User login
The Diagnosis: Cutaneous Collagenous Vasculopathy
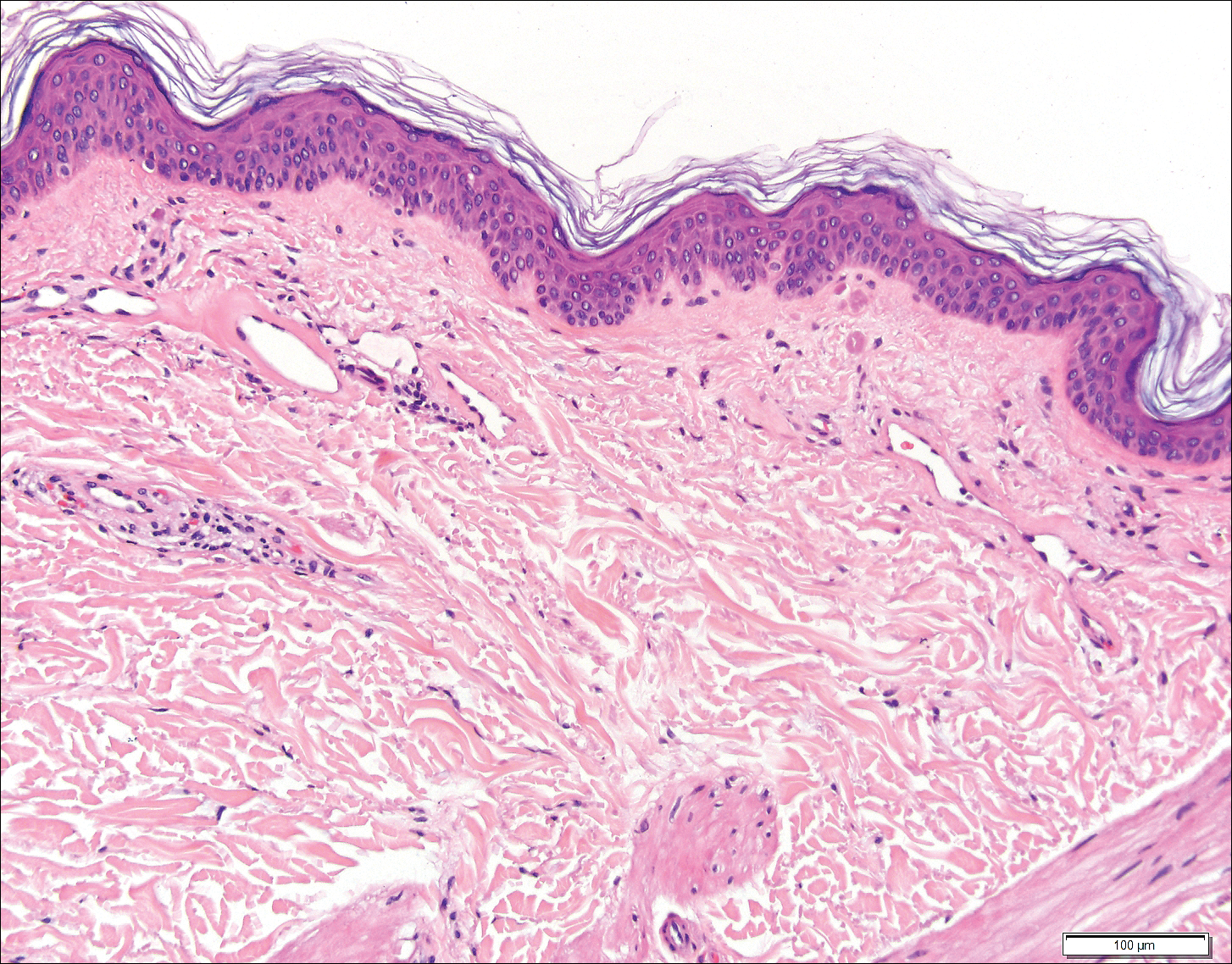
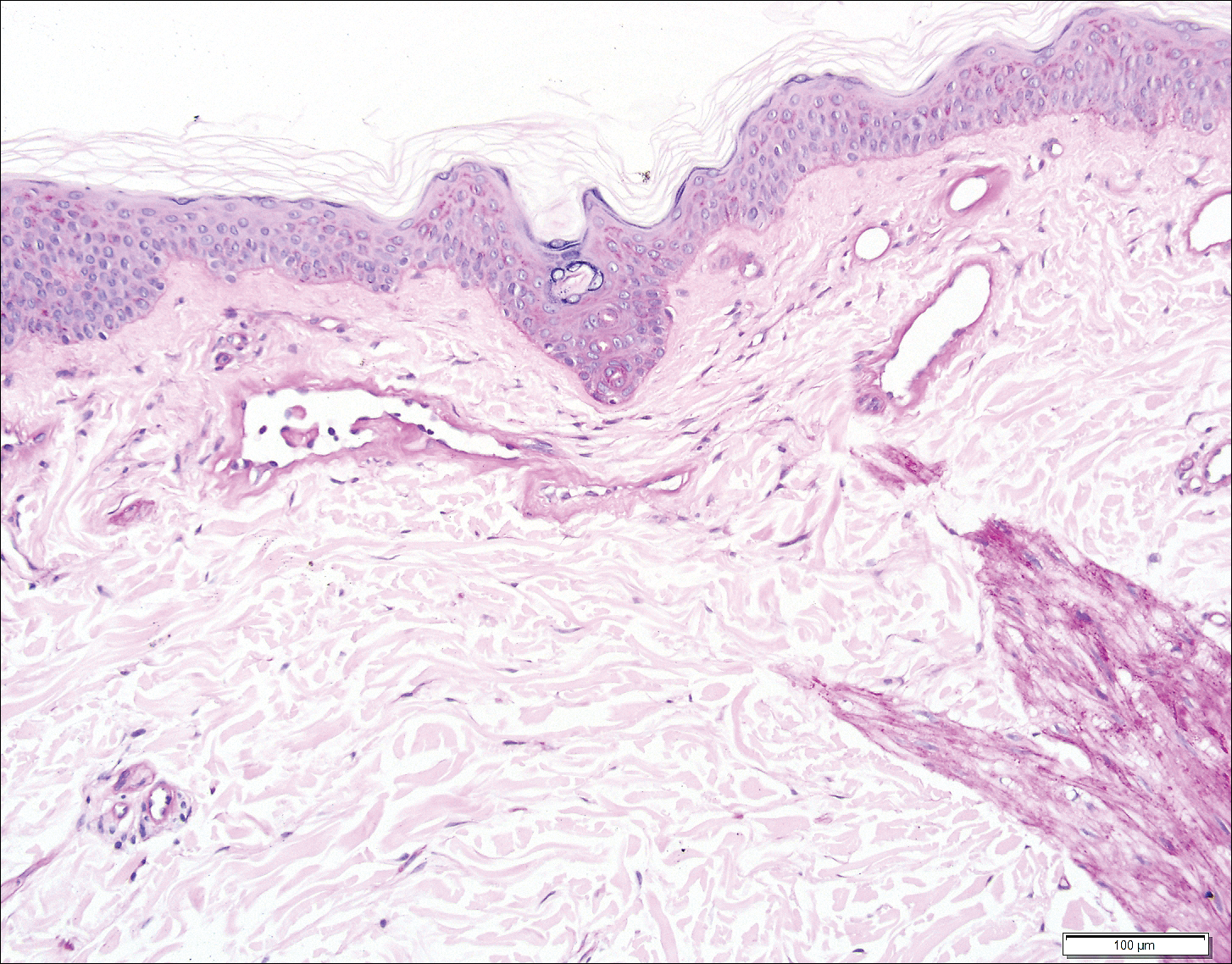
Histopathologic examination revealed ectatic blood vessels lined with unremarkable endothelial cells and thickened, hyalinized vessel walls scattered within the papillary dermis (Figure 1). The epidermis was unremarkable. There was minimal associated inflammation and no extravasation of erythrocytes. The hyalinized material was weakly positive on periodic acid-Schiff staining (Figure 2) and negative on Congo red staining, which supported of a diagnosis of cutaneous collagenous vasculopathy (CCV).
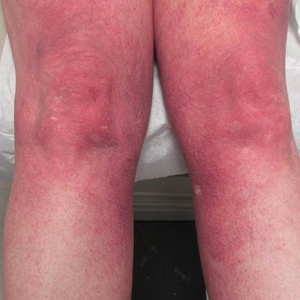
The patient previously had been given a suspected diagnosis of generalized essential telangiectasia by an outside dermatologist several years prior to the current presentation, as CCV had yet to be recognized as its own entity and therefore few cases had been described in the literature. She had a known history of obesity, hypertension, hyperlipidemia, and type 2 diabetes mellitus, which are associated with the condition. Multiple specialists concluded that the disease was too extensive for laser treatment. A review of PubMed articles indexed for MEDLINE yielded no established treatment options.
