User login
Case Report
A 27-year-old black man was admitted to the hospital with chills; night sweats; unintentional 25-lb weight loss; and multiple widespread, painful, progressively enlarging skin nodules of 3 months’ duration. The lesions had first developed on the back and later appeared on the face, trunk, arms, thighs, and genital region. He denied dysuria or urethral discharge. He had a remote history of adequately treated chlamydia infection but no other remarkable personal or family history.
| ||
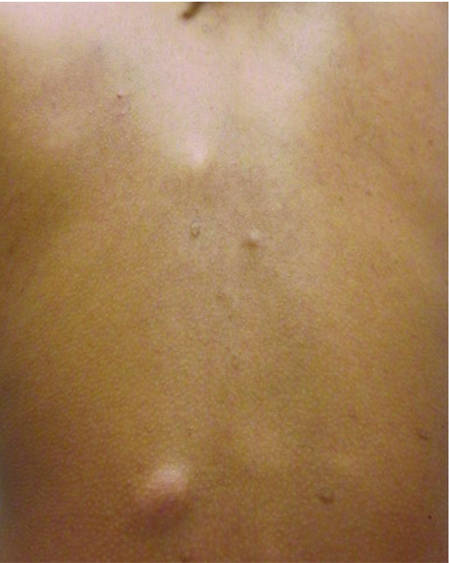
| Figure 1. Firm subcutaneous nodules on the back with no epidermal change. | ||
| ||
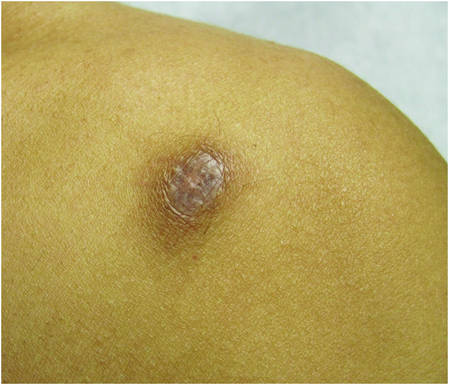
| Figure 2. Firm dermal papule on the anterior aspect of the left shoulder with violaceous hyperpigmentation (dermatofibromalike). |
Physical examination revealed a thin man with more than 20 lesions on the face, trunk, arms, thighs, and genital region ranging in size from 1 to 4 cm. Lesion morphologies varied greatly and included subcutaneous firm nodules with no epidermal change (Figure 1); dermatofibromalike nodules with overlying erythema and hyperpigmentation (Figure 2); condylomalike, verrucous, pink papulonodules (Figure 3); ulcerated angular plaques with rolled borders and palpable tumor extension deep (1–2 cm) to the subcutis (Figure 4); and a vegetative, eroded, exophytic tumor with palpable deep extension (Figure 4). A diffuse, erythematous, macular eruption also was noted on the trunk and bilateral arms and legs including the soles of both feet along with nontender cervical, axillary, and inguinal lymphadenopathy. The ocular, oral, and nasal mucosae were not affected.
The differential diagnosis for each lesion differed based on morphology. Infectious, inflammatory, and neoplastic processes were considered, including syphilis, dermatofibroma, dermatofibrosarcoma protuberans, metastatic disease, leukemia cutis, sarcoidosis, panniculitis, condyloma acuminatum, and vegetative herpes simplex virus infection (inguinal lesion).
Laboratory data revealed a reactive rapid plasma reagin with treponemal IgG titers of 1:64. Urine chlamydia RNA probe and lymphogranuloma venereum (LGV) serum antibodies also were positive. Human immunodeficiency virus screening was negative. Positron emission tomography–computerized tomography revealed enlarged and hypermetabolic lymphadenopathy above and below the diaphragm.
After therapy with intravenous penicillin G and oral doxycycline for concurrent secondary syphilis and LGV, the patient’s macular eruption and constitutional symptoms resolved within weeks of the initial presentation. His lymphadenopathy improved, his rapid plasma reagin titer decreased, and his chlamydia RNA became undetectable. However, the skin lesions remained unchanged.
Incisional biopsies of 4 clinically distinct skin lesions revealed well-delineated dermal proliferations of cells with eosinophilic granular cytoplasm and indistinct cell borders (Figure 5). Two specimens displayed marked epidermal hyperplasia (Figure 6).
