User login
The increasing incidence of nonmelanoma skin cancer (NMSC) is a serious public health concern.1 Lesions often are identified on routine total-body examination, and there is a considerate burden on dermatologists to diagnose these lesions, which is both costly and results in a long wait time to see a specialist. Furthermore, standard care requires patients to attend multiple visits for the diagnosis and treatment of NMSC.
In recent decades, diagnosing basal cell carcinoma (BCC) has been facilitated by the handheld dermatoscope. The advent of dermoscopy has led to increased sensitivity and specificity of the NMSC diagnosis (estimated at 95%–99%) and has helped facilitate earlier diagnosis of BCC and reduce unnecessary biopsy of benign lesions.2-5 Dermoscopy also can be useful in monitoring response to treatment.5 Lesions that are detected early tend to be easier and less expensive to treat, a strong argument for the use of early detection techniques.6-8
More recently, in vivo reflectance confocal microscopy (RCM)(Vivascope 1500 [Caliber I.D.]) has become an acceptable means for confirming a BCC diagnosis, offering an alternative to tissue biopsy. Reflectance confocal microscopy can be reimbursed under Category I Current Procedural Terminology codes 96931 to 96936.9 Reflectance confocal microscopy is a noninvasive diagnostic technique that uses an 830-nm diode laser to enable visualization of a 0.5×0.5-mm patch of skin to a depth of 200 to 300 μm, which corresponds roughly to the papillary dermis. Reflectance confocal microscopy has the advantage of providing real-time diagnosis, enabling same-day treatment of BCC, and providing an efficient alternative to biopsy. Ultimately, these advantages are beneficial and time-saving for patients because biopsies can be painful; create a delay in diagnosis; and require further follow-up visits for treatment, which may be of importance to patients who have trouble attending multiple appointments.
Optical coherence tomography (OCT) is another noninvasive imaging device that is useful in BCC management. It uses an infrared broadband light source to visualize skin architecture to 2-mm deep with a 6×6-mm field of view.10 Although OCT does not offer the same cellular clarity as RCM, it allows visualization of a greater depth of skin and a wider field of view, making it a useful tool both in marginating NMSCs prior to treatment and monitoring response to treatment over time.11-16 Optical coherence tomography has demonstrated a high negative predictive value (92.1%) for BCC, which makes it useful for ruling out residual tumor in lesions undergoing management.17-19
With all available options, BCC management benefits from care that is tailored to the individual and the lesion, taking into account size and subtype because not every available treatment is appropriate. Lasers, including solid state, diode, dye, and gas types, are emerging as promising minimally invasive treatment modalities.20,21
Nonablative laser therapy with a pulsed dye laser (PDL) and fractional laser is an example; the principal investigator (PI) of this study (O.M.) recently reported a 95.70% clearance rate utilizing a PDL and fractional laser protocol.22 The 1064-nm Nd:YAG laser also has been used with PDL and as a stand-alone treatment. Jalian et al23 used PDL and the Nd:YAG laser on 13 BCC lesions, with a 58% (7/12) clearance rate after 4 treatments; all nonresponders were taking an anticoagulant, which inhibited the laser’s mechanism of action, according to the researchers.
Moskalik et al24 published a report of 3346 facial BCC lesions treated with pulsed Nd and pulsed Nd:YAG lasers, and included follow-up for as long as 5 years, with a 3.7% recurrence rate. Another report by Moskalik et al25 recorded a recurrence rate of 2.2% to 3.1% for BCCs that were followed for at least 5 years.
Ortiz et al26 reported use of the long-pulsed 1064-nm Nd:YAG laser to treat 13 lesions with biopsy-confirmed BCC on the trunk and extremities, with a 92% (12/13) clearance rate based on histologic analysis 1 month after laser treatment. In an expanded study of 31 patients by Ortiz et al,27 the histologic clearance rate was 90.3% (28/31)—also obtained after 1 month—after 1 Nd:YAG laser treatment, also treating lesions on the trunk and extremities. A further retrospective review of Nd:YAG laser treatment of BCC revealed a 100% clearance rate for 16 lesions (including lesions on the face) that were monitored for at least 6 months (mean duration, 9 months; range, 6–15 months).28 Optical coherence tomography imaging was used for one of the review’s lesions before and after treatment and suggested that the Nd:YAG laser works by selectively destroying the vasculature supplying BCC tumors while preserving surrounding healthy tissue.28
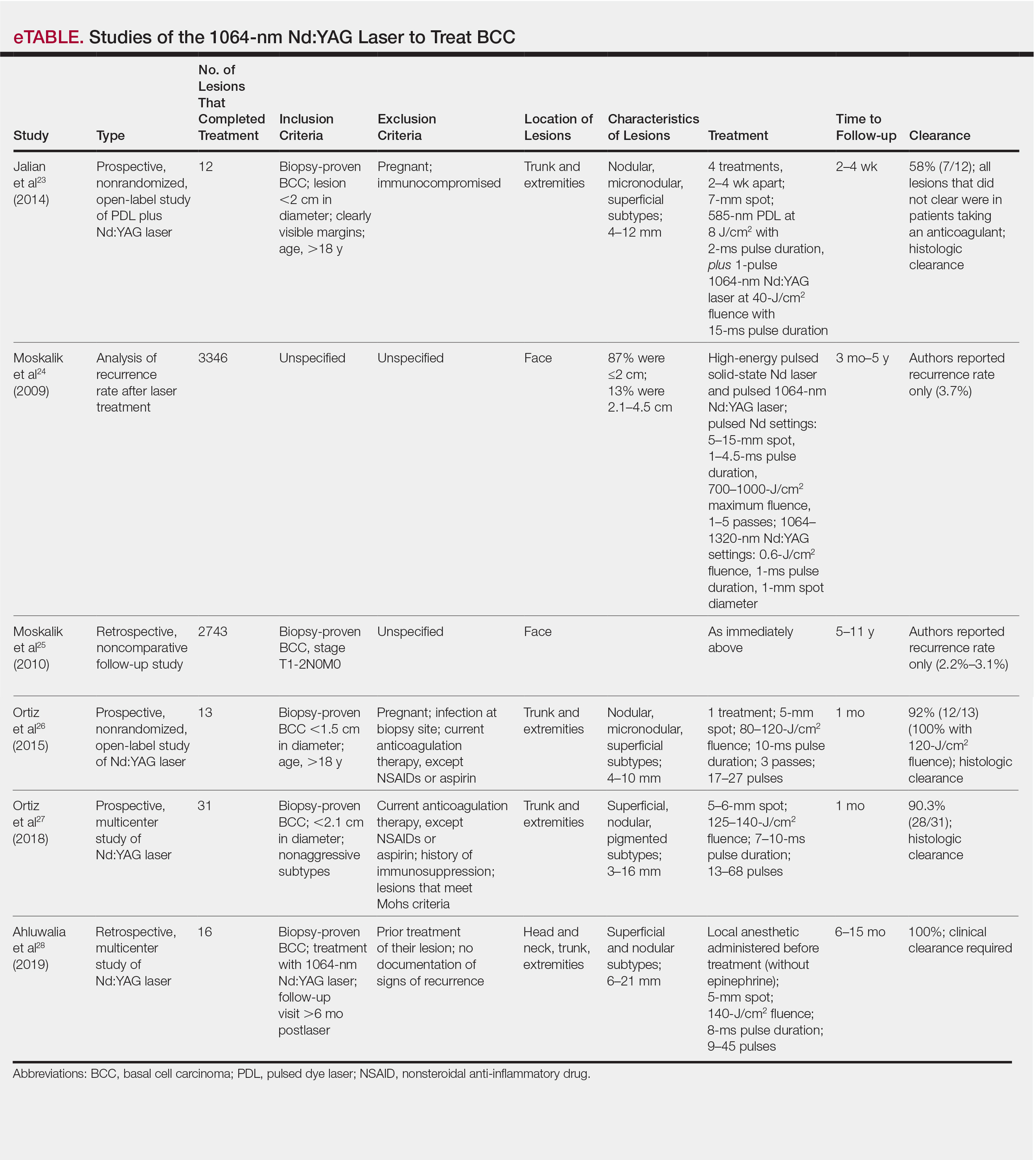
Apart from Moskalik et al,24,25 these studies are limited by a relatively short follow-up time to confirm tumor clearance. Prior studies utilizing the Nd:YAG laser to treat BCC are summarized in the eTable.
