User login
Twenty years ago, the dilemma in treating acute otitis media (AOM) was which among 10-plus antibiotics to prescribe. A recent column discussed the evolving pathogen distribution in AOM and its effects on antibiotic choices.1 But here we consider treatment duration. Until the past decade, AOM treatment (except azithromycin) involved 10-day courses. But lately, 10-day antibiotic regimens for uncomplicated infections are disappearing. Shorter-course recommendations are the new norm because of the evolving clinical data showing that an appropriately chosen antibiotic (in partnership with host defenses and source control) resolves infection faster than was previously thought. Shorter courses make sense because of fewer adverse effects, less distortion of normal flora, and less likely induction of pathogen resistance. Table 4.12 in the newest 2021-2024 SOID Redbook lists three antibiotic durations for AOM, and actually there are more than that.
Why so many duration options? Clinical data show that not all AOM is alike and short courses work for subsets of AOM because, besides antibiotics, key elements in AOM resolution are host anatomy and immunity. Bacterial AOM results from a combination of refluxed pathogens in the middle ear being trapped when the eustachian tube malfunctions (infection occurs when middle ear plumbing gets stopped up). If the eustachian tube spontaneously drains and the host immune response slows/stops pathogen growth, no antibiotics are needed. Indeed, a sizable proportion of mild/moderate AOM episodes spontaneously resolve, particularly in children over 2 years old. So a high likelihood of spontaneous remission allows an initial 0-days duration option (watchful waiting) or delayed antibiotics (rescue prescriptions) for older children.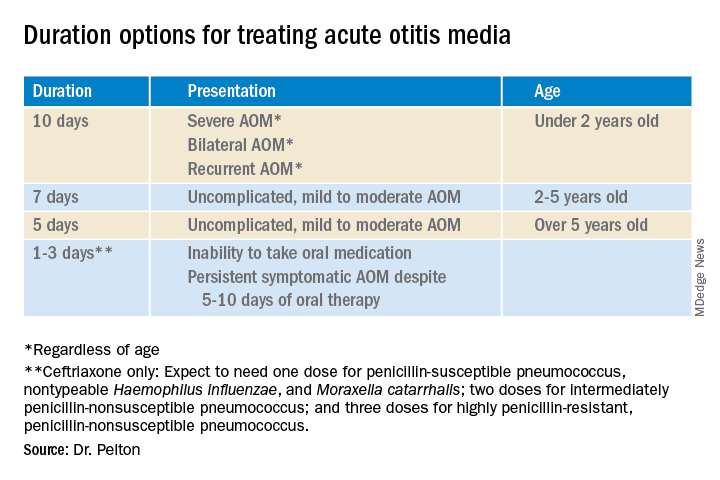
That said, when one chooses to initially prescribe antibiotics for AOM, different durations are recommended. Table 1 has my suggestions.
Data that gave me better microbiological understanding of why oral AOM trials less than 10 days were successful involved purulent AOM drainage from children who had pressure-equalizing (PE) tubes.2 The authors randomized children to either standard-dose amoxicillin-clavulanate or placebo. Of note, 95% of pathogens were susceptible to the antibiotic; 5% were pneumococcus intermediately resistant to penicillin. The authors sampled ear drainage daily for 7 days. Figure 1 shows that cultures remained positive in only around 5% of children by day 3-5 of antibiotics, but viable bacteria persisted through 7 days in over half of placebo recipients. Remember, both groups benefited from a form of source control (drainage of the middle ear via PE tubes). So, if antibiotics can do the job in 3-5 days, why continue antibiotics beyond 5 days?
Anatomy and severity. In children over 5 years old (reasonably mature eustachian tube anatomy) with nonrecurrent (no AOM in past month), nonsevere (no otalgia or high fever) AOM, 5 days is enough. But 2- to 5-year-olds (less mature anatomy) need 7 days and those <2 years old (least mature plumbing) need 10 days. Likewise, severe AOM usually warrants 10 days. Some experts recommend 10 days for bilateral AOM as well.
These age/severity differences make sense because failures are more frequent with:
1. Younger age.3 While not proven, my hypothesis is that “natural” source control (spontaneous internal draining the middle ear into the nasopharynx [NP]) is less frequent in younger children because they have less mature eustachian tube systems. Further, reflux of persisting NP organisms could restart a new AOM episode even if the original pathogen was eliminated by a short 5-day course.
