User login
Sleep disturbance in the critically ill has received much attention over recent years as this is a common result of intensive care unit (ICU) admission. Disruptions in sleep not only can, at a minimum, cause distress and lower patient satisfaction, but also inhibit recovery from illness and increase morbidity.1,2 Several studies have been conducted highlighting the altered sleep patterns of critically ill patients; although total sleep time may seem normal (7-9 hours), patients can experience multiple awakenings per hour, more time in light sleep (stages 1 and 2), and less time in restorative sleep (stages 3 and 4, [REM]rapid eye movement).2-5
There are several hypothesized physiologic detriments that contribute to slower ICU recovery with sleep deprivation. Research in noncritically ill subjects suggests that sleep deprivation contributes to hypoventilation and potentially prolonged time on the ventilator.6-9 Cardiovascular morbidity may be adversely affected by inflammatory cytokine release seen in sleep disruption.10,11 Studies of noncritically ill patients also suggest that immune response is impaired, potentially protracting infection recovery.12,13 Finally, although not directly investigated, sleep deprivation may contribute to ICU delirium, an independent adverse effect (AE) associated with increased mortality and worse long-term outcomes.14-16
The Society of Critical Care Medicine (SCCM) recently updated its consensus guidelines for the management of pain, agitation/sedation, delirium, immobility, and sleep disruption (PADIS) in adult patients.17 These guidelines offer limited interventions to promote sleep in ICU patients based on available evidence and steer the clinician toward minimizing exacerbating factors. Although factors that affect sleep patterns are multifactorial, such as noise levels, pain, mechanical ventilation, and inflammatory mediators, medication therapy is a known modifiable risk factor for sleep disturbance in critically ill patients.2 This focused review will specifically evaluate the effects of steroids on sleep deprivation, psychosis, delirium, and what is known about these effects in a critically ill population.
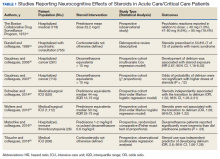
To include articles relevant to a critically ill population, a systematic search of MEDLINE and PubMed from 1966 to 2019 was performed using the following Medical Subject Headings (MeSH) terms: delirium/etiology, psychoses, substance-induced/etiology, sleep-wake disorders/chemically induced, neurocognitive disorders/chemically induced, dyssomnias/drug effects plus glucocorticoids/adverse effects, adrenal cortex hormones/adverse effects, prednisone/adverse effects, methylprednisolone/adverse effects, and hydrocortisone/adverse effects. The initial search produced 285 articles. Case reports, reviews, letters, and articles pertaining to primary care or palliative populations were excluded, leaving 8 relevant articles for inclusion (Table 1).18-25
ICU Steroid Use
Steroids are commonly used in the ICU and affect nearly every critically ill population. Common indications for steroids in the ICU include anaphylaxis, airway edema, septic shock, asthma and COPD exacerbations, pneumocystis pneumonia, adrenal crisis, antiemetic treatment, elevated intracranial pressure from tumors, autoimmune disorders, and stress doses needed for chronic steroid users before invasive procedures.26 Whether divided into glucocorticoid or mineralocorticoid subgroups, corticosteroids offer therapeutic benefit from their pharmacologic similarity to endogenously produced cortisol, which includes anti-inflammatory, immunosuppressive, antiproliferative, and vasoconstrictive effects.
Steroid receptors are present in most human tissue, and in varying degrees of binding affinity produce a wide variety of effects. After passive diffusion across cell membranes, steroid-receptor activation binds to various DNA sites, called glucocorticoid regulatory elements, which either stimulates or inhibits transcription of multiple nearby genes.
At the cellular level, corticosteroids inhibit the release of arachidonic acid through upstream production of lipocortin peptides and antagonism of phospholipase A2. This action decreases subsequent inflammatory mediators, including kinins, histamine, liposomal enzymes, and prostaglandins. Steroids also inhibit NF-κB, which further decreases expression of proinflammatory genes while promoting interleukin-10 and its anti-inflammatory properties. Antiproliferative effects of steroids are seen by triggering cell apoptosis and inhibition of fibroblast proliferation.27,28
By binding to mineralocorticoid receptors, steroids cause sodium retention coupled with hydrogen and potassium excretion in the distal renal tubule. Steroids also promote vasoconstriction by upregulating the production and sensitivity of β receptors in the endothelium while suppressing the production of vasodilators. Although rarely used for these physiologic effects, steroids also are involved in a number of metabolic pathways, including calcium regulation, gluconeogenesis, protein metabolism, and fat distribution. Given the similar structure to cortisol, exogenous steroids depress the hypothalamic-pituitary axis (HPA) and decrease the release of adrenocorticotropic hormone (ACTH). Tapering doses of steroid regimens is often required to allow natural androgen and cortisol synthesis and prevent steroid withdrawal.27,28
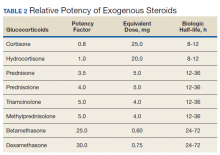
The potency of various exogenous steroids closely parallels their ability to retain sodium (Table 2). Prolonged activation of steroid receptors can have numerous systemic AEs, including unwanted neurocognitive effects (Table 3). Insomnia and psychosis are commonly described in corticosteroid clinical trials, and in one meta-analysis, both are associated with high costs per episode per year.29
Steroid-Induced Sleep Disruption and Psychosis
Sleep disruption caused by exogenous administration of steroids is thought to trigger other psychostimulant effects, such as mood swings, nervousness, psychoses, and delirium.30 Similarly, the SCCM PADIS guidelines included an ungraded statement: “although an association between sleep quality and delirium occurrence exists in critically ill adults, a cause-effect relationship has not been established.”17 For this review, these AEs will be discussed as related events.
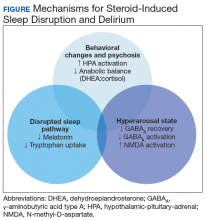
The medical literature proposes 3 pathways primarily responsible for neurocognitive AEs of steroids: behavior changes through modification of the HPA axis, changes in natural sleep-wake cycles, and hyperarousal caused by modification in neuroinhibitory pathways (Figure).
HPA Axis Modification
Under either physical or psychological stress, neural circuits in the brain release corticotropin-releasing hormone (CRH), dehydroepiandrosterone (DHEA), and arginine vasopressin, which go on to activate the sympathetic nervous system and the HPA axis. CRH from the hypothalamus goes on to stimulate ACTH release from the pituitary. ACTH then stimulates cortisol secretion from the adrenal glands. Circulating cortisol feeds into several structures of the brain, including the pituitary, hippocampus, and amygdala. Steroid-receptor complexes alter gene transcription in the central nervous system (CNS), affecting the production of neurotransmitters (eg, dopamine, serotonin) and neuropeptides (eg, somatostatin, β-endorphin). Feedback inhibition ensues, with downregulation of the HPA axis, which prevents depletion of endogenous production of steroids.31 DHEA has protective effects against excessive cortisol activity, but DHEA secretion declines with prolonged cortisol exposure. Exogenous steroids may have different effects than endogenous steroids, and neurocognitive sequelae stem from disruption and imbalance of these physiologic mechanisms.32,33
Steroid receptors are densely located in behavior centers in the brain: the amygdala, septum, and hippocampus. Pharmacologic changes in gene expression alter norepinephrine and serotonin levels in the brain as well as their receptors.32 Prolonged exposure to exogenous steroids has been shown to decrease amygdala and hippocampal volumes.34,35 Furthermore, prolonged corticosteroid exposure has been shown to decrease the number of steroid receptors in the hippocampus, pituitary gland, and amygdala.36 In a somewhat paradoxical finding, the production of CNS proinflammatory cytokines like interleuken-1β and tumor necrosis factor α has been seen after steroid administration, suggesting alternate gene signaling in the CNS.37 Although not proven conclusively, it is felt that these physiologic changes and hyperactivity of the HPA axis are predominantly responsible for changes in behavior, mood, memory, and eventually psychosis in steroid-treated patients.33,38
Finally, alterations in cognition and behavior may be related to steroid-induced changes in CNS carbohydrate, protein, and lipid metabolism with subsequent cellular neurotoxicity.32,38 Glucose uptake into the hippocampus is decreased with steroid exposure. Additionally, breakdown of metabolic compounds to produce energy can be destructive if left unchecked for prolonged periods. DHEA, growth hormone, and testosterone work to repair catabolic damage produced by cortisol, known as anabolic balance. A low anabolic balance (low DHEA levels to high cortisol levels) leads to a cascade of dysregulation in brain activity.39
Changes in Natural Sleep-Wake Cycles
Natural sleep pathways are also affected by steroids. The sleep-wake cycle is primarily regulated in the hypothalamus with circadian release of melatonin from the pineal gland. Melatonin release is highest at night, where it promotes sleep onset and continuity. Upstream, tryptophan is an amino acid that serves as a precursor to serotonin and melatonin.40 Both endogenous and exogenous corticosteroids decrease serum melatonin levels with a markedly diminished circadian rhythm secretion.41,42Demish and colleagues found a significant decrease in mean (SD) nocturnal melatonin plasma levels after the evening administration of oral dexamethasone 1 mg in 11 healthy volunteers: 127 (42) pg/mL before vs 73 (38) pg/mL after; P < .01.42 This result is likely due to decreased cellular metabolism and melatonin synthesis in the pineal gland. Of note, melatonin has neuroprotective affects, and the administration of melatonin has been shown to reverse some steroid-induced neurotoxicities in animal models.43
Steroids also reduce the uptake of tryptophan into the brain.33 Additionally, in animal models, dexamethasone administration caused a significant decrease in the gene expression of tryptophan hydroxylase, which is part of the multistep pathway in synthesizing serotonin from L-tryptophan. These effects upstream could inhibit the biosynthetic capacity of both melatonin and serotonin.44
A third pathway investigated in sleep regulation are the orexin neuropeptides. Orexins are produced in the hypothalamus and stimulate daytime wake activity in monoaminergic and cholinergic neurons. Subsequently, orexin receptor antagonists are a newer class of drugs aimed at mitigating nighttime hyperarousal and sleep disruption. Orexin overexpression may be a causal factor in steroid-induced sleep disturbance. However, this effect was specifically evaluated in a recent study in children with acute lymphoblastic leukemia, which showed that cerebral spinal fluid orexin levels (SD) were not significantly different from baseline after dexamethasone administration: 574 (26.6) pg/mL vs 580 (126.1) pg/mL; P = .8.45
Hyperarousal State
Finally, a hyperarousal state is thought to be produced by nongenomic changes to natural neuroinhibitory regulation seen with nonclassical steroid production called neurosteroids. Animal studies revealed that high levels of steroids were found in the CNS long after adrenalectomy, suggesting CNS de novo synthesis.46 In addition to altering gene expression at classic intercellular steroid receptors, neurosteroids can alter neurotransmission by direct interaction on ion-gated membranes and other receptors on the cell surface. Restlessness and insomnia could be due to γ-aminobutyric acid type A (GABAA) receptor modulation in the CNS where neuroactive steroids slow the rate of recovery of GABAA and potentially inhibit postsynaptic GABAergic transmission. It also is hypothesized that neuroactive steroids have excitatory action at nicotinic acetylcholine, 5HT3 receptors, and through increasing the fractional open time of the N-methyl-D-aspartate -activated channels.47 Allopregnanolone and DHEA are neurosteroids that act as GABAA agonists and have neuroprotective effects with anxiolytic, antidepressant, and antiaggressive properties.
