until the surgeon can remove the foreign body in the operating room and/or with aid of fluoroscopy at a later time. Depending on the size, material, and location of the foreign body, the surgeon might even elect to simply observe.
The bottom-line lesson from this case: depending on the mechanism of injury, EPs must maintain a high index of suspicion for retained foreign bodies in traumatic wounds. In addition to wound exploration, imaging studies should be used in patients at high risk for a retained foreign body, such as those injured with broken glass or porcelain, but in whom no foreign body is found on wound exploration.
Ruptured Esophagus
A 78-year-old man presented to the ED with symptoms of choking and chest discomfort. The patient stated that he had experienced a sudden onset of difficulty swallowing, along with chest pain, while he was eating dinner at a restaurant earlier that evening. The patient initially thought he had a piece of carrot stuck in his throat. He denied any previous history of similar symptoms. He complained of mild shortness of breath, but denied any drooling or vomiting. His medical history was significant for hypertension, which was controlled with medication. He denied tobacco or alcohol use and had no known drug allergies.
On physical examination, the patient’s vital signs were: heart rate (HR), 106 beats/minute; blood pressure (BP), 144/82 mm Hg; respiratory rate, 22 breaths/minute, and temperature, 98.6°F. Oxygen saturation was 95% on room air. The patient’s oropharynx appeared normal and without foreign body obstruction; his lungs were clear to auscultation bilaterally; and his HR was tachycardic but with a regular rhythm. Other than mild diaphoresis, the remainder of the physical examination was normal.
The EP ordered a complete blood count (CBC), a basic metabolic profile (BMP), and a portable chest X-ray, which the EP interpreted as normal. In addition, an intravenous (IV) saline lock was placed, and the patient was given morphine 4 mg IV and ondansetron 4 mg IV. He was also placed on 2 L of oxygen via nasal cannula. Since the patient continued to complain of chest pain and dysphagia, the EP consulted with a gastroenterologist; unfortunately, there was no documentation of this.
The EP admitted the patient to the floor with a diagnosis of esophageal obstruction, probably secondary to a piece of carrot. During the night, the patient’s shortness of breath worsened, requiring an increase in supplemental oxygen. The next morning, the patient’s HR increased to 120 beats/minute; his BP dropped to 96/50 mm Hg, and he developed a low-grade fever. He was transferred to the intensive care unit, where he was started on IV fluid resuscitation with normal saline and broad spectrum antibiotics. A CT scan of the chest was also ordered, which revealed an esophageal perforation. The patient was taken immediately to the operating room; surgery revealed a large esophageal perforation with evidence of mediastinitis and gross contamination of the left hemithorax. The patient died 2 days later.
The patient’s family sued the EP for failure to diagnose and treat the esophageal perforation in a timely manner. The EP argued that the patient’s symptoms were consistent with an obstruction, not esophageal perforation. The defendant also argued that the initial chest X-ray was normal. The case was resolved for $800,000 prior to going to trial.
Discussion
Esophageal perforation is a true medical emergency that requires timely diagnosis and management because morbidity and mortality are directly related to the time to treatment. Unfortunately, esophageal perforation can be a difficult diagnosis due to its relative rarity and variability in clinical presentation.
More than 50% of all esophageal perforations are iatrogenic, primarily as a complication of endoscopy.1 Other causes of perforation include spontaneous perforation or Boerhaave syndrome (15%), foreign body (12%), trauma (9%), and malignancy (1%).1 Anatomically, perforation tends to occur in the areas of the esophagus that are most narrow—eg, cricopharyngeus muscle, area of broncho-aortic constriction, and esophagogastric junction.1
Food impactions, not surprisingly, tend to occur in these same areas of the esophagus. In addition, there are structural esophageal abnormalities that increase the risk of food impaction, including diverticula, webs, rings, strictures, achalasia, and tumors.2 Since food impaction can result in an esophageal perforation, there is a significant overlap in the initial presentation of these two conditions. However, in cases of perforation, signs and symptoms of shock predominate as time progresses due to esophageal contents leaking into the mediastinal and pleural spaces.
Patients with a food impaction will often complain of an acute onset of dysphagia, difficulty in handling secretions, choking, drooling, retrosternal fullness, regurgitation of undigested food, and wheezing.2 Perforation can cause severe chest pain, tachypnea, dyspnea, fever, and shock.2
A chest X-ray is typically the initial imaging study for suspected esophageal perforation. Since most spontaneous perforations occur through the left posterolateral wall of the distal esophagus, a new left pleural effusion can frequently be seen on X-ray. Mediastinal emphysema is highly suspicious for perforation, but the condition takes time to develop; therefore, its absence on X-ray does not exclude perforation. In the setting of a normal chest X-ray and ongoing esophageal symptoms, further investigation is required, usually via CT scan or endoscopy. Computed tomography, because of its availability and speed, is usually the preferred study to confirm the diagnosis.
Once an esophageal perforation is confirmed or is highly suspected, the patient will require IV fluid resuscitation, IV broad-spectrum antibiotic treatment, and emergency surgical consultation. As previously stated, esophageal perforation is associated with a high mortality rate, and time is critical to successful management.
- Retained Foreign Body
1. Brancto JC. Minor wound preparation and irrigation. http://www.uptodate.com/contents/minor-wound-preparation-and-irrigation. Accessed June 1, 2016.
2. Pattamapaspong N, Srisuwan T, Sivasomboon C, et al. Accuracy of radiography, computed tomography and magnetic resonance imaging in diagnosing foreign bodies in the foot. Radiol Med. 2013;118(2):303-310.
3. Aras MH, Miloglu O, Barutcugil C, Kantarci M, Ozcan E, Harorli A. Comparison of the sensitivity for detecting foreign bodies among conventional plain radiography, computed tomography and ultrasonography. Dentomaxillofac Radiol. 2010;39(2):72-78.
4. Karabay N. US findings in traumatic wrist and hand injuries. Diagn Interv Radiol. 2013;19(4):320-325.
- Ruptured Esophagus
1. Raymond DP, Jones C. Surgical management of esophageal perforation. http://www.uptodate.com/contents/surgical-management-of-esophageal-perforation. Accessed June 27, 2016.
2. Triadafilopoulos G. Ingested foreign bodies and food impaction in adults. http://www.uptodate.com/contents/ingested-foreign-bodies-and-food-impactions-in-adults. Accessed June 27, 2016.
- Retained Foreign Body
1. Brancto JC. Minor wound preparation and irrigation. http://www.uptodate.com/contents/minor-wound-preparation-and-irrigation. Accessed June 1, 2016.
2. Pattamapaspong N, Srisuwan T, Sivasomboon C, et al. Accuracy of radiography, computed tomography and magnetic resonance imaging in diagnosing foreign bodies in the foot. Radiol Med. 2013;118(2):303-310.
3. Aras MH, Miloglu O, Barutcugil C, Kantarci M, Ozcan E, Harorli A. Comparison of the sensitivity for detecting foreign bodies among conventional plain radiography, computed tomography and ultrasonography. Dentomaxillofac Radiol. 2010;39(2):72-78.
4. Karabay N. US findings in traumatic wrist and hand injuries. Diagn Interv Radiol. 2013;19(4):320-325.
- Ruptured Esophagus
1. Raymond DP, Jones C. Surgical management of esophageal perforation. http://www.uptodate.com/contents/surgical-management-of-esophageal-perforation. Accessed June 27, 2016.
2. Triadafilopoulos G. Ingested foreign bodies and food impaction in adults. http://www.uptodate.com/contents/ingested-foreign-bodies-and-food-impactions-in-adults. Accessed June 27, 2016.
Malpractice Counsel: Too much medication, hot red knee
Too Much Medication, Too Little Monitoring
A 58-year-old man presented to the ED via emergency medical services (EMS) for evaluation of severe low-back pain. The patient said the pain started abruptly, approximately 1 hour earlier when he was picking up a 50-lb television set. He stated that the pain was so severe that he was unable to move and was forced to lie down on the floor. Although the patient noted that he had a history of a “bad back,” he said he never required surgery and never experienced an episode this severe. The patient denied any radiation of pain or lower extremity numbness or weakness. He denied any chest pain or abdominal pain. His medical history was significant for obstructive sleep apnea and hypertension for which he was taking hydrochlorothiazide. Regarding his social history, he denied any tobacco or alcohol use.
Upon presentation, the patient was found to be in extreme discomfort, rating his pain as an “11” on a scale of 0 to 10. His vital signs were heart rate (HR), 110 beats/minute; blood pressure (BP), 154/91 mm Hg; respiratory rate, 20 breaths/minute; and temperature, 98.6°F. Oxygen (O2) saturation was 98% on room air.
When the emergency physician (EP) entered the examination room, the patient was in bed, resting on his side and moaning from the pain. The head, eyes, ears, nose, and throat, cardiac, and lung examinations were all normal. The patient’s abdomen was soft and nontender and without guarding, rebound, or palpable mass. When the EP examined the patient’s back, there was no midline tenderness over the thoracic and lumbar spine. The patient did, however, exhibit bilateral paraspinal lumbar muscle tenderness to palpation and muscle spasm. After much prompting, he demonstrated 5/5 motor strength in his lower extremities bilaterally. The dorsalis pedis and posterior tibial pulses were 2+ and symmetrical.
To treat the patient’s severe pain, the EP had a saline lock placed and ordered intravenous (IV) hydromorphone 1 mg, ondansetron 4 mg, and diazepam 5 mg. No laboratory or imaging studies were ordered. Ninety minutes after receiving the analgesics, the patient continued to complain of severe pain without any improvement, and the EP ordered another two rounds of IV hydromorphone 1 mg and diazepam 5 mg. The EP did not return to check up on the patient, but rather relied solely on updates from the patient’s nurse.
Despite the additional doses of hydromorphone and diazepam, the patient continued to complain of severe pain, and the EP ordered IV hydromorphone 2 mg and diazepam 10 mg. Shortly after the patient received this third round of analgesics, his wife arrived at the ED asking to see her husband. When she entered his room, the patient was unresponsive. A code was called and the patient was found to be in asystole. Despite aggressive resuscitative efforts that included intubation, cardiopulmonary resuscitation, and advanced cardiac life support medications, the patient did not recover.
The patient’s wife sued the EP, the ED nurse, and the hospital for failure to appropriately monitor her husband while he received multiple doses of analgesic and sedative agents. The plaintiff argued that the patient’s death was caused by a cardiac arrest occurring secondary to a respiratory arrest, and that the respiratory arrest was secondary to the medications he was given in the ED. The defendants denied the allegations. A $2 million settlement was reached prior to trial.
Discussion
This was clearly a preventable death. Emergency physicians treat pain daily and should be knowledgeable about and experienced in managing acute pain. When evaluating and treating a patient’s pain, the EP must select the appropriate medication. Though we often talk about a tiered approach to pain in the ED, most of us would agree that opioids, usually via IV, are the first choice for managing severe pain.
In addition to prescribing the appropriate analgesics, the EP must identify which patients are at risk of opioid complications. This patient was at risk for opioid-induced respiratory depression based on his age (ie, >55 years old) and history of obstructive sleep apnea. These two risk factors, along with pre-existing chronic obstructive pulmonary disease, anatomic oral or airway abnormalities, and comorbidities (eg, renal or hepatic impairment), place patients at high risk for opioid-associated complications.1 Patients with any of these conditions must be closely monitored and, based on their response to the prescribed analgesia, the EP may need to decrease the analgesic dosage and increase dosage intervals. In addition to close monitoring, reversal agents such as naloxone should be readily available in case of respiratory depression.
The problem in this case was not the selection of hydromorphone as the initial analgesic agent. Hydromorphone is frequently used safely in the ED to treat severe pain associated with conditions such as sickle cell vaso-occlusive pain crisis, renal colic, and long-bone fracture. Issues arise when hydromorphone is combined with a benzodiazepine (in this case, diazepam), which by itself causes sedation and anxiolysis. Central nervous system (CNS) depression may be additive and occur when benzodiazepines are used concomitantly with drugs that also cause CNS depression (eg, opioids).1 This combination can lead to excessive sedation, resulting in partial airway obstruction and hypoxia.1 For example, in an investigation by Bailey et al,2 in human volunteers, neither hypoxemia nor apnea was evident after administration of .05 mg/kg of IV midazolam. In patients who received 2 mcg/kg of IV fentanyl alone, hypoxemia occurred in 50%, but apnea did not occur in any of the patients studied. However, when the same doses of these drugs were administered together, 92% of participants exhibited hypoxemia and 50% became apneic.2
When a combination of an opioid and benzodiazepine are given over frequent intervals, the clinician crosses over from treating pain to performing procedural sedation and analgesia—whether he intended to or not. As such, the patient in this case required proper monitoring, including cardiac monitoring and pulse oximetry; he also should have been placed on supplemental O2. Ideally, the patient would have benefited from end-tidal carbon dioxide (ETCO2), monitoring, if available. This is a noninvasive measurement of the partial pressure of CO2 in exhaled breath. Hypoventilation from respiratory depression results in an increase in ETCO2, and hyperventilation results in a decreased ETCO2. While pulse oximetry is excellent at monitoring O2 saturation, it is ineffective in the early detection of respiratory depression, hypoventilation, and apnea. The hypercarbia precedes the hypoxemia—by as much as 60 seconds (range 5-240 seconds), according to a study by Deitch et al.3
Finally, rather than relying solely on the reports from the nurse, the EP should have personally reassessed the patient at some point. Nursing updates are extremely helpful, but when ordering repeated doses of IV opioids and benzodiazepines, the EP should personally reassess the patient.
Hot Red Knee
64-year-old man presented to the ED with a chief complaint of right knee pain, which he stated began approximately 2 days earlier. He denied any injury or trauma or a recent history of fever, chills, or other joint complaints. He described the pain as constant, worse with weight bearing, and becoming progressively more painful. The patient had a history of gout; however, previous attacks had only affected his great toes and elbows. His medical history was also significant for hypertension, for which he was taking lisinopril and hydrochlorothiazide. He admitted to moderate alcohol consumption but denied tobacco use.
On physical examination, the patient appeared uncomfortable due to the knee pain. All of his vital signs were normal. A focused examination of the affected knee revealed a small effusion, diffuse tenderness to palpation, mild erythema, and slight increased warmth. The patient exhibited pain with flexion and extension of the right knee. The right ankle examination and right dorsalis pedis pulse and posterior tibial pulse were all normal. No laboratory or imaging studies were obtained.
Based on the patient’s history and physical examination, the EP believed the patient’s symptoms were due to an episode of gout. He prescribed oral colchicine, allopurinol, and acetaminophen/hydrocodone; he also advised the patient to apply warm compresses to the affected area and limit his activity. He discharged the patient home with instructions to follow up with his primary care physician.
Two days after discharge, the patient returned to the same ED via EMS. On this presentation, he was febrile, with a temperature of 102.6oF; a HR of 120 beats/minute; and a BP of 92/50 mm Hg. He also had altered mental status. The patient’s right knee appeared more swollen, and he would not flex it due to the severe pain. The EP was concerned for sepsis, and ordered blood cultures, a complete blood count, basic metabolic profile, and lactic acid evaluation. The patient was administered 2 L normal saline IV and broad-spectrum antibiotics. Despite the addition of vasopressors, he continued to deteriorate; he ultimately went into cardiac arrest and died.
The patient’s family sued the EP from the initial ED visit for failure to diagnose the right knee pain and swelling as septic arthritis (SA). The plaintiff’s attorney argued that this failure to diagnosis directly caused the patient’s sepsis and death. The EP argued that the patient’s history and physical examination were consistent with an acute gout attack, that there was no evidence of infection in the right knee, and that this was not the cause of the patient’s death. At trial, the jury returned a verdict in favor of the defense.
Discussion
Gout is caused by the precipitation of uric acid crystals into a joint. Attacks are usually monoarticular as opposed to polyarticular. The presence of hyperuricemia is variable; some patients have high serum uric acid levels and never experience gout, while other patients have normal serum uric acid levels and experience gout attacks. The condition is more common in men than in women. There are multiple risk factors for the development of gout, including obesity, hypertension, chronic kidney disease, regular excessive consumption of alcohol, taking diuretics, and consuming foods high in fructose corn syrup.1 The joints most often affected are the great toe and knee. Patients with gout typically complain of pain, swelling, redness, and increased warmth in the affected area.
Unfortunately, the clinical presentation of an acute gout attack and SA are indistinguishable.2 Risk factors for SA include IV drug abuse, diabetes mellitus, having a prosthetic joint, immunosuppression, and human immunodeficiency virus infection. The only reliable way to distinguish between gout and SA requires arthrocentesis with microscopic examination of the synovial fluid for bacteria, crystals, white blood cell (WBC) count, and culture.2
It is critical not to miss SA because it is associated with significant morbidity and a mortality rate of 11%.2 To further complicate the diagnosis, some patients can experience SA in the setting of an acute gout attack. In a study of all joint aspirations with crystals (both uric acid and calcium pyrophosphate), there was a 5.2% incidence of concomitant infection.2 Similarly, in patients with confirmed SA, crystals were present 21% of the time.2
A gram stain of the synovial fluid is highly specific, but only positive in 59% of cases of SA. Therefore, a negative gram stain does not exclude the diagnosis. Similarly, the presence of crystals does not exclude a coexisting joint infection. If there is high clinical suspicion for SA or an elevated synovial WBC, the patient should be presumed to have SA and treated as such until cultures prove otherwise.
It is unclear if this patient had SA. However, an EP is taking a risk in diagnosing an acute gout attack based solely on a patient’s history and physical examination. The EP should always be mindful that gout and SA can present with the identical signs and symptoms, and can present concomitantly.
- Too Much Medication, Too Little Monitoring
1. Jarzyna D, Jungquist CR, Pasero C, et al. American Society for Pain Management Nursing guidelines on monitoring for opioid induced sedation and respiratory depression. Pain Manag Nurs. 2011;12(3):118-145.e10.
2. Bailey PL, Pace NL, Ashburn MA, Moll JW, East KA, Stanley TH. Frequent hypoxemia and apnea after sedation with midazolam and fentanyl. Anesthesia. 1990;73(5):826-830.
3. Deitch K, Miner J, Chudnofsky CR, Dominici P, Latta D. Does end-tidal CO2 monitoring during emergency department procedural sedation and analgesia with propofol decrease the incidence of hypoxic events? A randomized, controlled trial. Ann Emerg Med. 2010;55(3):258-264.
- Hot Red Knee
1. Becker MA. Gout (beyond the basics). UpToDate.com. Available at http://www.uptodate.com/contents/gout-beyond-the-basics. Updated January 21, 2016. Accessed April 12, 2016.