2. Severe AOM. A rationale for longer courses in severe AOM (ear pain, high fever) is that high middle-ear pressures (indicated by degree of tympanic membrane bulging and ear pain) could impede antibiotic penetration, or that high initial bacterial loads (perhaps indicated by systemic fever) require more antibiotic. And finally, return to baseline eustachian tube function may take longer if severe AOM caused enhanced inflammation.
3. Recurrent AOM. (AOM within 1 prior month) – With recurrent AOM, the second “hit” to the eustachian tube may lead to more dysfunction, so a longer antibiotic course may be required to allow more complete source control and more time for more complete functional recovery after a repeated inflammatory injury.
4. Bilateral AOM. Two independent but infected sites mean twice the chance for failure. So, a longer course could allow more time for both sites to undergo “natural” source control.4
More bacteria – more antibiotic? So, is more antibiotic really needed for a higher bacterial load? In vitro this is known as the “inoculum effect,” particularly for beta-lactam drugs, for example, amoxicillin and cephalosporins. Laboratory susceptibility testing is performed with a specifically defined quantity of bacteria (105 bacteria/mL) and the minimum inhibitory concentration (MIC) is the lowest antibiotic concentration that stops bacterial growth. We know that drugs will likely fail if the MIC exceeds the achievable antibiotic concentration at the infection site. But is it as simple as just exceeding the MIC at the infection site? No, pharmacodynamics tell us that overall antibiotic exposure is also important. For example, to be successful, beta-lactam concentrations need to be above the MIC for 40%-50% of the day.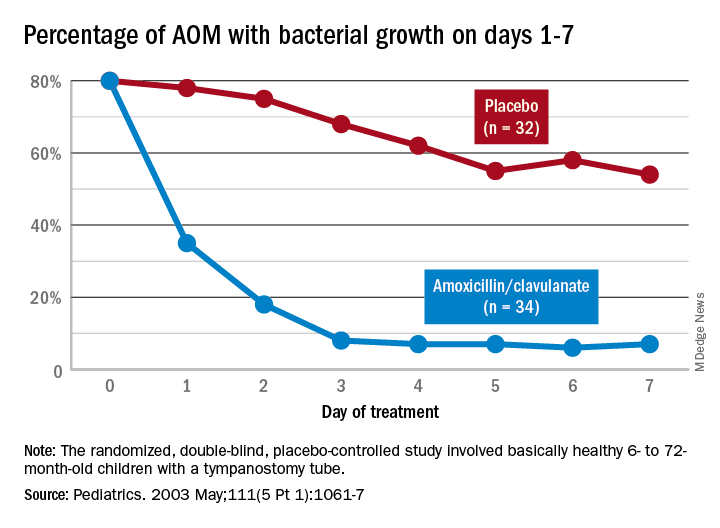
Higher MIC with higher bacterial load. Particularly for beta-lactams, testing with a quantity of bacteria >105/mL produces a higher MIC in vitro. This suggests that clinical failure could occur, even when our in vivo dosing leads to 40%-50% above the “standard” MIC that was obtained from testing the lab standard of 105/mL bacteria, when the infected site’s (middle ear) bacterial load is >105/mL (such higher bacterial loads occur in up to 30% of AOM).5 One way to negate inoculum effect is source control (drain the abscess or debridement), which reduces the bacterial load as well as allowing better antibiotic penetration– both favoring infection resolution. But with suboptimal source control, for example, the middle ear is not drained externally or internally, longer courses (more antibiotic exposure) could aid resolution. Whether the exposure can be administered as higher doses in fewer days or standard doses for more days is debatable but consider that a single parenteral dose of ceftriaxone successfully resolves AOM not attributable to penicillin-nonsusceptible pneumococcus.6Bottom line: Even though the number of potential antibiotics has contracted in the past 20 years, the need to individualize AOM treatment remains important and duration choices are more complex. Indeed, AOM comes in different flavors with patient age, clinical presentation, and episode frequency dictating the choice of duration.
Dr. Christopher J. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. Email him at [email protected].
