User login
To the Editor:
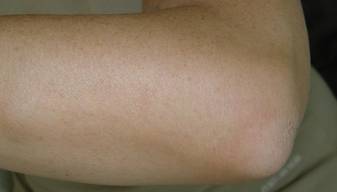
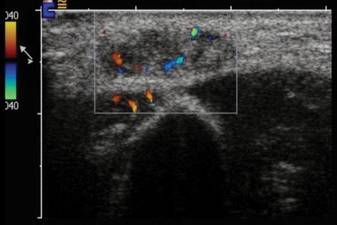
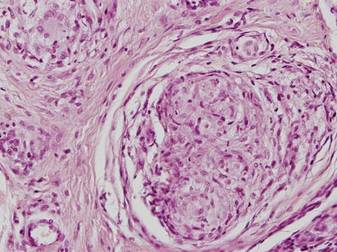
A 54-year-old woman presented with painless, firm, flesh-colored nodules measuring 1.0 to 1.5 cm in diameter on the extensor surface of the left forearm (Figure 1) and on the distal phalanx of the left thumb of 3 months’ duration. No other signs and symptoms were present. A detailed clinical examination revealed a slightly elevated erythrocyte sedimentation rate (24 mm/h [reference range, 0–20 mm/h]) and a high antinuclear antibody titer (1:3200 [reference range, <1:100])(anti–Sjögren syndrome anti-gen A, anti–Sjögren syndrome antigen B, anti-Ro52). Complete blood cell count, basic metabolic panel, liver function tests, urinalysis, pulmonary function tests, chest radiograph, and chest computed tomography all were normal. Hepatitis B antigen and antibody tests; hepatitis C antibody tests; and tuberculin test all were negative. An ophthalmic examination revealed no abnormalities. Ultrasonography of the nodules was performed with a system using an 8- to 12-MHz linear transducer and revealed 4 heterogenous hypoechoic lesions measuring up to 1.5 cm in size. Color Doppler images showed moderate hypervascularity (Figure 2). The largest nodule was excised. Histologic examination revealed noncaseating granulomas; special stains for microorganisms were negative. The histopathologic findings confirmed a diagnosis of sarcoidosis (Figure 3). The patient refused any medication. The nodules were stable at 6-month follow-up, then spontaneously resolved.
|
Subcutaneous sarcoidosis (SS) is a rare cutaneous expression of systemic sarcoidosis. The entity was first described by French physicians Darier and Roussy in 1904 as granulomatous panniculitis. Although their original study referred to a case of tuberculosis, the term Darier-Roussy sarcoid was coined and had been applied to a true sarcoid as well as to a variety of other forms of granulomatous panniculitis including those of infectious origin. A more accurate term subcutaneous sarcoidosis was established in 1984 by Vainsencher and Winkelmann.1
The most characteristic clinical picture of this disorder consists of the presence of multiple painless, firm, mobile nodules located on the extremities, most frequently the arms. However, other sites such as the trunk, buttocks, groin, head, face, and neck also have been reported.2,3
Marcoval et al2 demonstrated SS in only 2.1% of 480 patients with systemic sarcoidosis (10 patients). In the majority of these patients, subcutaneous nodules were the initial presentation of the disease.2 Ahmed and Harshad3 reported evidence of systemic involvement in 84.9% (45/53) of patients with SS. Chest involvement was the most common finding (eg, hilar lymphadenopathy, mediastinal adenopathy, interstitial pulmonary infiltration).3 Parotitis, uveitis, neuritis, and hepatosplenomegaly also have been noted systemically.4 The vast majority of reviews have suggested that SS has a relatively good prognosis. Ahmed and Harshad3 reported a satisfactory response to steroid treatment in all patients who received corticosteroids as the primary treatment. Subcutaneous sarcoidosis usually does not herald severe systemic involvement or chronic systemic complications. Both subcutaneous granulomas and hilar adenopathy may spontaneously resolve.
