User login
Direct-to-Consumer Teledermatology Growth: A Review and Outlook for the Future
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.
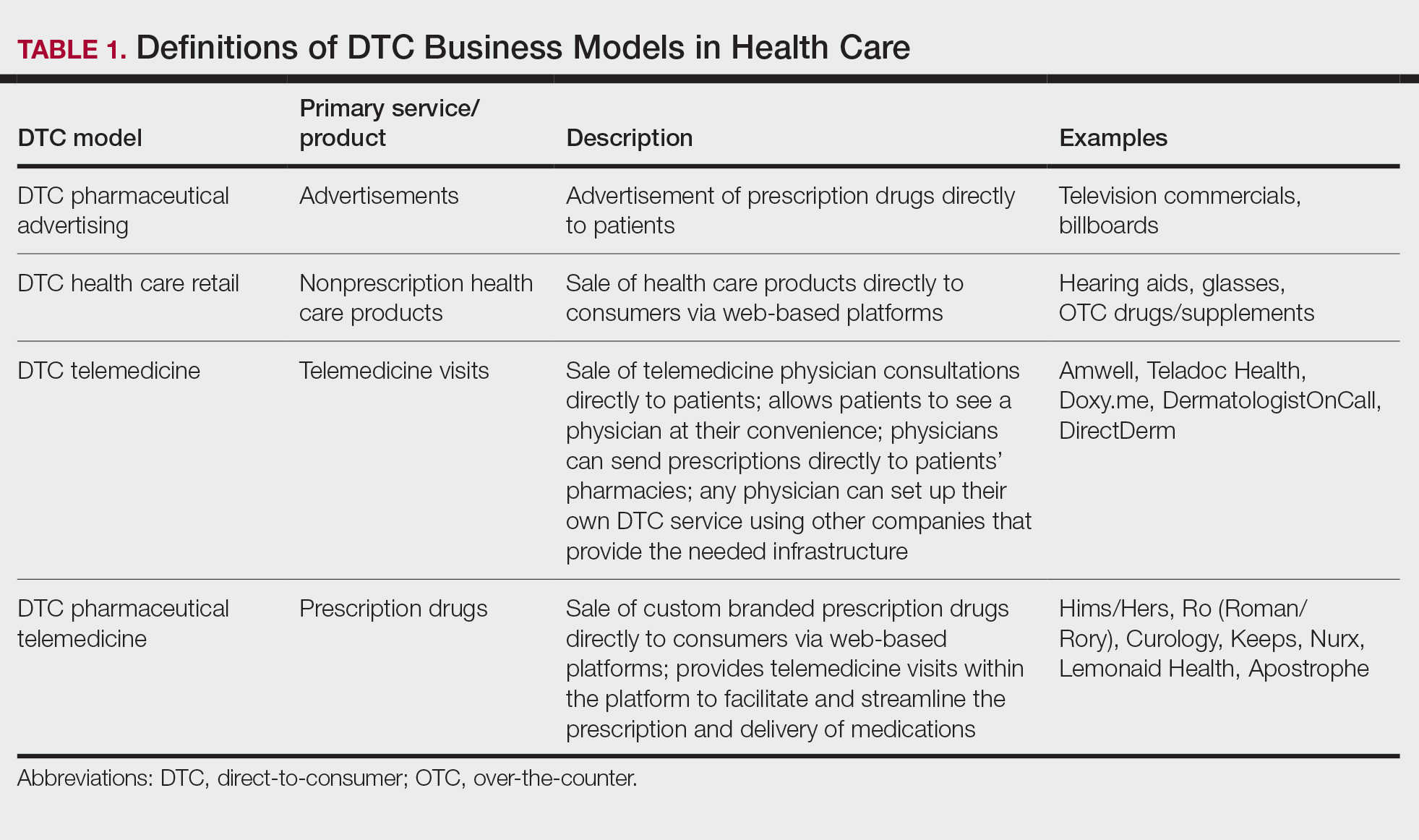
The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.
Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.
Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.

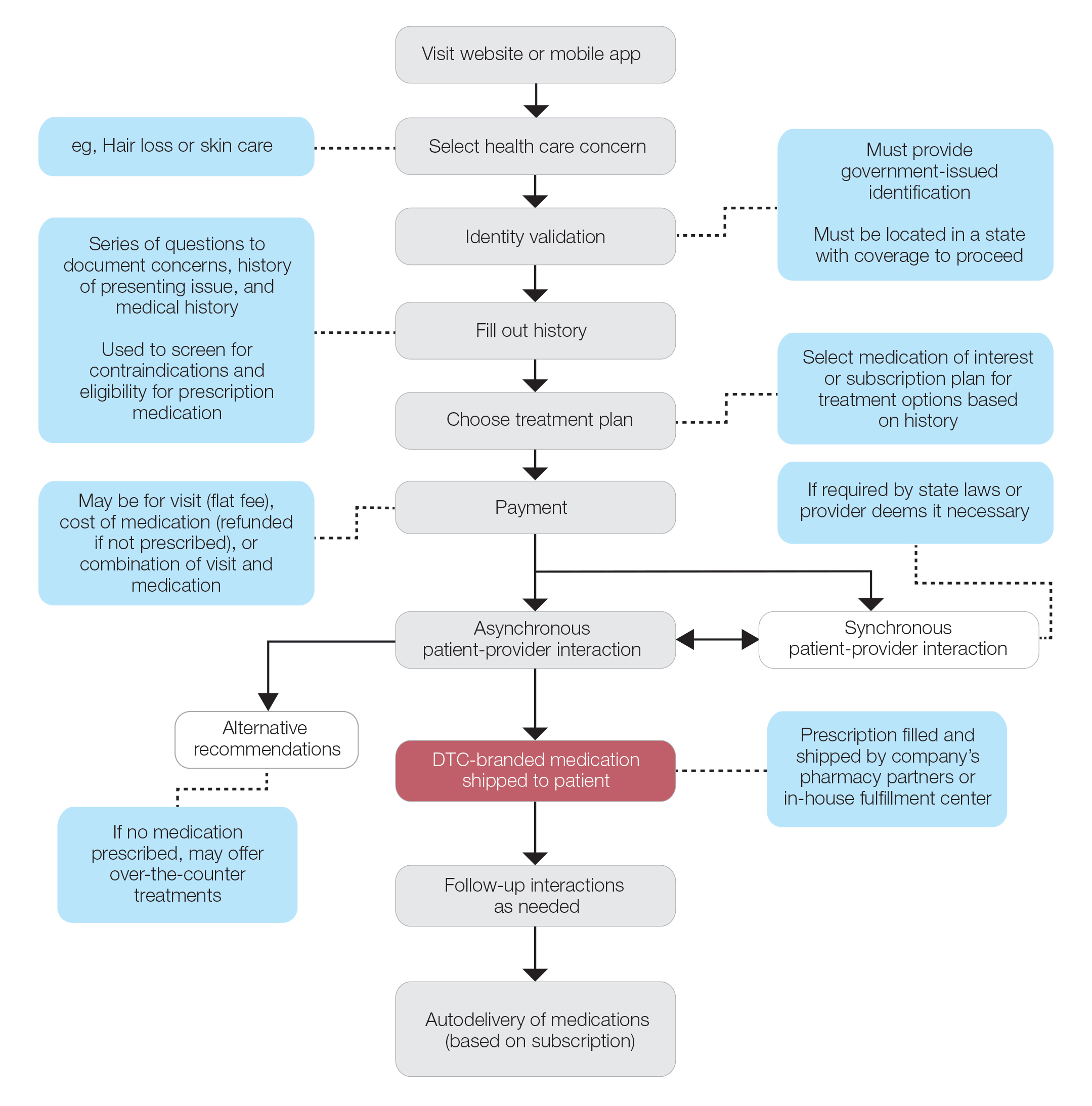
The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.

Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.

Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.

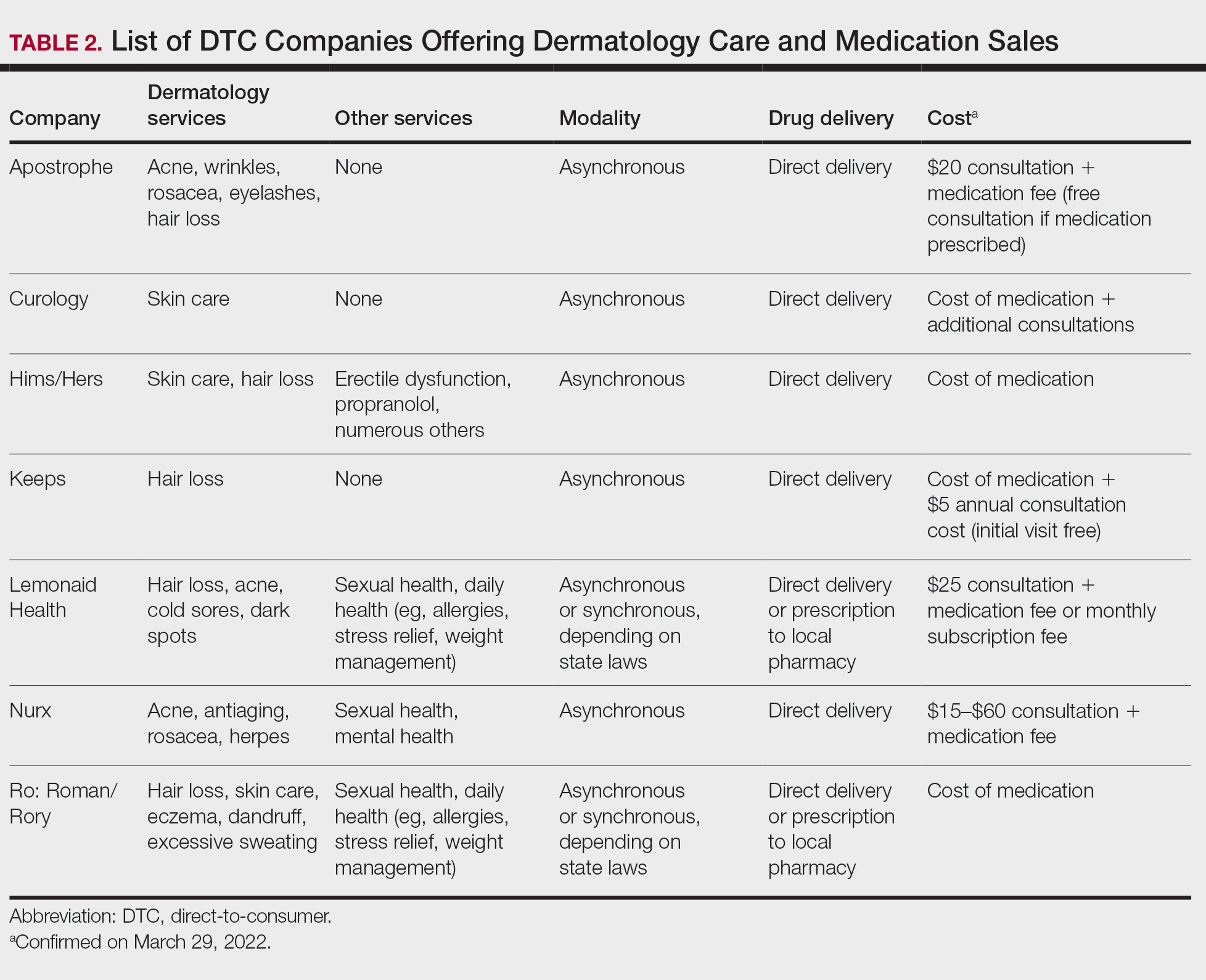
The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.

Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.

Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
Practice Points
- Direct-to-consumer (DTC) teledermatology platforms are for-profit companies that provide telemedicine visits and sell prescription drugs directly to patients.
- Although they are growing in popularity, DTC teledermatology platforms may lead to overdiagnosis, overtreatment, and fragmentation of health care. Knowledge of teledermatology will be vital to counsel patients on the risks and benefits of these platforms.
Teledermatology During the COVID-19 Pandemic: Lessons Learned and Future Directions
Although teledermatology utilization in the United States traditionally has lagged behind other countries,1,2 the COVID-19 pandemic upended this trend by creating the need for a massive teledermatology experiment. Recently reported survey results from a large representative sample of US dermatologists (5000 participants) on perceptions of teledermatology during COVID-19 indicated that only 14.1% of participants used teledermatology prior to the COVID-19 pandemic vs 54.1% of dermatologists in Europe.2,3 Since the pandemic started, 97% of US dermatologists reported teledermatology use,3 demonstrating a huge shift in utilization. This trend is notable, as teledermatology has been shown to increase access to dermatology in underserved areas, reduce patient travel times, improve patient triage, and even reduce carbon footprints.1,4 Thus, to sustain the momentum, insights from the recent teledermatology experience during the pandemic should inform future development.