References
1. Pichichero ME. MDedge. 2022 Jan 11.
2. Ruohola A et al. Pediatrics. 2003;111(5):1061-7.
3. Hoberman A et al. N Engl J Med. 2016;375(25):2446-56.
4. Pichichero ME et al. Otolaryngol Head Neck Surg. 2001;124(4):381-7.
5. Harrison CJ et al. Pediatr Infect Dis. 1985;4(6):641-6.
6. Leibovitz E et al. Pediatr Infect Dis. 2000;19(11):1040-5.
Twenty years ago, the dilemma in treating acute otitis media (AOM) was which among 10-plus antibiotics to prescribe. A recent column discussed the evolving pathogen distribution in AOM and its effects on antibiotic choices.1 But here we consider treatment duration. Until the past decade, AOM treatment (except azithromycin) involved 10-day courses. But lately, 10-day antibiotic regimens for uncomplicated infections are disappearing. Shorter-course recommendations are the new norm because of the evolving clinical data showing that an appropriately chosen antibiotic (in partnership with host defenses and source control) resolves infection faster than was previously thought. Shorter courses make sense because of fewer adverse effects, less distortion of normal flora, and less likely induction of pathogen resistance. Table 4.12 in the newest 2021-2024 SOID Redbook lists three antibiotic durations for AOM, and actually there are more than that.
Why so many duration options? Clinical data show that not all AOM is alike and short courses work for subsets of AOM because, besides antibiotics, key elements in AOM resolution are host anatomy and immunity. Bacterial AOM results from a combination of refluxed pathogens in the middle ear being trapped when the eustachian tube malfunctions (infection occurs when middle ear plumbing gets stopped up). If the eustachian tube spontaneously drains and the host immune response slows/stops pathogen growth, no antibiotics are needed. Indeed, a sizable proportion of mild/moderate AOM episodes spontaneously resolve, particularly in children over 2 years old. So a high likelihood of spontaneous remission allows an initial 0-days duration option (watchful waiting) or delayed antibiotics (rescue prescriptions) for older children.
That said, when one chooses to initially prescribe antibiotics for AOM, different durations are recommended. Table 1 has my suggestions.
Data that gave me better microbiological understanding of why oral AOM trials less than 10 days were successful involved purulent AOM drainage from children who had pressure-equalizing (PE) tubes.2 The authors randomized children to either standard-dose amoxicillin-clavulanate or placebo. Of note, 95% of pathogens were susceptible to the antibiotic; 5% were pneumococcus intermediately resistant to penicillin. The authors sampled ear drainage daily for 7 days. Figure 1 shows that cultures remained positive in only around 5% of children by day 3-5 of antibiotics, but viable bacteria persisted through 7 days in over half of placebo recipients. Remember, both groups benefited from a form of source control (drainage of the middle ear via PE tubes). So, if antibiotics can do the job in 3-5 days, why continue antibiotics beyond 5 days?
Anatomy and severity. In children over 5 years old (reasonably mature eustachian tube anatomy) with nonrecurrent (no AOM in past month), nonsevere (no otalgia or high fever) AOM, 5 days is enough. But 2- to 5-year-olds (less mature anatomy) need 7 days and those <2 years old (least mature plumbing) need 10 days. Likewise, severe AOM usually warrants 10 days. Some experts recommend 10 days for bilateral AOM as well.
These age/severity differences make sense because failures are more frequent with:
1. Younger age.3 While not proven, my hypothesis is that “natural” source control (spontaneous internal draining the middle ear into the nasopharynx [NP]) is less frequent in younger children because they have less mature eustachian tube systems. Further, reflux of persisting NP organisms could restart a new AOM episode even if the original pathogen was eliminated by a short 5-day course.
2. Severe AOM. A rationale for longer courses in severe AOM (ear pain, high fever) is that high middle-ear pressures (indicated by degree of tympanic membrane bulging and ear pain) could impede antibiotic penetration, or that high initial bacterial loads (perhaps indicated by systemic fever) require more antibiotic. And finally, return to baseline eustachian tube function may take longer if severe AOM caused enhanced inflammation.
3. Recurrent AOM. (AOM within 1 prior month) – With recurrent AOM, the second “hit” to the eustachian tube may lead to more dysfunction, so a longer antibiotic course may be required to allow more complete source control and more time for more complete functional recovery after a repeated inflammatory injury.
