User login
MelaFind, a noninvasive melanoma detection device, was approved by the Food and Drug Administration on Nov. 2.
The road to approval was marked by some controversy. It included a closely split advisory panel recommendation in November 2010, followed by the May 2011 submission of a citizen petition to the FDA seeking action by the manufacturer, Mela Sciences. The FDA deemed the device approvable in late September, pending agreement between the agency and Mela Sciences on how the device would be used and by whom.
The approval includes a long list of indications and caveats, according to the company. They include the following:
• "MelaFind is intended for use on clinically atypical cutaneous pigmented lesions with one or more clinical or historical characteristics of melanoma, excluding those with a clinical diagnosis of melanoma or likely melanoma.
• It is designed to be used when a dermatologist chooses to obtain additional information for a decision to biopsy. MelaFind should not be used to confirm a clinical diagnosis of melanoma.
• MelaFind is only for use by physicians trained in the clinical diagnosis and management of skin cancer [that is, dermatologists] who have also successfully completed a training program in the appropriate use of MelaFind."
• MelaFind positive lesions should be considered for biopsy. "Non-evaluable" lesions should be carefully reevaluated for biopsy.
• MelaFind is indicated for use only on the following lesions: those with a diameter between 2 mm and 22 mm; lesions accessible by the MelaFind imager; those sufficiently pigmented; lesions that do not contain a scar or fibrosis consistent with previous trauma; those in which the skin is intact; lesions greater than 1 cm away from the eye; and those that do not contain foreign matter. It should not be used on acral, palmar, plantar, mucosal, or subungual areas.
Dr. Darrell S. Rigel, a dermatologist at New York University and a consultant for Mela Sciences, said in a statement that the device "represents one of the most significant advances in early melanoma detection since the advent of the ABCD criteria that our group developed over a quarter century ago."
Dr. Joseph V. Gulfo, president and CEO of Mela Sciences, said that the company aims to launch MelaFind in the United States and Germany in the spring of 2012. "We’re planning a steadfast, deliberate and measured approach to the commercial launch to ensure that dermatologists in practices of all shapes and sizes are trained and set up to use MelaFind effectively on the patients who can benefit most from the objective information that the system provides during skin examinations," he said in the statement.
Initially, the device will be sold only to a handful of dermatologists in Connecticut, New Jersey, and New York. Mela Sciences will be working with the dermatologists to fine-tune the device before rolling it out to a larger number of practices.
The device received European Union approval in September. The company aims to have about 75 systems in Germany by next September, Dr. Gulfo said.
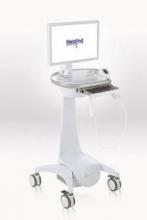
MelaFind is a multispectral computer vision system with a handheld imager that captures the image of a lesion; its software uses algorithms to analyze the image, indicating within 2 minutes whether a biopsy should be done.
The pivotal study of the device included 1,383 patients. According to the company, MelaFind had a sensitivity of 98%. The device’s sensitivity rating for malignant melanoma was significantly better than that of dermatologists, who showed a wide range of variability about which lesions would have been recommended for biopsy and which relegated to observation.
MelaFind, a noninvasive melanoma detection device, was approved by the Food and Drug Administration on Nov. 2.
The road to approval was marked by some controversy. It included a closely split advisory panel recommendation in November 2010, followed by the May 2011 submission of a citizen petition to the FDA seeking action by the manufacturer, Mela Sciences. The FDA deemed the device approvable in late September, pending agreement between the agency and Mela Sciences on how the device would be used and by whom.
The approval includes a long list of indications and caveats, according to the company. They include the following:
• "MelaFind is intended for use on clinically atypical cutaneous pigmented lesions with one or more clinical or historical characteristics of melanoma, excluding those with a clinical diagnosis of melanoma or likely melanoma.
• It is designed to be used when a dermatologist chooses to obtain additional information for a decision to biopsy. MelaFind should not be used to confirm a clinical diagnosis of melanoma.
• MelaFind is only for use by physicians trained in the clinical diagnosis and management of skin cancer [that is, dermatologists] who have also successfully completed a training program in the appropriate use of MelaFind."
• MelaFind positive lesions should be considered for biopsy. "Non-evaluable" lesions should be carefully reevaluated for biopsy.
• MelaFind is indicated for use only on the following lesions: those with a diameter between 2 mm and 22 mm; lesions accessible by the MelaFind imager; those sufficiently pigmented; lesions that do not contain a scar or fibrosis consistent with previous trauma; those in which the skin is intact; lesions greater than 1 cm away from the eye; and those that do not contain foreign matter. It should not be used on acral, palmar, plantar, mucosal, or subungual areas.