Notably, the COVID-19 pandemic led to a rapid shift in focus from store-and-forward teledermatology to live video–based models.1,2 Logistically, live video visits are challenging, require more time and resources, and often are diagnostically limited, with concerns regarding technology, connectivity, reimbursement, and appropriate use.3 Prior to COVID-19, formal Health Insurance Portability and Accountability Act–compliant teledermatology platforms often were costly to establish and maintain, largely relegating use to academic centers and Veterans Affairs hospitals. Thus, many fewer private practice dermatologists had used teledermatology compared to academic dermatologists in the United States (11.4% vs 27.6%).3 Government regulations—a key barrier to the adoption of teledermatology in private practice before COVID-19—were greatly relaxed during the pandemic. The Centers for Medicare and Medicaid Services removed restrictions on where patients could be seen, improved reimbursement for video visits, and allowed the use of platforms that are not Health Insurance Portability and Accountability Act compliant. Many states also relaxed medical licensing rules.
Overall, the general outlook on telehealth seems positive. Reimbursement has been found to be a primary factor in dermatologists’ willingness to use teledermatology.3 Thus, sustainable use of teledermatology likely will depend on continued reimbursement parity for live video as well as store-and-forward consultations, which have several advantages but currently are de-incentivized by low reimbursement. The survey also found that 70% of respondents felt that teledermatology use will continue after COVID-19, while 58% intended to continue use—nearly 5-fold more than before the pandemic.3 We suspect the discrepancy between participants’ predictions regarding future use of teledermatology and their personal intent to use it highlights perceived barriers and limitations of the long-term success of teledermatology. Aside from reimbursement, connectivity and functionality were common concerns, emphasizing the need for innovative technological solutions.3 Moving forward, we anticipate that dermatologists will need to establish consistent workflows to establish consistent triage for the most appropriate visit—in-person visits vs teledermatology, which may include augmented, intelligence-enhanced solutions. Similar to prior clinician perspectives about which types of visits are conducive to teledermatology,2 most survey participants believed virtual visits were effective for acne, routine follow-ups, medication monitoring, and some inflammatory conditions.3
Importantly, we must be mindful of patients who may be left behind by the digital divide, such as those with lack of access to a smartphone or the internet, language barriers, or limited telehealth experience.5 Systems should be designed to provide these patients with technologic and health literacy aid or alternate modalities to access care. For example, structured methods could be introduced to provide training and instructions on how to access phone applications, computer-based programs, and more. Likewise, for those with hearing or vision deficits, it will be important to improve sound amplification and accessibility for headphones or hearing aid connectivity, as well as appropriate font size, button size, and application navigation. In remote areas, existing clinics may be used to help field specialty consultation teleconferences. Certainly, applications and platforms devised for teledermatology must be designed to serve diverse patient groups, with special consideration for the elderly, those who speak languages other than English, and those with disabilities that may make telehealth use more challenging.
Large-scale regulatory changes and reimbursement parity can have a substantial impact on future teledermatology use. Advocacy efforts continue to push for fair valuation of telemedicine, coverage of store-and-forward teledermatology codes, and coverage for all models of care. It is imperative for the dermatology community to continue discussions on implementation and methodology to best leverage this technology for the most patient benefit.
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Moscarella E, Pasquali P, Cinotti E, et al. A survey on teledermatology use and doctors’ perception in times of COVID-19 [published online August 17, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E772-E773.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Bonsall A. Unleashing carbon emissions savings with regular teledermatology clinics. Clin Exp Dermatol. 2021;46:574-575.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
Although teledermatology utilization in the United States traditionally has lagged behind other countries,1,2 the COVID-19 pandemic upended this trend by creating the need for a massive teledermatology experiment. Recently reported survey results from a large representative sample of US dermatologists (5000 participants) on perceptions of teledermatology during COVID-19 indicated that only 14.1% of participants used teledermatology prior to the COVID-19 pandemic vs 54.1% of dermatologists in Europe.2,3 Since the pandemic started, 97% of US dermatologists reported teledermatology use,3 demonstrating a huge shift in utilization. This trend is notable, as teledermatology has been shown to increase access to dermatology in underserved areas, reduce patient travel times, improve patient triage, and even reduce carbon footprints.1,4 Thus, to sustain the momentum, insights from the recent teledermatology experience during the pandemic should inform future development.
Notably, the COVID-19 pandemic led to a rapid shift in focus from store-and-forward teledermatology to live video–based models.1,2 Logistically, live video visits are challenging, require more time and resources, and often are diagnostically limited, with concerns regarding technology, connectivity, reimbursement, and appropriate use.3 Prior to COVID-19, formal Health Insurance Portability and Accountability Act–compliant teledermatology platforms often were costly to establish and maintain, largely relegating use to academic centers and Veterans Affairs hospitals. Thus, many fewer private practice dermatologists had used teledermatology compared to academic dermatologists in the United States (11.4% vs 27.6%).3 Government regulations—a key barrier to the adoption of teledermatology in private practice before COVID-19—were greatly relaxed during the pandemic. The Centers for Medicare and Medicaid Services removed restrictions on where patients could be seen, improved reimbursement for video visits, and allowed the use of platforms that are not Health Insurance Portability and Accountability Act compliant. Many states also relaxed medical licensing rules.
Overall, the general outlook on telehealth seems positive. Reimbursement has been found to be a primary factor in dermatologists’ willingness to use teledermatology.3 Thus, sustainable use of teledermatology likely will depend on continued reimbursement parity for live video as well as store-and-forward consultations, which have several advantages but currently are de-incentivized by low reimbursement. The survey also found that 70% of respondents felt that teledermatology use will continue after COVID-19, while 58% intended to continue use—nearly 5-fold more than before the pandemic.3 We suspect the discrepancy between participants’ predictions regarding future use of teledermatology and their personal intent to use it highlights perceived barriers and limitations of the long-term success of teledermatology. Aside from reimbursement, connectivity and functionality were common concerns, emphasizing the need for innovative technological solutions.3 Moving forward, we anticipate that dermatologists will need to establish consistent workflows to establish consistent triage for the most appropriate visit—in-person visits vs teledermatology, which may include augmented, intelligence-enhanced solutions. Similar to prior clinician perspectives about which types of visits are conducive to teledermatology,2 most survey participants believed virtual visits were effective for acne, routine follow-ups, medication monitoring, and some inflammatory conditions.3
Importantly, we must be mindful of patients who may be left behind by the digital divide, such as those with lack of access to a smartphone or the internet, language barriers, or limited telehealth experience.5 Systems should be designed to provide these patients with technologic and health literacy aid or alternate modalities to access care. For example, structured methods could be introduced to provide training and instructions on how to access phone applications, computer-based programs, and more. Likewise, for those with hearing or vision deficits, it will be important to improve sound amplification and accessibility for headphones or hearing aid connectivity, as well as appropriate font size, button size, and application navigation. In remote areas, existing clinics may be used to help field specialty consultation teleconferences. Certainly, applications and platforms devised for teledermatology must be designed to serve diverse patient groups, with special consideration for the elderly, those who speak languages other than English, and those with disabilities that may make telehealth use more challenging.
Large-scale regulatory changes and reimbursement parity can have a substantial impact on future teledermatology use. Advocacy efforts continue to push for fair valuation of telemedicine, coverage of store-and-forward teledermatology codes, and coverage for all models of care. It is imperative for the dermatology community to continue discussions on implementation and methodology to best leverage this technology for the most patient benefit.
Although teledermatology utilization in the United States traditionally has lagged behind other countries,1,2 the COVID-19 pandemic upended this trend by creating the need for a massive teledermatology experiment. Recently reported survey results from a large representative sample of US dermatologists (5000 participants) on perceptions of teledermatology during COVID-19 indicated that only 14.1% of participants used teledermatology prior to the COVID-19 pandemic vs 54.1% of dermatologists in Europe.2,3 Since the pandemic started, 97% of US dermatologists reported teledermatology use,3 demonstrating a huge shift in utilization. This trend is notable, as teledermatology has been shown to increase access to dermatology in underserved areas, reduce patient travel times, improve patient triage, and even reduce carbon footprints.1,4 Thus, to sustain the momentum, insights from the recent teledermatology experience during the pandemic should inform future development.
Notably, the COVID-19 pandemic led to a rapid shift in focus from store-and-forward teledermatology to live video–based models.1,2 Logistically, live video visits are challenging, require more time and resources, and often are diagnostically limited, with concerns regarding technology, connectivity, reimbursement, and appropriate use.3 Prior to COVID-19, formal Health Insurance Portability and Accountability Act–compliant teledermatology platforms often were costly to establish and maintain, largely relegating use to academic centers and Veterans Affairs hospitals. Thus, many fewer private practice dermatologists had used teledermatology compared to academic dermatologists in the United States (11.4% vs 27.6%).3 Government regulations—a key barrier to the adoption of teledermatology in private practice before COVID-19—were greatly relaxed during the pandemic. The Centers for Medicare and Medicaid Services removed restrictions on where patients could be seen, improved reimbursement for video visits, and allowed the use of platforms that are not Health Insurance Portability and Accountability Act compliant. Many states also relaxed medical licensing rules.
Overall, the general outlook on telehealth seems positive. Reimbursement has been found to be a primary factor in dermatologists’ willingness to use teledermatology.3 Thus, sustainable use of teledermatology likely will depend on continued reimbursement parity for live video as well as store-and-forward consultations, which have several advantages but currently are de-incentivized by low reimbursement. The survey also found that 70% of respondents felt that teledermatology use will continue after COVID-19, while 58% intended to continue use—nearly 5-fold more than before the pandemic.3 We suspect the discrepancy between participants’ predictions regarding future use of teledermatology and their personal intent to use it highlights perceived barriers and limitations of the long-term success of teledermatology. Aside from reimbursement, connectivity and functionality were common concerns, emphasizing the need for innovative technological solutions.3 Moving forward, we anticipate that dermatologists will need to establish consistent workflows to establish consistent triage for the most appropriate visit—in-person visits vs teledermatology, which may include augmented, intelligence-enhanced solutions. Similar to prior clinician perspectives about which types of visits are conducive to teledermatology,2 most survey participants believed virtual visits were effective for acne, routine follow-ups, medication monitoring, and some inflammatory conditions.3
Importantly, we must be mindful of patients who may be left behind by the digital divide, such as those with lack of access to a smartphone or the internet, language barriers, or limited telehealth experience.5 Systems should be designed to provide these patients with technologic and health literacy aid or alternate modalities to access care. For example, structured methods could be introduced to provide training and instructions on how to access phone applications, computer-based programs, and more. Likewise, for those with hearing or vision deficits, it will be important to improve sound amplification and accessibility for headphones or hearing aid connectivity, as well as appropriate font size, button size, and application navigation. In remote areas, existing clinics may be used to help field specialty consultation teleconferences. Certainly, applications and platforms devised for teledermatology must be designed to serve diverse patient groups, with special consideration for the elderly, those who speak languages other than English, and those with disabilities that may make telehealth use more challenging.
Large-scale regulatory changes and reimbursement parity can have a substantial impact on future teledermatology use. Advocacy efforts continue to push for fair valuation of telemedicine, coverage of store-and-forward teledermatology codes, and coverage for all models of care. It is imperative for the dermatology community to continue discussions on implementation and methodology to best leverage this technology for the most patient benefit.
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Moscarella E, Pasquali P, Cinotti E, et al. A survey on teledermatology use and doctors’ perception in times of COVID-19 [published online August 17, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E772-E773.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Bonsall A. Unleashing carbon emissions savings with regular teledermatology clinics. Clin Exp Dermatol. 2021;46:574-575.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Moscarella E, Pasquali P, Cinotti E, et al. A survey on teledermatology use and doctors’ perception in times of COVID-19 [published online August 17, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E772-E773.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Bonsall A. Unleashing carbon emissions savings with regular teledermatology clinics. Clin Exp Dermatol. 2021;46:574-575.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
Persistent Panniculitis in Dermatomyositis
To the Editor:
A 62-year-old woman with a history of dermatomyositis (DM) presented to dermatology clinic for evaluation of multiple subcutaneous nodules. Two years prior to the current presentation, the patient was diagnosed by her primary care physician with DM based on clinical presentation. She initially developed body aches, muscle pain, and weakness of the upper extremities, specifically around the shoulders, and later the lower extremities, specifically around the thighs. The initial physical examination revealed pain with movement, tenderness to palpation, and proximal extremity weakness. The patient also noted a 50-lb weight loss. Over the next year, she noted dysphagia and developed multiple subcutaneous nodules on the right arm, chest, and left axilla. Subsequently, she developed a violaceous, hyperpigmented, periorbital rash and erythema of the anterior chest. She did not experience hair loss, oral ulcers, photosensitivity, or joint pain.
Laboratory testing in the months following the initial presentation revealed a creatine phosphokinase level of 436 U/L (reference range, 20–200 U/L), an erythrocyte sedimentation rate of 60 mm/h (reference range, <31 mm/h), and an aldolase level of 10.4 U/L (reference range, 1.0–8.0 U/L). Lactate dehydrogenase and thyroid function tests were within normal limits. Antinuclear antibodies, anti–double-stranded DNA, anti-Smith antibodies, anti-ribonucleoprotein, anti–Jo-1 antibodies, and anti–smooth muscle antibodies all were negative. Total blood complement levels were elevated, but complement C3 and C4 were within normal limits. Imaging demonstrated normal chest radiographs, and a modified barium swallow confirmed swallowing dysfunction. A right quadricep muscle biopsy confirmed the diagnosis of DM. A malignancy work-up including mammography, colonoscopy, and computed tomography of the chest, abdomen, and pelvis was negative aside from nodular opacities in the chest. She was treated with prednisone (60 mg, 0.9 mg/kg) daily and methotrexate (15–20 mg) weekly for several months. While the treatment attenuated the rash and improved weakness, the nodules persisted, prompting a referral to dermatology.
