User login
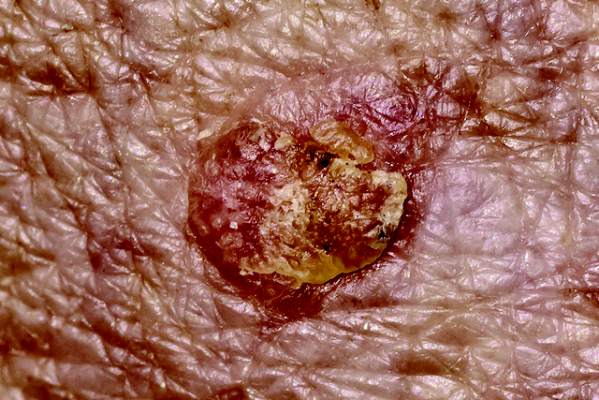
One course of topical fluorouracil cream reduced the need for localized treatments and the number of actinic keratoses (AK) over a mean follow-up of 2.6 years in the Veterans Affairs Keratinocyte Carcinoma Chemoprevention (VAKCC) trial.
These results “indicate that treating a patient with a single course of fluorouracil would reduce the subsequent number of spot treatments and benefit care of patients with multiple AKs for longer than 2 years,” concluded Dr. Hyemin Pomerantz of the department of dermatoepidemiology at the Providence (R.I.) VA Medical Center and his coauthors. Previous studies on treating AKs with topical fluorouracil have followed up participants for less than 6 months, they pointed out (JAMA Dermatol. 2015;9:952-60).
In the randomized, double-blinded, placebo-controlled study, conducted from 2009 to 2011 at 12 VA dermatology clinics, participants received topical fluorouracil cream, 5%, or a vehicle control cream, applied twice a day for 4 weeks, and were followed up for a mean of 2.6 years. There were no significant differences in the baseline characteristics of the 468 participants randomized to receive fluorouracil cream and the 464 participants randomized to receive the control cream.
The mean total AK count on the face and ears in both groups was about 11. At 6 months, the mean number of AKs per participant had dropped to 3 in the fluorouracil group (a 73% reduction from baseline) vs. a mean of 8.1 in the control group, a 24% reduction from baseline (P less than .001). Over the study period, those treated with fluorouracil had significantly fewer AKs. Over the entire study, there was not a significant difference in the number of hypertrophic AKs between the two groups, although the number of hypertrophic AKs was lower in the treatment group at 6 months.
During the study period, more participants treated with fluorouracil had complete clearance of AKs on the face and ears, compared with the control group (P less than .001). Finally, the fluorouracil group required 2 spot treatments per visit per participant vs. 3.9 in the control group (P less than .001).
“Our findings highlight the long-term efficacy of topical fluorouracil cream in treating and preventing AKs. A single course of topical fluorouracil cream, 5%, treatment led to a sustained reduction of the number of AKs and subsequent AK treatments,” in the population of patients at high risk for basal cell and squamous cell carcinomas, the authors wrote.
The study was supported by the Office of Research and Development Cooperative Studies Program at the Department of Veterans Affairs. Three of the authors reported several disclosures, including serving as a consultant to several pharmaceutical companies; the others, including the lead author, had no disclosures.
One course of topical fluorouracil cream reduced the need for localized treatments and the number of actinic keratoses (AK) over a mean follow-up of 2.6 years in the Veterans Affairs Keratinocyte Carcinoma Chemoprevention (VAKCC) trial.
These results “indicate that treating a patient with a single course of fluorouracil would reduce the subsequent number of spot treatments and benefit care of patients with multiple AKs for longer than 2 years,” concluded Dr. Hyemin Pomerantz of the department of dermatoepidemiology at the Providence (R.I.) VA Medical Center and his coauthors. Previous studies on treating AKs with topical fluorouracil have followed up participants for less than 6 months, they pointed out (JAMA Dermatol. 2015;9:952-60).
In the randomized, double-blinded, placebo-controlled study, conducted from 2009 to 2011 at 12 VA dermatology clinics, participants received topical fluorouracil cream, 5%, or a vehicle control cream, applied twice a day for 4 weeks, and were followed up for a mean of 2.6 years. There were no significant differences in the baseline characteristics of the 468 participants randomized to receive fluorouracil cream and the 464 participants randomized to receive the control cream.