Neurosteroids are synthesized from cholesterol in the hippocampus. Neurosteroids are upregulated in response to stress by CNS cortisol effects on various enzyme expressions.47 Whether exogenous steroid administration affects this biosynthesis vs the stress response in the HPA axis itself is not fully elucidated. Monteleone and colleagues found that dexamethasone 1 mg given orally significantly reduced cortisol and DHEA and allopregnanolone levels in both healthy volunteers and anorexia nervosa patients.48 Similarly, Genazzani and colleagues demonstrated that oral dexamethasone administration (0.5 mg every 6 hours) caused significant reductions in both serum allopregnanolone and DHEA levels.49
Outcomes Studies
The majority of reported data in steroid-induced insomnia and psychosis is in noncritically ill populations. In a randomized, prospective crossover study of healthy volunteers, dexamethasone administration (3 mg every 8 hours for 48 hours) resulted in significant changes in sleep patterns measured with polysomnography. Compared with placebo, steroid treatment showed significantly longer percentage (SD) of stage 0/awake times (11.7% [11.4] vs 2.9% [1.8]; P < .05); longer percentage (SD) of REM sleep latency (363.8 [74.5] minutes vs 202.8 [79.6] minutes; P < .01), and a reduced number (SD) of REM periods (3.8 [2.6] vs 9.7 [3.6]; P < .01).50 Insomnia was one of the most commonly self-reported AEs (> 60%) in a survey of 2,446 chronic steroid users, and the incidence increased as steroid doses increased.51
A prospective, open-label study of 240 patients with cancer demonstrated significant sleep disruptions using the Pittsburgh Sleep Quality Index with the use of high-dose steroids in chemotherapy.52 Naber and colleagues evaluated 50 previously healthy patients taking methylprednisolone 119 mg (41 mg/d) for retinitis and uveitis.53 They reported 26% to 34% of subjects experienced hypomanic syndrome based on a semistructured interview examination. Symptoms developed within 3 days and persisted for the 8-day course of therapy. Brown and colleagues prospectively evaluated 32 asthmatic patients prescribed bursts of prednisone > 40 mg daily. They observed significantly increased scores in the Young Mania Rating Scale within 3 to 7 days of starting therapy, which dissipated to baseline after stopping therapy.54
Despite a high reported incidence of neurologic AEs, outcomes in critically ill populations are mixed. Study methods are varied, and many were largely observational. No prospective, randomized studies exist to date specifically aimed and powered to evaluate the effects of steroids on sleep disturbances or delirium in a critically ill population. Furthermore, sleep quality is difficult to measure in this population, and self-reporting often is not an option. In critical care trials, if AEs such as insomnia, delirium, or psychosis are recorded at all, there is heterogeneity in the definitions, and these AEs are generally poorly defined (eg, psychiatric or neurologic disorder not otherwise specified), making pooled analysis of this outcome difficult.55
One of the largest observational studies in hospitalized patients was through the Boston Collaborative Drug Surveillance Program. A total of 718 consecutively enrolled inpatients who received prednisone were monitored for acute reactions. Psychiatric AEs were rare (1.3%) with low doses (< 40 mg/d), more prevalent (4.6%) with higher doses (41-80 mg/d), and most prevalent (18.4%) with the highest doses (> 80 mg/d), suggesting CNS AEs are dose dependent.18 A single-center, retrospective review of 755 psychiatric consults in hospitalized patients revealed that 54% of manic patients were due to corticosteroid administration.19 In a prospective observational study of 206 consecutive ICU admissions, steroid administration was an independent risk factor for development of ICU delirium, using the Confusion Assessment Method-ICU (CAM-ICU) at a single center (odds ratio [OR], 2.8; 95% CI, 1.05-7.28).25
Two studies in hospitalized oncology patients found conflicting results using the Nursing Delirium Screening Scale (Nu-DESC). One did not find a significant association between delirium and dexamethasone equivalent doses > 15 mg, while the second found an increased hazard ratio (HR) for a positive Nu-DESC score (HR, 2.67; 95% CI, 1.18-6.03).20,21 Similarly, conflicting results were found in 2 studies using first-order Markov models. In one prospective cohort study, 520 consecutive mechanically ventilated patients in 13 ICUs were monitored for the transition to delirium (CAM-ICU positive) from nondelirium states. Steroid administration was significantly associated with transitioning to delirium (OR, 1.52; 95% CI, 1.05-2.21).22 This conflicts with a similar study by Wolters and colleagues, which monitored 1,112 ICU patients who were given a median prednisone equivalent of 50 mg (interquartile range, 25-75 mg). Steroid administration was not significantly associated with the transition to delirium from an awake without delirium state (OR, 1.08; 95% CI, 0.89-1.32; adjusted OR, 1.00; 95% CI, 0.99-1.01 per 10-mg increase in prednisone equivalent).23
Mitigating Effects
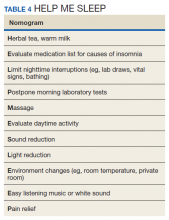
Although steroid therapy often cannot be altered in the critically ill population, research showed that steroid overuse is common in ICUs.56,57 Minimizing dosage and duration are important ways clinicians can mitigate unwanted effects. CNS AEs seen with steroids often can be reversed once therapy is discontinued. Avoiding split-dose administration has been proposed given the natural diurnal production of cortisol.58 A review by Flaherty discusses the importance of avoiding pharmacologic agents in hospitalized older patients if possible due to known risks (falls, dependency, hip fractures, rebound insomnia, and risk of delirium) and provides a HELP ME SLEEP nomogram for nonpharmacologic interventions in hospitalized patients (Table 4).59
Historically, lithium has been recommended for steroid-induced mania with chronic steroid use; however, given the large volume and electrolyte shifts seen in critically ill patients, this may not be a viable option. Antidepressants, especially tricyclics, should generally be avoided in steroid-induced psychosis as these may exacerbate symptoms. If symptoms are severe, either typical (haloperidol) or atypical (olanzapine, quetiapine, risperidone) antipsychotics have been used with success.60 Given the known depletion of serum melatonin levels, melatonin supplements are an attractive and relatively safe option for steroid-induced insomnia; however, there are no robust studies specifically aimed at this intervention for this population.
Conclusions
With known, multimodal foci driving sleep impairment in ICU patients, PADIS guidelines recommend myriad interventions for improvement. Recommendations include noise and light reduction with earplugs and/or eyeshades to improve sleep quality. Nocturnal assist-control ventilation may improve sleep quality in ventilated patients. Finally, the development of institutional protocols for promoting sleep quality in ICU patients is recommended.17
1. Simini B. Patients’ perceptions of intensive care. Lancet. 1999;354(9178):571-572. doi: 10.1016/S0140-6736(99)02728-2
2. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients—a clinical review. Ann Intensive Care. 2015;15:3. doi: 10.1186/s13613-015-0043-2
3. Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM. Quality and quantity of sleep in the surgical intensive care unit; are our patients sleeping? J Trauma. 2007;63(6):1210-1214. doi: 10.1097/TA.0b013e31815b83d7
4. Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care 2013;17(2):R46.
5. Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in patients receiving postoperative care. BJM (Clin Res Ed). 1985;290(6474)1029-1032. doi: 10.1136/bmj.290.6474.1029
6. White DP, Douglas NJ, Pickett CK, Zwillich CW, Weil JV. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128(6):984-986. doi: 10.1164/arrd.1983.128.6.984
7. Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481-485. doi: 10.1164/ajrccm.150.2.8049833
8. Tadjalli A, Peever J. Sleep loss reduces respiratory motor plasticity. Adv Exp Med Biol. 2010;669:289-292.
doi: 10.1007/978-1-4419-5692-7_59
9. Roche Campo F, Drouot X, Thille AW, et al. Poor sleep quality is associated with late noninvasive ventilation failure in patients with acute hypercapnic respiratory failure. Crit Care Med. 2010;38(2):447-485. doi: 10.1097/CCM.0b013e3181bc8243
10. Sauvet F, Leftheriotis G, Gomez-Merino D, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol (1985). 2010;108(1):68-75. doi: 10.1152/japplphysiol.00851.2009
11. Frey DJ, Fleshner M, Wright KP Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21(8):1050-1057. doi: 10.1016/j.bbi.2007.04.003
12. Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA 2002;288(12):1471-1472. doi: 10.1001/jama.288.12.1471-a
13. Dinges DF, Douglas SD, Zuagg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93(5):1930-1939. doi: 10.1172/JCI117184
14. Weinhouse GL, Schwab RJ, Watson PL, et al. Bench-to-bedside review: delirium in ICU patients— importance of sleep deprivation. Crit Care. 2009;13(6):234. doi: 10.1186/cc8131
15. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi: 10.1001/jama.291.14.1753
16. Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513-1520. doi: 10.1097/CCM.0b013e3181e47be1
17. Devlin JW, Skrobik Y, Gelinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873
18. The Boston Collaborative Drug Surveillance Program. Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther. 1972;13(5):694-698. doi: 10.1002/cpt1972135part1694
19. Rundell JR, Wise MG. Causes of organic mood disorder. J Neuropsychiatry Clin Neurosci. 1989;1(4):398-400. doi: 10.1176/jnp.1.4.398
20. Gaudreau JD, Gagnon P, Harel F, Roy MA, Tremblay A. Psychoactive medications and risk of delirium in hospitalized cancer patients. J Clin Oncol. 2005;23(27):6712-6718. doi: 10.1200/JCO.2005.05.140
21. Gaudreau JD, Gagnon P, Roy MA, Harel F, Tremblay A. Opioid medications and longitudinal risk of delirium in hospitalized cancer patients. Cancer. 2007;109(11):2365-2373.
doi: 10.1002/cncr.22665
22. Schreiber MP, Colantuoni E, Bienvenu OJ, et al. Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med. 2014;42(6):1480-1486. doi: 10.1097/CCM.0000000000000247
23. Wolters AE, Veldhuijzen DS, Zaal IJ, et al. Systemic corticosteroids and transition to delirium in critically ill patients. Crit Care Med. 2015;43(12):e585-e588. doi: 10.1097/CCM.0000000000001302
24. Matschke J, Muller-Beissenhirtz H, Novotny J, et al. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naïve adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol. 2016;136(2):101-107. doi: 10.1159/000445420
25. Tilouche N, Hassen M, Ali HBS, Jaoued AHO, Gharbi R, Atrous SS. Delirium in the intensive care unit: incidence, risk factors, and impact on outcome. Indian J Crit Care Med. 2018;22:144-149. doi: 10.4103/ijccm.IJCCM_244_17
26. Young A, Marsh S. Steroid use in critical care. BJA Education. 2018;18(5):129-134. doi: 10.1016/j.bjae.2018.01.005
27. DiPiro J, Talbert R, Yee G, Matzke GR, Wells BG, Posey M. Pharmacotherapy: A Pathophysiologic Approach. 4th ed. New York: McGraw-Hill; 1999:1277-1278.
