User login
The Diagnosis: Pigmented Dermatofibrosarcoma Protuberans
Pigmented dermatofibrosarcoma protuberans (PDFSP), also known as Bednar tumor, is an uncommon variant of dermatofibrosarcoma protuberans (DFSP). Pigmented dermatofibrosarcoma protuberans constitutes 1% to 5% of all DFSP cases and most commonly is seen in nonwhite adults in the fourth decade of life, with occasional cases seen in pediatric patients, including some congenital cases. Typical sites of involvement include the shoulders, trunk, arms, legs, head, and neck.1,2 It also has been reported at sites of prior immunization, trauma, and insect bites.3
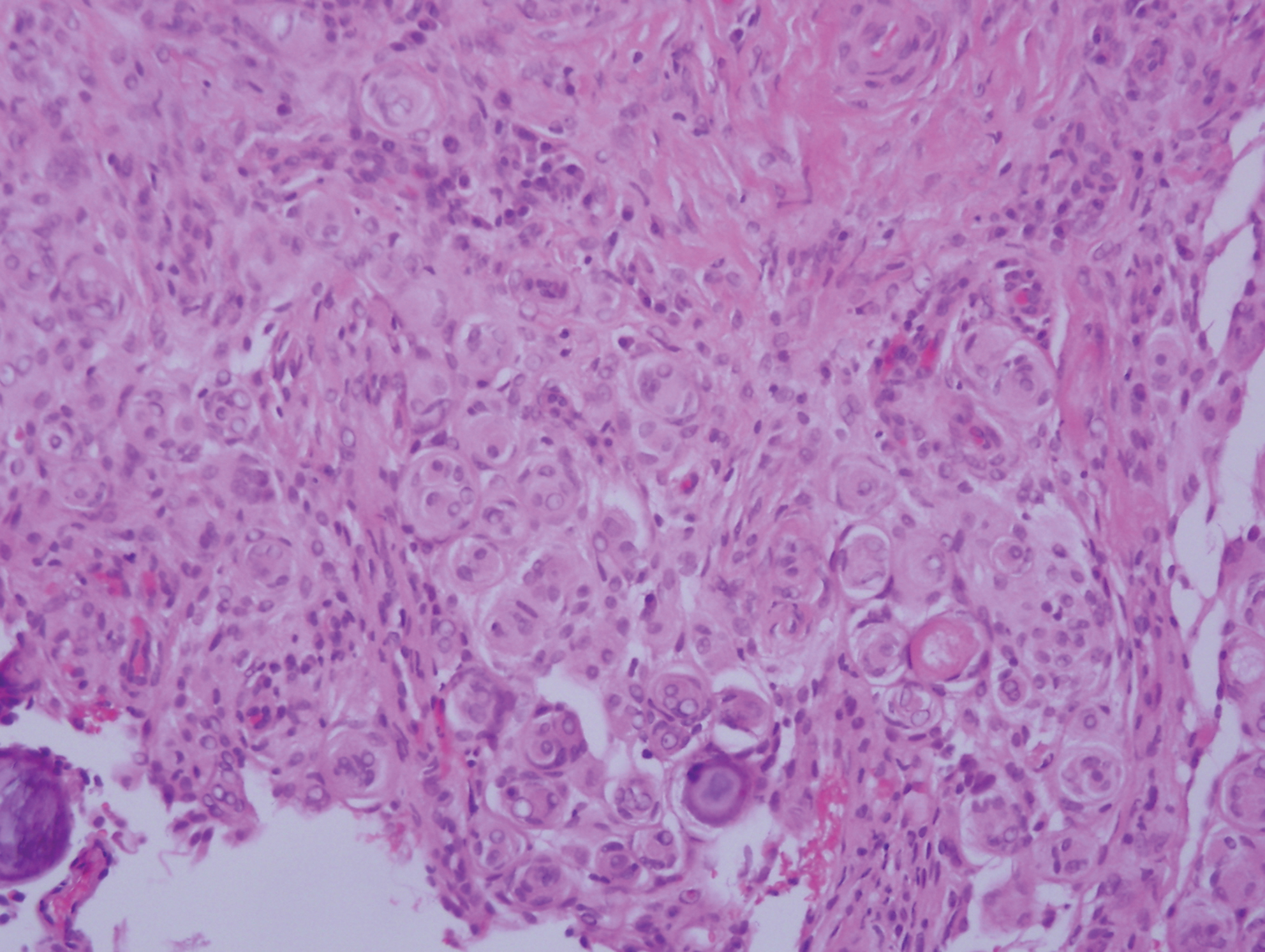
Histopathologic examination of our patient's shoulder nodule revealed an infiltrative neoplasm in the dermis and subcutaneous tissue composed of spindled cells with a storiform pattern and foci of scattered elongated dendritic pigmented cells. A narrow grenz zone separated the tumor from the epidermis, and characteristic honeycomb infiltration by tumor cells was noted in the subcutaneous fat. The nuclei were bland and monomorphous with areas of neuroid differentiation containing whorls and nerve cord-like structures (quiz image). The tumor cells were diffusely CD34 and vimentin positive, while S-100, SOX-10, neurofilament, smooth muscle actin, desmin, epithelial membrane antigen, and cytokeratins were negative. The immunophenotype excluded the possibility of neurogenic, pericytic, myofibroblastic, and myoid differentiation.