This pilot study describes a model of care that aims to alleviate some of the demand placed both on the specialty and on patients by utilizing a novel same-day approach to BCC management. We sought to evaluate management using noninvasive diagnosis with RCM; same-day laser treatment; and follow-up examination with clinical, dermoscopic, and noninvasive imaging using OCT. This method focuses on patient-driven health care from various perspectives. Patients are given real-time information about their diagnosis using RCM, leading to an increased level of information flow and immediate transparency regarding their diagnosis and management options. Patients also are receiving tailored care by incorporating noninvasive imaging and same-day laser treatment, allowing collaboration between patient and physician. Patients have more choices—to undergo surgical care; other at-home topical regimens; or laser management with potentially fewer visits, immediate results, a clearance rate similar to surgery, and improved cosmetic outcome.
Our study attempts to further evaluate the efficacy of the 1064-nm Nd:YAG laser in treating BCC while leveraging noninvasive imaging technology. The objective was to perform a retrospective review of medical records of a subgroup of patients with BCC diagnosed by RCM who were treated with the 1064-nm Nd:YAG laser and monitored for clearance using OCT imaging, in addition to clinical and dermoscopic examination. Similar to prior long-term Nd:YAG laser follow-up studies, we aimed to demonstrate the possibility of a minimally invasive BCC management approach—in our protocol, utilizing imaging instead of biopsy to facilitate long-term follow-up and by offering a model for patient-driven care.
Methods
Study Design
Institutional review board approval was received from Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects (New York, New York). We performed a retrospective review of medical records of patients diagnosed by RCM and treated with a 1064-nm Nd:YAG laser, as an alternative to surgery, at the Mount Sinai Faculty Practice Associates between March 2018 and August 2018. Included in this pilot study are 17 lesions in 16 patients.
Inclusion Criteria
Patients were enrolled based on the following criteria: BCCs diagnosed by clinical and dermoscopic examination followed by RCM imaging; treatment with the 1064-nm Nd:YAG laser, because of patients’ preference for this modality over surgery, superficial radiation therapy, topical regimens, and other laser therapies that require more visits; eligibility by PI included limited clinical ulceration or bleeding (or both) and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). The PI performed a detailed and thorough clinical and dermoscopic skin examination, enabling early detection of the BCCs. Basal cell carcinomas were not included if they exhibited rolled borders, visible ulceration, or oozing growths that allowed for treatment of less-advanced tumors. The PI utilized a clinical and dermoscopic color wheel algorithm to identify suspicious lesions combined with RCM for diagnostic confirmation.29
Two of 17 lesions that did not present as early lesions were included in the study due to patient refusal of surgery or radiation. We consider more advanced tumors to be exophytic, bleeding, crusting, nonhealing ulcerative growths. Patients who had received prior laser treatment with the PI’s PDL with fractional laser protocol with subsequent recurrence at the treatment site were included in the study. Lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor, were excluded.
Treatment Protocol
All patients attended the private clinic at Mount Sinai Hospital of a pigmented lesion expert (O.M.) for routine skin cancer screening. Patients with lesions suspicious for BCC—based on clinical and dermoscopic features—were offered tissue biopsy or RCM. Following diagnosis with RCM, treatment options were discussed, and patients were offered laser treatment when surgical options were declined. Topical treatment options were not emphasized because they require weeks of application to be effective and have been studied mainly in superficial BCC management.30,31
Patients with early lesions were offered either the PDL with fractional laser or Nd:YAG protocol, with their understanding that the Nd:YAG laser protocol would likely involve fewer treatments but a higher likelihood of residual hyperpigmentation or potential scarring (or both) than the more gentle PDL with fractional laser treatment.
All lesions on the face were premarginated using OCT by obtaining central scans and 4 additional scans—above, below, to the left, and to the right of the lesion—to ensure targeted laser treatment with desirable cosmetic results. Facial premargination scans were mandatory; however, patients with lesions on the trunk or extremities were offered the option to have pretreatment margination as an out-of-pocket expense. We did not require premargination of lesions on the body because of their location on less cosmetically critical areas. Most patients declined the optional scans.
This can be considered analogous to the situation in which more insurers reimburse Mohs surgery for cosmetically challenging areas such as the head and neck, while limiting reimbursement for treatment of lesions on the trunk and upper extremities to simple excision. Given cosmetic concerns on the head and neck compared to the body, some patients found it acceptable to have slightly increased dyschromia over a broader treatment area of non–cosmetically critical locations on the body.
Optical coherence tomography imaging was required for all anatomic locations at follow-up visits to detect residual disease or confirm clearance. All patients were given thorough information about the treatment, additional costs, treatment alternatives, potential adverse effects, and complications.
Clinical and dermoscopic images were obtained at every visit using a commercially available point-and-shoot digital single-lens reflex camera for clinical photographs, with an attached DermLite DL3N (3Gen) dermatoscope for all contact polarized dermoscopic photography.
Laser treatment was carried out with the 1064-nm Nd:YAG laser. Setting ranges were similar to previously published studies that used the 1064-nm Nd:YAG laser to treat BCCs (spot sizes, 5–6 mm; fluences, 125–140 J/cm2; pulse durations, 7–10 milliseconds).26-28 The exact settings and number of passes were tailored to the individual lesion based on skin type, anatomic location, extent of tumor involvement by depth (and margin on facial lesions), and posttreatment dermoscopic confirmation of clearance; additionally, for facial lesions, OCT confirmation of clearance.
Laser treatment was provided by the PI. Patients were instructed to apply a thick emollient (ie, formulation of petrolatum or 100% petrolatum) after treatment and until the area healed.
All tumors received 1 to 3 treatments at an interval of 1 to 2 months. The treatment end point was complete clearance, judged by absence of skin cancer clinically, dermoscopically, and on OCT scan. More specifically, the PI looked for vascular changes and echogenic changes on OCT consistent with tumor clearance as well as dermoscopic disappearance of recognized BCC features.
Patients were asked to return for follow-up visits 2 months after the final treatment to evaluate tumor clearance. They were asked to return subsequently every 6 to 12 months for routine care and long-term follow-up.
Results
Patient Characteristics
A total of 16 patients (6 female, 10 male) with 17 BCCs were included in this study. Mean age was 68 years (median, 71.5 years; range; 48–89 years). Mean lesion size was 7.1 mm (median, 6 mm; range, 3–15 mm). Eight lesions were on the face; 9 were on extrafacial sites. Two lesions had a history of laser treatment with the PI’s PDL with fractional laser treatment protocol and had locally recurred. Subtypes of lesions were not elicited by RCM.
Outcomes
Fourteen lesions (14/17 [82.4%]) required 1 treatment to achieve clearance, as confirmed clinically, dermoscopically, and by OCT scanning. One lesion on the back (1/17 [5.8%]) required 2 treatments (70 days between treatments). Two lesions (2/17 [11.8%]) required 3 treatments (time between treatments: 49 and 61 days [lesion 1]; 62 and 64 days [lesion 2]). Lesion 1 was on the face; lesion 2 was on the back. Mean time between last treatment and OCT clearance scan was 103 days (median, 64 days; range, 48–371 days).
Comment
Our study supports the notion that the 1064-nm Nd:YAG laser is a viable option for treating BCC. All (100%) lesions cleared, most (82.4%) with a single treatment. Of course, for patients who required more than 1 treatment (17.6%), we cannot make an argument for fewer patient visits because those patients had to return for multiple laser treatments, but they were able to avoid surgery, as they had wanted. Overall, our diagnostic approach utilizing RCM as opposed to traditional tissue biopsy meant that patients’ skin cancers were diagnosed and treated the same day.
A one-stop shop for diagnosis and treatment model has been reported by Kadouch et al32 as part of a randomized controlled trial in which patients were randomly assigned to receive standard care for BCC—biopsy followed by surgical excision—or RCM diagnosis followed by surgical excision. Their outcome was tumor-free margins after surgical treatment; the RCM approach was found to be noninferior to standard care.32 Our retrospective study differs, of course, in its laser treatment approach; however, both studies investigated a potentially more efficient pathway to BCC management, which becomes increasingly relevant given the rising incidence of NMSC.
A real-time, image-based diagnostic approach combined with laser treatment delivers patient-driven care, offering choice and convenience. It might be optimal for patients who have an extensive history of BCC, are poor surgical candidates, have difficulty with the logistics of the multiple visits required for surgical management, cannot (for practical reasons) spend multiple hours in office between Mohs stages, and do not want potentially disfiguring scars, making a minimally invasive treatment preferable.
As we found in our sample, not all patients are amenable to undergoing what is regarded now as the most definitive treatment—namely, surgical options. This subset of patients, whose lesions require more definitive treatment but who do not desire invasive management, need alternative approaches to BCC treatment. The present study proposes a model of patient-driven care that requires collaboration between physician and patient, offering more customized care that takes into account patient choice.