No atypical mitotic figures were identified. Immunohistochemistry for S-100 protein was diffusely positive in the neoplastic cells. Immunohistochemistry for Treponema pallidum was negative.
No mycobacterial or fungal organisms were identified in acid-fast bacillus, periodic acid–Schiff, or Gomori methenamine-silver–stained sections. All 4 lesions had histopathologic findings characteristic of granular cell tumors (GCTs). A lesion in the left inguinal region (Figure 4 [medial lesion]), which initially was thought to be condyloma latum or a squamous cell carcinoma (SCC), also was later confirmed to be a GCT.
Repeat positron emission tomography–computerized tomography several weeks later confirmed resolution of the previously noted lymphadenopathy. Although 2 GCTs have not recurred after biopsy, the other 2, which the patient refused to have completely excised, continued to grow. Follow-up 2.5 years after hospitalization revealed persistence of the lesions with no remarkable morphological changes.
| 
| |
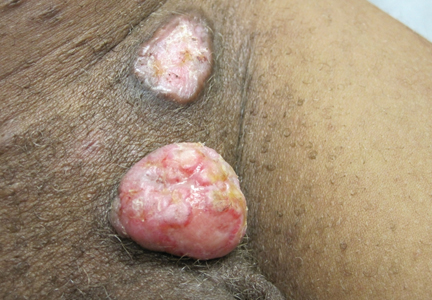
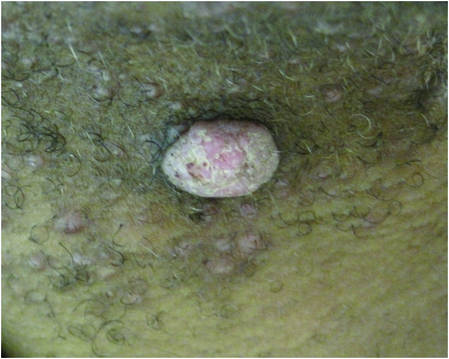
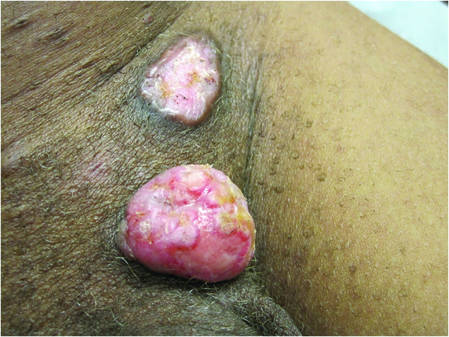
| Figure 3. Verrucous pink papule on the right side of the neck. | Figure 4. Ulcerated angular plaque in the left inguinal/genital area with rolled borders and tumor extension deep to the subcutis adjacent to a vegetative, eroded, exophytic tumor with palpable deep extension. | |
| 
| |
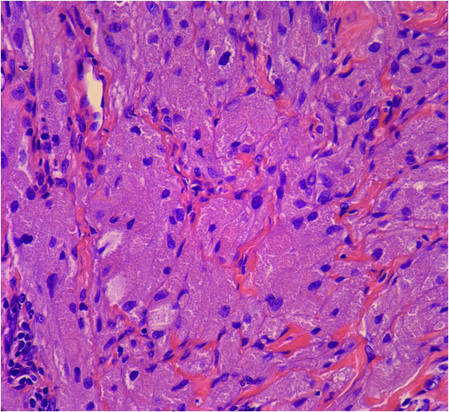
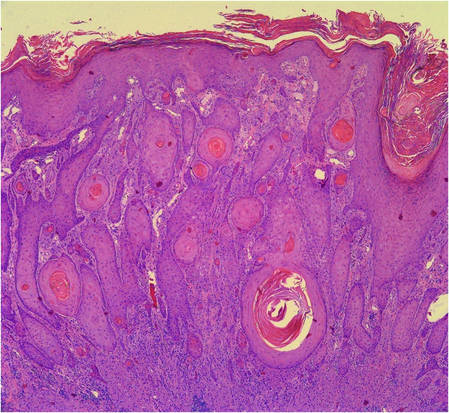
Figure 5. Large polygonal cells with eosinophilic granular cytoplasm, prominent bland nuclei, and indistinct cell borders (H&E, original magnification ×40). | Figure 6. Marked pseudoepitheliomatous hyperplasia (H&E, original magnification ×10). |
Comment
First described in 1854, GCTs are uncommon neoplasms of probable Schwann cell origin that can arise in almost any location of the body but most often appear on the skin and in the subcutaneous tissues and oral cavity.1,2 The commonly regarded rule of thirds describes its most favored locations: one-third on the tongue, one-third on the skin, and one-third in internal organs.3,4 Granular cell tumors occur with greater frequency in adults, females, and black individuals.1-5
Cutaneous GCTs usually present as solitary asymptomatic masses; however, multiple tumors have been noted in up to 25% of reported cases.4,6 In children, multiple cutaneous GCTs have been reported in the setting of neurofibromatosis type I as well as with other disorders.2,5,7-9
