User login
A 35-year-old woman with a history of hypothyroidism and idiopathic small fiber autonomic and sensory neuropathy presented to the emergency department (ED) 48 hours after IV immunoglobulin (IG) infusion with a severe headache, nausea, neck stiffness, photophobia, and episodes of intense positional eye pressure. The patient reported previous episodes of headaches post-IVIG infusion but not nearly as severe. On ED arrival, the patient was afebrile with vital signs within normal limits. Initial laboratory results were notable for levels within reference range parameters: 5.9 × 109/L white blood cell (WBC) count, 13.3 g/dL hemoglobin, 38.7% hematocrit, and 279 × 109/L platelet count; there were no abnormal urinalysis findings, and she was negative for human chorionic gonadotropin.
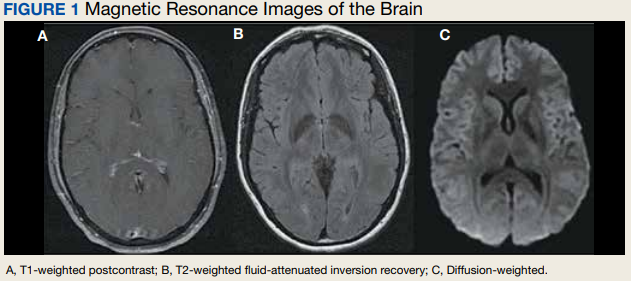
Due to the patient’s symptoms concerning for an acute intracranial process, a brain computed tomography (CT) without contrast was ordered. The CT demonstrated no intracranial abnormalities, but the patient’s symptoms continued to worsen. The patient was started on IV fluids and 1 g IV acetaminophen and underwent a lumbar puncture (LP). Her opening pressure was elevated at 29 cm H2O (reference range, 6-20 cm), and the fluid was notably clear. During the LP, 25 mL of cerebrospinal fluid (CSF) was collected for laboratory analysis to include a polymerase chain reaction (PCR) panel and cultures, and a closing pressure of 12 cm H2O was recorded at the end of the procedure with the patient reporting some relief of pressure. The patient was admitted to the medicine ward for further workup and observations.The patient’s meningitis/encephalitis PCR panel detected no pathogens in the CSF, but her WBC count was 84 × 109/L (reference range, 4-11) with 30 segmented neutrophils (reference range, 0-6) and red blood cell count of 24 (reference range, 0-1); her normal glucose at 60 mg/dL (reference range, 40-70) and protein of 33 mg/dL (reference range, 15-45) were within normal parameters. Brain magnetic resonance images with and without contrast was inconsistent with any acute intracranial pathology to include subarachnoid hemorrhage or central nervous system neoplasm (Figure 1). Bacterial and fungal cultures were negative.
- What is your diagnosis?
- How would you treat this patient?
Discussion
Aseptic meningitis presents with a typical clinical picture of meningitis to include headache, stiffened neck, and photophobia. In the event of negative CSF bacterial and fungal cultures and negative viral PCR, a diagnosis of aseptic meningitis is considered.1 Though the differential for aseptic meningitis is broad, in the immunocompetent patient, the most common etiology of aseptic meningitis in the United States is by far viral, and specifically, enterovirus (50.9%). It is less commonly caused by herpes simplex virus (8.3%), varicella zoster virus, and finally, the mosquito-borne St. Louis encephalitis and West Nile viruses typically acquired in the summer or early fall months. Other infectious agents that can present with aseptic meningitis are spirochetes (Lyme disease and syphilis), tuberculous meningitis, fungal infections (cryptococcal meningitis), and other bacterial infections that have a negative culture.
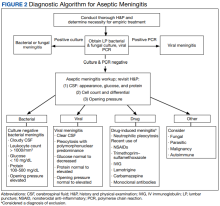
The patient’s history, physical examination, vital signs, imaging, and lumbar puncture findings were most concerning for drug-induced aseptic meningitis (DIAM) secondary to her recent IVIG infusion. An algorithm can be used to work through the diagnostic approach (Figure 2).3,4
Immediate and delayed adverse reactions to IVIG are known risks for IVIG therapy. About 1% to 15% of patients who receive IVIG will experience mild immediate reactions to the infusion.6 These immediate reactions include fever (78.6%), acrocyanosis (71.4%), rash (64.3%), headache (57.1%), shortness of breath (42.8%), hypotension (35.7%), and chest pain (21.4%).