2. Papanicolas LE, Hakendorf P, Gordon DL. Concomitant septic arthritis in crystal monoarthritis. J Rheumotal. 2012;39(1):157-160.
Too Much Medication, Too Little Monitoring
A 58-year-old man presented to the ED via emergency medical services (EMS) for evaluation of severe low-back pain. The patient said the pain started abruptly, approximately 1 hour earlier when he was picking up a 50-lb television set. He stated that the pain was so severe that he was unable to move and was forced to lie down on the floor. Although the patient noted that he had a history of a “bad back,” he said he never required surgery and never experienced an episode this severe. The patient denied any radiation of pain or lower extremity numbness or weakness. He denied any chest pain or abdominal pain. His medical history was significant for obstructive sleep apnea and hypertension for which he was taking hydrochlorothiazide. Regarding his social history, he denied any tobacco or alcohol use.
Upon presentation, the patient was found to be in extreme discomfort, rating his pain as an “11” on a scale of 0 to 10. His vital signs were heart rate (HR), 110 beats/minute; blood pressure (BP), 154/91 mm Hg; respiratory rate, 20 breaths/minute; and temperature, 98.6°F. Oxygen (O2) saturation was 98% on room air.
When the emergency physician (EP) entered the examination room, the patient was in bed, resting on his side and moaning from the pain. The head, eyes, ears, nose, and throat, cardiac, and lung examinations were all normal. The patient’s abdomen was soft and nontender and without guarding, rebound, or palpable mass. When the EP examined the patient’s back, there was no midline tenderness over the thoracic and lumbar spine. The patient did, however, exhibit bilateral paraspinal lumbar muscle tenderness to palpation and muscle spasm. After much prompting, he demonstrated 5/5 motor strength in his lower extremities bilaterally. The dorsalis pedis and posterior tibial pulses were 2+ and symmetrical.
To treat the patient’s severe pain, the EP had a saline lock placed and ordered intravenous (IV) hydromorphone 1 mg, ondansetron 4 mg, and diazepam 5 mg. No laboratory or imaging studies were ordered. Ninety minutes after receiving the analgesics, the patient continued to complain of severe pain without any improvement, and the EP ordered another two rounds of IV hydromorphone 1 mg and diazepam 5 mg. The EP did not return to check up on the patient, but rather relied solely on updates from the patient’s nurse.
Despite the additional doses of hydromorphone and diazepam, the patient continued to complain of severe pain, and the EP ordered IV hydromorphone 2 mg and diazepam 10 mg. Shortly after the patient received this third round of analgesics, his wife arrived at the ED asking to see her husband. When she entered his room, the patient was unresponsive. A code was called and the patient was found to be in asystole. Despite aggressive resuscitative efforts that included intubation, cardiopulmonary resuscitation, and advanced cardiac life support medications, the patient did not recover.
The patient’s wife sued the EP, the ED nurse, and the hospital for failure to appropriately monitor her husband while he received multiple doses of analgesic and sedative agents. The plaintiff argued that the patient’s death was caused by a cardiac arrest occurring secondary to a respiratory arrest, and that the respiratory arrest was secondary to the medications he was given in the ED. The defendants denied the allegations. A $2 million settlement was reached prior to trial.
Discussion
This was clearly a preventable death. Emergency physicians treat pain daily and should be knowledgeable about and experienced in managing acute pain. When evaluating and treating a patient’s pain, the EP must select the appropriate medication. Though we often talk about a tiered approach to pain in the ED, most of us would agree that opioids, usually via IV, are the first choice for managing severe pain.
In addition to prescribing the appropriate analgesics, the EP must identify which patients are at risk of opioid complications. This patient was at risk for opioid-induced respiratory depression based on his age (ie, >55 years old) and history of obstructive sleep apnea. These two risk factors, along with pre-existing chronic obstructive pulmonary disease, anatomic oral or airway abnormalities, and comorbidities (eg, renal or hepatic impairment), place patients at high risk for opioid-associated complications.1 Patients with any of these conditions must be closely monitored and, based on their response to the prescribed analgesia, the EP may need to decrease the analgesic dosage and increase dosage intervals. In addition to close monitoring, reversal agents such as naloxone should be readily available in case of respiratory depression.
The problem in this case was not the selection of hydromorphone as the initial analgesic agent. Hydromorphone is frequently used safely in the ED to treat severe pain associated with conditions such as sickle cell vaso-occlusive pain crisis, renal colic, and long-bone fracture. Issues arise when hydromorphone is combined with a benzodiazepine (in this case, diazepam), which by itself causes sedation and anxiolysis. Central nervous system (CNS) depression may be additive and occur when benzodiazepines are used concomitantly with drugs that also cause CNS depression (eg, opioids).1 This combination can lead to excessive sedation, resulting in partial airway obstruction and hypoxia.1 For example, in an investigation by Bailey et al,2 in human volunteers, neither hypoxemia nor apnea was evident after administration of .05 mg/kg of IV midazolam. In patients who received 2 mcg/kg of IV fentanyl alone, hypoxemia occurred in 50%, but apnea did not occur in any of the patients studied. However, when the same doses of these drugs were administered together, 92% of participants exhibited hypoxemia and 50% became apneic.2
When a combination of an opioid and benzodiazepine are given over frequent intervals, the clinician crosses over from treating pain to performing procedural sedation and analgesia—whether he intended to or not. As such, the patient in this case required proper monitoring, including cardiac monitoring and pulse oximetry; he also should have been placed on supplemental O2. Ideally, the patient would have benefited from end-tidal carbon dioxide (ETCO2), monitoring, if available. This is a noninvasive measurement of the partial pressure of CO2 in exhaled breath. Hypoventilation from respiratory depression results in an increase in ETCO2, and hyperventilation results in a decreased ETCO2. While pulse oximetry is excellent at monitoring O2 saturation, it is ineffective in the early detection of respiratory depression, hypoventilation, and apnea. The hypercarbia precedes the hypoxemia—by as much as 60 seconds (range 5-240 seconds), according to a study by Deitch et al.3
Finally, rather than relying solely on the reports from the nurse, the EP should have personally reassessed the patient at some point. Nursing updates are extremely helpful, but when ordering repeated doses of IV opioids and benzodiazepines, the EP should personally reassess the patient.
Hot Red Knee
64-year-old man presented to the ED with a chief complaint of right knee pain, which he stated began approximately 2 days earlier. He denied any injury or trauma or a recent history of fever, chills, or other joint complaints. He described the pain as constant, worse with weight bearing, and becoming progressively more painful. The patient had a history of gout; however, previous attacks had only affected his great toes and elbows. His medical history was also significant for hypertension, for which he was taking lisinopril and hydrochlorothiazide. He admitted to moderate alcohol consumption but denied tobacco use.
On physical examination, the patient appeared uncomfortable due to the knee pain. All of his vital signs were normal. A focused examination of the affected knee revealed a small effusion, diffuse tenderness to palpation, mild erythema, and slight increased warmth. The patient exhibited pain with flexion and extension of the right knee. The right ankle examination and right dorsalis pedis pulse and posterior tibial pulse were all normal. No laboratory or imaging studies were obtained.
Based on the patient’s history and physical examination, the EP believed the patient’s symptoms were due to an episode of gout. He prescribed oral colchicine, allopurinol, and acetaminophen/hydrocodone; he also advised the patient to apply warm compresses to the affected area and limit his activity. He discharged the patient home with instructions to follow up with his primary care physician.
Two days after discharge, the patient returned to the same ED via EMS. On this presentation, he was febrile, with a temperature of 102.6oF; a HR of 120 beats/minute; and a BP of 92/50 mm Hg. He also had altered mental status. The patient’s right knee appeared more swollen, and he would not flex it due to the severe pain. The EP was concerned for sepsis, and ordered blood cultures, a complete blood count, basic metabolic profile, and lactic acid evaluation. The patient was administered 2 L normal saline IV and broad-spectrum antibiotics. Despite the addition of vasopressors, he continued to deteriorate; he ultimately went into cardiac arrest and died.
The patient’s family sued the EP from the initial ED visit for failure to diagnose the right knee pain and swelling as septic arthritis (SA). The plaintiff’s attorney argued that this failure to diagnosis directly caused the patient’s sepsis and death. The EP argued that the patient’s history and physical examination were consistent with an acute gout attack, that there was no evidence of infection in the right knee, and that this was not the cause of the patient’s death. At trial, the jury returned a verdict in favor of the defense.
Discussion
Gout is caused by the precipitation of uric acid crystals into a joint. Attacks are usually monoarticular as opposed to polyarticular. The presence of hyperuricemia is variable; some patients have high serum uric acid levels and never experience gout, while other patients have normal serum uric acid levels and experience gout attacks. The condition is more common in men than in women. There are multiple risk factors for the development of gout, including obesity, hypertension, chronic kidney disease, regular excessive consumption of alcohol, taking diuretics, and consuming foods high in fructose corn syrup.1 The joints most often affected are the great toe and knee. Patients with gout typically complain of pain, swelling, redness, and increased warmth in the affected area.
Unfortunately, the clinical presentation of an acute gout attack and SA are indistinguishable.2 Risk factors for SA include IV drug abuse, diabetes mellitus, having a prosthetic joint, immunosuppression, and human immunodeficiency virus infection. The only reliable way to distinguish between gout and SA requires arthrocentesis with microscopic examination of the synovial fluid for bacteria, crystals, white blood cell (WBC) count, and culture.2
It is critical not to miss SA because it is associated with significant morbidity and a mortality rate of 11%.2 To further complicate the diagnosis, some patients can experience SA in the setting of an acute gout attack. In a study of all joint aspirations with crystals (both uric acid and calcium pyrophosphate), there was a 5.2% incidence of concomitant infection.2 Similarly, in patients with confirmed SA, crystals were present 21% of the time.2
A gram stain of the synovial fluid is highly specific, but only positive in 59% of cases of SA. Therefore, a negative gram stain does not exclude the diagnosis. Similarly, the presence of crystals does not exclude a coexisting joint infection. If there is high clinical suspicion for SA or an elevated synovial WBC, the patient should be presumed to have SA and treated as such until cultures prove otherwise.
It is unclear if this patient had SA. However, an EP is taking a risk in diagnosing an acute gout attack based solely on a patient’s history and physical examination. The EP should always be mindful that gout and SA can present with the identical signs and symptoms, and can present concomitantly.
Too Much Medication, Too Little Monitoring
A 58-year-old man presented to the ED via emergency medical services (EMS) for evaluation of severe low-back pain. The patient said the pain started abruptly, approximately 1 hour earlier when he was picking up a 50-lb television set. He stated that the pain was so severe that he was unable to move and was forced to lie down on the floor. Although the patient noted that he had a history of a “bad back,” he said he never required surgery and never experienced an episode this severe. The patient denied any radiation of pain or lower extremity numbness or weakness. He denied any chest pain or abdominal pain. His medical history was significant for obstructive sleep apnea and hypertension for which he was taking hydrochlorothiazide. Regarding his social history, he denied any tobacco or alcohol use.
Upon presentation, the patient was found to be in extreme discomfort, rating his pain as an “11” on a scale of 0 to 10. His vital signs were heart rate (HR), 110 beats/minute; blood pressure (BP), 154/91 mm Hg; respiratory rate, 20 breaths/minute; and temperature, 98.6°F. Oxygen (O2) saturation was 98% on room air.
When the emergency physician (EP) entered the examination room, the patient was in bed, resting on his side and moaning from the pain. The head, eyes, ears, nose, and throat, cardiac, and lung examinations were all normal. The patient’s abdomen was soft and nontender and without guarding, rebound, or palpable mass. When the EP examined the patient’s back, there was no midline tenderness over the thoracic and lumbar spine. The patient did, however, exhibit bilateral paraspinal lumbar muscle tenderness to palpation and muscle spasm. After much prompting, he demonstrated 5/5 motor strength in his lower extremities bilaterally. The dorsalis pedis and posterior tibial pulses were 2+ and symmetrical.
To treat the patient’s severe pain, the EP had a saline lock placed and ordered intravenous (IV) hydromorphone 1 mg, ondansetron 4 mg, and diazepam 5 mg. No laboratory or imaging studies were ordered. Ninety minutes after receiving the analgesics, the patient continued to complain of severe pain without any improvement, and the EP ordered another two rounds of IV hydromorphone 1 mg and diazepam 5 mg. The EP did not return to check up on the patient, but rather relied solely on updates from the patient’s nurse.
Despite the additional doses of hydromorphone and diazepam, the patient continued to complain of severe pain, and the EP ordered IV hydromorphone 2 mg and diazepam 10 mg. Shortly after the patient received this third round of analgesics, his wife arrived at the ED asking to see her husband. When she entered his room, the patient was unresponsive. A code was called and the patient was found to be in asystole. Despite aggressive resuscitative efforts that included intubation, cardiopulmonary resuscitation, and advanced cardiac life support medications, the patient did not recover.
The patient’s wife sued the EP, the ED nurse, and the hospital for failure to appropriately monitor her husband while he received multiple doses of analgesic and sedative agents. The plaintiff argued that the patient’s death was caused by a cardiac arrest occurring secondary to a respiratory arrest, and that the respiratory arrest was secondary to the medications he was given in the ED. The defendants denied the allegations. A $2 million settlement was reached prior to trial.
Discussion
This was clearly a preventable death. Emergency physicians treat pain daily and should be knowledgeable about and experienced in managing acute pain. When evaluating and treating a patient’s pain, the EP must select the appropriate medication. Though we often talk about a tiered approach to pain in the ED, most of us would agree that opioids, usually via IV, are the first choice for managing severe pain.
In addition to prescribing the appropriate analgesics, the EP must identify which patients are at risk of opioid complications. This patient was at risk for opioid-induced respiratory depression based on his age (ie, >55 years old) and history of obstructive sleep apnea. These two risk factors, along with pre-existing chronic obstructive pulmonary disease, anatomic oral or airway abnormalities, and comorbidities (eg, renal or hepatic impairment), place patients at high risk for opioid-associated complications.1 Patients with any of these conditions must be closely monitored and, based on their response to the prescribed analgesia, the EP may need to decrease the analgesic dosage and increase dosage intervals. In addition to close monitoring, reversal agents such as naloxone should be readily available in case of respiratory depression.
The problem in this case was not the selection of hydromorphone as the initial analgesic agent. Hydromorphone is frequently used safely in the ED to treat severe pain associated with conditions such as sickle cell vaso-occlusive pain crisis, renal colic, and long-bone fracture. Issues arise when hydromorphone is combined with a benzodiazepine (in this case, diazepam), which by itself causes sedation and anxiolysis. Central nervous system (CNS) depression may be additive and occur when benzodiazepines are used concomitantly with drugs that also cause CNS depression (eg, opioids).1 This combination can lead to excessive sedation, resulting in partial airway obstruction and hypoxia.1 For example, in an investigation by Bailey et al,2 in human volunteers, neither hypoxemia nor apnea was evident after administration of .05 mg/kg of IV midazolam. In patients who received 2 mcg/kg of IV fentanyl alone, hypoxemia occurred in 50%, but apnea did not occur in any of the patients studied. However, when the same doses of these drugs were administered together, 92% of participants exhibited hypoxemia and 50% became apneic.2
When a combination of an opioid and benzodiazepine are given over frequent intervals, the clinician crosses over from treating pain to performing procedural sedation and analgesia—whether he intended to or not. As such, the patient in this case required proper monitoring, including cardiac monitoring and pulse oximetry; he also should have been placed on supplemental O2. Ideally, the patient would have benefited from end-tidal carbon dioxide (ETCO2), monitoring, if available. This is a noninvasive measurement of the partial pressure of CO2 in exhaled breath. Hypoventilation from respiratory depression results in an increase in ETCO2, and hyperventilation results in a decreased ETCO2. While pulse oximetry is excellent at monitoring O2 saturation, it is ineffective in the early detection of respiratory depression, hypoventilation, and apnea. The hypercarbia precedes the hypoxemia—by as much as 60 seconds (range 5-240 seconds), according to a study by Deitch et al.3
Finally, rather than relying solely on the reports from the nurse, the EP should have personally reassessed the patient at some point. Nursing updates are extremely helpful, but when ordering repeated doses of IV opioids and benzodiazepines, the EP should personally reassess the patient.
Hot Red Knee
64-year-old man presented to the ED with a chief complaint of right knee pain, which he stated began approximately 2 days earlier. He denied any injury or trauma or a recent history of fever, chills, or other joint complaints. He described the pain as constant, worse with weight bearing, and becoming progressively more painful. The patient had a history of gout; however, previous attacks had only affected his great toes and elbows. His medical history was also significant for hypertension, for which he was taking lisinopril and hydrochlorothiazide. He admitted to moderate alcohol consumption but denied tobacco use.
On physical examination, the patient appeared uncomfortable due to the knee pain. All of his vital signs were normal. A focused examination of the affected knee revealed a small effusion, diffuse tenderness to palpation, mild erythema, and slight increased warmth. The patient exhibited pain with flexion and extension of the right knee. The right ankle examination and right dorsalis pedis pulse and posterior tibial pulse were all normal. No laboratory or imaging studies were obtained.
Based on the patient’s history and physical examination, the EP believed the patient’s symptoms were due to an episode of gout. He prescribed oral colchicine, allopurinol, and acetaminophen/hydrocodone; he also advised the patient to apply warm compresses to the affected area and limit his activity. He discharged the patient home with instructions to follow up with his primary care physician.
Two days after discharge, the patient returned to the same ED via EMS. On this presentation, he was febrile, with a temperature of 102.6oF; a HR of 120 beats/minute; and a BP of 92/50 mm Hg. He also had altered mental status. The patient’s right knee appeared more swollen, and he would not flex it due to the severe pain. The EP was concerned for sepsis, and ordered blood cultures, a complete blood count, basic metabolic profile, and lactic acid evaluation. The patient was administered 2 L normal saline IV and broad-spectrum antibiotics. Despite the addition of vasopressors, he continued to deteriorate; he ultimately went into cardiac arrest and died.
The patient’s family sued the EP from the initial ED visit for failure to diagnose the right knee pain and swelling as septic arthritis (SA). The plaintiff’s attorney argued that this failure to diagnosis directly caused the patient’s sepsis and death. The EP argued that the patient’s history and physical examination were consistent with an acute gout attack, that there was no evidence of infection in the right knee, and that this was not the cause of the patient’s death. At trial, the jury returned a verdict in favor of the defense.