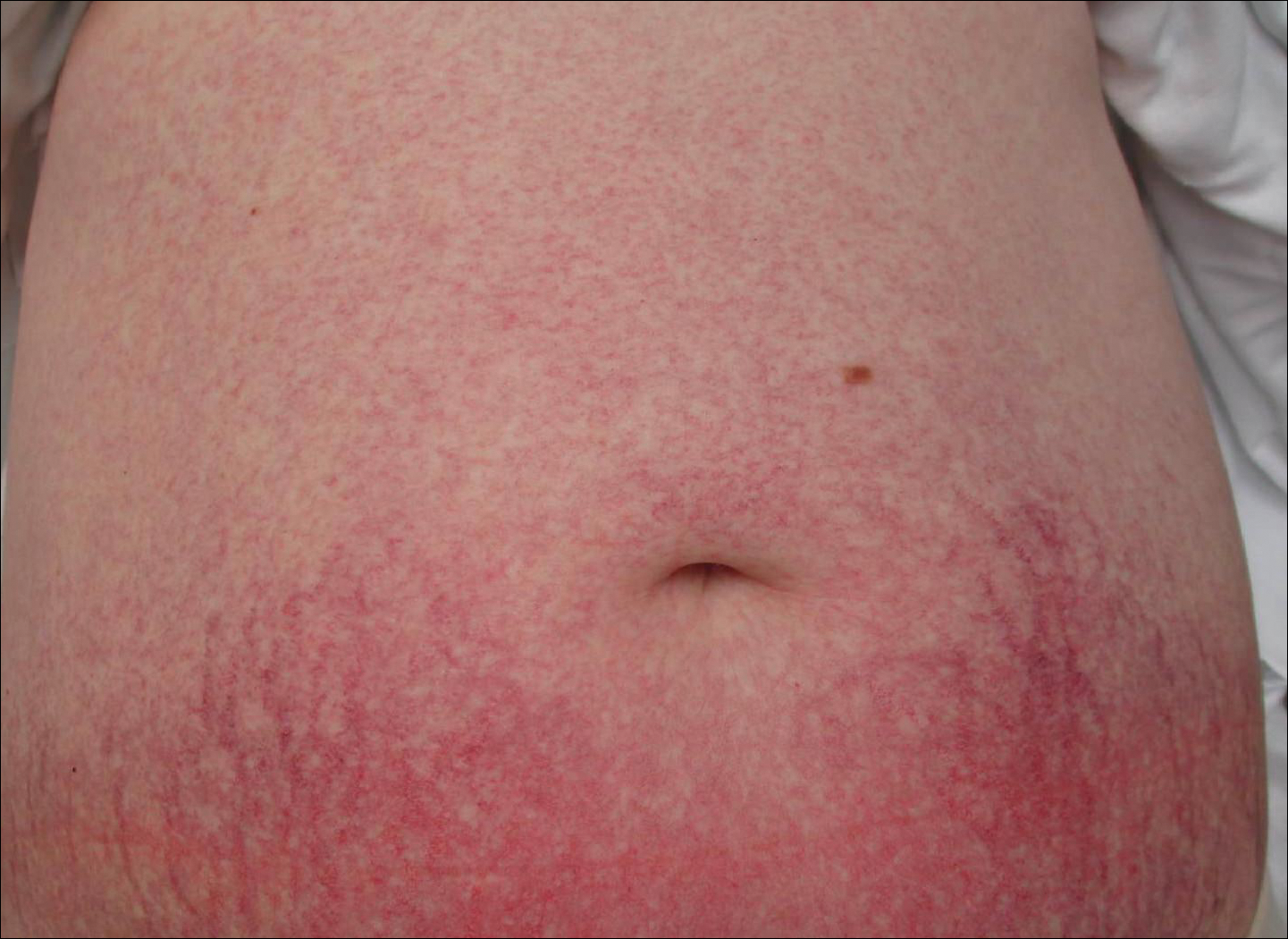
Cutaneous collagenous vasculopathy is a rare acquired microangiopathy involving the small vessels of the skin. Its clinical presentation is indistinguishable from that of generalized essential telangiectasia (GET). Patients generally present with asymptomatic, widespread, blanching, symmetric telangiectasias that classically begin on the legs and steadily progress upward with classic sparing of the face (Figure 3). Whereas GET has been reported to involve the oral and conjunctival mucosa, mucosal involvement is not typically observed in CCV and is considered to be a distinguishing factor between the 2 conditions.1,2 However, our patient reported oral symptoms, and oral erosions were seen on multiple physical examinations; therefore, ours is a rare case of mucosal involvement in conjunction with CCV. Given this finding, it is possible that more cases of CCV with mucosal involvement may exist but have been clinically misdiagnosed as GET.