Wang and Yang4 previously reported a case of PDFSP with prominent meningothelial-like whorls focally resembling extracranial meningioma; however, the tumor cells were CD34 positive and epithelial membrane antigen negative, weighing against a diagnosis of meningioma. Most cases of PDFSP demonstrate the COL1A1-PDGFB (collagen type I α; 1/platelet-derived growth factor B-chain) fusion protein caused by the translocation t(17;22)(q22;q13), as in classic DFSP.5
Cellular blue nevus (CBN) is a benign melanocytic neoplasm that can present at any age and often occurs on the buttocks and in the sacrococcygeal region. Clinically, CBN presents as a firm, bluish black to bluish gray, dome-shaped nodule. The size varies from a few millimeters to several centimeters.6,7 Histologically, CBN is located completely in the dermis, extending along the adnexae into the subcutaneous tissue with a dumbbell-shaped outline (Figure 1).6-8 The tumor demonstrates oval epithelioid melanocytes with vesicular nuclei and prominent nucleoli. Immunohistochemically, tumor cells stain positively for melanocytic markers such as S-100, SOX-10, MART-1, and human melanoma black 45. CD34 expression rarely is reported in a subset of CBN.9
Pigmented neurofibroma is a rare variant of neurofibroma that produces melanin pigment and has a strong association with neurofibromatosis.10 It occurs most frequently in dark-skinned populations (Fitzpatrick skin types IV-VI). The most common location is the head and neck region.11,12 Histologically, pigmented neurofibroma resembles a diffuse neurofibroma admixed with melanin-producing cells (Figure 2).12 Immunostaining shows positivity for S-100 in both pigmented and Schwann cells; however, the pigmented cells stain positively for human melanoma black 45, Melan-A, and tyrosinase.10 CD34 can be fingerprint positive in neurofibroma, but a distinction from DFSP can be made by S-100 and SOX-10 immunostaining.13
Desmoplastic melanoma (DM) is an uncommon variant of malignant melanoma and has a higher tendency for persistent local growth and less frequent metastases than other variants of melanoma. It has a predilection for chronically sun-exposed areas such as the head and neck and occurs later in life. Clinically, DM appears as nonspecific, often amelanotic nodules or plaques or as scarlike lesions.14 Histologically, DM can be classified as mixed or pure based on the degree of desmoplasia and cellularity. A paucicellular proliferation of malignant spindled melanocytes within a densely fibrotic stroma with lymphoid nodules in the dermis is characteristic (Figure 3); perineural involvement is common.14,15 The most reliable confirmative stains are S-100 and SOX-10.16
Cutaneous meningioma is a rare tumor and could be subtyped into 3 groups. Type I is primary cutaneous meningioma and usually is present at birth on the scalp and paravertebral regions with a relatively good prognosis. Type II is ectopic soft-tissue meningioma that extends into the skin from around the sensory organs on the face. Type III is local invasion or true metastasis from a central nervous system meningioma. Types II and III develop later in life and the prognosis is poor.17,18 Clinically, lesions present as firm subcutaneous nodules or swellings. Cutaneous meningioma has several histopathologic variants. The classic presentation reveals concentric wrapping of tumor cells with round-oval nuclei containing delicate chromatin. Psammoma bodies are a common finding (Figure 4). Immunohistochemically, tumor cells are diffusely positive for epithelial membrane antigen and vimentin.18,19
- Amonkar GP, Rupani A, Shah A, et al. Bednar tumor: an uncommon entity. Dermatopathology (Basel). 2016;3:36-38.
- El Hachem M, Diociaiuti A, Latella E, et al. Congenital myxoid and pigmented dermatofibrosarcoma protuberans: a case report. Pediatr Dermatol. 2013;30:E74-E77.
- Anon-Requena MJ, Pico-Valimana M, Munoz-Arias G. Bednar tumor (pigmented dermatofibrosarcoma protuberans). Actas Dermosifiliogr. 2016;107:618-620.
- Wang J, Yang W. Pigmented dermatofibrosarcoma protuberans with prominent meningothelial-like whorls. J Cutan Pathol. 2008;35(suppl 1):65-69.
- Zardawi IM, Kattampallil J, Rode J. An unusual pigmented skin tumour. Bednar tumour, dorsum of left foot (pigmented dermatofibrosarcoma protuberans). Pathology. 2004;36:358-361.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and "malignant blue nevus": a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2017;37:401-415.
- Zembowicz A, Granter SR, McKee PH, et al. Amelanotic cellular blue nevus: a hypopigmented variant of the cellular blue nevus: clinicopathologic analysis of 20 cases. Am J Surg Pathol. 2002;26:1493-1500.
