User login
Rejuvenation of the periocular area is in high demand among patients who want to look and feel their best. Physicians should understand the complicated anatomy surrounding the eyes before attempting to inject this area with facial fillers, both to understand the aging process and to minimize treatment complications.
Basic Oculoplastic and Orbital Anatomy
The injector should understand the anatomy of the periocular muscles, the orbital osteology, and the secretory and lacrimal system, in addition to the fat, ligaments, and vascular anatomy in this area.1
The eyes are surrounded by fat compartments that provide glide planes for the motion of the eyelids and globe. There are 2 upper eyelid fat-pads—nasal and central [preaponeurotic])—in the upper lid, leaving room for the lacrimal gland laterally. There are 3 fat compartments—nasal, central, and lateral—in the lower eyelid. The nasal and central compartments are separated by the inferior oblique muscle, which elevates and extorts the eye. The orbital septum holds the fat-pads in place in the orbit. The brow fat-pad is the retro-orbicularis oculi fat-pad (ROOF). There are fat compartments that lie in the subcutaneous space along the entire forehead and in the temple. The suborbicularis oculi fat-pad (SOOF) lies over the malar eminence. Superficial and deep submuscular fat compartments of the face have been described.2 Deep fat compartments also have been examined on computed tomography.3
Orbital circulation comes from the internal carotid artery and anastomoses with the supply from the external carotid artery to supply the orbit. The first branch off of the carotid artery is the ophthalmic artery, and the first branch off of the ophthalmic artery is the central retinal artery that enters the optic nerve sheath 1 cm behind the globe to supply the retina. The supraorbital and supratrochlear arteries branch off of the ophthalmic artery and supply the forehead. The supraorbital artery runs through the supraorbital notch (foramen in 8%)1 and can usually be palpated with one’s finger. There are 15 to 20 short posterior ciliary arteries leading to the choroid, 2 long posterior ciliary arteries to the iris circle, and 7 anterior ciliary arteries to the extraocular muscles. The superior and inferior venous systems drain into the cavernous sinus.4
The ligaments are important to signs of facial aging because tissue atrophy occurs along them. The main orbital ligaments are the lateral orbital thickening (known as the LOT) that adheres the eyelids to the lateral orbital rim and the orbitomalar ligament (orbicularis retaining ligament), which is a condensation fibrous tissue that attaches the skin to the inferior orbital rim and orbital septum along the arcus marginalis and defines the superior edge of the SOOF.5 The zygomatic ligament not only suspends the zygomaticus major and zygomaticus minor muscles to the malar eminence but there are osseocutaneous attachments that connect the skin over the zygoma’s malar eminence and demarcate the inferior edge of the SOOF.6
Periocular Aging
The skin, fat, muscles, and bones change and rotate with aging, and not all orbits age in the same manner. Older patients with dermatochalasis (excess skin fat and muscle) often undergo rejuvenation with blepharoplasty, a brow-lift, and a midface-lift, but many atrophic changes can be improved with facial fillers.7,8
As adults age, the soft tissue along the ligaments begins to show atrophy, prime signs of aging that are often improved with fillers. Atrophy along the orbitomalar ligament along the infraorbital rim creates a depressed tear trough, which is an early sign of aging. A 3-point grading system reported by Hirmand8 describes the severity of progressive hallowing. There also is atrophy along the zygomatic cutaneous ligament that creates the malar hollow. The SOOF appears to be more prominent when these areas above and below show atrophy, which creates the look of an unwanted bag known as a festoon. Additionally, there is atrophy along the superior orbital notch where the ophthalmic branch of the trigeminal nerve (V1) and the supraorbital artery traverse. Soft-tissue atrophy along the supraorbital notch resembles the peak at the top of the letter A, giving the slang term A-frame deformity.
Periocular fat can atrophy, hypertrophy, herniate forward as the septum weakens, or become ptotic. Some patients develop hypertrophy and herniation of the superior and inferior orbital fat-pads, while others develop unwanted atrophy leaving a hollow superior orbit and loss of support to the levator muscle that contributes to eyelid ptosis. The frontalis fat deflates, leaving veins, arteries, and the hypertrophied corrugators unwantedly visible. Loss of subcutaneous fat in the glabella contributes to the formation of frown lines between the brows (also called number 11’s). The ROOF deflates in some patients adding to brow ptosis. Loss of the facial frame occurs when temple fat atrophies.
Skeletal rotation also occurs. Throughout a patient’s life, the skeleton remodels itself via activity of osteoclasts and osteoblasts. Pessa et al9,10 has described the expansion of the anterior orbital aperture superomedially and inferolaterally as well as maxillary retrusion that results in angular changes of the midface in relation to the orbital rim. Lambros’ algorithm describes the rotational changes of the cranium where the superior orbit protrudes as the maxilla retreats posteriorly.9-11 The equator of the globe does not change its distance from the ROOF of the orbit, presumably because of its suspension in the orbit by the optic nerve after it passes through the optic canal and trochlea via the superior oblique muscle, but the distance of the inferior equator of the globe to the floor of the orbit increases as the floor of the orbit descends.12
Dermal Fillers for Periocular Rejuvenation
Hyaluronic acid (HA) was first pioneered for use in humans in the late 1970s by ophthalmologists for anterior segment surgery.13-15 Biocompatibility for orthopedic and dermal applications was explored in the early 1990s.16
At this time, no dermal filler is approved by the US Food and Drug Administration for use in the periorbital area. Some fillers are approved for subdermal areas extending to the preperiosteal plane and can be used in the midface such as HA fillers (eg, Restylane Lyft [Galderma Laboratories, LP]), Juvéderm Voluma XC [Allergan, Inc]), poly-L-lactic acid (PLLA), and calcium hydroxylapatite (CaHA). No dermal fillers are approved for use in the forehead, glabella, or temples. Their use is becoming increasingly popular but is considered off label. In addition, cannulas are not approved for use in these areas. Cannulas may be beneficial in that they are thought to create less bruising and have less chance of entering a vessel than needles, but some injectors prefer needles because they are stiffer and therefore more precise.
The ideal filler for the tear trough, superior sulcus, ROOF, over the orbitomalar ligament, forehead, and glabella is one that is somewhat moldable but does not migrate, is not hydrophilic, is smooth to inject, and is reversible should there be any complications. No single filler fits this ideal description, but HAs typically are the first choice.