First described by Salama and Rosenthal3 in 2000, CCV remains a rarely reported entity, with approximately 33 reported cases in the worldwide literature.2,4-7 The condition typically arises in adults with an equal predilection for males and females.2 The true incidence of CCV is unknown and likely is underreported given its close similarities to GET, which often is diagnosed clinically. The unique histopathologic finding of superficial ectatic vascular spaces with eosinophilic hyalinized vessel walls in CCV is key to distinguishing these similar entities, and even this finding can be subtle and is easily overlooked. Inflammation is sparse to absent. Deposited material is positive on periodic acid-Schiff and cytokeratin IV staining (representing reduplicated basement membrane-type collagen) and is diastase resistant. Smooth muscle actin staining is diminished or absent. Ultrastructural examination reveals reduplicated, laminated basement membrane; Luse bodies (abnormally long, widely spaced collagen fibers); and a decrease in or loss of pericytes. Of note, Luse bodies are nonspecific and their absence does not exclude a diagnosis of CCV.1
The etiology of CCV is unclear, and multiple pathogenetic mechanisms have been proposed. Ultimately, this entity is thought to arise from repeated endothelial cell damage, although the trigger for the endothelial cell injury is not completely understood. Diabetes mellitus sometimes is associated with microangiopathy and may be a confounding but not causative factor in some cases.1 Some investigators believe CCV is caused by a genetic defect that alters collagen production in the small vessels of the skin.5 Others have hypothesized that it is a secondary manifestation of an underlying disease or is associated with a medication; however, no disease or drug has been convincingly implicated in CCV.8
Cutaneous collagenous vasculopathy is limited to the skin, with no known reports of systemic involvement in the literature.7 There are no recommended laboratory studies to aid in diagnosis.1 It is critical to exclude hereditary hemorrhagic telangiectasia (HHT), as these patients can have life-threatening systemic involvement. Patients with CCV generally have no history of a bleeding diathesis, patients with HHT classically report recurrent epistaxis and gastrointestinal bleeding.7 A family history of HHT also is helpful for diagnosis, as the condition is autosomal dominant.1 Neither HHT or telangiectasia macularis eruptiva perstans, which also can be included in the differential diagnosis, demonstrate vessel wall hyalinization.
Treatment options for CCV are limited. Basso et al6 reported notable improvement in a patient with CCV treated with a combined 595-nm pulsed dye laser and 1064-nm Nd:YAG laser and optimized pulsed light. In one patient, treatment with a 585-nm pulsed dye laser produced a blanching response, suggesting that this may be a potential treatment option.7 Treatment with sclerotherapy has been ineffective.2
It is critical for both dermatologists and dermatopathologists to recognize and report this newly described entity, as the unique finding of vessel wall hyalinization in CCV may be indicative of a certain pathogenetic mechanism and effective treatment avenue that has yet to be established due to the relatively few number of reports that currently exist in the literature.
- Burdick LM, Lohser S, Somach SC, et al. Cutaneous collagenous vasculopathy: a rare cutaneous microangiopathy. J Cutan Pathol. 2012;39:741-746.
- Brady BG, Ortleb M, Boyd AS, et al. Cutaneous collagenous vasculopathy. J Clin Aesthet Dermatol. 2015;8:49-52.
- Salama S, Rosenthal D. Cutaneous collagenous vasculopathy with generalized telangiectasia: an immunohistochemical and structural study. J Cutan Pathol. 2000;27:40-48.
- Toda-Brito H, Resende C, Catorze G, et al. Cutaneous collagenous vasculopathy: a rare cause of generalised cutaneous telangiectasia. BMJ Case Rep. 2015. doi: 10.1136/bcr-2015-210635.
- Ma DL, Vano-Galvan S. Images in clinical medicine: cutaneous collagenous vasculopathy. N Engl J Med. 2015;373:E14.
