User login
Rattlesnakes are pit vipers with a rattle attached to the tip of the tail and facial pits located between the eyes and nose with a special organ that detects heat energy (infrared light) and is used for hunting prey. There are 2 genera of rattlesnakes, Sistrurus (3 species) and Crotalus (23 species). 1 The pigmy rattlesnake belongs to the Sistrurus miliarius species that is subdivided into 3 subspecies: the Carolina pigmy rattlesnake (Sistrurus miliarius miliarius), the Western pigmy rattlesnake (Sistrurus miliarius streckeri ), and the dusky pigmy rattlesnake (Sistrurus miliarius barbouri ). 1 The dusky pigmy rattlesnake is found in South Carolina, southern Georgia, southern Alabama, southeastern Mississippi, and Florida. 2 It is the most abundant venomous snake in Florida. 3 Its rattle is barely audible, and it is an aggressive small snake ranging in length from 38 to 56 cm. 4 Its venom is hemorrhagic, causing tissue damage but not containing neurotoxins. 4 Although bites can be painful, resulting in localized necrosis and rare loss of digits, it is unlikely for bites to be fatal given the snake’s small fangs, small size, and amount of envenomation. However, bites on children may require hospitalization. The venom contains proteins, polypeptides, and enzymes. 5 One such peptide, barbourin, inhibits a transmembrane receptor that plays a role in platelet aggregation. 6
We report a case of a 54-year-old man who was bitten on the left index finger by a dusky pigmy rattlesnake. We describe the clinical course and successful treatment with crotalidae polyvalent immune fab (CPIF) antivenom.
Case Report
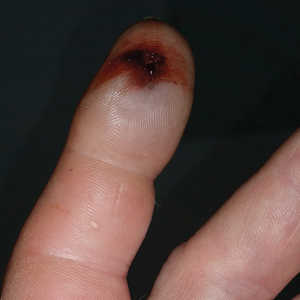
A 54-year-old man presented to the emergency department with a rapidly swelling and erythematous left hand following a snakebite to the left index fingertip while weeding in his yard (Figure 1). The patient was able to kill the snake with a shovel and photograph it, which helped identify it as a dusky pigmy rattlesnake (Figure 2). Vitals on presentation included a blood pressure of 161/98, pulse oximeter of 99%, temperature of 36.4°C, pulse of 84 beats per minute, and respiratory rate of 16 breaths per minute.

Given the poisonous snakebite, the patient was admitted to the intensive care unit. Laboratory test results at admission revealed the following values: platelet count, 235,000/µL (reference range, 150,000–450,000/µL); fibrinogen, 226.1 mg/dL (reference range, 185–410 mg/dL); fibrin degradation products, less than 10 µg/mL (reference range, <10 µg/mL); glucose, 145 mg/dL (reference range, 74–106 mg/dL). The remainder of the complete blood cell count and metabolic panel was unremarkable. His blood type was O Rh+. Radiography of the left second digit did not show any fractures, dislocations, or foreign object.