In vitro studies to determine the stiffness (G') and the ability to flow (viscosity) have been performed.17,18 Calcium hydroxylapatite has the most stiffness, while Belotero Balance (Merz Aesthetics) and Juvéderm Ultra XC (Allergan, Inc) are more soft17 (Table). These guidelines are important but may not correlate directly with how the fillers behave in vivo as demonstrated in animal models.18
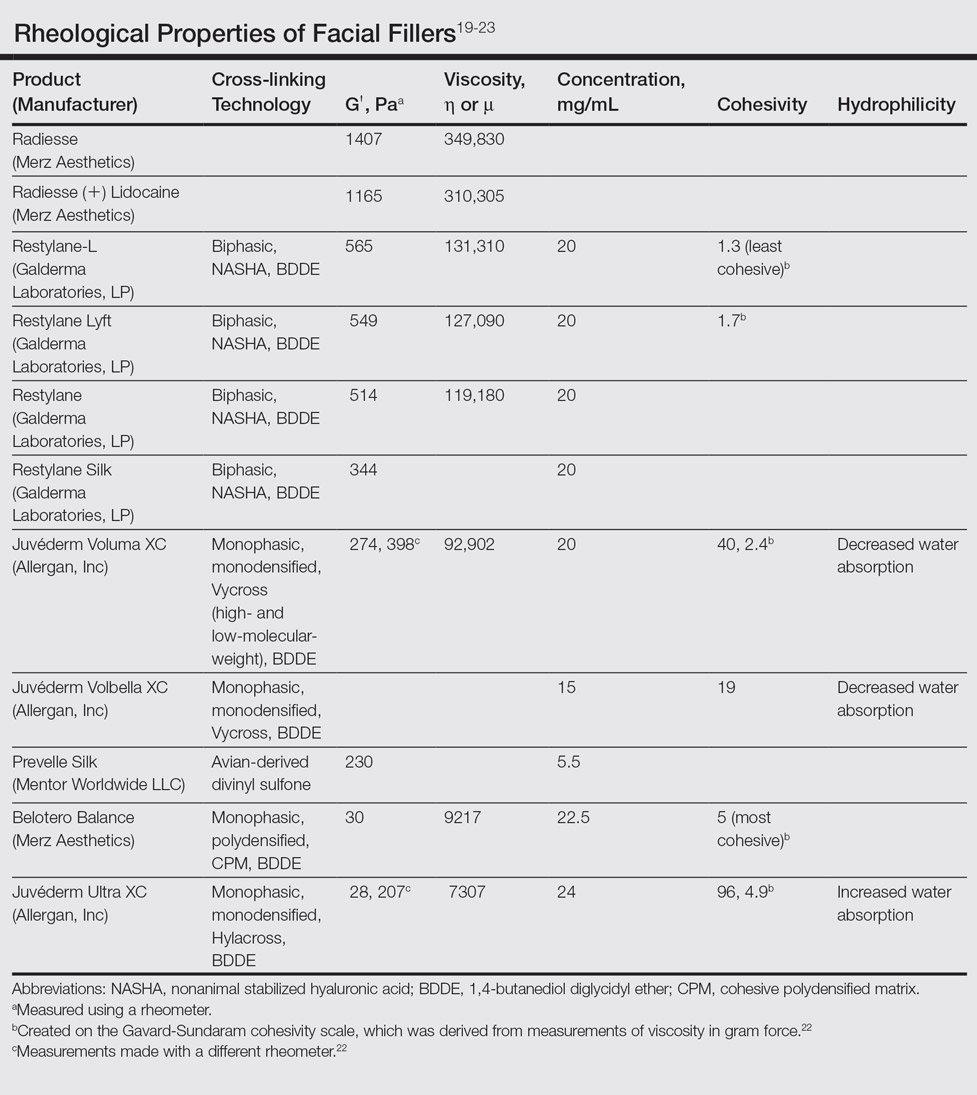
Hyaluronic acid fillers are produced by different technologies to create their cross-link patterns with 1,4-butanediol diglycidyl ether, which determines, to some degree, their behavior in human tissue. Fillers are either monophasic; monodensified; formed by Hylacross (Juvéderm), Vycross (Juvéderm Voluma XC, Juvéderm Volbella XC), or cohesive polydensified matrix technology (Belotero Balance), or biphasic, formed by nonanimal stabilized HA sieving technology (Restylane family). Biopsy has demonstrated that monophasic fillers tend to percolate through and integrate into the tissue, while biphasic fillers dissect tissue to the sides to create a potential space for the filler to reside (Table).24
Periocular Injection Considerations
An experienced injector is one who has developed not only an artistic eye for the face and excellent sense of anatomy but also has a sensitive ability to predict the filler-tissue interaction based on tactile feedback dependent on 3 main qualities: (1) stiffness and viscosity of the filler, (2) gauge of the needle or cannula, and (3) depth of the needle in the tissue. Periocular injections of dermal fillers can be delivered with needles or cannulas, diluted or undiluted. Smaller-gauge needles require more force than larger-gauge needles and cannulas that flow more freely. A needle in the dense dermis will require more force than one placed in the loose subcutaneous space.
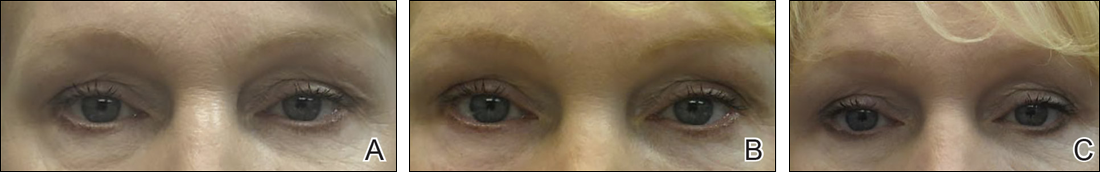
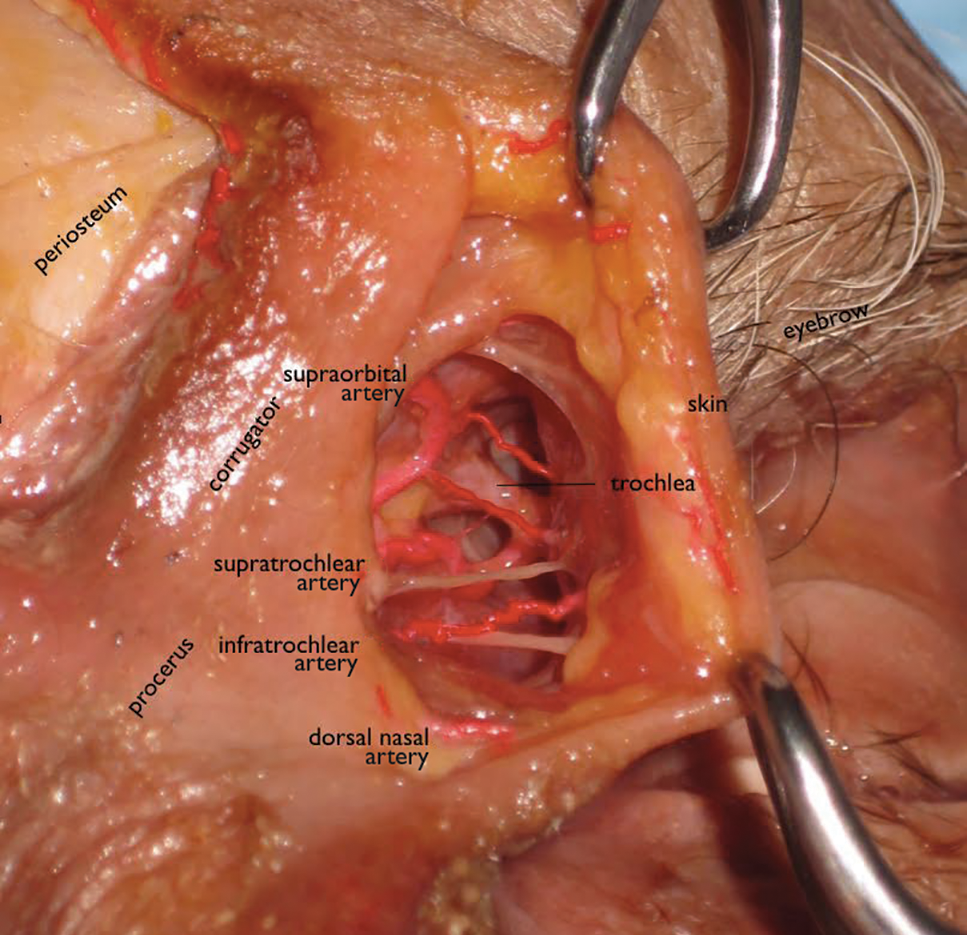
The tear trough is generally preferable to fill with a mid-level G' HA filler that is less apt to migrate. A neutral gaze during the injection is preferred because closing or moving the eyes can distort the position of the inferior orbital fat-pads (Figure 1). A needle or cannula can be used, diluted or undiluted. The tear trough can be filled with the injection directed horizontally or vertically via a fanning technique. If needles are used, the skin should be stretched to view the 3 to 5 vertical veins and then the needle should be advanced beneath them to avoid bruising. Avoidance of hydrophilic fillers in the tear trough is important to avoid edema. The superior sulcus can be filled both anteriorly and posteriorly to the septum, which is a highly advanced injection for experienced injectors because of the proximity to the supratrochlear and supraorbital arteries as well as the superior ophthalmic vein (Figure 2). Sharp creases such as deep lateral periocular rhytides known as crow’s-feet are nicely filled with intradermal HAs with a low G'.
Adding volume to the midface is important because it is the continuum of the lower eyelid. Fillers can be injected into multiple levels in this area: deep (to act as pillars to lift the malar eminence and replace bone that has rotated and soft tissue that has become atrophic or descended) and subcutaneous (to efface soft tissue along the zygomatic cutaneous ligament). Higher G' HA fillers and CaHA often are used in the midface along with PLLA. Facial framing of the temples, lateral cheeks, and preauricular area is often accomplished with PLLA but also can be done with mid to high G' HA fillers or CaHA. A cannula may be used to undermine and break apart the zygomatic cutaneous ligament’s cutaneous attachments prior to delivery of the filler in the subcutaneous plane.26 If not done, filler may track away from the hollow area where the ligament is attached and instead move to adjacent areas that will accentuate the hollow and make it look worse.
