User login
To the Editor:
A 62-year-old woman with a history of dermatomyositis (DM) presented to dermatology clinic for evaluation of multiple subcutaneous nodules. Two years prior to the current presentation, the patient was diagnosed by her primary care physician with DM based on clinical presentation. She initially developed body aches, muscle pain, and weakness of the upper extremities, specifically around the shoulders, and later the lower extremities, specifically around the thighs. The initial physical examination revealed pain with movement, tenderness to palpation, and proximal extremity weakness. The patient also noted a 50-lb weight loss. Over the next year, she noted dysphagia and developed multiple subcutaneous nodules on the right arm, chest, and left axilla. Subsequently, she developed a violaceous, hyperpigmented, periorbital rash and erythema of the anterior chest. She did not experience hair loss, oral ulcers, photosensitivity, or joint pain.
Laboratory testing in the months following the initial presentation revealed a creatine phosphokinase level of 436 U/L (reference range, 20–200 U/L), an erythrocyte sedimentation rate of 60 mm/h (reference range, <31 mm/h), and an aldolase level of 10.4 U/L (reference range, 1.0–8.0 U/L). Lactate dehydrogenase and thyroid function tests were within normal limits. Antinuclear antibodies, anti–double-stranded DNA, anti-Smith antibodies, anti-ribonucleoprotein, anti–Jo-1 antibodies, and anti–smooth muscle antibodies all were negative. Total blood complement levels were elevated, but complement C3 and C4 were within normal limits. Imaging demonstrated normal chest radiographs, and a modified barium swallow confirmed swallowing dysfunction. A right quadricep muscle biopsy confirmed the diagnosis of DM. A malignancy work-up including mammography, colonoscopy, and computed tomography of the chest, abdomen, and pelvis was negative aside from nodular opacities in the chest. She was treated with prednisone (60 mg, 0.9 mg/kg) daily and methotrexate (15–20 mg) weekly for several months. While the treatment attenuated the rash and improved weakness, the nodules persisted, prompting a referral to dermatology.
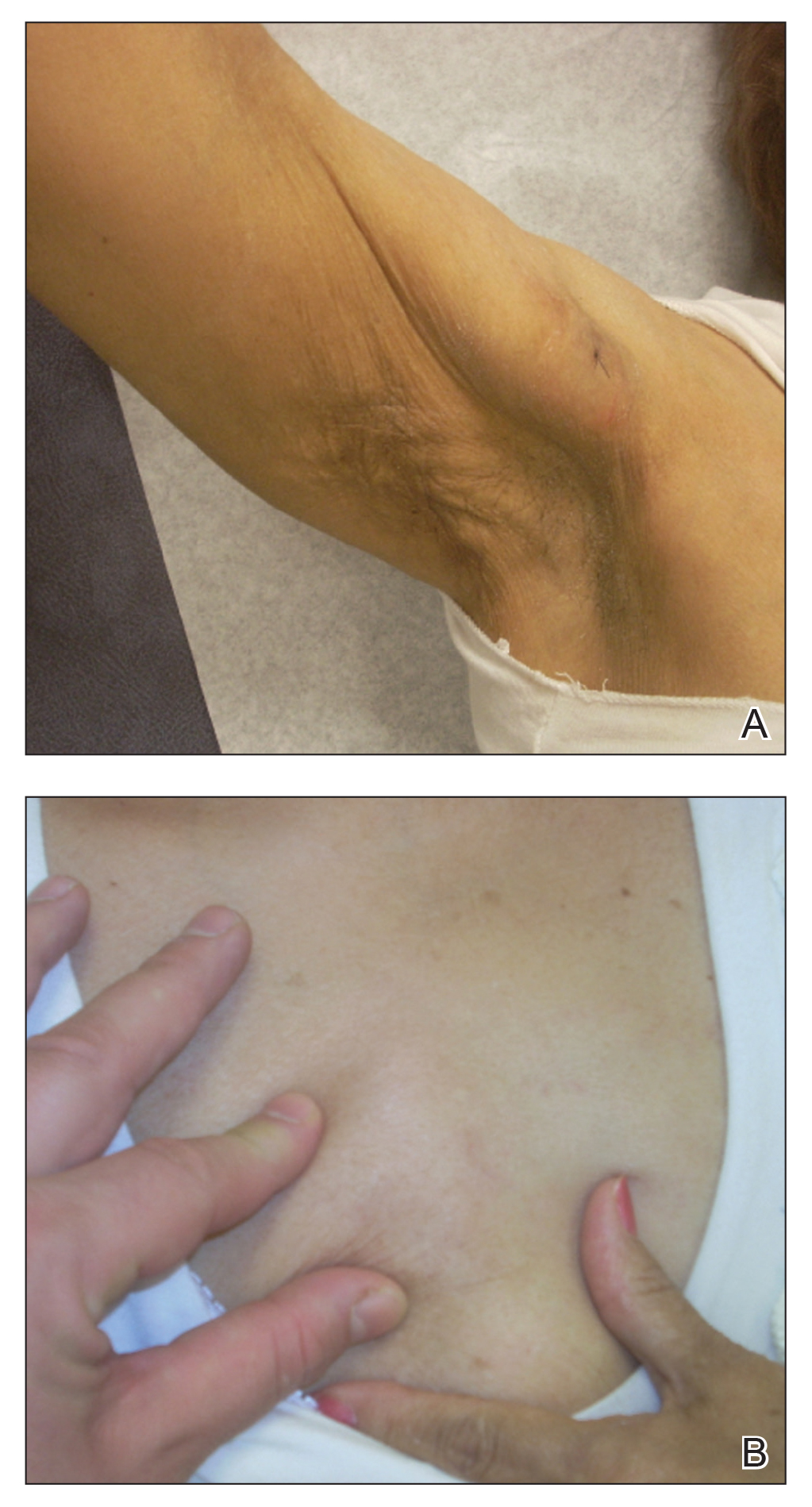
Physical examination at the dermatology clinic demonstrated the persistent subcutaneous nodules were indurated and bilaterally located on the arms, axillae, chest, abdomen, buttocks, and thighs with no pain or erythema (Figure). Laboratory tests demonstrated a normal creatine phosphokinase level, elevated erythrocyte sedimentation rate (70 mm/h), and elevated aldolase level (9.3 U/L). Complement levels were elevated, though complement C3 and C4 remained within normal limits. Histopathology of nodules from the medial right upper arm and left thigh showed lobular panniculitis with fat necrosis, calcification, and interface changes. The patient was treated for several months with daily mycophenolate mofetil (1 g increased to 3 g) and daily hydroxychloroquine (200 mg) without any effect on the nodules.