Discussion
Gout is caused by the precipitation of uric acid crystals into a joint. Attacks are usually monoarticular as opposed to polyarticular. The presence of hyperuricemia is variable; some patients have high serum uric acid levels and never experience gout, while other patients have normal serum uric acid levels and experience gout attacks. The condition is more common in men than in women. There are multiple risk factors for the development of gout, including obesity, hypertension, chronic kidney disease, regular excessive consumption of alcohol, taking diuretics, and consuming foods high in fructose corn syrup.1 The joints most often affected are the great toe and knee. Patients with gout typically complain of pain, swelling, redness, and increased warmth in the affected area.
Unfortunately, the clinical presentation of an acute gout attack and SA are indistinguishable.2 Risk factors for SA include IV drug abuse, diabetes mellitus, having a prosthetic joint, immunosuppression, and human immunodeficiency virus infection. The only reliable way to distinguish between gout and SA requires arthrocentesis with microscopic examination of the synovial fluid for bacteria, crystals, white blood cell (WBC) count, and culture.2
It is critical not to miss SA because it is associated with significant morbidity and a mortality rate of 11%.2 To further complicate the diagnosis, some patients can experience SA in the setting of an acute gout attack. In a study of all joint aspirations with crystals (both uric acid and calcium pyrophosphate), there was a 5.2% incidence of concomitant infection.2 Similarly, in patients with confirmed SA, crystals were present 21% of the time.2
A gram stain of the synovial fluid is highly specific, but only positive in 59% of cases of SA. Therefore, a negative gram stain does not exclude the diagnosis. Similarly, the presence of crystals does not exclude a coexisting joint infection. If there is high clinical suspicion for SA or an elevated synovial WBC, the patient should be presumed to have SA and treated as such until cultures prove otherwise.
It is unclear if this patient had SA. However, an EP is taking a risk in diagnosing an acute gout attack based solely on a patient’s history and physical examination. The EP should always be mindful that gout and SA can present with the identical signs and symptoms, and can present concomitantly.
- Too Much Medication, Too Little Monitoring
1. Jarzyna D, Jungquist CR, Pasero C, et al. American Society for Pain Management Nursing guidelines on monitoring for opioid induced sedation and respiratory depression. Pain Manag Nurs. 2011;12(3):118-145.e10.
2. Bailey PL, Pace NL, Ashburn MA, Moll JW, East KA, Stanley TH. Frequent hypoxemia and apnea after sedation with midazolam and fentanyl. Anesthesia. 1990;73(5):826-830.
3. Deitch K, Miner J, Chudnofsky CR, Dominici P, Latta D. Does end-tidal CO2 monitoring during emergency department procedural sedation and analgesia with propofol decrease the incidence of hypoxic events? A randomized, controlled trial. Ann Emerg Med. 2010;55(3):258-264.
- Hot Red Knee
1. Becker MA. Gout (beyond the basics). UpToDate.com. Available at http://www.uptodate.com/contents/gout-beyond-the-basics. Updated January 21, 2016. Accessed April 12, 2016.
2. Papanicolas LE, Hakendorf P, Gordon DL. Concomitant septic arthritis in crystal monoarthritis. J Rheumotal. 2012;39(1):157-160.
- Too Much Medication, Too Little Monitoring
1. Jarzyna D, Jungquist CR, Pasero C, et al. American Society for Pain Management Nursing guidelines on monitoring for opioid induced sedation and respiratory depression. Pain Manag Nurs. 2011;12(3):118-145.e10.
2. Bailey PL, Pace NL, Ashburn MA, Moll JW, East KA, Stanley TH. Frequent hypoxemia and apnea after sedation with midazolam and fentanyl. Anesthesia. 1990;73(5):826-830.
3. Deitch K, Miner J, Chudnofsky CR, Dominici P, Latta D. Does end-tidal CO2 monitoring during emergency department procedural sedation and analgesia with propofol decrease the incidence of hypoxic events? A randomized, controlled trial. Ann Emerg Med. 2010;55(3):258-264.
- Hot Red Knee
1. Becker MA. Gout (beyond the basics). UpToDate.com. Available at http://www.uptodate.com/contents/gout-beyond-the-basics. Updated January 21, 2016. Accessed April 12, 2016.
2. Papanicolas LE, Hakendorf P, Gordon DL. Concomitant septic arthritis in crystal monoarthritis. J Rheumotal. 2012;39(1):157-160.
Malpractice Counsel: Bicycle fall, hemodialysis complication
Fall
A 52-year-old man presented to the ED with complaints of left shoulder and left chest pain following a bicycle accident. The patient stated he had fallen from his bicycle and landed on his left side after he turned sharply to miss a speeding car. He denied head injury, loss of consciousness, or neck pain. The patient was ambulatory after the fall and had driven himself to the ED, and complained primarily of the left shoulder and left chest pain. He described the chest pain as sharp, worsening with movement and deep inspiration. The pain also was associated with mild shortness of breath. The patient denied headache, nausea, vomiting, abdominal pain, or back pain. He was otherwise in good health and on no medications.
The patient’s vital signs on presentation were normal and his head was atraumatic. He exhibited no midline posterior cervical tenderness to palpation. The head, eyes, ears, nose, and throat (HEENT) and mouth examinations were unremarkable. The patient did have tenderness to palpation over the left clavicle and left anterior chest; there was no crepitus or subcutaneous emphysema appreciated. Breath sounds were normal, and the heart had a regular rate and rhythm without murmurs, rubs, or gallops. The abdomen was soft and nontender, without guarding or rebound. The pelvis was stable, and the patient moved all four extremities with good strength. However, he did exhibit pain with movement of his left shoulder. Peripheral pulses were 2+ and symmetrical.
The emergency physician (EP) ordered an X-ray of the chest and left shoulder, as well as urinalysis. The X-rays revealed a small left pneumothorax, a minimally displaced left clavicular fracture, and fractures of the left fourth and fifth ribs. The urinalysis results were normal. The patient was administered intravenous (IV) morphine for pain and placed on 2 L/minute oxygen via nasal cannula, with 100% oxygen saturation on pulse oximetry.
The EP consulted a pulmonologist regarding management of the pneumothorax, who recommended a 4-hour observation period in the ED, followed by a repeat chest X-ray. During the observation period, the patient remained on oxygen and continued to deny any new complaints, including headache, dizziness, or abdominal pain. His vital signs remained normal throughout the entire observation period.
While in radiology services for a repeat chest X-ray, the patient fainted and struck his head on the floor. The EP immediately ordered a noncontrast computed tomography scan of the head, which demonstrated a large intracranial bleed. The patient was taken immediately to the operating room by neurosurgery. His recovery was uneventful, and he was discharged home without obvious sequelae.
The patient sued the EP and hospital for negligent care, claiming the EP underestimated the patient’s injuries and that additional testing was warranted. The defendants argued the patient was properly evaluated based on the history and physical examination. A defense verdict was returned.
Discussion
One possible criticism of this case is the consulting of a pulmonologist for the traumatic pneumothorax rather than a trauma surgeon or general surgeon. It is unclear if these specialists were not available for consult. Nevertheless, the pulmonologist’s advice to the EP was reasonable. Until just recently, it was dogma that all traumatic pneumothoraces required tube thoracostomy for management. This is still true for tension pneumothorax, hemothorax, moderate-to-large pneumothorax, symptomatic pneumothorax, or if mechanical ventilation is anticipated or needed.1 For small pneumothoraces, several management options exist, including close observation, needle or catheter aspiration, or placement of a pigtail catheter—in addition to the placement of a small (ie, 10-14 French) thoracostomy tube.2
Regardless, it does not appear the pneumothorax played a role in the patient’s hospital fall. More likely, the patient experienced a vasovagal episode. Interestingly, he never required treatment for the pneumothorax, despite requiring mechanical ventilation.
A Tragic Complication of Hemodialysis
A 58-year-old man presented to the ED with the chief complaint of bleeding from his dialysis fistula. The patient had end-stage renal disease and had been on hemodialysis (HD) for the past 3 years. He had an arteriovenous fistula (AVF) in his left arm for dialysis access, and received HD 3 days per week—every Tuesday, Thursday, and Saturday. He had completed a scheduled run of dialysis 5 hours prior to presentation, but had continued to bleed intermittently from the AVF site. The patient stated he had applied pressure multiple times to the site, but was unsuccessful in stopping the bleeding. His medical history was significant for hypertension and coronary artery disease. Regarding his social history, the patient admitted to smoking one pack of cigarettes per day and consuming alcohol on a regular basis.
The patient’s vital signs at presentation were: heart rate, 98 beats/minute; blood pressure, 146/85 mm Hg; respiratory rate, 20 breaths/minute; and temperature, 98.6°F. Oxygen saturation was 96% on room air. The HEENT examination was unremarkable. Examination of the heart revealed a normal rate and regular rhythm with a grade of 1/6 systolic murmur, heard best at the left sternal border. The breath sounds were equal bilaterally and clear to auscultation; the abdominal examination was unremarkable. The patient had an AVF in his left forearm that was not actively bleeding. There was a palpable thrill and a bruit present on auscultation over the site; there was no increased warmth or drainage.
The EP ordered a complete blood count (CBC) on the patient. The hemoglobin and hematocrit levels were essentially unchanged from a previous CBC 1 month prior, and the platelet count was normal. After approximately 1 hour of observation in the ED, there was no rebleeding at the site, and the patient was discharged home.
Unfortunately, the bleeding resumed the following day. The patient went into cardiac arrest and died at home prior to arrival of emergency medical services. The patient’s family sued the EP and hospital for discharging the patient home without first obtaining a surgical consult. The EP and hospital settled the case with the family for $2 million.
Discussion
Many patients who present to the ED with bleeding from the vascular access site can be managed simply with direct pressure, typically for a minimum of 5 to 10 minutes. In more severe cases, the EP can apply direct pressure with an absorbable gelatin sponge (eg, Gelfoam). If the patient presents soon after completion of dialysis, the EP should consider heparin anticoagulation as the etiology. In such cases, the use of IV protamine should be considered. One milligram of protamine can reverse 100 units of heparin. Since typically 1,000 to 2,000 units of heparin are administered at dialysis, a dose of 10 to 20 mg of protamine IV should be sufficient to reverse bleeding.
Other strategies to control hemorrhage from the access site include the use of topical thrombin or an IV drip of desmopressin. Once bleeding has been controlled, the patient should be observed for a minimum of 1 to 2 hours in the ED. If the bleeding still cannot be controlled, emergent consultation with vascular surgery services is required. Placing a suture at the site, or the use of a tourniquet proximal to the access site, can be used as a temporary measure until the surgeon arrives. The disadvantage of applying direct pressure is that it can cause thrombosis within the fistula or graft. However, given the alternative, this is an acceptable risk.
It is unfortunate that this case settled because it does not appear that any malpractice was committed. Vascular surgeons do not come to the ED to see functioning, nonbleeding AVFs. There was no published information explaining why the patient experienced rebleeding 10 to 12 hours after the initial event (perhaps some minor trauma precipitated it). Even if this patient had been observed in the ED for 8 hours, he would not have experienced rebleeding in the ED, but the tragic outcome would remain the same.
- Fall
- Legome E. Initial evaluation and management of blunt thoracic trauma in adults. UpToDate Web site. Available at http://www.uptodate.com/contents/initial-evaluation-and-management-of-blunt-thoracic-trauma-in-adults?source=search_result&search=Initial+evaluation+and+management+of+blunt+thoracic+trauma+in+adults.&selectedTitle=1~150. Updated September 21, 2015. Accessed February 21, 2016.
- Nicks BA, Manthey D. Pneumothorax. In: Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline DM, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw Hill Education; 2016:464-468.
- A Tragic Complication of Hemodialysis
- Stolic R. Most important chronic complications of arteriovenous fistulas for hemodialysis. Med Princ Pract. 2013;22(3):220-228.
- Lutz J, Menke J, Sollinger D, Schinzel H, Thurmel K. Haemostasis in chronic kidney disease. Nephrol Dial Transplant. 2014;29(1):29-40.
Fall
A 52-year-old man presented to the ED with complaints of left shoulder and left chest pain following a bicycle accident. The patient stated he had fallen from his bicycle and landed on his left side after he turned sharply to miss a speeding car. He denied head injury, loss of consciousness, or neck pain. The patient was ambulatory after the fall and had driven himself to the ED, and complained primarily of the left shoulder and left chest pain. He described the chest pain as sharp, worsening with movement and deep inspiration. The pain also was associated with mild shortness of breath. The patient denied headache, nausea, vomiting, abdominal pain, or back pain. He was otherwise in good health and on no medications.
The patient’s vital signs on presentation were normal and his head was atraumatic. He exhibited no midline posterior cervical tenderness to palpation. The head, eyes, ears, nose, and throat (HEENT) and mouth examinations were unremarkable. The patient did have tenderness to palpation over the left clavicle and left anterior chest; there was no crepitus or subcutaneous emphysema appreciated. Breath sounds were normal, and the heart had a regular rate and rhythm without murmurs, rubs, or gallops. The abdomen was soft and nontender, without guarding or rebound. The pelvis was stable, and the patient moved all four extremities with good strength. However, he did exhibit pain with movement of his left shoulder. Peripheral pulses were 2+ and symmetrical.
The emergency physician (EP) ordered an X-ray of the chest and left shoulder, as well as urinalysis. The X-rays revealed a small left pneumothorax, a minimally displaced left clavicular fracture, and fractures of the left fourth and fifth ribs. The urinalysis results were normal. The patient was administered intravenous (IV) morphine for pain and placed on 2 L/minute oxygen via nasal cannula, with 100% oxygen saturation on pulse oximetry.
The EP consulted a pulmonologist regarding management of the pneumothorax, who recommended a 4-hour observation period in the ED, followed by a repeat chest X-ray. During the observation period, the patient remained on oxygen and continued to deny any new complaints, including headache, dizziness, or abdominal pain. His vital signs remained normal throughout the entire observation period.
While in radiology services for a repeat chest X-ray, the patient fainted and struck his head on the floor. The EP immediately ordered a noncontrast computed tomography scan of the head, which demonstrated a large intracranial bleed. The patient was taken immediately to the operating room by neurosurgery. His recovery was uneventful, and he was discharged home without obvious sequelae.
The patient sued the EP and hospital for negligent care, claiming the EP underestimated the patient’s injuries and that additional testing was warranted. The defendants argued the patient was properly evaluated based on the history and physical examination. A defense verdict was returned.
Discussion
One possible criticism of this case is the consulting of a pulmonologist for the traumatic pneumothorax rather than a trauma surgeon or general surgeon. It is unclear if these specialists were not available for consult. Nevertheless, the pulmonologist’s advice to the EP was reasonable. Until just recently, it was dogma that all traumatic pneumothoraces required tube thoracostomy for management. This is still true for tension pneumothorax, hemothorax, moderate-to-large pneumothorax, symptomatic pneumothorax, or if mechanical ventilation is anticipated or needed.1 For small pneumothoraces, several management options exist, including close observation, needle or catheter aspiration, or placement of a pigtail catheter—in addition to the placement of a small (ie, 10-14 French) thoracostomy tube.2
Regardless, it does not appear the pneumothorax played a role in the patient’s hospital fall. More likely, the patient experienced a vasovagal episode. Interestingly, he never required treatment for the pneumothorax, despite requiring mechanical ventilation.
A Tragic Complication of Hemodialysis
A 58-year-old man presented to the ED with the chief complaint of bleeding from his dialysis fistula. The patient had end-stage renal disease and had been on hemodialysis (HD) for the past 3 years. He had an arteriovenous fistula (AVF) in his left arm for dialysis access, and received HD 3 days per week—every Tuesday, Thursday, and Saturday. He had completed a scheduled run of dialysis 5 hours prior to presentation, but had continued to bleed intermittently from the AVF site. The patient stated he had applied pressure multiple times to the site, but was unsuccessful in stopping the bleeding. His medical history was significant for hypertension and coronary artery disease. Regarding his social history, the patient admitted to smoking one pack of cigarettes per day and consuming alcohol on a regular basis.
The patient’s vital signs at presentation were: heart rate, 98 beats/minute; blood pressure, 146/85 mm Hg; respiratory rate, 20 breaths/minute; and temperature, 98.6°F. Oxygen saturation was 96% on room air. The HEENT examination was unremarkable. Examination of the heart revealed a normal rate and regular rhythm with a grade of 1/6 systolic murmur, heard best at the left sternal border. The breath sounds were equal bilaterally and clear to auscultation; the abdominal examination was unremarkable. The patient had an AVF in his left forearm that was not actively bleeding. There was a palpable thrill and a bruit present on auscultation over the site; there was no increased warmth or drainage.
The EP ordered a complete blood count (CBC) on the patient. The hemoglobin and hematocrit levels were essentially unchanged from a previous CBC 1 month prior, and the platelet count was normal. After approximately 1 hour of observation in the ED, there was no rebleeding at the site, and the patient was discharged home.
Unfortunately, the bleeding resumed the following day. The patient went into cardiac arrest and died at home prior to arrival of emergency medical services. The patient’s family sued the EP and hospital for discharging the patient home without first obtaining a surgical consult. The EP and hospital settled the case with the family for $2 million.
Discussion
Many patients who present to the ED with bleeding from the vascular access site can be managed simply with direct pressure, typically for a minimum of 5 to 10 minutes. In more severe cases, the EP can apply direct pressure with an absorbable gelatin sponge (eg, Gelfoam). If the patient presents soon after completion of dialysis, the EP should consider heparin anticoagulation as the etiology. In such cases, the use of IV protamine should be considered. One milligram of protamine can reverse 100 units of heparin. Since typically 1,000 to 2,000 units of heparin are administered at dialysis, a dose of 10 to 20 mg of protamine IV should be sufficient to reverse bleeding.
Other strategies to control hemorrhage from the access site include the use of topical thrombin or an IV drip of desmopressin. Once bleeding has been controlled, the patient should be observed for a minimum of 1 to 2 hours in the ED. If the bleeding still cannot be controlled, emergent consultation with vascular surgery services is required. Placing a suture at the site, or the use of a tourniquet proximal to the access site, can be used as a temporary measure until the surgeon arrives. The disadvantage of applying direct pressure is that it can cause thrombosis within the fistula or graft. However, given the alternative, this is an acceptable risk.
It is unfortunate that this case settled because it does not appear that any malpractice was committed. Vascular surgeons do not come to the ED to see functioning, nonbleeding AVFs. There was no published information explaining why the patient experienced rebleeding 10 to 12 hours after the initial event (perhaps some minor trauma precipitated it). Even if this patient had been observed in the ED for 8 hours, he would not have experienced rebleeding in the ED, but the tragic outcome would remain the same.