Physical examination at the dermatology clinic demonstrated the persistent subcutaneous nodules were indurated and bilaterally located on the arms, axillae, chest, abdomen, buttocks, and thighs with no pain or erythema (Figure). Laboratory tests demonstrated a normal creatine phosphokinase level, elevated erythrocyte sedimentation rate (70 mm/h), and elevated aldolase level (9.3 U/L). Complement levels were elevated, though complement C3 and C4 remained within normal limits. Histopathology of nodules from the medial right upper arm and left thigh showed lobular panniculitis with fat necrosis, calcification, and interface changes. The patient was treated for several months with daily mycophenolate mofetil (1 g increased to 3 g) and daily hydroxychloroquine (200 mg) without any effect on the nodules.
The histologic features of panniculitis in lupus and DM are similar and include multifocal hyalinization of the subcuticular fat and diffuse lobular infiltrates of mature lymphocytes without nuclear atypia.1 Though clinical panniculitis is a rare finding in DM, histologic panniculitis is a relatively common finding.2 Despite the similar histopathology of lupus and DM, the presence of typical DM clinical and laboratory features in our patient (body aches, muscle pain, proximal weakness, cutaneous manifestations, elevated creatine phosphokinase, normal complement C3 and C4) made a diagnosis of DM more likely.
Clinical panniculitis is a rare subcutaneous manifestation of DM with around 50 cases reported in the literature (Table). A PubMed search of articles indexed for MEDLINE was conducted using the terms dermatomyositis and panniculitis through July 2019. Additionally, a full-text review and search of references within these articles was used to identify all cases of patients presenting with panniculitis in the setting of DM. Exclusion criteria were cases in which another etiology was considered likely (infectious panniculitis and lupus panniculitis) as well as those without an English translation. We identified 43 cases; the average age of the patients was 39.6 years, and 36 (83.7%) of the cases were women. Patients typically presented with persistent, indurated, painful, erythematous, nodular lesions localized to the arms, abdomen, buttocks, and thighs.
While panniculitis has been reported preceding and concurrent with a diagnosis of DM, a number of cases described presentation as late as 5 years following onset of classic DM symptoms.12,13,31 In some cases (3/43 [7.0%]), panniculitis was the only cutaneous manifestation of DM.15,33,36 However, it occurred more commonly with other characteristic skin findings, such as heliotrope rash or Gottron sign.Some investigators have recommended that panniculitis be included as a diagnostic feature of DM and that DM be considered in the differential diagnosis in isolated cases of panniculitis.25,33
Though it seems panniculitis in DM may correlate with a better prognosis, we identified underlying malignancies in 3 cases. Malignancies associated with panniculitis in DM included ovarian adenocarcinoma, nasopharyngeal carcinoma, and parotid carcinoma, indicating that appropriate cancer screening still is critical in the diagnostic workup.2,11,22
A majority of the reported panniculitis cases in DM have responded to treatment with prednisone; however, treatment with prednisone has been more recalcitrant in other cases. Reports of successful additional therapies include methotrexate, cyclosporine, azathioprine, hydroxychloroquine, intravenous immunoglobulin, mepacrine, or a combination of these entities.19,22 In most cases, improvement of the panniculitis and other DM symptoms occurred simultaneously.25 It is noteworthy that the muscular symptoms often resolved more rapidly than cutaneous manifestations.33 Few reported cases (6 including the current case) found a persistent panniculitis despite improvement and remission of the myositis.3,5,10,11,30
Our patient was treated with both prednisone and methotrexate for several months, leading to remission of muscular symptoms (along with return to baseline of creatine phosphokinase), yet the panniculitis did not improve. The subcutaneous nodules also did not respond to treatment with mycophenolate mofetil and hydroxychloroquine.
Recent immunohistochemical studies have suggested that panniculitic lesions show better outcomes with immunosuppressive therapy when compared with other DM-related skin lesions.40 However, this was not the case for our patient, who after months of immunosuppressive therapy showed complete resolution of the periorbital and chest rashes with persistence of multiple indurated subcutaneous nodules.
Our case adds to a number of reports of DM presenting with panniculitis. Our patient fit the classic demographic of previously reported cases, as she was an adult woman without evidence of underlying malignancy; however, our case remains an example of the therapeutic challenge that exists when encountering a persistent, treatment-resistant panniculitis despite resolution of all other features of DM.
- Wick MR. Panniculitis: a summary. Semin Diagn Pathol. 2017;34:261-272.
- Girouard SD, Velez NF, Penson RT, et al. Panniculitis associated with dermatomyositis and recurrent ovarian cancer. Arch Dermatol. 2012;148:740-744.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:E187-E188.
- Choi YJ, Yoo WH. Panniculitis, a rare presentation of onset and exacerbation of juvenile dermatomyositis: a case report and literature review. Arch Rheumatol. 2018;33:367-371.
- Azevedo PO, Castellen NR, Salai AF, et al. Panniculitis associated with amyopathic dermatomyositis. An Bras Dermatol. 2018;93:119-121.
- Agulló A, Hinds B, Larrea M, et al. Livedo racemosa, reticulated ulcerations, panniculitis and violaceous plaques in a 46-year-old woman. Indian Dermatol Online J. 2018;9:47-49.
- Hattori Y, Matsuyama K, Takahashi T, et al. Anti-MDA5 antibody-positive dermatomyositis presenting with cellulitis-like erythema on the mandible as an initial symptom. Case Rep Dermatol. 2018;10:110-114.
- Hasegawa A, Shimomura Y, Kibune N, et al. Panniculitis as the initial manifestation of dermatomyositis with anti-MDA5 antibody. Clin Exp Dermatol. 2017;42:551-553.
- Salman A, Kasapcopur O, Ergun T, et al. Panniculitis in juvenile dermatomyositis: report of a case and review of the published work. J Dermatol. 2016;43:951-953.
- Carroll M, Mellick N, Wagner G. Dermatomyositis panniculitis: a case report. Australas J Dermatol. 2015;56:224‐226.
- Chairatchaneeboon M, Kulthanan K, Manapajon A. Calcific panniculitis and nasopharyngeal cancer-associated adult-onset dermatomyositis: a case report and literature review. Springerplus. 2015;4:201.
- Otero Rivas MM, Vicente Villa A, González Lara L, et al. Panniculitis in juvenile dermatomyositis. Clin Exp Dermatol. 2015;40:574-575.
- Yanaba K, Tanito K, Hamaguchi Y, et al. Anti‐transcription intermediary factor‐1γ/α/β antibody‐positive dermatomyositis associated with multiple panniculitis lesions. Int J Rheum Dis. 2015;20:1831-1834.
- Pau-Charles I, Moreno PJ, Ortiz-Ibanez K, et al. Anti-MDA5 positive clinically amyopathic dermatomyositis presenting with severe cardiomyopathy. J Eur Acad Dermatol Venereol. 2014;28:1097-1102.
- Lamb R, Digby S, Stewart W, et al. Cutaneous ulceration: more than skin deep? Clin Exp Dermatol. 2013;38:443-445.
- Arias M, Hernández MI, Cunha LG, et al. Panniculitis in a patient with dermatomyositis. An Bras Dermatol. 2011;86:146-148.
- Hemmi S, Kushida R, Nishimura H, et al. Magnetic resonance imaging diagnosis of panniculitis in dermatomyositis. Muscle Nerve. 2010;41:151-153.
- Geddes MR, Sinnreich M, Chalk C. Minocycline-induced dermatomyositis. Muscle Nerve. 2010;41:547-549.
- Abdul‐Wahab A, Holden CA, Harland C, et al Calcific panniculitis in adult‐onset dermatomyositis. Clin Exp Dermatol. 2009;34:E854-E856.
- Carneiro S, Alvim G, Resende P, et al. Dermatomyositis with panniculitis. Skinmed. 2007;6:46-47.
- Carrera E, Lobrinus JA, Spertini O, et al. Dermatomyositis, lobarpanniculitis and inflammatory myopathy with abundant macrophages. Neuromuscul Disord. 2006;16:468-471.
- Lin JH, Chu CY, Lin RY. Panniculitis in adult onset dermatomyositis: report of two cases and review of the literature. Dermatol Sinica. 2006;24:194-200.
- Chen GY, Liu MF, Lee JY, et al. Combination of massive mucinosis, dermatomyositis, pyoderma gangrenosum-like ulcer, bullae and fatal intestinal vasculopathy in a young female. Eur J Dermatol. 2005;15:396-400.
- Nakamori A, Yamaguchi Y, Kurimoto I, et al. Vesiculobullous dermatomyositis with panniculitis without muscle disease. J Am Acad Dermatol. 2003;49:1136-1139.
- Solans R, Cortés J, Selva A, et al. Panniculitis: a cutaneous manifestation of dermatomyositis. J Am Acad Dermatol. 2002;46:S148-S150.
- Chao YY, Yang LJ. Dermatomyositis presenting as panniculitis. Int J Dermatol. 2000;39:141-144.
- Lee MW, Lim YS, Choi JH, et al. Panniculitis showing membranocystic changes in the dermatomyositis. J Dermatol. 1999;26:608‐610.
- Ghali FE, Reed AM, Groben PA, et al. Panniculitis in juvenile dermatomyositis. Pediatr Dermatol. 1999;16:270-272.
- Molnar K, Kemeny L, Korom I, et al. Panniculitis in dermatomyositis: report of two cases. Br J Dermatol. 1998;139:161‐163.
- Ishikawa O, Tamura A, Ryuzaki K, et al. Membranocystic changes in the panniculitis of dermatomyositis. Br J Dermatol. 1996;134:773-776.
- Sabroe RA, Wallington TB, Kennedy CT. Dermatomyositis treated with high-dose intravenous immunoglobulins and associated with panniculitis. Clin Exp Dermatol. 1995;20:164-167.
- Neidenbach PJ, Sahn EE, Helton J. Panniculitis in juvenile dermatomyositis. J Am Acad Dermatol. 1995;33:305-307.
- Fusade T, Belanyi P, Joly P, et al. Subcutaneous changes in dermatomyositis. Br J Dermatol. 1993;128:451-453.
- Winkelmann WJ, Billick RC, Srolovitz H. Dermatomyositis presenting as panniculitis. J Am Acad Dermatol. 1990;23:127-128.
- Commens C, O’Neill P, Walker G. Dermatomyositis associated with multifocal lipoatrophy. J Am Acad Dermatol. 1990;22:966-969.
- Raimer SS, Solomon AR, Daniels JC. Polymyositis presenting with panniculitis. J Am Acad Dermatol. 1985;13(2 pt 2):366‐369.
- Feldman D, Hochberg MC, Zizic TM, et al. Cutaneous vasculitis in adult polymyositis/dermatomyositis. J Rheumatol. 1983;10:85-89.
- Kimura S, Fukuyama Y. Tubular cytoplasmic inclusions in a case of childhood dermatomyositis with migratory subcutaneous nodules. Eur J Pediatr. 1977;125:275-283.
- Weber FP, Gray AMH. Chronic relapsing polydermatomyositis with predominant involvement of the subcutaneous fat. Br J Dermatol. 1924;36:544-560.
- Santos‐Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
To the Editor:
A 62-year-old woman with a history of dermatomyositis (DM) presented to dermatology clinic for evaluation of multiple subcutaneous nodules. Two years prior to the current presentation, the patient was diagnosed by her primary care physician with DM based on clinical presentation. She initially developed body aches, muscle pain, and weakness of the upper extremities, specifically around the shoulders, and later the lower extremities, specifically around the thighs. The initial physical examination revealed pain with movement, tenderness to palpation, and proximal extremity weakness. The patient also noted a 50-lb weight loss. Over the next year, she noted dysphagia and developed multiple subcutaneous nodules on the right arm, chest, and left axilla. Subsequently, she developed a violaceous, hyperpigmented, periorbital rash and erythema of the anterior chest. She did not experience hair loss, oral ulcers, photosensitivity, or joint pain.
Laboratory testing in the months following the initial presentation revealed a creatine phosphokinase level of 436 U/L (reference range, 20–200 U/L), an erythrocyte sedimentation rate of 60 mm/h (reference range, <31 mm/h), and an aldolase level of 10.4 U/L (reference range, 1.0–8.0 U/L). Lactate dehydrogenase and thyroid function tests were within normal limits. Antinuclear antibodies, anti–double-stranded DNA, anti-Smith antibodies, anti-ribonucleoprotein, anti–Jo-1 antibodies, and anti–smooth muscle antibodies all were negative. Total blood complement levels were elevated, but complement C3 and C4 were within normal limits. Imaging demonstrated normal chest radiographs, and a modified barium swallow confirmed swallowing dysfunction. A right quadricep muscle biopsy confirmed the diagnosis of DM. A malignancy work-up including mammography, colonoscopy, and computed tomography of the chest, abdomen, and pelvis was negative aside from nodular opacities in the chest. She was treated with prednisone (60 mg, 0.9 mg/kg) daily and methotrexate (15–20 mg) weekly for several months. While the treatment attenuated the rash and improved weakness, the nodules persisted, prompting a referral to dermatology.