- Basso D, Ribero S, Blazek C, et al. Cutaneous collagenous vasculopathy: a rare form of microangiopathy successfully treated with a combination of multiplex laser and optimized pulsed light with a review of the literature. Dermatology. 2016;232:107-111.
- Moteqi SI, Yasuda M, Yamanaka M, et al. Cutaneous collagenous vasculopathy: report of first Japanese case and review of the literature. Australas J Dermatol. 2017;58:145-149.
- González Fernández D, Gómez Bernal S, Vivanco Allende B, et al. Cutaneous collagenous vasculopathy: description of two new cases in elderly women and review of the literature. Dermatology. 2012;225:1-8.
The Diagnosis: Cutaneous Collagenous Vasculopathy
Histopathologic examination revealed ectatic blood vessels lined with unremarkable endothelial cells and thickened, hyalinized vessel walls scattered within the papillary dermis (Figure 1). The epidermis was unremarkable. There was minimal associated inflammation and no extravasation of erythrocytes. The hyalinized material was weakly positive on periodic acid-Schiff staining (Figure 2) and negative on Congo red staining, which supported of a diagnosis of cutaneous collagenous vasculopathy (CCV).


The patient previously had been given a suspected diagnosis of generalized essential telangiectasia by an outside dermatologist several years prior to the current presentation, as CCV had yet to be recognized as its own entity and therefore few cases had been described in the literature. She had a known history of obesity, hypertension, hyperlipidemia, and type 2 diabetes mellitus, which are associated with the condition. Multiple specialists concluded that the disease was too extensive for laser treatment. A review of PubMed articles indexed for MEDLINE yielded no established treatment options.
Cutaneous collagenous vasculopathy is a rare acquired microangiopathy involving the small vessels of the skin. Its clinical presentation is indistinguishable from that of generalized essential telangiectasia (GET). Patients generally present with asymptomatic, widespread, blanching, symmetric telangiectasias that classically begin on the legs and steadily progress upward with classic sparing of the face (Figure 3). Whereas GET has been reported to involve the oral and conjunctival mucosa, mucosal involvement is not typically observed in CCV and is considered to be a distinguishing factor between the 2 conditions.1,2 However, our patient reported oral symptoms, and oral erosions were seen on multiple physical examinations; therefore, ours is a rare case of mucosal involvement in conjunction with CCV. Given this finding, it is possible that more cases of CCV with mucosal involvement may exist but have been clinically misdiagnosed as GET.

First described by Salama and Rosenthal3 in 2000, CCV remains a rarely reported entity, with approximately 33 reported cases in the worldwide literature.2,4-7 The condition typically arises in adults with an equal predilection for males and females.2 The true incidence of CCV is unknown and likely is underreported given its close similarities to GET, which often is diagnosed clinically. The unique histopathologic finding of superficial ectatic vascular spaces with eosinophilic hyalinized vessel walls in CCV is key to distinguishing these similar entities, and even this finding can be subtle and is easily overlooked. Inflammation is sparse to absent. Deposited material is positive on periodic acid-Schiff and cytokeratin IV staining (representing reduplicated basement membrane-type collagen) and is diastase resistant. Smooth muscle actin staining is diminished or absent. Ultrastructural examination reveals reduplicated, laminated basement membrane; Luse bodies (abnormally long, widely spaced collagen fibers); and a decrease in or loss of pericytes. Of note, Luse bodies are nonspecific and their absence does not exclude a diagnosis of CCV.1
The etiology of CCV is unclear, and multiple pathogenetic mechanisms have been proposed. Ultimately, this entity is thought to arise from repeated endothelial cell damage, although the trigger for the endothelial cell injury is not completely understood. Diabetes mellitus sometimes is associated with microangiopathy and may be a confounding but not causative factor in some cases.1 Some investigators believe CCV is caused by a genetic defect that alters collagen production in the small vessels of the skin.5 Others have hypothesized that it is a secondary manifestation of an underlying disease or is associated with a medication; however, no disease or drug has been convincingly implicated in CCV.8
Cutaneous collagenous vasculopathy is limited to the skin, with no known reports of systemic involvement in the literature.7 There are no recommended laboratory studies to aid in diagnosis.1 It is critical to exclude hereditary hemorrhagic telangiectasia (HHT), as these patients can have life-threatening systemic involvement. Patients with CCV generally have no history of a bleeding diathesis, patients with HHT classically report recurrent epistaxis and gastrointestinal bleeding.7 A family history of HHT also is helpful for diagnosis, as the condition is autosomal dominant.1 Neither HHT or telangiectasia macularis eruptiva perstans, which also can be included in the differential diagnosis, demonstrate vessel wall hyalinization.