The histologic features of panniculitis in lupus and DM are similar and include multifocal hyalinization of the subcuticular fat and diffuse lobular infiltrates of mature lymphocytes without nuclear atypia.1 Though clinical panniculitis is a rare finding in DM, histologic panniculitis is a relatively common finding.2 Despite the similar histopathology of lupus and DM, the presence of typical DM clinical and laboratory features in our patient (body aches, muscle pain, proximal weakness, cutaneous manifestations, elevated creatine phosphokinase, normal complement C3 and C4) made a diagnosis of DM more likely.
Clinical panniculitis is a rare subcutaneous manifestation of DM with around 50 cases reported in the literature (Table). A PubMed search of articles indexed for MEDLINE was conducted using the terms dermatomyositis and panniculitis through July 2019. Additionally, a full-text review and search of references within these articles was used to identify all cases of patients presenting with panniculitis in the setting of DM. Exclusion criteria were cases in which another etiology was considered likely (infectious panniculitis and lupus panniculitis) as well as those without an English translation. We identified 43 cases; the average age of the patients was 39.6 years, and 36 (83.7%) of the cases were women. Patients typically presented with persistent, indurated, painful, erythematous, nodular lesions localized to the arms, abdomen, buttocks, and thighs.
While panniculitis has been reported preceding and concurrent with a diagnosis of DM, a number of cases described presentation as late as 5 years following onset of classic DM symptoms.12,13,31 In some cases (3/43 [7.0%]), panniculitis was the only cutaneous manifestation of DM.15,33,36 However, it occurred more commonly with other characteristic skin findings, such as heliotrope rash or Gottron sign.Some investigators have recommended that panniculitis be included as a diagnostic feature of DM and that DM be considered in the differential diagnosis in isolated cases of panniculitis.25,33
Though it seems panniculitis in DM may correlate with a better prognosis, we identified underlying malignancies in 3 cases. Malignancies associated with panniculitis in DM included ovarian adenocarcinoma, nasopharyngeal carcinoma, and parotid carcinoma, indicating that appropriate cancer screening still is critical in the diagnostic workup.2,11,22
A majority of the reported panniculitis cases in DM have responded to treatment with prednisone; however, treatment with prednisone has been more recalcitrant in other cases. Reports of successful additional therapies include methotrexate, cyclosporine, azathioprine, hydroxychloroquine, intravenous immunoglobulin, mepacrine, or a combination of these entities.19,22 In most cases, improvement of the panniculitis and other DM symptoms occurred simultaneously.25 It is noteworthy that the muscular symptoms often resolved more rapidly than cutaneous manifestations.33 Few reported cases (6 including the current case) found a persistent panniculitis despite improvement and remission of the myositis.3,5,10,11,30
Our patient was treated with both prednisone and methotrexate for several months, leading to remission of muscular symptoms (along with return to baseline of creatine phosphokinase), yet the panniculitis did not improve. The subcutaneous nodules also did not respond to treatment with mycophenolate mofetil and hydroxychloroquine.
Recent immunohistochemical studies have suggested that panniculitic lesions show better outcomes with immunosuppressive therapy when compared with other DM-related skin lesions.40 However, this was not the case for our patient, who after months of immunosuppressive therapy showed complete resolution of the periorbital and chest rashes with persistence of multiple indurated subcutaneous nodules.
Our case adds to a number of reports of DM presenting with panniculitis. Our patient fit the classic demographic of previously reported cases, as she was an adult woman without evidence of underlying malignancy; however, our case remains an example of the therapeutic challenge that exists when encountering a persistent, treatment-resistant panniculitis despite resolution of all other features of DM.
- Wick MR. Panniculitis: a summary. Semin Diagn Pathol. 2017;34:261-272.
- Girouard SD, Velez NF, Penson RT, et al. Panniculitis associated with dermatomyositis and recurrent ovarian cancer. Arch Dermatol. 2012;148:740-744.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:E187-E188.
- Choi YJ, Yoo WH. Panniculitis, a rare presentation of onset and exacerbation of juvenile dermatomyositis: a case report and literature review. Arch Rheumatol. 2018;33:367-371.
- Azevedo PO, Castellen NR, Salai AF, et al. Panniculitis associated with amyopathic dermatomyositis. An Bras Dermatol. 2018;93:119-121.
- Agulló A, Hinds B, Larrea M, et al. Livedo racemosa, reticulated ulcerations, panniculitis and violaceous plaques in a 46-year-old woman. Indian Dermatol Online J. 2018;9:47-49.