Physical examination at the dermatology clinic demonstrated the persistent subcutaneous nodules were indurated and bilaterally located on the arms, axillae, chest, abdomen, buttocks, and thighs with no pain or erythema (Figure). Laboratory tests demonstrated a normal creatine phosphokinase level, elevated erythrocyte sedimentation rate (70 mm/h), and elevated aldolase level (9.3 U/L). Complement levels were elevated, though complement C3 and C4 remained within normal limits. Histopathology of nodules from the medial right upper arm and left thigh showed lobular panniculitis with fat necrosis, calcification, and interface changes. The patient was treated for several months with daily mycophenolate mofetil (1 g increased to 3 g) and daily hydroxychloroquine (200 mg) without any effect on the nodules.

The histologic features of panniculitis in lupus and DM are similar and include multifocal hyalinization of the subcuticular fat and diffuse lobular infiltrates of mature lymphocytes without nuclear atypia.1 Though clinical panniculitis is a rare finding in DM, histologic panniculitis is a relatively common finding.2 Despite the similar histopathology of lupus and DM, the presence of typical DM clinical and laboratory features in our patient (body aches, muscle pain, proximal weakness, cutaneous manifestations, elevated creatine phosphokinase, normal complement C3 and C4) made a diagnosis of DM more likely.
Clinical panniculitis is a rare subcutaneous manifestation of DM with around 50 cases reported in the literature (Table). A PubMed search of articles indexed for MEDLINE was conducted using the terms dermatomyositis and panniculitis through July 2019. Additionally, a full-text review and search of references within these articles was used to identify all cases of patients presenting with panniculitis in the setting of DM. Exclusion criteria were cases in which another etiology was considered likely (infectious panniculitis and lupus panniculitis) as well as those without an English translation. We identified 43 cases; the average age of the patients was 39.6 years, and 36 (83.7%) of the cases were women. Patients typically presented with persistent, indurated, painful, erythematous, nodular lesions localized to the arms, abdomen, buttocks, and thighs.
While panniculitis has been reported preceding and concurrent with a diagnosis of DM, a number of cases described presentation as late as 5 years following onset of classic DM symptoms.12,13,31 In some cases (3/43 [7.0%]), panniculitis was the only cutaneous manifestation of DM.15,33,36 However, it occurred more commonly with other characteristic skin findings, such as heliotrope rash or Gottron sign.Some investigators have recommended that panniculitis be included as a diagnostic feature of DM and that DM be considered in the differential diagnosis in isolated cases of panniculitis.25,33
Though it seems panniculitis in DM may correlate with a better prognosis, we identified underlying malignancies in 3 cases. Malignancies associated with panniculitis in DM included ovarian adenocarcinoma, nasopharyngeal carcinoma, and parotid carcinoma, indicating that appropriate cancer screening still is critical in the diagnostic workup.2,11,22
A majority of the reported panniculitis cases in DM have responded to treatment with prednisone; however, treatment with prednisone has been more recalcitrant in other cases. Reports of successful additional therapies include methotrexate, cyclosporine, azathioprine, hydroxychloroquine, intravenous immunoglobulin, mepacrine, or a combination of these entities.19,22 In most cases, improvement of the panniculitis and other DM symptoms occurred simultaneously.25 It is noteworthy that the muscular symptoms often resolved more rapidly than cutaneous manifestations.33 Few reported cases (6 including the current case) found a persistent panniculitis despite improvement and remission of the myositis.3,5,10,11,30
Our patient was treated with both prednisone and methotrexate for several months, leading to remission of muscular symptoms (along with return to baseline of creatine phosphokinase), yet the panniculitis did not improve. The subcutaneous nodules also did not respond to treatment with mycophenolate mofetil and hydroxychloroquine.
Recent immunohistochemical studies have suggested that panniculitic lesions show better outcomes with immunosuppressive therapy when compared with other DM-related skin lesions.40 However, this was not the case for our patient, who after months of immunosuppressive therapy showed complete resolution of the periorbital and chest rashes with persistence of multiple indurated subcutaneous nodules.
Our case adds to a number of reports of DM presenting with panniculitis. Our patient fit the classic demographic of previously reported cases, as she was an adult woman without evidence of underlying malignancy; however, our case remains an example of the therapeutic challenge that exists when encountering a persistent, treatment-resistant panniculitis despite resolution of all other features of DM.
To the Editor:
A 62-year-old woman with a history of dermatomyositis (DM) presented to dermatology clinic for evaluation of multiple subcutaneous nodules. Two years prior to the current presentation, the patient was diagnosed by her primary care physician with DM based on clinical presentation. She initially developed body aches, muscle pain, and weakness of the upper extremities, specifically around the shoulders, and later the lower extremities, specifically around the thighs. The initial physical examination revealed pain with movement, tenderness to palpation, and proximal extremity weakness. The patient also noted a 50-lb weight loss. Over the next year, she noted dysphagia and developed multiple subcutaneous nodules on the right arm, chest, and left axilla. Subsequently, she developed a violaceous, hyperpigmented, periorbital rash and erythema of the anterior chest. She did not experience hair loss, oral ulcers, photosensitivity, or joint pain.
Laboratory testing in the months following the initial presentation revealed a creatine phosphokinase level of 436 U/L (reference range, 20–200 U/L), an erythrocyte sedimentation rate of 60 mm/h (reference range, <31 mm/h), and an aldolase level of 10.4 U/L (reference range, 1.0–8.0 U/L). Lactate dehydrogenase and thyroid function tests were within normal limits. Antinuclear antibodies, anti–double-stranded DNA, anti-Smith antibodies, anti-ribonucleoprotein, anti–Jo-1 antibodies, and anti–smooth muscle antibodies all were negative. Total blood complement levels were elevated, but complement C3 and C4 were within normal limits. Imaging demonstrated normal chest radiographs, and a modified barium swallow confirmed swallowing dysfunction. A right quadricep muscle biopsy confirmed the diagnosis of DM. A malignancy work-up including mammography, colonoscopy, and computed tomography of the chest, abdomen, and pelvis was negative aside from nodular opacities in the chest. She was treated with prednisone (60 mg, 0.9 mg/kg) daily and methotrexate (15–20 mg) weekly for several months. While the treatment attenuated the rash and improved weakness, the nodules persisted, prompting a referral to dermatology.
Physical examination at the dermatology clinic demonstrated the persistent subcutaneous nodules were indurated and bilaterally located on the arms, axillae, chest, abdomen, buttocks, and thighs with no pain or erythema (Figure). Laboratory tests demonstrated a normal creatine phosphokinase level, elevated erythrocyte sedimentation rate (70 mm/h), and elevated aldolase level (9.3 U/L). Complement levels were elevated, though complement C3 and C4 remained within normal limits. Histopathology of nodules from the medial right upper arm and left thigh showed lobular panniculitis with fat necrosis, calcification, and interface changes. The patient was treated for several months with daily mycophenolate mofetil (1 g increased to 3 g) and daily hydroxychloroquine (200 mg) without any effect on the nodules.

The histologic features of panniculitis in lupus and DM are similar and include multifocal hyalinization of the subcuticular fat and diffuse lobular infiltrates of mature lymphocytes without nuclear atypia.1 Though clinical panniculitis is a rare finding in DM, histologic panniculitis is a relatively common finding.2 Despite the similar histopathology of lupus and DM, the presence of typical DM clinical and laboratory features in our patient (body aches, muscle pain, proximal weakness, cutaneous manifestations, elevated creatine phosphokinase, normal complement C3 and C4) made a diagnosis of DM more likely.
Clinical panniculitis is a rare subcutaneous manifestation of DM with around 50 cases reported in the literature (Table). A PubMed search of articles indexed for MEDLINE was conducted using the terms dermatomyositis and panniculitis through July 2019. Additionally, a full-text review and search of references within these articles was used to identify all cases of patients presenting with panniculitis in the setting of DM. Exclusion criteria were cases in which another etiology was considered likely (infectious panniculitis and lupus panniculitis) as well as those without an English translation. We identified 43 cases; the average age of the patients was 39.6 years, and 36 (83.7%) of the cases were women. Patients typically presented with persistent, indurated, painful, erythematous, nodular lesions localized to the arms, abdomen, buttocks, and thighs.
While panniculitis has been reported preceding and concurrent with a diagnosis of DM, a number of cases described presentation as late as 5 years following onset of classic DM symptoms.12,13,31 In some cases (3/43 [7.0%]), panniculitis was the only cutaneous manifestation of DM.15,33,36 However, it occurred more commonly with other characteristic skin findings, such as heliotrope rash or Gottron sign.Some investigators have recommended that panniculitis be included as a diagnostic feature of DM and that DM be considered in the differential diagnosis in isolated cases of panniculitis.25,33
Though it seems panniculitis in DM may correlate with a better prognosis, we identified underlying malignancies in 3 cases. Malignancies associated with panniculitis in DM included ovarian adenocarcinoma, nasopharyngeal carcinoma, and parotid carcinoma, indicating that appropriate cancer screening still is critical in the diagnostic workup.2,11,22
A majority of the reported panniculitis cases in DM have responded to treatment with prednisone; however, treatment with prednisone has been more recalcitrant in other cases. Reports of successful additional therapies include methotrexate, cyclosporine, azathioprine, hydroxychloroquine, intravenous immunoglobulin, mepacrine, or a combination of these entities.19,22 In most cases, improvement of the panniculitis and other DM symptoms occurred simultaneously.25 It is noteworthy that the muscular symptoms often resolved more rapidly than cutaneous manifestations.33 Few reported cases (6 including the current case) found a persistent panniculitis despite improvement and remission of the myositis.3,5,10,11,30
Our patient was treated with both prednisone and methotrexate for several months, leading to remission of muscular symptoms (along with return to baseline of creatine phosphokinase), yet the panniculitis did not improve. The subcutaneous nodules also did not respond to treatment with mycophenolate mofetil and hydroxychloroquine.
Recent immunohistochemical studies have suggested that panniculitic lesions show better outcomes with immunosuppressive therapy when compared with other DM-related skin lesions.40 However, this was not the case for our patient, who after months of immunosuppressive therapy showed complete resolution of the periorbital and chest rashes with persistence of multiple indurated subcutaneous nodules.
Our case adds to a number of reports of DM presenting with panniculitis. Our patient fit the classic demographic of previously reported cases, as she was an adult woman without evidence of underlying malignancy; however, our case remains an example of the therapeutic challenge that exists when encountering a persistent, treatment-resistant panniculitis despite resolution of all other features of DM.
- Wick MR. Panniculitis: a summary. Semin Diagn Pathol. 2017;34:261-272.
- Girouard SD, Velez NF, Penson RT, et al. Panniculitis associated with dermatomyositis and recurrent ovarian cancer. Arch Dermatol. 2012;148:740-744.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:E187-E188.
- Choi YJ, Yoo WH. Panniculitis, a rare presentation of onset and exacerbation of juvenile dermatomyositis: a case report and literature review. Arch Rheumatol. 2018;33:367-371.
- Azevedo PO, Castellen NR, Salai AF, et al. Panniculitis associated with amyopathic dermatomyositis. An Bras Dermatol. 2018;93:119-121.
- Agulló A, Hinds B, Larrea M, et al. Livedo racemosa, reticulated ulcerations, panniculitis and violaceous plaques in a 46-year-old woman. Indian Dermatol Online J. 2018;9:47-49.
- Hattori Y, Matsuyama K, Takahashi T, et al. Anti-MDA5 antibody-positive dermatomyositis presenting with cellulitis-like erythema on the mandible as an initial symptom. Case Rep Dermatol. 2018;10:110-114.
- Hasegawa A, Shimomura Y, Kibune N, et al. Panniculitis as the initial manifestation of dermatomyositis with anti-MDA5 antibody. Clin Exp Dermatol. 2017;42:551-553.
- Salman A, Kasapcopur O, Ergun T, et al. Panniculitis in juvenile dermatomyositis: report of a case and review of the published work. J Dermatol. 2016;43:951-953.
- Carroll M, Mellick N, Wagner G. Dermatomyositis panniculitis: a case report. Australas J Dermatol. 2015;56:224‐226.
- Chairatchaneeboon M, Kulthanan K, Manapajon A. Calcific panniculitis and nasopharyngeal cancer-associated adult-onset dermatomyositis: a case report and literature review. Springerplus. 2015;4:201.
- Otero Rivas MM, Vicente Villa A, González Lara L, et al. Panniculitis in juvenile dermatomyositis. Clin Exp Dermatol. 2015;40:574-575.
- Yanaba K, Tanito K, Hamaguchi Y, et al. Anti‐transcription intermediary factor‐1γ/α/β antibody‐positive dermatomyositis associated with multiple panniculitis lesions. Int J Rheum Dis. 2015;20:1831-1834.
- Pau-Charles I, Moreno PJ, Ortiz-Ibanez K, et al. Anti-MDA5 positive clinically amyopathic dermatomyositis presenting with severe cardiomyopathy. J Eur Acad Dermatol Venereol. 2014;28:1097-1102.