Fall
A 52-year-old man presented to the ED with complaints of left shoulder and left chest pain following a bicycle accident. The patient stated he had fallen from his bicycle and landed on his left side after he turned sharply to miss a speeding car. He denied head injury, loss of consciousness, or neck pain. The patient was ambulatory after the fall and had driven himself to the ED, and complained primarily of the left shoulder and left chest pain. He described the chest pain as sharp, worsening with movement and deep inspiration. The pain also was associated with mild shortness of breath. The patient denied headache, nausea, vomiting, abdominal pain, or back pain. He was otherwise in good health and on no medications.
The patient’s vital signs on presentation were normal and his head was atraumatic. He exhibited no midline posterior cervical tenderness to palpation. The head, eyes, ears, nose, and throat (HEENT) and mouth examinations were unremarkable. The patient did have tenderness to palpation over the left clavicle and left anterior chest; there was no crepitus or subcutaneous emphysema appreciated. Breath sounds were normal, and the heart had a regular rate and rhythm without murmurs, rubs, or gallops. The abdomen was soft and nontender, without guarding or rebound. The pelvis was stable, and the patient moved all four extremities with good strength. However, he did exhibit pain with movement of his left shoulder. Peripheral pulses were 2+ and symmetrical.
The emergency physician (EP) ordered an X-ray of the chest and left shoulder, as well as urinalysis. The X-rays revealed a small left pneumothorax, a minimally displaced left clavicular fracture, and fractures of the left fourth and fifth ribs. The urinalysis results were normal. The patient was administered intravenous (IV) morphine for pain and placed on 2 L/minute oxygen via nasal cannula, with 100% oxygen saturation on pulse oximetry.
The EP consulted a pulmonologist regarding management of the pneumothorax, who recommended a 4-hour observation period in the ED, followed by a repeat chest X-ray. During the observation period, the patient remained on oxygen and continued to deny any new complaints, including headache, dizziness, or abdominal pain. His vital signs remained normal throughout the entire observation period.
While in radiology services for a repeat chest X-ray, the patient fainted and struck his head on the floor. The EP immediately ordered a noncontrast computed tomography scan of the head, which demonstrated a large intracranial bleed. The patient was taken immediately to the operating room by neurosurgery. His recovery was uneventful, and he was discharged home without obvious sequelae.
The patient sued the EP and hospital for negligent care, claiming the EP underestimated the patient’s injuries and that additional testing was warranted. The defendants argued the patient was properly evaluated based on the history and physical examination. A defense verdict was returned.
Discussion
One possible criticism of this case is the consulting of a pulmonologist for the traumatic pneumothorax rather than a trauma surgeon or general surgeon. It is unclear if these specialists were not available for consult. Nevertheless, the pulmonologist’s advice to the EP was reasonable. Until just recently, it was dogma that all traumatic pneumothoraces required tube thoracostomy for management. This is still true for tension pneumothorax, hemothorax, moderate-to-large pneumothorax, symptomatic pneumothorax, or if mechanical ventilation is anticipated or needed.1 For small pneumothoraces, several management options exist, including close observation, needle or catheter aspiration, or placement of a pigtail catheter—in addition to the placement of a small (ie, 10-14 French) thoracostomy tube.2
Regardless, it does not appear the pneumothorax played a role in the patient’s hospital fall. More likely, the patient experienced a vasovagal episode. Interestingly, he never required treatment for the pneumothorax, despite requiring mechanical ventilation.
A Tragic Complication of Hemodialysis
A 58-year-old man presented to the ED with the chief complaint of bleeding from his dialysis fistula. The patient had end-stage renal disease and had been on hemodialysis (HD) for the past 3 years. He had an arteriovenous fistula (AVF) in his left arm for dialysis access, and received HD 3 days per week—every Tuesday, Thursday, and Saturday. He had completed a scheduled run of dialysis 5 hours prior to presentation, but had continued to bleed intermittently from the AVF site. The patient stated he had applied pressure multiple times to the site, but was unsuccessful in stopping the bleeding. His medical history was significant for hypertension and coronary artery disease. Regarding his social history, the patient admitted to smoking one pack of cigarettes per day and consuming alcohol on a regular basis.
The patient’s vital signs at presentation were: heart rate, 98 beats/minute; blood pressure, 146/85 mm Hg; respiratory rate, 20 breaths/minute; and temperature, 98.6°F. Oxygen saturation was 96% on room air. The HEENT examination was unremarkable. Examination of the heart revealed a normal rate and regular rhythm with a grade of 1/6 systolic murmur, heard best at the left sternal border. The breath sounds were equal bilaterally and clear to auscultation; the abdominal examination was unremarkable. The patient had an AVF in his left forearm that was not actively bleeding. There was a palpable thrill and a bruit present on auscultation over the site; there was no increased warmth or drainage.
The EP ordered a complete blood count (CBC) on the patient. The hemoglobin and hematocrit levels were essentially unchanged from a previous CBC 1 month prior, and the platelet count was normal. After approximately 1 hour of observation in the ED, there was no rebleeding at the site, and the patient was discharged home.
Unfortunately, the bleeding resumed the following day. The patient went into cardiac arrest and died at home prior to arrival of emergency medical services. The patient’s family sued the EP and hospital for discharging the patient home without first obtaining a surgical consult. The EP and hospital settled the case with the family for $2 million.
Discussion
Many patients who present to the ED with bleeding from the vascular access site can be managed simply with direct pressure, typically for a minimum of 5 to 10 minutes. In more severe cases, the EP can apply direct pressure with an absorbable gelatin sponge (eg, Gelfoam). If the patient presents soon after completion of dialysis, the EP should consider heparin anticoagulation as the etiology. In such cases, the use of IV protamine should be considered. One milligram of protamine can reverse 100 units of heparin. Since typically 1,000 to 2,000 units of heparin are administered at dialysis, a dose of 10 to 20 mg of protamine IV should be sufficient to reverse bleeding.
Other strategies to control hemorrhage from the access site include the use of topical thrombin or an IV drip of desmopressin. Once bleeding has been controlled, the patient should be observed for a minimum of 1 to 2 hours in the ED. If the bleeding still cannot be controlled, emergent consultation with vascular surgery services is required. Placing a suture at the site, or the use of a tourniquet proximal to the access site, can be used as a temporary measure until the surgeon arrives. The disadvantage of applying direct pressure is that it can cause thrombosis within the fistula or graft. However, given the alternative, this is an acceptable risk.
It is unfortunate that this case settled because it does not appear that any malpractice was committed. Vascular surgeons do not come to the ED to see functioning, nonbleeding AVFs. There was no published information explaining why the patient experienced rebleeding 10 to 12 hours after the initial event (perhaps some minor trauma precipitated it). Even if this patient had been observed in the ED for 8 hours, he would not have experienced rebleeding in the ED, but the tragic outcome would remain the same.
- Fall
- Legome E. Initial evaluation and management of blunt thoracic trauma in adults. UpToDate Web site. Available at http://www.uptodate.com/contents/initial-evaluation-and-management-of-blunt-thoracic-trauma-in-adults?source=search_result&search=Initial+evaluation+and+management+of+blunt+thoracic+trauma+in+adults.&selectedTitle=1~150. Updated September 21, 2015. Accessed February 21, 2016.
- Nicks BA, Manthey D. Pneumothorax. In: Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline DM, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw Hill Education; 2016:464-468.
- A Tragic Complication of Hemodialysis
- Stolic R. Most important chronic complications of arteriovenous fistulas for hemodialysis. Med Princ Pract. 2013;22(3):220-228.
- Lutz J, Menke J, Sollinger D, Schinzel H, Thurmel K. Haemostasis in chronic kidney disease. Nephrol Dial Transplant. 2014;29(1):29-40.
- Fall
- Legome E. Initial evaluation and management of blunt thoracic trauma in adults. UpToDate Web site. Available at http://www.uptodate.com/contents/initial-evaluation-and-management-of-blunt-thoracic-trauma-in-adults?source=search_result&search=Initial+evaluation+and+management+of+blunt+thoracic+trauma+in+adults.&selectedTitle=1~150. Updated September 21, 2015. Accessed February 21, 2016.
- Nicks BA, Manthey D. Pneumothorax. In: Tintinalli JE, Stapczynski JS, Ma OJ, Yealy DM, Meckler GD, Cline DM, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 8th ed. New York, NY: McGraw Hill Education; 2016:464-468.
- A Tragic Complication of Hemodialysis
- Stolic R. Most important chronic complications of arteriovenous fistulas for hemodialysis. Med Princ Pract. 2013;22(3):220-228.
- Lutz J, Menke J, Sollinger D, Schinzel H, Thurmel K. Haemostasis in chronic kidney disease. Nephrol Dial Transplant. 2014;29(1):29-40.
Malpractice Counsel: Constipation, missing diabetes
Constipation
A 26-year-old woman presented to the ED with a chief complaint of chronic constipation. This was the patient’s fourth ED visit for the same complaint over the previous 12 days. The patient stated that, at the prior visits, she was prescribed stool softeners and instructed to increase the amount of green vegetables in her diet and to drink plenty of fluids. She further noted that although constipation had been a long-standing problem for her, the condition had become worse over the past several weeks.
The patient described some lower abdominal discomfort, but denied nausea, vomiting, fevers, or chills. She also denied any genitourinary complaints or flank pain. Her last menstrual period was 2 weeks prior and normal. Her medical history was unremarkable; she denied smoking cigarettes or drinking alcohol and had no known drug allergies.
On physical examination, the patient’s vital signs were normal and she did not appear to be in any distress. The lung and heart examinations were also normal. Her abdomen was found to be soft, with slight tenderness in the lower abdomen, but with no guarding, rebound, or distention. Bowel sounds were present and hypoactive. A rectal examination revealed minimal stool in the vault, which was heme negative.
The patient sued the EP and the hospital, stating that the enema was not only contraindicated, but also caused the colon perforation. She further alleged that the EP failed to properly diagnose the sigmoid volvulus. The defense argued that the patient suffered from an uncommon condition, and the treatment provided was appropriate given her symptoms. The defense further stated that the perforation was present prior to the administration of the enema. At trial, a defense verdict was returned.
Discussion
Sigmoid volvulus is a relatively rare cause of bowel obstruction, accounting for only 2% of intestinal obstructions in the United States between 2002 and 2010.1 The majority of cases occur in older patients (mean age, 70 years).1 Risk factors for development include a history of laxative abuse, chronic constipation, and institutionalized patients with underlying neurological or psychiatric disease. There also appears to be an increased incidence during pregnancy. When observed in the pediatric population and in young adults, sigmoid volvulus is frequently due to an underlying colonic motility disorder.
A volvulus occurs when the colon twists on its mesenteric axis with greater than 180° rotation, resulting in obstruction of the intestinal lumen and mesenteric vessels.2 The most common locations for volvulus are the sigmoid colon, followed by the cecum. Though rare, the condition can occur in other locations.
The patient in this case presented very atypically for someone with a sigmoid volvulus as the majority of patients present with progressive abdominal pain, nausea, vomiting, and constipation. On physical examination, the abdomen is frequently distended and tympanitic with diffuse tenderness. If perforation has occurred, then peritoneal signs predominate (eg, guarding, rigidity, rebound tenderness) and abnormal vital signs (eg, fever, tachycardia, hypotension) are frequently present.
While a diagnosis of sigmoid volvulus may be suspected through the history and physical examination, it is confirmed through imaging studies, with abdominal/pelvic CT being the modality of choice. On CT scan, the “whirl sign” is frequently present, representing the dilated sigmoid colon twisted around its mesocolon and vessels.3 The tightness of the whirl is proportional to the degree of torsion. If rectal contrast is administered, the “bird-beak” sign is often present, representing the afferent and efferent colonic segments.3
As with this patient, if the colon has been perforated, IV fluid resuscitation, IV antibiotics, and immediate surgery are indicated. In cases in which there is no evidence of gangrene or perforation, sigmoidoscopy can be attempted to detorse the twisted bowel segment. This technique is successful in correcting torsion in the majority of cases. However, if detorsion attempts fail, emergent surgery is indicated.
Even when nonsurgical detorsion is successful, controversy exists over its use as the sole treatment for sigmoid volvulus. Due to a 50% to 60% chance of recurrent sigmoid volvulus, some experts recommend surgery immediately following detorsion, while others advise a wait-and-see approach.
The risk of complications from administering a soapsuds enema to an immunocompetent ED patient without signs or symptoms of peritonitis is exceedingly low. While no good data exist on the rate of complications from enemas administered for constipation, perforation of the bowel from barium enemas occurs in only 0.02% to 0.04% of patients undergoing radiologic imaging.4 The jury appears to have come to the proper conclusion in this atypical presentation of an uncommon condition with a rare complication.
Missed Diabetes Mellitus
A 27-year-old man presented to the ED with a 3-day history of severe abdominal pain, nausea and vomiting. The patient denied fevers, chills, or diarrhea, as well as any sick contacts. The patient stated he was otherwise in good health, on no medications, and had no known drug allergies. He denied alcohol or tobacco use.
His vital signs at presentation were: temperature, 98.6°F; pulse, 116 beats/minute; blood pressure, 152/92 mm Hg; and respiratory rate, 24 breaths/minute. Oxygen saturation was 100% on room air. On head, eyes, ears, nose, and throat examination, the patient’s mucous membranes were noted to be dry. The lung examination revealed bilateral breath sounds clear to auscultation. The heart examination was remarkable for tachycardia, but the rhythm was regular and with no murmurs, rubs, or gallops. The abdomen was soft with slight diffuse tenderness, but no guarding, rebound, or masses.
The EP ordered 1 L normal saline IV and ondansetron 4 mg IV for the nausea and vomiting. No laboratory or imaging studies were ordered.
On reexamination approximately 1 hour later, the patient denied any abdominal pain and stated he felt improved and was no longer nauseous. The abdominal examination remained unchanged. The patient was discharged home with a prescription for ondansetron and instructed to return to the ED if his symptoms did not improve within the next 12 hours.
The patient did not return to the ED, but was found dead at home 3 days later. An autopsy revealed the patient died from metabolic consequences of diabetes mellitus (DM). The plaintiff’s family argued the standard of care required a complete set of laboratory studies, the results of which would have revealed the hyperglycemia, prompting further evaluation and treatment. The defense contended the standard of care did not require laboratory evaluation since the patient responded well to the IV fluids and ondansetron, reported an improvement in pain and nausea, and had no history of DM. At trial, a defense verdict was returned.
Discussion
Emergency physicians are well versed in diagnosing and treating DM and its complications. Typical symptoms of new-onset diabetes include polyuria, polydipsia, abdominal pain, nausea, vomiting, and lack of energy. Occasionally, the patient will present with more severe symptoms (eg, altered mental status) when diabetic ketoacidosis is the initial presentation of the disease. It is unclear from the medical records in this case whether additional history, such as polyuria, was obtained. If so, and the answers were in the affirmative, this information might have led the EP to order laboratory studies. Similarly, we do not know how many episodes of emesis the patient experienced—eg, only one to two episodes of emesis or more than 10. It is important to have an appreciation of the severity of the presenting symptoms.
Emergency physicians frequently diagnose and manage patients appropriately without ordering laboratory or imaging studies. Acute asthma attacks, migraine headaches, bronchitis, sprains, and upper respiratory tract infections are just a few examples of the many conditions that are frequently managed by EPs based solely on history and physical examination. However, it is important the EP take a thorough enough history and physical examination to ensure confidence in excluding more severe disease processes. The severity of the symptoms must also be considered in the decision to order laboratory or other evaluation.
In this day and age of point-of-care testing, one should consider checking the glucose and electrolytes in patients with symptoms consistent with fluid loss (ie, vomiting, diarrhea, decreased oral intake).
A Note about Diabetes Mellitus
Emergency physicians should be aware of the increasing incidence of DM in the United States and around the world. The global prevalence of diabetes in adults in 2013 was reportedly 8.3% (382 million people), with 14 million more men than women diagnosed with the disease.1
Broadly defined, diabetes is a group of metabolic diseases characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin action, or both.1 Type 1 DM constitutes approximately 5% to 10% of patients diagnosed with diabetes and is due to the destruction of beta cells in the pancreas.1 It accounts for approximately 80% to 90% of DM in children and adolescents, and is thought to be present in approximately 3 million patients in the United States in 2010.1 Type 2 DM is the most common form, with 90% to 95% of patients belonging to this category, most of whom are adults. The problem in type 2 DM is primarily insulin resistance, as opposed to a lack of insulin. Obesity is the most common cause of insulin resistance in type 2 DM.1
- Constipation
- Halabi WJ, Jafari MD, Kang CY, et al. Colonic volvulus in the United States: trends, outcomes, and predictors of mortality. Ann Surg. 2014;259(2):293-301.
- Weingrow D, McCague A, Shah R, Lalezarzadeh F. Delayed presentation of sigmoid volvulus in a young woman. West J Emerg Med. 2012;13(1):100-102.
- Catalano O. Computed tomographic appearance of sigmoid volvulus. Abdom Imaging. 1996;21(4):314-317.
- Williams SM, Harned RK. Recognition and prevention of barium enema complications. Curr Probl Diagn Radiol. 1991;20(4):123-151.
- Missed Diabetes Mellitus
- Kharroubi AT, Darwish HM. Diabetes mellitus: the epidemic of the century. World J Diabetes. 2015;6(6):850-867.
Constipation
A 26-year-old woman presented to the ED with a chief complaint of chronic constipation. This was the patient’s fourth ED visit for the same complaint over the previous 12 days. The patient stated that, at the prior visits, she was prescribed stool softeners and instructed to increase the amount of green vegetables in her diet and to drink plenty of fluids. She further noted that although constipation had been a long-standing problem for her, the condition had become worse over the past several weeks.
The patient described some lower abdominal discomfort, but denied nausea, vomiting, fevers, or chills. She also denied any genitourinary complaints or flank pain. Her last menstrual period was 2 weeks prior and normal. Her medical history was unremarkable; she denied smoking cigarettes or drinking alcohol and had no known drug allergies.
On physical examination, the patient’s vital signs were normal and she did not appear to be in any distress. The lung and heart examinations were also normal. Her abdomen was found to be soft, with slight tenderness in the lower abdomen, but with no guarding, rebound, or distention. Bowel sounds were present and hypoactive. A rectal examination revealed minimal stool in the vault, which was heme negative.
The patient sued the EP and the hospital, stating that the enema was not only contraindicated, but also caused the colon perforation. She further alleged that the EP failed to properly diagnose the sigmoid volvulus. The defense argued that the patient suffered from an uncommon condition, and the treatment provided was appropriate given her symptoms. The defense further stated that the perforation was present prior to the administration of the enema. At trial, a defense verdict was returned.