IVIG is an increasingly used biologic pharmacologic agent used for a variety of medical conditions. This can be attributed to its multifaceted properties and ability to fight infection when given as replacement therapy and provide immunomodulation in conjunction with its more well-known anti-inflammatory properties.8 The number of conditions that can potentially benefit from IVIG is so vast that the American Academy of Allergy, Asthma and Immunology had to divide the indication for IVIG therapy into definitely beneficial, probably beneficial, may provide benefit, and unlikely to provide benefit categories.8
Conclusions
We encourage heightened clinical suspicion of DIAM in patients who have recently undergone IVIG infusion and present with meningeal signs (stiff neck, headache, photophobia, and ear/eye pressure) without any evidence of infection on physical examination or laboratory results. With such, we hope to improve clinician suspicion, detection, as well as patient education and outcomes in cases of DIAM.
1. Kareva L, Mironska K, Stavric K, Hasani A. Adverse reactions to intravenous immunoglobulins—our experience. Open Access Maced J Med Sci. 2018;6(12):2359-2362. doi:10.3889/oamjms.2018.513
2. Mount HR, Boyle SD. Aseptic and bacterial meningitis: evaluation, treatment, and prevention. Am Fam Physician. 2017;96(5):314-322.
3. Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003;68(6):1103-1108.
4. Connolly KJ, Hammer SM. The acute aseptic meningitis syndrome. Infect Dis Clin North Am. 1990;4(4):599-622.
5. Jolles S, Sewell WA, Leighton C. Drug-induced aseptic meningitis: diagnosis and management. Drug Saf. 2000;22(3):215-226. doi:10.2165/00002018-200022030-00005
6. Yelehe-Okouma M, Czmil-Garon J, Pape E, Petitpain N, Gillet P. Drug-induced aseptic meningitis: a mini-review. Fundam Clin Pharmacol. 2018;32(3):252-260. doi:10.1111/fcp.12349
7. Kepa L, Oczko-Grzesik B, Stolarz W, Sobala-Szczygiel B. Drug-induced aseptic meningitis in suspected central nervous system infections. J Clin Neurosci. 2005;12(5):562-564. doi:10.1016/j.jocn.2004.08.024
8. Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1-S46. doi:10.1016/j.jaci.2016.09.023
9. Kaarthigeyan K, Burli VV. Aseptic meningitis following intravenous immunoglobulin therapy of common variable immunodeficiency. J Pediatr Neurosci. 2011;6(2):160-161. doi:10.4103/1817-1745.92858
A 35-year-old woman with a history of hypothyroidism and idiopathic small fiber autonomic and sensory neuropathy presented to the emergency department (ED) 48 hours after IV immunoglobulin (IG) infusion with a severe headache, nausea, neck stiffness, photophobia, and episodes of intense positional eye pressure. The patient reported previous episodes of headaches post-IVIG infusion but not nearly as severe. On ED arrival, the patient was afebrile with vital signs within normal limits. Initial laboratory results were notable for levels within reference range parameters: 5.9 × 109/L white blood cell (WBC) count, 13.3 g/dL hemoglobin, 38.7% hematocrit, and 279 × 109/L platelet count; there were no abnormal urinalysis findings, and she was negative for human chorionic gonadotropin.
Due to the patient’s symptoms concerning for an acute intracranial process, a brain computed tomography (CT) without contrast was ordered. The CT demonstrated no intracranial abnormalities, but the patient’s symptoms continued to worsen. The patient was started on IV fluids and 1 g IV acetaminophen and underwent a lumbar puncture (LP). Her opening pressure was elevated at 29 cm H2O (reference range, 6-20 cm), and the fluid was notably clear. During the LP, 25 mL of cerebrospinal fluid (CSF) was collected for laboratory analysis to include a polymerase chain reaction (PCR) panel and cultures, and a closing pressure of 12 cm H2O was recorded at the end of the procedure with the patient reporting some relief of pressure. The patient was admitted to the medicine ward for further workup and observations.The patient’s meningitis/encephalitis PCR panel detected no pathogens in the CSF, but her WBC count was 84 × 109/L (reference range, 4-11) with 30 segmented neutrophils (reference range, 0-6) and red blood cell count of 24 (reference range, 0-1); her normal glucose at 60 mg/dL (reference range, 40-70) and protein of 33 mg/dL (reference range, 15-45) were within normal parameters. Brain magnetic resonance images with and without contrast was inconsistent with any acute intracranial pathology to include subarachnoid hemorrhage or central nervous system neoplasm (Figure 1). Bacterial and fungal cultures were negative.
- What is your diagnosis?
- How would you treat this patient?

Discussion
Aseptic meningitis presents with a typical clinical picture of meningitis to include headache, stiffened neck, and photophobia. In the event of negative CSF bacterial and fungal cultures and negative viral PCR, a diagnosis of aseptic meningitis is considered.1 Though the differential for aseptic meningitis is broad, in the immunocompetent patient, the most common etiology of aseptic meningitis in the United States is by far viral, and specifically, enterovirus (50.9%). It is less commonly caused by herpes simplex virus (8.3%), varicella zoster virus, and finally, the mosquito-borne St. Louis encephalitis and West Nile viruses typically acquired in the summer or early fall months. Other infectious agents that can present with aseptic meningitis are spirochetes (Lyme disease and syphilis), tuberculous meningitis, fungal infections (cryptococcal meningitis), and other bacterial infections that have a negative culture.