- Lamb R, Digby S, Stewart W, et al. Cutaneous ulceration: more than skin deep? Clin Exp Dermatol. 2013;38:443-445.
- Arias M, Hernández MI, Cunha LG, et al. Panniculitis in a patient with dermatomyositis. An Bras Dermatol. 2011;86:146-148.
- Hemmi S, Kushida R, Nishimura H, et al. Magnetic resonance imaging diagnosis of panniculitis in dermatomyositis. Muscle Nerve. 2010;41:151-153.
- Geddes MR, Sinnreich M, Chalk C. Minocycline-induced dermatomyositis. Muscle Nerve. 2010;41:547-549.
- Abdul‐Wahab A, Holden CA, Harland C, et al Calcific panniculitis in adult‐onset dermatomyositis. Clin Exp Dermatol. 2009;34:E854-E856.
- Carneiro S, Alvim G, Resende P, et al. Dermatomyositis with panniculitis. Skinmed. 2007;6:46-47.
- Carrera E, Lobrinus JA, Spertini O, et al. Dermatomyositis, lobarpanniculitis and inflammatory myopathy with abundant macrophages. Neuromuscul Disord. 2006;16:468-471.
- Lin JH, Chu CY, Lin RY. Panniculitis in adult onset dermatomyositis: report of two cases and review of the literature. Dermatol Sinica. 2006;24:194-200.
- Chen GY, Liu MF, Lee JY, et al. Combination of massive mucinosis, dermatomyositis, pyoderma gangrenosum-like ulcer, bullae and fatal intestinal vasculopathy in a young female. Eur J Dermatol. 2005;15:396-400.
- Nakamori A, Yamaguchi Y, Kurimoto I, et al. Vesiculobullous dermatomyositis with panniculitis without muscle disease. J Am Acad Dermatol. 2003;49:1136-1139.
- Solans R, Cortés J, Selva A, et al. Panniculitis: a cutaneous manifestation of dermatomyositis. J Am Acad Dermatol. 2002;46:S148-S150.
- Chao YY, Yang LJ. Dermatomyositis presenting as panniculitis. Int J Dermatol. 2000;39:141-144.
- Lee MW, Lim YS, Choi JH, et al. Panniculitis showing membranocystic changes in the dermatomyositis. J Dermatol. 1999;26:608‐610.
- Ghali FE, Reed AM, Groben PA, et al. Panniculitis in juvenile dermatomyositis. Pediatr Dermatol. 1999;16:270-272.
- Molnar K, Kemeny L, Korom I, et al. Panniculitis in dermatomyositis: report of two cases. Br J Dermatol. 1998;139:161‐163.
- Ishikawa O, Tamura A, Ryuzaki K, et al. Membranocystic changes in the panniculitis of dermatomyositis. Br J Dermatol. 1996;134:773-776.
- Sabroe RA, Wallington TB, Kennedy CT. Dermatomyositis treated with high-dose intravenous immunoglobulins and associated with panniculitis. Clin Exp Dermatol. 1995;20:164-167.
- Neidenbach PJ, Sahn EE, Helton J. Panniculitis in juvenile dermatomyositis. J Am Acad Dermatol. 1995;33:305-307.
- Fusade T, Belanyi P, Joly P, et al. Subcutaneous changes in dermatomyositis. Br J Dermatol. 1993;128:451-453.
- Winkelmann WJ, Billick RC, Srolovitz H. Dermatomyositis presenting as panniculitis. J Am Acad Dermatol. 1990;23:127-128.
- Commens C, O’Neill P, Walker G. Dermatomyositis associated with multifocal lipoatrophy. J Am Acad Dermatol. 1990;22:966-969.
- Raimer SS, Solomon AR, Daniels JC. Polymyositis presenting with panniculitis. J Am Acad Dermatol. 1985;13(2 pt 2):366‐369.
- Feldman D, Hochberg MC, Zizic TM, et al. Cutaneous vasculitis in adult polymyositis/dermatomyositis. J Rheumatol. 1983;10:85-89.
- Kimura S, Fukuyama Y. Tubular cytoplasmic inclusions in a case of childhood dermatomyositis with migratory subcutaneous nodules. Eur J Pediatr. 1977;125:275-283.
- Weber FP, Gray AMH. Chronic relapsing polydermatomyositis with predominant involvement of the subcutaneous fat. Br J Dermatol. 1924;36:544-560.
- Santos‐Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Wick MR. Panniculitis: a summary. Semin Diagn Pathol. 2017;34:261-272.
- Girouard SD, Velez NF, Penson RT, et al. Panniculitis associated with dermatomyositis and recurrent ovarian cancer. Arch Dermatol. 2012;148:740-744.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:E187-E188.
- Choi YJ, Yoo WH. Panniculitis, a rare presentation of onset and exacerbation of juvenile dermatomyositis: a case report and literature review. Arch Rheumatol. 2018;33:367-371.
- Azevedo PO, Castellen NR, Salai AF, et al. Panniculitis associated with amyopathic dermatomyositis. An Bras Dermatol. 2018;93:119-121.
- Agulló A, Hinds B, Larrea M, et al. Livedo racemosa, reticulated ulcerations, panniculitis and violaceous plaques in a 46-year-old woman. Indian Dermatol Online J. 2018;9:47-49.
- Hattori Y, Matsuyama K, Takahashi T, et al. Anti-MDA5 antibody-positive dermatomyositis presenting with cellulitis-like erythema on the mandible as an initial symptom. Case Rep Dermatol. 2018;10:110-114.
- Hasegawa A, Shimomura Y, Kibune N, et al. Panniculitis as the initial manifestation of dermatomyositis with anti-MDA5 antibody. Clin Exp Dermatol. 2017;42:551-553.
- Salman A, Kasapcopur O, Ergun T, et al. Panniculitis in juvenile dermatomyositis: report of a case and review of the published work. J Dermatol. 2016;43:951-953.
- Carroll M, Mellick N, Wagner G. Dermatomyositis panniculitis: a case report. Australas J Dermatol. 2015;56:224‐226.
- Chairatchaneeboon M, Kulthanan K, Manapajon A. Calcific panniculitis and nasopharyngeal cancer-associated adult-onset dermatomyositis: a case report and literature review. Springerplus. 2015;4:201.
- Otero Rivas MM, Vicente Villa A, González Lara L, et al. Panniculitis in juvenile dermatomyositis. Clin Exp Dermatol. 2015;40:574-575.
- Yanaba K, Tanito K, Hamaguchi Y, et al. Anti‐transcription intermediary factor‐1γ/α/β antibody‐positive dermatomyositis associated with multiple panniculitis lesions. Int J Rheum Dis. 2015;20:1831-1834.
- Pau-Charles I, Moreno PJ, Ortiz-Ibanez K, et al. Anti-MDA5 positive clinically amyopathic dermatomyositis presenting with severe cardiomyopathy. J Eur Acad Dermatol Venereol. 2014;28:1097-1102.
- Lamb R, Digby S, Stewart W, et al. Cutaneous ulceration: more than skin deep? Clin Exp Dermatol. 2013;38:443-445.
- Arias M, Hernández MI, Cunha LG, et al. Panniculitis in a patient with dermatomyositis. An Bras Dermatol. 2011;86:146-148.
- Hemmi S, Kushida R, Nishimura H, et al. Magnetic resonance imaging diagnosis of panniculitis in dermatomyositis. Muscle Nerve. 2010;41:151-153.
- Geddes MR, Sinnreich M, Chalk C. Minocycline-induced dermatomyositis. Muscle Nerve. 2010;41:547-549.
- Abdul‐Wahab A, Holden CA, Harland C, et al Calcific panniculitis in adult‐onset dermatomyositis. Clin Exp Dermatol. 2009;34:E854-E856.
- Carneiro S, Alvim G, Resende P, et al. Dermatomyositis with panniculitis. Skinmed. 2007;6:46-47.
- Carrera E, Lobrinus JA, Spertini O, et al. Dermatomyositis, lobarpanniculitis and inflammatory myopathy with abundant macrophages. Neuromuscul Disord. 2006;16:468-471.
- Lin JH, Chu CY, Lin RY. Panniculitis in adult onset dermatomyositis: report of two cases and review of the literature. Dermatol Sinica. 2006;24:194-200.
- Chen GY, Liu MF, Lee JY, et al. Combination of massive mucinosis, dermatomyositis, pyoderma gangrenosum-like ulcer, bullae and fatal intestinal vasculopathy in a young female. Eur J Dermatol. 2005;15:396-400.
- Nakamori A, Yamaguchi Y, Kurimoto I, et al. Vesiculobullous dermatomyositis with panniculitis without muscle disease. J Am Acad Dermatol. 2003;49:1136-1139.
- Solans R, Cortés J, Selva A, et al. Panniculitis: a cutaneous manifestation of dermatomyositis. J Am Acad Dermatol. 2002;46:S148-S150.
- Chao YY, Yang LJ. Dermatomyositis presenting as panniculitis. Int J Dermatol. 2000;39:141-144.
- Lee MW, Lim YS, Choi JH, et al. Panniculitis showing membranocystic changes in the dermatomyositis. J Dermatol. 1999;26:608‐610.
- Ghali FE, Reed AM, Groben PA, et al. Panniculitis in juvenile dermatomyositis. Pediatr Dermatol. 1999;16:270-272.
- Molnar K, Kemeny L, Korom I, et al. Panniculitis in dermatomyositis: report of two cases. Br J Dermatol. 1998;139:161‐163.
- Ishikawa O, Tamura A, Ryuzaki K, et al. Membranocystic changes in the panniculitis of dermatomyositis. Br J Dermatol. 1996;134:773-776.
- Sabroe RA, Wallington TB, Kennedy CT. Dermatomyositis treated with high-dose intravenous immunoglobulins and associated with panniculitis. Clin Exp Dermatol. 1995;20:164-167.
- Neidenbach PJ, Sahn EE, Helton J. Panniculitis in juvenile dermatomyositis. J Am Acad Dermatol. 1995;33:305-307.
- Fusade T, Belanyi P, Joly P, et al. Subcutaneous changes in dermatomyositis. Br J Dermatol. 1993;128:451-453.
- Winkelmann WJ, Billick RC, Srolovitz H. Dermatomyositis presenting as panniculitis. J Am Acad Dermatol. 1990;23:127-128.
- Commens C, O’Neill P, Walker G. Dermatomyositis associated with multifocal lipoatrophy. J Am Acad Dermatol. 1990;22:966-969.
- Raimer SS, Solomon AR, Daniels JC. Polymyositis presenting with panniculitis. J Am Acad Dermatol. 1985;13(2 pt 2):366‐369.
- Feldman D, Hochberg MC, Zizic TM, et al. Cutaneous vasculitis in adult polymyositis/dermatomyositis. J Rheumatol. 1983;10:85-89.
- Kimura S, Fukuyama Y. Tubular cytoplasmic inclusions in a case of childhood dermatomyositis with migratory subcutaneous nodules. Eur J Pediatr. 1977;125:275-283.
- Weber FP, Gray AMH. Chronic relapsing polydermatomyositis with predominant involvement of the subcutaneous fat. Br J Dermatol. 1924;36:544-560.
- Santos‐Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
Practice Points
- Clinical panniculitis is a rare subcutaneous manifestation of dermatomyositis (DM) that dermatologists must consider when evaluating patients with this condition.
- Panniculitis can precede, occur simultaneously with, or develop up to 5 years after onset of DM.
- Many patients suffer from treatment-resistant panniculitis in DM, suggesting that therapeutic management of this condition may require long-term and more aggressive treatment modalities.
Distribution of Skin-Type Diversity in Photographs in AAD Online Educational Modules
Recent studies have found poor representation of darker skin types (defined as Fitzpatrick skin types V–VI) in dermatology textbooks and online resources.1,2 We sought to evaluate representation of darker skin types in the Basic Dermatology Curriculum of the American Academy of Dermatology (AAD), an online curriculum of 35 lectures that serves as a standard curriculum for dermatologic education, particularly for medical students and residents without a home dermatology program.3 Although core dermatology knowledge was specified as a curricular goal, knowledge of how dermatologic conditions manifest across various skin types was not.3
Methods
Photographs from all Basic Dermatology Curriculum online lectures showing background skin were independently labeled by 3 investigators (B.C., R.F., and G.O.) as light skin (Fitzpatrick types I–IV) or dark skin (Fitzpatrick types V–VI), along with the associated diagnosis. Photographs without visible background skin were excluded (eg, mucous membranes, palms and soles, genitalia, scalp, dermoscopic images). Photographs with indeterminate skin type were evaluated by consensus and excluded if consensus could not be reached. Inter-rater reliability for labeling skin type was determined on an overlapping sample of 24 photographs (Fleiss’s κ, 0.80).
Results
Of 666 included photographs, 104 (15.6%) featured dark skin. Of all photographs of light skin (Fitzpatrick type I–IV), 80.8% were Fitzpatrick types I and II. One-quarter of lectures featured no photographs of dark skin (Figure 1). When the associated diagnoses of photographs were organized into 20 categories, 4 categories—pigmentary disorders, HIV infection, sexually transmitted infections and warts, and papulosquamous eruptions (Figure 2)—each featured 25% or more photographs of dark skin.