- Smith K, Germain M, Williams J, et al. CD34-positive cellular blue nevi. J Cutan Pathol. 2001;28:145-150.
- Inaba M, Yamamoto T, Minami R, et al. Pigmented neurofibroma: report of two cases and literature review. Pathol Int. 2001;51:565-569.
- Fetsch JF, Michal M, Miettinen M. Pigmented (melanotic) neurofibroma: a clinicopathologic and immunohistochemical analysis of 19 lesions from 17 patients. Am J Surg Pathol. 2000;24:331-343.
- Motoi T, Ishida T, Kawato A, et al. Pigmented neurofibroma: review of Japanese patients with an analysis of melanogenesis demonstrating coexpression of c-met protooncogene and microphthalmia-associated transcription factor. Hum Pathol. 2005;36:871-877.
- Yeh I, McCalmont TH. Distinguishing neurofibroma from desmoplastic melanoma: the value of the CD34 fingerprint. J Cutan Pathol. 2011;38:625-630.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- Busam KJ. Desmoplastic melanoma. Clin Lab Med. 2011;31:321-330.
- Schleich C, Ferringer T. Desmoplastic melanoma. Cutis. 2015;96:306, 313-314, 335.
- Lopez DA, Silvers DN, Helwig EB. Cutaneous meningiomas--a clinicopathologic study. Cancer. 1974;34:728-744.
- Miedema JR, Zedek D. Cutaneous meningioma. Arch Pathol Lab Med. 2012;136:208-211.
- Bhanusali DG, Heath C, Gur D, et al. Metastatic meningioma of the scalp. Cutis. 2018;101:386-389.
The Diagnosis: Pigmented Dermatofibrosarcoma Protuberans
Pigmented dermatofibrosarcoma protuberans (PDFSP), also known as Bednar tumor, is an uncommon variant of dermatofibrosarcoma protuberans (DFSP). Pigmented dermatofibrosarcoma protuberans constitutes 1% to 5% of all DFSP cases and most commonly is seen in nonwhite adults in the fourth decade of life, with occasional cases seen in pediatric patients, including some congenital cases. Typical sites of involvement include the shoulders, trunk, arms, legs, head, and neck.1,2 It also has been reported at sites of prior immunization, trauma, and insect bites.3
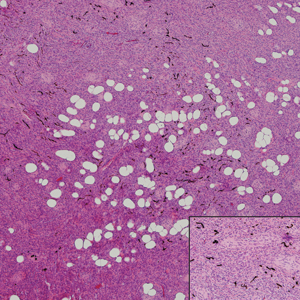