- Hattori Y, Matsuyama K, Takahashi T, et al. Anti-MDA5 antibody-positive dermatomyositis presenting with cellulitis-like erythema on the mandible as an initial symptom. Case Rep Dermatol. 2018;10:110-114.
- Hasegawa A, Shimomura Y, Kibune N, et al. Panniculitis as the initial manifestation of dermatomyositis with anti-MDA5 antibody. Clin Exp Dermatol. 2017;42:551-553.
- Salman A, Kasapcopur O, Ergun T, et al. Panniculitis in juvenile dermatomyositis: report of a case and review of the published work. J Dermatol. 2016;43:951-953.
- Carroll M, Mellick N, Wagner G. Dermatomyositis panniculitis: a case report. Australas J Dermatol. 2015;56:224‐226.
- Chairatchaneeboon M, Kulthanan K, Manapajon A. Calcific panniculitis and nasopharyngeal cancer-associated adult-onset dermatomyositis: a case report and literature review. Springerplus. 2015;4:201.
- Otero Rivas MM, Vicente Villa A, González Lara L, et al. Panniculitis in juvenile dermatomyositis. Clin Exp Dermatol. 2015;40:574-575.
- Yanaba K, Tanito K, Hamaguchi Y, et al. Anti‐transcription intermediary factor‐1γ/α/β antibody‐positive dermatomyositis associated with multiple panniculitis lesions. Int J Rheum Dis. 2015;20:1831-1834.
- Pau-Charles I, Moreno PJ, Ortiz-Ibanez K, et al. Anti-MDA5 positive clinically amyopathic dermatomyositis presenting with severe cardiomyopathy. J Eur Acad Dermatol Venereol. 2014;28:1097-1102.
- Lamb R, Digby S, Stewart W, et al. Cutaneous ulceration: more than skin deep? Clin Exp Dermatol. 2013;38:443-445.
- Arias M, Hernández MI, Cunha LG, et al. Panniculitis in a patient with dermatomyositis. An Bras Dermatol. 2011;86:146-148.
- Hemmi S, Kushida R, Nishimura H, et al. Magnetic resonance imaging diagnosis of panniculitis in dermatomyositis. Muscle Nerve. 2010;41:151-153.
- Geddes MR, Sinnreich M, Chalk C. Minocycline-induced dermatomyositis. Muscle Nerve. 2010;41:547-549.
- Abdul‐Wahab A, Holden CA, Harland C, et al Calcific panniculitis in adult‐onset dermatomyositis. Clin Exp Dermatol. 2009;34:E854-E856.
- Carneiro S, Alvim G, Resende P, et al. Dermatomyositis with panniculitis. Skinmed. 2007;6:46-47.
- Carrera E, Lobrinus JA, Spertini O, et al. Dermatomyositis, lobarpanniculitis and inflammatory myopathy with abundant macrophages. Neuromuscul Disord. 2006;16:468-471.
- Lin JH, Chu CY, Lin RY. Panniculitis in adult onset dermatomyositis: report of two cases and review of the literature. Dermatol Sinica. 2006;24:194-200.
- Chen GY, Liu MF, Lee JY, et al. Combination of massive mucinosis, dermatomyositis, pyoderma gangrenosum-like ulcer, bullae and fatal intestinal vasculopathy in a young female. Eur J Dermatol. 2005;15:396-400.
- Nakamori A, Yamaguchi Y, Kurimoto I, et al. Vesiculobullous dermatomyositis with panniculitis without muscle disease. J Am Acad Dermatol. 2003;49:1136-1139.
- Solans R, Cortés J, Selva A, et al. Panniculitis: a cutaneous manifestation of dermatomyositis. J Am Acad Dermatol. 2002;46:S148-S150.
- Chao YY, Yang LJ. Dermatomyositis presenting as panniculitis. Int J Dermatol. 2000;39:141-144.
- Lee MW, Lim YS, Choi JH, et al. Panniculitis showing membranocystic changes in the dermatomyositis. J Dermatol. 1999;26:608‐610.
- Ghali FE, Reed AM, Groben PA, et al. Panniculitis in juvenile dermatomyositis. Pediatr Dermatol. 1999;16:270-272.
- Molnar K, Kemeny L, Korom I, et al. Panniculitis in dermatomyositis: report of two cases. Br J Dermatol. 1998;139:161‐163.
- Ishikawa O, Tamura A, Ryuzaki K, et al. Membranocystic changes in the panniculitis of dermatomyositis. Br J Dermatol. 1996;134:773-776.
- Sabroe RA, Wallington TB, Kennedy CT. Dermatomyositis treated with high-dose intravenous immunoglobulins and associated with panniculitis. Clin Exp Dermatol. 1995;20:164-167.
- Neidenbach PJ, Sahn EE, Helton J. Panniculitis in juvenile dermatomyositis. J Am Acad Dermatol. 1995;33:305-307.
- Fusade T, Belanyi P, Joly P, et al. Subcutaneous changes in dermatomyositis. Br J Dermatol. 1993;128:451-453.
- Winkelmann WJ, Billick RC, Srolovitz H. Dermatomyositis presenting as panniculitis. J Am Acad Dermatol. 1990;23:127-128.
- Commens C, O’Neill P, Walker G. Dermatomyositis associated with multifocal lipoatrophy. J Am Acad Dermatol. 1990;22:966-969.
- Raimer SS, Solomon AR, Daniels JC. Polymyositis presenting with panniculitis. J Am Acad Dermatol. 1985;13(2 pt 2):366‐369.