After consulting with the Tampa General Hospital Florida Poison Information Center, 6 vials of CPIF antivenom in 250 mL of sodium chloride initially were infused intravenously, followed by 2 additional vials each at 6, 12, and 18 hours. Serial laboratory test results revealed white blood cell counts of 13,600, 10,000, 6800, 6100, and 6800/µL at 4, 15, 43, 65, and 88 hours postadmission, respectively. Platelet counts were 222,000, 159,000, 116,000, 99,000, and 129,000/µL at 4, 15, 43, 65, and 88 hours postadmission, respectively. The hemoglobin level was 14.8, 13.1, 13.8, 13.7, and 14.3 g/dL at 4, 15, 43, 65, and 88 hours postadmission, respectively. Other laboratory test results including prothrombin time (10.0 s), fibrinogen (226.1 mg/dL), and fibrin degradation products (<10 µg/mL) at 4 hours postadmission remained within reference range during serial monitoring.
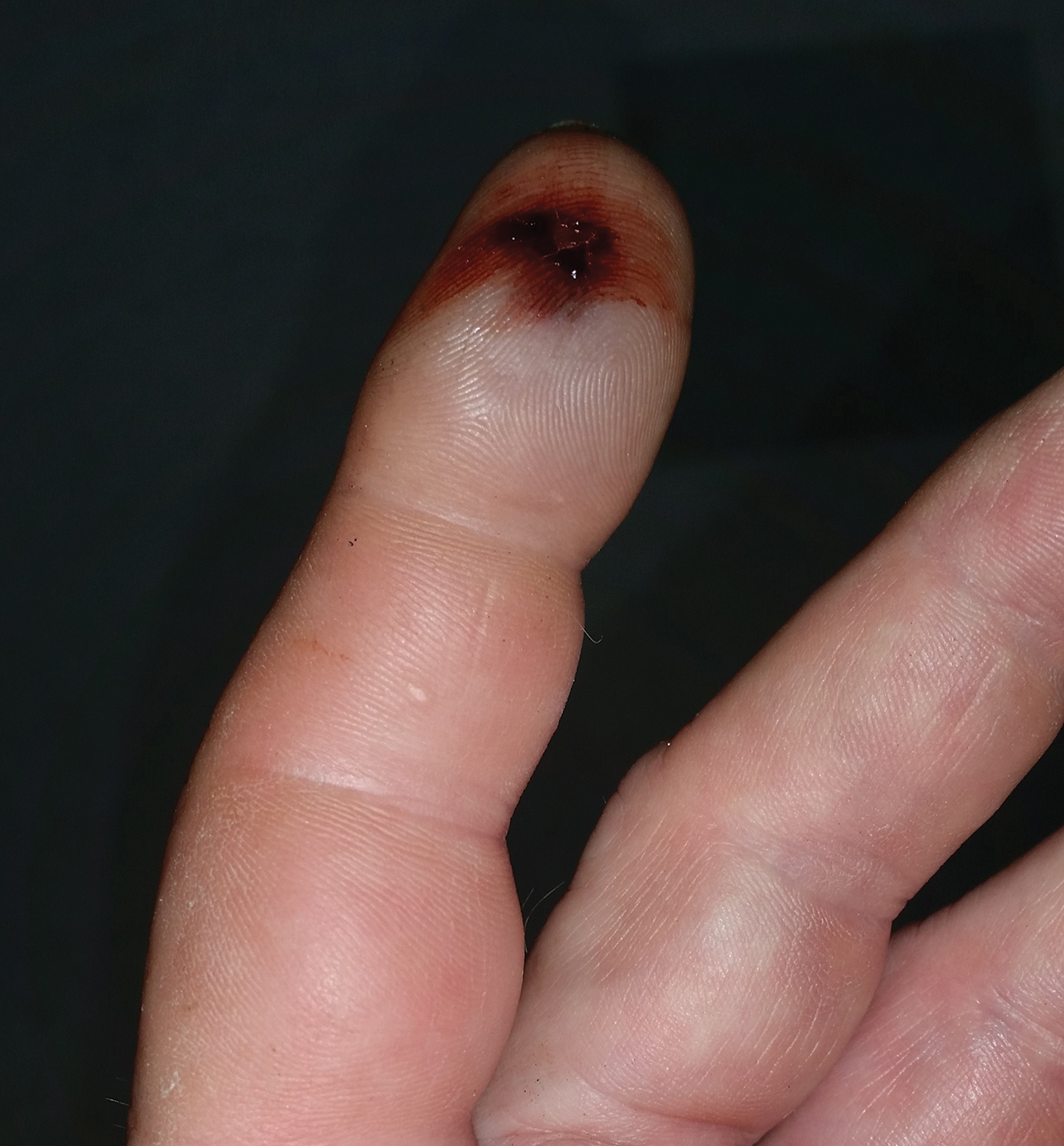
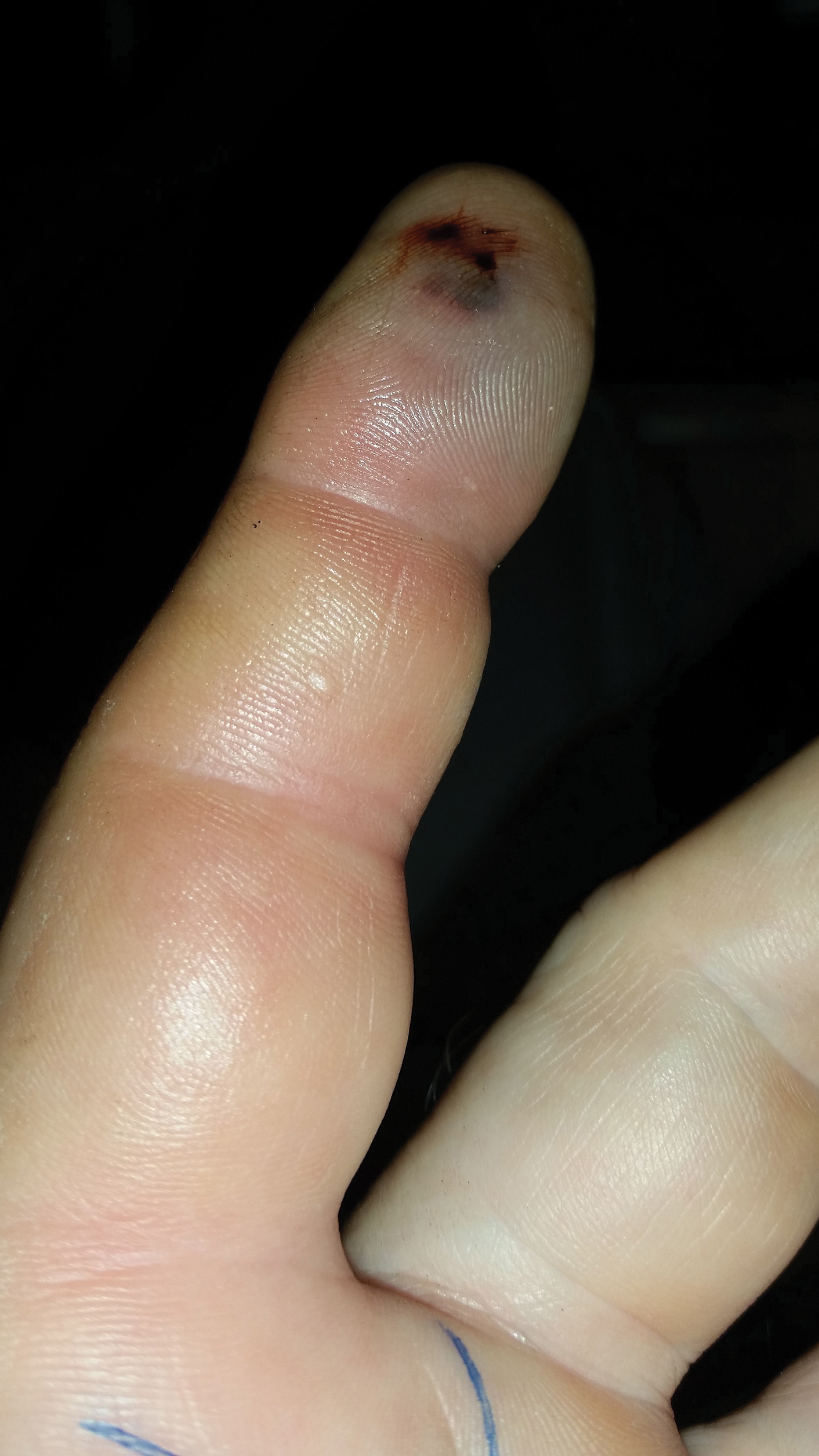
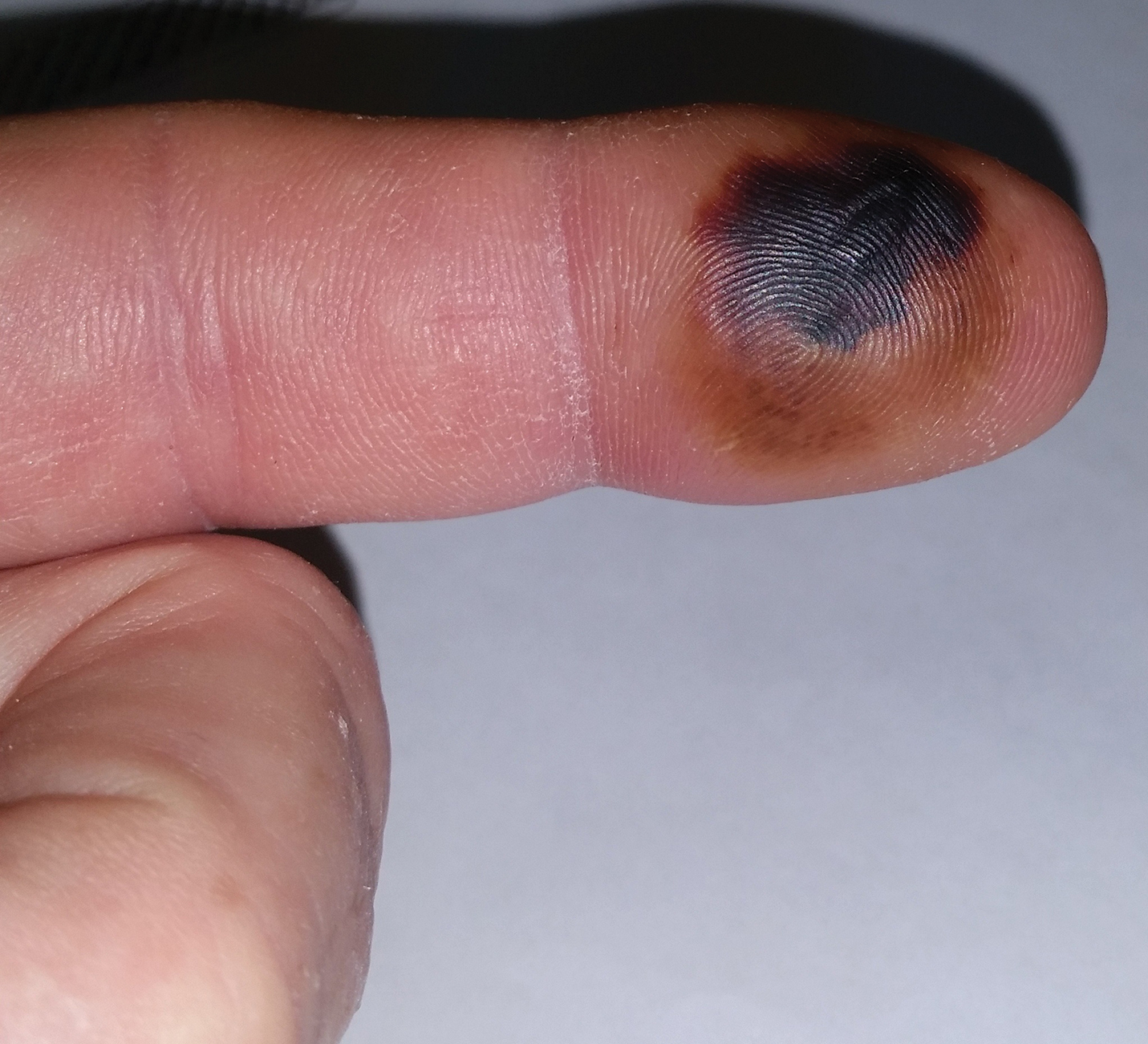
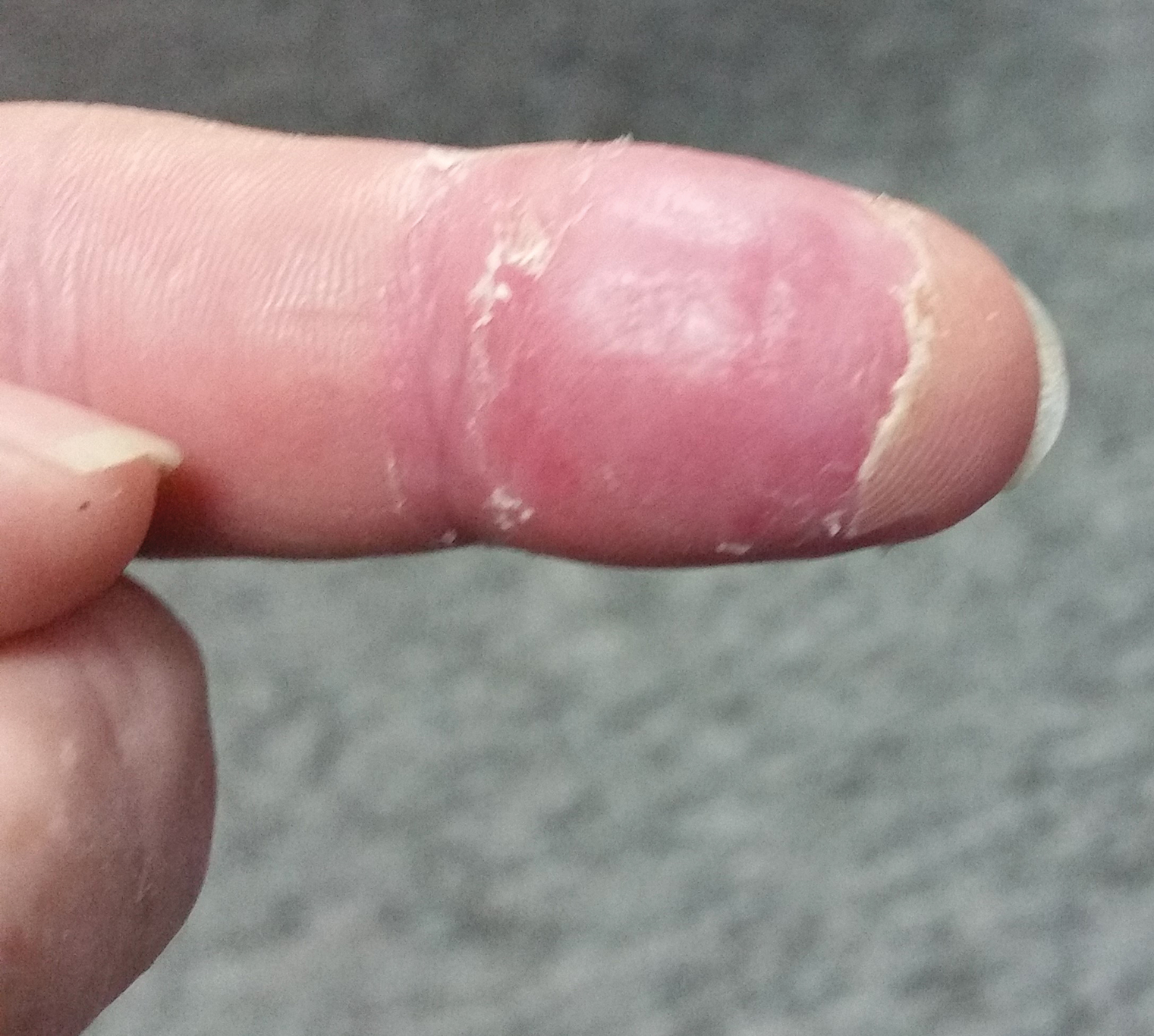
The patient was hospitalized for 4 days until the erythema of the left arm receded and only involved the left second phalanx where he eventually experienced localized skin necrosis (Figures 3 and 4). His thrombocytopenia trended upward from a low of 99,000/µL to 121,000/µL at the time of discharge. During his hospital stay, the patient developed hypertension from which he remained asymptomatic and was treated with lisinopril. The patient was treated with intravenous cefazolin and discharged on oral cephalexin due to an elevation in his white blood cell count on admission (13,600/µL). His cultures remained negative, and on discharge his white blood cell count had normalized (6800/µL) and he was transitioned to oral antibiotics to complete his treatment course. Following a surgical consultation, it was decided that skin debridement of the localized area of necrosis of the index fingertip was not necessary. The area of skin necrosis sloughed uneventfully with no residual functional impairment; however, the patient was left with residual numbness of the left second digit (Figure 5). He did not experience recurrent coagulopathy.
Comment
Envenomation
Snakebites and envenomation are a complex and broad subject beyond the scope of this article and further reading on this subject is highly encouraged. The clinical findings from snakebites range from mild local tissue reactions to severe systemic symptoms depending on the volume of venom injected, snake species, age and health of victim, and location of bite. Severe systemic symptoms include disseminated intravascular coagulation, acute renal failure, hypovolemic shock, and death.5 Venom can be hemotoxic and/or neurotoxic. Hemotoxic symptoms include pain, edema, swelling, ecchymoses, necrosis, and hemolysis. Neurotoxic symptoms may encompass diplopia, dysphagia, sweating, salivation, diaphoresis, respiratory depression, and paralysis.5 The pigmy rattlesnake venom is only hemotoxic, not neurotoxic.4 Eastern and western variety rattlesnakes account for most snake deaths due to their potent venom. Water moccasins (cottonmouths) have intermediate-potency venom, and copperhead snakes have the least-potent venom.5 Coral snakes are not pit vipers and require a different antivenom.