In our study, most patients had lesions that were detected early in their evolution; these lesions might be particularly amenable to laser management. The 2 resistant lesions in our set—requiring 3 treatments—appeared more aggressive clinically at initial evaluation but still had posttreatment outcomes with mild dyschromia similar to the lesions only treated once (Figure, A–D). Of those 2 lesions, the 9-mm lesion on the back (Figure, C and D) might have been larger than clinically apparent; in hindsight, it might have responded to a single treatment had it been premarginated. (An additional factor to have considered is the patient’s immunosuppressed status, which might have led to a more resistant lesion. Larger trials would help elucidate whether an immunosuppressed patient requires a different treatment approach, broader treatment area, OCT premargination regardless of anatomic location, or a greater number of treatments.) Nevertheless, the 2 aforementioned patients were offered treatment with the 1064-nm Nd:YAG laser because they refused surgery, radiation, and other more aggressive modalities. The patients were given advanced warning of an increased possibility of recurrence or nonclearance.
The lesion that required 2 treatments did not appear to be an aggressive subtype; however, it was considerably larger than most other treated lesions (1.5 cm)(Figure, E and F). In this patient, as with the others, we utilized milder (700–1000 J) fluence settings than those used in the Moskalik et al24 study; however, we were optimizing for patient comfort, overall downtime, and cosmetic outcomes.
Clearance in this study was assessed by OCT scanning. Scans were obtained 2 months after the last treatment to avoid detecting inflammation and early scar tissue. We opted not to perform biopsies to determine clearance, as done in prior studies, because we were investigating a fully nonsurgical protocol and wanted to enable patients to avoid surgical intervention, as they had requested. Clinical and dermoscopic examinations by a world expert in dermoscopy and OCT (O.M.) provided additional reassurance of lesion clearance.
Limitations
The retrospective study design with a limited sample size was a main limitation of our study. Our limited data suggest that there is value in further investigation and prospective trials of minimally invasive skin cancer management with the pulsed 1064-nm Nd:YAG laser.
Limitations or disadvantages of this nonablative laser treatment include dyschromia and minimal scarring. Furthermore, at fluence settings utilized, treatment can be painful. Without use of a local anesthetic, treatment is limited to what patients can tolerate.
The percentage of BCCs located on the body (53%) was higher in our study than in the general population, estimated in a study to be approximately 20%.33 This percentage might have been an effect of the larger Vivascope 1500 RCM probe, which made certain areas of the face difficult to access, therefore excluding certain facial lesions encountered in our practice from the initial noninvasive diagnosis.
Most lesions in our study have not been followed long-term; median noninvasive OCT follow-up was 64 days; however, the longest follow-up from our data set is longer than 1 year posttreatment (371 days). We have used OCT to establish clearance, which also will allow us to continue using imaging to monitor for changes that might indicate recurrence. Although OCT is not approved by the US Food and Drug Administration as a validated means of diagnosing and detecting BCC, numerous studies have suggested that this modality has high sensitivity (95.7%) and specificity (75.3%) for features of BCC as well as the more critical high negative predictive value (92.1%) for noninvasive management.22-24
Furthermore, setting up the lesions to be monitored long-term using OCT is likely to be more sensitive than monitoring lesions by clinical examination alone, as they have been followed in studies to date. In fact, an earlier study of 115 lesions by the PI found that utilizing OCT significantly improved sensitivity and specificity for detecting BCC (P<.01); improved diagnostic certainty by a factor of 4 compared to clinical examination alone; and improved overall diagnostic accuracy by 50% compared to clinical and dermoscopic examinations.19
Conclusion
Traditional approaches to BCC management usually involve multiple visits: the initial encounter, which might or might not include biopsy, and a return visit for more definitive management. Reflectance confocal microscopy enables live diagnosis and facilitates targeted same-day treatment of BCC. Our pilot study has contributed data to support the further investigation and use of the Nd:YAG laser to treat BCC in combination with early detection with noninvasive diagnosis for a more patient-driven approach. For some patients as well as for dermatologists, the potential for increased efficiency of same-day diagnosis and treatment might provide a clear advantage.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Menzies SW, Westerhoff K, Rabinovitz H, et al. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136:1012-1016.
- Altamura D, Menzies SW, Argenziano G, et al. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62:67-75.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Lallas A, Apalla Z, Ioannides D, et al. Dermoscopy in the diagnosis and management of basal cell carcinoma. Futur Oncol. 2015;11:2975-2984.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:202a10.
- Kauvar AN, Cronin T Jr, Roenigk R, et al; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41:550-571.
- Hoorens I, Vossaert K, Ongenae K, et al. Is early detection of basal cell carcinoma worthwhile? Systematic review based on the WHO criteria for screening. Br J Dermatol. 2016;174:1258-1265.
- Levine A, Markowitz O. In vivo reflectance confocal microscopy. Cutis. 2017;99:399-402.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer - a practical approach. Exp Dermatol. 2013;22:547-551.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- van Manen L, Dijkstra J, Boccara C, et al. The clinical usefulness of optical coherence tomography during cancer interventions. J Cancer Res Clin Oncol. 2018;144:1967-1990.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Markowitz O, Schwartz M. The use of noninvasive optical coherence tomography to monitor the treatment progress of vismodegib and imiquimod 5% cream in a transplant patient with advanced basal cell carcinoma of the nose. J Clin Aesthet Dermatol. 2016;9:37-41.
- Cheng HM, Guitera P. Systematic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Mirza FN, Khatri KA. The use of lasers in the treatment of skin cancer: a review. J Cosmet Laser Ther. 2017;19:451-458.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297.
- Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Moskalik K, Kozlow A, Demin E, et al. Powerful neodymium laser radiation for the treatment of facial carcinoma: 5 year follow-up data. Eur J Dermatol. 2010;20:738-742.
- Ortiz AE, Anderson RR, Avram MM. 1064 nm long-pulsed Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2015;47:106-110.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Papakostas D, Stockfleth E. Topical treatment of basal cell carcinoma with the immune response modifier imiquimod. Futur Oncol. 2015;11:2985-2990.
- Jansen MHE, Mosterd K, Arits AHMM, et al. Five-year results of a randomized controlled trial comparing effectiveness of photodynamic therapy, topical imiquimod, and topical 5-fluorouracil in patients with superficial basal cell carcinoma. J Invest Dermatol. 2018;138:527-533.
- Kadouch DJ, Elshot YS, Zupan-Kajcovski B, et al. One-stop-shop with confocal microscopy imaging vs. standard care for surgical treatment of basal cell carcinoma: an open-label, noninferiority, randomized controlled multicentre trial. Br J Dermatol. 2017;177:735-741.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
The increasing incidence of nonmelanoma skin cancer (NMSC) is a serious public health concern.1 Lesions often are identified on routine total-body examination, and there is a considerate burden on dermatologists to diagnose these lesions, which is both costly and results in a long wait time to see a specialist. Furthermore, standard care requires patients to attend multiple visits for the diagnosis and treatment of NMSC.
In recent decades, diagnosing basal cell carcinoma (BCC) has been facilitated by the handheld dermatoscope. The advent of dermoscopy has led to increased sensitivity and specificity of the NMSC diagnosis (estimated at 95%–99%) and has helped facilitate earlier diagnosis of BCC and reduce unnecessary biopsy of benign lesions.2-5 Dermoscopy also can be useful in monitoring response to treatment.5 Lesions that are detected early tend to be easier and less expensive to treat, a strong argument for the use of early detection techniques.6-8
More recently, in vivo reflectance confocal microscopy (RCM)(Vivascope 1500 [Caliber I.D.]) has become an acceptable means for confirming a BCC diagnosis, offering an alternative to tissue biopsy. Reflectance confocal microscopy can be reimbursed under Category I Current Procedural Terminology codes 96931 to 96936.9 Reflectance confocal microscopy is a noninvasive diagnostic technique that uses an 830-nm diode laser to enable visualization of a 0.5×0.5-mm patch of skin to a depth of 200 to 300 μm, which corresponds roughly to the papillary dermis. Reflectance confocal microscopy has the advantage of providing real-time diagnosis, enabling same-day treatment of BCC, and providing an efficient alternative to biopsy. Ultimately, these advantages are beneficial and time-saving for patients because biopsies can be painful; create a delay in diagnosis; and require further follow-up visits for treatment, which may be of importance to patients who have trouble attending multiple appointments.
