User login
LAS VEGAS – The development of the positron emission tomography radiotracer known as AV-45, or florbetapir F18, for the diagnosis of Alzheimer’s disease carries within a certain "curse," said Dr. Jeffrey L. Cummings of the Cleveland Clinic’s Lou Rubo Center for Brain Health.
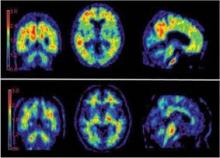
The PET scan can show positive results 5-10 years before Alzheimer’s symptoms appear, which raises a multilayered dilemma for clinicians.
"Let’s say someone comes to you and says, ‘My mom had Alzheimer’s at age 75 years. I’m now age 70, and I want to know if I’m going to have it.’ You can tell them," Dr. Cummings said at the conference. "There is going to be a moral, ethical, and cost dilemma that is brought with this scan.
"I don’t think we will use it on [cognitively] ‘normal’ people except in very special circumstances. I think we will use it to sort out those patients with mild cognitive impairment who are in the earliest stage of Alzheimer’s disease. This is going to be a great point of dialogue in the coming year."
For decades, clinicians have informed families of patients affected by Alzheimer’s disease that an autopsy is the only test that can confirm a diagnosis. The AV-45 has the potential to revolutionize the way clinicians diagnose Alzheimer’s disease. The radiotracer, developed by Avid Radiopharmaceuticals and currently under Food and Drug Administration review, enables clinicians to visualize on PET scan deposits of beta-amyloid plaques* in the brain – a hallmark feature of the illness.
"We now have a PET scan that uses ligands that were originally used to label the [amyloid] plaques at autopsy," Dr. Cummings, also chair of neurotherapeutics at the Cleveland Clinic’s Neurological Institute, said in an interview during a psychopharmacology conference sponsored by the Nevada Psychiatric Association. "It was realized that you could label these with positron imaging substances so that you could map the presence of amyloid in the brain.
"Now we can make a confident diagnosis of Alzheimer’s disease in the dementia patient. More importantly, we can make a diagnosis in the patient who has mild cognitive impairment. Some of those patients have Alzheimer’s disease; some do not. The new amyloid PET scan will help us know which of the mild cognitive impairment patients have Alzheimer’s disease, and therefore we would consider therapy."
In the meantime, clinicians are using PET in combination with the radiotracer fluorodeoxyglucose (FDG) to distinguish Alzheimer’s disease from frontotemporal dementia. This differential diagnosis is the only one which the Centers for Medicare and Medicaid Services will pay for," Dr. Cummings said. "CMS will pay for a PET scan if you write on the PET scan request, ‘I am trying to distinguish Alzheimer’s disease from frontotemporal dementia.’ If you request use of the scan to diagnose early Alzheimer’s disease, it usually will be denied." FDG PET is approved for differential diagnosis of these two similar conditions but not for the wider range of diagnostic questions such as early identification of Alzheimer’s disease.
Dr. Cummings disclosed that he has provided consultation to Abbott, Acadia, Adamas, Anavex, Astellas, Avanir, Bayer, BMS, Eisai, EnVivo, ExonHit, Janssen, Forest, Genentech, GlaxoSmithKline, Lundbeck, Merck, Neurokos, Novartis, Otsuka, Pfizer, Prana, QR Pharma, Sanofi-Aventis, and Takeda pharmaceutical companies.
*Clarification, 3/14/2012: An earlier version of this story used a less specific term.
LAS VEGAS – The development of the positron emission tomography radiotracer known as AV-45, or florbetapir F18, for the diagnosis of Alzheimer’s disease carries within a certain "curse," said Dr. Jeffrey L. Cummings of the Cleveland Clinic’s Lou Rubo Center for Brain Health.
The PET scan can show positive results 5-10 years before Alzheimer’s symptoms appear, which raises a multilayered dilemma for clinicians.
"Let’s say someone comes to you and says, ‘My mom had Alzheimer’s at age 75 years. I’m now age 70, and I want to know if I’m going to have it.’ You can tell them," Dr. Cummings said at the conference. "There is going to be a moral, ethical, and cost dilemma that is brought with this scan.
"I don’t think we will use it on [cognitively] ‘normal’ people except in very special circumstances. I think we will use it to sort out those patients with mild cognitive impairment who are in the earliest stage of Alzheimer’s disease. This is going to be a great point of dialogue in the coming year."
For decades, clinicians have informed families of patients affected by Alzheimer’s disease that an autopsy is the only test that can confirm a diagnosis. The AV-45 has the potential to revolutionize the way clinicians diagnose Alzheimer’s disease. The radiotracer, developed by Avid Radiopharmaceuticals and currently under Food and Drug Administration review, enables clinicians to visualize on PET scan deposits of beta-amyloid plaques* in the brain – a hallmark feature of the illness.
"We now have a PET scan that uses ligands that were originally used to label the [amyloid] plaques at autopsy," Dr. Cummings, also chair of neurotherapeutics at the Cleveland Clinic’s Neurological Institute, said in an interview during a psychopharmacology conference sponsored by the Nevada Psychiatric Association. "It was realized that you could label these with positron imaging substances so that you could map the presence of amyloid in the brain.