Cutaneous GCTs have been reported to range from sessile, pedunculated, or verrucous nodules to subcutaneous papules and nodules with no epidermal change. Our case not only illustrated the diverse clinical appearance of cutaneous GCTs but also demonstrated multiple morphologically distinct cutaneous GCTs occurring in a single patient. Of particular interest is our patient’s coexisting secondary syphilis and LGV infections, which can pose a diagnostic dilemma to the unsuspecting clinician. The manifold appearances of this patient’s GCTs resulted in a broad differential diagnosis. Syphilis (condyloma latum), condyloma acuminatum, LGV, metastatic disease, Kaposi sarcoma, lymphoma, dermatofibrosarcoma protuberans, leiomyoma, SCC, and deep fungal and atypical mycobacterial infection were all considerations. In 1981, Apisarnthanarax1 reviewed 88 cases of GCTs seen over a 15-year period and discovered that the preoperative clinical diagnoses were incorrect in all cases. Skin biopsy is necessary to diagnose GCT, and our patient’s case underscores the need for a thorough history, physical examination, and laboratory evaluation to rule out coexisting diseases.
Histopathology of cutaneous GCTs shows an unencapsulated dermal proliferation of large monotonous polygonal cells with blurred cell borders and fine, granular, eosinophilic cytoplasm arranged in irregular sheets and nests. Nuclei are small, uniform, round, centrally located, and rarely contain mitoses.3 The presence of mitotic activity on histopathology does not necessarily portend malignant biological behavior.5 Overlying pseudoepitheliomatous hyperplasia has been reported in as many as 85% of GCTs and may mimic SCC.10 The neoplastic cells stain positively with S-100 protein, neuron-specific enolase, and peripheral nerve myelin proteins.3,4 The cytoplasmic granules are positive on periodic acid–Schiff staining and diastase resistant and will sometimes stain for CD68.1 Electron microscopy shows degraded myelinated axons intracellularly.4
Malignancy is rare and reportedly occurs in 1% to 3% of cases.4,5 Consideration of both clinical behavior and histopathology is important in distinguishing benign from malignant lesions. According to published reports, in GCTs that were regarded as malignant, size tended to be greater than 4 cm, growth was rapid, and metastases to regional lymph nodes were observed.4,5 Histologically, nuclear pleomorphism and atypia, cell spindling, vesicular nuclei with prominent nucleoli, necrosis, and high mitotic activity favor malignancy.1,3
Treatment is complete surgical excision. Observation is acceptable if tumors are asymptomatic and do not impede function. Regression of some GCTs has been induced with use of intralesional corticosteroids.5 Spontaneous regression is rare. Prior reports have emphasized the importance of long-term follow-up in patients with multiple GCTs to monitor for development of systemic lesions.4
1. Apisarnthanarax P. Granular cell tumor. an analysis of 16 cases and review of the literature. J Am Acad Dermatol. 1981;5:171-182.
2. Guiglia MC, Prendiville JS. Multiple granular cell tumors associated with giant speckled lentiginous nevus and nevus flammeus in a child. J Am Acad Dermatol. 1991;24(2, pt 2):359-363.