Optical coherence tomography (OCT) is another noninvasive imaging device that is useful in BCC management. It uses an infrared broadband light source to visualize skin architecture to 2-mm deep with a 6×6-mm field of view.10 Although OCT does not offer the same cellular clarity as RCM, it allows visualization of a greater depth of skin and a wider field of view, making it a useful tool both in marginating NMSCs prior to treatment and monitoring response to treatment over time.11-16 Optical coherence tomography has demonstrated a high negative predictive value (92.1%) for BCC, which makes it useful for ruling out residual tumor in lesions undergoing management.17-19
With all available options, BCC management benefits from care that is tailored to the individual and the lesion, taking into account size and subtype because not every available treatment is appropriate. Lasers, including solid state, diode, dye, and gas types, are emerging as promising minimally invasive treatment modalities.20,21
Nonablative laser therapy with a pulsed dye laser (PDL) and fractional laser is an example; the principal investigator (PI) of this study (O.M.) recently reported a 95.70% clearance rate utilizing a PDL and fractional laser protocol.22 The 1064-nm Nd:YAG laser also has been used with PDL and as a stand-alone treatment. Jalian et al23 used PDL and the Nd:YAG laser on 13 BCC lesions, with a 58% (7/12) clearance rate after 4 treatments; all nonresponders were taking an anticoagulant, which inhibited the laser’s mechanism of action, according to the researchers.
Moskalik et al24 published a report of 3346 facial BCC lesions treated with pulsed Nd and pulsed Nd:YAG lasers, and included follow-up for as long as 5 years, with a 3.7% recurrence rate. Another report by Moskalik et al25 recorded a recurrence rate of 2.2% to 3.1% for BCCs that were followed for at least 5 years.
Ortiz et al26 reported use of the long-pulsed 1064-nm Nd:YAG laser to treat 13 lesions with biopsy-confirmed BCC on the trunk and extremities, with a 92% (12/13) clearance rate based on histologic analysis 1 month after laser treatment. In an expanded study of 31 patients by Ortiz et al,27 the histologic clearance rate was 90.3% (28/31)—also obtained after 1 month—after 1 Nd:YAG laser treatment, also treating lesions on the trunk and extremities. A further retrospective review of Nd:YAG laser treatment of BCC revealed a 100% clearance rate for 16 lesions (including lesions on the face) that were monitored for at least 6 months (mean duration, 9 months; range, 6–15 months).28 Optical coherence tomography imaging was used for one of the review’s lesions before and after treatment and suggested that the Nd:YAG laser works by selectively destroying the vasculature supplying BCC tumors while preserving surrounding healthy tissue.28
Apart from Moskalik et al,24,25 these studies are limited by a relatively short follow-up time to confirm tumor clearance. Prior studies utilizing the Nd:YAG laser to treat BCC are summarized in the eTable.

This pilot study describes a model of care that aims to alleviate some of the demand placed both on the specialty and on patients by utilizing a novel same-day approach to BCC management. We sought to evaluate management using noninvasive diagnosis with RCM; same-day laser treatment; and follow-up examination with clinical, dermoscopic, and noninvasive imaging using OCT. This method focuses on patient-driven health care from various perspectives. Patients are given real-time information about their diagnosis using RCM, leading to an increased level of information flow and immediate transparency regarding their diagnosis and management options. Patients also are receiving tailored care by incorporating noninvasive imaging and same-day laser treatment, allowing collaboration between patient and physician. Patients have more choices—to undergo surgical care; other at-home topical regimens; or laser management with potentially fewer visits, immediate results, a clearance rate similar to surgery, and improved cosmetic outcome.
Our study attempts to further evaluate the efficacy of the 1064-nm Nd:YAG laser in treating BCC while leveraging noninvasive imaging technology. The objective was to perform a retrospective review of medical records of a subgroup of patients with BCC diagnosed by RCM who were treated with the 1064-nm Nd:YAG laser and monitored for clearance using OCT imaging, in addition to clinical and dermoscopic examination. Similar to prior long-term Nd:YAG laser follow-up studies, we aimed to demonstrate the possibility of a minimally invasive BCC management approach—in our protocol, utilizing imaging instead of biopsy to facilitate long-term follow-up and by offering a model for patient-driven care.
Methods
Study Design
Institutional review board approval was received from Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects (New York, New York). We performed a retrospective review of medical records of patients diagnosed by RCM and treated with a 1064-nm Nd:YAG laser, as an alternative to surgery, at the Mount Sinai Faculty Practice Associates between March 2018 and August 2018. Included in this pilot study are 17 lesions in 16 patients.
Inclusion Criteria
Patients were enrolled based on the following criteria: BCCs diagnosed by clinical and dermoscopic examination followed by RCM imaging; treatment with the 1064-nm Nd:YAG laser, because of patients’ preference for this modality over surgery, superficial radiation therapy, topical regimens, and other laser therapies that require more visits; eligibility by PI included limited clinical ulceration or bleeding (or both) and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). The PI performed a detailed and thorough clinical and dermoscopic skin examination, enabling early detection of the BCCs. Basal cell carcinomas were not included if they exhibited rolled borders, visible ulceration, or oozing growths that allowed for treatment of less-advanced tumors. The PI utilized a clinical and dermoscopic color wheel algorithm to identify suspicious lesions combined with RCM for diagnostic confirmation.29
Two of 17 lesions that did not present as early lesions were included in the study due to patient refusal of surgery or radiation. We consider more advanced tumors to be exophytic, bleeding, crusting, nonhealing ulcerative growths. Patients who had received prior laser treatment with the PI’s PDL with fractional laser protocol with subsequent recurrence at the treatment site were included in the study. Lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor, were excluded.
Treatment Protocol
All patients attended the private clinic at Mount Sinai Hospital of a pigmented lesion expert (O.M.) for routine skin cancer screening. Patients with lesions suspicious for BCC—based on clinical and dermoscopic features—were offered tissue biopsy or RCM. Following diagnosis with RCM, treatment options were discussed, and patients were offered laser treatment when surgical options were declined. Topical treatment options were not emphasized because they require weeks of application to be effective and have been studied mainly in superficial BCC management.30,31
Patients with early lesions were offered either the PDL with fractional laser or Nd:YAG protocol, with their understanding that the Nd:YAG laser protocol would likely involve fewer treatments but a higher likelihood of residual hyperpigmentation or potential scarring (or both) than the more gentle PDL with fractional laser treatment.
All lesions on the face were premarginated using OCT by obtaining central scans and 4 additional scans—above, below, to the left, and to the right of the lesion—to ensure targeted laser treatment with desirable cosmetic results. Facial premargination scans were mandatory; however, patients with lesions on the trunk or extremities were offered the option to have pretreatment margination as an out-of-pocket expense. We did not require premargination of lesions on the body because of their location on less cosmetically critical areas. Most patients declined the optional scans.
This can be considered analogous to the situation in which more insurers reimburse Mohs surgery for cosmetically challenging areas such as the head and neck, while limiting reimbursement for treatment of lesions on the trunk and upper extremities to simple excision. Given cosmetic concerns on the head and neck compared to the body, some patients found it acceptable to have slightly increased dyschromia over a broader treatment area of non–cosmetically critical locations on the body.
Optical coherence tomography imaging was required for all anatomic locations at follow-up visits to detect residual disease or confirm clearance. All patients were given thorough information about the treatment, additional costs, treatment alternatives, potential adverse effects, and complications.
Clinical and dermoscopic images were obtained at every visit using a commercially available point-and-shoot digital single-lens reflex camera for clinical photographs, with an attached DermLite DL3N (3Gen) dermatoscope for all contact polarized dermoscopic photography.
Laser treatment was carried out with the 1064-nm Nd:YAG laser. Setting ranges were similar to previously published studies that used the 1064-nm Nd:YAG laser to treat BCCs (spot sizes, 5–6 mm; fluences, 125–140 J/cm2; pulse durations, 7–10 milliseconds).26-28 The exact settings and number of passes were tailored to the individual lesion based on skin type, anatomic location, extent of tumor involvement by depth (and margin on facial lesions), and posttreatment dermoscopic confirmation of clearance; additionally, for facial lesions, OCT confirmation of clearance.
Laser treatment was provided by the PI. Patients were instructed to apply a thick emollient (ie, formulation of petrolatum or 100% petrolatum) after treatment and until the area healed.
All tumors received 1 to 3 treatments at an interval of 1 to 2 months. The treatment end point was complete clearance, judged by absence of skin cancer clinically, dermoscopically, and on OCT scan. More specifically, the PI looked for vascular changes and echogenic changes on OCT consistent with tumor clearance as well as dermoscopic disappearance of recognized BCC features.