Treatment options for CCV are limited. Basso et al6 reported notable improvement in a patient with CCV treated with a combined 595-nm pulsed dye laser and 1064-nm Nd:YAG laser and optimized pulsed light. In one patient, treatment with a 585-nm pulsed dye laser produced a blanching response, suggesting that this may be a potential treatment option.7 Treatment with sclerotherapy has been ineffective.2
It is critical for both dermatologists and dermatopathologists to recognize and report this newly described entity, as the unique finding of vessel wall hyalinization in CCV may be indicative of a certain pathogenetic mechanism and effective treatment avenue that has yet to be established due to the relatively few number of reports that currently exist in the literature.
The Diagnosis: Cutaneous Collagenous Vasculopathy
Histopathologic examination revealed ectatic blood vessels lined with unremarkable endothelial cells and thickened, hyalinized vessel walls scattered within the papillary dermis (Figure 1). The epidermis was unremarkable. There was minimal associated inflammation and no extravasation of erythrocytes. The hyalinized material was weakly positive on periodic acid-Schiff staining (Figure 2) and negative on Congo red staining, which supported of a diagnosis of cutaneous collagenous vasculopathy (CCV).


The patient previously had been given a suspected diagnosis of generalized essential telangiectasia by an outside dermatologist several years prior to the current presentation, as CCV had yet to be recognized as its own entity and therefore few cases had been described in the literature. She had a known history of obesity, hypertension, hyperlipidemia, and type 2 diabetes mellitus, which are associated with the condition. Multiple specialists concluded that the disease was too extensive for laser treatment. A review of PubMed articles indexed for MEDLINE yielded no established treatment options.
Cutaneous collagenous vasculopathy is a rare acquired microangiopathy involving the small vessels of the skin. Its clinical presentation is indistinguishable from that of generalized essential telangiectasia (GET). Patients generally present with asymptomatic, widespread, blanching, symmetric telangiectasias that classically begin on the legs and steadily progress upward with classic sparing of the face (Figure 3). Whereas GET has been reported to involve the oral and conjunctival mucosa, mucosal involvement is not typically observed in CCV and is considered to be a distinguishing factor between the 2 conditions.1,2 However, our patient reported oral symptoms, and oral erosions were seen on multiple physical examinations; therefore, ours is a rare case of mucosal involvement in conjunction with CCV. Given this finding, it is possible that more cases of CCV with mucosal involvement may exist but have been clinically misdiagnosed as GET.

First described by Salama and Rosenthal3 in 2000, CCV remains a rarely reported entity, with approximately 33 reported cases in the worldwide literature.2,4-7 The condition typically arises in adults with an equal predilection for males and females.2 The true incidence of CCV is unknown and likely is underreported given its close similarities to GET, which often is diagnosed clinically. The unique histopathologic finding of superficial ectatic vascular spaces with eosinophilic hyalinized vessel walls in CCV is key to distinguishing these similar entities, and even this finding can be subtle and is easily overlooked. Inflammation is sparse to absent. Deposited material is positive on periodic acid-Schiff and cytokeratin IV staining (representing reduplicated basement membrane-type collagen) and is diastase resistant. Smooth muscle actin staining is diminished or absent. Ultrastructural examination reveals reduplicated, laminated basement membrane; Luse bodies (abnormally long, widely spaced collagen fibers); and a decrease in or loss of pericytes. Of note, Luse bodies are nonspecific and their absence does not exclude a diagnosis of CCV.1
The etiology of CCV is unclear, and multiple pathogenetic mechanisms have been proposed. Ultimately, this entity is thought to arise from repeated endothelial cell damage, although the trigger for the endothelial cell injury is not completely understood. Diabetes mellitus sometimes is associated with microangiopathy and may be a confounding but not causative factor in some cases.1 Some investigators believe CCV is caused by a genetic defect that alters collagen production in the small vessels of the skin.5 Others have hypothesized that it is a secondary manifestation of an underlying disease or is associated with a medication; however, no disease or drug has been convincingly implicated in CCV.8
Cutaneous collagenous vasculopathy is limited to the skin, with no known reports of systemic involvement in the literature.7 There are no recommended laboratory studies to aid in diagnosis.1 It is critical to exclude hereditary hemorrhagic telangiectasia (HHT), as these patients can have life-threatening systemic involvement. Patients with CCV generally have no history of a bleeding diathesis, patients with HHT classically report recurrent epistaxis and gastrointestinal bleeding.7 A family history of HHT also is helpful for diagnosis, as the condition is autosomal dominant.1 Neither HHT or telangiectasia macularis eruptiva perstans, which also can be included in the differential diagnosis, demonstrate vessel wall hyalinization.