The patient’s history, physical examination, vital signs, imaging, and lumbar puncture findings were most concerning for drug-induced aseptic meningitis (DIAM) secondary to her recent IVIG infusion. An algorithm can be used to work through the diagnostic approach (Figure 2).3,4
Immediate and delayed adverse reactions to IVIG are known risks for IVIG therapy. About 1% to 15% of patients who receive IVIG will experience mild immediate reactions to the infusion.6 These immediate reactions include fever (78.6%), acrocyanosis (71.4%), rash (64.3%), headache (57.1%), shortness of breath (42.8%), hypotension (35.7%), and chest pain (21.4%).
IVIG is an increasingly used biologic pharmacologic agent used for a variety of medical conditions. This can be attributed to its multifaceted properties and ability to fight infection when given as replacement therapy and provide immunomodulation in conjunction with its more well-known anti-inflammatory properties.8 The number of conditions that can potentially benefit from IVIG is so vast that the American Academy of Allergy, Asthma and Immunology had to divide the indication for IVIG therapy into definitely beneficial, probably beneficial, may provide benefit, and unlikely to provide benefit categories.8
Conclusions
We encourage heightened clinical suspicion of DIAM in patients who have recently undergone IVIG infusion and present with meningeal signs (stiff neck, headache, photophobia, and ear/eye pressure) without any evidence of infection on physical examination or laboratory results. With such, we hope to improve clinician suspicion, detection, as well as patient education and outcomes in cases of DIAM.
A 35-year-old woman with a history of hypothyroidism and idiopathic small fiber autonomic and sensory neuropathy presented to the emergency department (ED) 48 hours after IV immunoglobulin (IG) infusion with a severe headache, nausea, neck stiffness, photophobia, and episodes of intense positional eye pressure. The patient reported previous episodes of headaches post-IVIG infusion but not nearly as severe. On ED arrival, the patient was afebrile with vital signs within normal limits. Initial laboratory results were notable for levels within reference range parameters: 5.9 × 109/L white blood cell (WBC) count, 13.3 g/dL hemoglobin, 38.7% hematocrit, and 279 × 109/L platelet count; there were no abnormal urinalysis findings, and she was negative for human chorionic gonadotropin.
Due to the patient’s symptoms concerning for an acute intracranial process, a brain computed tomography (CT) without contrast was ordered. The CT demonstrated no intracranial abnormalities, but the patient’s symptoms continued to worsen. The patient was started on IV fluids and 1 g IV acetaminophen and underwent a lumbar puncture (LP). Her opening pressure was elevated at 29 cm H2O (reference range, 6-20 cm), and the fluid was notably clear. During the LP, 25 mL of cerebrospinal fluid (CSF) was collected for laboratory analysis to include a polymerase chain reaction (PCR) panel and cultures, and a closing pressure of 12 cm H2O was recorded at the end of the procedure with the patient reporting some relief of pressure. The patient was admitted to the medicine ward for further workup and observations.The patient’s meningitis/encephalitis PCR panel detected no pathogens in the CSF, but her WBC count was 84 × 109/L (reference range, 4-11) with 30 segmented neutrophils (reference range, 0-6) and red blood cell count of 24 (reference range, 0-1); her normal glucose at 60 mg/dL (reference range, 40-70) and protein of 33 mg/dL (reference range, 15-45) were within normal parameters. Brain magnetic resonance images with and without contrast was inconsistent with any acute intracranial pathology to include subarachnoid hemorrhage or central nervous system neoplasm (Figure 1). Bacterial and fungal cultures were negative.
- What is your diagnosis?
- How would you treat this patient?

Discussion
Aseptic meningitis presents with a typical clinical picture of meningitis to include headache, stiffened neck, and photophobia. In the event of negative CSF bacterial and fungal cultures and negative viral PCR, a diagnosis of aseptic meningitis is considered.1 Though the differential for aseptic meningitis is broad, in the immunocompetent patient, the most common etiology of aseptic meningitis in the United States is by far viral, and specifically, enterovirus (50.9%). It is less commonly caused by herpes simplex virus (8.3%), varicella zoster virus, and finally, the mosquito-borne St. Louis encephalitis and West Nile viruses typically acquired in the summer or early fall months. Other infectious agents that can present with aseptic meningitis are spirochetes (Lyme disease and syphilis), tuberculous meningitis, fungal infections (cryptococcal meningitis), and other bacterial infections that have a negative culture.
