User login
ROME – A new risk-stratification formula for atrial fibrillation patients starting oral anticoagulant therapy helps sort out their potential net benefit on edoxaban, compared with warfarin.
This risk score “could help guide selection of treatment” with a vitamin K antagonist such as warfarin or a new oral anticoagulant (NOAC) such as edoxaban, Christina L. Fanola, MD, said at the annual congress of the European Society of Cardiology.
“It’s a great time to think about this type of score, because so many more patients are being diagnosed with atrial fibrillation and there is a lot of clinical equipoise” over which anticoagulant to start patients on, said Dr. Fanola, a cardiologist at Brigham and Women’s Hospital in Boston. She said she and her associates hope to externally validate the score and test it in cohorts that received other NOACs, such as apixaban (Eliquis), dabigatran (Pradaxa), or rivaroxaban (Xarelto), but it is very possible that scoring might differ from one NOAC to the next. “Each NOAC may need its own scoring formula,” Dr. Fanola said in an interview.
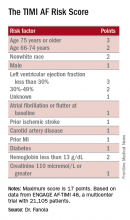
A Cox proportional hazards model identified 10 demographic, clinical, and laboratory features that had significant, independent correlations to a primary outcome of disabling stroke, life-threatening bleeding, or death. After weighing the point allocation for each item by the strength of its association, the researchers developed a scoring formula in a model that could account for about 69% of the three combined adverse outcomes.
An analysis that applied the scoring formula back to the ENGAGE AF-TIMI 48 database showed that a low-risk score of 0-6 correlated with a 4% per year rate of disabling stroke, life-threatening bleed, or death; an intermediate-risk score of 7-9 correlated with a 10% per year incidence of this combined outcome, and a high-risk score of 10 or greater linked with a 21% annual event rate.
Dr. Fanola and her associates ran a further analysis that evaluated the efficacy of edoxaban, compared with warfarin, among the patients in each of these risk strata. The high-risk patients received a major benefit from edoxaban, with a 30% overall incidence of the combined endpoint during 3 years of follow-up, compared with a 51% rate among patients on warfarin, a 21-percentage-point reduction in adverse events. Intermediate-risk patients also received a significant benefit, with a 26% event rate on warfarin and an 18% rate on edoxaban. But low-risk patients had identical 10% event rates with either treatment.
These findings suggest that atrial fibrillation patients with a TIMI AF score that is high or intermediate would have a better chance for a good outcome on edoxaban, or perhaps a different NOAC, than on warfarin. Low-risk patients seem to have similar outcomes on edoxaban or warfarin, so other considerations can come into play for choosing between these drug options, such as the cost of treatment and the inconvenience of regular warfarin monitoring, Dr. Fanola said.
ENGAGE AF-TIMI 48 was sponsored by Daiichi Sankyo, the company that markets edoxaban. Dr. Fanola had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
ROME – A new risk-stratification formula for atrial fibrillation patients starting oral anticoagulant therapy helps sort out their potential net benefit on edoxaban, compared with warfarin.
This risk score “could help guide selection of treatment” with a vitamin K antagonist such as warfarin or a new oral anticoagulant (NOAC) such as edoxaban, Christina L. Fanola, MD, said at the annual congress of the European Society of Cardiology.
“It’s a great time to think about this type of score, because so many more patients are being diagnosed with atrial fibrillation and there is a lot of clinical equipoise” over which anticoagulant to start patients on, said Dr. Fanola, a cardiologist at Brigham and Women’s Hospital in Boston. She said she and her associates hope to externally validate the score and test it in cohorts that received other NOACs, such as apixaban (Eliquis), dabigatran (Pradaxa), or rivaroxaban (Xarelto), but it is very possible that scoring might differ from one NOAC to the next. “Each NOAC may need its own scoring formula,” Dr. Fanola said in an interview.
A Cox proportional hazards model identified 10 demographic, clinical, and laboratory features that had significant, independent correlations to a primary outcome of disabling stroke, life-threatening bleeding, or death. After weighing the point allocation for each item by the strength of its association, the researchers developed a scoring formula in a model that could account for about 69% of the three combined adverse outcomes.
An analysis that applied the scoring formula back to the ENGAGE AF-TIMI 48 database showed that a low-risk score of 0-6 correlated with a 4% per year rate of disabling stroke, life-threatening bleed, or death; an intermediate-risk score of 7-9 correlated with a 10% per year incidence of this combined outcome, and a high-risk score of 10 or greater linked with a 21% annual event rate.
