User login
In the presentation of secondary amenorrhea, pregnancy is the No. 1 differential diagnosis. Once this has been excluded, an algorithm is initiated to determine the etiology, including an assessment of the hypothalamic-pituitary-ovarian axis. While the early onset of ovarian failure can be physically and psychologically disrupting, the effect on fertility is an especially devastating event. Previously identified by terms including premature ovarian failure and premature menopause, “primary ovarian insufficiency” (POI) is now the preferred designation. This month’s article will address the diagnosis, evaluation, and management of POI.
The definition of POI is the development of primary hypogonadism before the age of 40 years. Spontaneous POI occurs in approximately 1 in 250 women by age 35 years and 1 in 100 by age 40 years. After excluding pregnancy, the clinician should determine signs and symptoms that can lead to expedited and cost-efficient testing.
Consequences
POI is an important risk factor for bone loss and osteoporosis, especially in young women who develop ovarian dysfunction before they achieve peak adult bone mass. At the time of diagnosis of POI, a bone density test (dual-energy x-ray absorptiometry) should be obtained. Women with POI may also develop depression and anxiety as well as experience an increased risk for cardiovascular morbidity and mortality, possibly related to endothelial dysfunction.
Young women with spontaneous POI are at increased risk of developing autoimmune adrenal insufficiency (AAI), a potentially fatal disorder. Consequently, to diagnose AAI, serum adrenal cortical and 21-hydroxylase antibodies should be measured in all women who have a karyotype of 46,XX and experience spontaneous POI. Women with AAI have a 50% risk of developing adrenal insufficiency. Despite initial normal adrenal function, women with positive adrenal cortical antibodies should be followed annually.
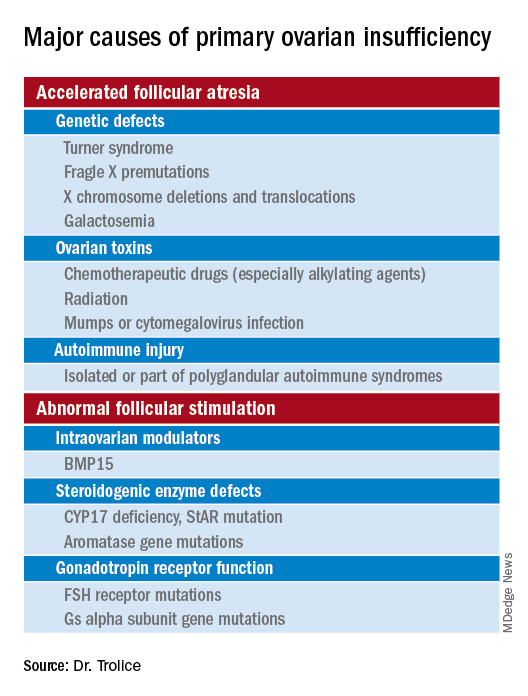
Causes (see table for a more complete list)
Iatrogenic
Known causes of POI include chemotherapy/radiation often in the setting of cancer treatment. The three most commonly used drugs, cyclophosphamide, cisplatin, and doxorubicin, cause POI by inducing death and/or accelerated activation of primordial follicles and increased atresia of growing follicles. The most damaging agents are alkylating drugs. A cyclophosphamide equivalent dose calculator has been established for ovarian failure risk stratification from chemotherapy based on the cumulative dose of alkylating agents received.
One study estimated the radiosensitivity of the oocyte to be less than 2 Gy. Based upon this estimate, the authors calculated the dose of radiotherapy that would result in immediate and permanent ovarian failure in 97.5% of patients as follows:
- 20.3 Gy at birth
- 18.4 Gy at age 10 years
- 16.5 Gy at age 20 years
- 14.3 Gy at age 30 years
Genetic
Approximately 10% of cases are familial. A family history of POI raises concern for a fragile X premutation. Fragile X syndrome is an X-linked form of intellectual disability that is one of the most common causes of mental retardation worldwide. There is a strong relationship between age at menopause, including POI, and premutations for fragile X syndrome. The American College of Obstetricians and Gynecologists recommends that women with POI or an elevated follicle-stimulating hormone (FSH) level before age 40 years without known cause be screened for FMR1 premutations. Approximately 6% of cases of POI are associated with premutations in the FMR1 gene.
Turner syndrome is one of the most common causes of POI and results from the lack of a second X chromosome. The most common chromosomal defect in humans, TS occurs in up to 1.5% of conceptions, 10% of spontaneous abortions, and 1 of 2,500 live births.