Interestingly, various autoimmune disease associations were seen in 6 of 21 patients (29%) in the study by Ahmed and Harshad3 including Hashimoto thyroiditis, rheumatoid arthritis, ulcerative colitis, systemic lupus erythematosus, and sicca syndrome. Barnadas et al5 reported a case of SS associated with vitiligo, pernicious anemia, and Hashimoto thyroiditis. Although our patient was not diagnosed with any particular autoimmune disease, an antinuclear antibody test was positive at a titer of 1:3200.
Our case is interesting for 2 reasons. First, it is a rare case of isolated SS. Thorough systemic evaluation showed no evidence of extracutaneous involvement. The literature only provides a few instances of isolated SS.6,7 Second, the sonographic appearance of SS is rare.8,9 Chen et al9 reported that gray-scale sonography revealed heterogenous, hypoechoic, well-demarcated plaquelike lesions with an intensive vascular pattern indicating Doppler hypervascularization. We obtained similar findings.
It has been widely acknowledged that sonographic findings of subcutaneous nodules tend to be nonspecific and overlapping. Color Doppler examination may show internal vessels both in malignant soft-tissue masses (eg, lymphoma, synovial sarcoma, liposarcoma, malignant fibrohistocytoma, metastases) and in benign lesions (eg, schwannoma, hemangioma, fibromatosis). However, the application of Doppler ultrasonography may restrict the diagnostic field, as it excludes nonvascularized benign masses such as lipomas as well as ganglion or epidermoid cysts. The ultimate diagnosis can only be made based on histopathology.
1. Vainsencher D, Winkelmann RK. Subcutaneous sarcoidosis. Arch Dermatol. 1984;120:1028-1031.
2. Marcoval J, Maña J, Moreno A, et al. Subcutaneous sarcoidosis—clinicopathological study of 10 cases. Br J Dermatol. 2005;153:790-794.
3. Ahmed I, Harshad SR. Subcutaneous sarcoidosis: is it a specific subset of cutaneous sarcoidosis frequently associated with systemic disease [published online ahead of print December 2, 2005]? J Am Acad Dermatol. 2006;54:55-60.
4. Dalle Vedove C, Colato C, Girolomoni G. Subcutaneous sarcoidosis: report of two cases and review of the literature [published online ahead of print April 2, 2011]. Clin Rheumatol. 2011;30:1123-1128.
5. Barnadas MA, Rodríguez-Arias JM, Alomar A. Subcutaneous sarcoidosis associated with vitiligo, pernicious anaemia and autoimmune thyroiditis. Clin Exp Dermatol. 2000;25:55-56.
6. Higgins EM, Salisbury JR, Du Vivier AW. Subcutaneous sarcoidosis. Clin Exp Dermatol. 1993;18:65-66.
7. Heller M, Soldano AC. Sarcoidosis with subcutaneous lesions. Dermatol Online J. 2008;14:1.
8. Bosni´c D, Baresi´c M, Bagatin D, et al. Subcutaneous sarcoidosis of the face [published online ahead of print March 15, 2010]. Intern Med. 2010;49:589-592.
9. Chen HH, Chen YM, Lan HH, et al. Sonographic appearance of subcutaneous sarcoidosis. J Ultrasound Med. 2009;28:813-816.
To the Editor:
A 54-year-old woman presented with painless, firm, flesh-colored nodules measuring 1.0 to 1.5 cm in diameter on the extensor surface of the left forearm (Figure 1) and on the distal phalanx of the left thumb of 3 months’ duration. No other signs and symptoms were present. A detailed clinical examination revealed a slightly elevated erythrocyte sedimentation rate (24 mm/h [reference range, 0–20 mm/h]) and a high antinuclear antibody titer (1:3200 [reference range, <1:100])(anti–Sjögren syndrome anti-gen A, anti–Sjögren syndrome antigen B, anti-Ro52). Complete blood cell count, basic metabolic panel, liver function tests, urinalysis, pulmonary function tests, chest radiograph, and chest computed tomography all were normal. Hepatitis B antigen and antibody tests; hepatitis C antibody tests; and tuberculin test all were negative. An ophthalmic examination revealed no abnormalities. Ultrasonography of the nodules was performed with a system using an 8- to 12-MHz linear transducer and revealed 4 heterogenous hypoechoic lesions measuring up to 1.5 cm in size. Color Doppler images showed moderate hypervascularity (Figure 2). The largest nodule was excised. Histologic examination revealed noncaseating granulomas; special stains for microorganisms were negative. The histopathologic findings confirmed a diagnosis of sarcoidosis (Figure 3). The patient refused any medication. The nodules were stable at 6-month follow-up, then spontaneously resolved.
