User login
Case Presentation: A 45-year-old US Coast Guard veteran with a medical history of asthma and chronic back pain was referred to the VA Boston Healthcare System (VABHS) for evaluation of progressive, unexplained dyspnea. Two years prior to presentation, the patient was an avid outdoorsman and highly active. At the time of his initial primary care physician (PCP) evaluation he reported dyspnea on exertion, and symptoms consistent with an upper respiratory tract infection (URTI) and a recent tick bite with an associated rash. He was treated with intranasal fluticasone and a course of antibiotics. His URTI symptoms and rash improved; however the dyspnea persisted and progressed over the ensuing winter and he was referred for pulmonary function testing. Additional history included a 20 pack-year history of smoking (resolved 10 years prior to the first VABHS clinical encounter) and a family history of premature coronary artery disease (CAD) in his father and 2 paternal uncles. He lived in northern New England where he previously worked as a cemetery groundskeeper.
►Kristopher Clark, MD, Chief Medical Resident, VABHS and Boston University/Boston Medical Center: Dr. Goldstein, how do you approach a patient who presents with progressive dyspnea?
►Ronald Goldstein, MD, Chief of Pulmonary and Critical Care VABHS: The evaluation of dyspnea is a common problem for pulmonary physicians. The sensation of dyspnea may originate from a wide variety of etiologies that involve pulmonary and cardiovascular disorders, neuromuscular impairment, deconditioning, or psychological issues. It is important to characterize the temporal pattern, severity, progression, relation to exertion or other triggers, the smoking history, environmental and occupational exposures to pulmonary toxins, associated symptoms, and the history of pulmonary problems.1
The physical examination may help to identify an airway or parenchymal disorder. Wheezing on chest examination would point to an obstructive defect and crackles to a possible restrictive problem, including pulmonary fibrosis. A cardiac examination should be performed to assess for evidence of heart failure, valvular heart disease, or the presence of loud P2 suggestive of pulmonary hypertension (PH). Laboratory studies, including complete blood counts are indicated.
A more complete pulmonary evaluation usually involves pulmonary function tests (PFTs), oximetry with exertion, and chest imaging. Additional cardiac testing might include electrocardiogram (ECG) and cardiac echocardiogram, followed by an exercise study, if needed. A B-natriuretic peptide determination could be considered if there is concern for congestive heart failure.2
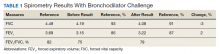
►Dr. Clark: The initial physical examination was normal and laboratory tests were unrevealing. Given his history of asthma, he underwent spirometry testing (Table 1).
Dr. Goldstein, aside from unexplained dyspnea, what are other indications for spirometry and when should we consider ordering a full PFT, including lung volumes and diffusion capacity? Can you interpret this patient’s spirometry results?
►Dr. Goldstein: Spirometry is indicated to evaluate for a suspected obstructive defect. The test is usually performed with and without a bronchodilator to assess airway reactivity. A change in > 12% and > 200 mL suggests acute bronchodilator responsiveness. Periodic spirometry determinations are useful to assess the effect of medications or progression of disease. A reduction in forced vital capacity (FVC) may suggest a restrictive component. This possibility requires measure of lung volumes.
A full set of PFTs (ie, spirometry plus assessment of lung volumes and diffusion capacity) is required to evaluate the abnormalities associated with chronic obstructive pulmonary disease (COPD), interstitial diseases, vascular abnormalities (particularly PH), as well as for certain preoperative assessments. The single breath diffusing capacity for carbon monoxide is a measure of the overall capillary alveolar surface area of the lung. It is decreased in emphysema and interstitial disease as well as pulmonary vascular disorders. It would be particularly useful in this case as the spirometry studies were normal.
In this case, the normal FVC renders a significant restrictive disorder unlikely and his normal forced expiratory volume (FEV1) and FEV1/FVC make a significant obstructive disorder unlikely. He did not show any bronchodilator response; however, this finding does not exclude the presence of underlying asthma or reactive airway disease as patients often will not show a bronchodilator response at time of testing if they are not experiencing active bronchospasm or constriction. Further provocative testing with a methacholine challenge could be used to assess for reactive airway disease.
►Dr. Clark: The patient continued to have dyspnea when he returned to his PCP. Given his family history of premature CAD, an ECG was obtained that showed normal sinus rhythm at a rate of 70 beats per minute. A cardiology consult was placed, and he was referred for cardiac stress testing.
Dr. Maron, there are many forms of cardiac stress tests. In this case, the patient is referred for a stress test due his dyspnea. Does that symptom help you decide which test to order? How often does dyspnea present as an anginal equivalent in the absence of other cardiovascular symptoms or known cardiovascular disease?
►Bradley Maron, MD, Codirector, Pulmonary Vascular Disease Center, VABHS: In this case, stress testing should include a functional (ie, exercise) assessment if possible. Exercise capacity is a critical determinant of prognosis across the spectrum of cardiovascular disease and in a young person can be particularly informative on global health status. Furthermore, the chief complaint from this patient is dyspnea on exertion, and therefore, exercise testing is likely to be needed to reproduce or provoke the main symptom in this case. Estimates for dyspnea as a presenting symptom for ischemic heart disease vary but may be as high as 25%.3 It should be noted that cardiopulmonary exercise testing is useful for evaluating patients with unexplained dyspnea, as exercise hypoxemia, blunted decrease in VD/VT (ventilatory dead space/tidal volume), and evidence of a pulmonary mechanical limit to physical activity can inform the differential diagnosis.