Discussion
Sigmoid volvulus is a relatively rare cause of bowel obstruction, accounting for only 2% of intestinal obstructions in the United States between 2002 and 2010.1 The majority of cases occur in older patients (mean age, 70 years).1 Risk factors for development include a history of laxative abuse, chronic constipation, and institutionalized patients with underlying neurological or psychiatric disease. There also appears to be an increased incidence during pregnancy. When observed in the pediatric population and in young adults, sigmoid volvulus is frequently due to an underlying colonic motility disorder.
A volvulus occurs when the colon twists on its mesenteric axis with greater than 180° rotation, resulting in obstruction of the intestinal lumen and mesenteric vessels.2 The most common locations for volvulus are the sigmoid colon, followed by the cecum. Though rare, the condition can occur in other locations.
The patient in this case presented very atypically for someone with a sigmoid volvulus as the majority of patients present with progressive abdominal pain, nausea, vomiting, and constipation. On physical examination, the abdomen is frequently distended and tympanitic with diffuse tenderness. If perforation has occurred, then peritoneal signs predominate (eg, guarding, rigidity, rebound tenderness) and abnormal vital signs (eg, fever, tachycardia, hypotension) are frequently present.
While a diagnosis of sigmoid volvulus may be suspected through the history and physical examination, it is confirmed through imaging studies, with abdominal/pelvic CT being the modality of choice. On CT scan, the “whirl sign” is frequently present, representing the dilated sigmoid colon twisted around its mesocolon and vessels.3 The tightness of the whirl is proportional to the degree of torsion. If rectal contrast is administered, the “bird-beak” sign is often present, representing the afferent and efferent colonic segments.3
As with this patient, if the colon has been perforated, IV fluid resuscitation, IV antibiotics, and immediate surgery are indicated. In cases in which there is no evidence of gangrene or perforation, sigmoidoscopy can be attempted to detorse the twisted bowel segment. This technique is successful in correcting torsion in the majority of cases. However, if detorsion attempts fail, emergent surgery is indicated.
Even when nonsurgical detorsion is successful, controversy exists over its use as the sole treatment for sigmoid volvulus. Due to a 50% to 60% chance of recurrent sigmoid volvulus, some experts recommend surgery immediately following detorsion, while others advise a wait-and-see approach.
The risk of complications from administering a soapsuds enema to an immunocompetent ED patient without signs or symptoms of peritonitis is exceedingly low. While no good data exist on the rate of complications from enemas administered for constipation, perforation of the bowel from barium enemas occurs in only 0.02% to 0.04% of patients undergoing radiologic imaging.4 The jury appears to have come to the proper conclusion in this atypical presentation of an uncommon condition with a rare complication.
Missed Diabetes Mellitus
A 27-year-old man presented to the ED with a 3-day history of severe abdominal pain, nausea and vomiting. The patient denied fevers, chills, or diarrhea, as well as any sick contacts. The patient stated he was otherwise in good health, on no medications, and had no known drug allergies. He denied alcohol or tobacco use.
His vital signs at presentation were: temperature, 98.6°F; pulse, 116 beats/minute; blood pressure, 152/92 mm Hg; and respiratory rate, 24 breaths/minute. Oxygen saturation was 100% on room air. On head, eyes, ears, nose, and throat examination, the patient’s mucous membranes were noted to be dry. The lung examination revealed bilateral breath sounds clear to auscultation. The heart examination was remarkable for tachycardia, but the rhythm was regular and with no murmurs, rubs, or gallops. The abdomen was soft with slight diffuse tenderness, but no guarding, rebound, or masses.
The EP ordered 1 L normal saline IV and ondansetron 4 mg IV for the nausea and vomiting. No laboratory or imaging studies were ordered.
On reexamination approximately 1 hour later, the patient denied any abdominal pain and stated he felt improved and was no longer nauseous. The abdominal examination remained unchanged. The patient was discharged home with a prescription for ondansetron and instructed to return to the ED if his symptoms did not improve within the next 12 hours.
The patient did not return to the ED, but was found dead at home 3 days later. An autopsy revealed the patient died from metabolic consequences of diabetes mellitus (DM). The plaintiff’s family argued the standard of care required a complete set of laboratory studies, the results of which would have revealed the hyperglycemia, prompting further evaluation and treatment. The defense contended the standard of care did not require laboratory evaluation since the patient responded well to the IV fluids and ondansetron, reported an improvement in pain and nausea, and had no history of DM. At trial, a defense verdict was returned.
Discussion
Emergency physicians are well versed in diagnosing and treating DM and its complications. Typical symptoms of new-onset diabetes include polyuria, polydipsia, abdominal pain, nausea, vomiting, and lack of energy. Occasionally, the patient will present with more severe symptoms (eg, altered mental status) when diabetic ketoacidosis is the initial presentation of the disease. It is unclear from the medical records in this case whether additional history, such as polyuria, was obtained. If so, and the answers were in the affirmative, this information might have led the EP to order laboratory studies. Similarly, we do not know how many episodes of emesis the patient experienced—eg, only one to two episodes of emesis or more than 10. It is important to have an appreciation of the severity of the presenting symptoms.
Emergency physicians frequently diagnose and manage patients appropriately without ordering laboratory or imaging studies. Acute asthma attacks, migraine headaches, bronchitis, sprains, and upper respiratory tract infections are just a few examples of the many conditions that are frequently managed by EPs based solely on history and physical examination. However, it is important the EP take a thorough enough history and physical examination to ensure confidence in excluding more severe disease processes. The severity of the symptoms must also be considered in the decision to order laboratory or other evaluation.
In this day and age of point-of-care testing, one should consider checking the glucose and electrolytes in patients with symptoms consistent with fluid loss (ie, vomiting, diarrhea, decreased oral intake).
A Note about Diabetes Mellitus
Emergency physicians should be aware of the increasing incidence of DM in the United States and around the world. The global prevalence of diabetes in adults in 2013 was reportedly 8.3% (382 million people), with 14 million more men than women diagnosed with the disease.1
Broadly defined, diabetes is a group of metabolic diseases characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin action, or both.1 Type 1 DM constitutes approximately 5% to 10% of patients diagnosed with diabetes and is due to the destruction of beta cells in the pancreas.1 It accounts for approximately 80% to 90% of DM in children and adolescents, and is thought to be present in approximately 3 million patients in the United States in 2010.1 Type 2 DM is the most common form, with 90% to 95% of patients belonging to this category, most of whom are adults. The problem in type 2 DM is primarily insulin resistance, as opposed to a lack of insulin. Obesity is the most common cause of insulin resistance in type 2 DM.1
Constipation
A 26-year-old woman presented to the ED with a chief complaint of chronic constipation. This was the patient’s fourth ED visit for the same complaint over the previous 12 days. The patient stated that, at the prior visits, she was prescribed stool softeners and instructed to increase the amount of green vegetables in her diet and to drink plenty of fluids. She further noted that although constipation had been a long-standing problem for her, the condition had become worse over the past several weeks.
The patient described some lower abdominal discomfort, but denied nausea, vomiting, fevers, or chills. She also denied any genitourinary complaints or flank pain. Her last menstrual period was 2 weeks prior and normal. Her medical history was unremarkable; she denied smoking cigarettes or drinking alcohol and had no known drug allergies.
On physical examination, the patient’s vital signs were normal and she did not appear to be in any distress. The lung and heart examinations were also normal. Her abdomen was found to be soft, with slight tenderness in the lower abdomen, but with no guarding, rebound, or distention. Bowel sounds were present and hypoactive. A rectal examination revealed minimal stool in the vault, which was heme negative.
The patient sued the EP and the hospital, stating that the enema was not only contraindicated, but also caused the colon perforation. She further alleged that the EP failed to properly diagnose the sigmoid volvulus. The defense argued that the patient suffered from an uncommon condition, and the treatment provided was appropriate given her symptoms. The defense further stated that the perforation was present prior to the administration of the enema. At trial, a defense verdict was returned.
Discussion
Sigmoid volvulus is a relatively rare cause of bowel obstruction, accounting for only 2% of intestinal obstructions in the United States between 2002 and 2010.1 The majority of cases occur in older patients (mean age, 70 years).1 Risk factors for development include a history of laxative abuse, chronic constipation, and institutionalized patients with underlying neurological or psychiatric disease. There also appears to be an increased incidence during pregnancy. When observed in the pediatric population and in young adults, sigmoid volvulus is frequently due to an underlying colonic motility disorder.
A volvulus occurs when the colon twists on its mesenteric axis with greater than 180° rotation, resulting in obstruction of the intestinal lumen and mesenteric vessels.2 The most common locations for volvulus are the sigmoid colon, followed by the cecum. Though rare, the condition can occur in other locations.
The patient in this case presented very atypically for someone with a sigmoid volvulus as the majority of patients present with progressive abdominal pain, nausea, vomiting, and constipation. On physical examination, the abdomen is frequently distended and tympanitic with diffuse tenderness. If perforation has occurred, then peritoneal signs predominate (eg, guarding, rigidity, rebound tenderness) and abnormal vital signs (eg, fever, tachycardia, hypotension) are frequently present.
While a diagnosis of sigmoid volvulus may be suspected through the history and physical examination, it is confirmed through imaging studies, with abdominal/pelvic CT being the modality of choice. On CT scan, the “whirl sign” is frequently present, representing the dilated sigmoid colon twisted around its mesocolon and vessels.3 The tightness of the whirl is proportional to the degree of torsion. If rectal contrast is administered, the “bird-beak” sign is often present, representing the afferent and efferent colonic segments.3
As with this patient, if the colon has been perforated, IV fluid resuscitation, IV antibiotics, and immediate surgery are indicated. In cases in which there is no evidence of gangrene or perforation, sigmoidoscopy can be attempted to detorse the twisted bowel segment. This technique is successful in correcting torsion in the majority of cases. However, if detorsion attempts fail, emergent surgery is indicated.
Even when nonsurgical detorsion is successful, controversy exists over its use as the sole treatment for sigmoid volvulus. Due to a 50% to 60% chance of recurrent sigmoid volvulus, some experts recommend surgery immediately following detorsion, while others advise a wait-and-see approach.
The risk of complications from administering a soapsuds enema to an immunocompetent ED patient without signs or symptoms of peritonitis is exceedingly low. While no good data exist on the rate of complications from enemas administered for constipation, perforation of the bowel from barium enemas occurs in only 0.02% to 0.04% of patients undergoing radiologic imaging.4 The jury appears to have come to the proper conclusion in this atypical presentation of an uncommon condition with a rare complication.
Missed Diabetes Mellitus
A 27-year-old man presented to the ED with a 3-day history of severe abdominal pain, nausea and vomiting. The patient denied fevers, chills, or diarrhea, as well as any sick contacts. The patient stated he was otherwise in good health, on no medications, and had no known drug allergies. He denied alcohol or tobacco use.
His vital signs at presentation were: temperature, 98.6°F; pulse, 116 beats/minute; blood pressure, 152/92 mm Hg; and respiratory rate, 24 breaths/minute. Oxygen saturation was 100% on room air. On head, eyes, ears, nose, and throat examination, the patient’s mucous membranes were noted to be dry. The lung examination revealed bilateral breath sounds clear to auscultation. The heart examination was remarkable for tachycardia, but the rhythm was regular and with no murmurs, rubs, or gallops. The abdomen was soft with slight diffuse tenderness, but no guarding, rebound, or masses.
The EP ordered 1 L normal saline IV and ondansetron 4 mg IV for the nausea and vomiting. No laboratory or imaging studies were ordered.
On reexamination approximately 1 hour later, the patient denied any abdominal pain and stated he felt improved and was no longer nauseous. The abdominal examination remained unchanged. The patient was discharged home with a prescription for ondansetron and instructed to return to the ED if his symptoms did not improve within the next 12 hours.
The patient did not return to the ED, but was found dead at home 3 days later. An autopsy revealed the patient died from metabolic consequences of diabetes mellitus (DM). The plaintiff’s family argued the standard of care required a complete set of laboratory studies, the results of which would have revealed the hyperglycemia, prompting further evaluation and treatment. The defense contended the standard of care did not require laboratory evaluation since the patient responded well to the IV fluids and ondansetron, reported an improvement in pain and nausea, and had no history of DM. At trial, a defense verdict was returned.
Discussion
Emergency physicians are well versed in diagnosing and treating DM and its complications. Typical symptoms of new-onset diabetes include polyuria, polydipsia, abdominal pain, nausea, vomiting, and lack of energy. Occasionally, the patient will present with more severe symptoms (eg, altered mental status) when diabetic ketoacidosis is the initial presentation of the disease. It is unclear from the medical records in this case whether additional history, such as polyuria, was obtained. If so, and the answers were in the affirmative, this information might have led the EP to order laboratory studies. Similarly, we do not know how many episodes of emesis the patient experienced—eg, only one to two episodes of emesis or more than 10. It is important to have an appreciation of the severity of the presenting symptoms.
Emergency physicians frequently diagnose and manage patients appropriately without ordering laboratory or imaging studies. Acute asthma attacks, migraine headaches, bronchitis, sprains, and upper respiratory tract infections are just a few examples of the many conditions that are frequently managed by EPs based solely on history and physical examination. However, it is important the EP take a thorough enough history and physical examination to ensure confidence in excluding more severe disease processes. The severity of the symptoms must also be considered in the decision to order laboratory or other evaluation.
In this day and age of point-of-care testing, one should consider checking the glucose and electrolytes in patients with symptoms consistent with fluid loss (ie, vomiting, diarrhea, decreased oral intake).
A Note about Diabetes Mellitus
Emergency physicians should be aware of the increasing incidence of DM in the United States and around the world. The global prevalence of diabetes in adults in 2013 was reportedly 8.3% (382 million people), with 14 million more men than women diagnosed with the disease.1
Broadly defined, diabetes is a group of metabolic diseases characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin action, or both.1 Type 1 DM constitutes approximately 5% to 10% of patients diagnosed with diabetes and is due to the destruction of beta cells in the pancreas.1 It accounts for approximately 80% to 90% of DM in children and adolescents, and is thought to be present in approximately 3 million patients in the United States in 2010.1 Type 2 DM is the most common form, with 90% to 95% of patients belonging to this category, most of whom are adults. The problem in type 2 DM is primarily insulin resistance, as opposed to a lack of insulin. Obesity is the most common cause of insulin resistance in type 2 DM.1
- Constipation
- Halabi WJ, Jafari MD, Kang CY, et al. Colonic volvulus in the United States: trends, outcomes, and predictors of mortality. Ann Surg. 2014;259(2):293-301.
- Weingrow D, McCague A, Shah R, Lalezarzadeh F. Delayed presentation of sigmoid volvulus in a young woman. West J Emerg Med. 2012;13(1):100-102.
- Catalano O. Computed tomographic appearance of sigmoid volvulus. Abdom Imaging. 1996;21(4):314-317.
- Williams SM, Harned RK. Recognition and prevention of barium enema complications. Curr Probl Diagn Radiol. 1991;20(4):123-151.
- Missed Diabetes Mellitus
- Kharroubi AT, Darwish HM. Diabetes mellitus: the epidemic of the century. World J Diabetes. 2015;6(6):850-867.
- Constipation
- Halabi WJ, Jafari MD, Kang CY, et al. Colonic volvulus in the United States: trends, outcomes, and predictors of mortality. Ann Surg. 2014;259(2):293-301.
- Weingrow D, McCague A, Shah R, Lalezarzadeh F. Delayed presentation of sigmoid volvulus in a young woman. West J Emerg Med. 2012;13(1):100-102.
- Catalano O. Computed tomographic appearance of sigmoid volvulus. Abdom Imaging. 1996;21(4):314-317.
- Williams SM, Harned RK. Recognition and prevention of barium enema complications. Curr Probl Diagn Radiol. 1991;20(4):123-151.
- Missed Diabetes Mellitus
- Kharroubi AT, Darwish HM. Diabetes mellitus: the epidemic of the century. World J Diabetes. 2015;6(6):850-867.
Malpractice Counsel: Allergic Reaction Versus Anaphylaxis
Allergic Reaction Versus Anaphylaxis
A 34-year-old woman presented to ED with complaints of an allergic reaction, the onset of which began approximately 1 hour prior. The patient did not know what might have caused her symptoms. She complained of hives and itching all over; she denied difficulty swallowing, wheezing, and shortness of breath. Her medical history was unremarkable. She was on no medications, and she denied any alcohol or tobacco use. She had no known medication or food allergies.
Physical examination revealed a woman in mild discomfort, secondary to generalized itching. Her vital signs, including pulse oximetry, were normal. There was no swelling of the face, lips, or oropharynx. The lungs were clear to auscultation bilaterally. The heart and abdominal examinations were normal. Examination of the skin revealed diffuse urticaria without petechiae or purpura.
The emergency physician (EP) ordered 125 mg of methylprednisolone sodium succinate and 25 mg of diphenhydramine intravenously (IV). After approximately 1 hour, the hives and itching decreased and the patient felt improved. She was diagnosed with an allergic reaction and discharged home with a prescription for diphenhydramine and a methylprednisolone dose pack.
The following day, the patient collapsed at home and emergency medical services was called. Unfortunately, the patient could not be resuscitated and was pronounced dead at the scene. An autopsy revealed the patient had died from anaphylaxis and laryngeal edema, with an extremely elevated tryptase level of 200 ng/mL (normal, <11.5 ng/mL).
The patient’s family sued the EP for failure to diagnose and treat anaphylaxis, failure to treat with epinephrine, and failure to admit the patient to the hospital. The defense claimed the patient did not present with anaphylaxis, but rather simply a worsening of the hives and angioedema, and that the treatment provided was appropriate. The jury found in favor of the defendants.
Discussion
It does not appear the patient presented with anaphylaxis on the first visit, but may have had it on the second visit. In 2004, the National Institutes of Allergy and Infectious Disease (NIAID) panel and the Food Allergy and Anaphylaxis Network (FAAN) developed criteria for the diagnosis of anaphylaxis.1 According to the criteria, anaphylaxis is likely when any one of the following three criteria are present: (1) acute onset of symptoms involving the skin or mucosa (eg, pruritus, hives, angioedema), and either respiratory compromise (eg, dyspnea, wheezing, stridor, hypoxia) or hypotension/end-organ dysfunction (eg, syncope, incontinence); (2) two or more symptoms (eg, respiratory compromise, hypotension/end-organ dysfunction, persistent gastrointestinal [GI] symptoms such as vomiting, diarrhea, or crampy abdominal pain) that occur rapidly after exposure involving the skin or mucosa; or (3) hypotension from a known allergen to the patient. The accuracy of these criteria has been retrospectively evaluated in an ED study, and found to have a 97% sensitivity and an 82% specificity.2 The negative predictive value was good at 98%, but the positive predictive value was only 69%.2
When a patient presents with minimal or subtle symptoms, anaphylaxis can be a very difficult diagnosis to make in the ED early on in the process. While no EP will miss the diagnosis in a patient with hives, hypotension, and wheezing, it can be easy to miss when the predominant symptoms are GI, such as nausea, vomiting, or diarrhea. In addition, the differential diagnosis for the presentation of anaphylaxis in the ED can be extremely broad and include vasovagal reaction, asthma attack, myocardial infarction, gastroenteritis, panic attack, or airway obstruction.