"Now we can make a confident diagnosis of Alzheimer’s disease in the dementia patient. More importantly, we can make a diagnosis in the patient who has mild cognitive impairment. Some of those patients have Alzheimer’s disease; some do not. The new amyloid PET scan will help us know which of the mild cognitive impairment patients have Alzheimer’s disease, and therefore we would consider therapy."
In the meantime, clinicians are using PET in combination with the radiotracer fluorodeoxyglucose (FDG) to distinguish Alzheimer’s disease from frontotemporal dementia. This differential diagnosis is the only one which the Centers for Medicare and Medicaid Services will pay for," Dr. Cummings said. "CMS will pay for a PET scan if you write on the PET scan request, ‘I am trying to distinguish Alzheimer’s disease from frontotemporal dementia.’ If you request use of the scan to diagnose early Alzheimer’s disease, it usually will be denied." FDG PET is approved for differential diagnosis of these two similar conditions but not for the wider range of diagnostic questions such as early identification of Alzheimer’s disease.
Dr. Cummings disclosed that he has provided consultation to Abbott, Acadia, Adamas, Anavex, Astellas, Avanir, Bayer, BMS, Eisai, EnVivo, ExonHit, Janssen, Forest, Genentech, GlaxoSmithKline, Lundbeck, Merck, Neurokos, Novartis, Otsuka, Pfizer, Prana, QR Pharma, Sanofi-Aventis, and Takeda pharmaceutical companies.
*Clarification, 3/14/2012: An earlier version of this story used a less specific term.
LAS VEGAS – The development of the positron emission tomography radiotracer known as AV-45, or florbetapir F18, for the diagnosis of Alzheimer’s disease carries within a certain "curse," said Dr. Jeffrey L. Cummings of the Cleveland Clinic’s Lou Rubo Center for Brain Health.
The PET scan can show positive results 5-10 years before Alzheimer’s symptoms appear, which raises a multilayered dilemma for clinicians.
"Let’s say someone comes to you and says, ‘My mom had Alzheimer’s at age 75 years. I’m now age 70, and I want to know if I’m going to have it.’ You can tell them," Dr. Cummings said at the conference. "There is going to be a moral, ethical, and cost dilemma that is brought with this scan.
"I don’t think we will use it on [cognitively] ‘normal’ people except in very special circumstances. I think we will use it to sort out those patients with mild cognitive impairment who are in the earliest stage of Alzheimer’s disease. This is going to be a great point of dialogue in the coming year."
For decades, clinicians have informed families of patients affected by Alzheimer’s disease that an autopsy is the only test that can confirm a diagnosis. The AV-45 has the potential to revolutionize the way clinicians diagnose Alzheimer’s disease. The radiotracer, developed by Avid Radiopharmaceuticals and currently under Food and Drug Administration review, enables clinicians to visualize on PET scan deposits of beta-amyloid plaques* in the brain – a hallmark feature of the illness.
"We now have a PET scan that uses ligands that were originally used to label the [amyloid] plaques at autopsy," Dr. Cummings, also chair of neurotherapeutics at the Cleveland Clinic’s Neurological Institute, said in an interview during a psychopharmacology conference sponsored by the Nevada Psychiatric Association. "It was realized that you could label these with positron imaging substances so that you could map the presence of amyloid in the brain.
"Now we can make a confident diagnosis of Alzheimer’s disease in the dementia patient. More importantly, we can make a diagnosis in the patient who has mild cognitive impairment. Some of those patients have Alzheimer’s disease; some do not. The new amyloid PET scan will help us know which of the mild cognitive impairment patients have Alzheimer’s disease, and therefore we would consider therapy."
In the meantime, clinicians are using PET in combination with the radiotracer fluorodeoxyglucose (FDG) to distinguish Alzheimer’s disease from frontotemporal dementia. This differential diagnosis is the only one which the Centers for Medicare and Medicaid Services will pay for," Dr. Cummings said. "CMS will pay for a PET scan if you write on the PET scan request, ‘I am trying to distinguish Alzheimer’s disease from frontotemporal dementia.’ If you request use of the scan to diagnose early Alzheimer’s disease, it usually will be denied." FDG PET is approved for differential diagnosis of these two similar conditions but not for the wider range of diagnostic questions such as early identification of Alzheimer’s disease.
Dr. Cummings disclosed that he has provided consultation to Abbott, Acadia, Adamas, Anavex, Astellas, Avanir, Bayer, BMS, Eisai, EnVivo, ExonHit, Janssen, Forest, Genentech, GlaxoSmithKline, Lundbeck, Merck, Neurokos, Novartis, Otsuka, Pfizer, Prana, QR Pharma, Sanofi-Aventis, and Takeda pharmaceutical companies.
*Clarification, 3/14/2012: An earlier version of this story used a less specific term.
EXPERT ANALYSIS FROM A PSYCHOPHARMACOLOGY CONFERENCE SPONSORED BY THE NEVADA PSYCHIATRIC ASSOCIATION