3. Hazan C, Fangman W. Multiple cutaneous granular-cell tumors. Dermatol Online J. 2007;13:4.
4. Gross VL, Lynfield Y. Multiple cutaneous granular cell tumors: a case report and review of the literature. Cutis. 2002;69:343-346.
5. Martin RW 3rd, Neldner KH, Boyd AS, et al. Multiple cutaneous granular cell tumors and neurofibromatosis in childhood. a case report and review of the literature. Arch Dermatol. 1990;126:1051-1056.
6. Janousková G, Campr V, Konkol’ová R, et al. Multiple granular cell tumour. J Eur Acad Dermatol Venereol. 2004;18:347-349.
7. Gunson TH, Hashim N, Sharpe GR. Generalized lentiginosis, short stature, and multiple cutaneous nodules—quiz case. LEOPARD syndrome (LS) associated with multiple granular cell tumors (GCTs). Arch Dermatol. 2010;146:337-342.
8. De Raeve L, Roseeuw D, Otten J. Multiple cutaneous granular cell tumors in a child in remission for Hodgkin’s disease. J Am Acad Dermatol. 2002;47(2 suppl):S180-S182.
9. Ramaswamy PV, Storm CA, Filiano JJ, et al. Multiple granular cell tumors in a child with Noonan syndrome. Pediatr Dermatol. 2010;27:209-211.
10. Bangle R Jr. A morphological and histochemical study of the granular-cell myoblastoma. Cancer. 1952;5:950-965.
Case Report
A 27-year-old black man was admitted to the hospital with chills; night sweats; unintentional 25-lb weight loss; and multiple widespread, painful, progressively enlarging skin nodules of 3 months’ duration. The lesions had first developed on the back and later appeared on the face, trunk, arms, thighs, and genital region. He denied dysuria or urethral discharge. He had a remote history of adequately treated chlamydia infection but no other remarkable personal or family history.

| ||
| Figure 1. Firm subcutaneous nodules on the back with no epidermal change. | ||

| ||
| Figure 2. Firm dermal papule on the anterior aspect of the left shoulder with violaceous hyperpigmentation (dermatofibromalike). |
Physical examination revealed a thin man with more than 20 lesions on the face, trunk, arms, thighs, and genital region ranging in size from 1 to 4 cm. Lesion morphologies varied greatly and included subcutaneous firm nodules with no epidermal change (Figure 1); dermatofibromalike nodules with overlying erythema and hyperpigmentation (Figure 2); condylomalike, verrucous, pink papulonodules (Figure 3); ulcerated angular plaques with rolled borders and palpable tumor extension deep (1–2 cm) to the subcutis (Figure 4); and a vegetative, eroded, exophytic tumor with palpable deep extension (Figure 4). A diffuse, erythematous, macular eruption also was noted on the trunk and bilateral arms and legs including the soles of both feet along with nontender cervical, axillary, and inguinal lymphadenopathy. The ocular, oral, and nasal mucosae were not affected.
The differential diagnosis for each lesion differed based on morphology. Infectious, inflammatory, and neoplastic processes were considered, including syphilis, dermatofibroma, dermatofibrosarcoma protuberans, metastatic disease, leukemia cutis, sarcoidosis, panniculitis, condyloma acuminatum, and vegetative herpes simplex virus infection (inguinal lesion).
Laboratory data revealed a reactive rapid plasma reagin with treponemal IgG titers of 1:64. Urine chlamydia RNA probe and lymphogranuloma venereum (LGV) serum antibodies also were positive. Human immunodeficiency virus screening was negative. Positron emission tomography–computerized tomography revealed enlarged and hypermetabolic lymphadenopathy above and below the diaphragm.
After therapy with intravenous penicillin G and oral doxycycline for concurrent secondary syphilis and LGV, the patient’s macular eruption and constitutional symptoms resolved within weeks of the initial presentation. His lymphadenopathy improved, his rapid plasma reagin titer decreased, and his chlamydia RNA became undetectable. However, the skin lesions remained unchanged.
Incisional biopsies of 4 clinically distinct skin lesions revealed well-delineated dermal proliferations of cells with eosinophilic granular cytoplasm and indistinct cell borders (Figure 5). Two specimens displayed marked epidermal hyperplasia (Figure 6).