Due to the nature of emergency medicine, EPs must consider multiple etiologies before determining an evaluation and management plan. While recognizing there are limitations to the NIAID/FAAN criteria, EPs should be aware of them. We are very good at treating these types of symptoms with antihistamines and steroids; however, we frequently fail to give epinephrine when indicated. It is important to remember that epinephrine is the first-line treatment for anaphylaxis—not corticosteroids or antihistamines.3
Reasons for not administering epinephrine are multiple. First, as discussed above, if the diagnosis of anaphylaxis is not considered, the EP is not going to administer the drug of choice. Secondly, EPs have been taught to have a healthy respect for epinephrine and its effects, especially in older patients. Due to this cautious approach, epinephrine is frequently not given to patients with mild symptoms or to those who present early in the course of disease.
Emergency physicians have experience giving epinephrine subcutaneously, but not nearly as much with the intramuscular (IM) route. This is important, because an IM injection in the anterolateral thigh is the recommended location for the treatment of anaphylaxis. The dose should be weight based (0.01 mg/kg) to a maximum of 0.5 mg. This dose can be given every 5 to 15 minutes as necessary to control symptoms.3 The dosing is important to remember, since many EDs stock only autoinjectable epinephrine devices for use in anaphylaxis. These autoinjectors only contain 0.3 mg of epinephrine, so some patients may be underdosed if used.
In the management of allergic reactions and anaphylaxis, EPs frequently administer antihistamines and corticosteroids. While there is no direct evidence to support their use in the management of anaphylaxis, theoretical benefits do exist.3 This, combined with the excellent medication safety profile and lack of serious side effects, make these two medication classes appropriate for use in the ED.
- Allergic Reaction Versus Anaphylaxis
- Manivannan V, Decker WW, Stead LG, Li JT, Campbell RL. National Institute of Allergy and Infectious Disease and Food Allergy and Anaphylaxis Network criteria for anaphylaxis. Int J Emerg Med. 2009;2(1):3-5.
- Campbell RL, Hagan JB, Manivannan V, et al. Evaluation of National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network criteria for the diagnosis of anaphylaxis in emergency department patients. J Allergy Clin Immunol. 2012;129(3):748-752.
- Campbell RL, Li JT, Nicklas RA, Sadosty AT; Members of the Joint Task Force; Practice Parameter Workgroup. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol. 2014;113(6):599-608.
Allergic Reaction Versus Anaphylaxis
A 34-year-old woman presented to ED with complaints of an allergic reaction, the onset of which began approximately 1 hour prior. The patient did not know what might have caused her symptoms. She complained of hives and itching all over; she denied difficulty swallowing, wheezing, and shortness of breath. Her medical history was unremarkable. She was on no medications, and she denied any alcohol or tobacco use. She had no known medication or food allergies.
Physical examination revealed a woman in mild discomfort, secondary to generalized itching. Her vital signs, including pulse oximetry, were normal. There was no swelling of the face, lips, or oropharynx. The lungs were clear to auscultation bilaterally. The heart and abdominal examinations were normal. Examination of the skin revealed diffuse urticaria without petechiae or purpura.
The emergency physician (EP) ordered 125 mg of methylprednisolone sodium succinate and 25 mg of diphenhydramine intravenously (IV). After approximately 1 hour, the hives and itching decreased and the patient felt improved. She was diagnosed with an allergic reaction and discharged home with a prescription for diphenhydramine and a methylprednisolone dose pack.
The following day, the patient collapsed at home and emergency medical services was called. Unfortunately, the patient could not be resuscitated and was pronounced dead at the scene. An autopsy revealed the patient had died from anaphylaxis and laryngeal edema, with an extremely elevated tryptase level of 200 ng/mL (normal, <11.5 ng/mL).
The patient’s family sued the EP for failure to diagnose and treat anaphylaxis, failure to treat with epinephrine, and failure to admit the patient to the hospital. The defense claimed the patient did not present with anaphylaxis, but rather simply a worsening of the hives and angioedema, and that the treatment provided was appropriate. The jury found in favor of the defendants.
Discussion
It does not appear the patient presented with anaphylaxis on the first visit, but may have had it on the second visit. In 2004, the National Institutes of Allergy and Infectious Disease (NIAID) panel and the Food Allergy and Anaphylaxis Network (FAAN) developed criteria for the diagnosis of anaphylaxis.1 According to the criteria, anaphylaxis is likely when any one of the following three criteria are present: (1) acute onset of symptoms involving the skin or mucosa (eg, pruritus, hives, angioedema), and either respiratory compromise (eg, dyspnea, wheezing, stridor, hypoxia) or hypotension/end-organ dysfunction (eg, syncope, incontinence); (2) two or more symptoms (eg, respiratory compromise, hypotension/end-organ dysfunction, persistent gastrointestinal [GI] symptoms such as vomiting, diarrhea, or crampy abdominal pain) that occur rapidly after exposure involving the skin or mucosa; or (3) hypotension from a known allergen to the patient. The accuracy of these criteria has been retrospectively evaluated in an ED study, and found to have a 97% sensitivity and an 82% specificity.2 The negative predictive value was good at 98%, but the positive predictive value was only 69%.2
When a patient presents with minimal or subtle symptoms, anaphylaxis can be a very difficult diagnosis to make in the ED early on in the process. While no EP will miss the diagnosis in a patient with hives, hypotension, and wheezing, it can be easy to miss when the predominant symptoms are GI, such as nausea, vomiting, or diarrhea. In addition, the differential diagnosis for the presentation of anaphylaxis in the ED can be extremely broad and include vasovagal reaction, asthma attack, myocardial infarction, gastroenteritis, panic attack, or airway obstruction.
Due to the nature of emergency medicine, EPs must consider multiple etiologies before determining an evaluation and management plan. While recognizing there are limitations to the NIAID/FAAN criteria, EPs should be aware of them. We are very good at treating these types of symptoms with antihistamines and steroids; however, we frequently fail to give epinephrine when indicated. It is important to remember that epinephrine is the first-line treatment for anaphylaxis—not corticosteroids or antihistamines.3
Reasons for not administering epinephrine are multiple. First, as discussed above, if the diagnosis of anaphylaxis is not considered, the EP is not going to administer the drug of choice. Secondly, EPs have been taught to have a healthy respect for epinephrine and its effects, especially in older patients. Due to this cautious approach, epinephrine is frequently not given to patients with mild symptoms or to those who present early in the course of disease.
Emergency physicians have experience giving epinephrine subcutaneously, but not nearly as much with the intramuscular (IM) route. This is important, because an IM injection in the anterolateral thigh is the recommended location for the treatment of anaphylaxis. The dose should be weight based (0.01 mg/kg) to a maximum of 0.5 mg. This dose can be given every 5 to 15 minutes as necessary to control symptoms.3 The dosing is important to remember, since many EDs stock only autoinjectable epinephrine devices for use in anaphylaxis. These autoinjectors only contain 0.3 mg of epinephrine, so some patients may be underdosed if used.
In the management of allergic reactions and anaphylaxis, EPs frequently administer antihistamines and corticosteroids. While there is no direct evidence to support their use in the management of anaphylaxis, theoretical benefits do exist.3 This, combined with the excellent medication safety profile and lack of serious side effects, make these two medication classes appropriate for use in the ED.
Allergic Reaction Versus Anaphylaxis
A 34-year-old woman presented to ED with complaints of an allergic reaction, the onset of which began approximately 1 hour prior. The patient did not know what might have caused her symptoms. She complained of hives and itching all over; she denied difficulty swallowing, wheezing, and shortness of breath. Her medical history was unremarkable. She was on no medications, and she denied any alcohol or tobacco use. She had no known medication or food allergies.
Physical examination revealed a woman in mild discomfort, secondary to generalized itching. Her vital signs, including pulse oximetry, were normal. There was no swelling of the face, lips, or oropharynx. The lungs were clear to auscultation bilaterally. The heart and abdominal examinations were normal. Examination of the skin revealed diffuse urticaria without petechiae or purpura.
The emergency physician (EP) ordered 125 mg of methylprednisolone sodium succinate and 25 mg of diphenhydramine intravenously (IV). After approximately 1 hour, the hives and itching decreased and the patient felt improved. She was diagnosed with an allergic reaction and discharged home with a prescription for diphenhydramine and a methylprednisolone dose pack.
The following day, the patient collapsed at home and emergency medical services was called. Unfortunately, the patient could not be resuscitated and was pronounced dead at the scene. An autopsy revealed the patient had died from anaphylaxis and laryngeal edema, with an extremely elevated tryptase level of 200 ng/mL (normal, <11.5 ng/mL).
The patient’s family sued the EP for failure to diagnose and treat anaphylaxis, failure to treat with epinephrine, and failure to admit the patient to the hospital. The defense claimed the patient did not present with anaphylaxis, but rather simply a worsening of the hives and angioedema, and that the treatment provided was appropriate. The jury found in favor of the defendants.
Discussion
It does not appear the patient presented with anaphylaxis on the first visit, but may have had it on the second visit. In 2004, the National Institutes of Allergy and Infectious Disease (NIAID) panel and the Food Allergy and Anaphylaxis Network (FAAN) developed criteria for the diagnosis of anaphylaxis.1 According to the criteria, anaphylaxis is likely when any one of the following three criteria are present: (1) acute onset of symptoms involving the skin or mucosa (eg, pruritus, hives, angioedema), and either respiratory compromise (eg, dyspnea, wheezing, stridor, hypoxia) or hypotension/end-organ dysfunction (eg, syncope, incontinence); (2) two or more symptoms (eg, respiratory compromise, hypotension/end-organ dysfunction, persistent gastrointestinal [GI] symptoms such as vomiting, diarrhea, or crampy abdominal pain) that occur rapidly after exposure involving the skin or mucosa; or (3) hypotension from a known allergen to the patient. The accuracy of these criteria has been retrospectively evaluated in an ED study, and found to have a 97% sensitivity and an 82% specificity.2 The negative predictive value was good at 98%, but the positive predictive value was only 69%.2
When a patient presents with minimal or subtle symptoms, anaphylaxis can be a very difficult diagnosis to make in the ED early on in the process. While no EP will miss the diagnosis in a patient with hives, hypotension, and wheezing, it can be easy to miss when the predominant symptoms are GI, such as nausea, vomiting, or diarrhea. In addition, the differential diagnosis for the presentation of anaphylaxis in the ED can be extremely broad and include vasovagal reaction, asthma attack, myocardial infarction, gastroenteritis, panic attack, or airway obstruction.
Due to the nature of emergency medicine, EPs must consider multiple etiologies before determining an evaluation and management plan. While recognizing there are limitations to the NIAID/FAAN criteria, EPs should be aware of them. We are very good at treating these types of symptoms with antihistamines and steroids; however, we frequently fail to give epinephrine when indicated. It is important to remember that epinephrine is the first-line treatment for anaphylaxis—not corticosteroids or antihistamines.3
Reasons for not administering epinephrine are multiple. First, as discussed above, if the diagnosis of anaphylaxis is not considered, the EP is not going to administer the drug of choice. Secondly, EPs have been taught to have a healthy respect for epinephrine and its effects, especially in older patients. Due to this cautious approach, epinephrine is frequently not given to patients with mild symptoms or to those who present early in the course of disease.
Emergency physicians have experience giving epinephrine subcutaneously, but not nearly as much with the intramuscular (IM) route. This is important, because an IM injection in the anterolateral thigh is the recommended location for the treatment of anaphylaxis. The dose should be weight based (0.01 mg/kg) to a maximum of 0.5 mg. This dose can be given every 5 to 15 minutes as necessary to control symptoms.3 The dosing is important to remember, since many EDs stock only autoinjectable epinephrine devices for use in anaphylaxis. These autoinjectors only contain 0.3 mg of epinephrine, so some patients may be underdosed if used.
In the management of allergic reactions and anaphylaxis, EPs frequently administer antihistamines and corticosteroids. While there is no direct evidence to support their use in the management of anaphylaxis, theoretical benefits do exist.3 This, combined with the excellent medication safety profile and lack of serious side effects, make these two medication classes appropriate for use in the ED.
- Allergic Reaction Versus Anaphylaxis
- Manivannan V, Decker WW, Stead LG, Li JT, Campbell RL. National Institute of Allergy and Infectious Disease and Food Allergy and Anaphylaxis Network criteria for anaphylaxis. Int J Emerg Med. 2009;2(1):3-5.
- Campbell RL, Hagan JB, Manivannan V, et al. Evaluation of National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network criteria for the diagnosis of anaphylaxis in emergency department patients. J Allergy Clin Immunol. 2012;129(3):748-752.
- Campbell RL, Li JT, Nicklas RA, Sadosty AT; Members of the Joint Task Force; Practice Parameter Workgroup. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol. 2014;113(6):599-608.
- Allergic Reaction Versus Anaphylaxis
- Manivannan V, Decker WW, Stead LG, Li JT, Campbell RL. National Institute of Allergy and Infectious Disease and Food Allergy and Anaphylaxis Network criteria for anaphylaxis. Int J Emerg Med. 2009;2(1):3-5.
- Campbell RL, Hagan JB, Manivannan V, et al. Evaluation of National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network criteria for the diagnosis of anaphylaxis in emergency department patients. J Allergy Clin Immunol. 2012;129(3):748-752.
- Campbell RL, Li JT, Nicklas RA, Sadosty AT; Members of the Joint Task Force; Practice Parameter Workgroup. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol. 2014;113(6):599-608.
Malpractice Counsel: Necrotizing fasciitis, corneal abrasion
A Pain in the Butt
A 75-year-old man presented to the ED complaining of low back pain radiating to his right buttock and leg. There was no history of trauma or overuse. His medical history was significant for hypertension and benign prostatic hypertrophy. Regarding social history, the patient stated that he had quit smoking 20 years prior and only drank alcohol on occasion. He denied any medication allergies.
On physical examination, the patient’s vital signs were: pulse 98 beats/minute; blood pressure, 132/82 mm Hg; respiratory rate, 18 breaths/minute; and temperature, 99.8°F. The head, eyes, ears, nose, and throat examination, and pulmonary, cardiovascular, and abdominal examinations were all normal. On examination of the lower back, the patient exhibited mild tenderness of the right paraspinal lumbar muscles without midline tenderness. He had normal and equal strength of the lower extremities bilaterally. The patient’s buttocks were not examined during this visit.
The emergency physician (EP) felt the patient’s history and physical examination were consistent with a lumbar radiculopathy, and contacted the patient’s primary care physician (PCP) to communicate his impression. Although the PCP was aware that his patient had recently complained of chills, night sweats, and pain radiating from the hip to the anus, he did not communicate this information to the EP. Unfortunately, the patient also did not relate these complaints to the EP. After the EP’s consultation with the PCP, the patient was prescribed an analgesic, referred to an orthopedic surgeon, and discharged home.
Two days later, the patient returned to the same ED via emergency medical services, this time with altered mental status, fever, and hypotension. He was found to have necrotizing fasciitis of the right buttock. The patient received intravenous fluid resuscitation and broad-spectrum antibiotics, and was taken immediately to the operating room. Unfortunately, he did not survive.
The patient’s estate sued the first EP for failure to visualize and palpate the buttocks area and for failure to diagnose an infection of the right buttocks. The plaintiff further argued that the patient would have survived if he had been properly diagnosed and treated at the initial ED visit. The defense argued the patient received a thorough history and physical examination and that the diagnosis of lumbar radiculopathy was consistent with the patient’s clinical presentation. The defense also argued that the patient never reported any complaints or indication of a buttock infection. The case was settled prior to trial for nearly $600,000.
Discussion
The second important point, examining the area of primary complaint, may sound simple, straightforward, and just plain common sense. However, this can be very challenging in the reality of today’s practice of emergency medicine as EPs are often called upon to perform entire histories and physical examinations on patients in hallway beds. Gone are the days when every patient was undressed and placed in a gown in a private area or room. With the emphasis on maintaining patients with relatively minor complaints “vertical” to improve throughput times, the EP is frequently forced to examine such patients fully clothed. This places the EP at high risk for misdiagnosis or a missed diagnosis. In the same study by Kachalia et al,1 failure to perform an adequate medical history or physical examination accounted for 42% of all diagnostic errors in the ED. Recognizing the challenge of examining the point of complaint is easy—solving the issue, however, will take all of a hospital’s resources.
Stealing Is Bad for Your Health
A 19-year-old man presented to the ED complaining of left eye pain, foreign body sensation, and tearing. He stated that when he was cleaning his garage approximately 3 hours prior to presentation, a cardboard box had fallen on his head, scattering its contents. Immediately after this occurrence, the patient experienced onset of a foreign body sensation and pain in his left eye. He further noted that he had attempted to irrigate his left eye with tap water, but it did not relieve the pain. After several hours of discomfort, the patient decided to come to the ED. He denied any other injuries or complaints, was in good health, on no medications, and had no known medication allergies. He also denied wearing contacts or corrective lenses.
On physical examination, the patient’s left eye was closed, and he appeared to be somewhat uncomfortable. His vital signs were all normal. His visual acuity was 20/20 in the right eye and 20/60 in the left eye, which was due to tearing and blurriness from the injury. To facilitate the examination, the EP placed two drops of proparacaine hydrochloride ophthalmic solution 0.5% in both of the patient’s eyes, which provided immediate and near total relief of the left eye pain. On fluorescein and slit-lamp examination, the EP found a small foreign body at approximately the seven o’clock position on the cornea, as well as a surrounding abrasion. The corneal anterior chamber was normal. The EP removed the foreign body, prescribed a topical ophthalmic antibiotic cream. and instructed the patient to return to the ED in 24 hours for a recheck. The EP also instructed the patient to take ibuprofen for any associated pain.
While waiting to be discharged, the patient took the vial of proparacaine that had been left on the counter in the room. He did so without informing anyone and without permission. After the nurse returned to the room and gave the patient his discharge instructions, he was released home. Neither the nurse nor the physician realized the patient had stolen the topical anesthetic.
The patient sued the EP for leaving the “miracle” pain-relief medication in the room and thereby facilitating his actions. The plaintiff argued that the immediate and complete pain-relief effects of the medication were too tempting for a nonmedical person to resist, and that the drug should not have been made available to him under any circumstance. The EP argued the patient stole the medication and that any resulting injury was completely the result of the patient’s own actions. The suit was eventually dropped.