- Feldman D, Hochberg MC, Zizic TM, et al. Cutaneous vasculitis in adult polymyositis/dermatomyositis. J Rheumatol. 1983;10:85-89.
- Kimura S, Fukuyama Y. Tubular cytoplasmic inclusions in a case of childhood dermatomyositis with migratory subcutaneous nodules. Eur J Pediatr. 1977;125:275-283.
- Weber FP, Gray AMH. Chronic relapsing polydermatomyositis with predominant involvement of the subcutaneous fat. Br J Dermatol. 1924;36:544-560.
- Santos‐Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
To the Editor:
A 62-year-old woman with a history of dermatomyositis (DM) presented to dermatology clinic for evaluation of multiple subcutaneous nodules. Two years prior to the current presentation, the patient was diagnosed by her primary care physician with DM based on clinical presentation. She initially developed body aches, muscle pain, and weakness of the upper extremities, specifically around the shoulders, and later the lower extremities, specifically around the thighs. The initial physical examination revealed pain with movement, tenderness to palpation, and proximal extremity weakness. The patient also noted a 50-lb weight loss. Over the next year, she noted dysphagia and developed multiple subcutaneous nodules on the right arm, chest, and left axilla. Subsequently, she developed a violaceous, hyperpigmented, periorbital rash and erythema of the anterior chest. She did not experience hair loss, oral ulcers, photosensitivity, or joint pain.
Laboratory testing in the months following the initial presentation revealed a creatine phosphokinase level of 436 U/L (reference range, 20–200 U/L), an erythrocyte sedimentation rate of 60 mm/h (reference range, <31 mm/h), and an aldolase level of 10.4 U/L (reference range, 1.0–8.0 U/L). Lactate dehydrogenase and thyroid function tests were within normal limits. Antinuclear antibodies, anti–double-stranded DNA, anti-Smith antibodies, anti-ribonucleoprotein, anti–Jo-1 antibodies, and anti–smooth muscle antibodies all were negative. Total blood complement levels were elevated, but complement C3 and C4 were within normal limits. Imaging demonstrated normal chest radiographs, and a modified barium swallow confirmed swallowing dysfunction. A right quadricep muscle biopsy confirmed the diagnosis of DM. A malignancy work-up including mammography, colonoscopy, and computed tomography of the chest, abdomen, and pelvis was negative aside from nodular opacities in the chest. She was treated with prednisone (60 mg, 0.9 mg/kg) daily and methotrexate (15–20 mg) weekly for several months. While the treatment attenuated the rash and improved weakness, the nodules persisted, prompting a referral to dermatology.
Physical examination at the dermatology clinic demonstrated the persistent subcutaneous nodules were indurated and bilaterally located on the arms, axillae, chest, abdomen, buttocks, and thighs with no pain or erythema (Figure). Laboratory tests demonstrated a normal creatine phosphokinase level, elevated erythrocyte sedimentation rate (70 mm/h), and elevated aldolase level (9.3 U/L). Complement levels were elevated, though complement C3 and C4 remained within normal limits. Histopathology of nodules from the medial right upper arm and left thigh showed lobular panniculitis with fat necrosis, calcification, and interface changes. The patient was treated for several months with daily mycophenolate mofetil (1 g increased to 3 g) and daily hydroxychloroquine (200 mg) without any effect on the nodules.

The histologic features of panniculitis in lupus and DM are similar and include multifocal hyalinization of the subcuticular fat and diffuse lobular infiltrates of mature lymphocytes without nuclear atypia.1 Though clinical panniculitis is a rare finding in DM, histologic panniculitis is a relatively common finding.2 Despite the similar histopathology of lupus and DM, the presence of typical DM clinical and laboratory features in our patient (body aches, muscle pain, proximal weakness, cutaneous manifestations, elevated creatine phosphokinase, normal complement C3 and C4) made a diagnosis of DM more likely.
Clinical panniculitis is a rare subcutaneous manifestation of DM with around 50 cases reported in the literature (Table). A PubMed search of articles indexed for MEDLINE was conducted using the terms dermatomyositis and panniculitis through July 2019. Additionally, a full-text review and search of references within these articles was used to identify all cases of patients presenting with panniculitis in the setting of DM. Exclusion criteria were cases in which another etiology was considered likely (infectious panniculitis and lupus panniculitis) as well as those without an English translation. We identified 43 cases; the average age of the patients was 39.6 years, and 36 (83.7%) of the cases were women. Patients typically presented with persistent, indurated, painful, erythematous, nodular lesions localized to the arms, abdomen, buttocks, and thighs.
While panniculitis has been reported preceding and concurrent with a diagnosis of DM, a number of cases described presentation as late as 5 years following onset of classic DM symptoms.12,13,31 In some cases (3/43 [7.0%]), panniculitis was the only cutaneous manifestation of DM.15,33,36 However, it occurred more commonly with other characteristic skin findings, such as heliotrope rash or Gottron sign.Some investigators have recommended that panniculitis be included as a diagnostic feature of DM and that DM be considered in the differential diagnosis in isolated cases of panniculitis.25,33
Though it seems panniculitis in DM may correlate with a better prognosis, we identified underlying malignancies in 3 cases. Malignancies associated with panniculitis in DM included ovarian adenocarcinoma, nasopharyngeal carcinoma, and parotid carcinoma, indicating that appropriate cancer screening still is critical in the diagnostic workup.2,11,22
A majority of the reported panniculitis cases in DM have responded to treatment with prednisone; however, treatment with prednisone has been more recalcitrant in other cases. Reports of successful additional therapies include methotrexate, cyclosporine, azathioprine, hydroxychloroquine, intravenous immunoglobulin, mepacrine, or a combination of these entities.19,22 In most cases, improvement of the panniculitis and other DM symptoms occurred simultaneously.25 It is noteworthy that the muscular symptoms often resolved more rapidly than cutaneous manifestations.33 Few reported cases (6 including the current case) found a persistent panniculitis despite improvement and remission of the myositis.3,5,10,11,30
Our patient was treated with both prednisone and methotrexate for several months, leading to remission of muscular symptoms (along with return to baseline of creatine phosphokinase), yet the panniculitis did not improve. The subcutaneous nodules also did not respond to treatment with mycophenolate mofetil and hydroxychloroquine.