►Dr. Clark: The patient underwent exercise treadmill testing and was able reach the target heart rate (> 85% age-predicted maximal heart rate) and achieve 11 metabolic equivalents. He had no chest pain or diagnostic ECG changes. The report made no mention of whether he experienced dyspnea during the test and was read as negative for exercise-induced ischemia.
He was seen by a cardiologist who noted an increased intensity S2 heart sound on examination without any other cardiopulmonary findings. It was noted that his symptoms occurred when tamping the ground or starting to walk up a hill but resolved with rest. It was also noted that his symptoms did not occur with gradual increased activity such as that performed during an exercise tolerance test. A 2-view chest X-ray was obtained and read as normal. Given the data from this evaluation thus far, the patient was told that his symptoms were most likely a result of his asthma exacerbated by dirt and dust exposure. Continued use of albuterol inhaler therapy was recommended, and no further diagnostic assessment was pursued.
Approximately 11 months later, the patient presented again to his PCP and reported progressive dyspnea. He had delayed seeking further care as he started to “feel like my symptoms were possibly in my head” given his prior negative workup. His symptoms had escalated drastically to the point where he felt short of breath with minimal exertion in addition to feeling sweaty, dizzy, fatigued, and having near-syncope when standing.
He was referred for a transthoracic echocardiogram (TTE) that revealed a left ventricular ejection fraction (LVEF) of 55 to 60% with diastolic relaxation abnormality and a normal-sized left atrium. The TTE also showed (qualitatively) a moderately dilated right ventricle with reduced systolic function, moderately severe tricuspid regurgitation, and severe elevation (> 60 mm Hg) in estimated right ventricular systolic pressure.
Dr. Maron, can you comment on how these findings may explain the patient’s symptoms? What differential diagnoses would you now consider?
►Dr. Maron: These echocardiography results exclude left ventricular systolic dysfunction or primary left-sided valvular disease at rest as a cause of the patient’s symptoms. In light of the patient’s prior normal stress test, high grade coronary disease in the absence of LV systolic dysfunction on echocardiography also seems unlikely. Estimated pulmonary artery systolic pressure > 60 mm Hg by echocardiography is highly suggestive of PH, but in and of itself does not diagnose PH nor inform pulmonary artery wedge pressure or pulmonary vascular resistance. Along with a direct measurement of pulmonary artery (PA) pressure, these data are needed to establish, classify, and prognosticate PH clinically.
►Dr. Clark: The patient was referred to a pulmonologist. His examination included bibasilar crackles and an enhanced P2 heart sound. A comprehensive pulmonary history was obtained, which noted his smoking history, possible asbestos exposure while serving in the Coast Guard, nighttime snoring without witnessed apnea events, and no personal or family history of thromboembolism or connective tissue disease.
Dr. Goldstein, is there anything in this patient’s history that could explain his symptoms and echocardiograph findings? Which tests would you order next?
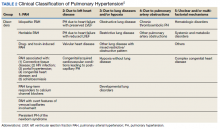
►Dr. Goldstein: PH may be secondary to a wide variety of disorders including left heart disease (Group 2), advanced COPD, interstitial fibrosis, obstructive sleep apnea (OSA), or other lung diseases (Group 3), thromboembolic disorders (Group 4), and other systemic diseases such as sarcoidosis (Group 5). Group 1 is pulmonary arterial hypertension. (Table 2).
A right heart catheterization should be done to confirm the PA pressures estimated by echocardiogram. As to a cause, clinically he does not have heart failure. The limited smoking history and spirometry data do not support advanced COPD. He was noted to have crackles on physical examination suggesting an interstitial disorder. To assess the extent of interstitial disease, we would obtain a noncontrast computed tomography (CT) of the chest. The history of snoring suggesting the possibility of OSA indicating the need for overnight oximetry as significant nocturnal hypoxemia is a possible contributing cause to PH. A polysomnogram would be required to fully evaluate a sleep disturbance. The possible asbestos exposure is not likely a contributing factor as asbestosis requires significant exposure. We would obtain a ventilation/perfusion (V/Q) scan to rule out chronic thromboembolic disease. Targeted tests for causes of Group 5 disease should also be done.
►Dr. Clark: The impression from his pulmonologist was that the patient has severe PH, though the specific etiology was not yet known. Dr. Maron, can you review for us the pathophysiology behind PH and describe how the disease is classified?
►Dr. Maron: Elevated mean pulmonary artery pressure (> 20 mm Hg) diagnosed by supine right heart catheterization is the sine qua non of PH.4 However, this alone does not inform pathophysiology. As Dr. Goldstein noted, elevated PA pressure may be due to left heart disease, primary parenchymal lung disease/sleep-disordered breathing, in situ thrombotic remodeling of pulmonary arterioles following prior luminal pulmonary embolism, or in the setting of various specific predisposing conditions, such as sickle cell disease and sarcoidosis among others.5
Alternatively, pulmonary arterial hypertension (PAH) is suspected in patients with no identifiable cause of PH, pulmonary artery wedge pressure 15 mm Hg and pulmonary vascular resistance of 3.0 Wood units.6 Importantly, PAH is not synonymous with PH but is a circumspect PH disease subgroup. In turn, PAH may be idiopathic, hereditary, or associated with other select, predisposing disorders, namely systemic sclerosis. In PAH, the interplay between genetic and molecular factors results in effacement of distal pulmonary arterioles due to plexigenic, fibrotic, and/or concentric hypertrophic remodeling. Increased vascular resistance promotes early right ventricular dilation and impaired systolic function. As a result, patients with PAH are at particularly elevated risk for cor pulmonale.