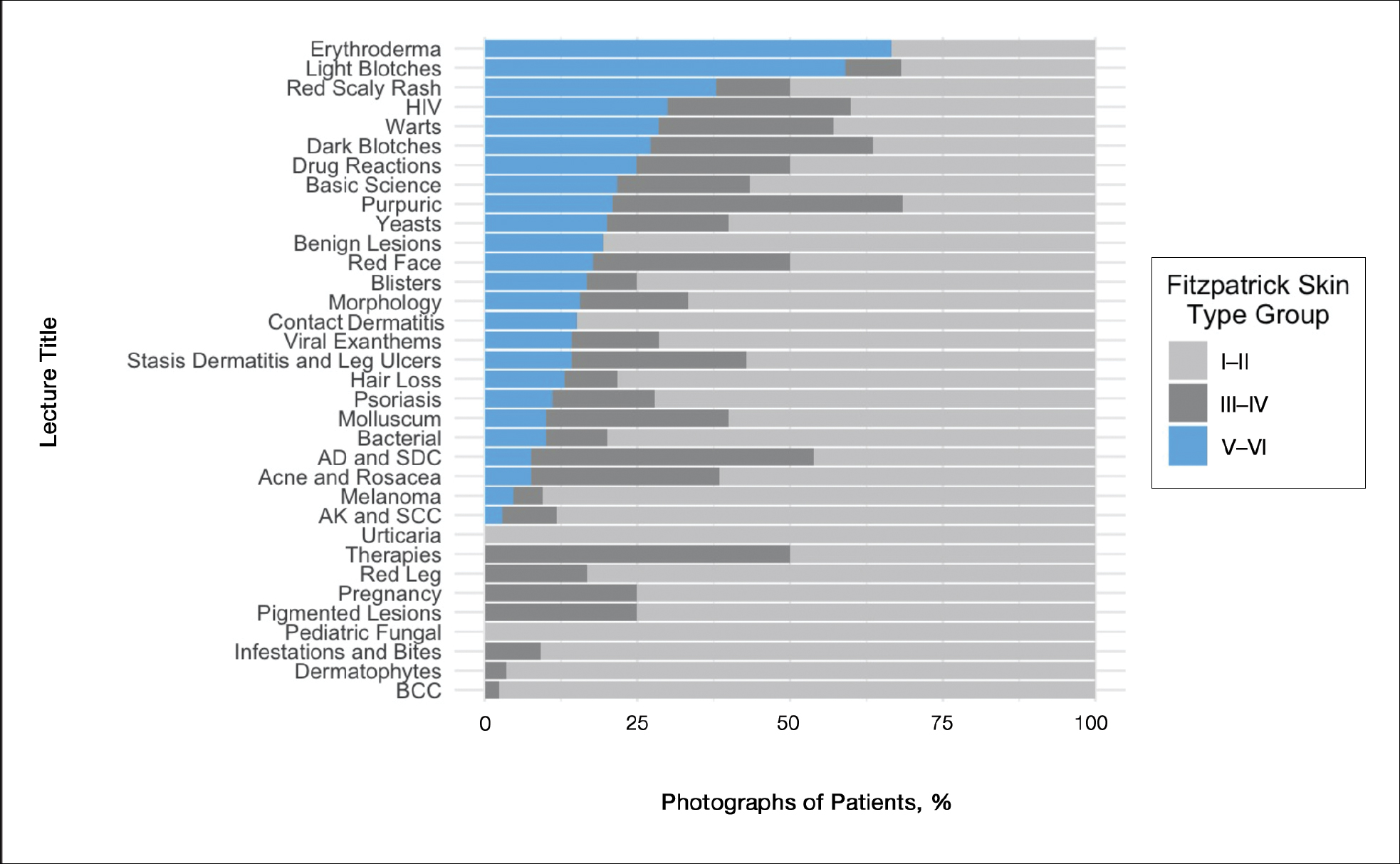
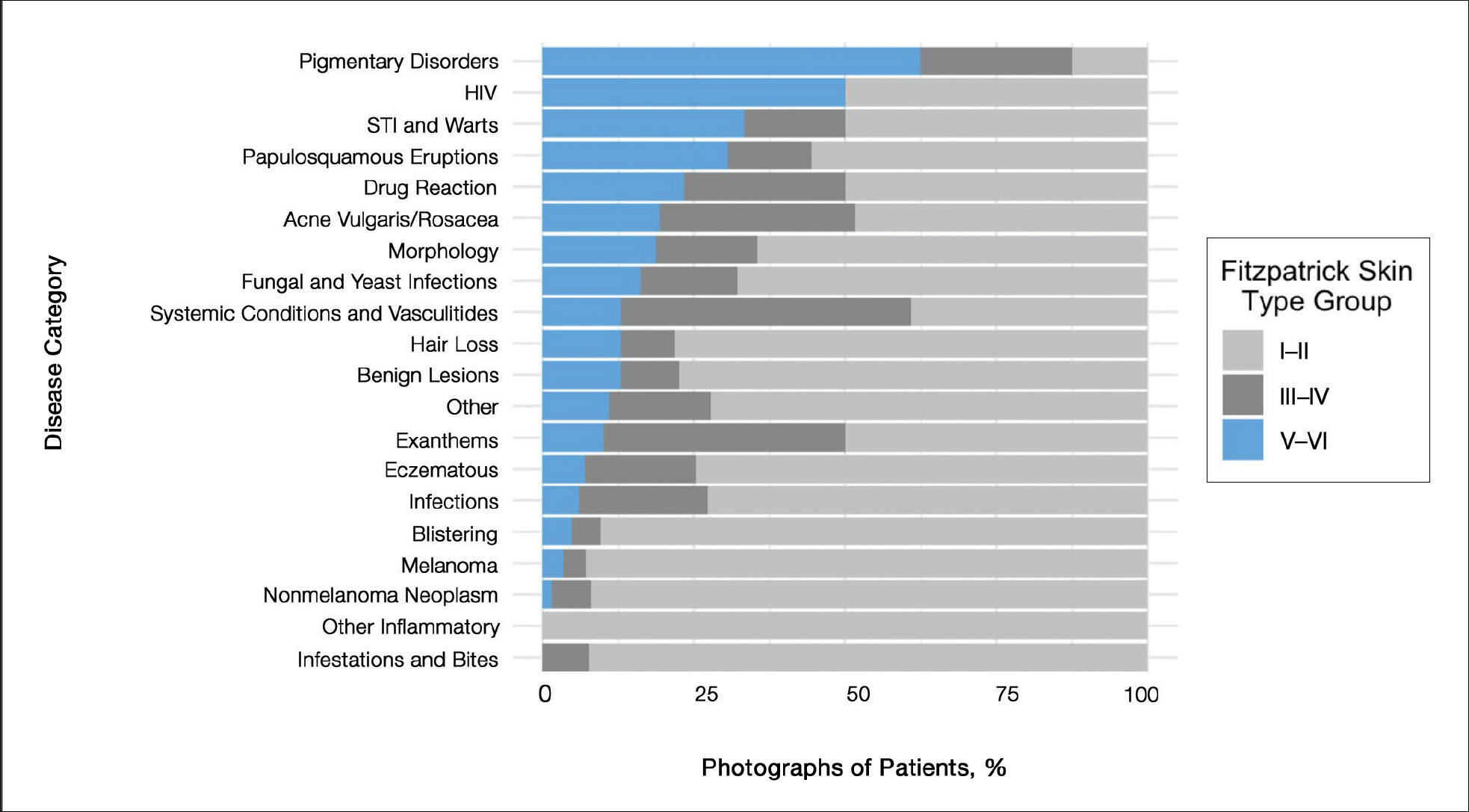
Comment
Our analysis of curricular photographs found dark skin representation in 16% of photographs, mirroring earlier findings in other educational resources.1,2 There was little (<5%) representation of skin cancer in individuals with darker skin, which may merely reflect lower incidence, but there is concern that lack of education about skin cancer might contribute to disparities in care, such as delayed diagnosis.2
For some conditions common in darker-skinned patients, such as acne vulgaris, representation was low; the lecture “Acne vulgaris” featured only 1 photograph of dark skin. In contrast, dark skin types were well represented in photographs of sexually transmitted infections, such as HIV infection, syphilis, and warts, which may suggest bias when dark skin is chosen to represent diseases, as noted in prior findings.1,2
Limitations of this study included individual judgment of skin type and use of the Fitzpatrick scale. Although inter-rater reliability was excellent, the validity of Fitzpatrick classification of skin color is controversial, given that it was intended to describe propensity for sunburn and that types V to VI were added later to describe darker skin.4
Suggestions for Improvement
Given the abundance of resources with depictions of skin of color in teaching materials (eg, Taylor and Kelly’s Dermatology for Skin of Color, Ethnic Dermatology: Principles and Practice) and digital resources (eg, VisualDx [https://www.visualdx.com]), a logical solution might be to add a greater percentage of photographs depicting darker skin from outside resources to address the imbalance. Still, this might be challenging with limited space. Often, there is only room for a single representative photograph. Therefore, greater effort must be made to consistently show how diseases might present variably on different background skin types or, at the least, to create new resources showing greater skin type diversity.
Furthermore, given the lack of representation of skin of color, authors of educational resources can prioritize capturing images of skin pathology presenting in darker skin during their clinical work. Authors who do not have access to a substantial census of patients with darker skin can collaborate with dermatologists who specialize in skin of color to gather such images.
Technical issues include difficulty capturing high-quality images of dermatologic conditions in darker skin because eruptions in these patients might have a narrower range of contrast. Although resources on taking high-quality clinical images are widely available, specific advice for photographing darker skin is lacking and warrants future research.5-7 Collaboration with professional photographers who are experienced with clients with darker skin might be useful in developing guidelines.
Conclusion
Given recent guidance by the AAD to “include common skin disorders and diseases requiring special consideration in people with skin of color” and highlight “current disparities in health outcomes within dermatology,”8 our findings might guide future improvements in curricula.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
- Lester JC, Taylor SC, Chren M‐M. Under‐representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Cipriano SD, Dybbro E, Boscardin CK, et al. Online learning in a dermatology clerkship: piloting the new American Academy of Dermatology Medical Student Core Curriculum. J Am Acad Dermatol. 2013;69:267-272.
- Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
- Muraco L. Improved medical photography: key tips for creating images of lasting value. JAMA Dermatol. 2020;156:121-123.
- Shainhouse T. Clinical photography best practices. Dermatology Times. May 13, 2016. Accessed January 10, 2021. https://www.dermatologytimes.com/view/clinical-photography-best-practices
- How to take the best photos for teledermatology. VisualDx. Accessed January 10, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
Recent studies have found poor representation of darker skin types (defined as Fitzpatrick skin types V–VI) in dermatology textbooks and online resources.1,2 We sought to evaluate representation of darker skin types in the Basic Dermatology Curriculum of the American Academy of Dermatology (AAD), an online curriculum of 35 lectures that serves as a standard curriculum for dermatologic education, particularly for medical students and residents without a home dermatology program.3 Although core dermatology knowledge was specified as a curricular goal, knowledge of how dermatologic conditions manifest across various skin types was not.3
Methods
Photographs from all Basic Dermatology Curriculum online lectures showing background skin were independently labeled by 3 investigators (B.C., R.F., and G.O.) as light skin (Fitzpatrick types I–IV) or dark skin (Fitzpatrick types V–VI), along with the associated diagnosis. Photographs without visible background skin were excluded (eg, mucous membranes, palms and soles, genitalia, scalp, dermoscopic images). Photographs with indeterminate skin type were evaluated by consensus and excluded if consensus could not be reached. Inter-rater reliability for labeling skin type was determined on an overlapping sample of 24 photographs (Fleiss’s κ, 0.80).
Results
Of 666 included photographs, 104 (15.6%) featured dark skin. Of all photographs of light skin (Fitzpatrick type I–IV), 80.8% were Fitzpatrick types I and II. One-quarter of lectures featured no photographs of dark skin (Figure 1). When the associated diagnoses of photographs were organized into 20 categories, 4 categories—pigmentary disorders, HIV infection, sexually transmitted infections and warts, and papulosquamous eruptions (Figure 2)—each featured 25% or more photographs of dark skin.


Comment
Our analysis of curricular photographs found dark skin representation in 16% of photographs, mirroring earlier findings in other educational resources.1,2 There was little (<5%) representation of skin cancer in individuals with darker skin, which may merely reflect lower incidence, but there is concern that lack of education about skin cancer might contribute to disparities in care, such as delayed diagnosis.2
For some conditions common in darker-skinned patients, such as acne vulgaris, representation was low; the lecture “Acne vulgaris” featured only 1 photograph of dark skin. In contrast, dark skin types were well represented in photographs of sexually transmitted infections, such as HIV infection, syphilis, and warts, which may suggest bias when dark skin is chosen to represent diseases, as noted in prior findings.1,2
Limitations of this study included individual judgment of skin type and use of the Fitzpatrick scale. Although inter-rater reliability was excellent, the validity of Fitzpatrick classification of skin color is controversial, given that it was intended to describe propensity for sunburn and that types V to VI were added later to describe darker skin.4
Suggestions for Improvement
Given the abundance of resources with depictions of skin of color in teaching materials (eg, Taylor and Kelly’s Dermatology for Skin of Color, Ethnic Dermatology: Principles and Practice) and digital resources (eg, VisualDx [https://www.visualdx.com]), a logical solution might be to add a greater percentage of photographs depicting darker skin from outside resources to address the imbalance. Still, this might be challenging with limited space. Often, there is only room for a single representative photograph. Therefore, greater effort must be made to consistently show how diseases might present variably on different background skin types or, at the least, to create new resources showing greater skin type diversity.