Recent immunohistochemical studies have suggested that panniculitic lesions show better outcomes with immunosuppressive therapy when compared with other DM-related skin lesions.40 However, this was not the case for our patient, who after months of immunosuppressive therapy showed complete resolution of the periorbital and chest rashes with persistence of multiple indurated subcutaneous nodules.
Our case adds to a number of reports of DM presenting with panniculitis. Our patient fit the classic demographic of previously reported cases, as she was an adult woman without evidence of underlying malignancy; however, our case remains an example of the therapeutic challenge that exists when encountering a persistent, treatment-resistant panniculitis despite resolution of all other features of DM.
To the Editor:
A 62-year-old woman with a history of dermatomyositis (DM) presented to dermatology clinic for evaluation of multiple subcutaneous nodules. Two years prior to the current presentation, the patient was diagnosed by her primary care physician with DM based on clinical presentation. She initially developed body aches, muscle pain, and weakness of the upper extremities, specifically around the shoulders, and later the lower extremities, specifically around the thighs. The initial physical examination revealed pain with movement, tenderness to palpation, and proximal extremity weakness. The patient also noted a 50-lb weight loss. Over the next year, she noted dysphagia and developed multiple subcutaneous nodules on the right arm, chest, and left axilla. Subsequently, she developed a violaceous, hyperpigmented, periorbital rash and erythema of the anterior chest. She did not experience hair loss, oral ulcers, photosensitivity, or joint pain.
Laboratory testing in the months following the initial presentation revealed a creatine phosphokinase level of 436 U/L (reference range, 20–200 U/L), an erythrocyte sedimentation rate of 60 mm/h (reference range, <31 mm/h), and an aldolase level of 10.4 U/L (reference range, 1.0–8.0 U/L). Lactate dehydrogenase and thyroid function tests were within normal limits. Antinuclear antibodies, anti–double-stranded DNA, anti-Smith antibodies, anti-ribonucleoprotein, anti–Jo-1 antibodies, and anti–smooth muscle antibodies all were negative. Total blood complement levels were elevated, but complement C3 and C4 were within normal limits. Imaging demonstrated normal chest radiographs, and a modified barium swallow confirmed swallowing dysfunction. A right quadricep muscle biopsy confirmed the diagnosis of DM. A malignancy work-up including mammography, colonoscopy, and computed tomography of the chest, abdomen, and pelvis was negative aside from nodular opacities in the chest. She was treated with prednisone (60 mg, 0.9 mg/kg) daily and methotrexate (15–20 mg) weekly for several months. While the treatment attenuated the rash and improved weakness, the nodules persisted, prompting a referral to dermatology.
Physical examination at the dermatology clinic demonstrated the persistent subcutaneous nodules were indurated and bilaterally located on the arms, axillae, chest, abdomen, buttocks, and thighs with no pain or erythema (Figure). Laboratory tests demonstrated a normal creatine phosphokinase level, elevated erythrocyte sedimentation rate (70 mm/h), and elevated aldolase level (9.3 U/L). Complement levels were elevated, though complement C3 and C4 remained within normal limits. Histopathology of nodules from the medial right upper arm and left thigh showed lobular panniculitis with fat necrosis, calcification, and interface changes. The patient was treated for several months with daily mycophenolate mofetil (1 g increased to 3 g) and daily hydroxychloroquine (200 mg) without any effect on the nodules.

The histologic features of panniculitis in lupus and DM are similar and include multifocal hyalinization of the subcuticular fat and diffuse lobular infiltrates of mature lymphocytes without nuclear atypia.1 Though clinical panniculitis is a rare finding in DM, histologic panniculitis is a relatively common finding.2 Despite the similar histopathology of lupus and DM, the presence of typical DM clinical and laboratory features in our patient (body aches, muscle pain, proximal weakness, cutaneous manifestations, elevated creatine phosphokinase, normal complement C3 and C4) made a diagnosis of DM more likely.
Clinical panniculitis is a rare subcutaneous manifestation of DM with around 50 cases reported in the literature (Table). A PubMed search of articles indexed for MEDLINE was conducted using the terms dermatomyositis and panniculitis through July 2019. Additionally, a full-text review and search of references within these articles was used to identify all cases of patients presenting with panniculitis in the setting of DM. Exclusion criteria were cases in which another etiology was considered likely (infectious panniculitis and lupus panniculitis) as well as those without an English translation. We identified 43 cases; the average age of the patients was 39.6 years, and 36 (83.7%) of the cases were women. Patients typically presented with persistent, indurated, painful, erythematous, nodular lesions localized to the arms, abdomen, buttocks, and thighs.