28. Schimmer
29. Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence of US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33(10):1413-1432.
30. Idzikowsi C, Shapiro CM. ABC of sleep disorders, non-psychotropic drugs and sleep. BMJ. 1993;306(6885):1118-1120. doi: 10.1136/bmj.306.6885.1118

31. Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14(4):398-406.
doi: 10.3109/10253890.2011.586446
32. Wolkowitz OM, Reus VI, Weingartner H, et al. Cognitive effects of corticosteroids. Am J Psychiatry 1990;147(10):1297-1303. doi: 10.1176/ajp.147.10.1297
33. McEwen BS, Davis PG, Parsons B, Pfaff DW. The brain as a target for steroid hormone action. Ann Rev Neurosci. 1979;2:65-112. doi: 10.1146/annurev.ne.02.030179.000433
34. Brown ES, Woolston DJ, Frol AM. Amygdala volume in patients receiving chronic corticosteroid therapy. Biol Psychiatry. 2008;63(7):705-709.
doi: 10.1016/j.biopsych.2007.09.014
35. Brown ES, Woolston D, Frol A, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid. Biol Psychiatry. 2004;55(5):538-545.
36. Sapolsky RM, McEwen BS. Down-regulation of neural corticosterone receptors by corticosterone and dexamethasone. Brain Res. 1985;339(1):161-165.
doi: 10.1016/0006-8993(85)90638-9
37. Sorrells SF, Caso JR, Munhoz CD, Spolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64(1):33-39.
doi: 10.1016/j.neuron.2009.09.032
38. Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids: mood, memory, and mechanisms. Ann NY Acad Sci. 2009;1179:19-40. doi: 10.1111/j.1749-6632.2009.04980.x
39. Wolkowitz OM, Epel ES, Reus VI. Stress hormone-related psychopathology: pathophysiological and treatment implications. World J Biol Psychiatry. 2001;2(3):115-143. doi: 10.3109/15622970109026799
40. Paredes S, Barriga C, Reiter R, Rodrigues A. Assessment of the potential role of tryptophan as the precursor of serotonin and melatonin for the aged sleep-wake cycle and immune function: Streptopelia Risoria as a model. Int J Tryptophan Res. 2009;2:23-36. doi: 10.4137/ijtr.s1129
41. Soszyński P, Stowińska-Srzednicka J, Kasperlik-Zatuska A, Zgliczyński S. Decreased melatonin concentration in Cushing’s Syndrome. Horm Metab Res. 1989;21(12):673-674. doi: 10.1055/s-2007-1009317
42. Demish L, Demish K, Neckelsen T. Influence of dexamethasone on nocturnal melatonin production in healthy adult subjects. J Pineal Res. 1988;5(3):317-321. doi: 10.1111/j.1600-079x.1988.tb00657.x
43. Assaf N, Shalby AB, Khalil WK, Ahmed HH. Biochemical and genetic alterations of oxidant/antioxidant status of the brain in rats treated with dexamethasone: protective roles of melatonin and acetyl-L-carnitine. J Physiol Biochem. 2012;68(1):77-90. doi: 10.1007/s13105-011-0121-3
44. Clark MS, Russo AF. Tissue-specific glucocorticoid regulation of tryptophan hydroxylase mRNA levels. Brain Res Mol Brain Res. 1997;48(2):346-54. doi: 10.1016/s0169-328x(97)00106-x
45. Kram DE, Krasnow SM, Levasseur PR, Zhu X, Stork LC, Marks DL. Dexamethasone chemotherapy does not disrupt orexin signaling. PLoS One. 2016;11(12):e0168731. doi: 10.1371/journal.pone.0168731
46. Mellon S. Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78(5):1003-1008. doi: 10.1210/jcem.78.5.8175951
47. Zorumski C, Paul SM, Izumi Y, Covey DF, Mennerick S . Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev. 2013;37(1):109-122. doi: 10.1016/j.neubiorev.2012.10.005
48. Monteleone P, Luisi M, Martiadis V, et al. Impaired reduction of enhanced levels of dehydroepiandrosterone by oral dexamethasone in anorexia nervosa. Psychoneuroendocrinology. 2006;31(4):537-542. doi: 10.1016/j.psyneuen.2005.08.015
49. Genazzani AR, Petraglia F, Bernardi F, et al. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83(6):2099-3103. doi: 10.1210/jcem.83.6.4905
50. Moser NJ, Phillips BA, Guthrie G, Barnett G. Effects of dexamethasone on sleep. Pharmacol Toxicol. 1996;79(2):100-102. doi: 10.1111/j.1600-0773.1996.tb00249.x
51. Curtis J, Westfall A, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420-426. doi: 10.1002/art.21984
52. Zhao J, Dai YH, Xi QS, Yu SY. A clinical study on insomnia in patients with cancer during chemotherapy containing high-dose glucocorticoids. Pharmazie. 2013;68(6):421-427
53. Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days corticosteroid treatment. A prospective study. Psychoneuroendocrinology. 1996;21(1):25-31. doi: 10.1016/0306-4530(95)00031-3
54. Brown ES, Suppes T, Khan DA, Carmody TJ 3rd. Mood changes during prednisone bursts in outpatients with asthma. J Clin Psychopharmacol. 2002;22(1):55-61.
doi: 10.1097/00004714-200202000-00009
55. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367. doi: 10.4065/81.10.1361
56. Britt RC, Devine A, Swallen KC et al. Corticosteroid use in the intensive care unit: at what cost? Arch Surg. 2006;141(2):145-159. doi:10.1001/archsurg.141.2.145
57. Kiser TH, Allen RR, Valuck RJ, Moss M, Vanivier RW. Outcomes associated with corticosteroid dosage in critically ill patients in acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):1052-1064. doi: 10.1164/rccm.201401-0058OC
58. Bourne RS, Mills GH. Sleep disruption in critically ill patients—pharmacological considerations. Anaesthesia. 2004;59(4):374-384. doi: 10.1111/j. 1365-2044.2004.03664.x
59. Flaherty JH. Insomnia among hospitalized older persons. Clin Geriatr Med. 2008;24(1):51-67. doi: 10.1016/j.cger.2007.08.012
60. Sirios F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25(1):27-33. doi: 10.1016/s0163-8343(02)00241-4
Sleep disturbance in the critically ill has received much attention over recent years as this is a common result of intensive care unit (ICU) admission. Disruptions in sleep not only can, at a minimum, cause distress and lower patient satisfaction, but also inhibit recovery from illness and increase morbidity.1,2 Several studies have been conducted highlighting the altered sleep patterns of critically ill patients; although total sleep time may seem normal (7-9 hours), patients can experience multiple awakenings per hour, more time in light sleep (stages 1 and 2), and less time in restorative sleep (stages 3 and 4, [REM]rapid eye movement).2-5
There are several hypothesized physiologic detriments that contribute to slower ICU recovery with sleep deprivation. Research in noncritically ill subjects suggests that sleep deprivation contributes to hypoventilation and potentially prolonged time on the ventilator.6-9 Cardiovascular morbidity may be adversely affected by inflammatory cytokine release seen in sleep disruption.10,11 Studies of noncritically ill patients also suggest that immune response is impaired, potentially protracting infection recovery.12,13 Finally, although not directly investigated, sleep deprivation may contribute to ICU delirium, an independent adverse effect (AE) associated with increased mortality and worse long-term outcomes.14-16
The Society of Critical Care Medicine (SCCM) recently updated its consensus guidelines for the management of pain, agitation/sedation, delirium, immobility, and sleep disruption (PADIS) in adult patients.17 These guidelines offer limited interventions to promote sleep in ICU patients based on available evidence and steer the clinician toward minimizing exacerbating factors. Although factors that affect sleep patterns are multifactorial, such as noise levels, pain, mechanical ventilation, and inflammatory mediators, medication therapy is a known modifiable risk factor for sleep disturbance in critically ill patients.2 This focused review will specifically evaluate the effects of steroids on sleep deprivation, psychosis, delirium, and what is known about these effects in a critically ill population.
To include articles relevant to a critically ill population, a systematic search of MEDLINE and PubMed from 1966 to 2019 was performed using the following Medical Subject Headings (MeSH) terms: delirium/etiology, psychoses, substance-induced/etiology, sleep-wake disorders/chemically induced, neurocognitive disorders/chemically induced, dyssomnias/drug effects plus glucocorticoids/adverse effects, adrenal cortex hormones/adverse effects, prednisone/adverse effects, methylprednisolone/adverse effects, and hydrocortisone/adverse effects. The initial search produced 285 articles. Case reports, reviews, letters, and articles pertaining to primary care or palliative populations were excluded, leaving 8 relevant articles for inclusion (Table 1).18-25
ICU Steroid Use
Steroids are commonly used in the ICU and affect nearly every critically ill population. Common indications for steroids in the ICU include anaphylaxis, airway edema, septic shock, asthma and COPD exacerbations, pneumocystis pneumonia, adrenal crisis, antiemetic treatment, elevated intracranial pressure from tumors, autoimmune disorders, and stress doses needed for chronic steroid users before invasive procedures.26 Whether divided into glucocorticoid or mineralocorticoid subgroups, corticosteroids offer therapeutic benefit from their pharmacologic similarity to endogenously produced cortisol, which includes anti-inflammatory, immunosuppressive, antiproliferative, and vasoconstrictive effects.
Steroid receptors are present in most human tissue, and in varying degrees of binding affinity produce a wide variety of effects. After passive diffusion across cell membranes, steroid-receptor activation binds to various DNA sites, called glucocorticoid regulatory elements, which either stimulates or inhibits transcription of multiple nearby genes.