Discussion
It is unfortunate that this ever became a malpractice case, since it is clear to any layman that the stealing of medications is inappropriate and illegal; moreover, any damages resulting from that action are completely the responsibility of the transgressor. With that said, a significant amount of time, discomfort, and inconvenience could have been avoided had the nurse or physician simply removed the medication from the room. While I certainly do not think this is required from a medical-legal standpoint, it is a good common-sense practice.
Corneal abrasions and corneal foreign bodies are very uncomfortable, and medications such as nonsteroidal anti-inflammatory drugs or acetaminophen only provide partial pain relief. It is easy to see how a young person, lacking any medical knowledge or the concepts of adverse side effects or toxicity, would be tempted in such a situation. In retrospect, it would probably have been best to have avoided leaving the medication within the patient’s reach in the first place.
With respect to evaluating and treating corneal abrasions, the most commonly used topical ophthalmic anesthetics are proparacaine and tetracaine. When used appropriately and in the hands of trained healthcare workers, these drugs are safe, effective, and exhibit nearly no side effects. However, as this case demonstrates, these drugs can be toxic when abused. The most common toxicities are to the ocular surface and include superficial punctate keratitis, persistent epithelial defects, stromal infiltrates, and corneal edema. More serious injuries can also occur, such as deep corneal infiltrates, ulceration, and eye perforation.1 The toxic effects associated with these drugs are the reason these medications are never prescribed for home use.
- A Pain in the Butt
- Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49(2):196-205.
- Stealing Is Bad for Your Health
- McGee HT, Fraunfelder FW. Toxicities of topical ophthalmic anesthetics. Expert Opin Drug Saf. 2007;5(6):637-640.
A Pain in the Butt
A 75-year-old man presented to the ED complaining of low back pain radiating to his right buttock and leg. There was no history of trauma or overuse. His medical history was significant for hypertension and benign prostatic hypertrophy. Regarding social history, the patient stated that he had quit smoking 20 years prior and only drank alcohol on occasion. He denied any medication allergies.
On physical examination, the patient’s vital signs were: pulse 98 beats/minute; blood pressure, 132/82 mm Hg; respiratory rate, 18 breaths/minute; and temperature, 99.8°F. The head, eyes, ears, nose, and throat examination, and pulmonary, cardiovascular, and abdominal examinations were all normal. On examination of the lower back, the patient exhibited mild tenderness of the right paraspinal lumbar muscles without midline tenderness. He had normal and equal strength of the lower extremities bilaterally. The patient’s buttocks were not examined during this visit.
The emergency physician (EP) felt the patient’s history and physical examination were consistent with a lumbar radiculopathy, and contacted the patient’s primary care physician (PCP) to communicate his impression. Although the PCP was aware that his patient had recently complained of chills, night sweats, and pain radiating from the hip to the anus, he did not communicate this information to the EP. Unfortunately, the patient also did not relate these complaints to the EP. After the EP’s consultation with the PCP, the patient was prescribed an analgesic, referred to an orthopedic surgeon, and discharged home.
Two days later, the patient returned to the same ED via emergency medical services, this time with altered mental status, fever, and hypotension. He was found to have necrotizing fasciitis of the right buttock. The patient received intravenous fluid resuscitation and broad-spectrum antibiotics, and was taken immediately to the operating room. Unfortunately, he did not survive.
The patient’s estate sued the first EP for failure to visualize and palpate the buttocks area and for failure to diagnose an infection of the right buttocks. The plaintiff further argued that the patient would have survived if he had been properly diagnosed and treated at the initial ED visit. The defense argued the patient received a thorough history and physical examination and that the diagnosis of lumbar radiculopathy was consistent with the patient’s clinical presentation. The defense also argued that the patient never reported any complaints or indication of a buttock infection. The case was settled prior to trial for nearly $600,000.
Discussion
The second important point, examining the area of primary complaint, may sound simple, straightforward, and just plain common sense. However, this can be very challenging in the reality of today’s practice of emergency medicine as EPs are often called upon to perform entire histories and physical examinations on patients in hallway beds. Gone are the days when every patient was undressed and placed in a gown in a private area or room. With the emphasis on maintaining patients with relatively minor complaints “vertical” to improve throughput times, the EP is frequently forced to examine such patients fully clothed. This places the EP at high risk for misdiagnosis or a missed diagnosis. In the same study by Kachalia et al,1 failure to perform an adequate medical history or physical examination accounted for 42% of all diagnostic errors in the ED. Recognizing the challenge of examining the point of complaint is easy—solving the issue, however, will take all of a hospital’s resources.
Stealing Is Bad for Your Health
A 19-year-old man presented to the ED complaining of left eye pain, foreign body sensation, and tearing. He stated that when he was cleaning his garage approximately 3 hours prior to presentation, a cardboard box had fallen on his head, scattering its contents. Immediately after this occurrence, the patient experienced onset of a foreign body sensation and pain in his left eye. He further noted that he had attempted to irrigate his left eye with tap water, but it did not relieve the pain. After several hours of discomfort, the patient decided to come to the ED. He denied any other injuries or complaints, was in good health, on no medications, and had no known medication allergies. He also denied wearing contacts or corrective lenses.
On physical examination, the patient’s left eye was closed, and he appeared to be somewhat uncomfortable. His vital signs were all normal. His visual acuity was 20/20 in the right eye and 20/60 in the left eye, which was due to tearing and blurriness from the injury. To facilitate the examination, the EP placed two drops of proparacaine hydrochloride ophthalmic solution 0.5% in both of the patient’s eyes, which provided immediate and near total relief of the left eye pain. On fluorescein and slit-lamp examination, the EP found a small foreign body at approximately the seven o’clock position on the cornea, as well as a surrounding abrasion. The corneal anterior chamber was normal. The EP removed the foreign body, prescribed a topical ophthalmic antibiotic cream. and instructed the patient to return to the ED in 24 hours for a recheck. The EP also instructed the patient to take ibuprofen for any associated pain.
While waiting to be discharged, the patient took the vial of proparacaine that had been left on the counter in the room. He did so without informing anyone and without permission. After the nurse returned to the room and gave the patient his discharge instructions, he was released home. Neither the nurse nor the physician realized the patient had stolen the topical anesthetic.
The patient sued the EP for leaving the “miracle” pain-relief medication in the room and thereby facilitating his actions. The plaintiff argued that the immediate and complete pain-relief effects of the medication were too tempting for a nonmedical person to resist, and that the drug should not have been made available to him under any circumstance. The EP argued the patient stole the medication and that any resulting injury was completely the result of the patient’s own actions. The suit was eventually dropped.
Discussion
It is unfortunate that this ever became a malpractice case, since it is clear to any layman that the stealing of medications is inappropriate and illegal; moreover, any damages resulting from that action are completely the responsibility of the transgressor. With that said, a significant amount of time, discomfort, and inconvenience could have been avoided had the nurse or physician simply removed the medication from the room. While I certainly do not think this is required from a medical-legal standpoint, it is a good common-sense practice.
Corneal abrasions and corneal foreign bodies are very uncomfortable, and medications such as nonsteroidal anti-inflammatory drugs or acetaminophen only provide partial pain relief. It is easy to see how a young person, lacking any medical knowledge or the concepts of adverse side effects or toxicity, would be tempted in such a situation. In retrospect, it would probably have been best to have avoided leaving the medication within the patient’s reach in the first place.
With respect to evaluating and treating corneal abrasions, the most commonly used topical ophthalmic anesthetics are proparacaine and tetracaine. When used appropriately and in the hands of trained healthcare workers, these drugs are safe, effective, and exhibit nearly no side effects. However, as this case demonstrates, these drugs can be toxic when abused. The most common toxicities are to the ocular surface and include superficial punctate keratitis, persistent epithelial defects, stromal infiltrates, and corneal edema. More serious injuries can also occur, such as deep corneal infiltrates, ulceration, and eye perforation.1 The toxic effects associated with these drugs are the reason these medications are never prescribed for home use.
A Pain in the Butt
A 75-year-old man presented to the ED complaining of low back pain radiating to his right buttock and leg. There was no history of trauma or overuse. His medical history was significant for hypertension and benign prostatic hypertrophy. Regarding social history, the patient stated that he had quit smoking 20 years prior and only drank alcohol on occasion. He denied any medication allergies.
On physical examination, the patient’s vital signs were: pulse 98 beats/minute; blood pressure, 132/82 mm Hg; respiratory rate, 18 breaths/minute; and temperature, 99.8°F. The head, eyes, ears, nose, and throat examination, and pulmonary, cardiovascular, and abdominal examinations were all normal. On examination of the lower back, the patient exhibited mild tenderness of the right paraspinal lumbar muscles without midline tenderness. He had normal and equal strength of the lower extremities bilaterally. The patient’s buttocks were not examined during this visit.
The emergency physician (EP) felt the patient’s history and physical examination were consistent with a lumbar radiculopathy, and contacted the patient’s primary care physician (PCP) to communicate his impression. Although the PCP was aware that his patient had recently complained of chills, night sweats, and pain radiating from the hip to the anus, he did not communicate this information to the EP. Unfortunately, the patient also did not relate these complaints to the EP. After the EP’s consultation with the PCP, the patient was prescribed an analgesic, referred to an orthopedic surgeon, and discharged home.
Two days later, the patient returned to the same ED via emergency medical services, this time with altered mental status, fever, and hypotension. He was found to have necrotizing fasciitis of the right buttock. The patient received intravenous fluid resuscitation and broad-spectrum antibiotics, and was taken immediately to the operating room. Unfortunately, he did not survive.
The patient’s estate sued the first EP for failure to visualize and palpate the buttocks area and for failure to diagnose an infection of the right buttocks. The plaintiff further argued that the patient would have survived if he had been properly diagnosed and treated at the initial ED visit. The defense argued the patient received a thorough history and physical examination and that the diagnosis of lumbar radiculopathy was consistent with the patient’s clinical presentation. The defense also argued that the patient never reported any complaints or indication of a buttock infection. The case was settled prior to trial for nearly $600,000.
Discussion
The second important point, examining the area of primary complaint, may sound simple, straightforward, and just plain common sense. However, this can be very challenging in the reality of today’s practice of emergency medicine as EPs are often called upon to perform entire histories and physical examinations on patients in hallway beds. Gone are the days when every patient was undressed and placed in a gown in a private area or room. With the emphasis on maintaining patients with relatively minor complaints “vertical” to improve throughput times, the EP is frequently forced to examine such patients fully clothed. This places the EP at high risk for misdiagnosis or a missed diagnosis. In the same study by Kachalia et al,1 failure to perform an adequate medical history or physical examination accounted for 42% of all diagnostic errors in the ED. Recognizing the challenge of examining the point of complaint is easy—solving the issue, however, will take all of a hospital’s resources.
Stealing Is Bad for Your Health
A 19-year-old man presented to the ED complaining of left eye pain, foreign body sensation, and tearing. He stated that when he was cleaning his garage approximately 3 hours prior to presentation, a cardboard box had fallen on his head, scattering its contents. Immediately after this occurrence, the patient experienced onset of a foreign body sensation and pain in his left eye. He further noted that he had attempted to irrigate his left eye with tap water, but it did not relieve the pain. After several hours of discomfort, the patient decided to come to the ED. He denied any other injuries or complaints, was in good health, on no medications, and had no known medication allergies. He also denied wearing contacts or corrective lenses.
On physical examination, the patient’s left eye was closed, and he appeared to be somewhat uncomfortable. His vital signs were all normal. His visual acuity was 20/20 in the right eye and 20/60 in the left eye, which was due to tearing and blurriness from the injury. To facilitate the examination, the EP placed two drops of proparacaine hydrochloride ophthalmic solution 0.5% in both of the patient’s eyes, which provided immediate and near total relief of the left eye pain. On fluorescein and slit-lamp examination, the EP found a small foreign body at approximately the seven o’clock position on the cornea, as well as a surrounding abrasion. The corneal anterior chamber was normal. The EP removed the foreign body, prescribed a topical ophthalmic antibiotic cream. and instructed the patient to return to the ED in 24 hours for a recheck. The EP also instructed the patient to take ibuprofen for any associated pain.
While waiting to be discharged, the patient took the vial of proparacaine that had been left on the counter in the room. He did so without informing anyone and without permission. After the nurse returned to the room and gave the patient his discharge instructions, he was released home. Neither the nurse nor the physician realized the patient had stolen the topical anesthetic.
The patient sued the EP for leaving the “miracle” pain-relief medication in the room and thereby facilitating his actions. The plaintiff argued that the immediate and complete pain-relief effects of the medication were too tempting for a nonmedical person to resist, and that the drug should not have been made available to him under any circumstance. The EP argued the patient stole the medication and that any resulting injury was completely the result of the patient’s own actions. The suit was eventually dropped.
Discussion
It is unfortunate that this ever became a malpractice case, since it is clear to any layman that the stealing of medications is inappropriate and illegal; moreover, any damages resulting from that action are completely the responsibility of the transgressor. With that said, a significant amount of time, discomfort, and inconvenience could have been avoided had the nurse or physician simply removed the medication from the room. While I certainly do not think this is required from a medical-legal standpoint, it is a good common-sense practice.
Corneal abrasions and corneal foreign bodies are very uncomfortable, and medications such as nonsteroidal anti-inflammatory drugs or acetaminophen only provide partial pain relief. It is easy to see how a young person, lacking any medical knowledge or the concepts of adverse side effects or toxicity, would be tempted in such a situation. In retrospect, it would probably have been best to have avoided leaving the medication within the patient’s reach in the first place.
With respect to evaluating and treating corneal abrasions, the most commonly used topical ophthalmic anesthetics are proparacaine and tetracaine. When used appropriately and in the hands of trained healthcare workers, these drugs are safe, effective, and exhibit nearly no side effects. However, as this case demonstrates, these drugs can be toxic when abused. The most common toxicities are to the ocular surface and include superficial punctate keratitis, persistent epithelial defects, stromal infiltrates, and corneal edema. More serious injuries can also occur, such as deep corneal infiltrates, ulceration, and eye perforation.1 The toxic effects associated with these drugs are the reason these medications are never prescribed for home use.
- A Pain in the Butt
- Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49(2):196-205.
- Stealing Is Bad for Your Health
- McGee HT, Fraunfelder FW. Toxicities of topical ophthalmic anesthetics. Expert Opin Drug Saf. 2007;5(6):637-640.
- A Pain in the Butt
- Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49(2):196-205.
- Stealing Is Bad for Your Health
- McGee HT, Fraunfelder FW. Toxicities of topical ophthalmic anesthetics. Expert Opin Drug Saf. 2007;5(6):637-640.
Malpractice Counsel: Aneurysm, Falls
Sued If You Do, Sued If You Don’t
A 52-year-old woman presented to the ED with complaints of abdominal pain, vaginal bleeding, and left leg pain. The patient stated that the symptoms, which she had been experiencing over the past few days, were becoming progressively worse. She denied fevers, chills, nausea, vomiting, diarrhea, or constipation. Her surgical history was
remarkable for an appendectomy 30 years prior. The patient was not currently on any medications. Regarding social history, she denied alcohol or tobacco use. She also denied any allergies to medications.
On physical examination, all of the patient’s vital signs were normal. The head, eyes, ears, nose, and throat, and lung and heart examinations were also normal; however, on abdominal examination, she exhibited tenderness throughout the lower abdomen, but without guarding or rebound. There was no costovertebral angle tenderness of the back. The pelvic examination was remarkable for a small amount of blood from the cervical os and a slightly enlarged uterus. The adnexa were normal and without tenderness.
The patient sued both the EP and the hospital, claiming that the CT scan was unnecessary and had it not been performed, she would not have experienced the stroke. The defense asserted that the CT scan with contrast was appropriate given the patient’s symptoms and physical findings, and that the contrast dye used was not the cause of the stroke. The jury awarded the plaintiff $3.6 million.
Discussion
This case is unique in that the EP was sued for ordering a CT scan. In the overwhelming majority of malpractice cases, EPs are sued for not obtaining a certain test—frequently a CT scan. It does not appear the jury in this case was correct in their judgment as there was no conceivable way the EP could have anticipated this type of unusual reaction, especially in a patient with no history of medication allergies.
This jury ruling places EPs in an untenable situation: If they order a test and anything bad happens, they will be sued. If they do not order a test and something bad happens, they will be sued. In legal theory, there must be proximal cause between what the physician did (ie, order the CT scan) and the bad outcome, or negligence (ie, SAH). For this case, the two events seem true-true and unrelated. The contrast dye clearly did not cause the cerebral aneurysm, which was a preexisting condition.
Nonidiosyncratic reactions are due to direct toxic or osmolar effects. Symptoms include bradycardia, hypotension, vasovagal reactions, sensation of warmth, metallic taste in the mouth, and nausea and vomiting.1
Ironically, the majority of adverse reactions to ICM involve hypotension, not hypertension. This includes cardiovascular reactions to ICM, which typically involve bradycardia, peripheral vasodilation, and hypotension.1 The incidence and severity of an adverse reaction to ICM also depends on whether ionic or nonionic ICM was used. (Unfortunately, the type of ICM administered to the patient in this case was not disclosed.)
The incidence and severity of adverse reactions to ICM are less with nonionic compared to ionic ICM. More than 90% of adverse reactions to nonionic ICM are anaphlyactoid.2 In general, adverse reactions occur in 4% to 12% of patients receiving ionic ICM compared to 1% to 3% of those receiving nonionic ICM.2 In a study of more than 300,000 contrast administrations, Katayama et al,3 found the overall risk for severe adverse reaction to be 0.2% for ionic ICM compared to 0.04% for nonionic ICM.
The bottom line in this case is that the patient’s event was a very rare and completely unforeseen result temporally related to the contrast CT scan ordered to evaluate the etiology of this patient’s abdominal pain.
Falls
A 67-year-old woman with a chief complaint of lightheadedness and dizziness was transferred from a dialysis center to the ED by emergency medical services (EMS). She stated that her symptoms came on suddenly right after she had completed her scheduled dialysis.
As the patient was being rolled on a stretcher from the ambulance to the ED entrance, the stretcher collapsed and tipped over, causing the patient to fall and strike her head on the pavement. The patient suffered a severe intraparenchymal brain hemorrhage, requiring intubation, ventilation, and admission to the intensive care unit. On the second day of admission, the patient’s family signed “do not resuscitate” orders and, in accordance with their wishes, life support was withdrawn and the patient died.