Patients were asked to return for follow-up visits 2 months after the final treatment to evaluate tumor clearance. They were asked to return subsequently every 6 to 12 months for routine care and long-term follow-up.
Results
Patient Characteristics
A total of 16 patients (6 female, 10 male) with 17 BCCs were included in this study. Mean age was 68 years (median, 71.5 years; range; 48–89 years). Mean lesion size was 7.1 mm (median, 6 mm; range, 3–15 mm). Eight lesions were on the face; 9 were on extrafacial sites. Two lesions had a history of laser treatment with the PI’s PDL with fractional laser treatment protocol and had locally recurred. Subtypes of lesions were not elicited by RCM.
Outcomes
Fourteen lesions (14/17 [82.4%]) required 1 treatment to achieve clearance, as confirmed clinically, dermoscopically, and by OCT scanning. One lesion on the back (1/17 [5.8%]) required 2 treatments (70 days between treatments). Two lesions (2/17 [11.8%]) required 3 treatments (time between treatments: 49 and 61 days [lesion 1]; 62 and 64 days [lesion 2]). Lesion 1 was on the face; lesion 2 was on the back. Mean time between last treatment and OCT clearance scan was 103 days (median, 64 days; range, 48–371 days).
Comment
Our study supports the notion that the 1064-nm Nd:YAG laser is a viable option for treating BCC. All (100%) lesions cleared, most (82.4%) with a single treatment. Of course, for patients who required more than 1 treatment (17.6%), we cannot make an argument for fewer patient visits because those patients had to return for multiple laser treatments, but they were able to avoid surgery, as they had wanted. Overall, our diagnostic approach utilizing RCM as opposed to traditional tissue biopsy meant that patients’ skin cancers were diagnosed and treated the same day.
A one-stop shop for diagnosis and treatment model has been reported by Kadouch et al32 as part of a randomized controlled trial in which patients were randomly assigned to receive standard care for BCC—biopsy followed by surgical excision—or RCM diagnosis followed by surgical excision. Their outcome was tumor-free margins after surgical treatment; the RCM approach was found to be noninferior to standard care.32 Our retrospective study differs, of course, in its laser treatment approach; however, both studies investigated a potentially more efficient pathway to BCC management, which becomes increasingly relevant given the rising incidence of NMSC.
A real-time, image-based diagnostic approach combined with laser treatment delivers patient-driven care, offering choice and convenience. It might be optimal for patients who have an extensive history of BCC, are poor surgical candidates, have difficulty with the logistics of the multiple visits required for surgical management, cannot (for practical reasons) spend multiple hours in office between Mohs stages, and do not want potentially disfiguring scars, making a minimally invasive treatment preferable.
As we found in our sample, not all patients are amenable to undergoing what is regarded now as the most definitive treatment—namely, surgical options. This subset of patients, whose lesions require more definitive treatment but who do not desire invasive management, need alternative approaches to BCC treatment. The present study proposes a model of patient-driven care that requires collaboration between physician and patient, offering more customized care that takes into account patient choice.
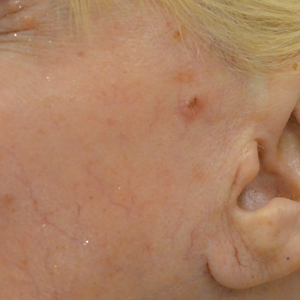
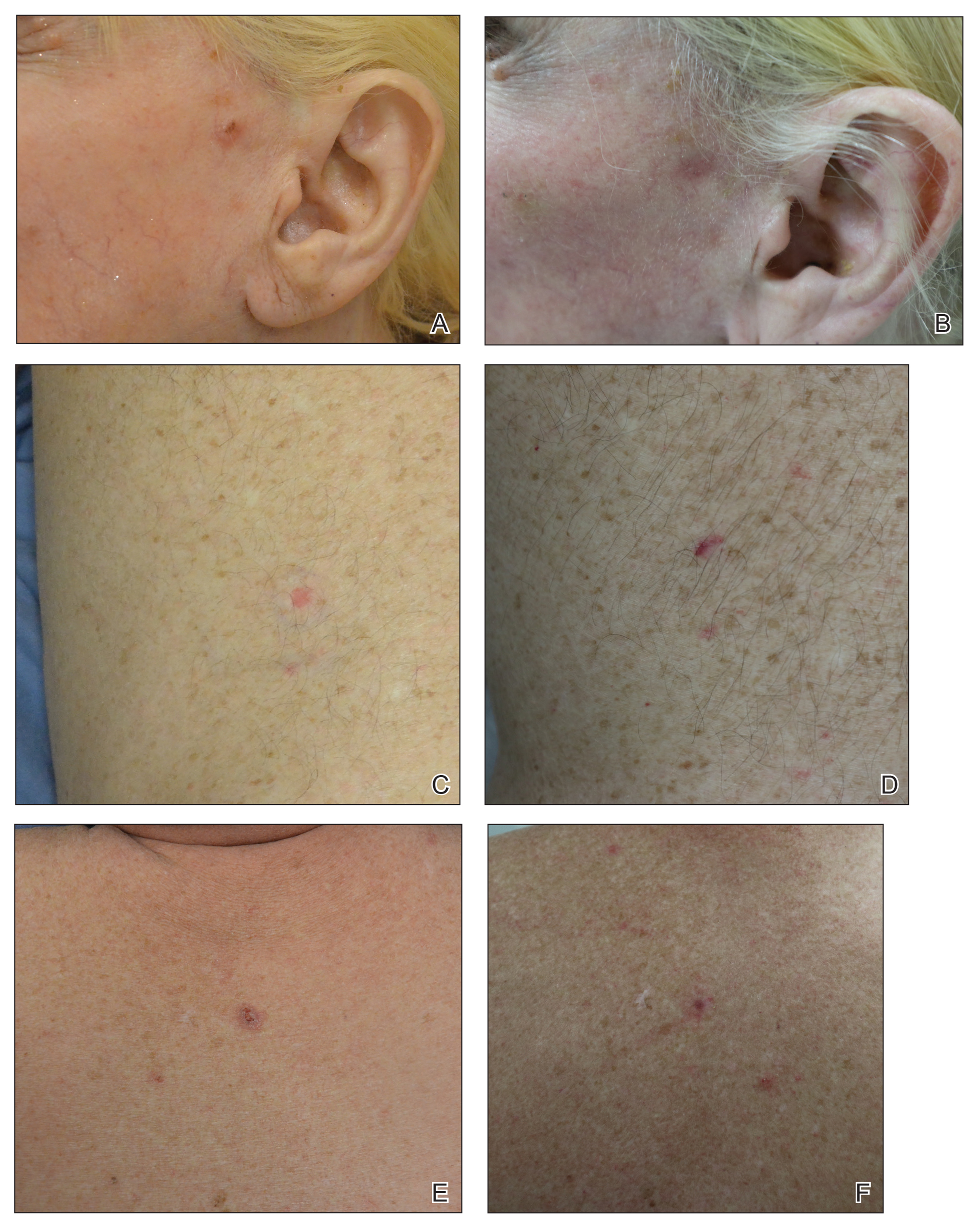
In our study, most patients had lesions that were detected early in their evolution; these lesions might be particularly amenable to laser management. The 2 resistant lesions in our set—requiring 3 treatments—appeared more aggressive clinically at initial evaluation but still had posttreatment outcomes with mild dyschromia similar to the lesions only treated once (Figure, A–D). Of those 2 lesions, the 9-mm lesion on the back (Figure, C and D) might have been larger than clinically apparent; in hindsight, it might have responded to a single treatment had it been premarginated. (An additional factor to have considered is the patient’s immunosuppressed status, which might have led to a more resistant lesion. Larger trials would help elucidate whether an immunosuppressed patient requires a different treatment approach, broader treatment area, OCT premargination regardless of anatomic location, or a greater number of treatments.) Nevertheless, the 2 aforementioned patients were offered treatment with the 1064-nm Nd:YAG laser because they refused surgery, radiation, and other more aggressive modalities. The patients were given advanced warning of an increased possibility of recurrence or nonclearance.

The lesion that required 2 treatments did not appear to be an aggressive subtype; however, it was considerably larger than most other treated lesions (1.5 cm)(Figure, E and F). In this patient, as with the others, we utilized milder (700–1000 J) fluence settings than those used in the Moskalik et al24 study; however, we were optimizing for patient comfort, overall downtime, and cosmetic outcomes.
Clearance in this study was assessed by OCT scanning. Scans were obtained 2 months after the last treatment to avoid detecting inflammation and early scar tissue. We opted not to perform biopsies to determine clearance, as done in prior studies, because we were investigating a fully nonsurgical protocol and wanted to enable patients to avoid surgical intervention, as they had requested. Clinical and dermoscopic examinations by a world expert in dermoscopy and OCT (O.M.) provided additional reassurance of lesion clearance.