Dr. Fanola and her associates ran a further analysis that evaluated the efficacy of edoxaban, compared with warfarin, among the patients in each of these risk strata. The high-risk patients received a major benefit from edoxaban, with a 30% overall incidence of the combined endpoint during 3 years of follow-up, compared with a 51% rate among patients on warfarin, a 21-percentage-point reduction in adverse events. Intermediate-risk patients also received a significant benefit, with a 26% event rate on warfarin and an 18% rate on edoxaban. But low-risk patients had identical 10% event rates with either treatment.
These findings suggest that atrial fibrillation patients with a TIMI AF score that is high or intermediate would have a better chance for a good outcome on edoxaban, or perhaps a different NOAC, than on warfarin. Low-risk patients seem to have similar outcomes on edoxaban or warfarin, so other considerations can come into play for choosing between these drug options, such as the cost of treatment and the inconvenience of regular warfarin monitoring, Dr. Fanola said.
ENGAGE AF-TIMI 48 was sponsored by Daiichi Sankyo, the company that markets edoxaban. Dr. Fanola had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
ROME – A new risk-stratification formula for atrial fibrillation patients starting oral anticoagulant therapy helps sort out their potential net benefit on edoxaban, compared with warfarin.
This risk score “could help guide selection of treatment” with a vitamin K antagonist such as warfarin or a new oral anticoagulant (NOAC) such as edoxaban, Christina L. Fanola, MD, said at the annual congress of the European Society of Cardiology.
“It’s a great time to think about this type of score, because so many more patients are being diagnosed with atrial fibrillation and there is a lot of clinical equipoise” over which anticoagulant to start patients on, said Dr. Fanola, a cardiologist at Brigham and Women’s Hospital in Boston. She said she and her associates hope to externally validate the score and test it in cohorts that received other NOACs, such as apixaban (Eliquis), dabigatran (Pradaxa), or rivaroxaban (Xarelto), but it is very possible that scoring might differ from one NOAC to the next. “Each NOAC may need its own scoring formula,” Dr. Fanola said in an interview.
A Cox proportional hazards model identified 10 demographic, clinical, and laboratory features that had significant, independent correlations to a primary outcome of disabling stroke, life-threatening bleeding, or death. After weighing the point allocation for each item by the strength of its association, the researchers developed a scoring formula in a model that could account for about 69% of the three combined adverse outcomes.
An analysis that applied the scoring formula back to the ENGAGE AF-TIMI 48 database showed that a low-risk score of 0-6 correlated with a 4% per year rate of disabling stroke, life-threatening bleed, or death; an intermediate-risk score of 7-9 correlated with a 10% per year incidence of this combined outcome, and a high-risk score of 10 or greater linked with a 21% annual event rate.
Dr. Fanola and her associates ran a further analysis that evaluated the efficacy of edoxaban, compared with warfarin, among the patients in each of these risk strata. The high-risk patients received a major benefit from edoxaban, with a 30% overall incidence of the combined endpoint during 3 years of follow-up, compared with a 51% rate among patients on warfarin, a 21-percentage-point reduction in adverse events. Intermediate-risk patients also received a significant benefit, with a 26% event rate on warfarin and an 18% rate on edoxaban. But low-risk patients had identical 10% event rates with either treatment.
These findings suggest that atrial fibrillation patients with a TIMI AF score that is high or intermediate would have a better chance for a good outcome on edoxaban, or perhaps a different NOAC, than on warfarin. Low-risk patients seem to have similar outcomes on edoxaban or warfarin, so other considerations can come into play for choosing between these drug options, such as the cost of treatment and the inconvenience of regular warfarin monitoring, Dr. Fanola said.
ENGAGE AF-TIMI 48 was sponsored by Daiichi Sankyo, the company that markets edoxaban. Dr. Fanola had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2016
Key clinical point:
Major finding: Among high-risk patients, edoxaban cut adverse events by 21 percentage points, compared with warfarin.
Data source: ENGAGE AF-TIMI 48, a multicenter trial with 21,105 patients.
Disclosures: ENGAGE AF-TIMI 48 was sponsored by Daiichi Sankyo, the company that markets edoxaban (Savaysa). Dr. Fanola had no relevant financial disclosures.