Furthermore, given the lack of representation of skin of color, authors of educational resources can prioritize capturing images of skin pathology presenting in darker skin during their clinical work. Authors who do not have access to a substantial census of patients with darker skin can collaborate with dermatologists who specialize in skin of color to gather such images.
Technical issues include difficulty capturing high-quality images of dermatologic conditions in darker skin because eruptions in these patients might have a narrower range of contrast. Although resources on taking high-quality clinical images are widely available, specific advice for photographing darker skin is lacking and warrants future research.5-7 Collaboration with professional photographers who are experienced with clients with darker skin might be useful in developing guidelines.
Conclusion
Given recent guidance by the AAD to “include common skin disorders and diseases requiring special consideration in people with skin of color” and highlight “current disparities in health outcomes within dermatology,”8 our findings might guide future improvements in curricula.
Recent studies have found poor representation of darker skin types (defined as Fitzpatrick skin types V–VI) in dermatology textbooks and online resources.1,2 We sought to evaluate representation of darker skin types in the Basic Dermatology Curriculum of the American Academy of Dermatology (AAD), an online curriculum of 35 lectures that serves as a standard curriculum for dermatologic education, particularly for medical students and residents without a home dermatology program.3 Although core dermatology knowledge was specified as a curricular goal, knowledge of how dermatologic conditions manifest across various skin types was not.3
Methods
Photographs from all Basic Dermatology Curriculum online lectures showing background skin were independently labeled by 3 investigators (B.C., R.F., and G.O.) as light skin (Fitzpatrick types I–IV) or dark skin (Fitzpatrick types V–VI), along with the associated diagnosis. Photographs without visible background skin were excluded (eg, mucous membranes, palms and soles, genitalia, scalp, dermoscopic images). Photographs with indeterminate skin type were evaluated by consensus and excluded if consensus could not be reached. Inter-rater reliability for labeling skin type was determined on an overlapping sample of 24 photographs (Fleiss’s κ, 0.80).
Results
Of 666 included photographs, 104 (15.6%) featured dark skin. Of all photographs of light skin (Fitzpatrick type I–IV), 80.8% were Fitzpatrick types I and II. One-quarter of lectures featured no photographs of dark skin (Figure 1). When the associated diagnoses of photographs were organized into 20 categories, 4 categories—pigmentary disorders, HIV infection, sexually transmitted infections and warts, and papulosquamous eruptions (Figure 2)—each featured 25% or more photographs of dark skin.


Comment
Our analysis of curricular photographs found dark skin representation in 16% of photographs, mirroring earlier findings in other educational resources.1,2 There was little (<5%) representation of skin cancer in individuals with darker skin, which may merely reflect lower incidence, but there is concern that lack of education about skin cancer might contribute to disparities in care, such as delayed diagnosis.2
For some conditions common in darker-skinned patients, such as acne vulgaris, representation was low; the lecture “Acne vulgaris” featured only 1 photograph of dark skin. In contrast, dark skin types were well represented in photographs of sexually transmitted infections, such as HIV infection, syphilis, and warts, which may suggest bias when dark skin is chosen to represent diseases, as noted in prior findings.1,2
Limitations of this study included individual judgment of skin type and use of the Fitzpatrick scale. Although inter-rater reliability was excellent, the validity of Fitzpatrick classification of skin color is controversial, given that it was intended to describe propensity for sunburn and that types V to VI were added later to describe darker skin.4
Suggestions for Improvement
Given the abundance of resources with depictions of skin of color in teaching materials (eg, Taylor and Kelly’s Dermatology for Skin of Color, Ethnic Dermatology: Principles and Practice) and digital resources (eg, VisualDx [https://www.visualdx.com]), a logical solution might be to add a greater percentage of photographs depicting darker skin from outside resources to address the imbalance. Still, this might be challenging with limited space. Often, there is only room for a single representative photograph. Therefore, greater effort must be made to consistently show how diseases might present variably on different background skin types or, at the least, to create new resources showing greater skin type diversity.
Furthermore, given the lack of representation of skin of color, authors of educational resources can prioritize capturing images of skin pathology presenting in darker skin during their clinical work. Authors who do not have access to a substantial census of patients with darker skin can collaborate with dermatologists who specialize in skin of color to gather such images.
Technical issues include difficulty capturing high-quality images of dermatologic conditions in darker skin because eruptions in these patients might have a narrower range of contrast. Although resources on taking high-quality clinical images are widely available, specific advice for photographing darker skin is lacking and warrants future research.5-7 Collaboration with professional photographers who are experienced with clients with darker skin might be useful in developing guidelines.
Conclusion
Given recent guidance by the AAD to “include common skin disorders and diseases requiring special consideration in people with skin of color” and highlight “current disparities in health outcomes within dermatology,”8 our findings might guide future improvements in curricula.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
- Lester JC, Taylor SC, Chren M‐M. Under‐representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Cipriano SD, Dybbro E, Boscardin CK, et al. Online learning in a dermatology clerkship: piloting the new American Academy of Dermatology Medical Student Core Curriculum. J Am Acad Dermatol. 2013;69:267-272.
- Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
- Muraco L. Improved medical photography: key tips for creating images of lasting value. JAMA Dermatol. 2020;156:121-123.
- Shainhouse T. Clinical photography best practices. Dermatology Times. May 13, 2016. Accessed January 10, 2021. https://www.dermatologytimes.com/view/clinical-photography-best-practices
- How to take the best photos for teledermatology. VisualDx. Accessed January 10, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
- Lester JC, Taylor SC, Chren M‐M. Under‐representation of skin of colour in dermatology images: not just an educational issue. Br J Dermatol. 2019;180:1521-1522.
- Cipriano SD, Dybbro E, Boscardin CK, et al. Online learning in a dermatology clerkship: piloting the new American Academy of Dermatology Medical Student Core Curriculum. J Am Acad Dermatol. 2013;69:267-272.
- Ware OR, Dawson JE, Shinohara MM, et al. Racial limitations of Fitzpatrick skin type. Cutis. 2020;105:77-80.
- Muraco L. Improved medical photography: key tips for creating images of lasting value. JAMA Dermatol. 2020;156:121-123.
- Shainhouse T. Clinical photography best practices. Dermatology Times. May 13, 2016. Accessed January 10, 2021. https://www.dermatologytimes.com/view/clinical-photography-best-practices
- How to take the best photos for teledermatology. VisualDx. Accessed January 10, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
PRACTICE POINTS
- Recent studies have highlighted poor representation of darker skin types in textbooks.
- The Basic Dermatology Curriculum of the American Academy of Dermatology has a low (16%) representation of darker skin types in photographs; more than one-quarter of curriculum lectures had no such images.
- Darker skin types were underrepresented for skin cancers and overrepresented for sexually transmitted infections, raising questions about how photographs were chosen.
- Educators should consider using existing resources of photographs of diverse skin types when designing future curricula.
Access to Inpatient Dermatology Care in Pennsylvania Hospitals
Access to care is a known issue in dermatology, and many patients may experience long waiting periods to see a physician.1 Previous research has evaluated access to outpatient dermatology services, but access to dermatology in inpatient medicine is also a growing problem.2 Reports depict a decrease in dermatologist involvement in inpatient care and an increase in nondermatologist physicians caring for inpatients with dermatologic needs.2,3 This lack of access could potentially lead to missed and/or incorrect diagnoses. One study showed that most cases in which dermatology was consulted required a change in treatment once correctly diagnosed by a dermatologist.4
Despite the known trend of decreasing involvement of dermatologists in inpatient care, there remains a paucity of data quantifying the current gap in access to care for inpatients with dermatologic needs. The purpose of this study was to evaluate differential access to inpatient dermatology services across licensed hospitals within the state of Pennsylvania.
Methods
In July 2014, an invitation to participate in an anonymous online survey was mailed to all 274 hospitals throughout Pennsylvania that were currently licensed by the US Department of Health. This study was declared exempt from review by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board. Study data were collected and managed using electronic data capture tools hosted by the University of Pennsylvania. Hospital administrators were encouraged to report dermatology access and details regardless of current status of inpatient dermatology services in order to inform efforts to improve access to care. Invitation letters to participate in the online survey were addressed to “Administrator” according to the contact method used by the US Department of Health for accreditation of state hospitals. Addresses for accredited state hospitals were obtained from the US Department of Health Web site and were supplemented with additional addresses of Veterans Administration hospitals obtained from public listings. Three weeks after initial survey invitations were sent, reminder letters were sent to nonresponsive hospitals. Only data from hospitals currently offering inpatient services were included in the analysis; exclusion criteria included psychiatric hospitals, substance abuse treatment centers, physical rehabilitation facilities, and outpatient centers.
Results
Of the 204 (74%) hospitals that met the inclusion criteria, 32 responded (16% response rate). Of the 32 hospitals that responded, 31 (97%) were privately owned facilities, 3 of which were specialty surgical centers. One (3%) hospital was a Veterans Administration hospital. Of the responders, 16 (50%) reported having any form of access to inpatient dermatology consultations. Of the 16 with reported access, 9 (56%) received their consultations through a local or private dermatology group, while 4 (25%) had a dermatologist on staff. The remaining 3 hospitals (19%) provided dermatology consultations through nondermatologist physicians on staff (a surgeon, an emergency care physician, and an internist, respectively).
The survey also sought to gain information about the various degrees of access to inpatient dermatology care that hospitals provide. Of the 16 hospitals that reported access to inpatient dermatology services, 11 (69%) provided specific details related to access (eg, coverage, anticipated response times) of dermatology consultations (Figure). The type of access to inpatient dermatology in relation to the type of hospital ownership is shown in the Table.
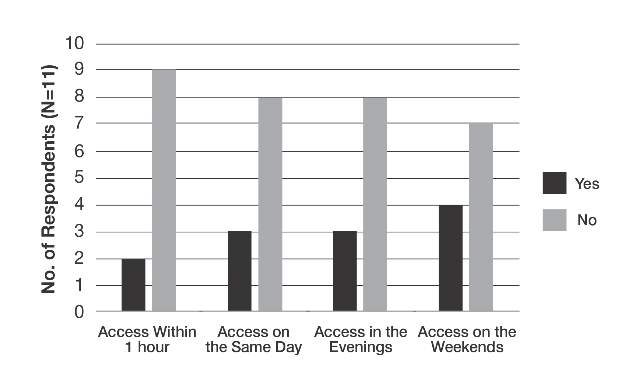
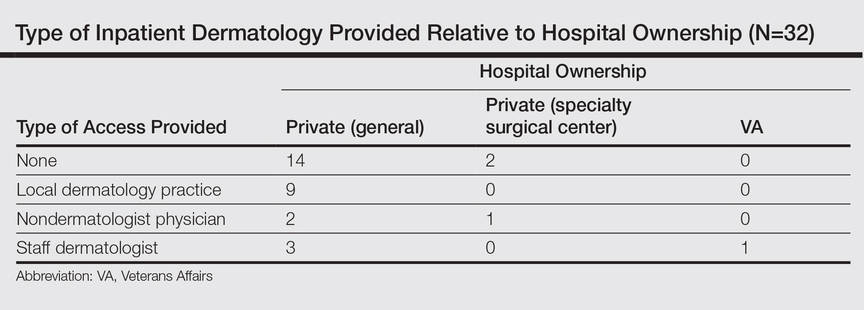
Comment
The survey results indicated suboptimal access to inpatient dermatology services in Pennsylvania hospitals. Only 50% (16/32) of respondents reported providing access to dermatology consultation, the majority of which appeared to have extremely limited same-day, evening, and weekend coverage. Although our study was limited by a low response rate (16%) and represents a narrow geographic distribution, these results suggested that lack of access to inpatient dermatology consultation may be a widespread problem and may be independent of the type of hospital ownership. Furthermore, the results of this study may offer insight into the different types and availability of inpatient dermatology services offered in hospitals across the United States.
The decrease in inpatient dermatology access has been driven by many factors. First, advances in medical research and pharmacotherapy may have decreased the need for dermatologic inpatient care, as patients who formerly would have required inpatient treatments are now able to receive therapies in an outpatient setting (eg, treatment of psoriasis).5 This may create less demand for hospitals to have a dermatologist on staff. Additionally, hospitals may be less able to incentivize dermatologists to provide inpatient dermatology consultations due to low reimbursement rates, time and distance required to visit inpatient facilities (taking away from outpatient clinic time), and the perception that inpatient cases carry greater liability given their greater complexity.6-8 Together, these factors may have contributed to the current lack of inpatient dermatology services in Pennsylvania hospitals and likely in hospitals throughout the United States.