Management of Snakebites
Venomous snakes may bite a person without injecting venom. In fact, as many as 20% to 25% of all pit viper bites are dry.7 No attempts should be made to capture or kill the biting snake, but identifying it safely is helpful. Dead snakes should not be handled carelessly, as reflex biting after death has been reported.8 Cutting or suctioning the bite wound, nonsteroidal anti-inflammatory drugs, prophylactic antibiotics, tourniquets, prophylactic fasciotomy, and ice have not proven to be beneficial in the management of viper envenomation.5,9-11 The best management involves immobilizing the affected extremity, seeking immediate medical attention, and initiating antivenom therapy as soon as possible.
Antivenom Therapy
Crotalidae polyvalent immune fab is an antivenom comprised of purified, sheep-derived fab IgG fragments and was approved by the US Food and Drug Administration in 2000 for the treatment of North American crotalid envenomation.12,13 Its venom-specific fab fragments of IgG bind to and neutralize venom toxins and facilitate their elimination. Crotalidae polyvalent immune fab does not contain the Fc fragment of the IgG antibody, resulting in a low incidence of hypersensitivity reactions (8%) and serum sickness (13%).13 It is produced using 4 North American venoms: Crotalus atrox (western diamondback rattlesnake), Crotalus adamanteus (eastern diamondback rattlesnake), Crotalus scutulatus (Mojave rattlesnake), and Agkistrodon piscivorus (water moccasin).12 It has become the standard-of-care antivenom for pit viper bites and has replaced its equine-derived predecessor antivenin crotalidae polyvalent, which is known for high rates of acute allergic reactions (23%–56%) including anaphylaxis and delayed serum sickness.13,14 In postmarketing studies, CPIF has demonstrated control of envenomation regardless of severity. The most common adverse reactions reported include urticaria, rash, nausea, pruritus, and back pain.13 Anaphylaxis and anaphylactoid reactions may occur, and patients should be carefully observed during antivenom infusion. Appropriate management should be readily available including epinephrine, intravenous antihistamines, and/or albuterol. Contraindications include known hypersensitivity to papaya or papain, which is used to cleave the antibodies into fragments during the processing of CPIF. Patients also may react to it if allergic to other papaya extracts, chymopapain, or bromelain (pineapple enzyme), as well as some dust mite allergens and latex allergens that share antigenic structures with papain.15 Each vial of CPIF is reconstituted with 18 mL of 0.9% sodium chloride, as described in the package insert.13 The total dose (minimum of 4 to maximum of 12 vials initial dose) is then diluted in 250 mL of normal saline and infused over 1 hour starting for the first 10 minutes at a rate of 25 to 50 mL/h, and if tolerated, then increased to 250 mL/h until completion.