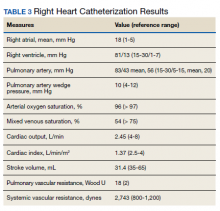
►Dr. Clark: Overnight oximetry revealed baseline oxygen saturation of 94%, an oxygen nadir of 84% with a total of 7 minutes with oxygen < 90%. On a 6-minute walk test, the patient had a max heart rate of 116 and oxygen nadir of 93%. Chest CT with and without contrast showed no evidence of pulmonary emboli but noted mild emphysematous changes. A V/Q revealed no evidence of acute or chronic pulmonary thromboembolic disease. Coronary catheterization showed normal coronary anatomy without significant CAD. A right heart catheterization showed findings consistent with severe PH with normal left-sided filling pressures (Table 3).
The patient returned a normal antinuclear antibody, C-reactive protein, HIV, and liver function panel. Based on these findings, a presumptive diagnosis of group 1 PH (idiopathic PAH) was made. Given the severity of his right heart dysfunction, he was transferred to the cardiac care unit and initiated on epoprostenol.
Dr. Maron, can you review the different treatment options for idiopathic PAH and explain why epoprostenol was chosen for this patient?
►Dr. Maron: There are 14 US Food and Drug Administration-approved drug therapies for patients with PAH, which all target either nitric oxide signaling, endothelin receptors, or the prostacyclin pathway. In the current era, treatment-naïve patients with PAH are generally initiated on calcium channel antagonist therapy if there is evidence of vasoreactivity during right heart catheterization (following nitric oxide administration), dual therapy most often with an endothelin receptor antagonist and phosphodiesterase inhibitor, or parenteral prostacyclin therapy. Since < 5% of patients will demonstrate vasoreactivity, the decision at point of care in incident patients with PAH often focuses on dual oral therapy or initiation of parenteral prostacyclin therapy. In this case, the patient reported presyncope with minimal physical activity (eg, bending over or walking up stairs) and severely decreased functional status (ie, New York Heart Association Functional [NYHA] Class III – IV), and he had a cardiac index within the range of cardiogenic shock (< 2.0 L/min/m2). Collectively, this clinical profile is considered particularly high risk, therefore, a recommendation for parenteral continuous prostacyclin therapy was made.
► Dr. Clark: The patient tolerated epoprostenol and reported improvement in his symptoms. He had a tunneled line catheter placed for continuous epoprostenol infusion. He was discharged home and scheduled for outpatient follow-up in a PH clinic. At 4 months following discharge, he was reporting steady clinical and functional improvement as well as improvement in his dyspnea. A second therapy (oral phosphodiesterase type-V inhibitor) was initiated and tolerated well. Overall, he reported resolution of presyncope, NYHA Functional Class II symptoms, and the absence of important drug effects.
1.. Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med. 1995;333(23):1547-1553. doi:10.1056/NEJM199512073332307
2. Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452. doi:10.1164/rccm.201111-2042ST
3. Phibbs B, Holmes RW, Lowe CR. Transient myocardial ischemia: the significance of dyspnea. Am J Med Sci. 1968;256(4):210-221. doi:10.1097/00000441-196810000-00002
4. Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation. 2016;133(13):1240-1248. doi:10.1161/CIRCULATIONAHA.115.020207
5. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. Published 2019 Jan 24. doi:10.1183/13993003.01913-2018
6. Maron BA, Galiè N. Diagnosis, Treatment, and Clinical Management of Pulmonary Arterial Hypertension in the Contemporary Era: A Review. JAMA Cardiol. 2016;1(9):1056-1065. doi:10.1001/jamacardio.2016.4471
Case Presentation: A 45-year-old US Coast Guard veteran with a medical history of asthma and chronic back pain was referred to the VA Boston Healthcare System (VABHS) for evaluation of progressive, unexplained dyspnea. Two years prior to presentation, the patient was an avid outdoorsman and highly active. At the time of his initial primary care physician (PCP) evaluation he reported dyspnea on exertion, and symptoms consistent with an upper respiratory tract infection (URTI) and a recent tick bite with an associated rash. He was treated with intranasal fluticasone and a course of antibiotics. His URTI symptoms and rash improved; however the dyspnea persisted and progressed over the ensuing winter and he was referred for pulmonary function testing. Additional history included a 20 pack-year history of smoking (resolved 10 years prior to the first VABHS clinical encounter) and a family history of premature coronary artery disease (CAD) in his father and 2 paternal uncles. He lived in northern New England where he previously worked as a cemetery groundskeeper.
►Kristopher Clark, MD, Chief Medical Resident, VABHS and Boston University/Boston Medical Center: Dr. Goldstein, how do you approach a patient who presents with progressive dyspnea?
►Ronald Goldstein, MD, Chief of Pulmonary and Critical Care VABHS: The evaluation of dyspnea is a common problem for pulmonary physicians. The sensation of dyspnea may originate from a wide variety of etiologies that involve pulmonary and cardiovascular disorders, neuromuscular impairment, deconditioning, or psychological issues. It is important to characterize the temporal pattern, severity, progression, relation to exertion or other triggers, the smoking history, environmental and occupational exposures to pulmonary toxins, associated symptoms, and the history of pulmonary problems.1
The physical examination may help to identify an airway or parenchymal disorder. Wheezing on chest examination would point to an obstructive defect and crackles to a possible restrictive problem, including pulmonary fibrosis. A cardiac examination should be performed to assess for evidence of heart failure, valvular heart disease, or the presence of loud P2 suggestive of pulmonary hypertension (PH). Laboratory studies, including complete blood counts are indicated.