No atypical mitotic figures were identified. Immunohistochemistry for S-100 protein was diffusely positive in the neoplastic cells. Immunohistochemistry for Treponema pallidum was negative.
No mycobacterial or fungal organisms were identified in acid-fast bacillus, periodic acid–Schiff, or Gomori methenamine-silver–stained sections. All 4 lesions had histopathologic findings characteristic of granular cell tumors (GCTs). A lesion in the left inguinal region (Figure 4 [medial lesion]), which initially was thought to be condyloma latum or a squamous cell carcinoma (SCC), also was later confirmed to be a GCT.
Repeat positron emission tomography–computerized tomography several weeks later confirmed resolution of the previously noted lymphadenopathy. Although 2 GCTs have not recurred after biopsy, the other 2, which the patient refused to have completely excised, continued to grow. Follow-up 2.5 years after hospitalization revealed persistence of the lesions with no remarkable morphological changes.

| 
| |
| Figure 3. Verrucous pink papule on the right side of the neck. | Figure 4. Ulcerated angular plaque in the left inguinal/genital area with rolled borders and tumor extension deep to the subcutis adjacent to a vegetative, eroded, exophytic tumor with palpable deep extension. | |

| 
| |
Figure 5. Large polygonal cells with eosinophilic granular cytoplasm, prominent bland nuclei, and indistinct cell borders (H&E, original magnification ×40). | Figure 6. Marked pseudoepitheliomatous hyperplasia (H&E, original magnification ×10). |
Comment
First described in 1854, GCTs are uncommon neoplasms of probable Schwann cell origin that can arise in almost any location of the body but most often appear on the skin and in the subcutaneous tissues and oral cavity.1,2 The commonly regarded rule of thirds describes its most favored locations: one-third on the tongue, one-third on the skin, and one-third in internal organs.3,4 Granular cell tumors occur with greater frequency in adults, females, and black individuals.1-5
Cutaneous GCTs usually present as solitary asymptomatic masses; however, multiple tumors have been noted in up to 25% of reported cases.4,6 In children, multiple cutaneous GCTs have been reported in the setting of neurofibromatosis type I as well as with other disorders.2,5,7-9
Cutaneous GCTs have been reported to range from sessile, pedunculated, or verrucous nodules to subcutaneous papules and nodules with no epidermal change. Our case not only illustrated the diverse clinical appearance of cutaneous GCTs but also demonstrated multiple morphologically distinct cutaneous GCTs occurring in a single patient. Of particular interest is our patient’s coexisting secondary syphilis and LGV infections, which can pose a diagnostic dilemma to the unsuspecting clinician. The manifold appearances of this patient’s GCTs resulted in a broad differential diagnosis. Syphilis (condyloma latum), condyloma acuminatum, LGV, metastatic disease, Kaposi sarcoma, lymphoma, dermatofibrosarcoma protuberans, leiomyoma, SCC, and deep fungal and atypical mycobacterial infection were all considerations. In 1981, Apisarnthanarax1 reviewed 88 cases of GCTs seen over a 15-year period and discovered that the preoperative clinical diagnoses were incorrect in all cases. Skin biopsy is necessary to diagnose GCT, and our patient’s case underscores the need for a thorough history, physical examination, and laboratory evaluation to rule out coexisting diseases.