Histopathologic examination of our patient's shoulder nodule revealed an infiltrative neoplasm in the dermis and subcutaneous tissue composed of spindled cells with a storiform pattern and foci of scattered elongated dendritic pigmented cells. A narrow grenz zone separated the tumor from the epidermis, and characteristic honeycomb infiltration by tumor cells was noted in the subcutaneous fat. The nuclei were bland and monomorphous with areas of neuroid differentiation containing whorls and nerve cord-like structures (quiz image). The tumor cells were diffusely CD34 and vimentin positive, while S-100, SOX-10, neurofilament, smooth muscle actin, desmin, epithelial membrane antigen, and cytokeratins were negative. The immunophenotype excluded the possibility of neurogenic, pericytic, myofibroblastic, and myoid differentiation.
Wang and Yang4 previously reported a case of PDFSP with prominent meningothelial-like whorls focally resembling extracranial meningioma; however, the tumor cells were CD34 positive and epithelial membrane antigen negative, weighing against a diagnosis of meningioma. Most cases of PDFSP demonstrate the COL1A1-PDGFB (collagen type I α; 1/platelet-derived growth factor B-chain) fusion protein caused by the translocation t(17;22)(q22;q13), as in classic DFSP.5
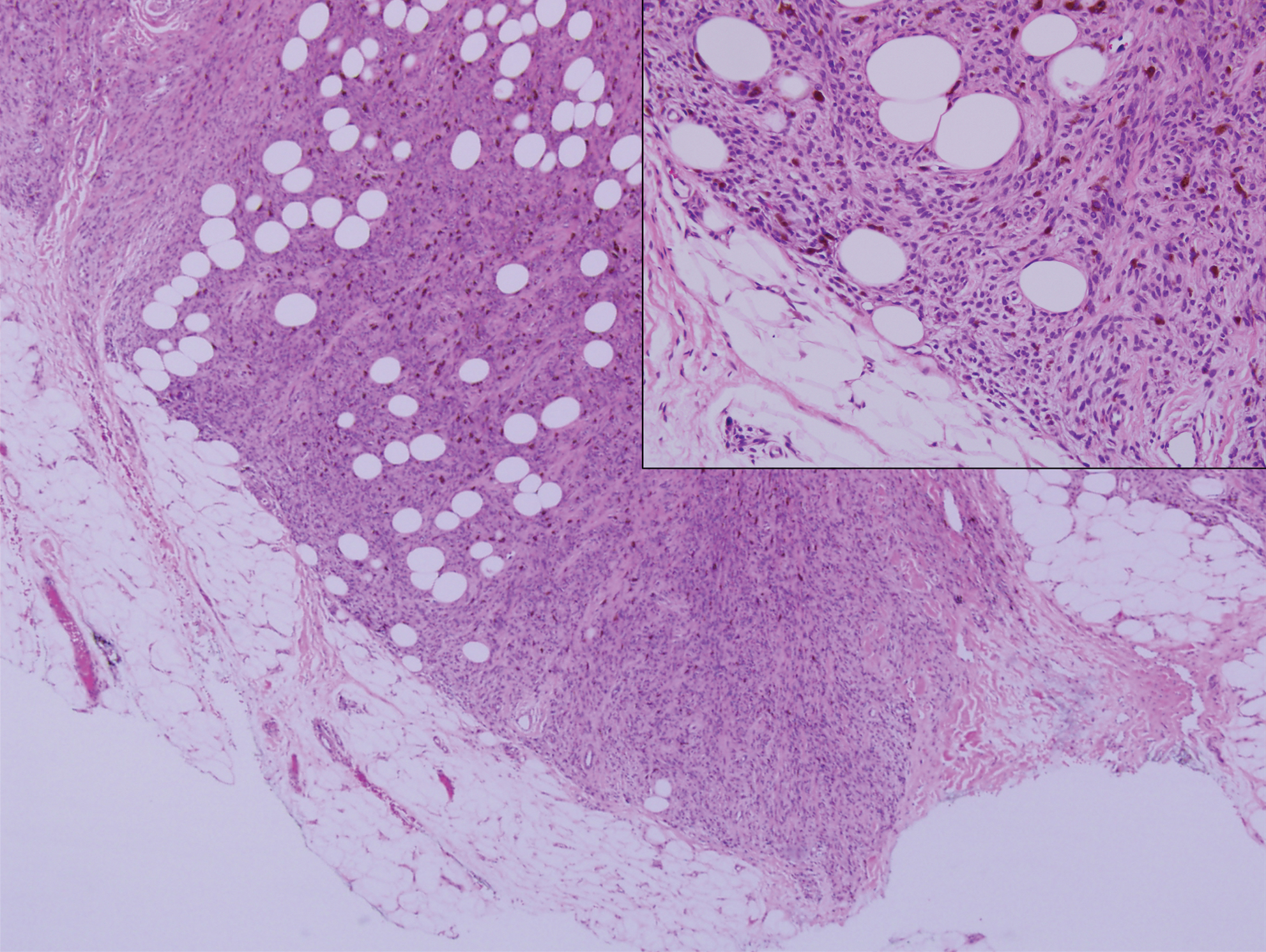
Cellular blue nevus (CBN) is a benign melanocytic neoplasm that can present at any age and often occurs on the buttocks and in the sacrococcygeal region. Clinically, CBN presents as a firm, bluish black to bluish gray, dome-shaped nodule. The size varies from a few millimeters to several centimeters.6,7 Histologically, CBN is located completely in the dermis, extending along the adnexae into the subcutaneous tissue with a dumbbell-shaped outline (Figure 1).6-8 The tumor demonstrates oval epithelioid melanocytes with vesicular nuclei and prominent nucleoli. Immunohistochemically, tumor cells stain positively for melanocytic markers such as S-100, SOX-10, MART-1, and human melanoma black 45. CD34 expression rarely is reported in a subset of CBN.9