Serum antiadrenal and/or anti–21-hydroxylase antibodies and antithyroid antiperoxidase antibodies, can aid in the diagnosis of adrenal gland, ovary, and thyroid autoimmune causes, which is found in 4% of women with spontaneous POI. Testing for the presence of 21-hydroxylase autoantibodies or adrenal autoantibodies is sufficient to make the diagnosis of autoimmune oophoritis in women with proven spontaneous POI.
The etiology of POI remains unknown in approximately 75%-90% of cases. However, studies using whole exome or whole genome sequencing have identified genetic variants in approximately 30%-35% of these patients.
Risk factors
Factors that are thought to play a role in determining the age of menopause, include genetics (e.g., FMR1 premutation and mosaic Turner syndrome), ethnicity (earlier among Hispanic women and later in Japanese American women when compared with White women), and smoking (reduced by approximately 2 years ).
Regarding ovarian aging, the holy grail of the reproductive life span is to predict menopause. While the definitive age eludes us, anti-Müllerian hormone levels appear to show promise. An ultrasensitive anti-Müllerian hormone assay (< 0.01 ng/mL) predicted a 79% probability of menopause within 12 months for women aged 51 and above; the probability was 51% for women below age 48.
Diagnosis
The three P’s of secondary amenorrhea are physiological, pharmacological, or pathological and can guide the clinician to a targeted evaluation. Physiological causes are pregnancy, the first 6 months of continuous breastfeeding (from elevated prolactin), and natural menopause. Pharmacological etiologies, excluding hormonal treatment that suppresses ovulation (combined oral contraceptives, gonadotropin-releasing hormone agonist/antagonist, or danazol), include agents that inhibit dopamine thereby increasing serum prolactin, such as metoclopramide; phenothiazine antipsychotics, such as haloperidol; and tardive dystonia dopamine-depleting medications, such as reserpine. Pathological causes include pituitary adenomas, thyroid disease, functional hypothalamic amenorrhea from changes in weight, exercise regimen, and stress.
Management
About 50%-75% of women with 46,XX spontaneous POI experience intermittent ovarian function and 5%-10% of women remain able to conceive. Anecdotally, a 32-year-old woman presented to me with primary infertility, secondary amenorrhea, and suspected POI based on vasomotor symptoms and elevated FSH levels. Pelvic ultrasound showed a hemorrhagic cyst, suspicious for a corpus luteum. Two weeks thereafter she reported a positive home urine human chorionic gonadotropin test and ultimately delivered twins. Her diagnosis of POI with amenorrhea remained postpartum.
Unless there is an absolute contraindication, estrogen therapy should be prescribed to women with POI to reduce the risk of osteoporosis, cardiovascular disease, and urogenital atrophy as well as to maintain sexual health and quality of life. For those with an intact uterus, women should receive progesterone because of the risk of endometrial hyperplasia from unopposed estrogen. Rather than oral estrogen, the use of transdermal or vaginal delivery of estrogen is a more physiological approach and provides lower risks of venous thromboembolism and gallbladder disease. Of note, standard postmenopausal hormone therapy, which has a much lower dose of estrogen than combined estrogen-progestin contraceptives, does not provide effective contraception. Per ACOG, systemic hormone treatment should be prescribed until age 50-51 years to all women with POI.
For fertility, women with spontaneous POI can be offered oocyte or embryo donation. The uterus does not age reproductively, unlike oocytes, therefore women can achieve reasonable pregnancy success rates through egg donation despite experiencing menopause.
Future potential options
Female germline stem cells have been isolated from neonatal mice and transplanted into sterile adult mice, who then were able to produce offspring. In a second study, oogonial stem cells were isolated from neonatal and adult mouse ovaries; pups were subsequently born from the oocytes. Further experiments are needed before the implications for humans can be determined.
Emotionally traumatic for most women, POI disrupts life plans, hopes, and dreams of raising a family. The approach to the patient with POI involves the above evidence-based testing along with empathy from the health care provider.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
In the presentation of secondary amenorrhea, pregnancy is the No. 1 differential diagnosis. Once this has been excluded, an algorithm is initiated to determine the etiology, including an assessment of the hypothalamic-pituitary-ovarian axis. While the early onset of ovarian failure can be physically and psychologically disrupting, the effect on fertility is an especially devastating event. Previously identified by terms including premature ovarian failure and premature menopause, “primary ovarian insufficiency” (POI) is now the preferred designation. This month’s article will address the diagnosis, evaluation, and management of POI.
The definition of POI is the development of primary hypogonadism before the age of 40 years. Spontaneous POI occurs in approximately 1 in 250 women by age 35 years and 1 in 100 by age 40 years. After excluding pregnancy, the clinician should determine signs and symptoms that can lead to expedited and cost-efficient testing.