A more complete pulmonary evaluation usually involves pulmonary function tests (PFTs), oximetry with exertion, and chest imaging. Additional cardiac testing might include electrocardiogram (ECG) and cardiac echocardiogram, followed by an exercise study, if needed. A B-natriuretic peptide determination could be considered if there is concern for congestive heart failure.2
►Dr. Clark: The initial physical examination was normal and laboratory tests were unrevealing. Given his history of asthma, he underwent spirometry testing (Table 1).
Dr. Goldstein, aside from unexplained dyspnea, what are other indications for spirometry and when should we consider ordering a full PFT, including lung volumes and diffusion capacity? Can you interpret this patient’s spirometry results?
►Dr. Goldstein: Spirometry is indicated to evaluate for a suspected obstructive defect. The test is usually performed with and without a bronchodilator to assess airway reactivity. A change in > 12% and > 200 mL suggests acute bronchodilator responsiveness. Periodic spirometry determinations are useful to assess the effect of medications or progression of disease. A reduction in forced vital capacity (FVC) may suggest a restrictive component. This possibility requires measure of lung volumes.
A full set of PFTs (ie, spirometry plus assessment of lung volumes and diffusion capacity) is required to evaluate the abnormalities associated with chronic obstructive pulmonary disease (COPD), interstitial diseases, vascular abnormalities (particularly PH), as well as for certain preoperative assessments. The single breath diffusing capacity for carbon monoxide is a measure of the overall capillary alveolar surface area of the lung. It is decreased in emphysema and interstitial disease as well as pulmonary vascular disorders. It would be particularly useful in this case as the spirometry studies were normal.
In this case, the normal FVC renders a significant restrictive disorder unlikely and his normal forced expiratory volume (FEV1) and FEV1/FVC make a significant obstructive disorder unlikely. He did not show any bronchodilator response; however, this finding does not exclude the presence of underlying asthma or reactive airway disease as patients often will not show a bronchodilator response at time of testing if they are not experiencing active bronchospasm or constriction. Further provocative testing with a methacholine challenge could be used to assess for reactive airway disease.
►Dr. Clark: The patient continued to have dyspnea when he returned to his PCP. Given his family history of premature CAD, an ECG was obtained that showed normal sinus rhythm at a rate of 70 beats per minute. A cardiology consult was placed, and he was referred for cardiac stress testing.
Dr. Maron, there are many forms of cardiac stress tests. In this case, the patient is referred for a stress test due his dyspnea. Does that symptom help you decide which test to order? How often does dyspnea present as an anginal equivalent in the absence of other cardiovascular symptoms or known cardiovascular disease?
►Bradley Maron, MD, Codirector, Pulmonary Vascular Disease Center, VABHS: In this case, stress testing should include a functional (ie, exercise) assessment if possible. Exercise capacity is a critical determinant of prognosis across the spectrum of cardiovascular disease and in a young person can be particularly informative on global health status. Furthermore, the chief complaint from this patient is dyspnea on exertion, and therefore, exercise testing is likely to be needed to reproduce or provoke the main symptom in this case. Estimates for dyspnea as a presenting symptom for ischemic heart disease vary but may be as high as 25%.3 It should be noted that cardiopulmonary exercise testing is useful for evaluating patients with unexplained dyspnea, as exercise hypoxemia, blunted decrease in VD/VT (ventilatory dead space/tidal volume), and evidence of a pulmonary mechanical limit to physical activity can inform the differential diagnosis.
►Dr. Clark: The patient underwent exercise treadmill testing and was able reach the target heart rate (> 85% age-predicted maximal heart rate) and achieve 11 metabolic equivalents. He had no chest pain or diagnostic ECG changes. The report made no mention of whether he experienced dyspnea during the test and was read as negative for exercise-induced ischemia.
He was seen by a cardiologist who noted an increased intensity S2 heart sound on examination without any other cardiopulmonary findings. It was noted that his symptoms occurred when tamping the ground or starting to walk up a hill but resolved with rest. It was also noted that his symptoms did not occur with gradual increased activity such as that performed during an exercise tolerance test. A 2-view chest X-ray was obtained and read as normal. Given the data from this evaluation thus far, the patient was told that his symptoms were most likely a result of his asthma exacerbated by dirt and dust exposure. Continued use of albuterol inhaler therapy was recommended, and no further diagnostic assessment was pursued.
Approximately 11 months later, the patient presented again to his PCP and reported progressive dyspnea. He had delayed seeking further care as he started to “feel like my symptoms were possibly in my head” given his prior negative workup. His symptoms had escalated drastically to the point where he felt short of breath with minimal exertion in addition to feeling sweaty, dizzy, fatigued, and having near-syncope when standing.
He was referred for a transthoracic echocardiogram (TTE) that revealed a left ventricular ejection fraction (LVEF) of 55 to 60% with diastolic relaxation abnormality and a normal-sized left atrium. The TTE also showed (qualitatively) a moderately dilated right ventricle with reduced systolic function, moderately severe tricuspid regurgitation, and severe elevation (> 60 mm Hg) in estimated right ventricular systolic pressure.