The temples and lateral face often are filled with PLLA for framing. Mid or high G' HA fillers and CaHA also are used in the temples both beneath the temporalis muscle and also above the deep temporalis fascia or sometimes in the subcutaneous plane.27
Prevention and Management of Periocular Complications
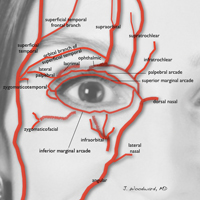
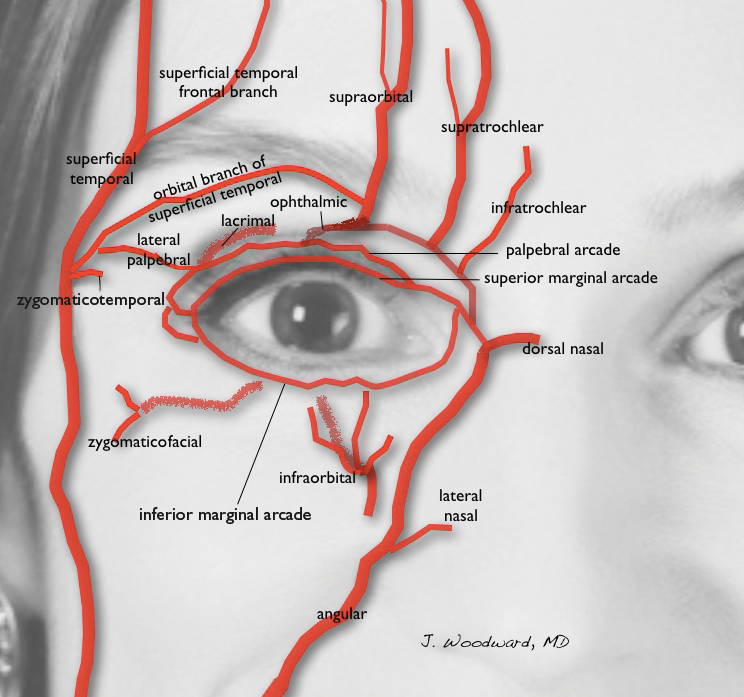
Blindness is the most devastating periocular complication of facial fillers, which is caused by retrograde arterial embolization followed by anterograde flow into the ophthalmic then central retinal arteries. Injectables that have caused blindness include (in descending order of frequency) fat, HA, collagen, paraffin, polymethyl methacrylate, silicone, PLLA, CaHA, polyacrylamide hydrogel, and micronized acellular dermal matrix. Of the 98 cases of blindness from periocular complications from dermal fillers reported in the world literature, the order of affected sites include the glabella (38 cases), nose (25), nasolabial folds (13), superior forehead (12), infraorbital rim (6), temples (1), malar area (1), lip (1), and chin (1). Prevention includes avoidance of danger zone arteries including the supratrochlear, supraorbital, dorsal nasal, angular, infraorbital, zygomaticofacial and zygomaticotemporal arteries.28
Avoiding the average critical volume of 0.84 in any single aliquot dispensed is key to avoid filling of these periocular arteries to the critical bifurcation point that can result in anterograde flow into the eye (Freudenthal Nicolau syndrome). The smallest supratrochlear artery’s volume in this study was 0.04 cc, so aliquots that do not exceed 0.03 cc are ideal.29,30
The injector should always be thinking about the anatomy of the danger zones (eg, infratrochlear and supratrochlear arteries, supraorbital artery, frontal branch of the superficial temporal artery, lacrimal artery, dorsal nasal artery, infraorbital artery, angular artery, zygomaticofacial artery, zygomaticotemporal artery)(Figure 3).
Hyaluronidase can be used off label to hydrolyze unwanted HA. It was first used to aid transcutaneous hydration and was used by ophthalmologists in the 1960s and 1970s to promote the spread of anesthetics by retrobulbar injection.31,32 It can penetrate through soft tissues and blood vessels.33 It is therefore hypothesized that a retrobulbar injection of hyaluronidase could aid in a case of impending blindness34 but has not been successfully accomplished to date. If vision is confirmed to be poor or there is no light perception, a retrobulbar injection of 300 U of hyaluronidase should be given immediately and then repeated in approximately 30 to 45 minutes. The retina begins to show permanent loss of function after being deprived of blood flow for just 97 minutes,35 so there may not be time for an immediate ophthalmology consultation, though such a consultation would be ideal.
Aside from common complications such as bruising and swelling, granulomas and biofilms are well documented in the literature. There are a variety of algorithms to treat such complications, which can happen many weeks after the injection of a dermal filler or years after the injection of a semipermanent filler.36 Postinjection periocular edema can occur years after the initial injection.37,38 Other periocular complications of dermal fillers include nonischemic (eg, bluish hue, filler migration, infection, inflammation, lumps) and ischemic (eg, blindness, necrosis, ophthalmoplegia, ptosis) disturbances.
Conclusion
In summary, periocular injections of facial fillers are useful tools for rejuvenation of the upper face when used with great caution and respect for anatomy.
- Foster J, ed. Orbit, Eyelids, and Lacrimal System. San Francisco, CA: American Academy of Ophthalmology; 2016. 2016-2017 Basic and Clinical Science Course; section 7.
- Rohrich RJ, Pessa JE. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119:2219-2227; discussion 2228-2231.
- Gierloff M, Stöhring C, Buder T, et al. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconst Surg. 2012;129:263-273.
- Zide BM, Jelks GW. Surgical Anatomy of the Orbit: The System of Zones. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
- Kikkawa DO, Lemke BN, Dortzbach RK. Relations of the superficial musculoaponeurotic system to the orbit and characterization of the oribitomalar ligament. Ophthal Plast Reconstr Surg. 1996;12:77-88.
- Furnas DW. The retaining ligaments of the cheek. Plast Reconstr Surg. 1989;83:11-16.
- Morley AM, Taban M, Malhotra R, et al. Use of hyaluronic acid gel for upper eyelid filling and contouring. Ophthal Plast Reconstr Surg. 2009;25:440-444.
- Hirmand H. Anatomy and nonsurgical correction of the tear trough deformity. Plast Reconstr Surg. 2010;125:699-708.
- Pessa JE, Zadoo VP, Mutimer KL, et al. Relative maxillary retrusion as a natural consequence of aging. Plast Reconstr Surg. 1998;102:205-212.
- Pessa JE, Desvigne LD, Lambros VS, et al. Changes in ocular globe-to-orbital rim position with age: implications for aesthetic blepharoplasty of the lower eyelids. Aesthet Plast Surg. 1999;23:337-345.
- Goldberg RA, Relan A, Hoenig J. Relationship of the eye to the bony orbit, with clinical correlations. Aust N Z J Ophthalmol. 1999;27:398-403.
- Richard MJ, Morris C, Deen BF, et al. Analysis of the anatomic changes of the aging facial skeleton using computer-assisted tomography. Ophthal Plast Reconstr Surg. 2009;25:382-386.
- Miller D, O’Connor P, Williams J. Use of Na-hyaluronate during intraocular lens implantation in rabbits. Ophthalmic Surg. 1977;8:58-61.
- Miller D, Stegmann R. Use of Na-hyaluronate in anterior segment eye surgery. J Am Intraocul Implant Soc. 1980;6:13-15.
- Pape LG, Balazs EA. The use of sodium hyaluronate (Healon) in human anterior segment surgery. Ophthalmology. 1980;87:699-705.
- Larsen NE, Pollak CT, Reiner K, et al. Hylan gel biomaterial: dermal and immunologic compatibility. J Biomed Mater Res. 1993;27:1129-1134.
- Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;132(4, suppl 2):5S-21S.
- Hee CK, Shumate GT, Narurkar V, et al. Rheological properties and in vivo performance characteristics of soft tissue fillers. Dermatol Surg. 2015;41(suppl 1):S373-S381.