While panniculitis has been reported preceding and concurrent with a diagnosis of DM, a number of cases described presentation as late as 5 years following onset of classic DM symptoms.12,13,31 In some cases (3/43 [7.0%]), panniculitis was the only cutaneous manifestation of DM.15,33,36 However, it occurred more commonly with other characteristic skin findings, such as heliotrope rash or Gottron sign.Some investigators have recommended that panniculitis be included as a diagnostic feature of DM and that DM be considered in the differential diagnosis in isolated cases of panniculitis.25,33
Though it seems panniculitis in DM may correlate with a better prognosis, we identified underlying malignancies in 3 cases. Malignancies associated with panniculitis in DM included ovarian adenocarcinoma, nasopharyngeal carcinoma, and parotid carcinoma, indicating that appropriate cancer screening still is critical in the diagnostic workup.2,11,22
A majority of the reported panniculitis cases in DM have responded to treatment with prednisone; however, treatment with prednisone has been more recalcitrant in other cases. Reports of successful additional therapies include methotrexate, cyclosporine, azathioprine, hydroxychloroquine, intravenous immunoglobulin, mepacrine, or a combination of these entities.19,22 In most cases, improvement of the panniculitis and other DM symptoms occurred simultaneously.25 It is noteworthy that the muscular symptoms often resolved more rapidly than cutaneous manifestations.33 Few reported cases (6 including the current case) found a persistent panniculitis despite improvement and remission of the myositis.3,5,10,11,30
Our patient was treated with both prednisone and methotrexate for several months, leading to remission of muscular symptoms (along with return to baseline of creatine phosphokinase), yet the panniculitis did not improve. The subcutaneous nodules also did not respond to treatment with mycophenolate mofetil and hydroxychloroquine.
Recent immunohistochemical studies have suggested that panniculitic lesions show better outcomes with immunosuppressive therapy when compared with other DM-related skin lesions.40 However, this was not the case for our patient, who after months of immunosuppressive therapy showed complete resolution of the periorbital and chest rashes with persistence of multiple indurated subcutaneous nodules.
Our case adds to a number of reports of DM presenting with panniculitis. Our patient fit the classic demographic of previously reported cases, as she was an adult woman without evidence of underlying malignancy; however, our case remains an example of the therapeutic challenge that exists when encountering a persistent, treatment-resistant panniculitis despite resolution of all other features of DM.
- Wick MR. Panniculitis: a summary. Semin Diagn Pathol. 2017;34:261-272.
- Girouard SD, Velez NF, Penson RT, et al. Panniculitis associated with dermatomyositis and recurrent ovarian cancer. Arch Dermatol. 2012;148:740-744.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:E187-E188.
- Choi YJ, Yoo WH. Panniculitis, a rare presentation of onset and exacerbation of juvenile dermatomyositis: a case report and literature review. Arch Rheumatol. 2018;33:367-371.
- Azevedo PO, Castellen NR, Salai AF, et al. Panniculitis associated with amyopathic dermatomyositis. An Bras Dermatol. 2018;93:119-121.
- Agulló A, Hinds B, Larrea M, et al. Livedo racemosa, reticulated ulcerations, panniculitis and violaceous plaques in a 46-year-old woman. Indian Dermatol Online J. 2018;9:47-49.
- Hattori Y, Matsuyama K, Takahashi T, et al. Anti-MDA5 antibody-positive dermatomyositis presenting with cellulitis-like erythema on the mandible as an initial symptom. Case Rep Dermatol. 2018;10:110-114.
- Hasegawa A, Shimomura Y, Kibune N, et al. Panniculitis as the initial manifestation of dermatomyositis with anti-MDA5 antibody. Clin Exp Dermatol. 2017;42:551-553.
- Salman A, Kasapcopur O, Ergun T, et al. Panniculitis in juvenile dermatomyositis: report of a case and review of the published work. J Dermatol. 2016;43:951-953.
- Carroll M, Mellick N, Wagner G. Dermatomyositis panniculitis: a case report. Australas J Dermatol. 2015;56:224‐226.
- Chairatchaneeboon M, Kulthanan K, Manapajon A. Calcific panniculitis and nasopharyngeal cancer-associated adult-onset dermatomyositis: a case report and literature review. Springerplus. 2015;4:201.
- Otero Rivas MM, Vicente Villa A, González Lara L, et al. Panniculitis in juvenile dermatomyositis. Clin Exp Dermatol. 2015;40:574-575.
- Yanaba K, Tanito K, Hamaguchi Y, et al. Anti‐transcription intermediary factor‐1γ/α/β antibody‐positive dermatomyositis associated with multiple panniculitis lesions. Int J Rheum Dis. 2015;20:1831-1834.
- Pau-Charles I, Moreno PJ, Ortiz-Ibanez K, et al. Anti-MDA5 positive clinically amyopathic dermatomyositis presenting with severe cardiomyopathy. J Eur Acad Dermatol Venereol. 2014;28:1097-1102.
- Lamb R, Digby S, Stewart W, et al. Cutaneous ulceration: more than skin deep? Clin Exp Dermatol. 2013;38:443-445.
- Arias M, Hernández MI, Cunha LG, et al. Panniculitis in a patient with dermatomyositis. An Bras Dermatol. 2011;86:146-148.
- Hemmi S, Kushida R, Nishimura H, et al. Magnetic resonance imaging diagnosis of panniculitis in dermatomyositis. Muscle Nerve. 2010;41:151-153.
- Geddes MR, Sinnreich M, Chalk C. Minocycline-induced dermatomyositis. Muscle Nerve. 2010;41:547-549.