Dr. Maron, can you comment on how these findings may explain the patient’s symptoms? What differential diagnoses would you now consider?
►Dr. Maron: These echocardiography results exclude left ventricular systolic dysfunction or primary left-sided valvular disease at rest as a cause of the patient’s symptoms. In light of the patient’s prior normal stress test, high grade coronary disease in the absence of LV systolic dysfunction on echocardiography also seems unlikely. Estimated pulmonary artery systolic pressure > 60 mm Hg by echocardiography is highly suggestive of PH, but in and of itself does not diagnose PH nor inform pulmonary artery wedge pressure or pulmonary vascular resistance. Along with a direct measurement of pulmonary artery (PA) pressure, these data are needed to establish, classify, and prognosticate PH clinically.
►Dr. Clark: The patient was referred to a pulmonologist. His examination included bibasilar crackles and an enhanced P2 heart sound. A comprehensive pulmonary history was obtained, which noted his smoking history, possible asbestos exposure while serving in the Coast Guard, nighttime snoring without witnessed apnea events, and no personal or family history of thromboembolism or connective tissue disease.
Dr. Goldstein, is there anything in this patient’s history that could explain his symptoms and echocardiograph findings? Which tests would you order next?
►Dr. Goldstein: PH may be secondary to a wide variety of disorders including left heart disease (Group 2), advanced COPD, interstitial fibrosis, obstructive sleep apnea (OSA), or other lung diseases (Group 3), thromboembolic disorders (Group 4), and other systemic diseases such as sarcoidosis (Group 5). Group 1 is pulmonary arterial hypertension. (Table 2).
A right heart catheterization should be done to confirm the PA pressures estimated by echocardiogram. As to a cause, clinically he does not have heart failure. The limited smoking history and spirometry data do not support advanced COPD. He was noted to have crackles on physical examination suggesting an interstitial disorder. To assess the extent of interstitial disease, we would obtain a noncontrast computed tomography (CT) of the chest. The history of snoring suggesting the possibility of OSA indicating the need for overnight oximetry as significant nocturnal hypoxemia is a possible contributing cause to PH. A polysomnogram would be required to fully evaluate a sleep disturbance. The possible asbestos exposure is not likely a contributing factor as asbestosis requires significant exposure. We would obtain a ventilation/perfusion (V/Q) scan to rule out chronic thromboembolic disease. Targeted tests for causes of Group 5 disease should also be done.
►Dr. Clark: The impression from his pulmonologist was that the patient has severe PH, though the specific etiology was not yet known. Dr. Maron, can you review for us the pathophysiology behind PH and describe how the disease is classified?
►Dr. Maron: Elevated mean pulmonary artery pressure (> 20 mm Hg) diagnosed by supine right heart catheterization is the sine qua non of PH.4 However, this alone does not inform pathophysiology. As Dr. Goldstein noted, elevated PA pressure may be due to left heart disease, primary parenchymal lung disease/sleep-disordered breathing, in situ thrombotic remodeling of pulmonary arterioles following prior luminal pulmonary embolism, or in the setting of various specific predisposing conditions, such as sickle cell disease and sarcoidosis among others.5
Alternatively, pulmonary arterial hypertension (PAH) is suspected in patients with no identifiable cause of PH, pulmonary artery wedge pressure 15 mm Hg and pulmonary vascular resistance of 3.0 Wood units.6 Importantly, PAH is not synonymous with PH but is a circumspect PH disease subgroup. In turn, PAH may be idiopathic, hereditary, or associated with other select, predisposing disorders, namely systemic sclerosis. In PAH, the interplay between genetic and molecular factors results in effacement of distal pulmonary arterioles due to plexigenic, fibrotic, and/or concentric hypertrophic remodeling. Increased vascular resistance promotes early right ventricular dilation and impaired systolic function. As a result, patients with PAH are at particularly elevated risk for cor pulmonale.
►Dr. Clark: Overnight oximetry revealed baseline oxygen saturation of 94%, an oxygen nadir of 84% with a total of 7 minutes with oxygen < 90%. On a 6-minute walk test, the patient had a max heart rate of 116 and oxygen nadir of 93%. Chest CT with and without contrast showed no evidence of pulmonary emboli but noted mild emphysematous changes. A V/Q revealed no evidence of acute or chronic pulmonary thromboembolic disease. Coronary catheterization showed normal coronary anatomy without significant CAD. A right heart catheterization showed findings consistent with severe PH with normal left-sided filling pressures (Table 3).
The patient returned a normal antinuclear antibody, C-reactive protein, HIV, and liver function panel. Based on these findings, a presumptive diagnosis of group 1 PH (idiopathic PAH) was made. Given the severity of his right heart dysfunction, he was transferred to the cardiac care unit and initiated on epoprostenol.
Dr. Maron, can you review the different treatment options for idiopathic PAH and explain why epoprostenol was chosen for this patient?