|
Subcutaneous sarcoidosis (SS) is a rare cutaneous expression of systemic sarcoidosis. The entity was first described by French physicians Darier and Roussy in 1904 as granulomatous panniculitis. Although their original study referred to a case of tuberculosis, the term Darier-Roussy sarcoid was coined and had been applied to a true sarcoid as well as to a variety of other forms of granulomatous panniculitis including those of infectious origin. A more accurate term subcutaneous sarcoidosis was established in 1984 by Vainsencher and Winkelmann.1
The most characteristic clinical picture of this disorder consists of the presence of multiple painless, firm, mobile nodules located on the extremities, most frequently the arms. However, other sites such as the trunk, buttocks, groin, head, face, and neck also have been reported.2,3
Marcoval et al2 demonstrated SS in only 2.1% of 480 patients with systemic sarcoidosis (10 patients). In the majority of these patients, subcutaneous nodules were the initial presentation of the disease.2 Ahmed and Harshad3 reported evidence of systemic involvement in 84.9% (45/53) of patients with SS. Chest involvement was the most common finding (eg, hilar lymphadenopathy, mediastinal adenopathy, interstitial pulmonary infiltration).3 Parotitis, uveitis, neuritis, and hepatosplenomegaly also have been noted systemically.4 The vast majority of reviews have suggested that SS has a relatively good prognosis. Ahmed and Harshad3 reported a satisfactory response to steroid treatment in all patients who received corticosteroids as the primary treatment. Subcutaneous sarcoidosis usually does not herald severe systemic involvement or chronic systemic complications. Both subcutaneous granulomas and hilar adenopathy may spontaneously resolve.
Interestingly, various autoimmune disease associations were seen in 6 of 21 patients (29%) in the study by Ahmed and Harshad3 including Hashimoto thyroiditis, rheumatoid arthritis, ulcerative colitis, systemic lupus erythematosus, and sicca syndrome. Barnadas et al5 reported a case of SS associated with vitiligo, pernicious anemia, and Hashimoto thyroiditis. Although our patient was not diagnosed with any particular autoimmune disease, an antinuclear antibody test was positive at a titer of 1:3200.
Our case is interesting for 2 reasons. First, it is a rare case of isolated SS. Thorough systemic evaluation showed no evidence of extracutaneous involvement. The literature only provides a few instances of isolated SS.6,7 Second, the sonographic appearance of SS is rare.8,9 Chen et al9 reported that gray-scale sonography revealed heterogenous, hypoechoic, well-demarcated plaquelike lesions with an intensive vascular pattern indicating Doppler hypervascularization. We obtained similar findings.
It has been widely acknowledged that sonographic findings of subcutaneous nodules tend to be nonspecific and overlapping. Color Doppler examination may show internal vessels both in malignant soft-tissue masses (eg, lymphoma, synovial sarcoma, liposarcoma, malignant fibrohistocytoma, metastases) and in benign lesions (eg, schwannoma, hemangioma, fibromatosis). However, the application of Doppler ultrasonography may restrict the diagnostic field, as it excludes nonvascularized benign masses such as lipomas as well as ganglion or epidermoid cysts. The ultimate diagnosis can only be made based on histopathology.