- Sundaram H, Voigts B, Beer K, et al. Comparison of the rheological properties of viscosity and elasticity in two categories of soft tissue fillers: calcium hydroxylapatite and hyaluronic acid. Dermatol Surg. 2010;36(suppl 3):1859-1865.
- Sundaram H. The new face of fillers: a multi-specialty CME initiative: supplement part II of II. J Drugs Dermatol. 2012;11(suppl 8):S8.
- Stocks D, Sundaram H, Michaels J, et al. Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J Drugs Dermatol. 2011;10:974-980.
- Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136(suppl 5):139S-148S.
- Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale and evaluation of six U.S. Food and Drug Administration–approved fillers. Plast Reconstr Surg. 2015;136:678-686.
- Flynn TC, Sarazin D, Bezzola A, et al. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637-643.
- Woodward JA, Langelier N. Filler enhancement of the superior periocular area [published online Jun 23, 2016]. JAMA Facial Plast Surg. doi:10.1001/jamafacial.2016.0636.
- Cotofana S, Schenck TL, Trevidic P, et al. Midface: clinical anatomy and regional approaches with injectable fillers. Plast Reconstr Surg. 2015;136(suppl 5):219S-234S.
- Buckingham ED, Glasgold R, Kontis T, et al. Volume rejuvenation of the facial upper third. Facial Plast Surg. 2015;31:43-54.
- Beleznay K, Carruthers JD, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097-1117.
- Coleman SR. Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J. 2002;22:555-557.
- Khan T, Colon-Acevedo B, Mettu P, et al. An anatomical analysis of the supratrochlear artery: considerations in facial filler injections and preventing vision loss [published online August 16, 2016]. Aesthet Surg J. pii: sjw132.
- Iserle J, Kumstat Z. Retrobulbar injections of hyaluronidase as a method of increasing safety in cataract surgery [in Czech]. Cesk Oftalmol. 1960;15:126-130.
- Wojtowicz S. Effect of retrobulbar injections of novocaine and lignocaine with adrenalin and hyaluronidase for the immobilization of the eye in electromyography [in Polish]. Klin Oczna. 1964;34:285-296.
- Delorenzi C. Transarterial degradation of hyaluronic acid filler by hyaluronidase. Dermatol Surg. 2014;40:832-841.
- Carruthers J, Fagien S, Dolman P. Retro or peribulbar injections techniques to reverse visual loss after filler injections. 2015;41(suppl 1):S354-S357.
- Hayreh SS, Zimmerman MB, Kimura A, et al. Central retinal artery occlusion. retinal survival time. Exp Eye Res. 2004;78:723-736.
- Woodward J, Khan T, Martin J. Facial filler complications. Facial Plast Surg Clin North Am. 2015;23:447-458.
- Khan TT, Woodward JA. Retained dermal filler in the upper eyelid masquerading as periorbital edema. Dermatol Surg. 2015;41:1182-1184.
- Chang JR, Baharestani S, Salek SS, et al. Delayed superficial migration of retained hyaluronic acid years following periocular injection [published online April 20, 2015]. Ophthal Plast Reconstr Surg. doi:10.1097/IOP.0000000000000434.
Rejuvenation of the periocular area is in high demand among patients who want to look and feel their best. Physicians should understand the complicated anatomy surrounding the eyes before attempting to inject this area with facial fillers, both to understand the aging process and to minimize treatment complications.
Basic Oculoplastic and Orbital Anatomy
The injector should understand the anatomy of the periocular muscles, the orbital osteology, and the secretory and lacrimal system, in addition to the fat, ligaments, and vascular anatomy in this area.1
The eyes are surrounded by fat compartments that provide glide planes for the motion of the eyelids and globe. There are 2 upper eyelid fat-pads—nasal and central [preaponeurotic])—in the upper lid, leaving room for the lacrimal gland laterally. There are 3 fat compartments—nasal, central, and lateral—in the lower eyelid. The nasal and central compartments are separated by the inferior oblique muscle, which elevates and extorts the eye. The orbital septum holds the fat-pads in place in the orbit. The brow fat-pad is the retro-orbicularis oculi fat-pad (ROOF). There are fat compartments that lie in the subcutaneous space along the entire forehead and in the temple. The suborbicularis oculi fat-pad (SOOF) lies over the malar eminence. Superficial and deep submuscular fat compartments of the face have been described.2 Deep fat compartments also have been examined on computed tomography.3
Orbital circulation comes from the internal carotid artery and anastomoses with the supply from the external carotid artery to supply the orbit. The first branch off of the carotid artery is the ophthalmic artery, and the first branch off of the ophthalmic artery is the central retinal artery that enters the optic nerve sheath 1 cm behind the globe to supply the retina. The supraorbital and supratrochlear arteries branch off of the ophthalmic artery and supply the forehead. The supraorbital artery runs through the supraorbital notch (foramen in 8%)1 and can usually be palpated with one’s finger. There are 15 to 20 short posterior ciliary arteries leading to the choroid, 2 long posterior ciliary arteries to the iris circle, and 7 anterior ciliary arteries to the extraocular muscles. The superior and inferior venous systems drain into the cavernous sinus.4
The ligaments are important to signs of facial aging because tissue atrophy occurs along them. The main orbital ligaments are the lateral orbital thickening (known as the LOT) that adheres the eyelids to the lateral orbital rim and the orbitomalar ligament (orbicularis retaining ligament), which is a condensation fibrous tissue that attaches the skin to the inferior orbital rim and orbital septum along the arcus marginalis and defines the superior edge of the SOOF.5 The zygomatic ligament not only suspends the zygomaticus major and zygomaticus minor muscles to the malar eminence but there are osseocutaneous attachments that connect the skin over the zygoma’s malar eminence and demarcate the inferior edge of the SOOF.6
Periocular Aging
The skin, fat, muscles, and bones change and rotate with aging, and not all orbits age in the same manner. Older patients with dermatochalasis (excess skin fat and muscle) often undergo rejuvenation with blepharoplasty, a brow-lift, and a midface-lift, but many atrophic changes can be improved with facial fillers.7,8
As adults age, the soft tissue along the ligaments begins to show atrophy, prime signs of aging that are often improved with fillers. Atrophy along the orbitomalar ligament along the infraorbital rim creates a depressed tear trough, which is an early sign of aging. A 3-point grading system reported by Hirmand8 describes the severity of progressive hallowing. There also is atrophy along the zygomatic cutaneous ligament that creates the malar hollow. The SOOF appears to be more prominent when these areas above and below show atrophy, which creates the look of an unwanted bag known as a festoon. Additionally, there is atrophy along the superior orbital notch where the ophthalmic branch of the trigeminal nerve (V1) and the supraorbital artery traverse. Soft-tissue atrophy along the supraorbital notch resembles the peak at the top of the letter A, giving the slang term A-frame deformity.
Periocular fat can atrophy, hypertrophy, herniate forward as the septum weakens, or become ptotic. Some patients develop hypertrophy and herniation of the superior and inferior orbital fat-pads, while others develop unwanted atrophy leaving a hollow superior orbit and loss of support to the levator muscle that contributes to eyelid ptosis. The frontalis fat deflates, leaving veins, arteries, and the hypertrophied corrugators unwantedly visible. Loss of subcutaneous fat in the glabella contributes to the formation of frown lines between the brows (also called number 11’s). The ROOF deflates in some patients adding to brow ptosis. Loss of the facial frame occurs when temple fat atrophies.
Skeletal rotation also occurs. Throughout a patient’s life, the skeleton remodels itself via activity of osteoclasts and osteoblasts. Pessa et al9,10 has described the expansion of the anterior orbital aperture superomedially and inferolaterally as well as maxillary retrusion that results in angular changes of the midface in relation to the orbital rim. Lambros’ algorithm describes the rotational changes of the cranium where the superior orbit protrudes as the maxilla retreats posteriorly.9-11 The equator of the globe does not change its distance from the ROOF of the orbit, presumably because of its suspension in the orbit by the optic nerve after it passes through the optic canal and trochlea via the superior oblique muscle, but the distance of the inferior equator of the globe to the floor of the orbit increases as the floor of the orbit descends.12
Dermal Fillers for Periocular Rejuvenation
Hyaluronic acid (HA) was first pioneered for use in humans in the late 1970s by ophthalmologists for anterior segment surgery.13-15 Biocompatibility for orthopedic and dermal applications was explored in the early 1990s.16
At this time, no dermal filler is approved by the US Food and Drug Administration for use in the periorbital area. Some fillers are approved for subdermal areas extending to the preperiosteal plane and can be used in the midface such as HA fillers (eg, Restylane Lyft [Galderma Laboratories, LP]), Juvéderm Voluma XC [Allergan, Inc]), poly-L-lactic acid (PLLA), and calcium hydroxylapatite (CaHA). No dermal fillers are approved for use in the forehead, glabella, or temples. Their use is becoming increasingly popular but is considered off label. In addition, cannulas are not approved for use in these areas. Cannulas may be beneficial in that they are thought to create less bruising and have less chance of entering a vessel than needles, but some injectors prefer needles because they are stiffer and therefore more precise.