Dr. Darrell S. Rigel, a dermatologist at New York University and a consultant for Mela Sciences, said in a statement that the device "represents one of the most significant advances in early melanoma detection since the advent of the ABCD criteria that our group developed over a quarter century ago."
Dr. Joseph V. Gulfo, president and CEO of Mela Sciences, said that the company aims to launch MelaFind in the United States and Germany in the spring of 2012. "We’re planning a steadfast, deliberate and measured approach to the commercial launch to ensure that dermatologists in practices of all shapes and sizes are trained and set up to use MelaFind effectively on the patients who can benefit most from the objective information that the system provides during skin examinations," he said in the statement.
Initially, the device will be sold only to a handful of dermatologists in Connecticut, New Jersey, and New York. Mela Sciences will be working with the dermatologists to fine-tune the device before rolling it out to a larger number of practices.
The device received European Union approval in September. The company aims to have about 75 systems in Germany by next September, Dr. Gulfo said.
MelaFind is a multispectral computer vision system with a handheld imager that captures the image of a lesion; its software uses algorithms to analyze the image, indicating within 2 minutes whether a biopsy should be done.
The pivotal study of the device included 1,383 patients. According to the company, MelaFind had a sensitivity of 98%. The device’s sensitivity rating for malignant melanoma was significantly better than that of dermatologists, who showed a wide range of variability about which lesions would have been recommended for biopsy and which relegated to observation.
MelaFind, a noninvasive melanoma detection device, was approved by the Food and Drug Administration on Nov. 2.
The road to approval was marked by some controversy. It included a closely split advisory panel recommendation in November 2010, followed by the May 2011 submission of a citizen petition to the FDA seeking action by the manufacturer, Mela Sciences. The FDA deemed the device approvable in late September, pending agreement between the agency and Mela Sciences on how the device would be used and by whom.
The approval includes a long list of indications and caveats, according to the company. They include the following:
• "MelaFind is intended for use on clinically atypical cutaneous pigmented lesions with one or more clinical or historical characteristics of melanoma, excluding those with a clinical diagnosis of melanoma or likely melanoma.
• It is designed to be used when a dermatologist chooses to obtain additional information for a decision to biopsy. MelaFind should not be used to confirm a clinical diagnosis of melanoma.
• MelaFind is only for use by physicians trained in the clinical diagnosis and management of skin cancer [that is, dermatologists] who have also successfully completed a training program in the appropriate use of MelaFind."
• MelaFind positive lesions should be considered for biopsy. "Non-evaluable" lesions should be carefully reevaluated for biopsy.
• MelaFind is indicated for use only on the following lesions: those with a diameter between 2 mm and 22 mm; lesions accessible by the MelaFind imager; those sufficiently pigmented; lesions that do not contain a scar or fibrosis consistent with previous trauma; those in which the skin is intact; lesions greater than 1 cm away from the eye; and those that do not contain foreign matter. It should not be used on acral, palmar, plantar, mucosal, or subungual areas.
Dr. Darrell S. Rigel, a dermatologist at New York University and a consultant for Mela Sciences, said in a statement that the device "represents one of the most significant advances in early melanoma detection since the advent of the ABCD criteria that our group developed over a quarter century ago."
Dr. Joseph V. Gulfo, president and CEO of Mela Sciences, said that the company aims to launch MelaFind in the United States and Germany in the spring of 2012. "We’re planning a steadfast, deliberate and measured approach to the commercial launch to ensure that dermatologists in practices of all shapes and sizes are trained and set up to use MelaFind effectively on the patients who can benefit most from the objective information that the system provides during skin examinations," he said in the statement.
Initially, the device will be sold only to a handful of dermatologists in Connecticut, New Jersey, and New York. Mela Sciences will be working with the dermatologists to fine-tune the device before rolling it out to a larger number of practices.
The device received European Union approval in September. The company aims to have about 75 systems in Germany by next September, Dr. Gulfo said.
MelaFind is a multispectral computer vision system with a handheld imager that captures the image of a lesion; its software uses algorithms to analyze the image, indicating within 2 minutes whether a biopsy should be done.
The pivotal study of the device included 1,383 patients. According to the company, MelaFind had a sensitivity of 98%. The device’s sensitivity rating for malignant melanoma was significantly better than that of dermatologists, who showed a wide range of variability about which lesions would have been recommended for biopsy and which relegated to observation.