To the Editor:
A 54-year-old woman presented with painless, firm, flesh-colored nodules measuring 1.0 to 1.5 cm in diameter on the extensor surface of the left forearm (Figure 1) and on the distal phalanx of the left thumb of 3 months’ duration. No other signs and symptoms were present. A detailed clinical examination revealed a slightly elevated erythrocyte sedimentation rate (24 mm/h [reference range, 0–20 mm/h]) and a high antinuclear antibody titer (1:3200 [reference range, <1:100])(anti–Sjögren syndrome anti-gen A, anti–Sjögren syndrome antigen B, anti-Ro52). Complete blood cell count, basic metabolic panel, liver function tests, urinalysis, pulmonary function tests, chest radiograph, and chest computed tomography all were normal. Hepatitis B antigen and antibody tests; hepatitis C antibody tests; and tuberculin test all were negative. An ophthalmic examination revealed no abnormalities. Ultrasonography of the nodules was performed with a system using an 8- to 12-MHz linear transducer and revealed 4 heterogenous hypoechoic lesions measuring up to 1.5 cm in size. Color Doppler images showed moderate hypervascularity (Figure 2). The largest nodule was excised. Histologic examination revealed noncaseating granulomas; special stains for microorganisms were negative. The histopathologic findings confirmed a diagnosis of sarcoidosis (Figure 3). The patient refused any medication. The nodules were stable at 6-month follow-up, then spontaneously resolved.
|
Subcutaneous sarcoidosis (SS) is a rare cutaneous expression of systemic sarcoidosis. The entity was first described by French physicians Darier and Roussy in 1904 as granulomatous panniculitis. Although their original study referred to a case of tuberculosis, the term Darier-Roussy sarcoid was coined and had been applied to a true sarcoid as well as to a variety of other forms of granulomatous panniculitis including those of infectious origin. A more accurate term subcutaneous sarcoidosis was established in 1984 by Vainsencher and Winkelmann.1
The most characteristic clinical picture of this disorder consists of the presence of multiple painless, firm, mobile nodules located on the extremities, most frequently the arms. However, other sites such as the trunk, buttocks, groin, head, face, and neck also have been reported.2,3
Marcoval et al2 demonstrated SS in only 2.1% of 480 patients with systemic sarcoidosis (10 patients). In the majority of these patients, subcutaneous nodules were the initial presentation of the disease.2 Ahmed and Harshad3 reported evidence of systemic involvement in 84.9% (45/53) of patients with SS. Chest involvement was the most common finding (eg, hilar lymphadenopathy, mediastinal adenopathy, interstitial pulmonary infiltration).3 Parotitis, uveitis, neuritis, and hepatosplenomegaly also have been noted systemically.4 The vast majority of reviews have suggested that SS has a relatively good prognosis. Ahmed and Harshad3 reported a satisfactory response to steroid treatment in all patients who received corticosteroids as the primary treatment. Subcutaneous sarcoidosis usually does not herald severe systemic involvement or chronic systemic complications. Both subcutaneous granulomas and hilar adenopathy may spontaneously resolve.
Interestingly, various autoimmune disease associations were seen in 6 of 21 patients (29%) in the study by Ahmed and Harshad3 including Hashimoto thyroiditis, rheumatoid arthritis, ulcerative colitis, systemic lupus erythematosus, and sicca syndrome. Barnadas et al5 reported a case of SS associated with vitiligo, pernicious anemia, and Hashimoto thyroiditis. Although our patient was not diagnosed with any particular autoimmune disease, an antinuclear antibody test was positive at a titer of 1:3200.
Our case is interesting for 2 reasons. First, it is a rare case of isolated SS. Thorough systemic evaluation showed no evidence of extracutaneous involvement. The literature only provides a few instances of isolated SS.6,7 Second, the sonographic appearance of SS is rare.8,9 Chen et al9 reported that gray-scale sonography revealed heterogenous, hypoechoic, well-demarcated plaquelike lesions with an intensive vascular pattern indicating Doppler hypervascularization. We obtained similar findings.