- Abdul‐Wahab A, Holden CA, Harland C, et al Calcific panniculitis in adult‐onset dermatomyositis. Clin Exp Dermatol. 2009;34:E854-E856.
- Carneiro S, Alvim G, Resende P, et al. Dermatomyositis with panniculitis. Skinmed. 2007;6:46-47.
- Carrera E, Lobrinus JA, Spertini O, et al. Dermatomyositis, lobarpanniculitis and inflammatory myopathy with abundant macrophages. Neuromuscul Disord. 2006;16:468-471.
- Lin JH, Chu CY, Lin RY. Panniculitis in adult onset dermatomyositis: report of two cases and review of the literature. Dermatol Sinica. 2006;24:194-200.
- Chen GY, Liu MF, Lee JY, et al. Combination of massive mucinosis, dermatomyositis, pyoderma gangrenosum-like ulcer, bullae and fatal intestinal vasculopathy in a young female. Eur J Dermatol. 2005;15:396-400.
- Nakamori A, Yamaguchi Y, Kurimoto I, et al. Vesiculobullous dermatomyositis with panniculitis without muscle disease. J Am Acad Dermatol. 2003;49:1136-1139.
- Solans R, Cortés J, Selva A, et al. Panniculitis: a cutaneous manifestation of dermatomyositis. J Am Acad Dermatol. 2002;46:S148-S150.
- Chao YY, Yang LJ. Dermatomyositis presenting as panniculitis. Int J Dermatol. 2000;39:141-144.
- Lee MW, Lim YS, Choi JH, et al. Panniculitis showing membranocystic changes in the dermatomyositis. J Dermatol. 1999;26:608‐610.
- Ghali FE, Reed AM, Groben PA, et al. Panniculitis in juvenile dermatomyositis. Pediatr Dermatol. 1999;16:270-272.
- Molnar K, Kemeny L, Korom I, et al. Panniculitis in dermatomyositis: report of two cases. Br J Dermatol. 1998;139:161‐163.
- Ishikawa O, Tamura A, Ryuzaki K, et al. Membranocystic changes in the panniculitis of dermatomyositis. Br J Dermatol. 1996;134:773-776.
- Sabroe RA, Wallington TB, Kennedy CT. Dermatomyositis treated with high-dose intravenous immunoglobulins and associated with panniculitis. Clin Exp Dermatol. 1995;20:164-167.
- Neidenbach PJ, Sahn EE, Helton J. Panniculitis in juvenile dermatomyositis. J Am Acad Dermatol. 1995;33:305-307.
- Fusade T, Belanyi P, Joly P, et al. Subcutaneous changes in dermatomyositis. Br J Dermatol. 1993;128:451-453.
- Winkelmann WJ, Billick RC, Srolovitz H. Dermatomyositis presenting as panniculitis. J Am Acad Dermatol. 1990;23:127-128.
- Commens C, O’Neill P, Walker G. Dermatomyositis associated with multifocal lipoatrophy. J Am Acad Dermatol. 1990;22:966-969.
- Raimer SS, Solomon AR, Daniels JC. Polymyositis presenting with panniculitis. J Am Acad Dermatol. 1985;13(2 pt 2):366‐369.
- Feldman D, Hochberg MC, Zizic TM, et al. Cutaneous vasculitis in adult polymyositis/dermatomyositis. J Rheumatol. 1983;10:85-89.
- Kimura S, Fukuyama Y. Tubular cytoplasmic inclusions in a case of childhood dermatomyositis with migratory subcutaneous nodules. Eur J Pediatr. 1977;125:275-283.
- Weber FP, Gray AMH. Chronic relapsing polydermatomyositis with predominant involvement of the subcutaneous fat. Br J Dermatol. 1924;36:544-560.
- Santos‐Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Wick MR. Panniculitis: a summary. Semin Diagn Pathol. 2017;34:261-272.
- Girouard SD, Velez NF, Penson RT, et al. Panniculitis associated with dermatomyositis and recurrent ovarian cancer. Arch Dermatol. 2012;148:740-744.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:E187-E188.
- Choi YJ, Yoo WH. Panniculitis, a rare presentation of onset and exacerbation of juvenile dermatomyositis: a case report and literature review. Arch Rheumatol. 2018;33:367-371.
- Azevedo PO, Castellen NR, Salai AF, et al. Panniculitis associated with amyopathic dermatomyositis. An Bras Dermatol. 2018;93:119-121.
- Agulló A, Hinds B, Larrea M, et al. Livedo racemosa, reticulated ulcerations, panniculitis and violaceous plaques in a 46-year-old woman. Indian Dermatol Online J. 2018;9:47-49.
- Hattori Y, Matsuyama K, Takahashi T, et al. Anti-MDA5 antibody-positive dermatomyositis presenting with cellulitis-like erythema on the mandible as an initial symptom. Case Rep Dermatol. 2018;10:110-114.
- Hasegawa A, Shimomura Y, Kibune N, et al. Panniculitis as the initial manifestation of dermatomyositis with anti-MDA5 antibody. Clin Exp Dermatol. 2017;42:551-553.
- Salman A, Kasapcopur O, Ergun T, et al. Panniculitis in juvenile dermatomyositis: report of a case and review of the published work. J Dermatol. 2016;43:951-953.
- Carroll M, Mellick N, Wagner G. Dermatomyositis panniculitis: a case report. Australas J Dermatol. 2015;56:224‐226.
- Chairatchaneeboon M, Kulthanan K, Manapajon A. Calcific panniculitis and nasopharyngeal cancer-associated adult-onset dermatomyositis: a case report and literature review. Springerplus. 2015;4:201.