Consequences
POI is an important risk factor for bone loss and osteoporosis, especially in young women who develop ovarian dysfunction before they achieve peak adult bone mass. At the time of diagnosis of POI, a bone density test (dual-energy x-ray absorptiometry) should be obtained. Women with POI may also develop depression and anxiety as well as experience an increased risk for cardiovascular morbidity and mortality, possibly related to endothelial dysfunction.
Young women with spontaneous POI are at increased risk of developing autoimmune adrenal insufficiency (AAI), a potentially fatal disorder. Consequently, to diagnose AAI, serum adrenal cortical and 21-hydroxylase antibodies should be measured in all women who have a karyotype of 46,XX and experience spontaneous POI. Women with AAI have a 50% risk of developing adrenal insufficiency. Despite initial normal adrenal function, women with positive adrenal cortical antibodies should be followed annually.
Causes (see table for a more complete list)
Iatrogenic
Known causes of POI include chemotherapy/radiation often in the setting of cancer treatment. The three most commonly used drugs, cyclophosphamide, cisplatin, and doxorubicin, cause POI by inducing death and/or accelerated activation of primordial follicles and increased atresia of growing follicles. The most damaging agents are alkylating drugs. A cyclophosphamide equivalent dose calculator has been established for ovarian failure risk stratification from chemotherapy based on the cumulative dose of alkylating agents received.
One study estimated the radiosensitivity of the oocyte to be less than 2 Gy. Based upon this estimate, the authors calculated the dose of radiotherapy that would result in immediate and permanent ovarian failure in 97.5% of patients as follows:
- 20.3 Gy at birth
- 18.4 Gy at age 10 years
- 16.5 Gy at age 20 years
- 14.3 Gy at age 30 years

Genetic
Approximately 10% of cases are familial. A family history of POI raises concern for a fragile X premutation. Fragile X syndrome is an X-linked form of intellectual disability that is one of the most common causes of mental retardation worldwide. There is a strong relationship between age at menopause, including POI, and premutations for fragile X syndrome. The American College of Obstetricians and Gynecologists recommends that women with POI or an elevated follicle-stimulating hormone (FSH) level before age 40 years without known cause be screened for FMR1 premutations. Approximately 6% of cases of POI are associated with premutations in the FMR1 gene.
Turner syndrome is one of the most common causes of POI and results from the lack of a second X chromosome. The most common chromosomal defect in humans, TS occurs in up to 1.5% of conceptions, 10% of spontaneous abortions, and 1 of 2,500 live births.
Serum antiadrenal and/or anti–21-hydroxylase antibodies and antithyroid antiperoxidase antibodies, can aid in the diagnosis of adrenal gland, ovary, and thyroid autoimmune causes, which is found in 4% of women with spontaneous POI. Testing for the presence of 21-hydroxylase autoantibodies or adrenal autoantibodies is sufficient to make the diagnosis of autoimmune oophoritis in women with proven spontaneous POI.
The etiology of POI remains unknown in approximately 75%-90% of cases. However, studies using whole exome or whole genome sequencing have identified genetic variants in approximately 30%-35% of these patients.
Risk factors
Factors that are thought to play a role in determining the age of menopause, include genetics (e.g., FMR1 premutation and mosaic Turner syndrome), ethnicity (earlier among Hispanic women and later in Japanese American women when compared with White women), and smoking (reduced by approximately 2 years ).
Regarding ovarian aging, the holy grail of the reproductive life span is to predict menopause. While the definitive age eludes us, anti-Müllerian hormone levels appear to show promise. An ultrasensitive anti-Müllerian hormone assay (< 0.01 ng/mL) predicted a 79% probability of menopause within 12 months for women aged 51 and above; the probability was 51% for women below age 48.
Diagnosis
The three P’s of secondary amenorrhea are physiological, pharmacological, or pathological and can guide the clinician to a targeted evaluation. Physiological causes are pregnancy, the first 6 months of continuous breastfeeding (from elevated prolactin), and natural menopause. Pharmacological etiologies, excluding hormonal treatment that suppresses ovulation (combined oral contraceptives, gonadotropin-releasing hormone agonist/antagonist, or danazol), include agents that inhibit dopamine thereby increasing serum prolactin, such as metoclopramide; phenothiazine antipsychotics, such as haloperidol; and tardive dystonia dopamine-depleting medications, such as reserpine. Pathological causes include pituitary adenomas, thyroid disease, functional hypothalamic amenorrhea from changes in weight, exercise regimen, and stress.