The patient’s history, physical examination, vital signs, imaging, and lumbar puncture findings were most concerning for drug-induced aseptic meningitis (DIAM) secondary to her recent IVIG infusion. An algorithm can be used to work through the diagnostic approach (Figure 2).3,4
Immediate and delayed adverse reactions to IVIG are known risks for IVIG therapy. About 1% to 15% of patients who receive IVIG will experience mild immediate reactions to the infusion.6 These immediate reactions include fever (78.6%), acrocyanosis (71.4%), rash (64.3%), headache (57.1%), shortness of breath (42.8%), hypotension (35.7%), and chest pain (21.4%).
IVIG is an increasingly used biologic pharmacologic agent used for a variety of medical conditions. This can be attributed to its multifaceted properties and ability to fight infection when given as replacement therapy and provide immunomodulation in conjunction with its more well-known anti-inflammatory properties.8 The number of conditions that can potentially benefit from IVIG is so vast that the American Academy of Allergy, Asthma and Immunology had to divide the indication for IVIG therapy into definitely beneficial, probably beneficial, may provide benefit, and unlikely to provide benefit categories.8
Conclusions
We encourage heightened clinical suspicion of DIAM in patients who have recently undergone IVIG infusion and present with meningeal signs (stiff neck, headache, photophobia, and ear/eye pressure) without any evidence of infection on physical examination or laboratory results. With such, we hope to improve clinician suspicion, detection, as well as patient education and outcomes in cases of DIAM.
1. Kareva L, Mironska K, Stavric K, Hasani A. Adverse reactions to intravenous immunoglobulins—our experience. Open Access Maced J Med Sci. 2018;6(12):2359-2362. doi:10.3889/oamjms.2018.513
2. Mount HR, Boyle SD. Aseptic and bacterial meningitis: evaluation, treatment, and prevention. Am Fam Physician. 2017;96(5):314-322.
3. Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003;68(6):1103-1108.
4. Connolly KJ, Hammer SM. The acute aseptic meningitis syndrome. Infect Dis Clin North Am. 1990;4(4):599-622.
5. Jolles S, Sewell WA, Leighton C. Drug-induced aseptic meningitis: diagnosis and management. Drug Saf. 2000;22(3):215-226. doi:10.2165/00002018-200022030-00005
6. Yelehe-Okouma M, Czmil-Garon J, Pape E, Petitpain N, Gillet P. Drug-induced aseptic meningitis: a mini-review. Fundam Clin Pharmacol. 2018;32(3):252-260. doi:10.1111/fcp.12349
7. Kepa L, Oczko-Grzesik B, Stolarz W, Sobala-Szczygiel B. Drug-induced aseptic meningitis in suspected central nervous system infections. J Clin Neurosci. 2005;12(5):562-564. doi:10.1016/j.jocn.2004.08.024
8. Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1-S46. doi:10.1016/j.jaci.2016.09.023
9. Kaarthigeyan K, Burli VV. Aseptic meningitis following intravenous immunoglobulin therapy of common variable immunodeficiency. J Pediatr Neurosci. 2011;6(2):160-161. doi:10.4103/1817-1745.92858
1. Kareva L, Mironska K, Stavric K, Hasani A. Adverse reactions to intravenous immunoglobulins—our experience. Open Access Maced J Med Sci. 2018;6(12):2359-2362. doi:10.3889/oamjms.2018.513
2. Mount HR, Boyle SD. Aseptic and bacterial meningitis: evaluation, treatment, and prevention. Am Fam Physician. 2017;96(5):314-322.
3. Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003;68(6):1103-1108.
4. Connolly KJ, Hammer SM. The acute aseptic meningitis syndrome. Infect Dis Clin North Am. 1990;4(4):599-622.
5. Jolles S, Sewell WA, Leighton C. Drug-induced aseptic meningitis: diagnosis and management. Drug Saf. 2000;22(3):215-226. doi:10.2165/00002018-200022030-00005
6. Yelehe-Okouma M, Czmil-Garon J, Pape E, Petitpain N, Gillet P. Drug-induced aseptic meningitis: a mini-review. Fundam Clin Pharmacol. 2018;32(3):252-260. doi:10.1111/fcp.12349
7. Kepa L, Oczko-Grzesik B, Stolarz W, Sobala-Szczygiel B. Drug-induced aseptic meningitis in suspected central nervous system infections. J Clin Neurosci. 2005;12(5):562-564. doi:10.1016/j.jocn.2004.08.024
8. Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1-S46. doi:10.1016/j.jaci.2016.09.023
9. Kaarthigeyan K, Burli VV. Aseptic meningitis following intravenous immunoglobulin therapy of common variable immunodeficiency. J Pediatr Neurosci. 2011;6(2):160-161. doi:10.4103/1817-1745.92858