- Otero Rivas MM, Vicente Villa A, González Lara L, et al. Panniculitis in juvenile dermatomyositis. Clin Exp Dermatol. 2015;40:574-575.
- Yanaba K, Tanito K, Hamaguchi Y, et al. Anti‐transcription intermediary factor‐1γ/α/β antibody‐positive dermatomyositis associated with multiple panniculitis lesions. Int J Rheum Dis. 2015;20:1831-1834.
- Pau-Charles I, Moreno PJ, Ortiz-Ibanez K, et al. Anti-MDA5 positive clinically amyopathic dermatomyositis presenting with severe cardiomyopathy. J Eur Acad Dermatol Venereol. 2014;28:1097-1102.
- Lamb R, Digby S, Stewart W, et al. Cutaneous ulceration: more than skin deep? Clin Exp Dermatol. 2013;38:443-445.
- Arias M, Hernández MI, Cunha LG, et al. Panniculitis in a patient with dermatomyositis. An Bras Dermatol. 2011;86:146-148.
- Hemmi S, Kushida R, Nishimura H, et al. Magnetic resonance imaging diagnosis of panniculitis in dermatomyositis. Muscle Nerve. 2010;41:151-153.
- Geddes MR, Sinnreich M, Chalk C. Minocycline-induced dermatomyositis. Muscle Nerve. 2010;41:547-549.
- Abdul‐Wahab A, Holden CA, Harland C, et al Calcific panniculitis in adult‐onset dermatomyositis. Clin Exp Dermatol. 2009;34:E854-E856.
- Carneiro S, Alvim G, Resende P, et al. Dermatomyositis with panniculitis. Skinmed. 2007;6:46-47.
- Carrera E, Lobrinus JA, Spertini O, et al. Dermatomyositis, lobarpanniculitis and inflammatory myopathy with abundant macrophages. Neuromuscul Disord. 2006;16:468-471.
- Lin JH, Chu CY, Lin RY. Panniculitis in adult onset dermatomyositis: report of two cases and review of the literature. Dermatol Sinica. 2006;24:194-200.
- Chen GY, Liu MF, Lee JY, et al. Combination of massive mucinosis, dermatomyositis, pyoderma gangrenosum-like ulcer, bullae and fatal intestinal vasculopathy in a young female. Eur J Dermatol. 2005;15:396-400.
- Nakamori A, Yamaguchi Y, Kurimoto I, et al. Vesiculobullous dermatomyositis with panniculitis without muscle disease. J Am Acad Dermatol. 2003;49:1136-1139.
- Solans R, Cortés J, Selva A, et al. Panniculitis: a cutaneous manifestation of dermatomyositis. J Am Acad Dermatol. 2002;46:S148-S150.
- Chao YY, Yang LJ. Dermatomyositis presenting as panniculitis. Int J Dermatol. 2000;39:141-144.
- Lee MW, Lim YS, Choi JH, et al. Panniculitis showing membranocystic changes in the dermatomyositis. J Dermatol. 1999;26:608‐610.
- Ghali FE, Reed AM, Groben PA, et al. Panniculitis in juvenile dermatomyositis. Pediatr Dermatol. 1999;16:270-272.
- Molnar K, Kemeny L, Korom I, et al. Panniculitis in dermatomyositis: report of two cases. Br J Dermatol. 1998;139:161‐163.
- Ishikawa O, Tamura A, Ryuzaki K, et al. Membranocystic changes in the panniculitis of dermatomyositis. Br J Dermatol. 1996;134:773-776.
- Sabroe RA, Wallington TB, Kennedy CT. Dermatomyositis treated with high-dose intravenous immunoglobulins and associated with panniculitis. Clin Exp Dermatol. 1995;20:164-167.
- Neidenbach PJ, Sahn EE, Helton J. Panniculitis in juvenile dermatomyositis. J Am Acad Dermatol. 1995;33:305-307.
- Fusade T, Belanyi P, Joly P, et al. Subcutaneous changes in dermatomyositis. Br J Dermatol. 1993;128:451-453.
- Winkelmann WJ, Billick RC, Srolovitz H. Dermatomyositis presenting as panniculitis. J Am Acad Dermatol. 1990;23:127-128.
- Commens C, O’Neill P, Walker G. Dermatomyositis associated with multifocal lipoatrophy. J Am Acad Dermatol. 1990;22:966-969.
- Raimer SS, Solomon AR, Daniels JC. Polymyositis presenting with panniculitis. J Am Acad Dermatol. 1985;13(2 pt 2):366‐369.
- Feldman D, Hochberg MC, Zizic TM, et al. Cutaneous vasculitis in adult polymyositis/dermatomyositis. J Rheumatol. 1983;10:85-89.
- Kimura S, Fukuyama Y. Tubular cytoplasmic inclusions in a case of childhood dermatomyositis with migratory subcutaneous nodules. Eur J Pediatr. 1977;125:275-283.
- Weber FP, Gray AMH. Chronic relapsing polydermatomyositis with predominant involvement of the subcutaneous fat. Br J Dermatol. 1924;36:544-560.
- Santos‐Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
Practice Points
- Clinical panniculitis is a rare subcutaneous manifestation of dermatomyositis (DM) that dermatologists must consider when evaluating patients with this condition.
- Panniculitis can precede, occur simultaneously with, or develop up to 5 years after onset of DM.
- Many patients suffer from treatment-resistant panniculitis in DM, suggesting that therapeutic management of this condition may require long-term and more aggressive treatment modalities.