Management
About 50%-75% of women with 46,XX spontaneous POI experience intermittent ovarian function and 5%-10% of women remain able to conceive. Anecdotally, a 32-year-old woman presented to me with primary infertility, secondary amenorrhea, and suspected POI based on vasomotor symptoms and elevated FSH levels. Pelvic ultrasound showed a hemorrhagic cyst, suspicious for a corpus luteum. Two weeks thereafter she reported a positive home urine human chorionic gonadotropin test and ultimately delivered twins. Her diagnosis of POI with amenorrhea remained postpartum.
Unless there is an absolute contraindication, estrogen therapy should be prescribed to women with POI to reduce the risk of osteoporosis, cardiovascular disease, and urogenital atrophy as well as to maintain sexual health and quality of life. For those with an intact uterus, women should receive progesterone because of the risk of endometrial hyperplasia from unopposed estrogen. Rather than oral estrogen, the use of transdermal or vaginal delivery of estrogen is a more physiological approach and provides lower risks of venous thromboembolism and gallbladder disease. Of note, standard postmenopausal hormone therapy, which has a much lower dose of estrogen than combined estrogen-progestin contraceptives, does not provide effective contraception. Per ACOG, systemic hormone treatment should be prescribed until age 50-51 years to all women with POI.
For fertility, women with spontaneous POI can be offered oocyte or embryo donation. The uterus does not age reproductively, unlike oocytes, therefore women can achieve reasonable pregnancy success rates through egg donation despite experiencing menopause.
Future potential options
Female germline stem cells have been isolated from neonatal mice and transplanted into sterile adult mice, who then were able to produce offspring. In a second study, oogonial stem cells were isolated from neonatal and adult mouse ovaries; pups were subsequently born from the oocytes. Further experiments are needed before the implications for humans can be determined.
Emotionally traumatic for most women, POI disrupts life plans, hopes, and dreams of raising a family. The approach to the patient with POI involves the above evidence-based testing along with empathy from the health care provider.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
In the presentation of secondary amenorrhea, pregnancy is the No. 1 differential diagnosis. Once this has been excluded, an algorithm is initiated to determine the etiology, including an assessment of the hypothalamic-pituitary-ovarian axis. While the early onset of ovarian failure can be physically and psychologically disrupting, the effect on fertility is an especially devastating event. Previously identified by terms including premature ovarian failure and premature menopause, “primary ovarian insufficiency” (POI) is now the preferred designation. This month’s article will address the diagnosis, evaluation, and management of POI.
The definition of POI is the development of primary hypogonadism before the age of 40 years. Spontaneous POI occurs in approximately 1 in 250 women by age 35 years and 1 in 100 by age 40 years. After excluding pregnancy, the clinician should determine signs and symptoms that can lead to expedited and cost-efficient testing.
Consequences
POI is an important risk factor for bone loss and osteoporosis, especially in young women who develop ovarian dysfunction before they achieve peak adult bone mass. At the time of diagnosis of POI, a bone density test (dual-energy x-ray absorptiometry) should be obtained. Women with POI may also develop depression and anxiety as well as experience an increased risk for cardiovascular morbidity and mortality, possibly related to endothelial dysfunction.
Young women with spontaneous POI are at increased risk of developing autoimmune adrenal insufficiency (AAI), a potentially fatal disorder. Consequently, to diagnose AAI, serum adrenal cortical and 21-hydroxylase antibodies should be measured in all women who have a karyotype of 46,XX and experience spontaneous POI. Women with AAI have a 50% risk of developing adrenal insufficiency. Despite initial normal adrenal function, women with positive adrenal cortical antibodies should be followed annually.
Causes (see table for a more complete list)
Iatrogenic
Known causes of POI include chemotherapy/radiation often in the setting of cancer treatment. The three most commonly used drugs, cyclophosphamide, cisplatin, and doxorubicin, cause POI by inducing death and/or accelerated activation of primordial follicles and increased atresia of growing follicles. The most damaging agents are alkylating drugs. A cyclophosphamide equivalent dose calculator has been established for ovarian failure risk stratification from chemotherapy based on the cumulative dose of alkylating agents received.
One study estimated the radiosensitivity of the oocyte to be less than 2 Gy. Based upon this estimate, the authors calculated the dose of radiotherapy that would result in immediate and permanent ovarian failure in 97.5% of patients as follows:
- 20.3 Gy at birth
- 18.4 Gy at age 10 years
- 16.5 Gy at age 20 years
- 14.3 Gy at age 30 years

Genetic
Approximately 10% of cases are familial. A family history of POI raises concern for a fragile X premutation. Fragile X syndrome is an X-linked form of intellectual disability that is one of the most common causes of mental retardation worldwide. There is a strong relationship between age at menopause, including POI, and premutations for fragile X syndrome. The American College of Obstetricians and Gynecologists recommends that women with POI or an elevated follicle-stimulating hormone (FSH) level before age 40 years without known cause be screened for FMR1 premutations. Approximately 6% of cases of POI are associated with premutations in the FMR1 gene.