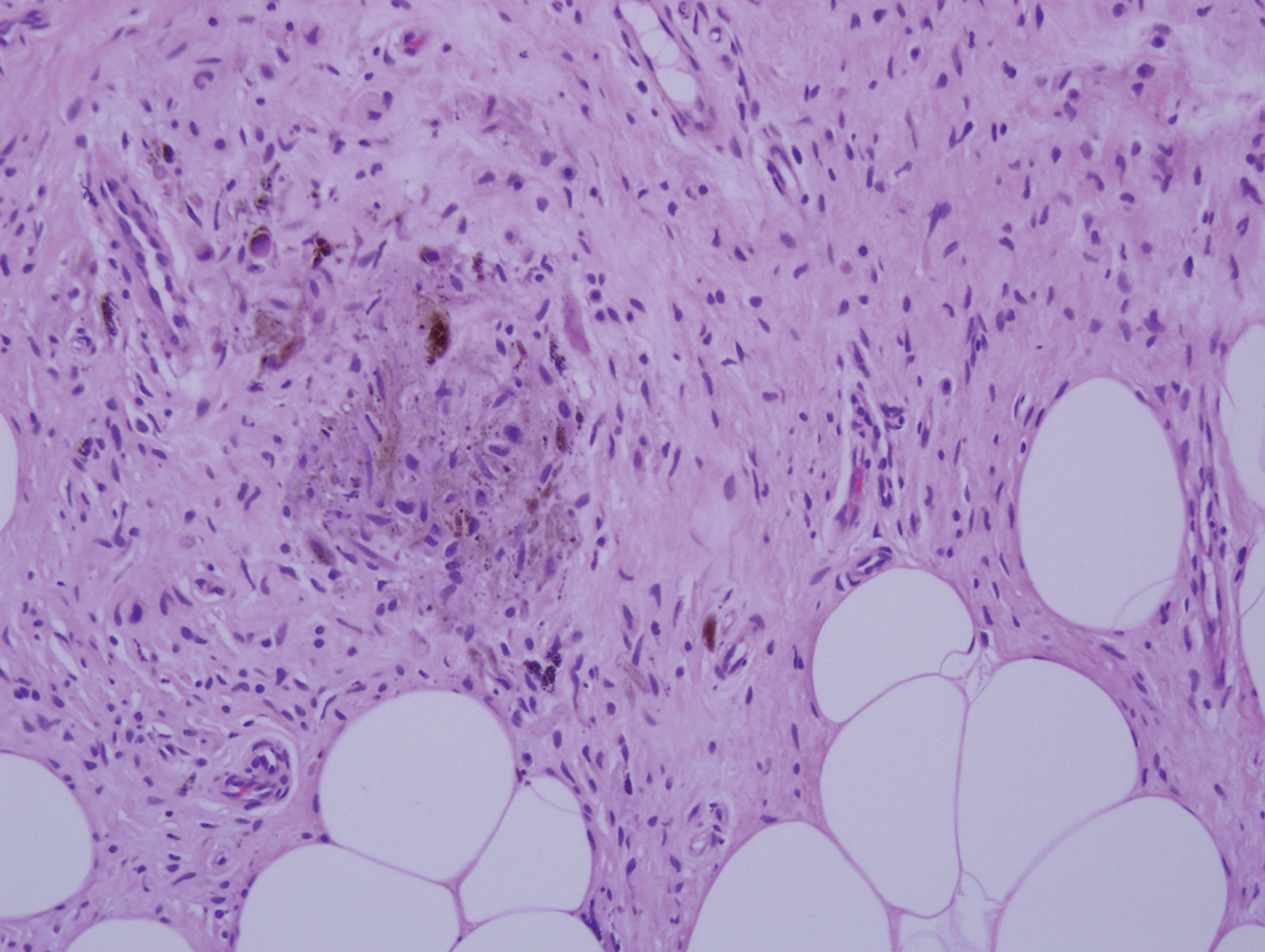
Pigmented neurofibroma is a rare variant of neurofibroma that produces melanin pigment and has a strong association with neurofibromatosis.10 It occurs most frequently in dark-skinned populations (Fitzpatrick skin types IV-VI). The most common location is the head and neck region.11,12 Histologically, pigmented neurofibroma resembles a diffuse neurofibroma admixed with melanin-producing cells (Figure 2).12 Immunostaining shows positivity for S-100 in both pigmented and Schwann cells; however, the pigmented cells stain positively for human melanoma black 45, Melan-A, and tyrosinase.10 CD34 can be fingerprint positive in neurofibroma, but a distinction from DFSP can be made by S-100 and SOX-10 immunostaining.13

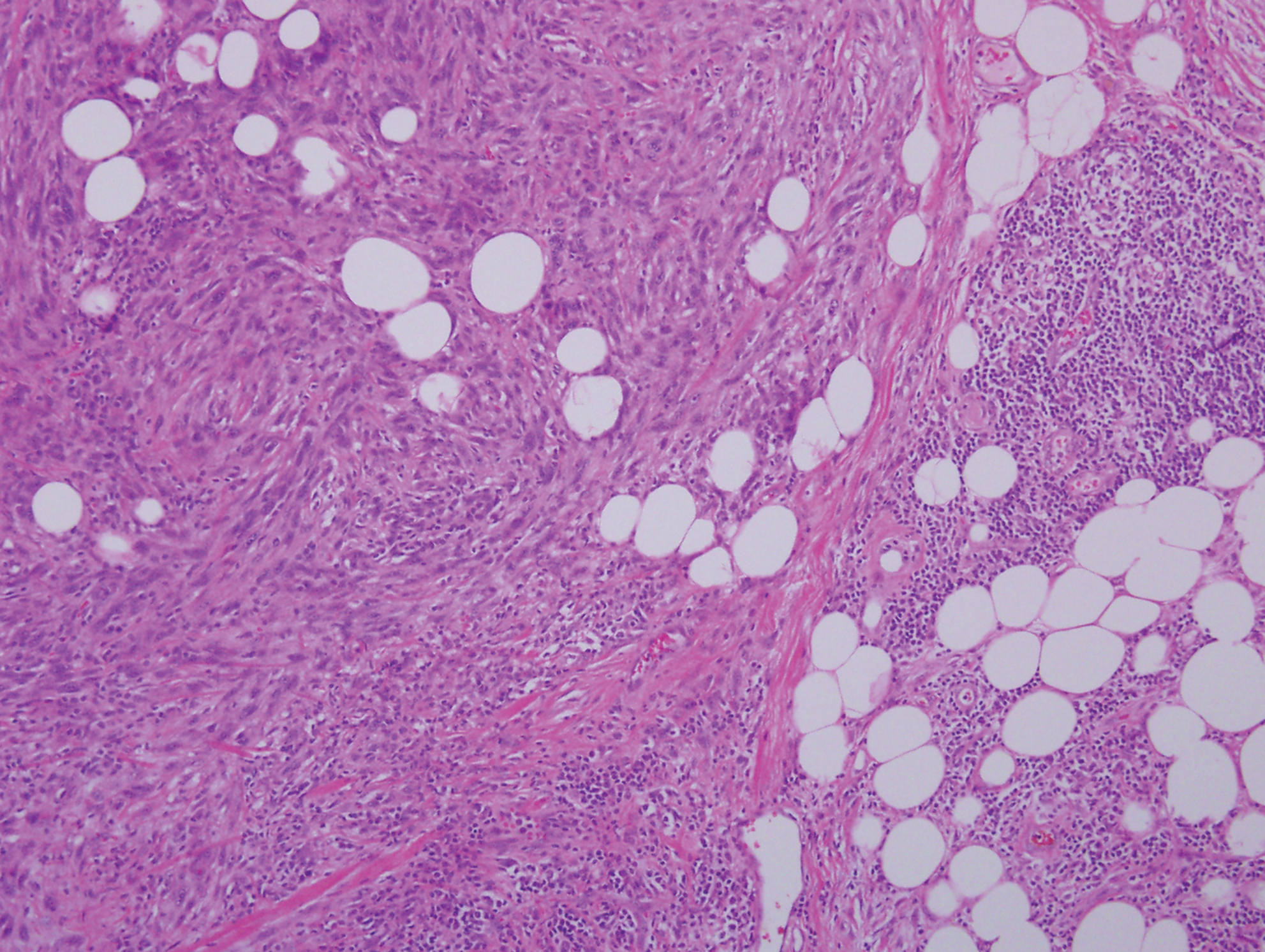
Desmoplastic melanoma (DM) is an uncommon variant of malignant melanoma and has a higher tendency for persistent local growth and less frequent metastases than other variants of melanoma. It has a predilection for chronically sun-exposed areas such as the head and neck and occurs later in life. Clinically, DM appears as nonspecific, often amelanotic nodules or plaques or as scarlike lesions.14 Histologically, DM can be classified as mixed or pure based on the degree of desmoplasia and cellularity. A paucicellular proliferation of malignant spindled melanocytes within a densely fibrotic stroma with lymphoid nodules in the dermis is characteristic (Figure 3); perineural involvement is common.14,15 The most reliable confirmative stains are S-100 and SOX-10.16

Cutaneous meningioma is a rare tumor and could be subtyped into 3 groups. Type I is primary cutaneous meningioma and usually is present at birth on the scalp and paravertebral regions with a relatively good prognosis. Type II is ectopic soft-tissue meningioma that extends into the skin from around the sensory organs on the face. Type III is local