►Dr. Maron: There are 14 US Food and Drug Administration-approved drug therapies for patients with PAH, which all target either nitric oxide signaling, endothelin receptors, or the prostacyclin pathway. In the current era, treatment-naïve patients with PAH are generally initiated on calcium channel antagonist therapy if there is evidence of vasoreactivity during right heart catheterization (following nitric oxide administration), dual therapy most often with an endothelin receptor antagonist and phosphodiesterase inhibitor, or parenteral prostacyclin therapy. Since < 5% of patients will demonstrate vasoreactivity, the decision at point of care in incident patients with PAH often focuses on dual oral therapy or initiation of parenteral prostacyclin therapy. In this case, the patient reported presyncope with minimal physical activity (eg, bending over or walking up stairs) and severely decreased functional status (ie, New York Heart Association Functional [NYHA] Class III – IV), and he had a cardiac index within the range of cardiogenic shock (< 2.0 L/min/m2). Collectively, this clinical profile is considered particularly high risk, therefore, a recommendation for parenteral continuous prostacyclin therapy was made.
► Dr. Clark: The patient tolerated epoprostenol and reported improvement in his symptoms. He had a tunneled line catheter placed for continuous epoprostenol infusion. He was discharged home and scheduled for outpatient follow-up in a PH clinic. At 4 months following discharge, he was reporting steady clinical and functional improvement as well as improvement in his dyspnea. A second therapy (oral phosphodiesterase type-V inhibitor) was initiated and tolerated well. Overall, he reported resolution of presyncope, NYHA Functional Class II symptoms, and the absence of important drug effects.
Case Presentation: A 45-year-old US Coast Guard veteran with a medical history of asthma and chronic back pain was referred to the VA Boston Healthcare System (VABHS) for evaluation of progressive, unexplained dyspnea. Two years prior to presentation, the patient was an avid outdoorsman and highly active. At the time of his initial primary care physician (PCP) evaluation he reported dyspnea on exertion, and symptoms consistent with an upper respiratory tract infection (URTI) and a recent tick bite with an associated rash. He was treated with intranasal fluticasone and a course of antibiotics. His URTI symptoms and rash improved; however the dyspnea persisted and progressed over the ensuing winter and he was referred for pulmonary function testing. Additional history included a 20 pack-year history of smoking (resolved 10 years prior to the first VABHS clinical encounter) and a family history of premature coronary artery disease (CAD) in his father and 2 paternal uncles. He lived in northern New England where he previously worked as a cemetery groundskeeper.
►Kristopher Clark, MD, Chief Medical Resident, VABHS and Boston University/Boston Medical Center: Dr. Goldstein, how do you approach a patient who presents with progressive dyspnea?
►Ronald Goldstein, MD, Chief of Pulmonary and Critical Care VABHS: The evaluation of dyspnea is a common problem for pulmonary physicians. The sensation of dyspnea may originate from a wide variety of etiologies that involve pulmonary and cardiovascular disorders, neuromuscular impairment, deconditioning, or psychological issues. It is important to characterize the temporal pattern, severity, progression, relation to exertion or other triggers, the smoking history, environmental and occupational exposures to pulmonary toxins, associated symptoms, and the history of pulmonary problems.1
The physical examination may help to identify an airway or parenchymal disorder. Wheezing on chest examination would point to an obstructive defect and crackles to a possible restrictive problem, including pulmonary fibrosis. A cardiac examination should be performed to assess for evidence of heart failure, valvular heart disease, or the presence of loud P2 suggestive of pulmonary hypertension (PH). Laboratory studies, including complete blood counts are indicated.
A more complete pulmonary evaluation usually involves pulmonary function tests (PFTs), oximetry with exertion, and chest imaging. Additional cardiac testing might include electrocardiogram (ECG) and cardiac echocardiogram, followed by an exercise study, if needed. A B-natriuretic peptide determination could be considered if there is concern for congestive heart failure.2
►Dr. Clark: The initial physical examination was normal and laboratory tests were unrevealing. Given his history of asthma, he underwent spirometry testing (Table 1).
Dr. Goldstein, aside from unexplained dyspnea, what are other indications for spirometry and when should we consider ordering a full PFT, including lung volumes and diffusion capacity? Can you interpret this patient’s spirometry results?
►Dr. Goldstein: Spirometry is indicated to evaluate for a suspected obstructive defect. The test is usually performed with and without a bronchodilator to assess airway reactivity. A change in > 12% and > 200 mL suggests acute bronchodilator responsiveness. Periodic spirometry determinations are useful to assess the effect of medications or progression of disease. A reduction in forced vital capacity (FVC) may suggest a restrictive component. This possibility requires measure of lung volumes.
A full set of PFTs (ie, spirometry plus assessment of lung volumes and diffusion capacity) is required to evaluate the abnormalities associated with chronic obstructive pulmonary disease (COPD), interstitial diseases, vascular abnormalities (particularly PH), as well as for certain preoperative assessments. The single breath diffusing capacity for carbon monoxide is a measure of the overall capillary alveolar surface area of the lung. It is decreased in emphysema and interstitial disease as well as pulmonary vascular disorders. It would be particularly useful in this case as the spirometry studies were normal.
In this case, the normal FVC renders a significant restrictive disorder unlikely and his normal forced expiratory volume (FEV1) and FEV1/FVC make a significant obstructive disorder unlikely. He did not show any bronchodilator response; however, this finding does not exclude the presence of underlying asthma or reactive airway disease as patients often will not show a bronchodilator response at time of testing if they are not experiencing active bronchospasm or constriction. Further provocative testing with a methacholine challenge could be used to assess for reactive airway disease.
►Dr. Clark: The patient continued to have dyspnea when he returned to his PCP. Given his family history of premature CAD, an ECG was obtained that showed normal sinus rhythm at a rate of 70 beats per minute. A cardiology consult was placed, and he was referred for cardiac stress testing.