At the cellular level, corticosteroids inhibit the release of arachidonic acid through upstream production of lipocortin peptides and antagonism of phospholipase A2. This action decreases subsequent inflammatory mediators, including kinins, histamine, liposomal enzymes, and prostaglandins. Steroids also inhibit NF-κB, which further decreases expression of proinflammatory genes while promoting interleukin-10 and its anti-inflammatory properties. Antiproliferative effects of steroids are seen by triggering cell apoptosis and inhibition of fibroblast proliferation.27,28
By binding to mineralocorticoid receptors, steroids cause sodium retention coupled with hydrogen and potassium excretion in the distal renal tubule. Steroids also promote vasoconstriction by upregulating the production and sensitivity of β receptors in the endothelium while suppressing the production of vasodilators. Although rarely used for these physiologic effects, steroids also are involved in a number of metabolic pathways, including calcium regulation, gluconeogenesis, protein metabolism, and fat distribution. Given the similar structure to cortisol, exogenous steroids depress the hypothalamic-pituitary axis (HPA) and decrease the release of adrenocorticotropic hormone (ACTH). Tapering doses of steroid regimens is often required to allow natural androgen and cortisol synthesis and prevent steroid withdrawal.27,28
The potency of various exogenous steroids closely parallels their ability to retain sodium (Table 2). Prolonged activation of steroid receptors can have numerous systemic AEs, including unwanted neurocognitive effects (Table 3). Insomnia and psychosis are commonly described in corticosteroid clinical trials, and in one meta-analysis, both are associated with high costs per episode per year.29
Steroid-Induced Sleep Disruption and Psychosis
Sleep disruption caused by exogenous administration of steroids is thought to trigger other psychostimulant effects, such as mood swings, nervousness, psychoses, and delirium.30 Similarly, the SCCM PADIS guidelines included an ungraded statement: “although an association between sleep quality and delirium occurrence exists in critically ill adults, a cause-effect relationship has not been established.”17 For this review, these AEs will be discussed as related events.
The medical literature proposes 3 pathways primarily responsible for neurocognitive AEs of steroids: behavior changes through modification of the HPA axis, changes in natural sleep-wake cycles, and hyperarousal caused by modification in neuroinhibitory pathways (Figure).
HPA Axis Modification
Under either physical or psychological stress, neural circuits in the brain release corticotropin-releasing hormone (CRH), dehydroepiandrosterone (DHEA), and arginine vasopressin, which go on to activate the sympathetic nervous system and the HPA axis. CRH from the hypothalamus goes on to stimulate ACTH release from the pituitary. ACTH then stimulates cortisol secretion from the adrenal glands. Circulating cortisol feeds into several structures of the brain, including the pituitary, hippocampus, and amygdala. Steroid-receptor complexes alter gene transcription in the central nervous system (CNS), affecting the production of neurotransmitters (eg, dopamine, serotonin) and neuropeptides (eg, somatostatin, β-endorphin). Feedback inhibition ensues, with downregulation of the HPA axis, which prevents depletion of endogenous production of steroids.31 DHEA has protective effects against excessive cortisol activity, but DHEA secretion declines with prolonged cortisol exposure. Exogenous steroids may have different effects than endogenous steroids, and neurocognitive sequelae stem from disruption and imbalance of these physiologic mechanisms.32,33
Steroid receptors are densely located in behavior centers in the brain: the amygdala, septum, and hippocampus. Pharmacologic changes in gene expression alter norepinephrine and serotonin levels in the brain as well as their receptors.32 Prolonged exposure to exogenous steroids has been shown to decrease amygdala and hippocampal volumes.34,35 Furthermore, prolonged corticosteroid exposure has been shown to decrease the number of steroid receptors in the hippocampus, pituitary gland, and amygdala.36 In a somewhat paradoxical finding, the production of CNS proinflammatory cytokines like interleuken-1β and tumor necrosis factor α has been seen after steroid administration, suggesting alternate gene signaling in the CNS.37 Although not proven conclusively, it is felt that these physiologic changes and hyperactivity of the HPA axis are predominantly responsible for changes in behavior, mood, memory, and eventually psychosis in steroid-treated patients.33,38
Finally, alterations in cognition and behavior may be related to steroid-induced changes in CNS carbohydrate, protein, and lipid metabolism with subsequent cellular neurotoxicity.32,38 Glucose uptake into the hippocampus is decreased with steroid exposure. Additionally, breakdown of metabolic compounds to produce energy can be destructive if left unchecked for prolonged periods. DHEA, growth hormone, and testosterone work to repair catabolic damage produced by cortisol, known as anabolic balance. A low anabolic balance (low DHEA levels to high cortisol levels) leads to a cascade of dysregulation in brain activity.39
Changes in Natural Sleep-Wake Cycles
Natural sleep pathways are also affected by steroids. The sleep-wake cycle is primarily regulated in the hypothalamus with circadian release of melatonin from the pineal gland. Melatonin release is highest at night, where it promotes sleep onset and continuity. Upstream, tryptophan is an amino acid that serves as a precursor to serotonin and melatonin.40 Both endogenous and exogenous corticosteroids decrease serum melatonin levels with a markedly diminished circadian rhythm secretion.41,42Demish and colleagues found a significant decrease in mean (SD) nocturnal melatonin plasma levels after the evening administration of oral dexamethasone 1 mg in 11 healthy volunteers: 127 (42) pg/mL before vs 73 (38) pg/mL after; P < .01.42 This result is likely due to decreased cellular metabolism and melatonin synthesis in the pineal gland. Of note, melatonin has neuroprotective affects, and the administration of melatonin has been shown to reverse some steroid-induced neurotoxicities in animal models.43
Steroids also reduce the uptake of tryptophan into the brain.33 Additionally, in animal models, dexamethasone administration caused a significant decrease in the gene expression of tryptophan hydroxylase, which is part of the multistep pathway in synthesizing serotonin from L-tryptophan. These effects upstream could inhibit the biosynthetic capacity of both melatonin and serotonin.44
A third pathway investigated in sleep regulation are the orexin neuropeptides. Orexins are produced in the hypothalamus and stimulate daytime wake activity in monoaminergic and cholinergic neurons. Subsequently, orexin receptor antagonists are a newer class of drugs aimed at mitigating nighttime hyperarousal and sleep disruption. Orexin overexpression may be a causal factor in steroid-induced sleep disturbance. However, this effect was specifically evaluated in a recent study in children with acute lymphoblastic leukemia, which showed that cerebral spinal fluid orexin levels (SD) were not significantly different from baseline after dexamethasone administration: 574 (26.6) pg/mL vs 580 (126.1) pg/mL; P = .8.45
Hyperarousal State
Finally, a hyperarousal state is thought to be produced by nongenomic changes to natural neuroinhibitory regulation seen with nonclassical steroid production called neurosteroids. Animal studies revealed that high levels of steroids were found in the CNS long after adrenalectomy, suggesting CNS de novo synthesis.46 In addition to altering gene expression at classic intercellular steroid receptors, neurosteroids can alter neurotransmission by direct interaction on ion-gated membranes and other receptors on the cell surface. Restlessness and insomnia could be due to γ-aminobutyric acid type A (GABAA) receptor modulation in the CNS where neuroactive steroids slow the rate of recovery of GABAA and potentially inhibit postsynaptic GABAergic transmission. It also is hypothesized that neuroactive steroids have excitatory action at nicotinic acetylcholine, 5HT3 receptors, and through increasing the fractional open time of the N-methyl-D-aspartate -activated channels.47 Allopregnanolone and DHEA are neurosteroids that act as GABAA agonists and have neuroprotective effects with anxiolytic, antidepressant, and antiaggressive properties.