The mean total AK count on the face and ears in both groups was about 11. At 6 months, the mean number of AKs per participant had dropped to 3 in the fluorouracil group (a 73% reduction from baseline) vs. a mean of 8.1 in the control group, a 24% reduction from baseline (P less than .001). Over the study period, those treated with fluorouracil had significantly fewer AKs. Over the entire study, there was not a significant difference in the number of hypertrophic AKs between the two groups, although the number of hypertrophic AKs was lower in the treatment group at 6 months.
During the study period, more participants treated with fluorouracil had complete clearance of AKs on the face and ears, compared with the control group (P less than .001). Finally, the fluorouracil group required 2 spot treatments per visit per participant vs. 3.9 in the control group (P less than .001).
“Our findings highlight the long-term efficacy of topical fluorouracil cream in treating and preventing AKs. A single course of topical fluorouracil cream, 5%, treatment led to a sustained reduction of the number of AKs and subsequent AK treatments,” in the population of patients at high risk for basal cell and squamous cell carcinomas, the authors wrote.
The study was supported by the Office of Research and Development Cooperative Studies Program at the Department of Veterans Affairs. Three of the authors reported several disclosures, including serving as a consultant to several pharmaceutical companies; the others, including the lead author, had no disclosures.
One course of topical fluorouracil cream reduced the need for localized treatments and the number of actinic keratoses (AK) over a mean follow-up of 2.6 years in the Veterans Affairs Keratinocyte Carcinoma Chemoprevention (VAKCC) trial.
These results “indicate that treating a patient with a single course of fluorouracil would reduce the subsequent number of spot treatments and benefit care of patients with multiple AKs for longer than 2 years,” concluded Dr. Hyemin Pomerantz of the department of dermatoepidemiology at the Providence (R.I.) VA Medical Center and his coauthors. Previous studies on treating AKs with topical fluorouracil have followed up participants for less than 6 months, they pointed out (JAMA Dermatol. 2015;9:952-60).
In the randomized, double-blinded, placebo-controlled study, conducted from 2009 to 2011 at 12 VA dermatology clinics, participants received topical fluorouracil cream, 5%, or a vehicle control cream, applied twice a day for 4 weeks, and were followed up for a mean of 2.6 years. There were no significant differences in the baseline characteristics of the 468 participants randomized to receive fluorouracil cream and the 464 participants randomized to receive the control cream.
The mean total AK count on the face and ears in both groups was about 11. At 6 months, the mean number of AKs per participant had dropped to 3 in the fluorouracil group (a 73% reduction from baseline) vs. a mean of 8.1 in the control group, a 24% reduction from baseline (P less than .001). Over the study period, those treated with fluorouracil had significantly fewer AKs. Over the entire study, there was not a significant difference in the number of hypertrophic AKs between the two groups, although the number of hypertrophic AKs was lower in the treatment group at 6 months.
During the study period, more participants treated with fluorouracil had complete clearance of AKs on the face and ears, compared with the control group (P less than .001). Finally, the fluorouracil group required 2 spot treatments per visit per participant vs. 3.9 in the control group (P less than .001).
“Our findings highlight the long-term efficacy of topical fluorouracil cream in treating and preventing AKs. A single course of topical fluorouracil cream, 5%, treatment led to a sustained reduction of the number of AKs and subsequent AK treatments,” in the population of patients at high risk for basal cell and squamous cell carcinomas, the authors wrote.
The study was supported by the Office of Research and Development Cooperative Studies Program at the Department of Veterans Affairs. Three of the authors reported several disclosures, including serving as a consultant to several pharmaceutical companies; the others, including the lead author, had no disclosures.
FROM JAMA DERMATOLOGY
Key clinical point:One course of topical fluorouracil cream, 5%, decreased the need for localized treatment and the number of actinic keratoses long term.
Major finding: Participants whose AKs were treated with a course of fluorouracil cream, 5%, had significantly fewer AKs and required fewer treatments, compared with the control group, for over 2 years.
Data source: The randomized, double-blind study compared the effect of a course of topical fluorouracil cream with a vehicle cream on the number of AKs and other measures, over a mean follow-up of 2.6 years, in 932 patients treated at 12 VA dermatology clinics.
Disclosures: The study was supported by the Office of Research and Development Cooperative Studies Program at the Department of Veterans Affairs. Three of the authors reported several disclosures, including serving as a consultant to several pharmaceutical companies; the others had no disclosures.