Dr. Maron, there are many forms of cardiac stress tests. In this case, the patient is referred for a stress test due his dyspnea. Does that symptom help you decide which test to order? How often does dyspnea present as an anginal equivalent in the absence of other cardiovascular symptoms or known cardiovascular disease?
►Bradley Maron, MD, Codirector, Pulmonary Vascular Disease Center, VABHS: In this case, stress testing should include a functional (ie, exercise) assessment if possible. Exercise capacity is a critical determinant of prognosis across the spectrum of cardiovascular disease and in a young person can be particularly informative on global health status. Furthermore, the chief complaint from this patient is dyspnea on exertion, and therefore, exercise testing is likely to be needed to reproduce or provoke the main symptom in this case. Estimates for dyspnea as a presenting symptom for ischemic heart disease vary but may be as high as 25%.3 It should be noted that cardiopulmonary exercise testing is useful for evaluating patients with unexplained dyspnea, as exercise hypoxemia, blunted decrease in VD/VT (ventilatory dead space/tidal volume), and evidence of a pulmonary mechanical limit to physical activity can inform the differential diagnosis.
►Dr. Clark: The patient underwent exercise treadmill testing and was able reach the target heart rate (> 85% age-predicted maximal heart rate) and achieve 11 metabolic equivalents. He had no chest pain or diagnostic ECG changes. The report made no mention of whether he experienced dyspnea during the test and was read as negative for exercise-induced ischemia.
He was seen by a cardiologist who noted an increased intensity S2 heart sound on examination without any other cardiopulmonary findings. It was noted that his symptoms occurred when tamping the ground or starting to walk up a hill but resolved with rest. It was also noted that his symptoms did not occur with gradual increased activity such as that performed during an exercise tolerance test. A 2-view chest X-ray was obtained and read as normal. Given the data from this evaluation thus far, the patient was told that his symptoms were most likely a result of his asthma exacerbated by dirt and dust exposure. Continued use of albuterol inhaler therapy was recommended, and no further diagnostic assessment was pursued.
Approximately 11 months later, the patient presented again to his PCP and reported progressive dyspnea. He had delayed seeking further care as he started to “feel like my symptoms were possibly in my head” given his prior negative workup. His symptoms had escalated drastically to the point where he felt short of breath with minimal exertion in addition to feeling sweaty, dizzy, fatigued, and having near-syncope when standing.
He was referred for a transthoracic echocardiogram (TTE) that revealed a left ventricular ejection fraction (LVEF) of 55 to 60% with diastolic relaxation abnormality and a normal-sized left atrium. The TTE also showed (qualitatively) a moderately dilated right ventricle with reduced systolic function, moderately severe tricuspid regurgitation, and severe elevation (> 60 mm Hg) in estimated right ventricular systolic pressure.
Dr. Maron, can you comment on how these findings may explain the patient’s symptoms? What differential diagnoses would you now consider?
►Dr. Maron: These echocardiography results exclude left ventricular systolic dysfunction or primary left-sided valvular disease at rest as a cause of the patient’s symptoms. In light of the patient’s prior normal stress test, high grade coronary disease in the absence of LV systolic dysfunction on echocardiography also seems unlikely. Estimated pulmonary artery systolic pressure > 60 mm Hg by echocardiography is highly suggestive of PH, but in and of itself does not diagnose PH nor inform pulmonary artery wedge pressure or pulmonary vascular resistance. Along with a direct measurement of pulmonary artery (PA) pressure, these data are needed to establish, classify, and prognosticate PH clinically.
►Dr. Clark: The patient was referred to a pulmonologist. His examination included bibasilar crackles and an enhanced P2 heart sound. A comprehensive pulmonary history was obtained, which noted his smoking history, possible asbestos exposure while serving in the Coast Guard, nighttime snoring without witnessed apnea events, and no personal or family history of thromboembolism or connective tissue disease.
Dr. Goldstein, is there anything in this patient’s history that could explain his symptoms and echocardiograph findings? Which tests would you order next?
►Dr. Goldstein: PH may be secondary to a wide variety of disorders including left heart disease (Group 2), advanced COPD, interstitial fibrosis, obstructive sleep apnea (OSA), or other lung diseases (Group 3), thromboembolic disorders (Group 4), and other systemic diseases such as sarcoidosis (Group 5). Group 1 is pulmonary arterial hypertension. (Table 2).
A right heart catheterization should be done to confirm the PA pressures estimated by echocardiogram. As to a cause, clinically he does not have heart failure. The limited smoking history and spirometry data do not support advanced COPD. He was noted to have crackles on physical examination suggesting an interstitial disorder. To assess the extent of interstitial disease, we would obtain a noncontrast computed tomography (CT) of the chest. The history of snoring suggesting the possibility of OSA indicating the need for overnight oximetry as significant nocturnal hypoxemia is a possible contributing cause to PH. A polysomnogram would be required to fully evaluate a sleep disturbance. The possible asbestos exposure is not likely a contributing factor as asbestosis requires significant exposure. We would obtain a ventilation/perfusion (V/Q) scan to rule out chronic thromboembolic disease. Targeted tests for causes of Group 5 disease should also be done.
►Dr. Clark: The impression from his pulmonologist was that the patient has severe PH, though the specific etiology was not yet known. Dr. Maron, can you review for us the pathophysiology behind PH and describe how the disease is classified?