Neurosteroids are synthesized from cholesterol in the hippocampus. Neurosteroids are upregulated in response to stress by CNS cortisol effects on various enzyme expressions.47 Whether exogenous steroid administration affects this biosynthesis vs the stress response in the HPA axis itself is not fully elucidated. Monteleone and colleagues found that dexamethasone 1 mg given orally significantly reduced cortisol and DHEA and allopregnanolone levels in both healthy volunteers and anorexia nervosa patients.48 Similarly, Genazzani and colleagues demonstrated that oral dexamethasone administration (0.5 mg every 6 hours) caused significant reductions in both serum allopregnanolone and DHEA levels.49
Outcomes Studies
The majority of reported data in steroid-induced insomnia and psychosis is in noncritically ill populations. In a randomized, prospective crossover study of healthy volunteers, dexamethasone administration (3 mg every 8 hours for 48 hours) resulted in significant changes in sleep patterns measured with polysomnography. Compared with placebo, steroid treatment showed significantly longer percentage (SD) of stage 0/awake times (11.7% [11.4] vs 2.9% [1.8]; P < .05); longer percentage (SD) of REM sleep latency (363.8 [74.5] minutes vs 202.8 [79.6] minutes; P < .01), and a reduced number (SD) of REM periods (3.8 [2.6] vs 9.7 [3.6]; P < .01).50 Insomnia was one of the most commonly self-reported AEs (> 60%) in a survey of 2,446 chronic steroid users, and the incidence increased as steroid doses increased.51
A prospective, open-label study of 240 patients with cancer demonstrated significant sleep disruptions using the Pittsburgh Sleep Quality Index with the use of high-dose steroids in chemotherapy.52 Naber and colleagues evaluated 50 previously healthy patients taking methylprednisolone 119 mg (41 mg/d) for retinitis and uveitis.53 They reported 26% to 34% of subjects experienced hypomanic syndrome based on a semistructured interview examination. Symptoms developed within 3 days and persisted for the 8-day course of therapy. Brown and colleagues prospectively evaluated 32 asthmatic patients prescribed bursts of prednisone > 40 mg daily. They observed significantly increased scores in the Young Mania Rating Scale within 3 to 7 days of starting therapy, which dissipated to baseline after stopping therapy.54
Despite a high reported incidence of neurologic AEs, outcomes in critically ill populations are mixed. Study methods are varied, and many were largely observational. No prospective, randomized studies exist to date specifically aimed and powered to evaluate the effects of steroids on sleep disturbances or delirium in a critically ill population. Furthermore, sleep quality is difficult to measure in this population, and self-reporting often is not an option. In critical care trials, if AEs such as insomnia, delirium, or psychosis are recorded at all, there is heterogeneity in the definitions, and these AEs are generally poorly defined (eg, psychiatric or neurologic disorder not otherwise specified), making pooled analysis of this outcome difficult.55
One of the largest observational studies in hospitalized patients was through the Boston Collaborative Drug Surveillance Program. A total of 718 consecutively enrolled inpatients who received prednisone were monitored for acute reactions. Psychiatric AEs were rare (1.3%) with low doses (< 40 mg/d), more prevalent (4.6%) with higher doses (41-80 mg/d), and most prevalent (18.4%) with the highest doses (> 80 mg/d), suggesting CNS AEs are dose dependent.18 A single-center, retrospective review of 755 psychiatric consults in hospitalized patients revealed that 54% of manic patients were due to corticosteroid administration.19 In a prospective observational study of 206 consecutive ICU admissions, steroid administration was an independent risk factor for development of ICU delirium, using the Confusion Assessment Method-ICU (CAM-ICU) at a single center (odds ratio [OR], 2.8; 95% CI, 1.05-7.28).25
Two studies in hospitalized oncology patients found conflicting results using the Nursing Delirium Screening Scale (Nu-DESC). One did not find a significant association between delirium and dexamethasone equivalent doses > 15 mg, while the second found an increased hazard ratio (HR) for a positive Nu-DESC score (HR, 2.67; 95% CI, 1.18-6.03).20,21 Similarly, conflicting results were found in 2 studies using first-order Markov models. In one prospective cohort study, 520 consecutive mechanically ventilated patients in 13 ICUs were monitored for the transition to delirium (CAM-ICU positive) from nondelirium states. Steroid administration was significantly associated with transitioning to delirium (OR, 1.52; 95% CI, 1.05-2.21).22 This conflicts with a similar study by Wolters and colleagues, which monitored 1,112 ICU patients who were given a median prednisone equivalent of 50 mg (interquartile range, 25-75 mg). Steroid administration was not significantly associated with the transition to delirium from an awake without delirium state (OR, 1.08; 95% CI, 0.89-1.32; adjusted OR, 1.00; 95% CI, 0.99-1.01 per 10-mg increase in prednisone equivalent).23
Mitigating Effects
Although steroid therapy often cannot be altered in the critically ill population, research showed that steroid overuse is common in ICUs.56,57 Minimizing dosage and duration are important ways clinicians can mitigate unwanted effects. CNS AEs seen with steroids often can be reversed once therapy is discontinued. Avoiding split-dose administration has been proposed given the natural diurnal production of cortisol.58 A review by Flaherty discusses the importance of avoiding pharmacologic agents in hospitalized older patients if possible due to known risks (falls, dependency, hip fractures, rebound insomnia, and risk of delirium) and provides a HELP ME SLEEP nomogram for nonpharmacologic interventions in hospitalized patients (Table 4).59
Historically, lithium has been recommended for steroid-induced mania with chronic steroid use; however, given the large volume and electrolyte shifts seen in critically ill patients, this may not be a viable option. Antidepressants, especially tricyclics, should generally be avoided in steroid-induced psychosis as these may exacerbate symptoms. If symptoms are severe, either typical (haloperidol) or atypical (olanzapine, quetiapine, risperidone) antipsychotics have been used with success.60 Given the known depletion of serum melatonin levels, melatonin supplements are an attractive and relatively safe option for steroid-induced insomnia; however, there are no robust studies specifically aimed at this intervention for this population.
Conclusions
With known, multimodal foci driving sleep impairment in ICU patients, PADIS guidelines recommend myriad interventions for improvement. Recommendations include noise and light reduction with earplugs and/or eyeshades to improve sleep quality. Nocturnal assist-control ventilation may improve sleep quality in ventilated patients. Finally, the development of institutional protocols for promoting sleep quality in ICU patients is recommended.17
Sleep disturbance in the critically ill has received much attention over recent years as this is a common result of intensive care unit (ICU) admission. Disruptions in sleep not only can, at a minimum, cause distress and lower patient satisfaction, but also inhibit recovery from illness and increase morbidity.1,2 Several studies have been conducted highlighting the altered sleep patterns of critically ill patients; although total sleep time may seem normal (7-9 hours), patients can experience multiple awakenings per hour, more time in light sleep (stages 1 and 2), and less time in restorative sleep (stages 3 and 4, [REM]rapid eye movement).2-5
There are several hypothesized physiologic detriments that contribute to slower ICU recovery with sleep deprivation. Research in noncritically ill subjects suggests that sleep deprivation contributes to hypoventilation and potentially prolonged time on the ventilator.6-9 Cardiovascular morbidity may be adversely affected by inflammatory cytokine release seen in sleep disruption.10,11 Studies of noncritically ill patients also suggest that immune response is impaired, potentially protracting infection recovery.12,13 Finally, although not directly investigated, sleep deprivation may contribute to ICU delirium, an independent adverse effect (AE) associated with increased mortality and worse long-term outcomes.14-16
The Society of Critical Care Medicine (SCCM) recently updated its consensus guidelines for the management of pain, agitation/sedation, delirium, immobility, and sleep disruption (PADIS) in adult patients.17 These guidelines offer limited interventions to promote sleep in ICU patients based on available evidence and steer the clinician toward minimizing exacerbating factors. Although factors that affect sleep patterns are multifactorial, such as noise levels, pain, mechanical ventilation, and inflammatory mediators, medication therapy is a known modifiable risk factor for sleep disturbance in critically ill patients.2 This focused review will specifically evaluate the effects of steroids on sleep deprivation, psychosis, delirium, and what is known about these effects in a critically ill population.
To include articles relevant to a critically ill population, a systematic search of MEDLINE and PubMed from 1966 to 2019 was performed using the following Medical Subject Headings (MeSH) terms: delirium/etiology, psychoses, substance-induced/etiology, sleep-wake disorders/chemically induced, neurocognitive disorders/chemically induced, dyssomnias/drug effects plus glucocorticoids/adverse effects, adrenal cortex hormones/adverse effects, prednisone/adverse effects, methylprednisolone/adverse effects, and hydrocortisone/adverse effects. The initial search produced 285 articles. Case reports, reviews, letters, and articles pertaining to primary care or palliative populations were excluded, leaving 8 relevant articles for inclusion (Table 1).18-25
ICU Steroid Use
Steroids are commonly used in the ICU and affect nearly every critically ill population. Common indications for steroids in the ICU include anaphylaxis, airway edema, septic shock, asthma and COPD exacerbations, pneumocystis pneumonia, adrenal crisis, antiemetic treatment, elevated intracranial pressure from tumors, autoimmune disorders, and stress doses needed for chronic steroid users before invasive procedures.26 Whether divided into glucocorticoid or mineralocorticoid subgroups, corticosteroids offer therapeutic benefit from their pharmacologic similarity to endogenously produced cortisol, which includes anti-inflammatory, immunosuppressive, antiproliferative, and vasoconstrictive effects.
Steroid receptors are present in most human tissue, and in varying degrees of binding affinity produce a wide variety of effects. After passive diffusion across cell membranes, steroid-receptor activation binds to various DNA sites, called glucocorticoid regulatory elements, which either stimulates or inhibits transcription of multiple nearby genes.
At the cellular level, corticosteroids inhibit the release of arachidonic acid through upstream production of lipocortin peptides and antagonism of phospholipase A2. This action decreases subsequent inflammatory mediators, including kinins, histamine, liposomal enzymes, and prostaglandins. Steroids also inhibit NF-κB, which further decreases expression of proinflammatory genes while promoting interleukin-10 and its anti-inflammatory properties. Antiproliferative effects of steroids are seen by triggering cell apoptosis and inhibition of fibroblast proliferation.27,28
By binding to mineralocorticoid receptors, steroids cause sodium retention coupled with hydrogen and potassium excretion in the distal renal tubule. Steroids also promote vasoconstriction by upregulating the production and sensitivity of β receptors in the endothelium while suppressing the production of vasodilators. Although rarely used for these physiologic effects, steroids also are involved in a number of metabolic pathways, including calcium regulation, gluconeogenesis, protein metabolism, and fat distribution. Given the similar structure to cortisol, exogenous steroids depress the hypothalamic-pituitary axis (HPA) and decrease the release of adrenocorticotropic hormone (ACTH). Tapering doses of steroid regimens is often required to allow natural androgen and cortisol synthesis and prevent steroid withdrawal.27,28
The potency of various exogenous steroids closely parallels their ability to retain sodium (Table 2). Prolonged activation of steroid receptors can have numerous systemic AEs, including unwanted neurocognitive effects (Table 3). Insomnia and psychosis are commonly described in corticosteroid clinical trials, and in one meta-analysis, both are associated with high costs per episode per year.29
Steroid-Induced Sleep Disruption and Psychosis
Sleep disruption caused by exogenous administration of steroids is thought to trigger other psychostimulant effects, such as mood swings, nervousness, psychoses, and delirium.30 Similarly, the SCCM PADIS guidelines included an ungraded statement: “although an association between sleep quality and delirium occurrence exists in critically ill adults, a cause-effect relationship has not been established.”17 For this review, these AEs will be discussed as related events.
The medical literature proposes 3 pathways primarily responsible for neurocognitive AEs of steroids: behavior changes through modification of the HPA axis, changes in natural sleep-wake cycles, and hyperarousal caused by modification in neuroinhibitory pathways (Figure).