Treatment options for CCV are limited. Basso et al6 reported notable improvement in a patient with CCV treated with a combined 595-nm pulsed dye laser and 1064-nm Nd:YAG laser and optimized pulsed light. In one patient, treatment with a 585-nm pulsed dye laser produced a blanching response, suggesting that this may be a potential treatment option.7 Treatment with sclerotherapy has been ineffective.2
It is critical for both dermatologists and dermatopathologists to recognize and report this newly described entity, as the unique finding of vessel wall hyalinization in CCV may be indicative of a certain pathogenetic mechanism and effective treatment avenue that has yet to be established due to the relatively few number of reports that currently exist in the literature.
- Burdick LM, Lohser S, Somach SC, et al. Cutaneous collagenous vasculopathy: a rare cutaneous microangiopathy. J Cutan Pathol. 2012;39:741-746.
- Brady BG, Ortleb M, Boyd AS, et al. Cutaneous collagenous vasculopathy. J Clin Aesthet Dermatol. 2015;8:49-52.
- Salama S, Rosenthal D. Cutaneous collagenous vasculopathy with generalized telangiectasia: an immunohistochemical and structural study. J Cutan Pathol. 2000;27:40-48.
- Toda-Brito H, Resende C, Catorze G, et al. Cutaneous collagenous vasculopathy: a rare cause of generalised cutaneous telangiectasia. BMJ Case Rep. 2015. doi: 10.1136/bcr-2015-210635.
- Ma DL, Vano-Galvan S. Images in clinical medicine: cutaneous collagenous vasculopathy. N Engl J Med. 2015;373:E14.
- Basso D, Ribero S, Blazek C, et al. Cutaneous collagenous vasculopathy: a rare form of microangiopathy successfully treated with a combination of multiplex laser and optimized pulsed light with a review of the literature. Dermatology. 2016;232:107-111.
- Moteqi SI, Yasuda M, Yamanaka M, et al. Cutaneous collagenous vasculopathy: report of first Japanese case and review of the literature. Australas J Dermatol. 2017;58:145-149.
- González Fernández D, Gómez Bernal S, Vivanco Allende B, et al. Cutaneous collagenous vasculopathy: description of two new cases in elderly women and review of the literature. Dermatology. 2012;225:1-8.
- Burdick LM, Lohser S, Somach SC, et al. Cutaneous collagenous vasculopathy: a rare cutaneous microangiopathy. J Cutan Pathol. 2012;39:741-746.
- Brady BG, Ortleb M, Boyd AS, et al. Cutaneous collagenous vasculopathy. J Clin Aesthet Dermatol. 2015;8:49-52.
- Salama S, Rosenthal D. Cutaneous collagenous vasculopathy with generalized telangiectasia: an immunohistochemical and structural study. J Cutan Pathol. 2000;27:40-48.
- Toda-Brito H, Resende C, Catorze G, et al. Cutaneous collagenous vasculopathy: a rare cause of generalised cutaneous telangiectasia. BMJ Case Rep. 2015. doi: 10.1136/bcr-2015-210635.
- Ma DL, Vano-Galvan S. Images in clinical medicine: cutaneous collagenous vasculopathy. N Engl J Med. 2015;373:E14.
- Basso D, Ribero S, Blazek C, et al. Cutaneous collagenous vasculopathy: a rare form of microangiopathy successfully treated with a combination of multiplex laser and optimized pulsed light with a review of the literature. Dermatology. 2016;232:107-111.
- Moteqi SI, Yasuda M, Yamanaka M, et al. Cutaneous collagenous vasculopathy: report of first Japanese case and review of the literature. Australas J Dermatol. 2017;58:145-149.
- González Fernández D, Gómez Bernal S, Vivanco Allende B, et al. Cutaneous collagenous vasculopathy: description of two new cases in elderly women and review of the literature. Dermatology. 2012;225:1-8.