Histopathology of cutaneous GCTs shows an unencapsulated dermal proliferation of large monotonous polygonal cells with blurred cell borders and fine, granular, eosinophilic cytoplasm arranged in irregular sheets and nests. Nuclei are small, uniform, round, centrally located, and rarely contain mitoses.3 The presence of mitotic activity on histopathology does not necessarily portend malignant biological behavior.5 Overlying pseudoepitheliomatous hyperplasia has been reported in as many as 85% of GCTs and may mimic SCC.10 The neoplastic cells stain positively with S-100 protein, neuron-specific enolase, and peripheral nerve myelin proteins.3,4 The cytoplasmic granules are positive on periodic acid–Schiff staining and diastase resistant and will sometimes stain for CD68.1 Electron microscopy shows degraded myelinated axons intracellularly.4
Malignancy is rare and reportedly occurs in 1% to 3% of cases.4,5 Consideration of both clinical behavior and histopathology is important in distinguishing benign from malignant lesions. According to published reports, in GCTs that were regarded as malignant, size tended to be greater than 4 cm, growth was rapid, and metastases to regional lymph nodes were observed.4,5 Histologically, nuclear pleomorphism and atypia, cell spindling, vesicular nuclei with prominent nucleoli, necrosis, and high mitotic activity favor malignancy.1,3
Treatment is complete surgical excision. Observation is acceptable if tumors are asymptomatic and do not impede function. Regression of some GCTs has been induced with use of intralesional corticosteroids.5 Spontaneous regression is rare. Prior reports have emphasized the importance of long-term follow-up in patients with multiple GCTs to monitor for development of systemic lesions.4
Case Report
A 27-year-old black man was admitted to the hospital with chills; night sweats; unintentional 25-lb weight loss; and multiple widespread, painful, progressively enlarging skin nodules of 3 months’ duration. The lesions had first developed on the back and later appeared on the face, trunk, arms, thighs, and genital region. He denied dysuria or urethral discharge. He had a remote history of adequately treated chlamydia infection but no other remarkable personal or family history.

| ||
| Figure 1. Firm subcutaneous nodules on the back with no epidermal change. | ||

| ||
| Figure 2. Firm dermal papule on the anterior aspect of the left shoulder with violaceous hyperpigmentation (dermatofibromalike). |
Physical examination revealed a thin man with more than 20 lesions on the face, trunk, arms, thighs, and genital region ranging in size from 1 to 4 cm. Lesion morphologies varied greatly and included subcutaneous firm nodules with no epidermal change (Figure 1); dermatofibromalike nodules with overlying erythema and hyperpigmentation (Figure 2); condylomalike, verrucous, pink papulonodules (Figure 3); ulcerated angular plaques with rolled borders and palpable tumor extension deep (1–2 cm) to the subcutis (Figure 4); and a vegetative, eroded, exophytic tumor with palpable deep extension (Figure 4). A diffuse, erythematous, macular eruption also was noted on the trunk and bilateral arms and legs including the soles of both feet along with nontender cervical, axillary, and inguinal lymphadenopathy. The ocular, oral, and nasal mucosae were not affected.
The differential diagnosis for each lesion differed based on morphology. Infectious, inflammatory, and neoplastic processes were considered, including syphilis, dermatofibroma, dermatofibrosarcoma protuberans, metastatic disease, leukemia cutis, sarcoidosis, panniculitis, condyloma acuminatum, and vegetative herpes simplex virus infection (inguinal lesion).
Laboratory data revealed a reactive rapid plasma reagin with treponemal IgG titers of 1:64. Urine chlamydia RNA probe and lymphogranuloma venereum (LGV) serum antibodies also were positive. Human immunodeficiency virus screening was negative. Positron emission tomography–computerized tomography revealed enlarged and hypermetabolic lymphadenopathy above and below the diaphragm.
After therapy with intravenous penicillin G and oral doxycycline for concurrent secondary syphilis and LGV, the patient’s macular eruption and constitutional symptoms resolved within weeks of the initial presentation. His lymphadenopathy improved, his rapid plasma reagin titer decreased, and his chlamydia RNA became undetectable. However, the skin lesions remained unchanged.
Incisional biopsies of 4 clinically distinct skin lesions revealed well-delineated dermal proliferations of cells with eosinophilic granular cytoplasm and indistinct cell borders (Figure 5). Two specimens displayed marked epidermal hyperplasia (Figure 6).
No atypical mitotic figures were identified. Immunohistochemistry for S-100 protein was diffusely positive in the neoplastic cells. Immunohistochemistry for Treponema pallidum was negative.
No mycobacterial or fungal organisms were identified in acid-fast bacillus, periodic acid–Schiff, or Gomori methenamine-silver–stained sections. All 4 lesions had histopathologic findings characteristic of granular cell tumors (GCTs). A lesion in the left inguinal region (Figure 4 [medial lesion]), which initially was thought to be condyloma latum or a squamous cell carcinoma (SCC), also was later confirmed to be a GCT.