The ideal filler for the tear trough, superior sulcus, ROOF, over the orbitomalar ligament, forehead, and glabella is one that is somewhat moldable but does not migrate, is not hydrophilic, is smooth to inject, and is reversible should there be any complications. No single filler fits this ideal description, but HAs typically are the first choice.
In vitro studies to determine the stiffness (G') and the ability to flow (viscosity) have been performed.17,18 Calcium hydroxylapatite has the most stiffness, while Belotero Balance (Merz Aesthetics) and Juvéderm Ultra XC (Allergan, Inc) are more soft17 (Table). These guidelines are important but may not correlate directly with how the fillers behave in vivo as demonstrated in animal models.18

Hyaluronic acid fillers are produced by different technologies to create their cross-link patterns with 1,4-butanediol diglycidyl ether, which determines, to some degree, their behavior in human tissue. Fillers are either monophasic; monodensified; formed by Hylacross (Juvéderm), Vycross (Juvéderm Voluma XC, Juvéderm Volbella XC), or cohesive polydensified matrix technology (Belotero Balance), or biphasic, formed by nonanimal stabilized HA sieving technology (Restylane family). Biopsy has demonstrated that monophasic fillers tend to percolate through and integrate into the tissue, while biphasic fillers dissect tissue to the sides to create a potential space for the filler to reside (Table).24
Periocular Injection Considerations
An experienced injector is one who has developed not only an artistic eye for the face and excellent sense of anatomy but also has a sensitive ability to predict the filler-tissue interaction based on tactile feedback dependent on 3 main qualities: (1) stiffness and viscosity of the filler, (2) gauge of the needle or cannula, and (3) depth of the needle in the tissue. Periocular injections of dermal fillers can be delivered with needles or cannulas, diluted or undiluted. Smaller-gauge needles require more force than larger-gauge needles and cannulas that flow more freely. A needle in the dense dermis will require more force than one placed in the loose subcutaneous space.
The tear trough is generally preferable to fill with a mid-level G' HA filler that is less apt to migrate. A neutral gaze during the injection is preferred because closing or moving the eyes can distort the position of the inferior orbital fat-pads (Figure 1). A needle or cannula can be used, diluted or undiluted. The tear trough can be filled with the injection directed horizontally or vertically via a fanning technique. If needles are used, the skin should be stretched to view the 3 to 5 vertical veins and then the needle should be advanced beneath them to avoid bruising. Avoidance of hydrophilic fillers in the tear trough is important to avoid edema. The superior sulcus can be filled both anteriorly and posteriorly to the septum, which is a highly advanced injection for experienced injectors because of the proximity to the supratrochlear and supraorbital arteries as well as the superior ophthalmic vein (Figure 2). Sharp creases such as deep lateral periocular rhytides known as crow’s-feet are nicely filled with intradermal HAs with a low G'.


Adding volume to the midface is important because it is the continuum of the lower eyelid. Fillers can be injected into multiple levels in this area: deep (to act as pillars to lift the malar eminence and replace bone that has rotated and soft tissue that has become atrophic or descended) and subcutaneous (to efface soft tissue along the zygomatic cutaneous ligament). Higher G' HA fillers and CaHA often are used in the midface along with PLLA. Facial framing of the temples, lateral cheeks, and preauricular area is often accomplished with PLLA but also can be done with mid to high G' HA fillers or CaHA. A cannula may be used to undermine and break apart the zygomatic cutaneous ligament’s cutaneous attachments prior to delivery of the filler in the subcutaneous plane.26 If not done, filler may track away from the hollow area where the ligament is attached and instead move to adjacent areas that will accentuate the hollow and make it look worse.
The temples and lateral face often are filled with PLLA for framing. Mid or high G' HA fillers and CaHA also are used in the temples both beneath the temporalis muscle and also above the deep temporalis fascia or sometimes in the subcutaneous plane.27
Prevention and Management of Periocular Complications
Blindness is the most devastating periocular complication of facial fillers, which is caused by retrograde arterial embolization followed by anterograde flow into the ophthalmic then central retinal arteries. Injectables that have caused blindness include (in descending order of frequency) fat, HA, collagen, paraffin, polymethyl methacrylate, silicone, PLLA, CaHA, polyacrylamide hydrogel, and micronized acellular dermal matrix. Of the 98 cases of blindness from periocular complications from dermal fillers reported in the world literature, the order of affected sites include the glabella (38 cases), nose (25), nasolabial folds (13), superior forehead (12), infraorbital rim (6), temples (1), malar area (1), lip (1), and chin (1). Prevention includes avoidance of danger zone arteries including the supratrochlear, supraorbital, dorsal nasal, angular, infraorbital, zygomaticofacial and zygomaticotemporal arteries.28
Avoiding the average critical volume of 0.84 in any single aliquot dispensed is key to avoid filling of these periocular arteries to the critical bifurcation point that can result in anterograde flow into the eye (Freudenthal Nicolau syndrome). The smallest supratrochlear artery’s volume in this study was 0.04 cc, so aliquots that do not exceed 0.03 cc are ideal.29,30
The injector should always be thinking about the anatomy of the danger zones (eg, infratrochlear and supratrochlear arteries, supraorbital artery, frontal branch of the superficial temporal artery, lacrimal artery, dorsal nasal artery, infraorbital artery, angular artery, zygomaticofacial artery, zygomaticotemporal artery)(Figure 3).

Hyaluronidase can be used off label to hydrolyze unwanted HA. It was first used to aid transcutaneous hydration and was used by ophthalmologists in the 1960s and 1970s to promote the spread of anesthetics by retrobulbar injection.31,32 It can penetrate through soft tissues and blood vessels.33 It is therefore hypothesized that a retrobulbar injection of hyaluronidase could aid in a case of impending blindness34 but has not been successfully accomplished to date. If vision is confirmed to be poor or there is no light perception, a retrobulbar injection of 300 U of hyaluronidase should be given immediately and then repeated in approximately 30 to 45 minutes. The retina begins to show permanent loss of function after being deprived of blood flow for just 97 minutes,35 so there may not be time for an immediate ophthalmology consultation, though such a consultation would be ideal.
Aside from common complications such as bruising and swelling, granulomas and biofilms are well documented in the literature. There are a variety of algorithms to treat such complications, which can happen many weeks after the injection of a dermal filler or years after the injection of a semipermanent filler.36 Postinjection periocular edema can occur years after the initial injection.37,38 Other periocular complications of dermal fillers include nonischemic (eg, bluish hue, filler migration, infection, inflammation, lumps) and ischemic (eg, blindness, necrosis, ophthalmoplegia, ptosis) disturbances.
Conclusion
In summary, periocular injections of facial fillers are useful tools for rejuvenation of the upper face when used with great caution and respect for anatomy.
Rejuvenation of the periocular area is in high demand among patients who want to look and feel their best. Physicians should understand the complicated anatomy surrounding the eyes before attempting to inject this area with facial fillers, both to understand the aging process and to minimize treatment complications.