Treatment Algorithm
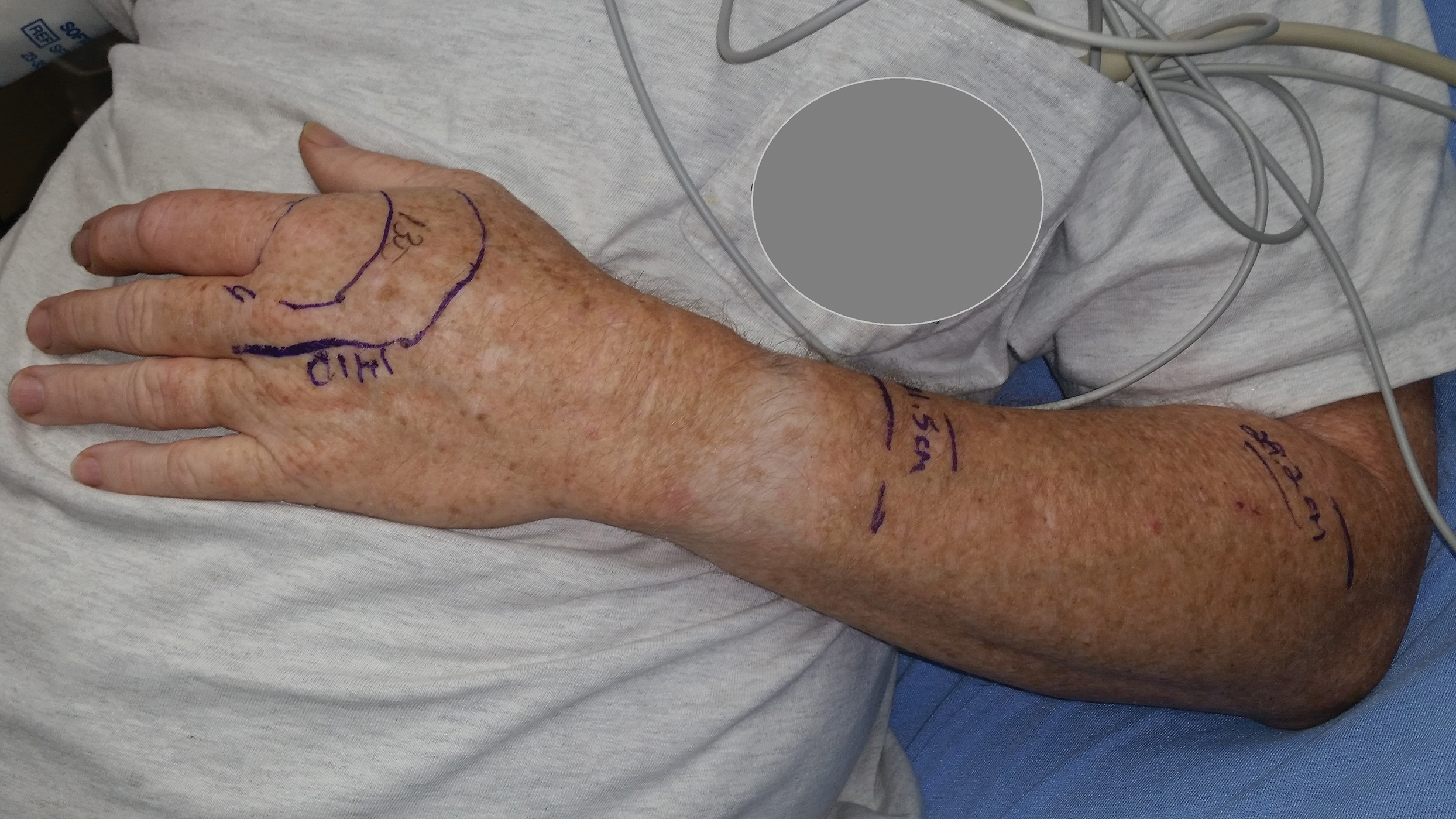
According to the envenomation consensus treatment algorithm, assess the site of the snakebite and mark leading edge of swelling every 15 to 30 minutes,16 as shown in our patient in Figure 6.Immobilize and elevate the affected extremity. Update tetanus vaccine and order initial laboratory tests to include prothrombin time, hemoglobin, platelets, and fibrinogen. If the patient does not exhibit local signs of envenomation such as redness, swelling, or ecchymosis, and the patient has no coagulation laboratory abnormalities and exhibits no systemic signs such as diarrhea, vomiting or angioedema, withhold CPIF and observe the patient for a minimum of 8 hours. Repeat laboratory tests prior to discharge. This clinical scenario most likely occurs in the setting of a dry bite or no bite at all. For minor envenomation, it also is possible to withhold CPIF and observe the patient for 12 to 24 hours if he/she remains stable and laboratory tests remain within reference range. If the patient has local or systemic signs of envenomation, start CPIF (4–6 vials and up to a maximum of 12 vials). The first dose of CPIF should be administered in the emergency department or intensive care unit. If after the first hour envenomation is worsening, an additional 4 to 6 vials may be infused. If after the first hour of observation the envenomation is controlled based on decreased swelling or lack of progression and there is improvement in laboratory values, then a maintenance regimen can be initiated. The maintenance dose consists of 2 vials every 6 hours for up to 18 hours (3 separate 2-vial doses). Patients can be discharged if stable and with no negative laboratory trends during the observation period.16 If CPIF was administered, follow-up laboratory tests results are dependent on prior findings of coagulation abnormalities, degree of envenomation, and signs and symptoms of coagulopathy postdischarge. Recurrent coagulopathy can occur in patients with coagulation abnormalities during initial envenomation, and patients should be monitored for possible re-treatment for at least 1 week or longer.17 Coagulopathy can present with decreased fibrinogen, decreased platelets, and elevated prothrombin time. Our patient experienced a drop in platelet count and hemoglobin level in addition to localized tissue effects, but he responded to the antivenom therapy. Lastly and importantly, no pediatric adjustments are necessary, and although mercury has been removed from the product’s manufacturing process, certain easily identifiable antivenom lots that have not expired contain ethyl mercury from thimerosal.13,18,19 Some side effects of thimerosal include redness and swelling of the injection site, but scientific research does not show a connection with autism.20
Conclusion
Dusky pigmy rattlesnake envenomations are clinically responsive to CPIF antivenom treatment.21
- The pigmy rattlesnake (Sistrurus miliarius). Stetson University website. http://www.stetson.edu/other/pigmy/pigmy-rattlesnake-information.php. Accessed October 18, 2019.
- Meadows A. Pigmy rattlesnake (Sistrurus miliarius)-Venomous. Savannah River Ecology Laboratory, University of Georgia website. https://srelherp.uga.edu/snakes/sismil.htm. Accessed October 21, 2019.
- Dusky pygmy rattlesnake. Central Florida Zoo & Botanical Gardens website. http://www.centralfloridazoo.org/animals/dusky-pygmy-rattlesnake/. Accessed October 21, 2019.
- Singha R. Facts about the pigmy rattlesnake that are sure to surprise you. AnimalSake website. https://animalsake.com/pygmy-rattlesnake. Updated August 1, 2017. Accessed October 21, 2019.
- Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1375.