Limitations
The retrospective study design with a limited sample size was a main limitation of our study. Our limited data suggest that there is value in further investigation and prospective trials of minimally invasive skin cancer management with the pulsed 1064-nm Nd:YAG laser.
Limitations or disadvantages of this nonablative laser treatment include dyschromia and minimal scarring. Furthermore, at fluence settings utilized, treatment can be painful. Without use of a local anesthetic, treatment is limited to what patients can tolerate.
The percentage of BCCs located on the body (53%) was higher in our study than in the general population, estimated in a study to be approximately 20%.33 This percentage might have been an effect of the larger Vivascope 1500 RCM probe, which made certain areas of the face difficult to access, therefore excluding certain facial lesions encountered in our practice from the initial noninvasive diagnosis.
Most lesions in our study have not been followed long-term; median noninvasive OCT follow-up was 64 days; however, the longest follow-up from our data set is longer than 1 year posttreatment (371 days). We have used OCT to establish clearance, which also will allow us to continue using imaging to monitor for changes that might indicate recurrence. Although OCT is not approved by the US Food and Drug Administration as a validated means of diagnosing and detecting BCC, numerous studies have suggested that this modality has high sensitivity (95.7%) and specificity (75.3%) for features of BCC as well as the more critical high negative predictive value (92.1%) for noninvasive management.22-24
Furthermore, setting up the lesions to be monitored long-term using OCT is likely to be more sensitive than monitoring lesions by clinical examination alone, as they have been followed in studies to date. In fact, an earlier study of 115 lesions by the PI found that utilizing OCT significantly improved sensitivity and specificity for detecting BCC (P<.01); improved diagnostic certainty by a factor of 4 compared to clinical examination alone; and improved overall diagnostic accuracy by 50% compared to clinical and dermoscopic examinations.19
Conclusion
Traditional approaches to BCC management usually involve multiple visits: the initial encounter, which might or might not include biopsy, and a return visit for more definitive management. Reflectance confocal microscopy enables live diagnosis and facilitates targeted same-day treatment of BCC. Our pilot study has contributed data to support the further investigation and use of the Nd:YAG laser to treat BCC in combination with early detection with noninvasive diagnosis for a more patient-driven approach. For some patients as well as for dermatologists, the potential for increased efficiency of same-day diagnosis and treatment might provide a clear advantage.
The increasing incidence of nonmelanoma skin cancer (NMSC) is a serious public health concern.1 Lesions often are identified on routine total-body examination, and there is a considerate burden on dermatologists to diagnose these lesions, which is both costly and results in a long wait time to see a specialist. Furthermore, standard care requires patients to attend multiple visits for the diagnosis and treatment of NMSC.
In recent decades, diagnosing basal cell carcinoma (BCC) has been facilitated by the handheld dermatoscope. The advent of dermoscopy has led to increased sensitivity and specificity of the NMSC diagnosis (estimated at 95%–99%) and has helped facilitate earlier diagnosis of BCC and reduce unnecessary biopsy of benign lesions.2-5 Dermoscopy also can be useful in monitoring response to treatment.5 Lesions that are detected early tend to be easier and less expensive to treat, a strong argument for the use of early detection techniques.6-8
More recently, in vivo reflectance confocal microscopy (RCM)(Vivascope 1500 [Caliber I.D.]) has become an acceptable means for confirming a BCC diagnosis, offering an alternative to tissue biopsy. Reflectance confocal microscopy can be reimbursed under Category I Current Procedural Terminology codes 96931 to 96936.9 Reflectance confocal microscopy is a noninvasive diagnostic technique that uses an 830-nm diode laser to enable visualization of a 0.5×0.5-mm patch of skin to a depth of 200 to 300 μm, which corresponds roughly to the papillary dermis. Reflectance confocal microscopy has the advantage of providing real-time diagnosis, enabling same-day treatment of BCC, and providing an efficient alternative to biopsy. Ultimately, these advantages are beneficial and time-saving for patients because biopsies can be painful; create a delay in diagnosis; and require further follow-up visits for treatment, which may be of importance to patients who have trouble attending multiple appointments.
Optical coherence tomography (OCT) is another noninvasive imaging device that is useful in BCC management. It uses an infrared broadband light source to visualize skin architecture to 2-mm deep with a 6×6-mm field of view.10 Although OCT does not offer the same cellular clarity as RCM, it allows visualization of a greater depth of skin and a wider field of view, making it a useful tool both in marginating NMSCs prior to treatment and monitoring response to treatment over time.11-16 Optical coherence tomography has demonstrated a high negative predictive value (92.1%) for BCC, which makes it useful for ruling out residual tumor in lesions undergoing management.17-19
With all available options, BCC management benefits from care that is tailored to the individual and the lesion, taking into account size and subtype because not every available treatment is appropriate. Lasers, including solid state, diode, dye, and gas types, are emerging as promising minimally invasive treatment modalities.20,21
Nonablative laser therapy with a pulsed dye laser (PDL) and fractional laser is an example; the principal investigator (PI) of this study (O.M.) recently reported a 95.70% clearance rate utilizing a PDL and fractional laser protocol.22 The 1064-nm Nd:YAG laser also has been used with PDL and as a stand-alone treatment. Jalian et al23 used PDL and the Nd:YAG laser on 13 BCC lesions, with a 58% (7/12) clearance rate after 4 treatments; all nonresponders were taking an anticoagulant, which inhibited the laser’s mechanism of action, according to the researchers.
Moskalik et al24 published a report of 3346 facial BCC lesions treated with pulsed Nd and pulsed Nd:YAG lasers, and included follow-up for as long as 5 years, with a 3.7% recurrence rate. Another report by Moskalik et al25 recorded a recurrence rate of 2.2% to 3.1% for BCCs that were followed for at least 5 years.
Ortiz et al26 reported use of the long-pulsed 1064-nm Nd:YAG laser to treat 13 lesions with biopsy-confirmed BCC on the trunk and extremities, with a 92% (12/13) clearance rate based on histologic analysis 1 month after laser treatment. In an expanded study of 31 patients by Ortiz et al,27 the histologic clearance rate was 90.3% (28/31)—also obtained after 1 month—after 1 Nd:YAG laser treatment, also treating lesions on the trunk and extremities. A further retrospective review of Nd:YAG laser treatment of BCC revealed a 100% clearance rate for 16 lesions (including lesions on the face) that were monitored for at least 6 months (mean duration, 9 months; range, 6–15 months).28 Optical coherence tomography imaging was used for one of the review’s lesions before and after treatment and suggested that the Nd:YAG laser works by selectively destroying the vasculature supplying BCC tumors while preserving surrounding healthy tissue.28
Apart from Moskalik et al,24,25 these studies are limited by a relatively short follow-up time to confirm tumor clearance. Prior studies utilizing the Nd:YAG laser to treat BCC are summarized in the eTable.

This pilot study describes a model of care that aims to alleviate some of the demand placed both on the specialty and on patients by utilizing a novel same-day approach to BCC management. We sought to evaluate management using noninvasive diagnosis with RCM; same-day laser treatment; and follow-up examination with clinical, dermoscopic, and noninvasive imaging using OCT. This method focuses on patient-driven health care from various perspectives. Patients are given real-time information about their diagnosis using RCM, leading to an increased level of information flow and immediate transparency regarding their diagnosis and management options. Patients also are receiving tailored care by incorporating noninvasive imaging and same-day laser treatment, allowing collaboration between patient and physician. Patients have more choices—to undergo surgical care; other at-home topical regimens; or laser management with potentially fewer visits, immediate results, a clearance rate similar to surgery, and improved cosmetic outcome.
Our study attempts to further evaluate the efficacy of the 1064-nm Nd:YAG laser in treating BCC while leveraging noninvasive imaging technology. The objective was to perform a retrospective review of medical records of a subgroup of patients with BCC diagnosed by RCM who were treated with the 1064-nm Nd:YAG laser and monitored for clearance using OCT imaging, in addition to clinical and dermoscopic examination. Similar to prior long-term Nd:YAG laser follow-up studies, we aimed to demonstrate the possibility of a minimally invasive BCC management approach—in our protocol, utilizing imaging instead of biopsy to facilitate long-term follow-up and by offering a model for patient-driven care.