Conclusion
Although a relatively small number of academic hospitals are experiencing an emergence of dermatology hospitalists, poor access to inpatient dermatology care continues to be a problem.8 Innovation (eg, the use of teledermatology to improve access to care9) and further studies are needed to address this gap in access to inpatient dermatology care.
- Kimball AB, Resneck JS. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Helms AE, Helms SE, Brodell RT. Hospital consultations: time to address an unmet need? J Am Acad Dermatol. 2009;60:308-311.
- Kirsner RS, Yang DG, Kerdel FA. The changing status of inpatient dermatology at American academic dermatology programs. J Am Acad Dermatol. 1999;40:755-757.
- Nahass GT, Meyer AJ, Campbell SF, et al. Prevalence of cutaneous findings in hospitalized medical patients. J Am Acad Dermatol. 1995;33:207-211.
- Steinke S, Peitsch WK, Ludwig A, et al. Cost-of-illness in psoriasis: comparing inpatient and outpatient therapy. PLoS One. 2013;8:e78152.
- Swerlick RA. Declining interest in medical dermatology. Arch Dermatol. 1998;134:1160-1162.
- Kirsner RS, Yang DG, Kerdel FA. Inpatient dermatology: the difficulties, the reality, and the future. Dermatol Clin. 2000;18:383-390.
- Fox LP, Cotliar J, Hughey L, et al. Hospitalist dermatology. J Am Acad Dermatol. 2009;61:153-154.
- Sharma P, Kovarik CL, Lipoff JB. Teledermatology as a means to improve access to inpatient dermatology care [published online ahead of print September 16, 2015]. J Telemed Telecare. PII: 1357633X15603298.
Access to care is a known issue in dermatology, and many patients may experience long waiting periods to see a physician.1 Previous research has evaluated access to outpatient dermatology services, but access to dermatology in inpatient medicine is also a growing problem.2 Reports depict a decrease in dermatologist involvement in inpatient care and an increase in nondermatologist physicians caring for inpatients with dermatologic needs.2,3 This lack of access could potentially lead to missed and/or incorrect diagnoses. One study showed that most cases in which dermatology was consulted required a change in treatment once correctly diagnosed by a dermatologist.4
Despite the known trend of decreasing involvement of dermatologists in inpatient care, there remains a paucity of data quantifying the current gap in access to care for inpatients with dermatologic needs. The purpose of this study was to evaluate differential access to inpatient dermatology services across licensed hospitals within the state of Pennsylvania.
Methods
In July 2014, an invitation to participate in an anonymous online survey was mailed to all 274 hospitals throughout Pennsylvania that were currently licensed by the US Department of Health. This study was declared exempt from review by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board. Study data were collected and managed using electronic data capture tools hosted by the University of Pennsylvania. Hospital administrators were encouraged to report dermatology access and details regardless of current status of inpatient dermatology services in order to inform efforts to improve access to care. Invitation letters to participate in the online survey were addressed to “Administrator” according to the contact method used by the US Department of Health for accreditation of state hospitals. Addresses for accredited state hospitals were obtained from the US Department of Health Web site and were supplemented with additional addresses of Veterans Administration hospitals obtained from public listings. Three weeks after initial survey invitations were sent, reminder letters were sent to nonresponsive hospitals. Only data from hospitals currently offering inpatient services were included in the analysis; exclusion criteria included psychiatric hospitals, substance abuse treatment centers, physical rehabilitation facilities, and outpatient centers.
Results
Of the 204 (74%) hospitals that met the inclusion criteria, 32 responded (16% response rate). Of the 32 hospitals that responded, 31 (97%) were privately owned facilities, 3 of which were specialty surgical centers. One (3%) hospital was a Veterans Administration hospital. Of the responders, 16 (50%) reported having any form of access to inpatient dermatology consultations. Of the 16 with reported access, 9 (56%) received their consultations through a local or private dermatology group, while 4 (25%) had a dermatologist on staff. The remaining 3 hospitals (19%) provided dermatology consultations through nondermatologist physicians on staff (a surgeon, an emergency care physician, and an internist, respectively).
The survey also sought to gain information about the various degrees of access to inpatient dermatology care that hospitals provide. Of the 16 hospitals that reported access to inpatient dermatology services, 11 (69%) provided specific details related to access (eg, coverage, anticipated response times) of dermatology consultations (Figure). The type of access to inpatient dermatology in relation to the type of hospital ownership is shown in the Table.


Comment
The survey results indicated suboptimal access to inpatient dermatology services in Pennsylvania hospitals. Only 50% (16/32) of respondents reported providing access to dermatology consultation, the majority of which appeared to have extremely limited same-day, evening, and weekend coverage. Although our study was limited by a low response rate (16%) and represents a narrow geographic distribution, these results suggested that lack of access to inpatient dermatology consultation may be a widespread problem and may be independent of the type of hospital ownership. Furthermore, the results of this study may offer insight into the different types and availability of inpatient dermatology services offered in hospitals across the United States.
The decrease in inpatient dermatology access has been driven by many factors. First, advances in medical research and pharmacotherapy may have decreased the need for dermatologic inpatient care, as patients who formerly would have required inpatient treatments are now able to receive therapies in an outpatient setting (eg, treatment of psoriasis).5 This may create less demand for hospitals to have a dermatologist on staff. Additionally, hospitals may be less able to incentivize dermatologists to provide inpatient dermatology consultations due to low reimbursement rates, time and distance required to visit inpatient facilities (taking away from outpatient clinic time), and the perception that inpatient cases carry greater liability given their greater complexity.6-8 Together, these factors may have contributed to the current lack of inpatient dermatology services in Pennsylvania hospitals and likely in hospitals throughout the United States.
Conclusion
Although a relatively small number of academic hospitals are experiencing an emergence of dermatology hospitalists, poor access to inpatient dermatology care continues to be a problem.8 Innovation (eg, the use of teledermatology to improve access to care9) and further studies are needed to address this gap in access to inpatient dermatology care.
Access to care is a known issue in dermatology, and many patients may experience long waiting periods to see a physician.1 Previous research has evaluated access to outpatient dermatology services, but access to dermatology in inpatient medicine is also a growing problem.2 Reports depict a decrease in dermatologist involvement in inpatient care and an increase in nondermatologist physicians caring for inpatients with dermatologic needs.2,3 This lack of access could potentially lead to missed and/or incorrect diagnoses. One study showed that most cases in which dermatology was consulted required a change in treatment once correctly diagnosed by a dermatologist.4
Despite the known trend of decreasing involvement of dermatologists in inpatient care, there remains a paucity of data quantifying the current gap in access to care for inpatients with dermatologic needs. The purpose of this study was to evaluate differential access to inpatient dermatology services across licensed hospitals within the state of Pennsylvania.
Methods
In July 2014, an invitation to participate in an anonymous online survey was mailed to all 274 hospitals throughout Pennsylvania that were currently licensed by the US Department of Health. This study was declared exempt from review by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board. Study data were collected and managed using electronic data capture tools hosted by the University of Pennsylvania. Hospital administrators were encouraged to report dermatology access and details regardless of current status of inpatient dermatology services in order to inform efforts to improve access to care. Invitation letters to participate in the online survey were addressed to “Administrator” according to the contact method used by the US Department of Health for accreditation of state hospitals. Addresses for accredited state hospitals were obtained from the US Department of Health Web site and were supplemented with additional addresses of Veterans Administration hospitals obtained from public listings. Three weeks after initial survey invitations were sent, reminder letters were sent to nonresponsive hospitals. Only data from hospitals currently offering inpatient services were included in the analysis; exclusion criteria included psychiatric hospitals, substance abuse treatment centers, physical rehabilitation facilities, and outpatient centers.
Results
Of the 204 (74%) hospitals that met the inclusion criteria, 32 responded (16% response rate). Of the 32 hospitals that responded, 31 (97%) were privately owned facilities, 3 of which were specialty surgical centers. One (3%) hospital was a Veterans Administration hospital. Of the responders, 16 (50%) reported having any form of access to inpatient dermatology consultations. Of the 16 with reported access, 9 (56%) received their consultations through a local or private dermatology group, while 4 (25%) had a dermatologist on staff. The remaining 3 hospitals (19%) provided dermatology consultations through nondermatologist physicians on staff (a surgeon, an emergency care physician, and an internist, respectively).
The survey also sought to gain information about the various degrees of access to inpatient dermatology care that hospitals provide. Of the 16 hospitals that reported access to inpatient dermatology services, 11 (69%) provided specific details related to access (eg, coverage, anticipated response times) of dermatology consultations (Figure). The type of access to inpatient dermatology in relation to the type of hospital ownership is shown in the Table.


Comment
The survey results indicated suboptimal access to inpatient dermatology services in Pennsylvania hospitals. Only 50% (16/32) of respondents reported providing access to dermatology consultation, the majority of which appeared to have extremely limited same-day, evening, and weekend coverage. Although our study was limited by a low response rate (16%) and represents a narrow geographic distribution, these results suggested that lack of access to inpatient dermatology consultation may be a widespread problem and may be independent of the type of hospital ownership. Furthermore, the results of this study may offer insight into the different types and availability of inpatient dermatology services offered in hospitals across the United States.
The decrease in inpatient dermatology access has been driven by many factors. First, advances in medical research and pharmacotherapy may have decreased the need for dermatologic inpatient care, as patients who formerly would have required inpatient treatments are now able to receive therapies in an outpatient setting (eg, treatment of psoriasis).5 This may create less demand for hospitals to have a dermatologist on staff. Additionally, hospitals may be less able to incentivize dermatologists to provide inpatient dermatology consultations due to low reimbursement rates, time and distance required to visit inpatient facilities (taking away from outpatient clinic time), and the perception that inpatient cases carry greater liability given their greater complexity.6-8 Together, these factors may have contributed to the current lack of inpatient dermatology services in Pennsylvania hospitals and likely in hospitals throughout the United States.
Conclusion
Although a relatively small number of academic hospitals are experiencing an emergence of dermatology hospitalists, poor access to inpatient dermatology care continues to be a problem.8 Innovation (eg, the use of teledermatology to improve access to care9) and further studies are needed to address this gap in access to inpatient dermatology care.
- Kimball AB, Resneck JS. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Helms AE, Helms SE, Brodell RT. Hospital consultations: time to address an unmet need? J Am Acad Dermatol. 2009;60:308-311.
- Kirsner RS, Yang DG, Kerdel FA. The changing status of inpatient dermatology at American academic dermatology programs. J Am Acad Dermatol. 1999;40:755-757.
- Nahass GT, Meyer AJ, Campbell SF, et al. Prevalence of cutaneous findings in hospitalized medical patients. J Am Acad Dermatol. 1995;33:207-211.
- Steinke S, Peitsch WK, Ludwig A, et al. Cost-of-illness in psoriasis: comparing inpatient and outpatient therapy. PLoS One. 2013;8:e78152.
- Swerlick RA. Declining interest in medical dermatology. Arch Dermatol. 1998;134:1160-1162.
- Kirsner RS, Yang DG, Kerdel FA. Inpatient dermatology: the difficulties, the reality, and the future. Dermatol Clin. 2000;18:383-390.
- Fox LP, Cotliar J, Hughey L, et al. Hospitalist dermatology. J Am Acad Dermatol. 2009;61:153-154.
- Sharma P, Kovarik CL, Lipoff JB. Teledermatology as a means to improve access to inpatient dermatology care [published online ahead of print September 16, 2015]. J Telemed Telecare. PII: 1357633X15603298.
- Kimball AB, Resneck JS. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Helms AE, Helms SE, Brodell RT. Hospital consultations: time to address an unmet need? J Am Acad Dermatol. 2009;60:308-311.
- Kirsner RS, Yang DG, Kerdel FA. The changing status of inpatient dermatology at American academic dermatology programs. J Am Acad Dermatol. 1999;40:755-757.
- Nahass GT, Meyer AJ, Campbell SF, et al. Prevalence of cutaneous findings in hospitalized medical patients. J Am Acad Dermatol. 1995;33:207-211.
- Steinke S, Peitsch WK, Ludwig A, et al. Cost-of-illness in psoriasis: comparing inpatient and outpatient therapy. PLoS One. 2013;8:e78152.
- Swerlick RA. Declining interest in medical dermatology. Arch Dermatol. 1998;134:1160-1162.
- Kirsner RS, Yang DG, Kerdel FA. Inpatient dermatology: the difficulties, the reality, and the future. Dermatol Clin. 2000;18:383-390.
- Fox LP, Cotliar J, Hughey L, et al. Hospitalist dermatology. J Am Acad Dermatol. 2009;61:153-154.
- Sharma P, Kovarik CL, Lipoff JB. Teledermatology as a means to improve access to inpatient dermatology care [published online ahead of print September 16, 2015]. J Telemed Telecare. PII: 1357633X15603298.
Practice Points
- Changes in inpatient dermatology care over the past few decades have led to barriers in patient access to care.
- Many hospitals currently lack access to inpatient dermatology care, and those that do provide access often have no same-day, evening, or weekend coverage or may only provide access to dermatology care via nondermatologist physicians.
- Intervention by a dermatologist may be essential in making correct dermatologic diagnoses and treatment recommendations in inpatient settings.