invasion or true metastasis from a central nervous system meningioma. Types II and III develop later in life and the prognosis is poor.17,18 Clinically, lesions present as firm subcutaneous nodules or swellings. Cutaneous meningioma has several histopathologic variants. The classic presentation reveals concentric wrapping of tumor cells with round-oval nuclei containing delicate chromatin. Psammoma bodies are a common finding (Figure 4). Immunohistochemically, tumor cells are diffusely positive for epithelial membrane antigen and vimentin.18,19

The Diagnosis: Pigmented Dermatofibrosarcoma Protuberans
Pigmented dermatofibrosarcoma protuberans (PDFSP), also known as Bednar tumor, is an uncommon variant of dermatofibrosarcoma protuberans (DFSP). Pigmented dermatofibrosarcoma protuberans constitutes 1% to 5% of all DFSP cases and most commonly is seen in nonwhite adults in the fourth decade of life, with occasional cases seen in pediatric patients, including some congenital cases. Typical sites of involvement include the shoulders, trunk, arms, legs, head, and neck.1,2 It also has been reported at sites of prior immunization, trauma, and insect bites.3
Histopathologic examination of our patient's shoulder nodule revealed an infiltrative neoplasm in the dermis and subcutaneous tissue composed of spindled cells with a storiform pattern and foci of scattered elongated dendritic pigmented cells. A narrow grenz zone separated the tumor from the epidermis, and characteristic honeycomb infiltration by tumor cells was noted in the subcutaneous fat. The nuclei were bland and monomorphous with areas of neuroid differentiation containing whorls and nerve cord-like structures (quiz image). The tumor cells were diffusely CD34 and vimentin positive, while S-100, SOX-10, neurofilament, smooth muscle actin, desmin, epithelial membrane antigen, and cytokeratins were negative. The immunophenotype excluded the possibility of neurogenic, pericytic, myofibroblastic, and myoid differentiation.