Repeat positron emission tomography–computerized tomography several weeks later confirmed resolution of the previously noted lymphadenopathy. Although 2 GCTs have not recurred after biopsy, the other 2, which the patient refused to have completely excised, continued to grow. Follow-up 2.5 years after hospitalization revealed persistence of the lesions with no remarkable morphological changes.

| 
| |
| Figure 3. Verrucous pink papule on the right side of the neck. | Figure 4. Ulcerated angular plaque in the left inguinal/genital area with rolled borders and tumor extension deep to the subcutis adjacent to a vegetative, eroded, exophytic tumor with palpable deep extension. | |

| 
| |
Figure 5. Large polygonal cells with eosinophilic granular cytoplasm, prominent bland nuclei, and indistinct cell borders (H&E, original magnification ×40). | Figure 6. Marked pseudoepitheliomatous hyperplasia (H&E, original magnification ×10). |
Comment
First described in 1854, GCTs are uncommon neoplasms of probable Schwann cell origin that can arise in almost any location of the body but most often appear on the skin and in the subcutaneous tissues and oral cavity.1,2 The commonly regarded rule of thirds describes its most favored locations: one-third on the tongue, one-third on the skin, and one-third in internal organs.3,4 Granular cell tumors occur with greater frequency in adults, females, and black individuals.1-5
Cutaneous GCTs usually present as solitary asymptomatic masses; however, multiple tumors have been noted in up to 25% of reported cases.4,6 In children, multiple cutaneous GCTs have been reported in the setting of neurofibromatosis type I as well as with other disorders.2,5,7-9
Cutaneous GCTs have been reported to range from sessile, pedunculated, or verrucous nodules to subcutaneous papules and nodules with no epidermal change. Our case not only illustrated the diverse clinical appearance of cutaneous GCTs but also demonstrated multiple morphologically distinct cutaneous GCTs occurring in a single patient. Of particular interest is our patient’s coexisting secondary syphilis and LGV infections, which can pose a diagnostic dilemma to the unsuspecting clinician. The manifold appearances of this patient’s GCTs resulted in a broad differential diagnosis. Syphilis (condyloma latum), condyloma acuminatum, LGV, metastatic disease, Kaposi sarcoma, lymphoma, dermatofibrosarcoma protuberans, leiomyoma, SCC, and deep fungal and atypical mycobacterial infection were all considerations. In 1981, Apisarnthanarax1 reviewed 88 cases of GCTs seen over a 15-year period and discovered that the preoperative clinical diagnoses were incorrect in all cases. Skin biopsy is necessary to diagnose GCT, and our patient’s case underscores the need for a thorough history, physical examination, and laboratory evaluation to rule out coexisting diseases.
Histopathology of cutaneous GCTs shows an unencapsulated dermal proliferation of large monotonous polygonal cells with blurred cell borders and fine, granular, eosinophilic cytoplasm arranged in irregular sheets and nests. Nuclei are small, uniform, round, centrally located, and rarely contain mitoses.3 The presence of mitotic activity on histopathology does not necessarily portend malignant biological behavior.5 Overlying pseudoepitheliomatous hyperplasia has been reported in as many as 85% of GCTs and may mimic SCC.10 The neoplastic cells stain positively with S-100 protein, neuron-specific enolase, and peripheral nerve myelin proteins.3,4 The cytoplasmic granules are positive on periodic acid–Schiff staining and diastase resistant and will sometimes stain for CD68.1 Electron microscopy shows degraded myelinated axons intracellularly.4
Malignancy is rare and reportedly occurs in 1% to 3% of cases.4,5 Consideration of both clinical behavior and histopathology is important in distinguishing benign from malignant lesions. According to published reports, in GCTs that were regarded as malignant, size tended to be greater than 4 cm, growth was rapid, and metastases to regional lymph nodes were observed.4,5 Histologically, nuclear pleomorphism and atypia, cell spindling, vesicular nuclei with prominent nucleoli, necrosis, and high mitotic activity favor malignancy.1,3
Treatment is complete surgical excision. Observation is acceptable if tumors are asymptomatic and do not impede function. Regression of some GCTs has been induced with use of intralesional corticosteroids.5 Spontaneous regression is rare. Prior reports have emphasized the importance of long-term follow-up in patients with multiple GCTs to monitor for development of systemic lesions.4
1. Apisarnthanarax P. Granular cell tumor. an analysis of 16 cases and review of the literature. J Am Acad Dermatol. 1981;5:171-182.