The family sued the ambulance company, stating the patient’s death was a direct result of negligent training and supervision of EMS personnel. The plaintiff further claimed the incident was caused by the failure to properly secure a locking mechanism on the stretcher, which caused it to tip. The ambulance company disputed the liability, asserting that what occurred was a tragic accident, not negligence. The jury found in favor of the plaintiff and awarded $1.5 million.
Discussion
While this is not a true ED case since the patient’s fall occurred just outside the ED, it does emphasize the importance of falls and the challenges of fall prevention within the hospital—including the ED. The incidence of falls within hospitals ranges from 1.3 to 9 falls per 1,000 occupied bed days (OBD).1 This incidence, however, is not evenly distributed across hospital departments. Not surprisingly, the highest rates are reported in areas such as geriatric, neurology, and rehabilitation units.1 The highest rates, 17 to 67 per 1,000 OBDs, appear to occur in geropsychiatric units,2,3 and a significant number of such patient falls are serious, with some type of injury resulting from the fall in 30% to 51% of cases.1 The percentage of falls resulting in a fracture ranges from 1% to 3%.1
As previously noted, the ED is not immune to patient falls. A review of one academic medical center ED with 75,000 annual visits found an incidence of 1.3 falls per month, 31% of which resulted in patient injury.4
Some relatively simple steps can be taken to reduce the incidence of falls. For example, identifying patients at high risk of falling (eg, patients who are elderly, confused, dizzy) and ensuring other care-team workers are aware of the risk, can be very helpful.4,5 In addition, brightly colored signs on the stretcher or colored wrist bands indicating the patient is at high-risk for falls helps to engage the entire healthcare team in fall-prevention measures.4 Sitters with high-risk patients can also help minimize fall risk.
Although side rails on hospital beds are intended to increase patient safety, their use is not without controversy. Most hospitals require staff to have side rails up for obvious reasons. Some hospitals, however, are concerned that the use of side rails can cause a fall from a higher position and increase the risk of injury when a patient attempts to get out of bed. Additional important steps include ensuring that all wet surfaces are quickly identified and cleaned, and making sure everyone is aware of the importance of fall-prevention measures.
The employment of the abovementioned fall-prevention measures is especially important in relation to the aging US population. As the number of elderly patients in the United States continues to grow, the risk of patient falls is expected to increase. Therefore, hospitals should be proactive in implementing preventive measures to reduce the risk of patient falls and injury.
- Sued If You Do, Sued If You Don't
- Siddiqi NH, Lin EC. Contrast medium reactions. http://emedicine.medscape.com/article. Updated September 29, 2015. Accessed October 8, 2015.
- Cochran ST. Anaphylactoid reactions to radiocontrast media. Curr Allergy Asthma Rep. 2005;5(1):28-31.
- Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990;175(3):621-128.
- Falls
- Oliver D, Healey F, Haines TP. Preventing falls and fall-related injuries in hospitals. Clin Geriatr Med. 2010;26(4):645-692.
- Nyberg L, Gustafson Y, Janson A, Sandman PO, Eriksson S. Incidence of falls in three different types of geriatric care. A Swedish prospective study. Scand J Soc Med. 1997;25(1):8-13.
- Weintraub D, Spurlock M. Change in the rate of restraint use and falls on a psychogeriatric inpatient unit: impact of the health care financing administration’s new restraint and seclusion standards for hospitals. J Geriatr Psychiatry Neurol. 2002;15(2):91-94.
- Rosenthal A. Preventing falls in the emergency department: a program that works (Abstract). Virginia Henderson Global Nursing e-Repository Web site. http://www.nursinglibrary.org/vhl/handle/10755/162669. Accessed October 7, 2015.
- Alexander D, Kinsley TL, Waszinski C. Journey to a safe environment: fall precaution in an emergency department at a level I trauma center. J Emerg Nurs. 2013;39(4):346-352.
Sued If You Do, Sued If You Don’t
A 52-year-old woman presented to the ED with complaints of abdominal pain, vaginal bleeding, and left leg pain. The patient stated that the symptoms, which she had been experiencing over the past few days, were becoming progressively worse. She denied fevers, chills, nausea, vomiting, diarrhea, or constipation. Her surgical history was
remarkable for an appendectomy 30 years prior. The patient was not currently on any medications. Regarding social history, she denied alcohol or tobacco use. She also denied any allergies to medications.
On physical examination, all of the patient’s vital signs were normal. The head, eyes, ears, nose, and throat, and lung and heart examinations were also normal; however, on abdominal examination, she exhibited tenderness throughout the lower abdomen, but without guarding or rebound. There was no costovertebral angle tenderness of the back. The pelvic examination was remarkable for a small amount of blood from the cervical os and a slightly enlarged uterus. The adnexa were normal and without tenderness.
The patient sued both the EP and the hospital, claiming that the CT scan was unnecessary and had it not been performed, she would not have experienced the stroke. The defense asserted that the CT scan with contrast was appropriate given the patient’s symptoms and physical findings, and that the contrast dye used was not the cause of the stroke. The jury awarded the plaintiff $3.6 million.
Discussion
This case is unique in that the EP was sued for ordering a CT scan. In the overwhelming majority of malpractice cases, EPs are sued for not obtaining a certain test—frequently a CT scan. It does not appear the jury in this case was correct in their judgment as there was no conceivable way the EP could have anticipated this type of unusual reaction, especially in a patient with no history of medication allergies.
This jury ruling places EPs in an untenable situation: If they order a test and anything bad happens, they will be sued. If they do not order a test and something bad happens, they will be sued. In legal theory, there must be proximal cause between what the physician did (ie, order the CT scan) and the bad outcome, or negligence (ie, SAH). For this case, the two events seem true-true and unrelated. The contrast dye clearly did not cause the cerebral aneurysm, which was a preexisting condition.
Nonidiosyncratic reactions are due to direct toxic or osmolar effects. Symptoms include bradycardia, hypotension, vasovagal reactions, sensation of warmth, metallic taste in the mouth, and nausea and vomiting.1
Ironically, the majority of adverse reactions to ICM involve hypotension, not hypertension. This includes cardiovascular reactions to ICM, which typically involve bradycardia, peripheral vasodilation, and hypotension.1 The incidence and severity of an adverse reaction to ICM also depends on whether ionic or nonionic ICM was used. (Unfortunately, the type of ICM administered to the patient in this case was not disclosed.)
The incidence and severity of adverse reactions to ICM are less with nonionic compared to ionic ICM. More than 90% of adverse reactions to nonionic ICM are anaphlyactoid.2 In general, adverse reactions occur in 4% to 12% of patients receiving ionic ICM compared to 1% to 3% of those receiving nonionic ICM.2 In a study of more than 300,000 contrast administrations, Katayama et al,3 found the overall risk for severe adverse reaction to be 0.2% for ionic ICM compared to 0.04% for nonionic ICM.
The bottom line in this case is that the patient’s event was a very rare and completely unforeseen result temporally related to the contrast CT scan ordered to evaluate the etiology of this patient’s abdominal pain.
Falls
A 67-year-old woman with a chief complaint of lightheadedness and dizziness was transferred from a dialysis center to the ED by emergency medical services (EMS). She stated that her symptoms came on suddenly right after she had completed her scheduled dialysis.
As the patient was being rolled on a stretcher from the ambulance to the ED entrance, the stretcher collapsed and tipped over, causing the patient to fall and strike her head on the pavement. The patient suffered a severe intraparenchymal brain hemorrhage, requiring intubation, ventilation, and admission to the intensive care unit. On the second day of admission, the patient’s family signed “do not resuscitate” orders and, in accordance with their wishes, life support was withdrawn and the patient died.
The family sued the ambulance company, stating the patient’s death was a direct result of negligent training and supervision of EMS personnel. The plaintiff further claimed the incident was caused by the failure to properly secure a locking mechanism on the stretcher, which caused it to tip. The ambulance company disputed the liability, asserting that what occurred was a tragic accident, not negligence. The jury found in favor of the plaintiff and awarded $1.5 million.
Discussion
While this is not a true ED case since the patient’s fall occurred just outside the ED, it does emphasize the importance of falls and the challenges of fall prevention within the hospital—including the ED. The incidence of falls within hospitals ranges from 1.3 to 9 falls per 1,000 occupied bed days (OBD).1 This incidence, however, is not evenly distributed across hospital departments. Not surprisingly, the highest rates are reported in areas such as geriatric, neurology, and rehabilitation units.1 The highest rates, 17 to 67 per 1,000 OBDs, appear to occur in geropsychiatric units,2,3 and a significant number of such patient falls are serious, with some type of injury resulting from the fall in 30% to 51% of cases.1 The percentage of falls resulting in a fracture ranges from 1% to 3%.1
As previously noted, the ED is not immune to patient falls. A review of one academic medical center ED with 75,000 annual visits found an incidence of 1.3 falls per month, 31% of which resulted in patient injury.4
Some relatively simple steps can be taken to reduce the incidence of falls. For example, identifying patients at high risk of falling (eg, patients who are elderly, confused, dizzy) and ensuring other care-team workers are aware of the risk, can be very helpful.4,5 In addition, brightly colored signs on the stretcher or colored wrist bands indicating the patient is at high-risk for falls helps to engage the entire healthcare team in fall-prevention measures.4 Sitters with high-risk patients can also help minimize fall risk.
Although side rails on hospital beds are intended to increase patient safety, their use is not without controversy. Most hospitals require staff to have side rails up for obvious reasons. Some hospitals, however, are concerned that the use of side rails can cause a fall from a higher position and increase the risk of injury when a patient attempts to get out of bed. Additional important steps include ensuring that all wet surfaces are quickly identified and cleaned, and making sure everyone is aware of the importance of fall-prevention measures.
The employment of the abovementioned fall-prevention measures is especially important in relation to the aging US population. As the number of elderly patients in the United States continues to grow, the risk of patient falls is expected to increase. Therefore, hospitals should be proactive in implementing preventive measures to reduce the risk of patient falls and injury.
Sued If You Do, Sued If You Don’t
A 52-year-old woman presented to the ED with complaints of abdominal pain, vaginal bleeding, and left leg pain. The patient stated that the symptoms, which she had been experiencing over the past few days, were becoming progressively worse. She denied fevers, chills, nausea, vomiting, diarrhea, or constipation. Her surgical history was
remarkable for an appendectomy 30 years prior. The patient was not currently on any medications. Regarding social history, she denied alcohol or tobacco use. She also denied any allergies to medications.
On physical examination, all of the patient’s vital signs were normal. The head, eyes, ears, nose, and throat, and lung and heart examinations were also normal; however, on abdominal examination, she exhibited tenderness throughout the lower abdomen, but without guarding or rebound. There was no costovertebral angle tenderness of the back. The pelvic examination was remarkable for a small amount of blood from the cervical os and a slightly enlarged uterus. The adnexa were normal and without tenderness.
The patient sued both the EP and the hospital, claiming that the CT scan was unnecessary and had it not been performed, she would not have experienced the stroke. The defense asserted that the CT scan with contrast was appropriate given the patient’s symptoms and physical findings, and that the contrast dye used was not the cause of the stroke. The jury awarded the plaintiff $3.6 million.
Discussion
This case is unique in that the EP was sued for ordering a CT scan. In the overwhelming majority of malpractice cases, EPs are sued for not obtaining a certain test—frequently a CT scan. It does not appear the jury in this case was correct in their judgment as there was no conceivable way the EP could have anticipated this type of unusual reaction, especially in a patient with no history of medication allergies.
This jury ruling places EPs in an untenable situation: If they order a test and anything bad happens, they will be sued. If they do not order a test and something bad happens, they will be sued. In legal theory, there must be proximal cause between what the physician did (ie, order the CT scan) and the bad outcome, or negligence (ie, SAH). For this case, the two events seem true-true and unrelated. The contrast dye clearly did not cause the cerebral aneurysm, which was a preexisting condition.
Nonidiosyncratic reactions are due to direct toxic or osmolar effects. Symptoms include bradycardia, hypotension, vasovagal reactions, sensation of warmth, metallic taste in the mouth, and nausea and vomiting.1
Ironically, the majority of adverse reactions to ICM involve hypotension, not hypertension. This includes cardiovascular reactions to ICM, which typically involve bradycardia, peripheral vasodilation, and hypotension.1 The incidence and severity of an adverse reaction to ICM also depends on whether ionic or nonionic ICM was used. (Unfortunately, the type of ICM administered to the patient in this case was not disclosed.)
The incidence and severity of adverse reactions to ICM are less with nonionic compared to ionic ICM. More than 90% of adverse reactions to nonionic ICM are anaphlyactoid.2 In general, adverse reactions occur in 4% to 12% of patients receiving ionic ICM compared to 1% to 3% of those receiving nonionic ICM.2 In a study of more than 300,000 contrast administrations, Katayama et al,3 found the overall risk for severe adverse reaction to be 0.2% for ionic ICM compared to 0.04% for nonionic ICM.
The bottom line in this case is that the patient’s event was a very rare and completely unforeseen result temporally related to the contrast CT scan ordered to evaluate the etiology of this patient’s abdominal pain.
Falls
A 67-year-old woman with a chief complaint of lightheadedness and dizziness was transferred from a dialysis center to the ED by emergency medical services (EMS). She stated that her symptoms came on suddenly right after she had completed her scheduled dialysis.
As the patient was being rolled on a stretcher from the ambulance to the ED entrance, the stretcher collapsed and tipped over, causing the patient to fall and strike her head on the pavement. The patient suffered a severe intraparenchymal brain hemorrhage, requiring intubation, ventilation, and admission to the intensive care unit. On the second day of admission, the patient’s family signed “do not resuscitate” orders and, in accordance with their wishes, life support was withdrawn and the patient died.
The family sued the ambulance company, stating the patient’s death was a direct result of negligent training and supervision of EMS personnel. The plaintiff further claimed the incident was caused by the failure to properly secure a locking mechanism on the stretcher, which caused it to tip. The ambulance company disputed the liability, asserting that what occurred was a tragic accident, not negligence. The jury found in favor of the plaintiff and awarded $1.5 million.
Discussion
While this is not a true ED case since the patient’s fall occurred just outside the ED, it does emphasize the importance of falls and the challenges of fall prevention within the hospital—including the ED. The incidence of falls within hospitals ranges from 1.3 to 9 falls per 1,000 occupied bed days (OBD).1 This incidence, however, is not evenly distributed across hospital departments. Not surprisingly, the highest rates are reported in areas such as geriatric, neurology, and rehabilitation units.1 The highest rates, 17 to 67 per 1,000 OBDs, appear to occur in geropsychiatric units,2,3 and a significant number of such patient falls are serious, with some type of injury resulting from the fall in 30% to 51% of cases.1 The percentage of falls resulting in a fracture ranges from 1% to 3%.1
As previously noted, the ED is not immune to patient falls. A review of one academic medical center ED with 75,000 annual visits found an incidence of 1.3 falls per month, 31% of which resulted in patient injury.4
Some relatively simple steps can be taken to reduce the incidence of falls. For example, identifying patients at high risk of falling (eg, patients who are elderly, confused, dizzy) and ensuring other care-team workers are aware of the risk, can be very helpful.4,5 In addition, brightly colored signs on the stretcher or colored wrist bands indicating the patient is at high-risk for falls helps to engage the entire healthcare team in fall-prevention measures.4 Sitters with high-risk patients can also help minimize fall risk.
Although side rails on hospital beds are intended to increase patient safety, their use is not without controversy. Most hospitals require staff to have side rails up for obvious reasons. Some hospitals, however, are concerned that the use of side rails can cause a fall from a higher position and increase the risk of injury when a patient attempts to get out of bed. Additional important steps include ensuring that all wet surfaces are quickly identified and cleaned, and making sure everyone is aware of the importance of fall-prevention measures.
The employment of the abovementioned fall-prevention measures is especially important in relation to the aging US population. As the number of elderly patients in the United States continues to grow, the risk of patient falls is expected to increase. Therefore, hospitals should be proactive in implementing preventive measures to reduce the risk of patient falls and injury.
- Sued If You Do, Sued If You Don't
- Siddiqi NH, Lin EC. Contrast medium reactions. http://emedicine.medscape.com/article. Updated September 29, 2015. Accessed October 8, 2015.
- Cochran ST. Anaphylactoid reactions to radiocontrast media. Curr Allergy Asthma Rep. 2005;5(1):28-31.
- Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990;175(3):621-128.
- Falls
- Oliver D, Healey F, Haines TP. Preventing falls and fall-related injuries in hospitals. Clin Geriatr Med. 2010;26(4):645-692.
- Nyberg L, Gustafson Y, Janson A, Sandman PO, Eriksson S. Incidence of falls in three different types of geriatric care. A Swedish prospective study. Scand J Soc Med. 1997;25(1):8-13.
- Weintraub D, Spurlock M. Change in the rate of restraint use and falls on a psychogeriatric inpatient unit: impact of the health care financing administration’s new restraint and seclusion standards for hospitals. J Geriatr Psychiatry Neurol. 2002;15(2):91-94.
- Rosenthal A. Preventing falls in the emergency department: a program that works (Abstract). Virginia Henderson Global Nursing e-Repository Web site. http://www.nursinglibrary.org/vhl/handle/10755/162669. Accessed October 7, 2015.
- Alexander D, Kinsley TL, Waszinski C. Journey to a safe environment: fall precaution in an emergency department at a level I trauma center. J Emerg Nurs. 2013;39(4):346-352.
- Sued If You Do, Sued If You Don't
- Siddiqi NH, Lin EC. Contrast medium reactions. http://emedicine.medscape.com/article. Updated September 29, 2015. Accessed October 8, 2015.
- Cochran ST. Anaphylactoid reactions to radiocontrast media. Curr Allergy Asthma Rep. 2005;5(1):28-31.
- Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990;175(3):621-128.
- Falls
- Oliver D, Healey F, Haines TP. Preventing falls and fall-related injuries in hospitals. Clin Geriatr Med. 2010;26(4):645-692.
- Nyberg L, Gustafson Y, Janson A, Sandman PO, Eriksson S. Incidence of falls in three different types of geriatric care. A Swedish prospective study. Scand J Soc Med. 1997;25(1):8-13.
- Weintraub D, Spurlock M. Change in the rate of restraint use and falls on a psychogeriatric inpatient unit: impact of the health care financing administration’s new restraint and seclusion standards for hospitals. J Geriatr Psychiatry Neurol. 2002;15(2):91-94.
- Rosenthal A. Preventing falls in the emergency department: a program that works (Abstract). Virginia Henderson Global Nursing e-Repository Web site. http://www.nursinglibrary.org/vhl/handle/10755/162669. Accessed October 7, 2015.
- Alexander D, Kinsley TL, Waszinski C. Journey to a safe environment: fall precaution in an emergency department at a level I trauma center. J Emerg Nurs. 2013;39(4):346-352.