It has been widely acknowledged that sonographic findings of subcutaneous nodules tend to be nonspecific and overlapping. Color Doppler examination may show internal vessels both in malignant soft-tissue masses (eg, lymphoma, synovial sarcoma, liposarcoma, malignant fibrohistocytoma, metastases) and in benign lesions (eg, schwannoma, hemangioma, fibromatosis). However, the application of Doppler ultrasonography may restrict the diagnostic field, as it excludes nonvascularized benign masses such as lipomas as well as ganglion or epidermoid cysts. The ultimate diagnosis can only be made based on histopathology.
1. Vainsencher D, Winkelmann RK. Subcutaneous sarcoidosis. Arch Dermatol. 1984;120:1028-1031.
2. Marcoval J, Maña J, Moreno A, et al. Subcutaneous sarcoidosis—clinicopathological study of 10 cases. Br J Dermatol. 2005;153:790-794.
3. Ahmed I, Harshad SR. Subcutaneous sarcoidosis: is it a specific subset of cutaneous sarcoidosis frequently associated with systemic disease [published online ahead of print December 2, 2005]? J Am Acad Dermatol. 2006;54:55-60.
4. Dalle Vedove C, Colato C, Girolomoni G. Subcutaneous sarcoidosis: report of two cases and review of the literature [published online ahead of print April 2, 2011]. Clin Rheumatol. 2011;30:1123-1128.
5. Barnadas MA, Rodríguez-Arias JM, Alomar A. Subcutaneous sarcoidosis associated with vitiligo, pernicious anaemia and autoimmune thyroiditis. Clin Exp Dermatol. 2000;25:55-56.
6. Higgins EM, Salisbury JR, Du Vivier AW. Subcutaneous sarcoidosis. Clin Exp Dermatol. 1993;18:65-66.
7. Heller M, Soldano AC. Sarcoidosis with subcutaneous lesions. Dermatol Online J. 2008;14:1.
8. Bosni´c D, Baresi´c M, Bagatin D, et al. Subcutaneous sarcoidosis of the face [published online ahead of print March 15, 2010]. Intern Med. 2010;49:589-592.
9. Chen HH, Chen YM, Lan HH, et al. Sonographic appearance of subcutaneous sarcoidosis. J Ultrasound Med. 2009;28:813-816.
1. Vainsencher D, Winkelmann RK. Subcutaneous sarcoidosis. Arch Dermatol. 1984;120:1028-1031.
2. Marcoval J, Maña J, Moreno A, et al. Subcutaneous sarcoidosis—clinicopathological study of 10 cases. Br J Dermatol. 2005;153:790-794.
3. Ahmed I, Harshad SR. Subcutaneous sarcoidosis: is it a specific subset of cutaneous sarcoidosis frequently associated with systemic disease [published online ahead of print December 2, 2005]? J Am Acad Dermatol. 2006;54:55-60.
4. Dalle Vedove C, Colato C, Girolomoni G. Subcutaneous sarcoidosis: report of two cases and review of the literature [published online ahead of print April 2, 2011]. Clin Rheumatol. 2011;30:1123-1128.
5. Barnadas MA, Rodríguez-Arias JM, Alomar A. Subcutaneous sarcoidosis associated with vitiligo, pernicious anaemia and autoimmune thyroiditis. Clin Exp Dermatol. 2000;25:55-56.
6. Higgins EM, Salisbury JR, Du Vivier AW. Subcutaneous sarcoidosis. Clin Exp Dermatol. 1993;18:65-66.
7. Heller M, Soldano AC. Sarcoidosis with subcutaneous lesions. Dermatol Online J. 2008;14:1.
8. Bosni´c D, Baresi´c M, Bagatin D, et al. Subcutaneous sarcoidosis of the face [published online ahead of print March 15, 2010]. Intern Med. 2010;49:589-592.
9. Chen HH, Chen YM, Lan HH, et al. Sonographic appearance of subcutaneous sarcoidosis. J Ultrasound Med. 2009;28:813-816.