Methods
Study Design
Institutional review board approval was received from Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects (New York, New York). We performed a retrospective review of medical records of patients diagnosed by RCM and treated with a 1064-nm Nd:YAG laser, as an alternative to surgery, at the Mount Sinai Faculty Practice Associates between March 2018 and August 2018. Included in this pilot study are 17 lesions in 16 patients.
Inclusion Criteria
Patients were enrolled based on the following criteria: BCCs diagnosed by clinical and dermoscopic examination followed by RCM imaging; treatment with the 1064-nm Nd:YAG laser, because of patients’ preference for this modality over surgery, superficial radiation therapy, topical regimens, and other laser therapies that require more visits; eligibility by PI included limited clinical ulceration or bleeding (or both) and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). The PI performed a detailed and thorough clinical and dermoscopic skin examination, enabling early detection of the BCCs. Basal cell carcinomas were not included if they exhibited rolled borders, visible ulceration, or oozing growths that allowed for treatment of less-advanced tumors. The PI utilized a clinical and dermoscopic color wheel algorithm to identify suspicious lesions combined with RCM for diagnostic confirmation.29
Two of 17 lesions that did not present as early lesions were included in the study due to patient refusal of surgery or radiation. We consider more advanced tumors to be exophytic, bleeding, crusting, nonhealing ulcerative growths. Patients who had received prior laser treatment with the PI’s PDL with fractional laser protocol with subsequent recurrence at the treatment site were included in the study. Lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor, were excluded.
Treatment Protocol
All patients attended the private clinic at Mount Sinai Hospital of a pigmented lesion expert (O.M.) for routine skin cancer screening. Patients with lesions suspicious for BCC—based on clinical and dermoscopic features—were offered tissue biopsy or RCM. Following diagnosis with RCM, treatment options were discussed, and patients were offered laser treatment when surgical options were declined. Topical treatment options were not emphasized because they require weeks of application to be effective and have been studied mainly in superficial BCC management.30,31
Patients with early lesions were offered either the PDL with fractional laser or Nd:YAG protocol, with their understanding that the Nd:YAG laser protocol would likely involve fewer treatments but a higher likelihood of residual hyperpigmentation or potential scarring (or both) than the more gentle PDL with fractional laser treatment.
All lesions on the face were premarginated using OCT by obtaining central scans and 4 additional scans—above, below, to the left, and to the right of the lesion—to ensure targeted laser treatment with desirable cosmetic results. Facial premargination scans were mandatory; however, patients with lesions on the trunk or extremities were offered the option to have pretreatment margination as an out-of-pocket expense. We did not require premargination of lesions on the body because of their location on less cosmetically critical areas. Most patients declined the optional scans.
This can be considered analogous to the situation in which more insurers reimburse Mohs surgery for cosmetically challenging areas such as the head and neck, while limiting reimbursement for treatment of lesions on the trunk and upper extremities to simple excision. Given cosmetic concerns on the head and neck compared to the body, some patients found it acceptable to have slightly increased dyschromia over a broader treatment area of non–cosmetically critical locations on the body.
Optical coherence tomography imaging was required for all anatomic locations at follow-up visits to detect residual disease or confirm clearance. All patients were given thorough information about the treatment, additional costs, treatment alternatives, potential adverse effects, and complications.
Clinical and dermoscopic images were obtained at every visit using a commercially available point-and-shoot digital single-lens reflex camera for clinical photographs, with an attached DermLite DL3N (3Gen) dermatoscope for all contact polarized dermoscopic photography.
Laser treatment was carried out with the 1064-nm Nd:YAG laser. Setting ranges were similar to previously published studies that used the 1064-nm Nd:YAG laser to treat BCCs (spot sizes, 5–6 mm; fluences, 125–140 J/cm2; pulse durations, 7–10 milliseconds).26-28 The exact settings and number of passes were tailored to the individual lesion based on skin type, anatomic location, extent of tumor involvement by depth (and margin on facial lesions), and posttreatment dermoscopic confirmation of clearance; additionally, for facial lesions, OCT confirmation of clearance.
Laser treatment was provided by the PI. Patients were instructed to apply a thick emollient (ie, formulation of petrolatum or 100% petrolatum) after treatment and until the area healed.
All tumors received 1 to 3 treatments at an interval of 1 to 2 months. The treatment end point was complete clearance, judged by absence of skin cancer clinically, dermoscopically, and on OCT scan. More specifically, the PI looked for vascular changes and echogenic changes on OCT consistent with tumor clearance as well as dermoscopic disappearance of recognized BCC features.
Patients were asked to return for follow-up visits 2 months after the final treatment to evaluate tumor clearance. They were asked to return subsequently every 6 to 12 months for routine care and long-term follow-up.
Results
Patient Characteristics
A total of 16 patients (6 female, 10 male) with 17 BCCs were included in this study. Mean age was 68 years (median, 71.5 years; range; 48–89 years). Mean lesion size was 7.1 mm (median, 6 mm; range, 3–15 mm). Eight lesions were on the face; 9 were on extrafacial sites. Two lesions had a history of laser treatment with the PI’s PDL with fractional laser treatment protocol and had locally recurred. Subtypes of lesions were not elicited by RCM.
Outcomes
Fourteen lesions (14/17 [82.4%]) required 1 treatment to achieve clearance, as confirmed clinically, dermoscopically, and by OCT scanning. One lesion on the back (1/17 [5.8%]) required 2 treatments (70 days between treatments). Two lesions (2/17 [11.8%]) required 3 treatments (time between treatments: 49 and 61 days [lesion 1]; 62 and 64 days [lesion 2]). Lesion 1 was on the face; lesion 2 was on the back. Mean time between last treatment and OCT clearance scan was 103 days (median, 64 days; range, 48–371 days).
Comment
Our study supports the notion that the 1064-nm Nd:YAG laser is a viable option for treating BCC. All (100%) lesions cleared, most (82.4%) with a single treatment. Of course, for patients who required more than 1 treatment (17.6%), we cannot make an argument for fewer patient visits because those patients had to return for multiple laser treatments, but they were able to avoid surgery, as they had wanted. Overall, our diagnostic approach utilizing RCM as opposed to traditional tissue biopsy meant that patients’ skin cancers were diagnosed and treated the same day.
A one-stop shop for diagnosis and treatment model has been reported by Kadouch et al32 as part of a randomized controlled trial in which patients were randomly assigned to receive standard care for BCC—biopsy followed by surgical excision—or RCM diagnosis followed by surgical excision. Their outcome was tumor-free margins after surgical treatment; the RCM approach was found to be noninferior to standard care.32 Our retrospective study differs, of course, in its laser treatment approach; however, both studies investigated a potentially more efficient pathway to BCC management, which becomes increasingly relevant given the rising incidence of NMSC.
A real-time, image-based diagnostic approach combined with laser treatment delivers patient-driven care, offering choice and convenience. It might be optimal for patients who have an extensive history of BCC, are poor surgical candidates, have difficulty with the logistics of the multiple visits required for surgical management, cannot (for practical reasons) spend multiple hours in office between Mohs stages, and do not want potentially disfiguring scars, making a minimally invasive treatment preferable.
As we found in our sample, not all patients are amenable to undergoing what is regarded now as the most definitive treatment—namely, surgical options. This subset of patients, whose lesions require more definitive treatment but who do not desire invasive management, need alternative approaches to BCC treatment. The present study proposes a model of patient-driven care that requires collaboration between physician and patient, offering more customized care that takes into account patient choice.
In our study, most patients had lesions that were detected early in their evolution; these lesions might be particularly amenable to laser management. The 2 resistant lesions in our set—requiring 3 treatments—appeared more aggressive clinically at initial evaluation but still had posttreatment outcomes with mild dyschromia similar to the lesions only treated once (Figure, A–D). Of those 2 lesions, the 9-mm lesion on the back (Figure, C and D) might have been larger than clinically apparent; in hindsight, it might have responded to a single treatment had it been premarginated. (An additional factor to have considered is the patient’s immunosuppressed status, which might have led to a more resistant lesion. Larger trials would help elucidate whether an immunosuppressed patient requires a different treatment approach, broader treatment area, OCT premargination regardless of anatomic location, or a greater number of treatments.) Nevertheless, the 2 aforementioned patients were offered treatment with the 1064-nm Nd:YAG laser because they refused surgery, radiation, and other more aggressive modalities. The patients were given advanced warning of an increased possibility of recurrence or nonclearance.

The lesion that required 2 treatments did not appear to be an aggressive subtype; however, it was considerably larger than most other treated lesions (1.5 cm)(Figure, E and F). In this patient, as with the others, we utilized milder (700–1000 J) fluence settings than those used in the Moskalik et al24 study; however, we were optimizing for patient comfort, overall downtime, and cosmetic outcomes.