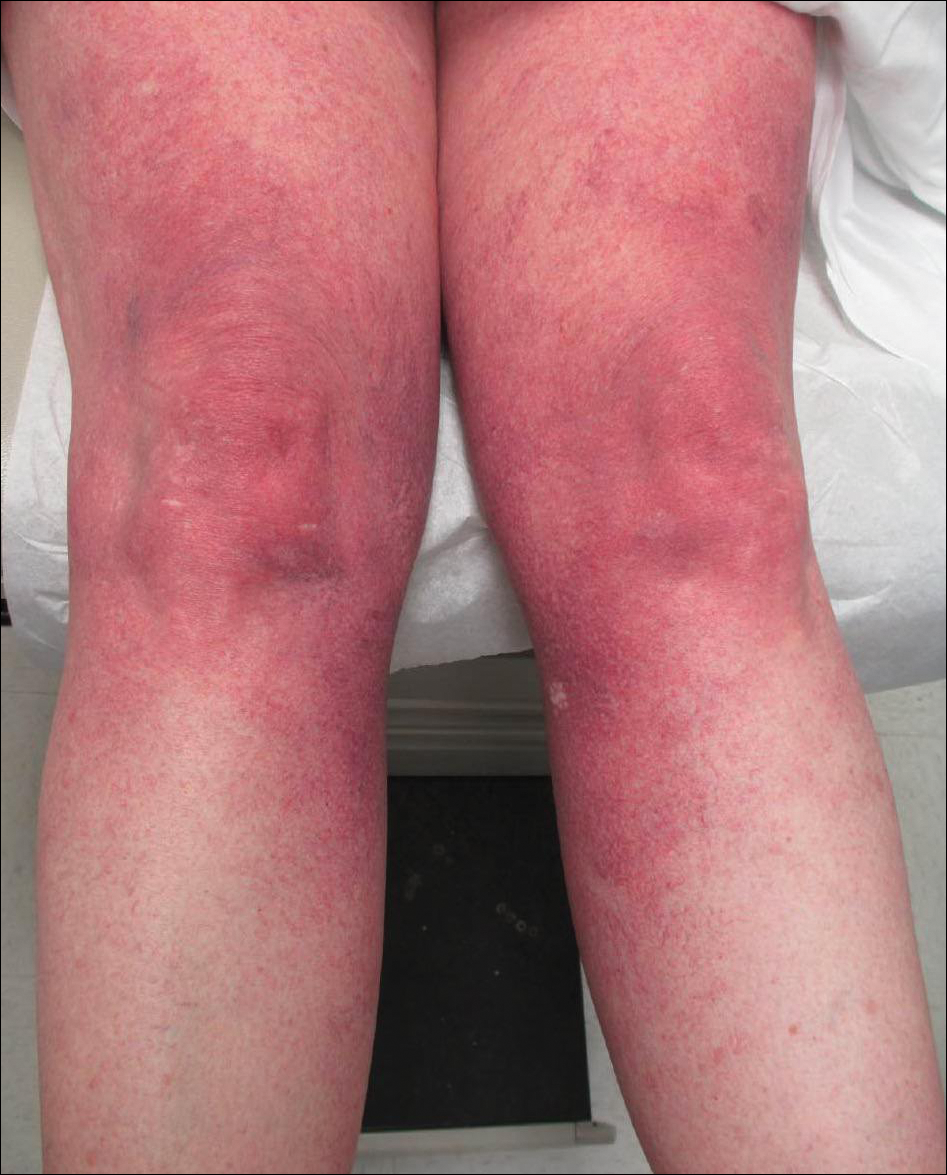
A 55-year-old woman presented for evaluation of widespread asymptomatic telangiectasias of several years' duration that first appeared on the legs and steadily progressed to involve the trunk and arms. A review of systems was remarkable for episodic glossitis and oral erosions that developed at the same time as the eruption. The patient had no history of bleeding diasthesis, and her family history was unremarkable. A laboratory workup (including autoimmune screening) and a malignancy workup were negative. Physical examination revealed confluent sheets of erythematous and purple blanching telangiectasias scattered symmetrically on the trunk, bilateral arms and legs, buttocks, and dorsal aspects of the feet with sparing of the palms, soles, and head and neck regions. A small, shallow erosion was present on the lateral aspect of the tongue. A 4-mm punch biopsy of a thigh lesion revealed ectatic blood vessels with hyalinized walls.