- Scarborough RM, Rose JW, Hsu MA, et al. Barbourin. A spIIb-IIIa-specific integrin antagonist from the venom of Sistrurus m. barbouri. J Biol Chem. 1991;266:9359-9362.
- Johnson SA. Frequently asked questions about venomous snakes. UF Wildlife website. http://ufwildlife.ifas.ufl.edu/venomous_snake_faqs.shtml. Accessed October 18, 2019.
- Suchard JR, LoVecchio F. Envenomations by rattlesnakes thought to be dead. N Engl J Med. 1999;20:659-661.
- Wingert WA, Chan L. Rattlesnake bites in southern California and rationale for recommended treatment. West J Med. 1988;37:175-180.
- Kerrigan KR, Mertz BL, Nelson SJ, et al. Antibiotic prophylaxis for pit viper envenomation: prospective, controlled trial. World J Surg. 1997;21:369-373.
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586.
- Keating GM. Crotalidae polyvalent immune fab: in patients with North American crotaline envenomation. Bio Drugs. 2011;25:69-76.
- CroFab [prescribing information]. BTG International Inc; 2018.
- Consroe P, Egen NB, Russell FE, et al. Comparison of a new antigen binding fragment (FAB) antivenin for United States crotalidae with the commercial antivenin for protection against venom induced lethality in mice. Am J Trop Med Hyg. 1995;53:507-510.
- Quarre JP, Lecomte J, Lauwers D, et al. Allergy to latex and papain. J Allergy Clin Immunol. 1995;95:922.
- Lavonas EJ, Ruha AM, Banner W, et al. Unified treatment algorithm for the management of crotaline snakebite in the United States: results of an evidence-informed consensus workshop. BMC Emerg Med. 2011;11:2-15.
- Lavonas EJ, Khatri V, Daugherty C, et al. Medically significant late bleeding after treated crotaline envenomation: a systematic review. Ann Emerg Med. 2014;63:71-78.
- Pizon AF, Riley BD, LoVecchio F, et al. Safety and efficacy of crotalidae polyvalent immune fab in pediatric crotaline envenomations. Acad Emerg Med. 2007;14:373-376.
- Offerman SR, Bush SP, Moynihan JA, Clark RF. Crotaline fab antivenom for the treatment of children with rattlesnake envenomation. Pediatrics. 2002;110:968-971.
- Centers for Disease Control and Prevention. Thimerosal in vaccines. https://www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Updated October 25, 2015. Accessed October 21, 2019.
- King AM, Crim WS, Menke NB. Pigmy rattlesnake envenomation treated with crotalidae polyvalent immune fab antivenom. Toxicon. 2012;60:1287-1289.
Rattlesnakes are pit vipers with a rattle attached to the tip of the tail and facial pits located between the eyes and nose with a special organ that detects heat energy (infrared light) and is used for hunting prey. There are 2 genera of rattlesnakes, Sistrurus (3 species) and Crotalus (23 species). 1 The pigmy rattlesnake belongs to the Sistrurus miliarius species that is subdivided into 3 subspecies: the Carolina pigmy rattlesnake (Sistrurus miliarius miliarius), the Western pigmy rattlesnake (Sistrurus miliarius streckeri ), and the dusky pigmy rattlesnake (Sistrurus miliarius barbouri ). 1 The dusky pigmy rattlesnake is found in South Carolina, southern Georgia, southern Alabama, southeastern Mississippi, and Florida. 2 It is the most abundant venomous snake in Florida. 3 Its rattle is barely audible, and it is an aggressive small snake ranging in length from 38 to 56 cm. 4 Its venom is hemorrhagic, causing tissue damage but not containing neurotoxins. 4 Although bites can be painful, resulting in localized necrosis and rare loss of digits, it is unlikely for bites to be fatal given the snake’s small fangs, small size, and amount of envenomation. However, bites on children may require hospitalization. The venom contains proteins, polypeptides, and enzymes. 5 One such peptide, barbourin, inhibits a transmembrane receptor that plays a role in platelet aggregation. 6
We report a case of a 54-year-old man who was bitten on the left index finger by a dusky pigmy rattlesnake. We describe the clinical course and successful treatment with crotalidae polyvalent immune fab (CPIF) antivenom.
Case Report
A 54-year-old man presented to the emergency department with a rapidly swelling and erythematous left hand following a snakebite to the left index fingertip while weeding in his yard (Figure 1). The patient was able to kill the snake with a shovel and photograph it, which helped identify it as a dusky pigmy rattlesnake (Figure 2). Vitals on presentation included a blood pressure of 161/98, pulse oximeter of 99%, temperature of 36.4°C, pulse of 84 beats per minute, and respiratory rate of 16 breaths per minute.


Given the poisonous snakebite, the patient was admitted to the intensive care unit. Laboratory test results at admission revealed the following values: platelet count, 235,000/µL (reference range, 150,000–450,000/µL); fibrinogen, 226.1 mg/dL (reference range, 185–410 mg/dL); fibrin degradation products, less than 10 µg/mL (reference range, <10 µg/mL); glucose, 145 mg/dL (reference range, 74–106 mg/dL). The remainder of the complete blood cell count and metabolic panel was unremarkable. His blood type was O Rh+. Radiography of the left second digit did not show any fractures, dislocations, or foreign object.
After consulting with the Tampa General Hospital Florida Poison Information Center, 6 vials of CPIF antivenom in 250 mL of sodium chloride initially were infused intravenously, followed by 2 additional vials each at 6, 12, and 18 hours. Serial laboratory test results revealed white blood cell counts of 13,600, 10,000, 6800, 6100, and 6800/µL at 4, 15, 43, 65, and 88 hours postadmission, respectively. Platelet counts were 222,000, 159,000, 116,000, 99,000, and 129,000/µL at 4, 15, 43, 65, and 88 hours postadmission, respectively. The hemoglobin level was 14.8, 13.1, 13.8, 13.7, and 14.3 g/dL at 4, 15, 43, 65, and 88 hours postadmission, respectively. Other laboratory test results including prothrombin time (10.0 s), fibrinogen (226.1 mg/dL), and fibrin degradation products (<10 µg/mL) at 4 hours postadmission remained within reference range during serial monitoring.
The patient was hospitalized for 4 days until the erythema of the left arm receded and only involved the left second phalanx where he eventually experienced localized skin necrosis (Figures 3 and 4). His thrombocytopenia trended upward from a low of 99,000/µL to 121,000/µL at the time of discharge. During his hospital stay, the patient developed hypertension from which he remained asymptomatic and was treated with lisinopril. The patient was treated with intravenous cefazolin and discharged on oral cephalexin due to an elevation in his white blood cell count on admission (13,600/µL). His cultures remained negative, and on discharge his white blood cell count had normalized (6800/µL) and he was transitioned to oral antibiotics to complete his treatment course. Following a surgical consultation, it was decided that skin debridement of the localized area of necrosis of the index fingertip was not necessary. The area of skin necrosis sloughed uneventfully with no residual functional impairment; however, the patient was left with residual numbness of the left second digit (Figure 5). He did not experience recurrent coagulopathy.



Comment
Envenomation
Snakebites and envenomation are a complex and broad subject beyond the scope of this article and further reading on this subject is highly encouraged. The clinical findings from snakebites range from mild local tissue reactions to severe systemic symptoms depending on the volume of venom injected, snake species, age and health of victim, and location of bite. Severe systemic symptoms include disseminated intravascular coagulation, acute renal failure, hypovolemic shock, and death.5 Venom can be hemotoxic and/or neurotoxic. Hemotoxic symptoms include pain, edema, swelling, ecchymoses, necrosis, and hemolysis. Neurotoxic symptoms may encompass diplopia, dysphagia, sweating, salivation, diaphoresis, respiratory depression, and paralysis.5 The pigmy rattlesnake venom is only hemotoxic, not neurotoxic.4 Eastern and western variety rattlesnakes account for most snake deaths due to their potent venom. Water moccasins (cottonmouths) have intermediate-potency venom, and copperhead snakes have the least-potent venom.5 Coral snakes are not pit vipers and require a different antivenom.