4. Bilateral AOM. Two independent but infected sites mean twice the chance for failure. So, a longer course could allow more time for both sites to undergo “natural” source control.4
More bacteria – more antibiotic? So, is more antibiotic really needed for a higher bacterial load? In vitro this is known as the “inoculum effect,” particularly for beta-lactam drugs, for example, amoxicillin and cephalosporins. Laboratory susceptibility testing is performed with a specifically defined quantity of bacteria (105 bacteria/mL) and the minimum inhibitory concentration (MIC) is the lowest antibiotic concentration that stops bacterial growth. We know that drugs will likely fail if the MIC exceeds the achievable antibiotic concentration at the infection site. But is it as simple as just exceeding the MIC at the infection site? No, pharmacodynamics tell us that overall antibiotic exposure is also important. For example, to be successful, beta-lactam concentrations need to be above the MIC for 40%-50% of the day.
Higher MIC with higher bacterial load. Particularly for beta-lactams, testing with a quantity of bacteria >105/mL produces a higher MIC in vitro. This suggests that clinical failure could occur, even when our in vivo dosing leads to 40%-50% above the “standard” MIC that was obtained from testing the lab standard of 105/mL bacteria, when the infected site’s (middle ear) bacterial load is >105/mL (such higher bacterial loads occur in up to 30% of AOM).5 One way to negate inoculum effect is source control (drain the abscess or debridement), which reduces the bacterial load as well as allowing better antibiotic penetration– both favoring infection resolution. But with suboptimal source control, for example, the middle ear is not drained externally or internally, longer courses (more antibiotic exposure) could aid resolution. Whether the exposure can be administered as higher doses in fewer days or standard doses for more days is debatable but consider that a single parenteral dose of ceftriaxone successfully resolves AOM not attributable to penicillin-nonsusceptible pneumococcus.6Bottom line: Even though the number of potential antibiotics has contracted in the past 20 years, the need to individualize AOM treatment remains important and duration choices are more complex. Indeed, AOM comes in different flavors with patient age, clinical presentation, and episode frequency dictating the choice of duration.
Dr. Christopher J. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. Email him at [email protected].
References
1. Pichichero ME. MDedge. 2022 Jan 11.
2. Ruohola A et al. Pediatrics. 2003;111(5):1061-7.
3. Hoberman A et al. N Engl J Med. 2016;375(25):2446-56.
4. Pichichero ME et al. Otolaryngol Head Neck Surg. 2001;124(4):381-7.
5. Harrison CJ et al. Pediatr Infect Dis. 1985;4(6):641-6.
6. Leibovitz E et al. Pediatr Infect Dis. 2000;19(11):1040-5.
Twenty years ago, the dilemma in treating acute otitis media (AOM) was which among 10-plus antibiotics to prescribe. A recent column discussed the evolving pathogen distribution in AOM and its effects on antibiotic choices.1 But here we consider treatment duration. Until the past decade, AOM treatment (except azithromycin) involved 10-day courses. But lately, 10-day antibiotic regimens for uncomplicated infections are disappearing. Shorter-course recommendations are the new norm because of the evolving clinical data showing that an appropriately chosen antibiotic (in partnership with host defenses and source control) resolves infection faster than was previously thought. Shorter courses make sense because of fewer adverse effects, less distortion of normal flora, and less likely induction of pathogen resistance. Table 4.12 in the newest 2021-2024 SOID Redbook lists three antibiotic durations for AOM, and actually there are more than that.
Why so many duration options? Clinical data show that not all AOM is alike and short courses work for subsets of AOM because, besides antibiotics, key elements in AOM resolution are host anatomy and immunity. Bacterial AOM results from a combination of refluxed pathogens in the middle ear being trapped when the eustachian tube malfunctions (infection occurs when middle ear plumbing gets stopped up). If the eustachian tube spontaneously drains and the host immune response slows/stops pathogen growth, no antibiotics are needed. Indeed, a sizable proportion of mild/moderate AOM episodes spontaneously resolve, particularly in children over 2 years old. So a high likelihood of spontaneous remission allows an initial 0-days duration option (watchful waiting) or delayed antibiotics (rescue prescriptions) for older children.
That said, when one chooses to initially prescribe antibiotics for AOM, different durations are recommended. Table 1 has my suggestions.
Data that gave me better microbiological understanding of why oral AOM trials less than 10 days were successful involved purulent AOM drainage from children who had pressure-equalizing (PE) tubes.2 The authors randomized children to either standard-dose amoxicillin-clavulanate or placebo. Of note, 95% of pathogens were susceptible to the antibiotic; 5% were pneumococcus intermediately resistant to penicillin. The authors sampled ear drainage daily for 7 days. Figure 1 shows that cultures remained positive in only around 5% of children by day 3-5 of antibiotics, but viable bacteria persisted through 7 days in over half of placebo recipients. Remember, both groups benefited from a form of source control (drainage of the middle ear via PE tubes). So, if antibiotics can do the job in 3-5 days, why continue antibiotics beyond 5 days?