HPA Axis Modification
Under either physical or psychological stress, neural circuits in the brain release corticotropin-releasing hormone (CRH), dehydroepiandrosterone (DHEA), and arginine vasopressin, which go on to activate the sympathetic nervous system and the HPA axis. CRH from the hypothalamus goes on to stimulate ACTH release from the pituitary. ACTH then stimulates cortisol secretion from the adrenal glands. Circulating cortisol feeds into several structures of the brain, including the pituitary, hippocampus, and amygdala. Steroid-receptor complexes alter gene transcription in the central nervous system (CNS), affecting the production of neurotransmitters (eg, dopamine, serotonin) and neuropeptides (eg, somatostatin, β-endorphin). Feedback inhibition ensues, with downregulation of the HPA axis, which prevents depletion of endogenous production of steroids.31 DHEA has protective effects against excessive cortisol activity, but DHEA secretion declines with prolonged cortisol exposure. Exogenous steroids may have different effects than endogenous steroids, and neurocognitive sequelae stem from disruption and imbalance of these physiologic mechanisms.32,33
Steroid receptors are densely located in behavior centers in the brain: the amygdala, septum, and hippocampus. Pharmacologic changes in gene expression alter norepinephrine and serotonin levels in the brain as well as their receptors.32 Prolonged exposure to exogenous steroids has been shown to decrease amygdala and hippocampal volumes.34,35 Furthermore, prolonged corticosteroid exposure has been shown to decrease the number of steroid receptors in the hippocampus, pituitary gland, and amygdala.36 In a somewhat paradoxical finding, the production of CNS proinflammatory cytokines like interleuken-1β and tumor necrosis factor α has been seen after steroid administration, suggesting alternate gene signaling in the CNS.37 Although not proven conclusively, it is felt that these physiologic changes and hyperactivity of the HPA axis are predominantly responsible for changes in behavior, mood, memory, and eventually psychosis in steroid-treated patients.33,38
Finally, alterations in cognition and behavior may be related to steroid-induced changes in CNS carbohydrate, protein, and lipid metabolism with subsequent cellular neurotoxicity.32,38 Glucose uptake into the hippocampus is decreased with steroid exposure. Additionally, breakdown of metabolic compounds to produce energy can be destructive if left unchecked for prolonged periods. DHEA, growth hormone, and testosterone work to repair catabolic damage produced by cortisol, known as anabolic balance. A low anabolic balance (low DHEA levels to high cortisol levels) leads to a cascade of dysregulation in brain activity.39
Changes in Natural Sleep-Wake Cycles
Natural sleep pathways are also affected by steroids. The sleep-wake cycle is primarily regulated in the hypothalamus with circadian release of melatonin from the pineal gland. Melatonin release is highest at night, where it promotes sleep onset and continuity. Upstream, tryptophan is an amino acid that serves as a precursor to serotonin and melatonin.40 Both endogenous and exogenous corticosteroids decrease serum melatonin levels with a markedly diminished circadian rhythm secretion.41,42Demish and colleagues found a significant decrease in mean (SD) nocturnal melatonin plasma levels after the evening administration of oral dexamethasone 1 mg in 11 healthy volunteers: 127 (42) pg/mL before vs 73 (38) pg/mL after; P < .01.42 This result is likely due to decreased cellular metabolism and melatonin synthesis in the pineal gland. Of note, melatonin has neuroprotective affects, and the administration of melatonin has been shown to reverse some steroid-induced neurotoxicities in animal models.43
Steroids also reduce the uptake of tryptophan into the brain.33 Additionally, in animal models, dexamethasone administration caused a significant decrease in the gene expression of tryptophan hydroxylase, which is part of the multistep pathway in synthesizing serotonin from L-tryptophan. These effects upstream could inhibit the biosynthetic capacity of both melatonin and serotonin.44
A third pathway investigated in sleep regulation are the orexin neuropeptides. Orexins are produced in the hypothalamus and stimulate daytime wake activity in monoaminergic and cholinergic neurons. Subsequently, orexin receptor antagonists are a newer class of drugs aimed at mitigating nighttime hyperarousal and sleep disruption. Orexin overexpression may be a causal factor in steroid-induced sleep disturbance. However, this effect was specifically evaluated in a recent study in children with acute lymphoblastic leukemia, which showed that cerebral spinal fluid orexin levels (SD) were not significantly different from baseline after dexamethasone administration: 574 (26.6) pg/mL vs 580 (126.1) pg/mL; P = .8.45
Hyperarousal State
Finally, a hyperarousal state is thought to be produced by nongenomic changes to natural neuroinhibitory regulation seen with nonclassical steroid production called neurosteroids. Animal studies revealed that high levels of steroids were found in the CNS long after adrenalectomy, suggesting CNS de novo synthesis.46 In addition to altering gene expression at classic intercellular steroid receptors, neurosteroids can alter neurotransmission by direct interaction on ion-gated membranes and other receptors on the cell surface. Restlessness and insomnia could be due to γ-aminobutyric acid type A (GABAA) receptor modulation in the CNS where neuroactive steroids slow the rate of recovery of GABAA and potentially inhibit postsynaptic GABAergic transmission. It also is hypothesized that neuroactive steroids have excitatory action at nicotinic acetylcholine, 5HT3 receptors, and through increasing the fractional open time of the N-methyl-D-aspartate -activated channels.47 Allopregnanolone and DHEA are neurosteroids that act as GABAA agonists and have neuroprotective effects with anxiolytic, antidepressant, and antiaggressive properties.
Neurosteroids are synthesized from cholesterol in the hippocampus. Neurosteroids are upregulated in response to stress by CNS cortisol effects on various enzyme expressions.47 Whether exogenous steroid administration affects this biosynthesis vs the stress response in the HPA axis itself is not fully elucidated. Monteleone and colleagues found that dexamethasone 1 mg given orally significantly reduced cortisol and DHEA and allopregnanolone levels in both healthy volunteers and anorexia nervosa patients.48 Similarly, Genazzani and colleagues demonstrated that oral dexamethasone administration (0.5 mg every 6 hours) caused significant reductions in both serum allopregnanolone and DHEA levels.49
Outcomes Studies
The majority of reported data in steroid-induced insomnia and psychosis is in noncritically ill populations. In a randomized, prospective crossover study of healthy volunteers, dexamethasone administration (3 mg every 8 hours for 48 hours) resulted in significant changes in sleep patterns measured with polysomnography. Compared with placebo, steroid treatment showed significantly longer percentage (SD) of stage 0/awake times (11.7% [11.4] vs 2.9% [1.8]; P < .05); longer percentage (SD) of REM sleep latency (363.8 [74.5] minutes vs 202.8 [79.6] minutes; P < .01), and a reduced number (SD) of REM periods (3.8 [2.6] vs 9.7 [3.6]; P < .01).50 Insomnia was one of the most commonly self-reported AEs (> 60%) in a survey of 2,446 chronic steroid users, and the incidence increased as steroid doses increased.51
A prospective, open-label study of 240 patients with cancer demonstrated significant sleep disruptions using the Pittsburgh Sleep Quality Index with the use of high-dose steroids in chemotherapy.52 Naber and colleagues evaluated 50 previously healthy patients taking methylprednisolone 119 mg (41 mg/d) for retinitis and uveitis.53 They reported 26% to 34% of subjects experienced hypomanic syndrome based on a semistructured interview examination. Symptoms developed within 3 days and persisted for the 8-day course of therapy. Brown and colleagues prospectively evaluated 32 asthmatic patients prescribed bursts of prednisone > 40 mg daily. They observed significantly increased scores in the Young Mania Rating Scale within 3 to 7 days of starting therapy, which dissipated to baseline after stopping therapy.54
Despite a high reported incidence of neurologic AEs, outcomes in critically ill populations are mixed. Study methods are varied, and many were largely observational. No prospective, randomized studies exist to date specifically aimed and powered to evaluate the effects of steroids on sleep disturbances or delirium in a critically ill population. Furthermore, sleep quality is difficult to measure in this population, and self-reporting often is not an option. In critical care trials, if AEs such as insomnia, delirium, or psychosis are recorded at all, there is heterogeneity in the definitions, and these AEs are generally poorly defined (eg, psychiatric or neurologic disorder not otherwise specified), making pooled analysis of this outcome difficult.55
One of the largest observational studies in hospitalized patients was through the Boston Collaborative Drug Surveillance Program. A total of 718 consecutively enrolled inpatients who received prednisone were monitored for acute reactions. Psychiatric AEs were rare (1.3%) with low doses (< 40 mg/d), more prevalent (4.6%) with higher doses (41-80 mg/d), and most prevalent (18.4%) with the highest doses (> 80 mg/d), suggesting CNS AEs are dose dependent.18 A single-center, retrospective review of 755 psychiatric consults in hospitalized patients revealed that 54% of manic patients were due to corticosteroid administration.19 In a prospective observational study of 206 consecutive ICU admissions, steroid administration was an independent risk factor for development of ICU delirium, using the Confusion Assessment Method-ICU (CAM-ICU) at a single center (odds ratio [OR], 2.8; 95% CI, 1.05-7.28).25
Two studies in hospitalized oncology patients found conflicting results using the Nursing Delirium Screening Scale (Nu-DESC). One did not find a significant association between delirium and dexamethasone equivalent doses > 15 mg, while the second found an increased hazard ratio (HR) for a positive Nu-DESC score (HR, 2.67; 95% CI, 1.18-6.03).20,21 Similarly, conflicting results were found in 2 studies using first-order Markov models. In one prospective cohort study, 520 consecutive mechanically ventilated patients in 13 ICUs were monitored for the transition to delirium (CAM-ICU positive) from nondelirium states. Steroid administration was significantly associated with transitioning to delirium (OR, 1.52; 95% CI, 1.05-2.21).22 This conflicts with a similar study by Wolters and colleagues, which monitored 1,112 ICU patients who were given a median prednisone equivalent of 50 mg (interquartile range, 25-75 mg). Steroid administration was not significantly associated with the transition to delirium from an awake without delirium state (OR, 1.08; 95% CI, 0.89-1.32; adjusted OR, 1.00; 95% CI, 0.99-1.01 per 10-mg increase in prednisone equivalent).23
Mitigating Effects
Although steroid therapy often cannot be altered in the critically ill population, research showed that steroid overuse is common in ICUs.56,57 Minimizing dosage and duration are important ways clinicians can mitigate unwanted effects. CNS AEs seen with steroids often can be reversed once therapy is discontinued. Avoiding split-dose administration has been proposed given the natural diurnal production of cortisol.58 A review by Flaherty discusses the importance of avoiding pharmacologic agents in hospitalized older patients if possible due to known risks (falls, dependency, hip fractures, rebound insomnia, and risk of delirium) and provides a HELP ME SLEEP nomogram for nonpharmacologic interventions in hospitalized patients (Table 4).59
Historically, lithium has been recommended for steroid-induced mania with chronic steroid use; however, given the large volume and electrolyte shifts seen in critically ill patients, this may not be a viable option. Antidepressants, especially tricyclics, should generally be avoided in steroid-induced psychosis as these may exacerbate symptoms. If symptoms are severe, either typical (haloperidol) or atypical (olanzapine, quetiapine, risperidone) antipsychotics have been used with success.60 Given the known depletion of serum melatonin levels, melatonin supplements are an attractive and relatively safe option for steroid-induced insomnia; however, there are no robust studies specifically aimed at this intervention for this population.
Conclusions
With known, multimodal foci driving sleep impairment in ICU patients, PADIS guidelines recommend myriad interventions for improvement. Recommendations include noise and light reduction with earplugs and/or eyeshades to improve sleep quality. Nocturnal assist-control ventilation may improve sleep quality in ventilated patients. Finally, the development of institutional protocols for promoting sleep quality in ICU patients is recommended.17
1. Simini B. Patients’ perceptions of intensive care. Lancet. 1999;354(9178):571-572. doi: 10.1016/S0140-6736(99)02728-2
2. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients—a clinical review. Ann Intensive Care. 2015;15:3. doi: 10.1186/s13613-015-0043-2
3. Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM. Quality and quantity of sleep in the surgical intensive care unit; are our patients sleeping? J Trauma. 2007;63(6):1210-1214. doi: 10.1097/TA.0b013e31815b83d7
4. Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care 2013;17(2):R46.
5. Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in patients receiving postoperative care. BJM (Clin Res Ed). 1985;290(6474)1029-1032. doi: 10.1136/bmj.290.6474.1029
6. White DP, Douglas NJ, Pickett CK, Zwillich CW, Weil JV. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128(6):984-986. doi: 10.1164/arrd.1983.128.6.984
7. Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481-485. doi: 10.1164/ajrccm.150.2.8049833
8. Tadjalli A, Peever J. Sleep loss reduces respiratory motor plasticity. Adv Exp Med Biol. 2010;669:289-292.
doi: 10.1007/978-1-4419-5692-7_59
9. Roche Campo F, Drouot X, Thille AW, et al. Poor sleep quality is associated with late noninvasive ventilation failure in patients with acute hypercapnic respiratory failure. Crit Care Med. 2010;38(2):447-485. doi: 10.1097/CCM.0b013e3181bc8243
10. Sauvet F, Leftheriotis G, Gomez-Merino D, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol (1985). 2010;108(1):68-75. doi: 10.1152/japplphysiol.00851.2009
11. Frey DJ, Fleshner M, Wright KP Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21(8):1050-1057. doi: 10.1016/j.bbi.2007.04.003
12. Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA 2002;288(12):1471-1472. doi: 10.1001/jama.288.12.1471-a
13. Dinges DF, Douglas SD, Zuagg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93(5):1930-1939. doi: 10.1172/JCI117184
14. Weinhouse GL, Schwab RJ, Watson PL, et al. Bench-to-bedside review: delirium in ICU patients— importance of sleep deprivation. Crit Care. 2009;13(6):234. doi: 10.1186/cc8131
15. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi: 10.1001/jama.291.14.1753
16. Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513-1520. doi: 10.1097/CCM.0b013e3181e47be1
17. Devlin JW, Skrobik Y, Gelinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873
18. The Boston Collaborative Drug Surveillance Program. Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther. 1972;13(5):694-698. doi: 10.1002/cpt1972135part1694
19. Rundell JR, Wise MG. Causes of organic mood disorder. J Neuropsychiatry Clin Neurosci. 1989;1(4):398-400. doi: 10.1176/jnp.1.4.398
20. Gaudreau JD, Gagnon P, Harel F, Roy MA, Tremblay A. Psychoactive medications and risk of delirium in hospitalized cancer patients. J Clin Oncol. 2005;23(27):6712-6718. doi: 10.1200/JCO.2005.05.140
21. Gaudreau JD, Gagnon P, Roy MA, Harel F, Tremblay A. Opioid medications and longitudinal risk of delirium in hospitalized cancer patients. Cancer. 2007;109(11):2365-2373.
doi: 10.1002/cncr.22665
22. Schreiber MP, Colantuoni E, Bienvenu OJ, et al. Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med. 2014;42(6):1480-1486. doi: 10.1097/CCM.0000000000000247
23. Wolters AE, Veldhuijzen DS, Zaal IJ, et al. Systemic corticosteroids and transition to delirium in critically ill patients. Crit Care Med. 2015;43(12):e585-e588. doi: 10.1097/CCM.0000000000001302
24. Matschke J, Muller-Beissenhirtz H, Novotny J, et al. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naïve adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol. 2016;136(2):101-107. doi: 10.1159/000445420
25. Tilouche N, Hassen M, Ali HBS, Jaoued AHO, Gharbi R, Atrous SS. Delirium in the intensive care unit: incidence, risk factors, and impact on outcome. Indian J Crit Care Med. 2018;22:144-149. doi: 10.4103/ijccm.IJCCM_244_17
26. Young A, Marsh S. Steroid use in critical care. BJA Education. 2018;18(5):129-134. doi: 10.1016/j.bjae.2018.01.005
27. DiPiro J, Talbert R, Yee G, Matzke GR, Wells BG, Posey M. Pharmacotherapy: A Pathophysiologic Approach. 4th ed. New York: McGraw-Hill; 1999:1277-1278.
28. Schimmer
29. Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence of US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33(10):1413-1432.
30. Idzikowsi C, Shapiro CM. ABC of sleep disorders, non-psychotropic drugs and sleep. BMJ. 1993;306(6885):1118-1120. doi: 10.1136/bmj.306.6885.1118

31. Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14(4):398-406.
doi: 10.3109/10253890.2011.586446
32. Wolkowitz OM, Reus VI, Weingartner H, et al. Cognitive effects of corticosteroids. Am J Psychiatry 1990;147(10):1297-1303. doi: 10.1176/ajp.147.10.1297
33. McEwen BS, Davis PG, Parsons B, Pfaff DW. The brain as a target for steroid hormone action. Ann Rev Neurosci. 1979;2:65-112. doi: 10.1146/annurev.ne.02.030179.000433
34. Brown ES, Woolston DJ, Frol AM. Amygdala volume in patients receiving chronic corticosteroid therapy. Biol Psychiatry. 2008;63(7):705-709.
doi: 10.1016/j.biopsych.2007.09.014
35. Brown ES, Woolston D, Frol A, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid. Biol Psychiatry. 2004;55(5):538-545.
36. Sapolsky RM, McEwen BS. Down-regulation of neural corticosterone receptors by corticosterone and dexamethasone. Brain Res. 1985;339(1):161-165.
doi: 10.1016/0006-8993(85)90638-9
37. Sorrells SF, Caso JR, Munhoz CD, Spolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64(1):33-39.
doi: 10.1016/j.neuron.2009.09.032
38. Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids: mood, memory, and mechanisms. Ann NY Acad Sci. 2009;1179:19-40. doi: 10.1111/j.1749-6632.2009.04980.x
39. Wolkowitz OM, Epel ES, Reus VI. Stress hormone-related psychopathology: pathophysiological and treatment implications. World J Biol Psychiatry. 2001;2(3):115-143. doi: 10.3109/15622970109026799
40. Paredes S, Barriga C, Reiter R, Rodrigues A. Assessment of the potential role of tryptophan as the precursor of serotonin and melatonin for the aged sleep-wake cycle and immune function: Streptopelia Risoria as a model. Int J Tryptophan Res. 2009;2:23-36. doi: 10.4137/ijtr.s1129
41. Soszyński P, Stowińska-Srzednicka J, Kasperlik-Zatuska A, Zgliczyński S. Decreased melatonin concentration in Cushing’s Syndrome. Horm Metab Res. 1989;21(12):673-674. doi: 10.1055/s-2007-1009317
42. Demish L, Demish K, Neckelsen T. Influence of dexamethasone on nocturnal melatonin production in healthy adult subjects. J Pineal Res. 1988;5(3):317-321. doi: 10.1111/j.1600-079x.1988.tb00657.x
43. Assaf N, Shalby AB, Khalil WK, Ahmed HH. Biochemical and genetic alterations of oxidant/antioxidant status of the brain in rats treated with dexamethasone: protective roles of melatonin and acetyl-L-carnitine. J Physiol Biochem. 2012;68(1):77-90. doi: 10.1007/s13105-011-0121-3
44. Clark MS, Russo AF. Tissue-specific glucocorticoid regulation of tryptophan hydroxylase mRNA levels. Brain Res Mol Brain Res. 1997;48(2):346-54. doi: 10.1016/s0169-328x(97)00106-x
45. Kram DE, Krasnow SM, Levasseur PR, Zhu X, Stork LC, Marks DL. Dexamethasone chemotherapy does not disrupt orexin signaling. PLoS One. 2016;11(12):e0168731. doi: 10.1371/journal.pone.0168731
46. Mellon S. Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78(5):1003-1008. doi: 10.1210/jcem.78.5.8175951
47. Zorumski C, Paul SM, Izumi Y, Covey DF, Mennerick S . Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev. 2013;37(1):109-122. doi: 10.1016/j.neubiorev.2012.10.005
48. Monteleone P, Luisi M, Martiadis V, et al. Impaired reduction of enhanced levels of dehydroepiandrosterone by oral dexamethasone in anorexia nervosa. Psychoneuroendocrinology. 2006;31(4):537-542. doi: 10.1016/j.psyneuen.2005.08.015
49. Genazzani AR, Petraglia F, Bernardi F, et al. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83(6):2099-3103. doi: 10.1210/jcem.83.6.4905
50. Moser NJ, Phillips BA, Guthrie G, Barnett G. Effects of dexamethasone on sleep. Pharmacol Toxicol. 1996;79(2):100-102. doi: 10.1111/j.1600-0773.1996.tb00249.x
51. Curtis J, Westfall A, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420-426. doi: 10.1002/art.21984
52. Zhao J, Dai YH, Xi QS, Yu SY. A clinical study on insomnia in patients with cancer during chemotherapy containing high-dose glucocorticoids. Pharmazie. 2013;68(6):421-427
53. Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days corticosteroid treatment. A prospective study. Psychoneuroendocrinology. 1996;21(1):25-31. doi: 10.1016/0306-4530(95)00031-3
54. Brown ES, Suppes T, Khan DA, Carmody TJ 3rd. Mood changes during prednisone bursts in outpatients with asthma. J Clin Psychopharmacol. 2002;22(1):55-61.
doi: 10.1097/00004714-200202000-00009
55. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367. doi: 10.4065/81.10.1361
56. Britt RC, Devine A, Swallen KC et al. Corticosteroid use in the intensive care unit: at what cost? Arch Surg. 2006;141(2):145-159. doi:10.1001/archsurg.141.2.145
57. Kiser TH, Allen RR, Valuck RJ, Moss M, Vanivier RW. Outcomes associated with corticosteroid dosage in critically ill patients in acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):1052-1064. doi: 10.1164/rccm.201401-0058OC
58. Bourne RS, Mills GH. Sleep disruption in critically ill patients—pharmacological considerations. Anaesthesia. 2004;59(4):374-384. doi: 10.1111/j. 1365-2044.2004.03664.x
59. Flaherty JH. Insomnia among hospitalized older persons. Clin Geriatr Med. 2008;24(1):51-67. doi: 10.1016/j.cger.2007.08.012
60. Sirios F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25(1):27-33. doi: 10.1016/s0163-8343(02)00241-4
1. Simini B. Patients’ perceptions of intensive care. Lancet. 1999;354(9178):571-572. doi: 10.1016/S0140-6736(99)02728-2
2. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients—a clinical review. Ann Intensive Care. 2015;15:3. doi: 10.1186/s13613-015-0043-2
3. Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM. Quality and quantity of sleep in the surgical intensive care unit; are our patients sleeping? J Trauma. 2007;63(6):1210-1214. doi: 10.1097/TA.0b013e31815b83d7
4. Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care 2013;17(2):R46.
5. Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in patients receiving postoperative care. BJM (Clin Res Ed). 1985;290(6474)1029-1032. doi: 10.1136/bmj.290.6474.1029
6. White DP, Douglas NJ, Pickett CK, Zwillich CW, Weil JV. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128(6):984-986. doi: 10.1164/arrd.1983.128.6.984
7. Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481-485. doi: 10.1164/ajrccm.150.2.8049833
8. Tadjalli A, Peever J. Sleep loss reduces respiratory motor plasticity. Adv Exp Med Biol. 2010;669:289-292.
doi: 10.1007/978-1-4419-5692-7_59
9. Roche Campo F, Drouot X, Thille AW, et al. Poor sleep quality is associated with late noninvasive ventilation failure in patients with acute hypercapnic respiratory failure. Crit Care Med. 2010;38(2):447-485. doi: 10.1097/CCM.0b013e3181bc8243
10. Sauvet F, Leftheriotis G, Gomez-Merino D, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol (1985). 2010;108(1):68-75. doi: 10.1152/japplphysiol.00851.2009
11. Frey DJ, Fleshner M, Wright KP Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21(8):1050-1057. doi: 10.1016/j.bbi.2007.04.003
12. Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA 2002;288(12):1471-1472. doi: 10.1001/jama.288.12.1471-a
13. Dinges DF, Douglas SD, Zuagg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93(5):1930-1939. doi: 10.1172/JCI117184
14. Weinhouse GL, Schwab RJ, Watson PL, et al. Bench-to-bedside review: delirium in ICU patients— importance of sleep deprivation. Crit Care. 2009;13(6):234. doi: 10.1186/cc8131
15. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753-1762. doi: 10.1001/jama.291.14.1753
16. Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513-1520. doi: 10.1097/CCM.0b013e3181e47be1
17. Devlin JW, Skrobik Y, Gelinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873
18. The Boston Collaborative Drug Surveillance Program. Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther. 1972;13(5):694-698. doi: 10.1002/cpt1972135part1694
19. Rundell JR, Wise MG. Causes of organic mood disorder. J Neuropsychiatry Clin Neurosci. 1989;1(4):398-400. doi: 10.1176/jnp.1.4.398
20. Gaudreau JD, Gagnon P, Harel F, Roy MA, Tremblay A. Psychoactive medications and risk of delirium in hospitalized cancer patients. J Clin Oncol. 2005;23(27):6712-6718. doi: 10.1200/JCO.2005.05.140
21. Gaudreau JD, Gagnon P, Roy MA, Harel F, Tremblay A. Opioid medications and longitudinal risk of delirium in hospitalized cancer patients. Cancer. 2007;109(11):2365-2373.
doi: 10.1002/cncr.22665
22. Schreiber MP, Colantuoni E, Bienvenu OJ, et al. Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med. 2014;42(6):1480-1486. doi: 10.1097/CCM.0000000000000247
23. Wolters AE, Veldhuijzen DS, Zaal IJ, et al. Systemic corticosteroids and transition to delirium in critically ill patients. Crit Care Med. 2015;43(12):e585-e588. doi: 10.1097/CCM.0000000000001302
24. Matschke J, Muller-Beissenhirtz H, Novotny J, et al. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naïve adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol. 2016;136(2):101-107. doi: 10.1159/000445420
25. Tilouche N, Hassen M, Ali HBS, Jaoued AHO, Gharbi R, Atrous SS. Delirium in the intensive care unit: incidence, risk factors, and impact on outcome. Indian J Crit Care Med. 2018;22:144-149. doi: 10.4103/ijccm.IJCCM_244_17
26. Young A, Marsh S. Steroid use in critical care. BJA Education. 2018;18(5):129-134. doi: 10.1016/j.bjae.2018.01.005
27. DiPiro J, Talbert R, Yee G, Matzke GR, Wells BG, Posey M. Pharmacotherapy: A Pathophysiologic Approach. 4th ed. New York: McGraw-Hill; 1999:1277-1278.
28. Schimmer
29. Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence of US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33(10):1413-1432.
30. Idzikowsi C, Shapiro CM. ABC of sleep disorders, non-psychotropic drugs and sleep. BMJ. 1993;306(6885):1118-1120. doi: 10.1136/bmj.306.6885.1118

31. Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14(4):398-406.
doi: 10.3109/10253890.2011.586446
32. Wolkowitz OM, Reus VI, Weingartner H, et al. Cognitive effects of corticosteroids. Am J Psychiatry 1990;147(10):1297-1303. doi: 10.1176/ajp.147.10.1297
33. McEwen BS, Davis PG, Parsons B, Pfaff DW. The brain as a target for steroid hormone action. Ann Rev Neurosci. 1979;2:65-112. doi: 10.1146/annurev.ne.02.030179.000433
34. Brown ES, Woolston DJ, Frol AM. Amygdala volume in patients receiving chronic corticosteroid therapy. Biol Psychiatry. 2008;63(7):705-709.
doi: 10.1016/j.biopsych.2007.09.014
35. Brown ES, Woolston D, Frol A, et al. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid. Biol Psychiatry. 2004;55(5):538-545.
36. Sapolsky RM, McEwen BS. Down-regulation of neural corticosterone receptors by corticosterone and dexamethasone. Brain Res. 1985;339(1):161-165.
doi: 10.1016/0006-8993(85)90638-9
37. Sorrells SF, Caso JR, Munhoz CD, Spolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64(1):33-39.
doi: 10.1016/j.neuron.2009.09.032
38. Wolkowitz OM, Burke H, Epel ES, Reus VI. Glucocorticoids: mood, memory, and mechanisms. Ann NY Acad Sci. 2009;1179:19-40. doi: 10.1111/j.1749-6632.2009.04980.x
39. Wolkowitz OM, Epel ES, Reus VI. Stress hormone-related psychopathology: pathophysiological and treatment implications. World J Biol Psychiatry. 2001;2(3):115-143. doi: 10.3109/15622970109026799
40. Paredes S, Barriga C, Reiter R, Rodrigues A. Assessment of the potential role of tryptophan as the precursor of serotonin and melatonin for the aged sleep-wake cycle and immune function: Streptopelia Risoria as a model. Int J Tryptophan Res. 2009;2:23-36. doi: 10.4137/ijtr.s1129
41. Soszyński P, Stowińska-Srzednicka J, Kasperlik-Zatuska A, Zgliczyński S. Decreased melatonin concentration in Cushing’s Syndrome. Horm Metab Res. 1989;21(12):673-674. doi: 10.1055/s-2007-1009317
42. Demish L, Demish K, Neckelsen T. Influence of dexamethasone on nocturnal melatonin production in healthy adult subjects. J Pineal Res. 1988;5(3):317-321. doi: 10.1111/j.1600-079x.1988.tb00657.x
43. Assaf N, Shalby AB, Khalil WK, Ahmed HH. Biochemical and genetic alterations of oxidant/antioxidant status of the brain in rats treated with dexamethasone: protective roles of melatonin and acetyl-L-carnitine. J Physiol Biochem. 2012;68(1):77-90. doi: 10.1007/s13105-011-0121-3
44. Clark MS, Russo AF. Tissue-specific glucocorticoid regulation of tryptophan hydroxylase mRNA levels. Brain Res Mol Brain Res. 1997;48(2):346-54. doi: 10.1016/s0169-328x(97)00106-x
45. Kram DE, Krasnow SM, Levasseur PR, Zhu X, Stork LC, Marks DL. Dexamethasone chemotherapy does not disrupt orexin signaling. PLoS One. 2016;11(12):e0168731. doi: 10.1371/journal.pone.0168731
46. Mellon S. Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab. 1994;78(5):1003-1008. doi: 10.1210/jcem.78.5.8175951
47. Zorumski C, Paul SM, Izumi Y, Covey DF, Mennerick S . Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev. 2013;37(1):109-122. doi: 10.1016/j.neubiorev.2012.10.005
48. Monteleone P, Luisi M, Martiadis V, et al. Impaired reduction of enhanced levels of dehydroepiandrosterone by oral dexamethasone in anorexia nervosa. Psychoneuroendocrinology. 2006;31(4):537-542. doi: 10.1016/j.psyneuen.2005.08.015
49. Genazzani AR, Petraglia F, Bernardi F, et al. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83(6):2099-3103. doi: 10.1210/jcem.83.6.4905
50. Moser NJ, Phillips BA, Guthrie G, Barnett G. Effects of dexamethasone on sleep. Pharmacol Toxicol. 1996;79(2):100-102. doi: 10.1111/j.1600-0773.1996.tb00249.x
51. Curtis J, Westfall A, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420-426. doi: 10.1002/art.21984
52. Zhao J, Dai YH, Xi QS, Yu SY. A clinical study on insomnia in patients with cancer during chemotherapy containing high-dose glucocorticoids. Pharmazie. 2013;68(6):421-427
53. Naber D, Sand P, Heigl B. Psychopathological and neuropsychological effects of 8-days corticosteroid treatment. A prospective study. Psychoneuroendocrinology. 1996;21(1):25-31. doi: 10.1016/0306-4530(95)00031-3
54. Brown ES, Suppes T, Khan DA, Carmody TJ 3rd. Mood changes during prednisone bursts in outpatients with asthma. J Clin Psychopharmacol. 2002;22(1):55-61.
doi: 10.1097/00004714-200202000-00009
55. Warrington TP, Bostwick JM. Psychiatric adverse effects of corticosteroids. Mayo Clin Proc. 2006;81(10):1361-1367. doi: 10.4065/81.10.1361
56. Britt RC, Devine A, Swallen KC et al. Corticosteroid use in the intensive care unit: at what cost? Arch Surg. 2006;141(2):145-159. doi:10.1001/archsurg.141.2.145
57. Kiser TH, Allen RR, Valuck RJ, Moss M, Vanivier RW. Outcomes associated with corticosteroid dosage in critically ill patients in acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):1052-1064. doi: 10.1164/rccm.201401-0058OC
58. Bourne RS, Mills GH. Sleep disruption in critically ill patients—pharmacological considerations. Anaesthesia. 2004;59(4):374-384. doi: 10.1111/j. 1365-2044.2004.03664.x
59. Flaherty JH. Insomnia among hospitalized older persons. Clin Geriatr Med. 2008;24(1):51-67. doi: 10.1016/j.cger.2007.08.012
60. Sirios F. Steroid psychosis: a review. Gen Hosp Psychiatry. 2003;25(1):27-33. doi: 10.1016/s0163-8343(02)00241-4