Wang and Yang4 previously reported a case of PDFSP with prominent meningothelial-like whorls focally resembling extracranial meningioma; however, the tumor cells were CD34 positive and epithelial membrane antigen negative, weighing against a diagnosis of meningioma. Most cases of PDFSP demonstrate the COL1A1-PDGFB (collagen type I α; 1/platelet-derived growth factor B-chain) fusion protein caused by the translocation t(17;22)(q22;q13), as in classic DFSP.5
Cellular blue nevus (CBN) is a benign melanocytic neoplasm that can present at any age and often occurs on the buttocks and in the sacrococcygeal region. Clinically, CBN presents as a firm, bluish black to bluish gray, dome-shaped nodule. The size varies from a few millimeters to several centimeters.6,7 Histologically, CBN is located completely in the dermis, extending along the adnexae into the subcutaneous tissue with a dumbbell-shaped outline (Figure 1).6-8 The tumor demonstrates oval epithelioid melanocytes with vesicular nuclei and prominent nucleoli. Immunohistochemically, tumor cells stain positively for melanocytic markers such as S-100, SOX-10, MART-1, and human melanoma black 45. CD34 expression rarely is reported in a subset of CBN.9

Pigmented neurofibroma is a rare variant of neurofibroma that produces melanin pigment and has a strong association with neurofibromatosis.10 It occurs most frequently in dark-skinned populations (Fitzpatrick skin types IV-VI). The most common location is the head and neck region.11,12 Histologically, pigmented neurofibroma resembles a diffuse neurofibroma admixed with melanin-producing cells (Figure 2).12 Immunostaining shows positivity for S-100 in both pigmented and Schwann cells; however, the pigmented cells stain positively for human melanoma black 45, Melan-A, and tyrosinase.10 CD34 can be fingerprint positive in neurofibroma, but a distinction from DFSP can be made by S-100 and SOX-10 immunostaining.13

Desmoplastic melanoma (DM) is an uncommon variant of malignant melanoma and has a higher tendency for persistent local growth and less frequent metastases than other variants of melanoma. It has a predilection for chronically sun-exposed areas such as the head and neck and occurs later in life. Clinically, DM appears as nonspecific, often amelanotic nodules or plaques or as scarlike lesions.14 Histologically, DM can be classified as mixed or pure based on the degree of desmoplasia and cellularity. A paucicellular proliferation of malignant spindled melanocytes within a densely fibrotic stroma with lymphoid nodules in the dermis is characteristic (Figure 3); perineural involvement is common.14,15 The most reliable confirmative stains are S-100 and SOX-10.16

Cutaneous meningioma is a rare tumor and could be subtyped into 3 groups. Type I is primary cutaneous meningioma and usually is present at birth on the scalp and paravertebral regions with a relatively good prognosis. Type II is ectopic soft-tissue meningioma that extends into the skin from around the sensory organs on the face. Type III is local invasion or true metastasis from a central nervous system meningioma. Types II and III develop later in life and the prognosis is poor.17,18 Clinically, lesions present as firm subcutaneous nodules or swellings. Cutaneous meningioma has several histopathologic variants. The classic presentation reveals concentric wrapping of tumor cells with round-oval nuclei containing delicate chromatin. Psammoma bodies are a common finding (Figure 4). Immunohistochemically, tumor cells are diffusely positive for epithelial membrane antigen and vimentin.18,19

- Amonkar GP, Rupani A, Shah A, et al. Bednar tumor: an uncommon entity. Dermatopathology (Basel). 2016;3:36-38.
- El Hachem M, Diociaiuti A, Latella E, et al. Congenital myxoid and pigmented dermatofibrosarcoma protuberans: a case report. Pediatr Dermatol. 2013;30:E74-E77.
- Anon-Requena MJ, Pico-Valimana M, Munoz-Arias G. Bednar tumor (pigmented dermatofibrosarcoma protuberans). Actas Dermosifiliogr. 2016;107:618-620.
- Wang J, Yang W. Pigmented dermatofibrosarcoma protuberans with prominent meningothelial-like whorls. J Cutan Pathol. 2008;35(suppl 1):65-69.
- Zardawi IM, Kattampallil J, Rode J. An unusual pigmented skin tumour. Bednar tumour, dorsum of left foot (pigmented dermatofibrosarcoma protuberans). Pathology. 2004;36:358-361.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and "malignant blue nevus": a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2017;37:401-415.
- Zembowicz A, Granter SR, McKee PH, et al. Amelanotic cellular blue nevus: a hypopigmented variant of the cellular blue nevus: clinicopathologic analysis of 20 cases. Am J Surg Pathol. 2002;26:1493-1500.
- Smith K, Germain M, Williams J, et al. CD34-positive cellular blue nevi. J Cutan Pathol. 2001;28:145-150.