Basic Oculoplastic and Orbital Anatomy
The injector should understand the anatomy of the periocular muscles, the orbital osteology, and the secretory and lacrimal system, in addition to the fat, ligaments, and vascular anatomy in this area.1
The eyes are surrounded by fat compartments that provide glide planes for the motion of the eyelids and globe. There are 2 upper eyelid fat-pads—nasal and central [preaponeurotic])—in the upper lid, leaving room for the lacrimal gland laterally. There are 3 fat compartments—nasal, central, and lateral—in the lower eyelid. The nasal and central compartments are separated by the inferior oblique muscle, which elevates and extorts the eye. The orbital septum holds the fat-pads in place in the orbit. The brow fat-pad is the retro-orbicularis oculi fat-pad (ROOF). There are fat compartments that lie in the subcutaneous space along the entire forehead and in the temple. The suborbicularis oculi fat-pad (SOOF) lies over the malar eminence. Superficial and deep submuscular fat compartments of the face have been described.2 Deep fat compartments also have been examined on computed tomography.3
Orbital circulation comes from the internal carotid artery and anastomoses with the supply from the external carotid artery to supply the orbit. The first branch off of the carotid artery is the ophthalmic artery, and the first branch off of the ophthalmic artery is the central retinal artery that enters the optic nerve sheath 1 cm behind the globe to supply the retina. The supraorbital and supratrochlear arteries branch off of the ophthalmic artery and supply the forehead. The supraorbital artery runs through the supraorbital notch (foramen in 8%)1 and can usually be palpated with one’s finger. There are 15 to 20 short posterior ciliary arteries leading to the choroid, 2 long posterior ciliary arteries to the iris circle, and 7 anterior ciliary arteries to the extraocular muscles. The superior and inferior venous systems drain into the cavernous sinus.4
The ligaments are important to signs of facial aging because tissue atrophy occurs along them. The main orbital ligaments are the lateral orbital thickening (known as the LOT) that adheres the eyelids to the lateral orbital rim and the orbitomalar ligament (orbicularis retaining ligament), which is a condensation fibrous tissue that attaches the skin to the inferior orbital rim and orbital septum along the arcus marginalis and defines the superior edge of the SOOF.5 The zygomatic ligament not only suspends the zygomaticus major and zygomaticus minor muscles to the malar eminence but there are osseocutaneous attachments that connect the skin over the zygoma’s malar eminence and demarcate the inferior edge of the SOOF.6
Periocular Aging
The skin, fat, muscles, and bones change and rotate with aging, and not all orbits age in the same manner. Older patients with dermatochalasis (excess skin fat and muscle) often undergo rejuvenation with blepharoplasty, a brow-lift, and a midface-lift, but many atrophic changes can be improved with facial fillers.7,8
As adults age, the soft tissue along the ligaments begins to show atrophy, prime signs of aging that are often improved with fillers. Atrophy along the orbitomalar ligament along the infraorbital rim creates a depressed tear trough, which is an early sign of aging. A 3-point grading system reported by Hirmand8 describes the severity of progressive hallowing. There also is atrophy along the zygomatic cutaneous ligament that creates the malar hollow. The SOOF appears to be more prominent when these areas above and below show atrophy, which creates the look of an unwanted bag known as a festoon. Additionally, there is atrophy along the superior orbital notch where the ophthalmic branch of the trigeminal nerve (V1) and the supraorbital artery traverse. Soft-tissue atrophy along the supraorbital notch resembles the peak at the top of the letter A, giving the slang term A-frame deformity.
Periocular fat can atrophy, hypertrophy, herniate forward as the septum weakens, or become ptotic. Some patients develop hypertrophy and herniation of the superior and inferior orbital fat-pads, while others develop unwanted atrophy leaving a hollow superior orbit and loss of support to the levator muscle that contributes to eyelid ptosis. The frontalis fat deflates, leaving veins, arteries, and the hypertrophied corrugators unwantedly visible. Loss of subcutaneous fat in the glabella contributes to the formation of frown lines between the brows (also called number 11’s). The ROOF deflates in some patients adding to brow ptosis. Loss of the facial frame occurs when temple fat atrophies.
Skeletal rotation also occurs. Throughout a patient’s life, the skeleton remodels itself via activity of osteoclasts and osteoblasts. Pessa et al9,10 has described the expansion of the anterior orbital aperture superomedially and inferolaterally as well as maxillary retrusion that results in angular changes of the midface in relation to the orbital rim. Lambros’ algorithm describes the rotational changes of the cranium where the superior orbit protrudes as the maxilla retreats posteriorly.9-11 The equator of the globe does not change its distance from the ROOF of the orbit, presumably because of its suspension in the orbit by the optic nerve after it passes through the optic canal and trochlea via the superior oblique muscle, but the distance of the inferior equator of the globe to the floor of the orbit increases as the floor of the orbit descends.12
Dermal Fillers for Periocular Rejuvenation
Hyaluronic acid (HA) was first pioneered for use in humans in the late 1970s by ophthalmologists for anterior segment surgery.13-15 Biocompatibility for orthopedic and dermal applications was explored in the early 1990s.16
At this time, no dermal filler is approved by the US Food and Drug Administration for use in the periorbital area. Some fillers are approved for subdermal areas extending to the preperiosteal plane and can be used in the midface such as HA fillers (eg, Restylane Lyft [Galderma Laboratories, LP]), Juvéderm Voluma XC [Allergan, Inc]), poly-L-lactic acid (PLLA), and calcium hydroxylapatite (CaHA). No dermal fillers are approved for use in the forehead, glabella, or temples. Their use is becoming increasingly popular but is considered off label. In addition, cannulas are not approved for use in these areas. Cannulas may be beneficial in that they are thought to create less bruising and have less chance of entering a vessel than needles, but some injectors prefer needles because they are stiffer and therefore more precise.
The ideal filler for the tear trough, superior sulcus, ROOF, over the orbitomalar ligament, forehead, and glabella is one that is somewhat moldable but does not migrate, is not hydrophilic, is smooth to inject, and is reversible should there be any complications. No single filler fits this ideal description, but HAs typically are the first choice.
In vitro studies to determine the stiffness (G') and the ability to flow (viscosity) have been performed.17,18 Calcium hydroxylapatite has the most stiffness, while Belotero Balance (Merz Aesthetics) and Juvéderm Ultra XC (Allergan, Inc) are more soft17 (Table). These guidelines are important but may not correlate directly with how the fillers behave in vivo as demonstrated in animal models.18

Hyaluronic acid fillers are produced by different technologies to create their cross-link patterns with 1,4-butanediol diglycidyl ether, which determines, to some degree, their behavior in human tissue. Fillers are either monophasic; monodensified; formed by Hylacross (Juvéderm), Vycross (Juvéderm Voluma XC, Juvéderm Volbella XC), or cohesive polydensified matrix technology (Belotero Balance), or biphasic, formed by nonanimal stabilized HA sieving technology (Restylane family). Biopsy has demonstrated that monophasic fillers tend to percolate through and integrate into the tissue, while biphasic fillers dissect tissue to the sides to create a potential space for the filler to reside (Table).24
Periocular Injection Considerations
An experienced injector is one who has developed not only an artistic eye for the face and excellent sense of anatomy but also has a sensitive ability to predict the filler-tissue interaction based on tactile feedback dependent on 3 main qualities: (1) stiffness and viscosity of the filler, (2) gauge of the needle or cannula, and (3) depth of the needle in the tissue. Periocular injections of dermal fillers can be delivered with needles or cannulas, diluted or undiluted. Smaller-gauge needles require more force than larger-gauge needles and cannulas that flow more freely. A needle in the dense dermis will require more force than one placed in the loose subcutaneous space.
The tear trough is generally preferable to fill with a mid-level G' HA filler that is less apt to migrate. A neutral gaze during the injection is preferred because closing or moving the eyes can distort the position of the inferior orbital fat-pads (Figure 1). A needle or cannula can be used, diluted or undiluted. The tear trough can be filled with the injection directed horizontally or vertically via a fanning technique. If needles are used, the skin should be stretched to view the 3 to 5 vertical veins and then the needle should be advanced beneath them to avoid bruising. Avoidance of hydrophilic fillers in the tear trough is important to avoid edema. The superior sulcus can be filled both anteriorly and posteriorly to the septum, which is a highly advanced injection for experienced injectors because of the proximity to the supratrochlear and supraorbital arteries as well as the superior ophthalmic vein (Figure 2). Sharp creases such as deep lateral periocular rhytides known as crow’s-feet are nicely filled with intradermal HAs with a low G'.