2. Guiglia MC, Prendiville JS. Multiple granular cell tumors associated with giant speckled lentiginous nevus and nevus flammeus in a child. J Am Acad Dermatol. 1991;24(2, pt 2):359-363.
3. Hazan C, Fangman W. Multiple cutaneous granular-cell tumors. Dermatol Online J. 2007;13:4.
4. Gross VL, Lynfield Y. Multiple cutaneous granular cell tumors: a case report and review of the literature. Cutis. 2002;69:343-346.
5. Martin RW 3rd, Neldner KH, Boyd AS, et al. Multiple cutaneous granular cell tumors and neurofibromatosis in childhood. a case report and review of the literature. Arch Dermatol. 1990;126:1051-1056.
6. Janousková G, Campr V, Konkol’ová R, et al. Multiple granular cell tumour. J Eur Acad Dermatol Venereol. 2004;18:347-349.
7. Gunson TH, Hashim N, Sharpe GR. Generalized lentiginosis, short stature, and multiple cutaneous nodules—quiz case. LEOPARD syndrome (LS) associated with multiple granular cell tumors (GCTs). Arch Dermatol. 2010;146:337-342.
8. De Raeve L, Roseeuw D, Otten J. Multiple cutaneous granular cell tumors in a child in remission for Hodgkin’s disease. J Am Acad Dermatol. 2002;47(2 suppl):S180-S182.
9. Ramaswamy PV, Storm CA, Filiano JJ, et al. Multiple granular cell tumors in a child with Noonan syndrome. Pediatr Dermatol. 2010;27:209-211.
10. Bangle R Jr. A morphological and histochemical study of the granular-cell myoblastoma. Cancer. 1952;5:950-965.
1. Apisarnthanarax P. Granular cell tumor. an analysis of 16 cases and review of the literature. J Am Acad Dermatol. 1981;5:171-182.
2. Guiglia MC, Prendiville JS. Multiple granular cell tumors associated with giant speckled lentiginous nevus and nevus flammeus in a child. J Am Acad Dermatol. 1991;24(2, pt 2):359-363.
3. Hazan C, Fangman W. Multiple cutaneous granular-cell tumors. Dermatol Online J. 2007;13:4.
4. Gross VL, Lynfield Y. Multiple cutaneous granular cell tumors: a case report and review of the literature. Cutis. 2002;69:343-346.
5. Martin RW 3rd, Neldner KH, Boyd AS, et al. Multiple cutaneous granular cell tumors and neurofibromatosis in childhood. a case report and review of the literature. Arch Dermatol. 1990;126:1051-1056.
6. Janousková G, Campr V, Konkol’ová R, et al. Multiple granular cell tumour. J Eur Acad Dermatol Venereol. 2004;18:347-349.
7. Gunson TH, Hashim N, Sharpe GR. Generalized lentiginosis, short stature, and multiple cutaneous nodules—quiz case. LEOPARD syndrome (LS) associated with multiple granular cell tumors (GCTs). Arch Dermatol. 2010;146:337-342.
8. De Raeve L, Roseeuw D, Otten J. Multiple cutaneous granular cell tumors in a child in remission for Hodgkin’s disease. J Am Acad Dermatol. 2002;47(2 suppl):S180-S182.
9. Ramaswamy PV, Storm CA, Filiano JJ, et al. Multiple granular cell tumors in a child with Noonan syndrome. Pediatr Dermatol. 2010;27:209-211.
10. Bangle R Jr. A morphological and histochemical study of the granular-cell myoblastoma. Cancer. 1952;5:950-965.
Practice Points
- Granular cell tumors (GCTs) typically present as solitary lesions; however, multiple lesions occur in approximately 25% of cases.
- Granular cell tumors have a variable clinical appearance and may mimic malignant neoplasms (eg, squamous cell carcinoma) as well as infectious diseases (eg, condyloma, syphilis).
- The histological features of GCTs are distinctive, including an unencapsulated dermal proliferation of monotonous polygonal cells with indistinct borders and fine, granular, eosinophilic cytoplasm arranged in irregular sheets and nests.