►Dr. Maron: Elevated mean pulmonary artery pressure (> 20 mm Hg) diagnosed by supine right heart catheterization is the sine qua non of PH.4 However, this alone does not inform pathophysiology. As Dr. Goldstein noted, elevated PA pressure may be due to left heart disease, primary parenchymal lung disease/sleep-disordered breathing, in situ thrombotic remodeling of pulmonary arterioles following prior luminal pulmonary embolism, or in the setting of various specific predisposing conditions, such as sickle cell disease and sarcoidosis among others.5
Alternatively, pulmonary arterial hypertension (PAH) is suspected in patients with no identifiable cause of PH, pulmonary artery wedge pressure 15 mm Hg and pulmonary vascular resistance of 3.0 Wood units.6 Importantly, PAH is not synonymous with PH but is a circumspect PH disease subgroup. In turn, PAH may be idiopathic, hereditary, or associated with other select, predisposing disorders, namely systemic sclerosis. In PAH, the interplay between genetic and molecular factors results in effacement of distal pulmonary arterioles due to plexigenic, fibrotic, and/or concentric hypertrophic remodeling. Increased vascular resistance promotes early right ventricular dilation and impaired systolic function. As a result, patients with PAH are at particularly elevated risk for cor pulmonale.
►Dr. Clark: Overnight oximetry revealed baseline oxygen saturation of 94%, an oxygen nadir of 84% with a total of 7 minutes with oxygen < 90%. On a 6-minute walk test, the patient had a max heart rate of 116 and oxygen nadir of 93%. Chest CT with and without contrast showed no evidence of pulmonary emboli but noted mild emphysematous changes. A V/Q revealed no evidence of acute or chronic pulmonary thromboembolic disease. Coronary catheterization showed normal coronary anatomy without significant CAD. A right heart catheterization showed findings consistent with severe PH with normal left-sided filling pressures (Table 3).
The patient returned a normal antinuclear antibody, C-reactive protein, HIV, and liver function panel. Based on these findings, a presumptive diagnosis of group 1 PH (idiopathic PAH) was made. Given the severity of his right heart dysfunction, he was transferred to the cardiac care unit and initiated on epoprostenol.
Dr. Maron, can you review the different treatment options for idiopathic PAH and explain why epoprostenol was chosen for this patient?
►Dr. Maron: There are 14 US Food and Drug Administration-approved drug therapies for patients with PAH, which all target either nitric oxide signaling, endothelin receptors, or the prostacyclin pathway. In the current era, treatment-naïve patients with PAH are generally initiated on calcium channel antagonist therapy if there is evidence of vasoreactivity during right heart catheterization (following nitric oxide administration), dual therapy most often with an endothelin receptor antagonist and phosphodiesterase inhibitor, or parenteral prostacyclin therapy. Since < 5% of patients will demonstrate vasoreactivity, the decision at point of care in incident patients with PAH often focuses on dual oral therapy or initiation of parenteral prostacyclin therapy. In this case, the patient reported presyncope with minimal physical activity (eg, bending over or walking up stairs) and severely decreased functional status (ie, New York Heart Association Functional [NYHA] Class III – IV), and he had a cardiac index within the range of cardiogenic shock (< 2.0 L/min/m2). Collectively, this clinical profile is considered particularly high risk, therefore, a recommendation for parenteral continuous prostacyclin therapy was made.
► Dr. Clark: The patient tolerated epoprostenol and reported improvement in his symptoms. He had a tunneled line catheter placed for continuous epoprostenol infusion. He was discharged home and scheduled for outpatient follow-up in a PH clinic. At 4 months following discharge, he was reporting steady clinical and functional improvement as well as improvement in his dyspnea. A second therapy (oral phosphodiesterase type-V inhibitor) was initiated and tolerated well. Overall, he reported resolution of presyncope, NYHA Functional Class II symptoms, and the absence of important drug effects.
1.. Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med. 1995;333(23):1547-1553. doi:10.1056/NEJM199512073332307
2. Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452. doi:10.1164/rccm.201111-2042ST
3. Phibbs B, Holmes RW, Lowe CR. Transient myocardial ischemia: the significance of dyspnea. Am J Med Sci. 1968;256(4):210-221. doi:10.1097/00000441-196810000-00002
4. Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation. 2016;133(13):1240-1248. doi:10.1161/CIRCULATIONAHA.115.020207
5. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. Published 2019 Jan 24. doi:10.1183/13993003.01913-2018
6. Maron BA, Galiè N. Diagnosis, Treatment, and Clinical Management of Pulmonary Arterial Hypertension in the Contemporary Era: A Review. JAMA Cardiol. 2016;1(9):1056-1065. doi:10.1001/jamacardio.2016.4471
1.. Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med. 1995;333(23):1547-1553. doi:10.1056/NEJM199512073332307
2. Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452. doi:10.1164/rccm.201111-2042ST
3. Phibbs B, Holmes RW, Lowe CR. Transient myocardial ischemia: the significance of dyspnea. Am J Med Sci. 1968;256(4):210-221. doi:10.1097/00000441-196810000-00002
4. Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation. 2016;133(13):1240-1248. doi:10.1161/CIRCULATIONAHA.115.020207
5. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. Published 2019 Jan 24. doi:10.1183/13993003.01913-2018
6. Maron BA, Galiè N. Diagnosis, Treatment, and Clinical Management of Pulmonary Arterial Hypertension in the Contemporary Era: A Review. JAMA Cardiol. 2016;1(9):1056-1065. doi:10.1001/jamacardio.2016.4471