Management of Snakebites
Venomous snakes may bite a person without injecting venom. In fact, as many as 20% to 25% of all pit viper bites are dry.7 No attempts should be made to capture or kill the biting snake, but identifying it safely is helpful. Dead snakes should not be handled carelessly, as reflex biting after death has been reported.8 Cutting or suctioning the bite wound, nonsteroidal anti-inflammatory drugs, prophylactic antibiotics, tourniquets, prophylactic fasciotomy, and ice have not proven to be beneficial in the management of viper envenomation.5,9-11 The best management involves immobilizing the affected extremity, seeking immediate medical attention, and initiating antivenom therapy as soon as possible.
Antivenom Therapy
Crotalidae polyvalent immune fab is an antivenom comprised of purified, sheep-derived fab IgG fragments and was approved by the US Food and Drug Administration in 2000 for the treatment of North American crotalid envenomation.12,13 Its venom-specific fab fragments of IgG bind to and neutralize venom toxins and facilitate their elimination. Crotalidae polyvalent immune fab does not contain the Fc fragment of the IgG antibody, resulting in a low incidence of hypersensitivity reactions (8%) and serum sickness (13%).13 It is produced using 4 North American venoms: Crotalus atrox (western diamondback rattlesnake), Crotalus adamanteus (eastern diamondback rattlesnake), Crotalus scutulatus (Mojave rattlesnake), and Agkistrodon piscivorus (water moccasin).12 It has become the standard-of-care antivenom for pit viper bites and has replaced its equine-derived predecessor antivenin crotalidae polyvalent, which is known for high rates of acute allergic reactions (23%–56%) including anaphylaxis and delayed serum sickness.13,14 In postmarketing studies, CPIF has demonstrated control of envenomation regardless of severity. The most common adverse reactions reported include urticaria, rash, nausea, pruritus, and back pain.13 Anaphylaxis and anaphylactoid reactions may occur, and patients should be carefully observed during antivenom infusion. Appropriate management should be readily available including epinephrine, intravenous antihistamines, and/or albuterol. Contraindications include known hypersensitivity to papaya or papain, which is used to cleave the antibodies into fragments during the processing of CPIF. Patients also may react to it if allergic to other papaya extracts, chymopapain, or bromelain (pineapple enzyme), as well as some dust mite allergens and latex allergens that share antigenic structures with papain.15 Each vial of CPIF is reconstituted with 18 mL of 0.9% sodium chloride, as described in the package insert.13 The total dose (minimum of 4 to maximum of 12 vials initial dose) is then diluted in 250 mL of normal saline and infused over 1 hour starting for the first 10 minutes at a rate of 25 to 50 mL/h, and if tolerated, then increased to 250 mL/h until completion.
Treatment Algorithm
According to the envenomation consensus treatment algorithm, assess the site of the snakebite and mark leading edge of swelling every 15 to 30 minutes,16 as shown in our patient in Figure 6.Immobilize and elevate the affected extremity. Update tetanus vaccine and order initial laboratory tests to include prothrombin time, hemoglobin, platelets, and fibrinogen. If the patient does not exhibit local signs of envenomation such as redness, swelling, or ecchymosis, and the patient has no coagulation laboratory abnormalities and exhibits no systemic signs such as diarrhea, vomiting or angioedema, withhold CPIF and observe the patient for a minimum of 8 hours. Repeat laboratory tests prior to discharge. This clinical scenario most likely occurs in the setting of a dry bite or no bite at all. For minor envenomation, it also is possible to withhold CPIF and observe the patient for 12 to 24 hours if he/she remains stable and laboratory tests remain within reference range. If the patient has local or systemic signs of envenomation, start CPIF (4–6 vials and up to a maximum of 12 vials). The first dose of CPIF should be administered in the emergency department or intensive care unit. If after the first hour envenomation is worsening, an additional 4 to 6 vials may be infused. If after the first hour of observation the envenomation is controlled based on decreased swelling or lack of progression and there is improvement in laboratory values, then a maintenance regimen can be initiated. The maintenance dose consists of 2 vials every 6 hours for up to 18 hours (3 separate 2-vial doses). Patients can be discharged if stable and with no negative laboratory trends during the observation period.16 If CPIF was administered, follow-up laboratory tests results are dependent on prior findings of coagulation abnormalities, degree of envenomation, and signs and symptoms of coagulopathy postdischarge. Recurrent coagulopathy can occur in patients with coagulation abnormalities during initial envenomation, and patients should be monitored for possible re-treatment for at least 1 week or longer.17 Coagulopathy can present with decreased fibrinogen, decreased platelets, and elevated prothrombin time. Our patient experienced a drop in platelet count and hemoglobin level in addition to localized tissue effects, but he responded to the antivenom therapy. Lastly and importantly, no pediatric adjustments are necessary, and although mercury has been removed from the product’s manufacturing process, certain easily identifiable antivenom lots that have not expired contain ethyl mercury from thimerosal.13,18,19 Some side effects of thimerosal include redness and swelling of the injection site, but scientific research does not show a connection with autism.20

Conclusion
Dusky pigmy rattlesnake envenomations are clinically responsive to CPIF antivenom treatment.21
Rattlesnakes are pit vipers with a rattle attached to the tip of the tail and facial pits located between the eyes and nose with a special organ that detects heat energy (infrared light) and is used for hunting prey. There are 2 genera of rattlesnakes, Sistrurus (3 species) and Crotalus (23 species). 1 The pigmy rattlesnake belongs to the Sistrurus miliarius species that is subdivided into 3 subspecies: the Carolina pigmy rattlesnake (Sistrurus miliarius miliarius), the Western pigmy rattlesnake (Sistrurus miliarius streckeri ), and the dusky pigmy rattlesnake (Sistrurus miliarius barbouri ). 1 The dusky pigmy rattlesnake is found in South Carolina, southern Georgia, southern Alabama, southeastern Mississippi, and Florida. 2 It is the most abundant venomous snake in Florida. 3 Its rattle is barely audible, and it is an aggressive small snake ranging in length from 38 to 56 cm. 4 Its venom is hemorrhagic, causing tissue damage but not containing neurotoxins. 4 Although bites can be painful, resulting in localized necrosis and rare loss of digits, it is unlikely for bites to be fatal given the snake’s small fangs, small size, and amount of envenomation. However, bites on children may require hospitalization. The venom contains proteins, polypeptides, and enzymes. 5 One such peptide, barbourin, inhibits a transmembrane receptor that plays a role in platelet aggregation. 6
We report a case of a 54-year-old man who was bitten on the left index finger by a dusky pigmy rattlesnake. We describe the clinical course and successful treatment with crotalidae polyvalent immune fab (CPIF) antivenom.
Case Report
A 54-year-old man presented to the emergency department with a rapidly swelling and erythematous left hand following a snakebite to the left index fingertip while weeding in his yard (Figure 1). The patient was able to kill the snake with a shovel and photograph it, which helped identify it as a dusky pigmy rattlesnake (Figure 2). Vitals on presentation included a blood pressure of 161/98, pulse oximeter of 99%, temperature of 36.4°C, pulse of 84 beats per minute, and respiratory rate of 16 breaths per minute.


Given the poisonous snakebite, the patient was admitted to the intensive care unit. Laboratory test results at admission revealed the following values: platelet count, 235,000/µL (reference range, 150,000–450,000/µL); fibrinogen, 226.1 mg/dL (reference range, 185–410 mg/dL); fibrin degradation products, less than 10 µg/mL (reference range, <10 µg/mL); glucose, 145 mg/dL (reference range, 74–106 mg/dL). The remainder of the complete blood cell count and metabolic panel was unremarkable. His blood type was O Rh+. Radiography of the left second digit did not show any fractures, dislocations, or foreign object.