Clearance in this study was assessed by OCT scanning. Scans were obtained 2 months after the last treatment to avoid detecting inflammation and early scar tissue. We opted not to perform biopsies to determine clearance, as done in prior studies, because we were investigating a fully nonsurgical protocol and wanted to enable patients to avoid surgical intervention, as they had requested. Clinical and dermoscopic examinations by a world expert in dermoscopy and OCT (O.M.) provided additional reassurance of lesion clearance.
Limitations
The retrospective study design with a limited sample size was a main limitation of our study. Our limited data suggest that there is value in further investigation and prospective trials of minimally invasive skin cancer management with the pulsed 1064-nm Nd:YAG laser.
Limitations or disadvantages of this nonablative laser treatment include dyschromia and minimal scarring. Furthermore, at fluence settings utilized, treatment can be painful. Without use of a local anesthetic, treatment is limited to what patients can tolerate.
The percentage of BCCs located on the body (53%) was higher in our study than in the general population, estimated in a study to be approximately 20%.33 This percentage might have been an effect of the larger Vivascope 1500 RCM probe, which made certain areas of the face difficult to access, therefore excluding certain facial lesions encountered in our practice from the initial noninvasive diagnosis.
Most lesions in our study have not been followed long-term; median noninvasive OCT follow-up was 64 days; however, the longest follow-up from our data set is longer than 1 year posttreatment (371 days). We have used OCT to establish clearance, which also will allow us to continue using imaging to monitor for changes that might indicate recurrence. Although OCT is not approved by the US Food and Drug Administration as a validated means of diagnosing and detecting BCC, numerous studies have suggested that this modality has high sensitivity (95.7%) and specificity (75.3%) for features of BCC as well as the more critical high negative predictive value (92.1%) for noninvasive management.22-24
Furthermore, setting up the lesions to be monitored long-term using OCT is likely to be more sensitive than monitoring lesions by clinical examination alone, as they have been followed in studies to date. In fact, an earlier study of 115 lesions by the PI found that utilizing OCT significantly improved sensitivity and specificity for detecting BCC (P<.01); improved diagnostic certainty by a factor of 4 compared to clinical examination alone; and improved overall diagnostic accuracy by 50% compared to clinical and dermoscopic examinations.19
Conclusion
Traditional approaches to BCC management usually involve multiple visits: the initial encounter, which might or might not include biopsy, and a return visit for more definitive management. Reflectance confocal microscopy enables live diagnosis and facilitates targeted same-day treatment of BCC. Our pilot study has contributed data to support the further investigation and use of the Nd:YAG laser to treat BCC in combination with early detection with noninvasive diagnosis for a more patient-driven approach. For some patients as well as for dermatologists, the potential for increased efficiency of same-day diagnosis and treatment might provide a clear advantage.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Menzies SW, Westerhoff K, Rabinovitz H, et al. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136:1012-1016.
- Altamura D, Menzies SW, Argenziano G, et al. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62:67-75.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Lallas A, Apalla Z, Ioannides D, et al. Dermoscopy in the diagnosis and management of basal cell carcinoma. Futur Oncol. 2015;11:2975-2984.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:202a10.
- Kauvar AN, Cronin T Jr, Roenigk R, et al; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41:550-571.
- Hoorens I, Vossaert K, Ongenae K, et al. Is early detection of basal cell carcinoma worthwhile? Systematic review based on the WHO criteria for screening. Br J Dermatol. 2016;174:1258-1265.
- Levine A, Markowitz O. In vivo reflectance confocal microscopy. Cutis. 2017;99:399-402.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer - a practical approach. Exp Dermatol. 2013;22:547-551.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- van Manen L, Dijkstra J, Boccara C, et al. The clinical usefulness of optical coherence tomography during cancer interventions. J Cancer Res Clin Oncol. 2018;144:1967-1990.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Markowitz O, Schwartz M. The use of noninvasive optical coherence tomography to monitor the treatment progress of vismodegib and imiquimod 5% cream in a transplant patient with advanced basal cell carcinoma of the nose. J Clin Aesthet Dermatol. 2016;9:37-41.
- Cheng HM, Guitera P. Systematic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Mirza FN, Khatri KA. The use of lasers in the treatment of skin cancer: a review. J Cosmet Laser Ther. 2017;19:451-458.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297.
- Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Moskalik K, Kozlow A, Demin E, et al. Powerful neodymium laser radiation for the treatment of facial carcinoma: 5 year follow-up data. Eur J Dermatol. 2010;20:738-742.
- Ortiz AE, Anderson RR, Avram MM. 1064 nm long-pulsed Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2015;47:106-110.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Papakostas D, Stockfleth E. Topical treatment of basal cell carcinoma with the immune response modifier imiquimod. Futur Oncol. 2015;11:2985-2990.
- Jansen MHE, Mosterd K, Arits AHMM, et al. Five-year results of a randomized controlled trial comparing effectiveness of photodynamic therapy, topical imiquimod, and topical 5-fluorouracil in patients with superficial basal cell carcinoma. J Invest Dermatol. 2018;138:527-533.
- Kadouch DJ, Elshot YS, Zupan-Kajcovski B, et al. One-stop-shop with confocal microscopy imaging vs. standard care for surgical treatment of basal cell carcinoma: an open-label, noninferiority, randomized controlled multicentre trial. Br J Dermatol. 2017;177:735-741.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Menzies SW, Westerhoff K, Rabinovitz H, et al. Surface microscopy of pigmented basal cell carcinoma. Arch Dermatol. 2000;136:1012-1016.
- Altamura D, Menzies SW, Argenziano G, et al. Dermatoscopy of basal cell carcinoma: morphologic variability of global and local features and accuracy of diagnosis. J Am Acad Dermatol. 2010;62:67-75.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Lallas A, Apalla Z, Ioannides D, et al. Dermoscopy in the diagnosis and management of basal cell carcinoma. Futur Oncol. 2015;11:2975-2984.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:202a10.
- Kauvar AN, Cronin T Jr, Roenigk R, et al; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41:550-571.
- Hoorens I, Vossaert K, Ongenae K, et al. Is early detection of basal cell carcinoma worthwhile? Systematic review based on the WHO criteria for screening. Br J Dermatol. 2016;174:1258-1265.
- Levine A, Markowitz O. In vivo reflectance confocal microscopy. Cutis. 2017;99:399-402.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer - a practical approach. Exp Dermatol. 2013;22:547-551.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- van Manen L, Dijkstra J, Boccara C, et al. The clinical usefulness of optical coherence tomography during cancer interventions. J Cancer Res Clin Oncol. 2018;144:1967-1990.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Markowitz O, Schwartz M. The use of noninvasive optical coherence tomography to monitor the treatment progress of vismodegib and imiquimod 5% cream in a transplant patient with advanced basal cell carcinoma of the nose. J Clin Aesthet Dermatol. 2016;9:37-41.
- Cheng HM, Guitera P. Systematic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Mirza FN, Khatri KA. The use of lasers in the treatment of skin cancer: a review. J Cosmet Laser Ther. 2017;19:451-458.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297.
- Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Moskalik K, Kozlow A, Demin E, et al. Powerful neodymium laser radiation for the treatment of facial carcinoma: 5 year follow-up data. Eur J Dermatol. 2010;20:738-742.
- Ortiz AE, Anderson RR, Avram MM. 1064 nm long-pulsed Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2015;47:106-110.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Papakostas D, Stockfleth E. Topical treatment of basal cell carcinoma with the immune response modifier imiquimod. Futur Oncol. 2015;11:2985-2990.
- Jansen MHE, Mosterd K, Arits AHMM, et al. Five-year results of a randomized controlled trial comparing effectiveness of photodynamic therapy, topical imiquimod, and topical 5-fluorouracil in patients with superficial basal cell carcinoma. J Invest Dermatol. 2018;138:527-533.
- Kadouch DJ, Elshot YS, Zupan-Kajcovski B, et al. One-stop-shop with confocal microscopy imaging vs. standard care for surgical treatment of basal cell carcinoma: an open-label, noninferiority, randomized controlled multicentre trial. Br J Dermatol. 2017;177:735-741.
- Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41-47.
Practice Points
- Novel imaging modalities such as reflectance confocal microscopy and optical coherence tomography can be used to diagnose, monitor treatment response, and confirm clearance of basal cell carcinoma.
- Leveraging new imaging technologies can enable a streamlined patient experience, with same-day diagnosis and management of skin cancer.
- For patients who do not desire surgical management of nonmelanoma skin cancer, laser treatment with Nd:YAG is a promising emerging therapeutic option.