Turner syndrome is one of the most common causes of POI and results from the lack of a second X chromosome. The most common chromosomal defect in humans, TS occurs in up to 1.5% of conceptions, 10% of spontaneous abortions, and 1 of 2,500 live births.
Serum antiadrenal and/or anti–21-hydroxylase antibodies and antithyroid antiperoxidase antibodies, can aid in the diagnosis of adrenal gland, ovary, and thyroid autoimmune causes, which is found in 4% of women with spontaneous POI. Testing for the presence of 21-hydroxylase autoantibodies or adrenal autoantibodies is sufficient to make the diagnosis of autoimmune oophoritis in women with proven spontaneous POI.
The etiology of POI remains unknown in approximately 75%-90% of cases. However, studies using whole exome or whole genome sequencing have identified genetic variants in approximately 30%-35% of these patients.
Risk factors
Factors that are thought to play a role in determining the age of menopause, include genetics (e.g., FMR1 premutation and mosaic Turner syndrome), ethnicity (earlier among Hispanic women and later in Japanese American women when compared with White women), and smoking (reduced by approximately 2 years ).
Regarding ovarian aging, the holy grail of the reproductive life span is to predict menopause. While the definitive age eludes us, anti-Müllerian hormone levels appear to show promise. An ultrasensitive anti-Müllerian hormone assay (< 0.01 ng/mL) predicted a 79% probability of menopause within 12 months for women aged 51 and above; the probability was 51% for women below age 48.
Diagnosis
The three P’s of secondary amenorrhea are physiological, pharmacological, or pathological and can guide the clinician to a targeted evaluation. Physiological causes are pregnancy, the first 6 months of continuous breastfeeding (from elevated prolactin), and natural menopause. Pharmacological etiologies, excluding hormonal treatment that suppresses ovulation (combined oral contraceptives, gonadotropin-releasing hormone agonist/antagonist, or danazol), include agents that inhibit dopamine thereby increasing serum prolactin, such as metoclopramide; phenothiazine antipsychotics, such as haloperidol; and tardive dystonia dopamine-depleting medications, such as reserpine. Pathological causes include pituitary adenomas, thyroid disease, functional hypothalamic amenorrhea from changes in weight, exercise regimen, and stress.
Management
About 50%-75% of women with 46,XX spontaneous POI experience intermittent ovarian function and 5%-10% of women remain able to conceive. Anecdotally, a 32-year-old woman presented to me with primary infertility, secondary amenorrhea, and suspected POI based on vasomotor symptoms and elevated FSH levels. Pelvic ultrasound showed a hemorrhagic cyst, suspicious for a corpus luteum. Two weeks thereafter she reported a positive home urine human chorionic gonadotropin test and ultimately delivered twins. Her diagnosis of POI with amenorrhea remained postpartum.
Unless there is an absolute contraindication, estrogen therapy should be prescribed to women with POI to reduce the risk of osteoporosis, cardiovascular disease, and urogenital atrophy as well as to maintain sexual health and quality of life. For those with an intact uterus, women should receive progesterone because of the risk of endometrial hyperplasia from unopposed estrogen. Rather than oral estrogen, the use of transdermal or vaginal delivery of estrogen is a more physiological approach and provides lower risks of venous thromboembolism and gallbladder disease. Of note, standard postmenopausal hormone therapy, which has a much lower dose of estrogen than combined estrogen-progestin contraceptives, does not provide effective contraception. Per ACOG, systemic hormone treatment should be prescribed until age 50-51 years to all women with POI.
For fertility, women with spontaneous POI can be offered oocyte or embryo donation. The uterus does not age reproductively, unlike oocytes, therefore women can achieve reasonable pregnancy success rates through egg donation despite experiencing menopause.
Future potential options
Female germline stem cells have been isolated from neonatal mice and transplanted into sterile adult mice, who then were able to produce offspring. In a second study, oogonial stem cells were isolated from neonatal and adult mouse ovaries; pups were subsequently born from the oocytes. Further experiments are needed before the implications for humans can be determined.
Emotionally traumatic for most women, POI disrupts life plans, hopes, and dreams of raising a family. The approach to the patient with POI involves the above evidence-based testing along with empathy from the health care provider.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.