After consulting with the Tampa General Hospital Florida Poison Information Center, 6 vials of CPIF antivenom in 250 mL of sodium chloride initially were infused intravenously, followed by 2 additional vials each at 6, 12, and 18 hours. Serial laboratory test results revealed white blood cell counts of 13,600, 10,000, 6800, 6100, and 6800/µL at 4, 15, 43, 65, and 88 hours postadmission, respectively. Platelet counts were 222,000, 159,000, 116,000, 99,000, and 129,000/µL at 4, 15, 43, 65, and 88 hours postadmission, respectively. The hemoglobin level was 14.8, 13.1, 13.8, 13.7, and 14.3 g/dL at 4, 15, 43, 65, and 88 hours postadmission, respectively. Other laboratory test results including prothrombin time (10.0 s), fibrinogen (226.1 mg/dL), and fibrin degradation products (<10 µg/mL) at 4 hours postadmission remained within reference range during serial monitoring.
The patient was hospitalized for 4 days until the erythema of the left arm receded and only involved the left second phalanx where he eventually experienced localized skin necrosis (Figures 3 and 4). His thrombocytopenia trended upward from a low of 99,000/µL to 121,000/µL at the time of discharge. During his hospital stay, the patient developed hypertension from which he remained asymptomatic and was treated with lisinopril. The patient was treated with intravenous cefazolin and discharged on oral cephalexin due to an elevation in his white blood cell count on admission (13,600/µL). His cultures remained negative, and on discharge his white blood cell count had normalized (6800/µL) and he was transitioned to oral antibiotics to complete his treatment course. Following a surgical consultation, it was decided that skin debridement of the localized area of necrosis of the index fingertip was not necessary. The area of skin necrosis sloughed uneventfully with no residual functional impairment; however, the patient was left with residual numbness of the left second digit (Figure 5). He did not experience recurrent coagulopathy.



Comment
Envenomation
Snakebites and envenomation are a complex and broad subject beyond the scope of this article and further reading on this subject is highly encouraged. The clinical findings from snakebites range from mild local tissue reactions to severe systemic symptoms depending on the volume of venom injected, snake species, age and health of victim, and location of bite. Severe systemic symptoms include disseminated intravascular coagulation, acute renal failure, hypovolemic shock, and death.5 Venom can be hemotoxic and/or neurotoxic. Hemotoxic symptoms include pain, edema, swelling, ecchymoses, necrosis, and hemolysis. Neurotoxic symptoms may encompass diplopia, dysphagia, sweating, salivation, diaphoresis, respiratory depression, and paralysis.5 The pigmy rattlesnake venom is only hemotoxic, not neurotoxic.4 Eastern and western variety rattlesnakes account for most snake deaths due to their potent venom. Water moccasins (cottonmouths) have intermediate-potency venom, and copperhead snakes have the least-potent venom.5 Coral snakes are not pit vipers and require a different antivenom.
Management of Snakebites
Venomous snakes may bite a person without injecting venom. In fact, as many as 20% to 25% of all pit viper bites are dry.7 No attempts should be made to capture or kill the biting snake, but identifying it safely is helpful. Dead snakes should not be handled carelessly, as reflex biting after death has been reported.8 Cutting or suctioning the bite wound, nonsteroidal anti-inflammatory drugs, prophylactic antibiotics, tourniquets, prophylactic fasciotomy, and ice have not proven to be beneficial in the management of viper envenomation.5,9-11 The best management involves immobilizing the affected extremity, seeking immediate medical attention, and initiating antivenom therapy as soon as possible.
Antivenom Therapy
Crotalidae polyvalent immune fab is an antivenom comprised of purified, sheep-derived fab IgG fragments and was approved by the US Food and Drug Administration in 2000 for the treatment of North American crotalid envenomation.12,13 Its venom-specific fab fragments of IgG bind to and neutralize venom toxins and facilitate their elimination. Crotalidae polyvalent immune fab does not contain the Fc fragment of the IgG antibody, resulting in a low incidence of hypersensitivity reactions (8%) and serum sickness (13%).13 It is produced using 4 North American venoms: Crotalus atrox (western diamondback rattlesnake), Crotalus adamanteus (eastern diamondback rattlesnake), Crotalus scutulatus (Mojave rattlesnake), and Agkistrodon piscivorus (water moccasin).12 It has become the standard-of-care antivenom for pit viper bites and has replaced its equine-derived predecessor antivenin crotalidae polyvalent, which is known for high rates of acute allergic reactions (23%–56%) including anaphylaxis and delayed serum sickness.13,14 In postmarketing studies, CPIF has demonstrated control of envenomation regardless of severity. The most common adverse reactions reported include urticaria, rash, nausea, pruritus, and back pain.13 Anaphylaxis and anaphylactoid reactions may occur, and patients should be carefully observed during antivenom infusion. Appropriate management should be readily available including epinephrine, intravenous antihistamines, and/or albuterol. Contraindications include known hypersensitivity to papaya or papain, which is used to cleave the antibodies into fragments during the processing of CPIF. Patients also may react to it if allergic to other papaya extracts, chymopapain, or bromelain (pineapple enzyme), as well as some dust mite allergens and latex allergens that share antigenic structures with papain.15 Each vial of CPIF is reconstituted with 18 mL of 0.9% sodium chloride, as described in the package insert.13 The total dose (minimum of 4 to maximum of 12 vials initial dose) is then diluted in 250 mL of normal saline and infused over 1 hour starting for the first 10 minutes at a rate of 25 to 50 mL/h, and if tolerated, then increased to 250 mL/h until completion.
Treatment Algorithm
According to the envenomation consensus treatment algorithm, assess the site of the snakebite and mark leading edge of swelling every 15 to 30 minutes,16 as shown in our patient in Figure 6.Immobilize and elevate the affected extremity. Update tetanus vaccine and order initial laboratory tests to include prothrombin time, hemoglobin, platelets, and fibrinogen. If the patient does not exhibit local signs of envenomation such as redness, swelling, or ecchymosis, and the patient has no coagulation laboratory abnormalities and exhibits no systemic signs such as diarrhea, vomiting or angioedema, withhold CPIF and observe the patient for a minimum of 8 hours. Repeat laboratory tests prior to discharge. This clinical scenario most likely occurs in the setting of a dry bite or no bite at all. For minor envenomation, it also is possible to withhold CPIF and observe the patient for 12 to 24 hours if he/she remains stable and laboratory tests remain within reference range. If the patient has local or systemic signs of envenomation, start CPIF (4–6 vials and up to a maximum of 12 vials). The first dose of CPIF should be administered in the emergency department or intensive care unit. If after the first hour envenomation is worsening, an additional 4 to 6 vials may be infused. If after the first hour of observation the envenomation is controlled based on decreased swelling or lack of progression and there is improvement in laboratory values, then a maintenance regimen can be initiated. The maintenance dose consists of 2 vials every 6 hours for up to 18 hours (3 separate 2-vial doses). Patients can be discharged if stable and with no negative laboratory trends during the observation period.16 If CPIF was administered, follow-up laboratory tests results are dependent on prior findings of coagulation abnormalities, degree of envenomation, and signs and symptoms of coagulopathy postdischarge. Recurrent coagulopathy can occur in patients with coagulation abnormalities during initial envenomation, and patients should be monitored for possible re-treatment for at least 1 week or longer.17 Coagulopathy can present with decreased fibrinogen, decreased platelets, and elevated prothrombin time. Our patient experienced a drop in platelet count and hemoglobin level in addition to localized tissue effects, but he responded to the antivenom therapy. Lastly and importantly, no pediatric adjustments are necessary, and although mercury has been removed from the product’s manufacturing process, certain easily identifiable antivenom lots that have not expired contain ethyl mercury from thimerosal.13,18,19 Some side effects of thimerosal include redness and swelling of the injection site, but scientific research does not show a connection with autism.20

Conclusion
Dusky pigmy rattlesnake envenomations are clinically responsive to CPIF antivenom treatment.21
- The pigmy rattlesnake (Sistrurus miliarius). Stetson University website. http://www.stetson.edu/other/pigmy/pigmy-rattlesnake-information.php. Accessed October 18, 2019.