Adding volume to the midface is important because it is the continuum of the lower eyelid. Fillers can be injected into multiple levels in this area: deep (to act as pillars to lift the malar eminence and replace bone that has rotated and soft tissue that has become atrophic or descended) and subcutaneous (to efface soft tissue along the zygomatic cutaneous ligament). Higher G' HA fillers and CaHA often are used in the midface along with PLLA. Facial framing of the temples, lateral cheeks, and preauricular area is often accomplished with PLLA but also can be done with mid to high G' HA fillers or CaHA. A cannula may be used to undermine and break apart the zygomatic cutaneous ligament’s cutaneous attachments prior to delivery of the filler in the subcutaneous plane.26 If not done, filler may track away from the hollow area where the ligament is attached and instead move to adjacent areas that will accentuate the hollow and make it look worse.
The temples and lateral face often are filled with PLLA for framing. Mid or high G' HA fillers and CaHA also are used in the temples both beneath the temporalis muscle and also above the deep temporalis fascia or sometimes in the subcutaneous plane.27
Prevention and Management of Periocular Complications
Blindness is the most devastating periocular complication of facial fillers, which is caused by retrograde arterial embolization followed by anterograde flow into the ophthalmic then central retinal arteries. Injectables that have caused blindness include (in descending order of frequency) fat, HA, collagen, paraffin, polymethyl methacrylate, silicone, PLLA, CaHA, polyacrylamide hydrogel, and micronized acellular dermal matrix. Of the 98 cases of blindness from periocular complications from dermal fillers reported in the world literature, the order of affected sites include the glabella (38 cases), nose (25), nasolabial folds (13), superior forehead (12), infraorbital rim (6), temples (1), malar area (1), lip (1), and chin (1). Prevention includes avoidance of danger zone arteries including the supratrochlear, supraorbital, dorsal nasal, angular, infraorbital, zygomaticofacial and zygomaticotemporal arteries.28
Avoiding the average critical volume of 0.84 in any single aliquot dispensed is key to avoid filling of these periocular arteries to the critical bifurcation point that can result in anterograde flow into the eye (Freudenthal Nicolau syndrome). The smallest supratrochlear artery’s volume in this study was 0.04 cc, so aliquots that do not exceed 0.03 cc are ideal.29,30
The injector should always be thinking about the anatomy of the danger zones (eg, infratrochlear and supratrochlear arteries, supraorbital artery, frontal branch of the superficial temporal artery, lacrimal artery, dorsal nasal artery, infraorbital artery, angular artery, zygomaticofacial artery, zygomaticotemporal artery)(Figure 3).

Hyaluronidase can be used off label to hydrolyze unwanted HA. It was first used to aid transcutaneous hydration and was used by ophthalmologists in the 1960s and 1970s to promote the spread of anesthetics by retrobulbar injection.31,32 It can penetrate through soft tissues and blood vessels.33 It is therefore hypothesized that a retrobulbar injection of hyaluronidase could aid in a case of impending blindness34 but has not been successfully accomplished to date. If vision is confirmed to be poor or there is no light perception, a retrobulbar injection of 300 U of hyaluronidase should be given immediately and then repeated in approximately 30 to 45 minutes. The retina begins to show permanent loss of function after being deprived of blood flow for just 97 minutes,35 so there may not be time for an immediate ophthalmology consultation, though such a consultation would be ideal.
Aside from common complications such as bruising and swelling, granulomas and biofilms are well documented in the literature. There are a variety of algorithms to treat such complications, which can happen many weeks after the injection of a dermal filler or years after the injection of a semipermanent filler.36 Postinjection periocular edema can occur years after the initial injection.37,38 Other periocular complications of dermal fillers include nonischemic (eg, bluish hue, filler migration, infection, inflammation, lumps) and ischemic (eg, blindness, necrosis, ophthalmoplegia, ptosis) disturbances.
Conclusion
In summary, periocular injections of facial fillers are useful tools for rejuvenation of the upper face when used with great caution and respect for anatomy.
- Foster J, ed. Orbit, Eyelids, and Lacrimal System. San Francisco, CA: American Academy of Ophthalmology; 2016. 2016-2017 Basic and Clinical Science Course; section 7.
- Rohrich RJ, Pessa JE. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119:2219-2227; discussion 2228-2231.
- Gierloff M, Stöhring C, Buder T, et al. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconst Surg. 2012;129:263-273.
- Zide BM, Jelks GW. Surgical Anatomy of the Orbit: The System of Zones. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
- Kikkawa DO, Lemke BN, Dortzbach RK. Relations of the superficial musculoaponeurotic system to the orbit and characterization of the oribitomalar ligament. Ophthal Plast Reconstr Surg. 1996;12:77-88.
- Furnas DW. The retaining ligaments of the cheek. Plast Reconstr Surg. 1989;83:11-16.
- Morley AM, Taban M, Malhotra R, et al. Use of hyaluronic acid gel for upper eyelid filling and contouring. Ophthal Plast Reconstr Surg. 2009;25:440-444.
- Hirmand H. Anatomy and nonsurgical correction of the tear trough deformity. Plast Reconstr Surg. 2010;125:699-708.
- Pessa JE, Zadoo VP, Mutimer KL, et al. Relative maxillary retrusion as a natural consequence of aging. Plast Reconstr Surg. 1998;102:205-212.
- Pessa JE, Desvigne LD, Lambros VS, et al. Changes in ocular globe-to-orbital rim position with age: implications for aesthetic blepharoplasty of the lower eyelids. Aesthet Plast Surg. 1999;23:337-345.
- Goldberg RA, Relan A, Hoenig J. Relationship of the eye to the bony orbit, with clinical correlations. Aust N Z J Ophthalmol. 1999;27:398-403.
- Richard MJ, Morris C, Deen BF, et al. Analysis of the anatomic changes of the aging facial skeleton using computer-assisted tomography. Ophthal Plast Reconstr Surg. 2009;25:382-386.
- Miller D, O’Connor P, Williams J. Use of Na-hyaluronate during intraocular lens implantation in rabbits. Ophthalmic Surg. 1977;8:58-61.
- Miller D, Stegmann R. Use of Na-hyaluronate in anterior segment eye surgery. J Am Intraocul Implant Soc. 1980;6:13-15.
- Pape LG, Balazs EA. The use of sodium hyaluronate (Healon) in human anterior segment surgery. Ophthalmology. 1980;87:699-705.
- Larsen NE, Pollak CT, Reiner K, et al. Hylan gel biomaterial: dermal and immunologic compatibility. J Biomed Mater Res. 1993;27:1129-1134.
- Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;132(4, suppl 2):5S-21S.
- Hee CK, Shumate GT, Narurkar V, et al. Rheological properties and in vivo performance characteristics of soft tissue fillers. Dermatol Surg. 2015;41(suppl 1):S373-S381.
- Sundaram H, Voigts B, Beer K, et al. Comparison of the rheological properties of viscosity and elasticity in two categories of soft tissue fillers: calcium hydroxylapatite and hyaluronic acid. Dermatol Surg. 2010;36(suppl 3):1859-1865.
- Sundaram H. The new face of fillers: a multi-specialty CME initiative: supplement part II of II. J Drugs Dermatol. 2012;11(suppl 8):S8.
- Stocks D, Sundaram H, Michaels J, et al. Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J Drugs Dermatol. 2011;10:974-980.
- Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136(suppl 5):139S-148S.
- Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale and evaluation of six U.S. Food and Drug Administration–approved fillers. Plast Reconstr Surg. 2015;136:678-686.
- Flynn TC, Sarazin D, Bezzola A, et al. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637-643.
- Woodward JA, Langelier N. Filler enhancement of the superior periocular area [published online Jun 23, 2016]. JAMA Facial Plast Surg. doi:10.1001/jamafacial.2016.0636.
- Cotofana S, Schenck TL, Trevidic P, et al. Midface: clinical anatomy and regional approaches with injectable fillers. Plast Reconstr Surg. 2015;136(suppl 5):219S-234S.
- Buckingham ED, Glasgold R, Kontis T, et al. Volume rejuvenation of the facial upper third. Facial Plast Surg. 2015;31:43-54.
- Beleznay K, Carruthers JD, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097-1117.
- Coleman SR. Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J. 2002;22:555-557.