Anatomy and severity. In children over 5 years old (reasonably mature eustachian tube anatomy) with nonrecurrent (no AOM in past month), nonsevere (no otalgia or high fever) AOM, 5 days is enough. But 2- to 5-year-olds (less mature anatomy) need 7 days and those <2 years old (least mature plumbing) need 10 days. Likewise, severe AOM usually warrants 10 days. Some experts recommend 10 days for bilateral AOM as well.
These age/severity differences make sense because failures are more frequent with:
1. Younger age.3 While not proven, my hypothesis is that “natural” source control (spontaneous internal draining the middle ear into the nasopharynx [NP]) is less frequent in younger children because they have less mature eustachian tube systems. Further, reflux of persisting NP organisms could restart a new AOM episode even if the original pathogen was eliminated by a short 5-day course.
2. Severe AOM. A rationale for longer courses in severe AOM (ear pain, high fever) is that high middle-ear pressures (indicated by degree of tympanic membrane bulging and ear pain) could impede antibiotic penetration, or that high initial bacterial loads (perhaps indicated by systemic fever) require more antibiotic. And finally, return to baseline eustachian tube function may take longer if severe AOM caused enhanced inflammation.
3. Recurrent AOM. (AOM within 1 prior month) – With recurrent AOM, the second “hit” to the eustachian tube may lead to more dysfunction, so a longer antibiotic course may be required to allow more complete source control and more time for more complete functional recovery after a repeated inflammatory injury.
4. Bilateral AOM. Two independent but infected sites mean twice the chance for failure. So, a longer course could allow more time for both sites to undergo “natural” source control.4
More bacteria – more antibiotic? So, is more antibiotic really needed for a higher bacterial load? In vitro this is known as the “inoculum effect,” particularly for beta-lactam drugs, for example, amoxicillin and cephalosporins. Laboratory susceptibility testing is performed with a specifically defined quantity of bacteria (105 bacteria/mL) and the minimum inhibitory concentration (MIC) is the lowest antibiotic concentration that stops bacterial growth. We know that drugs will likely fail if the MIC exceeds the achievable antibiotic concentration at the infection site. But is it as simple as just exceeding the MIC at the infection site? No, pharmacodynamics tell us that overall antibiotic exposure is also important. For example, to be successful, beta-lactam concentrations need to be above the MIC for 40%-50% of the day.
Higher MIC with higher bacterial load. Particularly for beta-lactams, testing with a quantity of bacteria >105/mL produces a higher MIC in vitro. This suggests that clinical failure could occur, even when our in vivo dosing leads to 40%-50% above the “standard” MIC that was obtained from testing the lab standard of 105/mL bacteria, when the infected site’s (middle ear) bacterial load is >105/mL (such higher bacterial loads occur in up to 30% of AOM).5 One way to negate inoculum effect is source control (drain the abscess or debridement), which reduces the bacterial load as well as allowing better antibiotic penetration– both favoring infection resolution. But with suboptimal source control, for example, the middle ear is not drained externally or internally, longer courses (more antibiotic exposure) could aid resolution. Whether the exposure can be administered as higher doses in fewer days or standard doses for more days is debatable but consider that a single parenteral dose of ceftriaxone successfully resolves AOM not attributable to penicillin-nonsusceptible pneumococcus.6Bottom line: Even though the number of potential antibiotics has contracted in the past 20 years, the need to individualize AOM treatment remains important and duration choices are more complex. Indeed, AOM comes in different flavors with patient age, clinical presentation, and episode frequency dictating the choice of duration.
Dr. Christopher J. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. Email him at [email protected].
References
1. Pichichero ME. MDedge. 2022 Jan 11.
2. Ruohola A et al. Pediatrics. 2003;111(5):1061-7.
3. Hoberman A et al. N Engl J Med. 2016;375(25):2446-56.
4. Pichichero ME et al. Otolaryngol Head Neck Surg. 2001;124(4):381-7.
5. Harrison CJ et al. Pediatr Infect Dis. 1985;4(6):641-6.
6. Leibovitz E et al. Pediatr Infect Dis. 2000;19(11):1040-5.