- Meadows A. Pigmy rattlesnake (Sistrurus miliarius)-Venomous. Savannah River Ecology Laboratory, University of Georgia website. https://srelherp.uga.edu/snakes/sismil.htm. Accessed October 21, 2019.
- Dusky pygmy rattlesnake. Central Florida Zoo & Botanical Gardens website. http://www.centralfloridazoo.org/animals/dusky-pygmy-rattlesnake/. Accessed October 21, 2019.
- Singha R. Facts about the pigmy rattlesnake that are sure to surprise you. AnimalSake website. https://animalsake.com/pygmy-rattlesnake. Updated August 1, 2017. Accessed October 21, 2019.
- Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1375.
- Scarborough RM, Rose JW, Hsu MA, et al. Barbourin. A spIIb-IIIa-specific integrin antagonist from the venom of Sistrurus m. barbouri. J Biol Chem. 1991;266:9359-9362.
- Johnson SA. Frequently asked questions about venomous snakes. UF Wildlife website. http://ufwildlife.ifas.ufl.edu/venomous_snake_faqs.shtml. Accessed October 18, 2019.
- Suchard JR, LoVecchio F. Envenomations by rattlesnakes thought to be dead. N Engl J Med. 1999;20:659-661.
- Wingert WA, Chan L. Rattlesnake bites in southern California and rationale for recommended treatment. West J Med. 1988;37:175-180.
- Kerrigan KR, Mertz BL, Nelson SJ, et al. Antibiotic prophylaxis for pit viper envenomation: prospective, controlled trial. World J Surg. 1997;21:369-373.
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586.
- Keating GM. Crotalidae polyvalent immune fab: in patients with North American crotaline envenomation. Bio Drugs. 2011;25:69-76.
- CroFab [prescribing information]. BTG International Inc; 2018.
- Consroe P, Egen NB, Russell FE, et al. Comparison of a new antigen binding fragment (FAB) antivenin for United States crotalidae with the commercial antivenin for protection against venom induced lethality in mice. Am J Trop Med Hyg. 1995;53:507-510.
- Quarre JP, Lecomte J, Lauwers D, et al. Allergy to latex and papain. J Allergy Clin Immunol. 1995;95:922.
- Lavonas EJ, Ruha AM, Banner W, et al. Unified treatment algorithm for the management of crotaline snakebite in the United States: results of an evidence-informed consensus workshop. BMC Emerg Med. 2011;11:2-15.
- Lavonas EJ, Khatri V, Daugherty C, et al. Medically significant late bleeding after treated crotaline envenomation: a systematic review. Ann Emerg Med. 2014;63:71-78.
- Pizon AF, Riley BD, LoVecchio F, et al. Safety and efficacy of crotalidae polyvalent immune fab in pediatric crotaline envenomations. Acad Emerg Med. 2007;14:373-376.
- Offerman SR, Bush SP, Moynihan JA, Clark RF. Crotaline fab antivenom for the treatment of children with rattlesnake envenomation. Pediatrics. 2002;110:968-971.
- Centers for Disease Control and Prevention. Thimerosal in vaccines. https://www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Updated October 25, 2015. Accessed October 21, 2019.
- King AM, Crim WS, Menke NB. Pigmy rattlesnake envenomation treated with crotalidae polyvalent immune fab antivenom. Toxicon. 2012;60:1287-1289.
- The pigmy rattlesnake (Sistrurus miliarius). Stetson University website. http://www.stetson.edu/other/pigmy/pigmy-rattlesnake-information.php. Accessed October 18, 2019.
- Meadows A. Pigmy rattlesnake (Sistrurus miliarius)-Venomous. Savannah River Ecology Laboratory, University of Georgia website. https://srelherp.uga.edu/snakes/sismil.htm. Accessed October 21, 2019.
- Dusky pygmy rattlesnake. Central Florida Zoo & Botanical Gardens website. http://www.centralfloridazoo.org/animals/dusky-pygmy-rattlesnake/. Accessed October 21, 2019.
- Singha R. Facts about the pigmy rattlesnake that are sure to surprise you. AnimalSake website. https://animalsake.com/pygmy-rattlesnake. Updated August 1, 2017. Accessed October 21, 2019.
- Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1375.
- Scarborough RM, Rose JW, Hsu MA, et al. Barbourin. A spIIb-IIIa-specific integrin antagonist from the venom of Sistrurus m. barbouri. J Biol Chem. 1991;266:9359-9362.
- Johnson SA. Frequently asked questions about venomous snakes. UF Wildlife website. http://ufwildlife.ifas.ufl.edu/venomous_snake_faqs.shtml. Accessed October 18, 2019.
- Suchard JR, LoVecchio F. Envenomations by rattlesnakes thought to be dead. N Engl J Med. 1999;20:659-661.
- Wingert WA, Chan L. Rattlesnake bites in southern California and rationale for recommended treatment. West J Med. 1988;37:175-180.
- Kerrigan KR, Mertz BL, Nelson SJ, et al. Antibiotic prophylaxis for pit viper envenomation: prospective, controlled trial. World J Surg. 1997;21:369-373.
- Clark RF, Selden BS, Furbee B. The incidence of wound infection following crotalid envenomation. J Emerg Med. 1993;11:583-586.
- Keating GM. Crotalidae polyvalent immune fab: in patients with North American crotaline envenomation. Bio Drugs. 2011;25:69-76.
- CroFab [prescribing information]. BTG International Inc; 2018.
- Consroe P, Egen NB, Russell FE, et al. Comparison of a new antigen binding fragment (FAB) antivenin for United States crotalidae with the commercial antivenin for protection against venom induced lethality in mice. Am J Trop Med Hyg. 1995;53:507-510.
- Quarre JP, Lecomte J, Lauwers D, et al. Allergy to latex and papain. J Allergy Clin Immunol. 1995;95:922.
- Lavonas EJ, Ruha AM, Banner W, et al. Unified treatment algorithm for the management of crotaline snakebite in the United States: results of an evidence-informed consensus workshop. BMC Emerg Med. 2011;11:2-15.
- Lavonas EJ, Khatri V, Daugherty C, et al. Medically significant late bleeding after treated crotaline envenomation: a systematic review. Ann Emerg Med. 2014;63:71-78.
- Pizon AF, Riley BD, LoVecchio F, et al. Safety and efficacy of crotalidae polyvalent immune fab in pediatric crotaline envenomations. Acad Emerg Med. 2007;14:373-376.
- Offerman SR, Bush SP, Moynihan JA, Clark RF. Crotaline fab antivenom for the treatment of children with rattlesnake envenomation. Pediatrics. 2002;110:968-971.
- Centers for Disease Control and Prevention. Thimerosal in vaccines. https://www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Updated October 25, 2015. Accessed October 21, 2019.
- King AM, Crim WS, Menke NB. Pigmy rattlesnake envenomation treated with crotalidae polyvalent immune fab antivenom. Toxicon. 2012;60:1287-1289.
Practice Points
- Avoid icing, cutting, and suctioning a snakebite wound or using tourniquets.
- Immobilize and elevate the affected extremity and seek medical attention immediately for early initiation of antivenom treatment.
- Remove rings or constrictive items in the event of swelling.