- Khan T, Colon-Acevedo B, Mettu P, et al. An anatomical analysis of the supratrochlear artery: considerations in facial filler injections and preventing vision loss [published online August 16, 2016]. Aesthet Surg J. pii: sjw132.
- Iserle J, Kumstat Z. Retrobulbar injections of hyaluronidase as a method of increasing safety in cataract surgery [in Czech]. Cesk Oftalmol. 1960;15:126-130.
- Wojtowicz S. Effect of retrobulbar injections of novocaine and lignocaine with adrenalin and hyaluronidase for the immobilization of the eye in electromyography [in Polish]. Klin Oczna. 1964;34:285-296.
- Delorenzi C. Transarterial degradation of hyaluronic acid filler by hyaluronidase. Dermatol Surg. 2014;40:832-841.
- Carruthers J, Fagien S, Dolman P. Retro or peribulbar injections techniques to reverse visual loss after filler injections. 2015;41(suppl 1):S354-S357.
- Hayreh SS, Zimmerman MB, Kimura A, et al. Central retinal artery occlusion. retinal survival time. Exp Eye Res. 2004;78:723-736.
- Woodward J, Khan T, Martin J. Facial filler complications. Facial Plast Surg Clin North Am. 2015;23:447-458.
- Khan TT, Woodward JA. Retained dermal filler in the upper eyelid masquerading as periorbital edema. Dermatol Surg. 2015;41:1182-1184.
- Chang JR, Baharestani S, Salek SS, et al. Delayed superficial migration of retained hyaluronic acid years following periocular injection [published online April 20, 2015]. Ophthal Plast Reconstr Surg. doi:10.1097/IOP.0000000000000434.
- Foster J, ed. Orbit, Eyelids, and Lacrimal System. San Francisco, CA: American Academy of Ophthalmology; 2016. 2016-2017 Basic and Clinical Science Course; section 7.
- Rohrich RJ, Pessa JE. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119:2219-2227; discussion 2228-2231.
- Gierloff M, Stöhring C, Buder T, et al. Aging changes of the midfacial fat compartments: a computed tomographic study. Plast Reconst Surg. 2012;129:263-273.
- Zide BM, Jelks GW. Surgical Anatomy of the Orbit: The System of Zones. Philadelphia, PA: Lippincott Williams & Wilkins; 2006.
- Kikkawa DO, Lemke BN, Dortzbach RK. Relations of the superficial musculoaponeurotic system to the orbit and characterization of the oribitomalar ligament. Ophthal Plast Reconstr Surg. 1996;12:77-88.
- Furnas DW. The retaining ligaments of the cheek. Plast Reconstr Surg. 1989;83:11-16.
- Morley AM, Taban M, Malhotra R, et al. Use of hyaluronic acid gel for upper eyelid filling and contouring. Ophthal Plast Reconstr Surg. 2009;25:440-444.
- Hirmand H. Anatomy and nonsurgical correction of the tear trough deformity. Plast Reconstr Surg. 2010;125:699-708.
- Pessa JE, Zadoo VP, Mutimer KL, et al. Relative maxillary retrusion as a natural consequence of aging. Plast Reconstr Surg. 1998;102:205-212.
- Pessa JE, Desvigne LD, Lambros VS, et al. Changes in ocular globe-to-orbital rim position with age: implications for aesthetic blepharoplasty of the lower eyelids. Aesthet Plast Surg. 1999;23:337-345.
- Goldberg RA, Relan A, Hoenig J. Relationship of the eye to the bony orbit, with clinical correlations. Aust N Z J Ophthalmol. 1999;27:398-403.
- Richard MJ, Morris C, Deen BF, et al. Analysis of the anatomic changes of the aging facial skeleton using computer-assisted tomography. Ophthal Plast Reconstr Surg. 2009;25:382-386.
- Miller D, O’Connor P, Williams J. Use of Na-hyaluronate during intraocular lens implantation in rabbits. Ophthalmic Surg. 1977;8:58-61.
- Miller D, Stegmann R. Use of Na-hyaluronate in anterior segment eye surgery. J Am Intraocul Implant Soc. 1980;6:13-15.
- Pape LG, Balazs EA. The use of sodium hyaluronate (Healon) in human anterior segment surgery. Ophthalmology. 1980;87:699-705.
- Larsen NE, Pollak CT, Reiner K, et al. Hylan gel biomaterial: dermal and immunologic compatibility. J Biomed Mater Res. 1993;27:1129-1134.
- Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;132(4, suppl 2):5S-21S.
- Hee CK, Shumate GT, Narurkar V, et al. Rheological properties and in vivo performance characteristics of soft tissue fillers. Dermatol Surg. 2015;41(suppl 1):S373-S381.
- Sundaram H, Voigts B, Beer K, et al. Comparison of the rheological properties of viscosity and elasticity in two categories of soft tissue fillers: calcium hydroxylapatite and hyaluronic acid. Dermatol Surg. 2010;36(suppl 3):1859-1865.
- Sundaram H. The new face of fillers: a multi-specialty CME initiative: supplement part II of II. J Drugs Dermatol. 2012;11(suppl 8):S8.
- Stocks D, Sundaram H, Michaels J, et al. Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J Drugs Dermatol. 2011;10:974-980.
- Goodman GJ, Swift A, Remington BK. Current concepts in the use of Voluma, Volift, and Volbella. Plast Reconstr Surg. 2015;136(suppl 5):139S-148S.
- Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale and evaluation of six U.S. Food and Drug Administration–approved fillers. Plast Reconstr Surg. 2015;136:678-686.
- Flynn TC, Sarazin D, Bezzola A, et al. Comparative histology of intradermal implantation of mono and biphasic hyaluronic acid fillers. Dermatol Surg. 2011;37:637-643.
- Woodward JA, Langelier N. Filler enhancement of the superior periocular area [published online Jun 23, 2016]. JAMA Facial Plast Surg. doi:10.1001/jamafacial.2016.0636.
- Cotofana S, Schenck TL, Trevidic P, et al. Midface: clinical anatomy and regional approaches with injectable fillers. Plast Reconstr Surg. 2015;136(suppl 5):219S-234S.
- Buckingham ED, Glasgold R, Kontis T, et al. Volume rejuvenation of the facial upper third. Facial Plast Surg. 2015;31:43-54.
- Beleznay K, Carruthers JD, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097-1117.
- Coleman SR. Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J. 2002;22:555-557.
- Khan T, Colon-Acevedo B, Mettu P, et al. An anatomical analysis of the supratrochlear artery: considerations in facial filler injections and preventing vision loss [published online August 16, 2016]. Aesthet Surg J. pii: sjw132.
- Iserle J, Kumstat Z. Retrobulbar injections of hyaluronidase as a method of increasing safety in cataract surgery [in Czech]. Cesk Oftalmol. 1960;15:126-130.
- Wojtowicz S. Effect of retrobulbar injections of novocaine and lignocaine with adrenalin and hyaluronidase for the immobilization of the eye in electromyography [in Polish]. Klin Oczna. 1964;34:285-296.
- Delorenzi C. Transarterial degradation of hyaluronic acid filler by hyaluronidase. Dermatol Surg. 2014;40:832-841.
- Carruthers J, Fagien S, Dolman P. Retro or peribulbar injections techniques to reverse visual loss after filler injections. 2015;41(suppl 1):S354-S357.
- Hayreh SS, Zimmerman MB, Kimura A, et al. Central retinal artery occlusion. retinal survival time. Exp Eye Res. 2004;78:723-736.
- Woodward J, Khan T, Martin J. Facial filler complications. Facial Plast Surg Clin North Am. 2015;23:447-458.
- Khan TT, Woodward JA. Retained dermal filler in the upper eyelid masquerading as periorbital edema. Dermatol Surg. 2015;41:1182-1184.
- Chang JR, Baharestani S, Salek SS, et al. Delayed superficial migration of retained hyaluronic acid years following periocular injection [published online April 20, 2015]. Ophthal Plast Reconstr Surg. doi:10.1097/IOP.0000000000000434.
Practice Points
- When performing periocular dermal injections, physicians should understand the complicated anatomy surrounding the eyes and related changes with upper face aging.
- The different rheological properties of facial fillers impact product selection for various areas of the upper face.
- Physicians should be aware of the anatomical danger zones to avoid intravascular embolization.