- Inaba M, Yamamoto T, Minami R, et al. Pigmented neurofibroma: report of two cases and literature review. Pathol Int. 2001;51:565-569.
- Fetsch JF, Michal M, Miettinen M. Pigmented (melanotic) neurofibroma: a clinicopathologic and immunohistochemical analysis of 19 lesions from 17 patients. Am J Surg Pathol. 2000;24:331-343.
- Motoi T, Ishida T, Kawato A, et al. Pigmented neurofibroma: review of Japanese patients with an analysis of melanogenesis demonstrating coexpression of c-met protooncogene and microphthalmia-associated transcription factor. Hum Pathol. 2005;36:871-877.
- Yeh I, McCalmont TH. Distinguishing neurofibroma from desmoplastic melanoma: the value of the CD34 fingerprint. J Cutan Pathol. 2011;38:625-630.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- Busam KJ. Desmoplastic melanoma. Clin Lab Med. 2011;31:321-330.
- Schleich C, Ferringer T. Desmoplastic melanoma. Cutis. 2015;96:306, 313-314, 335.
- Lopez DA, Silvers DN, Helwig EB. Cutaneous meningiomas--a clinicopathologic study. Cancer. 1974;34:728-744.
- Miedema JR, Zedek D. Cutaneous meningioma. Arch Pathol Lab Med. 2012;136:208-211.
- Bhanusali DG, Heath C, Gur D, et al. Metastatic meningioma of the scalp. Cutis. 2018;101:386-389.
- Amonkar GP, Rupani A, Shah A, et al. Bednar tumor: an uncommon entity. Dermatopathology (Basel). 2016;3:36-38.
- El Hachem M, Diociaiuti A, Latella E, et al. Congenital myxoid and pigmented dermatofibrosarcoma protuberans: a case report. Pediatr Dermatol. 2013;30:E74-E77.
- Anon-Requena MJ, Pico-Valimana M, Munoz-Arias G. Bednar tumor (pigmented dermatofibrosarcoma protuberans). Actas Dermosifiliogr. 2016;107:618-620.
- Wang J, Yang W. Pigmented dermatofibrosarcoma protuberans with prominent meningothelial-like whorls. J Cutan Pathol. 2008;35(suppl 1):65-69.
- Zardawi IM, Kattampallil J, Rode J. An unusual pigmented skin tumour. Bednar tumour, dorsum of left foot (pigmented dermatofibrosarcoma protuberans). Pathology. 2004;36:358-361.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and "malignant blue nevus": a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2017;37:401-415.
- Zembowicz A, Granter SR, McKee PH, et al. Amelanotic cellular blue nevus: a hypopigmented variant of the cellular blue nevus: clinicopathologic analysis of 20 cases. Am J Surg Pathol. 2002;26:1493-1500.
- Smith K, Germain M, Williams J, et al. CD34-positive cellular blue nevi. J Cutan Pathol. 2001;28:145-150.
- Inaba M, Yamamoto T, Minami R, et al. Pigmented neurofibroma: report of two cases and literature review. Pathol Int. 2001;51:565-569.
- Fetsch JF, Michal M, Miettinen M. Pigmented (melanotic) neurofibroma: a clinicopathologic and immunohistochemical analysis of 19 lesions from 17 patients. Am J Surg Pathol. 2000;24:331-343.
- Motoi T, Ishida T, Kawato A, et al. Pigmented neurofibroma: review of Japanese patients with an analysis of melanogenesis demonstrating coexpression of c-met protooncogene and microphthalmia-associated transcription factor. Hum Pathol. 2005;36:871-877.
- Yeh I, McCalmont TH. Distinguishing neurofibroma from desmoplastic melanoma: the value of the CD34 fingerprint. J Cutan Pathol. 2011;38:625-630.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- Busam KJ. Desmoplastic melanoma. Clin Lab Med. 2011;31:321-330.
- Schleich C, Ferringer T. Desmoplastic melanoma. Cutis. 2015;96:306, 313-314, 335.
- Lopez DA, Silvers DN, Helwig EB. Cutaneous meningiomas--a clinicopathologic study. Cancer. 1974;34:728-744.
- Miedema JR, Zedek D. Cutaneous meningioma. Arch Pathol Lab Med. 2012;136:208-211.
- Bhanusali DG, Heath C, Gur D, et al. Metastatic meningioma of the scalp. Cutis. 2018;101:386-389.

A 37-year-old woman presented with an asymptomatic, indurated, pigmented, subcutaneous nodule on the right shoulder of more than 3 years' duration. The lesion had gradually increased in size with no associated symptoms. The patient had a history of endometrial adenocarcinoma and papillary thyroid carcinoma, which had been treated by hysterectomy-oophorectomy and right thyroidectomy, respectively. She had no other notable systemic abnormalities, and there was no family history of genetic disease or cancer. Physical examination demonstrated a 1.2×1.8-cm nontender, pigmented, subcutaneous nodule with a rough surface and indistinct borders. An excisional biopsy was performed.